WO2010050578A1 - カチオン性ナノゲルを用いる粘膜ワクチン - Google Patents
カチオン性ナノゲルを用いる粘膜ワクチン Download PDFInfo
- Publication number
- WO2010050578A1 WO2010050578A1 PCT/JP2009/068647 JP2009068647W WO2010050578A1 WO 2010050578 A1 WO2010050578 A1 WO 2010050578A1 JP 2009068647 W JP2009068647 W JP 2009068647W WO 2010050578 A1 WO2010050578 A1 WO 2010050578A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- vaccine
- antigen
- mucosal
- nanogel
- preparation according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/06—Ointments; Bases therefor; Other semi-solid forms, e.g. creams, sticks, gels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/08—Clostridium, e.g. Clostridium tetani
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/39—Medicinal preparations containing antigens or antibodies characterised by the immunostimulating additives, e.g. chemical adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/554—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound the modifying agent being a steroid plant sterol, glycyrrhetic acid, enoxolone or bile acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/61—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule the organic macromolecular compound being a polysaccharide or a derivative thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6903—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being semi-solid, e.g. an ointment, a gel, a hydrogel or a solidifying gel
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
- A61K9/5107—Excipients; Inactive ingredients
- A61K9/513—Organic macromolecular compounds; Dendrimers
- A61K9/5161—Polysaccharides, e.g. alginate, chitosan, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/10—Antimycotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
- A61K2039/541—Mucosal route
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
- A61K2039/541—Mucosal route
- A61K2039/543—Mucosal route intranasal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55583—Polysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/60—Medicinal preparations containing antigens or antibodies characteristics by the carrier linked to the antigen
- A61K2039/6087—Polysaccharides; Lipopolysaccharides [LPS]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0043—Nose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/006—Oral mucosa, e.g. mucoadhesive forms, sublingual droplets; Buccal patches or films; Buccal sprays
Definitions
- the present invention relates to a mucosal vaccine comprising a complex of a vaccine antigen and a cationic nanogel administered by nasal administration or oral administration.
- the mucosal vaccine not based on injection is regarded as a safe and simple next-generation vaccine.
- Toxin-related proteins such as cholera toxin CT and detoxified cholera toxin mCT are known as mucosal adjuvants.
- mucosal adjuvants By adding these mucosal adjuvants to the mucosal vaccine, it is possible to induce not only systemic IgG antigen-specific to the nasal vaccine but also mucosal IgA.
- the above mucosal adjuvant has a risk of moving into the brain, and has a problem in safety to the living body.
- the present inventors have developed a nanogel composed of molecules such as cholesterol-bearing pulullan (CHP) in which hydrophobic cholesterol is added as a side chain to a hydrophilic polysaccharide as a DDS substrate (Patent Documents 1 to 6). And non-patent document 1). That is, CHP self-assembles in a water environment to form a colloid (nanogel) having a diameter of 20 to 30 nm, and various substances can be encapsulated therein.
- One of the excellent features of CHP is the “molecular chaperone effect”. This is because when protein-like molecules are encapsulated in the CHP nanogel and then released, refolding occurs during the release, acquiring a physiological three-dimensional structure and exhibiting normal activity. .
- the present invention is a mucosa for nasal or oral administration that can induce a vaccine antigen-specific immune response in a living body without adding a mucosal adjuvant such as a toxin-related protein (cholera toxin CT, detoxified cholera toxin mCT, etc.)
- a mucosal adjuvant such as a toxin-related protein (cholera toxin CT, detoxified cholera toxin mCT, etc.)
- the purpose is to provide a vaccine.
- the present inventors have previously developed a nanogel obtained by adding hydrophobic cholesterol as a side chain to a hydrophilic polysaccharide that can be used for delivery of a substance such as a physiologically active protein.
- nanogels with cationic functional groups such as amino groups are combined with vaccine antigens, which are viral and bacterial proteins, and administered via the nasal mucosa and intestinal mucosa, which is more effective than using liposomes.
- vaccine antigens which are viral and bacterial proteins
- the present invention has been found to cause systemic immune reaction and mucosal immune reaction and to help prevent or treat viral and bacterial infections.
- the present invention is as follows.
