WO2011096349A1 - フラーレン誘導体とその製造方法、並びにこれを用いたアレルゲン吸着剤 - Google Patents
フラーレン誘導体とその製造方法、並びにこれを用いたアレルゲン吸着剤 Download PDFInfo
- Publication number
- WO2011096349A1 WO2011096349A1 PCT/JP2011/051864 JP2011051864W WO2011096349A1 WO 2011096349 A1 WO2011096349 A1 WO 2011096349A1 JP 2011051864 W JP2011051864 W JP 2011051864W WO 2011096349 A1 WO2011096349 A1 WO 2011096349A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- fullerene
- halogen group
- fullerene derivative
- derivative according
- hydroxylated
- Prior art date
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/22—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising organic material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y40/00—Manufacture or treatment of nanostructures
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/15—Nano-sized carbon materials
- C01B32/152—Fullerenes
- C01B32/156—After-treatment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C17/00—Preparation of halogenated hydrocarbons
- C07C17/013—Preparation of halogenated hydrocarbons by addition of halogens
- C07C17/02—Preparation of halogenated hydrocarbons by addition of halogens to unsaturated hydrocarbons
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C25/00—Compounds containing at least one halogen atom bound to a six-membered aromatic ring
- C07C25/18—Polycyclic aromatic halogenated hydrocarbons
- C07C25/22—Polycyclic aromatic halogenated hydrocarbons with condensed rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C29/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring
- C07C29/09—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by hydrolysis
- C07C29/12—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by hydrolysis of esters of mineral acids
- C07C29/124—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by hydrolysis of esters of mineral acids of halides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C29/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring
- C07C29/48—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by oxidation reactions with formation of hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C29/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring
- C07C29/62—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by introduction of halogen; by substitution of halogen atoms by other halogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C35/00—Compounds having at least one hydroxy or O-metal group bound to a carbon atom of a ring other than a six-membered aromatic ring
- C07C35/48—Halogenated derivatives
- C07C35/52—Alcohols with a condensed ring system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C2604/00—Fullerenes, e.g. C60 buckminsterfullerene or C70
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/70—Nanostructure
- Y10S977/734—Fullerenes, i.e. graphene-based structures, such as nanohorns, nanococoons, nanoscrolls or fullerene-like structures, e.g. WS2 or MoS2 chalcogenide nanotubes, planar C3N4, etc.
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/70—Nanostructure
- Y10S977/734—Fullerenes, i.e. graphene-based structures, such as nanohorns, nanococoons, nanoscrolls or fullerene-like structures, e.g. WS2 or MoS2 chalcogenide nanotubes, planar C3N4, etc.
- Y10S977/735—Carbon buckyball
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/70—Nanostructure
- Y10S977/734—Fullerenes, i.e. graphene-based structures, such as nanohorns, nanococoons, nanoscrolls or fullerene-like structures, e.g. WS2 or MoS2 chalcogenide nanotubes, planar C3N4, etc.
- Y10S977/735—Carbon buckyball
- Y10S977/736—Carbon buckyball having atoms interior to the carbon cage
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/70—Nanostructure
- Y10S977/734—Fullerenes, i.e. graphene-based structures, such as nanohorns, nanococoons, nanoscrolls or fullerene-like structures, e.g. WS2 or MoS2 chalcogenide nanotubes, planar C3N4, etc.
- Y10S977/735—Carbon buckyball
- Y10S977/737—Carbon buckyball having a modified surface
- Y10S977/74—Modified with atoms or molecules bonded to the surface
Definitions
- the present invention relates to a fullerene derivative that rapidly adsorbs an allergen and a method for producing the same.
- Patent Document 1 proposes that tea polyphenols inactivate allergenic activity of allergen substances.
- Patent Document 2 discloses that an inorganic fine particle such as silica or titanium oxide is impregnated and supported on a nonwoven fabric to adsorb an allergen.
- Patent Document 3 proposes a pollen adsorbent using a polymer fiber having a positively charged functional group such as a quaternary ammonium salt.
- the present invention provides an adsorbent that does not efficiently adsorb and re-release allergens that cause hay fever in a short time and does not contain metals that adversely affect the human body.
- the purpose is to do.
- the present invention aims to provide a method for producing such an adsorbent or fullerene derivative.
- the present invention has achieved the above object with a fullerene derivative characterized by having both a hydroxyl group and a halogen group.
- the present invention is a fullerene derivative in which both a hydroxyl group and a halogen group are directly bonded to a fullerene nucleus, and has a general formula CpXn (OH) m (p is an even number of 60 or more, X is a halogen group, and n is greater than 0 (
- the above-mentioned object was achieved by a fullerene derivative characterized by being represented by a number of 48 or less (not including 0) and m being a number of 44 or less greater than 0).
- the halogen group is not particularly limited, but is preferably chlorine, bromine or fluorine.
- the present invention also provides a halogenated fullerene in which a halogen group is bonded to a fullerene nucleus, and a hydroxyl group is bonded to the fullerene nucleus while leaving a part of the halogen group, thereby producing a partially halogenated hydroxylated fullerene.
- the above object was achieved by a method for producing a fullerene derivative characterized by the following.
- chlorinated fullerene in which the halogen group is chlorine, it can be reacted with hydrogen peroxide, sodium hydroxide or potassium hydroxide to produce partially chlorinated fullerene hydroxide.
- a partially brominated fullerene hydroxide can be produced by reacting brominated fullerenes whose halogen group is bromine with sodium hydroxide or potassium hydroxide.
- the present invention provides that a partially halogenated hydroxylated fullerene is produced by bonding a fullerene hydroxide having a hydroxyl group bonded to a fullerene nucleus to a halogen group bonded to the fullerene nucleus while leaving a part of the hydroxyl groups.
- the above object has been achieved by the production method of the characteristic fullerene derivative.
- the present invention reacts a fullerene hydroxide having a hydroxyl group bonded to a fullerene nucleus with iodine chloride to leave a partial hydroxyl group to bond chlorine to the fullerene nucleus, thereby partially chlorinated hydroxylating.
- an allergen adsorbent containing the above-mentioned fullerene derivative is provided.
- the fullerene derivative of the present invention has a hydroxyl group and a halogen group directly bonded to the fullerene nucleus, and does not contain a metal that adversely affects the human body.
- the fullerene derivative of the present invention can efficiently adsorb allergens causing hay fever in a short time, and does not re-release the adsorbed allergens once again. That is, proteins such as allergens have many amino groups and carboxyl groups, so they easily interact chemically with functional groups with high polarizability such as hydroxyl groups and halogen groups. Can be adsorbed.
- a protein such as an allergen has a hydrophilic functional group. Therefore, it is considered necessary for the fullerene to have a hydrophilic surface.
- the fullerene derivative to which only is bonded was examined, but sufficient results were not obtained.
- the fullerene derivative having both a hydroxyl group and a halogen group of the present invention sufficient results have been obtained with respect to allergen adsorption.
- the surface area can be increased and it is suitable as an adsorbent. That is, if the weight of the adsorbent is the same, the particle size and surface area of the adsorbent are in an inversely proportional relationship, and the surface area of adsorption increases as the particle size decreases, so the fullerene derivative of the present invention is suitable as an adsorbent. ing.
- the fullerene derivative of the present invention has both a hydroxyl group and a halogen group, and has an amphiphilic property having both hydrophilicity and hydrophobicity. For this reason, the fullerene derivative of the present invention can be applied, impregnated, or chemically bonded to the surface of various materials.
- the fullerene derivative of the present invention can be used as an adsorbent for allergens that cause pollenosis (in the case of cedar pollen, the protein Cry j 1 ⁇ is the main substance) and can be used as a mask for removing pollen and for air cleaner filters. Can be applied.
- a novel fullerene derivative having both a large number of hydroxyl groups and halogen groups can be produced by a relatively simple method. That is, it can be synthesized by partial hydroxylation of a halogenated fullerene or partial halogenation of a fullerene hydroxide.
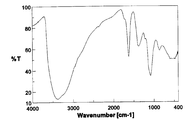
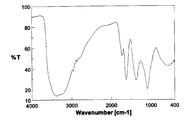
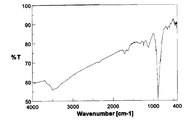
- FIG. 4 is an FT-IR spectrum diagram of partially chlorinated hydroxylated fullerene C 60 Cl 10 (OH) 30 ⁇ 5H 2 O. It is a FT-IR spectrum of the partially chlorinated fullerene C 60 Cl 2 (OH) 38 ⁇ 6H 2 O. It is a FT-IR spectrum of the hydroxylated fullerene C 60 (OH) 12 ⁇ 5H 2 O.
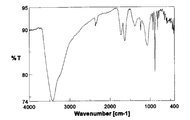
- FIG. 4 is an FT-IR spectrum diagram of chlorinated fullerene C 60 Cl 8 . Partially chlorinated fullerene C 60 Cl 0. Is 5 (OH) 35. 5 ⁇ 8H 2 FT-IR spectrum of the O.
- FIG. 4 is an FT-IR spectrum diagram of chlorinated fullerene C 60 Cl 28 . It is a FT-IR spectrum of the partially chlorinated fullerene C 60 Cl 3 (OH) 25 ⁇ 6H 2 O.
- the novel fullerene derivative according to the present invention has a halogen group as well as a hydroxyl group in the fullerene nucleus.
- fullerene represented by the general formula CpXn (OH) m (p is an even number of 60 or more, X is a halogen group, n is a number of 48 or less greater than 0, and m is a number of 44 or less greater than 0). Is a derivative.
- the fullerene derivative of the present invention may or may not contain secondary bond water.
- secondary bond water When the number of hydroxyl groups is small, secondary bond water is not included, and as the number increases, secondary bond water tends to be included.
- the fullerene used in the production of the fullerene derivative of the present invention is not particularly limited as long as it is a spherical carbon molecule, but preferably C 60 , C 70, or C 60 and C 70 or higher fullerenes (for example, C 76 , C 70 78 , C80 , C84 , C86, etc.).
- fullerene examples are C 60, not only the fullerene C 60, mixed fullerene chemical and physical properties including or similar fullerene C 70 or C 60, (C 60, C 70, a mixture of higher fullerenes )
- a compound having a similar structure and similar properties can be obtained.
- the halogen group (X) in the present invention is preferably fluorine (F), chlorine (Cl) and bromine (Br) among monovalent elements belonging to Group 7B of the periodic table.
- the fullerene derivative of the present invention is produced by partial hydroxylation or partial halogenation by hydroxylation (or hydrolysis) of a halogenated fullerene or halogenation of a fullerene hydroxide (or halogen group substitution reaction).
- substitution reaction is carried out, it is carried out only to a moderate degree of progress (ie partial hydroxylation or partial halogenation).
- the reaction may theoretically be performed until the reaction is completed.
- hydroxylation of the halogenated fullerene is mainly carried out by a substitution reaction, the reaction is allowed to proceed only until a part of the halogen groups remain (can be controlled by reaction conditions, time, reagent equivalents, etc.).
- a hydroxyl group addition reaction may be carried out at the same time or may be contained as secondary bond water.
- Halogenation of hydroxylated fullerene is mainly carried out by an addition reaction. According to the examples, when the number of hydroxyl groups in the starting fullerene hydroxide is large, the number of hydroxyl groups in the product after the reaction is reduced. It is thought that the introduction of the group was performed by a substitution reaction.
- halogenated fullerene or hydroxylated fullerene that is the starting material for the fullerene derivative of the present invention is already known.
- Non-Patent Document 1 J. Am. Chem. Soc., 1991, 113, 9900
- Non-Patent Document 2 J. Chem. Soc., Chem. Commun., 1993, 1230
- Patent Document 3 Eur. J. Org. Chem., 2005, 4951
- Patent Document 4 Japanese Patent Application Laid-Open No.
- Non-Patent Document 4 discloses a method for producing fluorinated fullerene C 60 F 48 .
- Non-Patent Document 4 Angew. Chem. Int. Ed. 2001, 40, 2285
- Non-Patent Document 5 discloses a method for producing brominated fullerene C 60 Br 16 .
- Non-Patent Document 5 Science, 1992, 256, 822
- the manufacturing method of the fullerene derivative of this invention the halogenated fullerene used as a starting material may be manufactured by what kind of method.
- Hydroxylene fullerene C 60 (OH) m is obtained by hydroxylating fullerene C 60 as a starting material, and its production method is already known.
- Patent Document 5 Japanese Patent Application Laid-Open No. 7-48302
- Patent Document 6 International Publication WO 2008/096763
- Non-Patent Document 6 J. Org. Chem., 1994, 59, 3960
- Non-Patent Document 7 Synth. Commun., 2005, 35, 1803
- Non-Patent Document 8 ACS Nano, 2008, 2, 327 It can manufacture by the method currently disclosed by these.
- the fullerene hydroxide used as a starting material may be produced by any method.
- Non-Patent Document 9 Fullerenes, Nanotubes, and Carbon Nanostructures, 2005, 13, 331
- halogenated fullerenes and hydroxylated fullerenes are conventionally known.
- partially halogenated hydroxylated fullerene derivatives in which a halogen group and a hydroxyl group coexist are not known so far, such as the fullerene derivatives of the present invention. .
- the fullerene derivative of the present invention can be obtained by partial hydroxylation of a chlorinated fullerene after the fullerene is converted into a chlorinated fullerene or a hydroxylated fullerene or by using a known chlorinated fullerene or a hydroxylated fullerene. It can be synthesized by partial chlorination (method B) of (method A) or fullerene hydroxide. For example, when the fullerene is C 60 , the following formula 1 is obtained.
- Partial hydroxylation methods include a general hydrolysis reaction using a base catalyst such as sodium hydroxide and potassium hydroxide, and a hydroxylation reaction using a hydrogen peroxide solution, but these methods are particularly limited. is not.
- hydroxylating reagent for partial hydroxylation in addition to sodium hydroxide and potassium hydroxide, LiOH, RbOH, CsOH, Ca (OH) 2 , Sr (OH) 2 , Ba (OH) 2 , TlOH , NBuN (OH), Triton B, and the like.