- a vaccine antigen and the nanogel comprising mixing a nanogel obtained by adding hydrophobic cholesterol as a side chain to a hydrophilic polysaccharide having a cationic functional group and a vaccine antigen at 4 to 37 ° C. for 2 to 48 hours.
- a method for producing a mucosal vaccine preparation comprising the complex of
- the mucosal vaccine prepared by combining the vaccine antigen of the present invention with a cationic nanogel effectively induces systemic and mucosal immune responses in animals by nasal or oral mucosal administration. Since the mucosal vaccine of the present invention uses a cationic nanogel, the vaccine antigen is efficiently delivered to the immune system, and the immune response is more effective than when non-cationic nanogels or cationic liposomes are used. To induce.
- the mucosal vaccine of the present invention can be effectively used for prevention or treatment of animal viruses and bacterial infections.
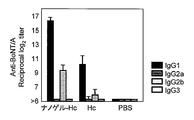
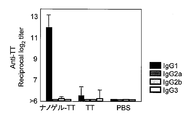
- IgG1, IgG2a, IgG2b, and IgG3 show the antibody titer of IgG1, IgG2a, IgG2b, and IgG3 with respect to TT in the serum of the mouse
- IgG1, IgG2a, IgG2b, and IgG3 are shown from the left, respectively.
- nanogel means a hydrophobic polymer gel nanoparticle having a structure in which hydrophobic cholesterol is added as a side chain to a hydrophilic polysaccharide.
- the nanogel to be used can be produced, for example, by the method described in International Publication No. WO00 / 12564 (high purity hydrophobic group-containing polysaccharide and production method thereof).
- a hydroxyl group-containing hydrocarbon or sterol having 12 to 50 carbon atoms is reacted with a diisocyanate compound represented by 0CN-R1 NCO (wherein R1 is a hydrocarbon group having 1 to 50 carbon atoms).
- R1 is a hydrocarbon group having 1 to 50 carbon atoms.
- an isocyanate group-containing hydrophobic compound obtained by reacting one molecule of a hydroxyl group-containing hydrocarbon or sterol having 12 to 50 carbon atoms is produced.
- the obtained isocyanate group-containing hydrophobic compound and polysaccharide are further reacted to produce a hydrophobic group-containing polysaccharide containing a hydrocarbon group having 12 to 50 carbon atoms or a steryl group as the hydrophobic group. .
- the resulting reaction product can be purified with a ketone solvent to produce a high purity hydrophobic group-containing polysaccharide.
- the polysaccharide include pullulan, amylopectin, amylose, dextran, hydroxyethyldextran, mannan, levan, inulin, chitin, chitosan, xyloglucan and water-soluble cellulose.
- nanogels examples include cholesterol-substituted pullulan (hereinafter referred to as CHP) and CHP derivatives.
- CHP has a structure in which 1 to 10, preferably 1 to several cholesterols are substituted per 100 monosaccharides in pullulan having a molecular weight of 30,000 to 200,000, for example, a molecular weight of 100,000.
- the properties of CHP can be changed by changing the amount of cholesterol substitution depending on the size of the protein and the degree of hydrophobicity.
- an alkyl group having 10 to 30 carbon atoms preferably about 12 to 20 carbon atoms may be introduced.
- the nanogel used in the present invention has a particle size of 10 to 40 nm, preferably 20 to 30 nm. Nanogels have already been widely marketed, and in the present invention, these commercially available products can be widely used.
- the mucosal vaccine uses a nanogel into which a functional group having a positive charge, such as an amino group, is introduced.
- the introduction rate of amino groups into the nanogel is 1-50, more preferably 5-30, per 100 glucose monosaccharides of CHP.
- a method for introducing an amino group into the nanogel a method using cholesterol pullulan (CHPNH 2 ) to which an amino group has been added is preferably mentioned as follows.
- the mucosal vaccine preparation of the present invention can efficiently induce systemic and mucosal immune responses specific to vaccine antigens to animals without adding other mucosal adjuvants.
- vaccine antigens used in the mucosal vaccine of the present invention include antigens of microorganisms such as bacteria, viruses, fungi, and protozoa that cause infectious diseases in animals.
- the antigen is an antigen that induces an animal to have a specific immune response to the antigen, and can be used as a vaccine, and is called a vaccine antigen.