- the number m of hydroxyl groups introduced is the same as the number m 'of substituents in the starting fullerene hydroxide, or is reduced by a substitution reaction with chlorine, or It may be increased by an operation during the reaction process.
- chlorination reaction using iodine chloride (ICl) is shown in the examples as the method of partial chlorination, it is not particularly limited to this reagent.
- ICl iodine chloride
- POCl 3 , PCl 5 , SbCl 5 , VCl 4 , VOCl 3 , MoCl 5 , KICl 4 and the like can be mentioned.
- Solvents usable in the production of the starting material (halogenated fullerene or hydroxylated fullerene) of the present invention hydroxylation of halogenated fullerene or halogenation of hydroxylated fullerene include, for example, o-dichlorobenzene, chlorobenzene, trimethylbenzene, xylene , Aromatic solvents such as toluene and benzene, Halogen solvents such as methylene chloride, chloroform, carbon tetrachloride, dichloroethane, tetrachloroethane, Aprotic polar solvents such as THF, ether, ethyl acetate, dioxane, DMF, DMSO, Other examples include carbon disulfide and acetonitrile.
- n and m are at least larger than 0, and n is smaller than the maximum number 30 known in C 60 Cln ′ (see Non-Patent Document 3 above).
- M is smaller than the maximum number 44 known in C 60 (OH) m ′ (see the aforementioned Patent Document 5).
- native to one type of isomer may be sufficient, and the average number of many isomer mixtures may be sufficient.
- the position of introduction of these substituents on the surface of the fullerene nucleus is not particularly specified.
- brominated fullerene or fluorinated fullerene is subjected to a general hydrolysis reaction using a base catalyst such as sodium hydroxide or potassium hydroxide, hydrogen peroxide Partial hydroxylation may be performed by a hydroxylation reaction using water.
- the number n of bromine or fluorine substituents in the synthesized fullerene derivative is the same as the number of substituents n ′ in the starting material, or is reduced by a substitution reaction with a hydroxyl group. Since C 60 F 48 is known as described in Non-patent Document 4 and Patent Document 4 as a brominated fullerene or fluorinated fullerene as a starting material having a large number of substituents, the fullerene of the present invention The number n of halogen substituents in the derivative is 48 or less at the maximum.
- Synthesis methods of the present invention is not limited to the fullerene C 60, chemical and physical properties and similar fullerene C 70, or C 60 mixed fullerenes containing (C 60, C 70, a mixture of higher fullerenes) starting material It is considered that a compound having a similar structure and similar properties can be obtained.
- the aqueous layer after removal of the toluene layer was added dropwise to 85 mL of a solution in which hexane, diethyl ether and 2-propanol were mixed at a ratio of 5: 5: 7, respectively, while irradiating with ultrasonic waves to precipitate a pale yellow solid.
- the resulting precipitate was allowed to settle by centrifugation, and then the supernatant was removed by decantation.
- This solid was washed with 60 mL of diethyl ether and re-precipitated, and then the supernatant was removed and dried in vacuo at room temperature overnight.
- the reaction crude product, fullerene hydroxide was obtained as a pale yellow powder.
- this solid was dissolved in 3 mL of water, and passed through column chromatography packed with a length of about 6 cm with about 1 g of Florisil (60-100 mesh).
- the aqueous solution after removing the catalyst was passed through a 0.45 ⁇ m membrane filter to completely remove Florisil. Hexane, diethyl ether and 2-propanol were added to this aqueous solution at a ratio of 5: 5: 7 with respect to the volume of water to precipitate a pale yellow solid.
- This solid was vacuum-dried overnight at room temperature to obtain a purified product, fullerene hydroxide C 60 (OH) 4 4H 2 O, as a pale yellow powder (yield 149 mg, 67%).
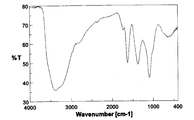
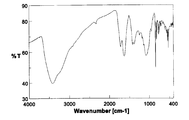
- the infrared absorption (IR) spectrum of the obtained product is shown in FIG.
- the infrared absorption (IR) spectrum of the product thus obtained is shown in FIG.
- the IR spectrum shown in FIG. 2 is slightly different from the spectrum of fullerene hydroxide C 60 (OH) 4 4H 2 O used as a starting material (FIG. 1), suggesting that the reaction has progressed, (with large broad absorption around 3400 cm -1 based on O-H stretching of the hydroxyl group, broad absorption around 1620,1380,1080Cm -1 based on C-C and C-O stretch) characteristic of the spectrum of oxidized fullerenes I left it. Further, in the thermogravimetric analysis of this product, a weight reduction of 5.0 wt% was observed while heating from room temperature to around 100 ° C.
- This weight loss was estimated as the amount of secondary bound water contained in the product.
- the elemental analysis values were C; 41.87%, H; 3.00%, Cl; 21.84%.
- the IR spectrum of the product thus obtained is shown in FIG.
- the IR spectrum shown in FIG. 3 is slightly different from the IR spectrum shown in FIG. 1 of the fullerene hydroxide C 60 (OH) 44 ⁇ 8H 2 O used as the starting material, suggesting that the reaction has proceeded, The spectral characteristics of the fullerene hydroxide remained. Further, according to the thermogravimetric analysis of this product, a weight loss of 7.4 wt% was observed during heating from room temperature to around 120 ° C. This weight loss was estimated as the amount of secondary bound water contained in the product.
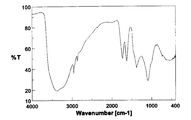
- the IR spectrum of the product is shown in FIG. IR spectrum in FIG. 6, the spectrum of the hydroxylated fullerene C 60 (OH) 12 ⁇ 5H 2 O was used as the starting material is different (see FIG. 4) and is slightly with it suggests that the reaction proceeded, water The spectral characteristics of fullerene oxide were retained. Moreover, according to the thermogravimetric analysis of the product, a weight loss of 7.9 wt% was observed during heating from room temperature to around 110 ° C. This weight loss was estimated as the amount of secondary bound water contained in the product.
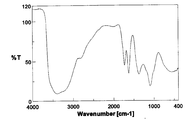
- the IR spectrum of this product is shown in FIG.
- the IR spectrum of FIG. 8 is greatly different from the IR spectrum of chlorinated fullerene C 60 Cl 8 used as the starting material (see FIG. 7), suggesting that the reaction has progressed, and also the characteristics of the spectrum of fullerene hydroxide. Was leaving. Further, in the thermogravimetric analysis, a weight loss of 9.8 wt% was observed during heating from room temperature to around 100 ° C. This weight loss was estimated as the amount of secondary bound water contained in the product. Elemental value analysis C; 48.29%, H; 3.10 %, Cl; becomes 1.06%, C 60 Cl 0 5 (OH) 35 5 ⁇ 8H 2 O Calculated (C;.. 48 .49%, H; 3.49%, Cl; 1.19%, water; 9.7 wt%).
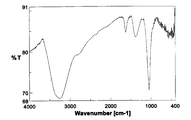
- the IR spectrum of this product is shown in FIG.
- the IR spectrum in FIG. 9 shows a broad C—Cl stretching vibration at 883 cm ⁇ 1 , which is very similar to the spectrum of chlorinated fullerene C 60 Cl 28 described in Non-Patent Document 3.
- the elemental analysis values were C; 40.96%, Cl; 58.28%, which was in good agreement with the calculated value of C 60 Cl 28 (C; 42.06%, Cl; 57.94%).
- the IR spectrum of this product is shown in FIG.
- the IR spectrum in FIG. 10 is significantly different from the spectrum of chlorinated fullerene C 60 Cl 28 used as a starting material (see FIG. 9), suggesting that the reaction has progressed, and that the hydroxylated fullerene and chlorinated fullerene It had both spectral features.
- the thermogravimetric analysis showed that a weight loss of 8.2 wt% was observed during heating from room temperature to around 115 ° C., so this weight loss was estimated as the amount of secondary bound water contained in the product. .
- the IR spectrum of the product is shown in FIG.
- the IR spectrum of FIG. 11 shows a sharp and large C—Br stretching vibration at 848 cm ⁇ 1 , which is very similar to the spectrum of brominated fullerenes C 60 Br 8 and C 60 Br 24 described in Non-Patent Document 5, It showed the grounds for the average structure of 60 Br 16 .
- the elemental analysis values were C; 35.48%, H; 0.45%, Br; 62.47%, and the calculated value of C 60 Br 16 (C; 36.05%, Br; 63.95%) Matched well.
- the IR spectrum of this product is shown in FIG.
- the IR spectrum in FIG. 12 is significantly different from the spectrum of brominated fullerene C 60 Br 16 used as the starting material (see FIG. 11), suggesting that the reaction has progressed, and that the hydroxylated fullerene and brominated fullerene It had both spectral features.
- the thermogravimetric analysis showed that a weight reduction of 5.0 wt% was observed during heating from room temperature to around 100 ° C., so this weight reduction was estimated as the amount of secondary bound water contained in the product. . Elemental value analysis C; 55.26%, H; 1.43 %, Br;. Becomes 27.12%, C 60 Br 4 5 (OH) 9 ⁇ 4H 2 O becomes Calculated (C; 55.21 %, H; 1.31%, Br; 27.55%, water; 5.5 wt%).
- Anti-Cry j 1 antibody is immobilized on each well of the microplate, washed and post-coated. Further, after washing, a sample solution or a standard allergen solution is added to perform a primary reaction. After washing, anti-Cry j 1 biotin-labeled antibody is added and a secondary reaction is performed. Further, after washing, the enzyme reagent streptavidin HRP is added, and after washing, the substrate o-phenylenediamine is added to perform a color reaction. After stopping the reaction by adding dilute sulfuric acid, the absorbance at a wavelength of 490 nm is measured using a microplate reader. The allergen concentration in each sample is obtained from a calibration curve prepared in advance using a standard allergen solution.
- the fullerene derivative of the present invention has antiallergen / antivirus performance and can be used in masks and filter products.
- the fullerene derivative of the present invention has an amphiphilic property having both hydrophilicity and hydrophobicity, and can be applied, impregnated, or chemically bonded to the surface of various materials. Therefore, it can be used in new organic synthesis, polymer modification, surface modification, medical field and the like.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Nanotechnology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Materials Engineering (AREA)
- Analytical Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Composite Materials (AREA)
- Inorganic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Carbon And Carbon Compounds (AREA)
Abstract
Description
〔非特許文献1〕J.Am.Chem.Soc.,1991,113,9900
〔非特許文献2〕J.Chem.Soc.,Chem.Commun.,1993,1230
〔非特許文献3〕Eur.J.Org.Chem.,2005,4951
フッ素化フラーレン、塩素化フラーレンおよび臭素化フラーレンの製造方法が下記特許文献4に開示されている。
〔特許文献4〕 特開2002-193861号公報
下記非特許文献4にはフッ素化フラーレンC60F48の製造方法が開示されている。
〔非特許文献4〕Angew.Chem.Int.Ed.2001,40,2285
下記非特許文献5には臭素化フラーレンC60Br16の製造方法が開示されている。
〔非特許文献5〕Science,1992,256,822
なお、本発明のフラーレン誘導体の製造方法において、出発原料となるハロゲン化フラーレンは、どのような方法で製造したものであってもよい。
〔特許文献5〕 特開平7-48302号公報
〔特許文献6〕 国際公開WO2008/096763号公報
〔非特許文献6〕J.Org.Chem.,1994,59,3960
〔非特許文献7〕Synth.Commun.,2005,35,1803
〔非特許文献8〕ACS Nano,2008,2,327
などに開示されている方法により製造することができる。
〔特許文献4〕 特開2002-193861号公報
〔非特許文献9〕Fullerenes,Nanotubes,and Carbon Nanostructures, 2005,13,331
上記のように従来からハロゲン化フラーレンや水酸化フラーレンは知られているが、本発明のフラーレン誘導体のようにハロゲン基と水酸基が共存した部分ハロゲン化水酸化フラーレン誘導体はこれまでに知られていない。