- the antigens of microorganisms include influenza virus A, influenza virus B, hepatitis C virus, hepatitis A virus, hepatitis B virus, rotavirus, cytomegalovirus, RS virus, adenovirus, HIV , Varicella-zoster virus, herpes simplex virus type 1 and 2, ATL (adult T-cell leukemia) virus, coxsackie virus, enterovirus, idiopathic rash virus (HHV-6), measles virus, rubella virus, mumps virus , Poliovirus, Japanese encephalitis virus, rabies virus, hepatitis C virus, noo Oak virus, rabies virus, RS virus, cytomegalovirus, foot-and-mouth disease virus, infectious gastroenteritis virus, rubella virus, ATL virus, adenovirus ,echo Virus, herpes virus, smallpox virus, proboscis fever virus, yellow fever virus, West Nile virus, SARS (cor
- Viruses such as enterohaemorrhagic E. coli; pathogenic E. coli such as enterohemorrhagic E. coli; Staphylococcus such as Staphylococcus aureus; Streptococcus pneumoniae, Bordetella pertussis, Diphtheria, Tetanus, Haemophilus influenzae, Plague, Clostridium botulinum, Bacillus anthracis, Wild boar, Salmonella, VRE (Enterococcus), Mycobacterium tuberculosis, Shigella, Salmonella typhi, Paratyphi, Chlamydia , Amoeba dysentery, Legionella, Lyme disease Borrelia, Brucella disease (wavy fever) Q fever rickettsia, rickettsia, such chlamydia; pathogenic bacteria etc.
- enterohaemorrhagic E. coli pathogenic E. coli
- pathogenic E. coli such
- malaria pathogens insects, protozoa Cryptosporidium like can include a protein antigen of microorganisms such as fungi such as Cryptococcus Aspergillus.
- Proteins derived from pathogenic microorganisms include proteins or peptides that constitute pathogenic microorganisms (for example, surface proteins, capsid proteins, cilia proteins, etc.), proteins or peptides produced by pathogenic microorganisms (for example, toxins, enzymes, hormones) , Immunomodulators, receptors and their ligands), fragments or domains thereof, and the like.
- pathogenic microorganisms for example, surface proteins, capsid proteins, cilia proteins, etc.
- proteins or peptides produced by pathogenic microorganisms for example, toxins, enzymes, hormones
- Immunomodulators, receptors and their ligands fragments or domains thereof, and the like.
- what is necessary is just to use the protein antigen which can induce the antibody production which can carry out
- the protein antigen is not limited to one type, and the mucosal vaccine of the present invention may contain a plurality of vaccine antigens derived from the same or different microorganisms.
- a mucosal vaccine complexed with a cationic nanogel can be produced by using hemagglutinin (HA), neuraminidase (NA), etc., which are receptors, alone or in a mixture.
- Vaccine antigens can be obtained by processing from microorganisms or purification. It can also be chemically synthesized and can be obtained as a recombinant protein by genetic engineering techniques.
- the molecular weight of the vaccine antigen contained in the mucosal vaccine preparation of the present invention is not limited, but is, for example, about 500 to 1,000,000, preferably about 1,000 to 200,000.
- the complex of the vaccine antigen and the cationic nanogel can be prepared by allowing the cationic nanogel and the vaccine antigen to coexist and interact with each other and incorporating the vaccine antigen into the cationic nanogel. Making a composite is called composite.
- the mixing ratio of the vaccine antigen and the cationic nanogel can be appropriately determined according to the type of vaccine antigen and cationic nanogel used. For example, CHPNH 2 may be mixed with the vaccine antigen at a molar ratio of 1: 1 to 1: 100, preferably 1: 1 to 1:10.
- vaccine antigen and cationic nanogel are mixed in a buffer and mixed at 4 to 37 ° C. for 2 to 48 hours, preferably 20 to 30 hours. do it.
- the buffer used for forming the vaccine antigen-cationic nanogel complex can be appropriately prepared depending on the type of protein and nanogel. Examples of the buffer include Tris-HCl buffer (50 mM, pH 7.6).