塩素化フラーレンを部分水酸化(method A)する場合、導入される塩素置換基の数nは出発原料の塩素化フラーレン中の塩素置換基数n’と同じか、または水酸基への置換反応により減少している。部分水酸化の方法は、水酸化ナトリウムや水酸化カリウムなどの塩基触媒を用いた一般的な加水分解反応や、過酸化水素水を用いる水酸化反応があるが、これら手法に特に限定されるものではない。例えば、部分水酸化するための水酸化試薬としては、水酸化ナトリウムや水酸化カリウムの他に、LiOH、RbOH、CsOH、Ca(OH)2、Sr(OH)2、Ba(OH)2、TlOH、nBuN(OH)、Triton Bなどが挙げられる。
o-ジクロロベンゼン、クロロベンゼン、トリメチルベンゼン、キシレン、トルエン、ベンゼンなどの芳香族溶媒、
塩化メチレン、クロロホルム、四塩化炭素、ジクロロエタン、テトラクロロエタンなどのハロゲン溶媒、
THF、エーテル、酢酸エチル、ジオキサン、DMF、DMSOなどの非プロトン性極性溶媒、
その他、二硫化炭素、アセトニトリルなどが考えられる。
特許文献6に開示された方法により合成した。すなわち、C60(市販品:商品名「nanom purple」フロンティアカーボン社製)100mgをトルエン(50mL)に溶解させ、30%過酸化水素水5mLおよび相間移動触媒として水酸化テトラn-ブチルアンモニウム(40%水溶液、500μL)を加え、60℃で16時間攪拌した。この溶液から無色になったトルエン層を除去した。トルエン層除去後の水層を、ヘキサン、ジエチルエーテル、2-プロパノールをそれぞれ5:5:7の割合で混合した溶液85mLに超音波照射しながら滴下し、淡黄色固体を析出させた。生じた沈殿を遠心分離により沈降させた後、デカンテーションにより上澄み液を除いた。この固体を60mLのジエチルエーテルを用いて洗浄し、再沈降させた後、上澄み液を除き、室温で終夜真空乾燥した。これにより反応粗生成物の水酸化フラーレンを淡黄色粉末として得た。
上記の方法により得たC60(OH)44・8H2O(200mg)をテトラヒドロフラン(THF)2.5mL中に超音波を5分間照射することでよく分散させて、ICl(0.5mL)を加え、室温(rt)で2.5時間反応を行った(下記化2)。固体が消失し、赤褐色のクリアな溶液になったことを確認した後、減圧下においてTHF、IClを、エバポレータを用いて留去した。更に、残渣中に黒紫色固体として含まれる副生したヨウ素を除去するため、ヘキサンを用いて約20回洗浄することを繰り返し、ろ液の色が薄い赤色になったところで褐色固体を遠心分離により取り出し、室温で終夜真空乾燥を行った(収量208mg、収率100%)。
このようにして得られた生成物の赤外線吸収(IR)スペクトルを図2に示した。図2に示すIRスペクトルは、出発原料として用いた水酸化フラーレンC60(OH)44・8H2Oのスペクトル(図1)とは若干異なっており、反応が進行したことを示唆するとともに、水酸化フラーレンのスペクトルの特徴(水酸基のO-H伸縮に基づく3400cm-1付近の大きなブロードな吸収とともに、C-CおよびC-O伸縮に基づく1620、1380、1080cm-1付近にブロードな吸収)を残していた。また、この生成物の熱重量分析において、室温から100℃付近まで加熱する間に重量減少が5.0wt%見られた。この重量減少分を生成物に含まれる二次結合水の量と見積もった。元素分析の値はC;41.87%,H;3.00%,Cl;21.84%となり、C60Cl10(OH)30・5H2Oの計算値(C;43.01%,H;2.41%,Cl;21.16%,水;5.4wt%)とよく一致した。
実施例1の方法により得た水酸化フラーレンC60(OH)44・8H2Oの(200mg)をICl(1mL)と、室温で24時間反応を行った(下記化3)。黒色の粘性の高いスラリー状残渣をヘキサンにて約15回洗浄することを繰り返し、メタノールに溶解させ、減圧下においてメタノールを、エバポレータを用いて留去した。この茶色固体をエタノール中に加え、超音波照射することでよく分散させ、これにヘキサンを加えた。析出した黄色固体を遠心分離により取り出し、ジエチルエーテルで洗浄後、室温で終夜真空乾燥を行った(収量120mg、収率63%)。
このようにして得られた生成物のIRスペクトルを図3に示した。図3のIRスペクトルは、出発原料として用いた水酸化フラーレンC60(OH)44・8H2Oの図1に示したIRスペクトルとは若干異なっており、反応が進行したことを示唆するとともに、水酸化フラーレンのスペクトルの特徴を残していた。また、この生成物の熱重量分析によれば、室温から120℃付近まで加熱する間に重量減少が7.4wt%見られた。この重量減少分を生成物に含まれる二次結合水の量と見積もった。元素分析の値はC;46.73%,H;2.56%,Cl;4.76%となり、C60Cl2(OH)38・6H2Oの計算値(C;46.61%,H;3.26%,Cl;4.59%,水;7.0wt%)とよく一致した。
非特許文献6に開示された方法により合成した。すなわち、C60(10g)と60%発煙硫酸(150mL)を窒素雰囲気下に55~60℃で3日間攪拌した。得られた反応混合物を氷浴中のジエチルエーテル中に激しく攪拌しながら滴下し、生成した沈殿物を遠心分離にて分離した。得られた沈殿物をジエチルエーテルで洗浄し、遠心分離にて分離した後、更に、ジエチルエーテル/アセトニトリル混合溶媒で洗浄し、遠心分離にて分離し、これを40℃で真空乾燥して、ポリシクロ硫酸化フラーレン13gを赤橙色粉末として得た。このポリシクロ硫酸化フラーレン(5.0g)と蒸留水(100mL)を窒素雰囲気下、85℃で10時間攪拌し、生成した沈殿物を遠心分離にて分離した。得られた沈殿物を水で洗浄し、遠心分離した後、40℃で真空乾燥して、水酸化フラーレンC60(OH)12・5H2Oを茶褐色粉末として得た(収量4.5g)。得られた生成物のIRスペクトルを図4に示す。
上記の方法により得たC60(OH)12・5H2O(100mg)をICl(1mL)と室温で24時間反応を行った(下記化4)。黒色の粘性の高いスラリー状残渣をヘキサンにて約15回洗浄することを繰り返し、THFを加え、超音波照射することでよく分散させ、これにヘキサンを加えた。ヘキサンを加えたことにより析出した黄色固体を遠心分離により取り出し、室温で終夜真空乾燥を行った(収量117mg、収率71%)。
この生成物のIRスペクトルを図5に示した。図5に示したIRスペクトルは、出発原料として用いた水酸化フラーレンC60(OH)12・5H2OのIRスペクトル(図4参照)とは若干異なっており、反応が進行したことを示唆するとともに、水酸化フラーレンのスペクトルの特徴を残していた。また、その熱重量分析において、室温から110℃付近まで加熱する間に重量減少が9.7wt%見られたことから、この重量減少分を生成物に含まれる二次結合水の量と見積もった。元素分析の値はC;42.68%,H;1.60%,Cl;32.57%となり、C60Cl15(OH)15・9H2Oの計算値(C;42.68%,H;1.99%,Cl;31.85%,水;9.7wt%)とよく一致した。
実施例1の方法により得た水酸化フラーレンC60(OH)12・5H2O(100mg)をTHF2.5mL中に超音波を5分間照射することでよく分散させ、ICl(0.5mL)を加え、室温で24時間反応を行った(下記化5)。反応終了後、減圧下においてTHF、ヨウ素を留去した。残渣をヘキサンにて約10回洗浄することを繰り返し、酢酸エチルを加え、超音波照射することでよく分散させた。得られた橙色固体にさらにヘキサンを加えて超音波照射しながら、3回洗浄した。その後、橙色固体を遠心分離により取り出し、室温で終夜真空乾燥を行った(収量123mg、収率100%)。
生成物のIRスペクトルを図6に示した。図6のIRスペクトルは、出発原料として用いた水酸化フラーレンC60(OH)12・5H2Oのスペクトル(図4参照)とは若干異なっており、反応が進行したことを示唆するとともに、水酸化フラーレンのスペクトルの特徴を残していた。また、生成物の熱重量分析によれば、室温から110℃付近まで加熱する間に重量減少が7.9wt%見られた。この重量減少分を生成物に含まれる二次結合水の量と見積もった。元素分析の値はC;58.89%,H;3.86%,Cl;15.22%となり、C60Cl5(OH)15・5H2Oの計算値(C;57.97%,H;2.03%,Cl;14.26%,水;7.2wt%)とよく一致した。
非特許文献2に開示された方法により合成した。すなわち、C60(2.33g)のo-ジクロロベンゼン(ODCB)溶液60mLに、アルゴン雰囲気下、ICl(7.5g)のo-ジクロロベンゼン溶液20mLを滴下し、室温にて6時間反応を行った(下記化6)。反応が終了したことを高速液体クロマトグラフィー(HPLC)にて確認した後、o-ジクロロベンゼンおよび副生するヨウ素を、エバポレータを用いて留去した。残渣をヘキサンで洗浄後、遠心分離にて固体を取り出し、さらにもう一度ペンタンで洗浄後、遠心分離によりオレンジ色の固体を取り出した。これを室温で終夜真空乾燥を行った(収量2.53g、収率78%)。
液体クロマトグラフィー質量分析(LCMS)測定により、ほぼ1本の大きな生成物ピークの中に、C60Cl5(M=895)に相当すると思われるM=897のフラグメントピークを得た。生成物のIRスペクトルを図7に示した。図7のIRスペクトルは、非特許文献2に記載の塩素化フラーレンC60Cl6のIRスペクトルとよく類似していた。元素分析の値はC;72.53%,Cl;28.24%となり、C60Cl8の計算値(C;71.76%,Cl;28.55%)とよく一致した。
上記で作成したC60Cl8(1g)を1,3,4-トリメチルベンゼン(TMB)50mLに溶解させ、相間移動触媒として水酸化テトラn-ブチルアンモニウム(TBAH)の40%水溶液(5mL)存在下、30%の過酸化水素水溶液(H2O2aq)(30mL)と70℃で20時間反応を行った(下記化7)。上層の有機相の赤色がほぼ消失したことを確認した後、下層の黄褐色水溶液(約30mL)を取り出した。これに2-プロパノール、酢酸エチル、ヘキサンをそれぞれ加えた。沈殿させた黄色固体を遠心分離により取り出し、室温で終夜真空乾燥を行った(収量980mg、収率66%)。
この生成物のIRスペクトルを図8に示した。図8のIRスペクトルは、出発原料として用いた塩素化フラーレンC60Cl8のIRスペクトル(図7参照)と大きく異なっており、反応が進行したことを示唆するとともに、水酸化フラーレンのスペクトルの特徴を残していた。また、その熱重量分析において、室温から100℃付近まで加熱する間に重量減少が9.8wt%見られた。この重量減少分を生成物に含まれる二次結合水の量と見積もった。元素分析の値はC;48.29%,H;3.10%,Cl;1.06%となり、C60Cl0.5(OH)35.5・8H2Oの計算値(C;48.49%,H;3.49%,Cl;1.19%,水;9.7wt%)とよく一致した。
非特許文献3に開示された方法により合成した。すなわち、C60(400mg)にICl(2mL)を加え、アルゴン雰囲気下、120℃にて40時間反応を行った(下記化8)。反応終了後、反応器上部に析出した黒紫色のヨウ素の結晶を除去し、得られた茶色固体を室温で終夜真空乾燥を行った(収量931mg、収率98%)。
上記で作成したC60Cl28(50mg)に、フラーレン核に対し14当量の濃度となるよう調製した水酸化ナトリウム水溶液(40.8mM、10mL)を加え、超音波照射により水に分散させ、60℃で1時間反応を行った(下記化9)。pH試験紙を用いて溶液が中性になったことを確認した後、メタノールを加えて沈殿させた茶色固体を遠心分離により取り出し、エーテルで洗浄した。その後、室温で終夜真空乾燥を行った(収量31.4mg、収率79%)。
非特許文献5に開示された方法により合成した。すなわち、C60(700mg)にBr2(12mL)を加え、アルゴン雰囲気下、室温にて10日間反応を行った(下記化10)。反応終了後、ヘキサン中に反応溶液を加えて、生じた茶色固体を遠心分離により取り出し、少量のクロロホルムに溶解させた。その後、ヘキサンを加えて再沈殿させた。更に、エーテルで洗浄した後、室温で終夜真空乾燥を行った(収量1587mg、収率82%)。
上記で作成したC60Br16(50mg)に、フラーレン核に対し8当量の濃度となるよう調製した水酸化ナトリウムの水溶液(20.0mM、10mL)を加え、超音波照射により水に分散させ、60℃で30分反応を行った(下記化11)。pH試験紙を用いて溶液が中性になったことを確認した後、これに水の体積に対して5:6:7の比でヘキサン、ジエチルエーテル、2-プロパノールを加えて沈殿させた茶色固体を遠心分離により取り出した。これをエーテルで洗浄した後、室温で終夜真空乾燥を行った(収量32.4mg、収率99%)。
<スギ花粉アレルゲン(Cry j 1)に対する試料の反応試験>
実施例1~5で合成した化合物の1%(w/v)溶液試料を調製し、リン酸緩衝液に溶解したアレルゲン(Cry j 1)を100ng/mLとなるよう加え、ボルテックスで混合後、4℃で振とうしながら反応させた。所定時間(5分および30分後)毎に溶液を回収し、遠心処理をした上清について、サンドイッチELISA法(酵素免疫測定法Enzyme-Linked Immunosorbent Assay)を用いてアレルゲン濃度(A)を測定した。比較としてフラーレン試料を加えなかったアレルゲン溶液の濃度(B)を用い、下記の式からアレルゲン減少率(%)を求めた。
実施例1で合成した水酸化フラーレンC60(OH)44・8H2Oを用いて、上記試験例1と同じアレルゲン吸着試験を同条件下で行った。結果は、60分後でも6.2%の減少に止まった。
Claims (17)
- 水酸基およびハロゲン基を有することを特徴とするフラーレン誘導体。
- 一般式CpXn(OH)m (pは60以上の偶数、Xはハロゲン基、nは0より大きな48以下の数、m は0より大きな44以下の数)で表わされることを特徴とする請求項1記載のフラーレン誘導体。
- ハロゲン基が塩素であることを特徴とする請求項1または2記載のフラーレン誘導体。
- ハロゲン基が臭素であることを特徴とする請求項1または2記載のフラーレン誘導体。
- ハロゲン基がフッ素であることを特徴とする請求項1または2記載のフラーレン誘導体。
- フラーレン核がC60であることを特徴とする請求項1~5の何れか1項に記載のフラーレン誘導体。
- フラーレン核がC70であることを特徴とする請求項1~5の何れか1項に記載のフラーレン誘導体。
- フラーレン核がC60を含む、C70以上の高次フラーレンとの混合物であることを特徴とする請求項1~5の何れか1項に記載のフラーレン誘導体。
- フラーレン核にハロゲン基が結合しているハロゲン化フラーレンを、一部のハロゲン基を残したまま、水酸基をフラーレン核に結合させて、部分ハロゲン化水酸化フラーレンを生成することを特徴とするフラーレン誘導体の製造方法。
- ハロゲン基が塩素である塩素化フラーレンと過酸化水素とを反応させることを特徴とする請求項9記載のフラーレン誘導体の製造方法。
- ハロゲン基が塩素である塩素化フラーレンと水酸化ナトリウムとを反応させることを特徴とする請求項9記載のフラーレン誘導体の製造方法。
- ハロゲン基が塩素である塩素化フラーレンと水酸化カリウムとを反応させることを特徴とする請求項9記載のフラーレン誘導体の製造方法。
- ハロゲン基が臭素である臭素化フラーレンと水酸化ナトリウムとを反応させることを特徴とする請求項9記載のフラーレン誘導体の製造方法。
- ハロゲン基が臭素である臭素化フラーレンと水酸化カリウムとを反応させることを特徴とする請求項9記載のフラーレン誘導体の製造方法。
- フラーレン核に水酸基が結合している水酸化フラーレンを、一部の水酸基を残したまま、ハロゲン基をフラーレン核に結合させて、部分ハロゲン化水酸化フラーレンを生成することを特徴とするフラーレン誘導体の製造方法。
- フラーレン核に水酸基が結合している水酸化フラーレンを、塩化ヨウ素と反応させて、一部の水酸基を残したまま、塩素をフラーレン核に結合させて、部分塩素化水酸化フラーレンを生成することを特徴とするフラーレン誘導体の製造方法。
- 請求項1から8の何れかのフラーレン誘導体を含んでいることを特徴とするアレルゲン吸着剤。
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11739697.8A EP2535313B1 (en) | 2010-02-08 | 2011-01-31 | Fullerene derivative, process for production of same, and allergen adsorbent comprising same |
| US13/577,868 US8957261B2 (en) | 2010-02-08 | 2011-01-31 | Method for producing partially halogenated, hydroxylated fullerene |
| CN201180006254.9A CN102858686B (zh) | 2010-02-08 | 2011-01-31 | 富勒烯衍生物及其制备方法、以及使用其的变应原吸附剂 |
| US14/495,438 US8987526B2 (en) | 2010-02-08 | 2014-09-24 | Partially halogenated, hydroxylated fullerene and allergen adsorbent using the same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010-025303 | 2010-02-08 | ||
| JP2010025303A JP4980437B2 (ja) | 2010-02-08 | 2010-02-08 | フラーレン誘導体とその製造方法、並びにこれを用いたアレルゲン吸着剤 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/577,868 A-371-Of-International US8957261B2 (en) | 2010-02-08 | 2011-01-31 | Method for producing partially halogenated, hydroxylated fullerene |
| US14/495,438 Division US8987526B2 (en) | 2010-02-08 | 2014-09-24 | Partially halogenated, hydroxylated fullerene and allergen adsorbent using the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011096349A1 true WO2011096349A1 (ja) | 2011-08-11 |
Family
ID=44355347
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/051864 WO2011096349A1 (ja) | 2010-02-08 | 2011-01-31 | フラーレン誘導体とその製造方法、並びにこれを用いたアレルゲン吸着剤 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US8957261B2 (ja) |
| EP (1) | EP2535313B1 (ja) |
| JP (1) | JP4980437B2 (ja) |
| CN (1) | CN102858686B (ja) |
| WO (1) | WO2011096349A1 (ja) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013026155A (ja) | 2011-07-25 | 2013-02-04 | Yazaki Corp | 防水コネクタ用端子 |
| JP5806077B2 (ja) * | 2011-10-11 | 2015-11-10 | 本荘ケミカル株式会社 | フラーレン内包シリカゲルの製造方法 |
| US20150333124A1 (en) * | 2012-12-20 | 2015-11-19 | Basf Se | Edge halogenation of graphene materials |
| JP2016017063A (ja) * | 2014-07-10 | 2016-02-01 | 国立大学法人大阪大学 | 長鎖アルキルエーテル化フラーレン誘導体およびその製造方法、並びにそれを用いた樹脂組成物 |
| KR20200010806A (ko) | 2018-07-23 | 2020-01-31 | 삼성전자주식회사 | 연마 슬러리 및 그 제조 방법과 반도체 소자의 제조 방법 |
| KR102653892B1 (ko) | 2018-08-30 | 2024-04-02 | 삼성전자주식회사 | 화학적 기계적 연마용 슬러리 조성물, 그의 제조 방법, 및 그를 이용한 반도체 소자의 제조 방법 |
| CN110963480A (zh) * | 2018-09-29 | 2020-04-07 | 蔡蓼芸 | 一种富勒烯衍生物、制备方法及其应用 |
| US10934168B1 (en) * | 2020-04-21 | 2021-03-02 | Terry Earl Brady | Synthetic, multifaceted halogenated, functionalized fullerenes engineered for microbicidal effects employing controlled contact for safe therapeutic and environmental utility |
| US11638720B1 (en) * | 2022-08-15 | 2023-05-02 | Terry Earl Brady | Risk mitigation of infectious disease transmission from incidental and intimate contact using atomic scale molecular disruption and biocidal halo-fullerenes delivered via topical, flushing and enteral mechanisms |
| US12005132B1 (en) * | 2023-08-11 | 2024-06-11 | Terry Earl Brady | Atomic scale topical composition with enhanced interstitial cellular uptake for increased moisturizing, fluidity, antioxidant and radiation protection, antimicrobial cleansing and therapeutics for optimal dermal integrity and homeostasis |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0748302A (ja) | 1993-07-30 | 1995-02-21 | Tokyo Gas Co Ltd | フラロールの合成方法 |
| JP2000005531A (ja) | 1998-06-19 | 2000-01-11 | Matsushita Seiko Co Ltd | 抗アレルゲンフィルターと該フィルターを設けた空気清浄機および換気装置およびマスク |
| JP2002167332A (ja) | 2000-11-30 | 2002-06-11 | Lion Corp | アレルゲン吸着組成物 |
| JP2002193861A (ja) | 2000-12-25 | 2002-07-10 | Sony Corp | フラーレン誘導体の製造方法及びそのフラーレン誘導体、プロトン伝導体、並びに電気化学デバイス |
| JP2004204401A (ja) | 2002-12-26 | 2004-07-22 | Ebara Corp | 花粉吸着材 |
| JP2005104907A (ja) * | 2003-09-30 | 2005-04-21 | Neos Co Ltd | 含フッ素フラレノール誘導体及びその製造方法 |
| JP2007254246A (ja) * | 2006-03-27 | 2007-10-04 | Central Glass Co Ltd | フッ化フラーレンを含有する溶解液、または分散液 |
| JP2008094656A (ja) * | 2006-10-11 | 2008-04-24 | Chemicals Evaluation & Research Institute | フラーレン類の水分散方法 |
| WO2008096763A1 (ja) | 2007-02-09 | 2008-08-14 | Osaka University | 相間移動触媒およびポリ水酸化フラーレンの製造方法 |
| JP2008280290A (ja) * | 2007-05-10 | 2008-11-20 | Mitsubishi Chemicals Corp | フラーレン誘導体及びその製造方法 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1874981A (zh) * | 2003-10-28 | 2006-12-06 | 理想星株式会社 | 内含式富勒烯衍生物、质子导体和燃料电池 |
| JPWO2005049538A1 (ja) * | 2003-10-28 | 2007-06-07 | 株式会社イデアルスター | 内包フラーレン誘導体、プロトン伝導体、及び、燃料電池 |
-
2010
- 2010-02-08 JP JP2010025303A patent/JP4980437B2/ja active Active
-
2011
- 2011-01-31 US US13/577,868 patent/US8957261B2/en active Active
- 2011-01-31 EP EP11739697.8A patent/EP2535313B1/en not_active Not-in-force
- 2011-01-31 CN CN201180006254.9A patent/CN102858686B/zh active Active
- 2011-01-31 WO PCT/JP2011/051864 patent/WO2011096349A1/ja active Application Filing
-
2014
- 2014-09-24 US US14/495,438 patent/US8987526B2/en not_active Expired - Fee Related
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0748302A (ja) | 1993-07-30 | 1995-02-21 | Tokyo Gas Co Ltd | フラロールの合成方法 |
| JP2000005531A (ja) | 1998-06-19 | 2000-01-11 | Matsushita Seiko Co Ltd | 抗アレルゲンフィルターと該フィルターを設けた空気清浄機および換気装置およびマスク |
| JP2002167332A (ja) | 2000-11-30 | 2002-06-11 | Lion Corp | アレルゲン吸着組成物 |
| JP2002193861A (ja) | 2000-12-25 | 2002-07-10 | Sony Corp | フラーレン誘導体の製造方法及びそのフラーレン誘導体、プロトン伝導体、並びに電気化学デバイス |
| JP2004204401A (ja) | 2002-12-26 | 2004-07-22 | Ebara Corp | 花粉吸着材 |
| JP2005104907A (ja) * | 2003-09-30 | 2005-04-21 | Neos Co Ltd | 含フッ素フラレノール誘導体及びその製造方法 |
| JP2007254246A (ja) * | 2006-03-27 | 2007-10-04 | Central Glass Co Ltd | フッ化フラーレンを含有する溶解液、または分散液 |
| JP2008094656A (ja) * | 2006-10-11 | 2008-04-24 | Chemicals Evaluation & Research Institute | フラーレン類の水分散方法 |
| WO2008096763A1 (ja) | 2007-02-09 | 2008-08-14 | Osaka University | 相間移動触媒およびポリ水酸化フラーレンの製造方法 |
| JP2008280290A (ja) * | 2007-05-10 | 2008-11-20 | Mitsubishi Chemicals Corp | フラーレン誘導体及びその製造方法 |
Non-Patent Citations (12)
| Title |
|---|
| A.ABDUL-SADA ET AL.: "Isolation and characterisation of fluorinated derivatives of [76]- and [78]fullerenes", JOURNAL OF THE CHEMICAL SOCIETY, PERKIN TRANSACTIONS 2, vol. 12, no. 12, 1999, pages 2659 - 2666, XP008165185 * |
| A.AVENT ET AL.: "In the first proven SN2' fullerene reaction, both C3 and C1 C6oF36 hydrolyse to C1 isomers of C6oF35OH that eliminate HF to give epoxides C6oF340; C6oF360 oxides are shown to be ethers, and a fourth isomer of C6oF36 exists", ORGANIC & BIOMOLECULAR CHEMISTRY, vol. 1, no. 6, 21 March 2003 (2003-03-21), pages 1026 - 1033, XP008165183 * |
| ACS NANO, vol. 2, 2008, pages 37 |
| ANGEW. CHEM. INT. ED., vol. 40, 2001, pages 2285 |
| EUR. J. ORG. CHEM., 2005, pages 4951 |
| FULLERENES, NANOTUBES, AND CARBON NANOSTRUCTURES, vol. 13, 2005, pages 331 |
| J. AM. CHEM. SOC., vol. 113, 1991, pages 9900 |
| J. CHEM. SOC., CHEM. COMMUN., 1993, pages 1230 |
| J. ORG. CHEM., vol. 59, 1994, pages 3960 |
| SCIENCE, vol. 256, 1992, pages 822 |
| See also references of EP2535313A4 * |
| SYNTH. COMMUN., vol. 35, 2005, pages 1803 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2011162468A (ja) | 2011-08-25 |
| CN102858686A (zh) | 2013-01-02 |
| EP2535313B1 (en) | 2017-11-29 |
| JP4980437B2 (ja) | 2012-07-18 |
| US20150011802A1 (en) | 2015-01-08 |
| CN102858686B (zh) | 2016-01-20 |
| US20130041185A1 (en) | 2013-02-14 |
| US8957261B2 (en) | 2015-02-17 |
| EP2535313A1 (en) | 2012-12-19 |
| EP2535313A4 (en) | 2014-05-07 |
| US8987526B2 (en) | 2015-03-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4980437B2 (ja) | フラーレン誘導体とその製造方法、並びにこれを用いたアレルゲン吸着剤 | |
| US8410196B2 (en) | Surface-modified nanodiamond and its producing method | |
| Gao et al. | Preparation, characterization, and adsorption evaluation of chitosan-functionalized mesoporous composites | |
| Pontón et al. | The effects of the chemical composition of titanate nanotubes and solvent type on 3-aminopropyltriethoxysilane grafting efficiency | |
| Jin et al. | Controlled drug delivery from mesoporous silica using a pH-response release system | |
| KR102418329B1 (ko) | 삼중 응답 나노 모터, 이의 제조에 사용되는 조성물 및 이의 제조방법 | |
| CN110183773A (zh) | 壳聚糖季铵盐有机插层蒙脱土复合材料的制备方法 | |
| Vieira et al. | Synthesis and characterization of 3-[(thiourea)-propyl]-functionalized silica gel and its application in adsorption and catalysis | |
| Prabu et al. | Non-covalent polyhedral oligomeric silsesquioxane-polyoxometalates as inorganic–organic–inorganic hybrid materials for visible-light photocatalytic splitting of water | |
| CN104448321B (zh) | 一种改性有序介孔有机硅材料及其制备方法和应用 | |
| CN113173575A (zh) | 一种铜纳米颗粒/富勒醇纳米复合材料及其制备方法和应用 | |
| JP6910602B2 (ja) | 表面修飾ナノダイヤモンドの製造方法、及び表面修飾ナノダイヤモンド | |
| Paris et al. | Phthalocyanine–titanate nanotubes: a promising nanocarrier detectable by optical imaging in the so-called imaging window | |
| JP2014172781A (ja) | 表面修飾グラフェン | |
| RU2548971C2 (ru) | Способ получения водных нанодисперсий фуллерена | |
| Jamasbi et al. | Highly silver-selective fluorescent sensor based on functionalized nanoporous silica (SBA-Pr-NMP) in aqueous media | |
| JP2005239863A (ja) | キラル配向構造を有する有機/無機複合体及びその製造法 | |
| CN115254073A (zh) | 一种表面增强型色谱填料的制备方法 | |
| WO2023275787A1 (en) | Zirconium-based metal organic framework for using as a heavy metal adsorbent in condensate and preparation method thereof | |
| US20220241419A1 (en) | Highly dispersible zinc phthalocyanine-silica nanotubes and preparation method therefor | |
| Calhau et al. | One‐Pot Intercalation Strategy for the Encapsulation of a CO‐Releasing Organometallic Molecule in a Layered Double Hydroxide | |
| CN102786046B (zh) | 一种基于n负离子的共价键修饰石墨烯的方法 | |
| Nosach et al. | Gas-phase crosslinking of the lignin on the nanoscale fumed silica surface | |
| KR100840544B1 (ko) | 작용기로 치환된 알킬기, 또는 페닐기를 함유한트리알콕시실란을 이용한 실리카 입자의 표면개질방법 및그 실리카 입자 | |
| Sabrana et al. | Systematic Study of Calcination Temperature on Photocatalytic Activity of Luminescent Copper (I) Pyrazolate Complex/Titanium Oxide Composites |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180006254.9 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11739697 Country of ref document: EP Kind code of ref document: A1 |
|
| REEP | Request for entry into the european phase |
Ref document number: 2011739697 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011739697 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13577868 Country of ref document: US |