- the prepared vaccine antigen-nanogel complex can be analyzed by a known method. For example, it can be analyzed by gel permeation chromatography (GPC), atomic force microscope (AFM), fluorescence microscope and confocal laser fluorescence microscope.
- the mucosal vaccine preparation of the present invention is administered via the mucosa.
- Administration via the mucosa is preferably via the nasal mucosa or the intestinal mucosa.
- it is administered nasally as a nasal vaccine formulation
- it is administered orally as an oral vaccine formulation.
- Nasal vaccine formulations induce an immune response intranasally by nasal administration. That is, it can induce an immune mechanism in the local mucosa of the respiratory tract (particularly the upper respiratory tract), which is the infection route of microorganisms that cause infections such as viruses.
- the nasal vaccine preparation may be administered to the nasal cavity by spraying, coating, dripping or the like.
- Oral vaccine formulations induce an immune response in the intestine by oral administration.
- the mucosal vaccine preparation stays in the administered mucosa, nasal cavity-related lymphoid tissue (NALT) and intestinal tract-related lymphoid tissue (GALT), and releases the vaccine antigen gradually.
- Both nasal vaccine preparations and oral vaccine preparations cause systemic immunity, producing virus-specific IgG in the body and mucosal immunity, producing IgA antibodies in the mucosa, systemic immunity mechanism, mucosal immunity mechanism Both mechanisms can protect against infection and treat infections.
- the mucosal vaccine preparation may contain known pharmaceutically acceptable stabilizers, preservatives, antioxidants and the like.
- stabilizer include gelatin, dextran, sorbitol and the like.
- preservatives include thimerosal and ⁇ -propiolactone.
- antioxidant include ⁇ -tocopherol.
- the administration target of the mucosal vaccine preparation of the present invention includes mammals such as humans, monkeys, mice, rats, rabbits, cats, cows, dogs, horses, goats, and birds such as chickens.
- the dose of the mucosal vaccine preparation can be appropriately determined depending on the type of immunogen, the age and weight of the administration target, etc., and includes a pharmaceutically effective amount of vaccine antigen.
- a pharmaceutically effective amount refers to the amount of antigen necessary to induce an immune response against the vaccine antigen.
- a single dose of vaccine antigen of several ⁇ g to several tens of mg may be administered once to several times a day, and may be administered several times at intervals of 1 to several weeks, for example, 1 to 5 times.
- Example 1 Production of Mucosal Vaccine Cationic nanogel (cationic CHP) used was one in which cholesterol was replaced with 1.4 and ethylenediamine was replaced with 18 per 100 monosaccharides (CHPNH 2 nanogel).
- CHP derivatives or cationic pullulan were dissolved in 1 mg / ml phosphate buffer solution (PBS). The CHPNH 2 nanogel was sonicated for 15 minutes and then passed through a filter (0.22 mm).
- Hc molecular weight 45000
- TT molecular weight 150000
- AIDS virus membrane antigen molecule gag p24, molecular weight 24000
- the complex of the obtained antigen and cationic nanogel was used as a cationic nanogel mucosal vaccine.
- the heavy chain C-terminal non-toxic region of the purified botulinum toxin was inserted into the GST fusion protein expression vector pGEX-6P3 (GE healthcare), transduced into Escherichia coli Rossetta2 (Novagen), and 0.1 mM IPTG was added. Expression was induced by addition.
- Hc cells suspended in PBS are sonicated, centrifuged, and the supernatant is anion exchange chromatography (DAEA ⁇ Sepharose; GE Healthcare), affinity chromatography (Glutathione Sepharose; GE healthcare), gel filtration chromatography. It was purified by subjecting it to a graphic (Sephacryl S-100; GE healthcare).
- Tetanus toxoid was obtained from the Osaka University Microbial Disease Research Group, and gag p24 was obtained from Yokota Yokota's Director, National Institute of Infectious Diseases, Immunity.
- Example 2 Nasal Immunization Cationic nanogel mucosal vaccine prepared in Example 1 or antigen alone was applied to 6-8 week old Balb / c mice (female) per Hc 10 ⁇ g (nanogel 88.9 ⁇ g), TT 30 ⁇ g ( The nasal immunization was performed by administering nanogel 80.0 ⁇ g) or gag p24 10 ⁇ g (nanogel 166.7 ⁇ g) into the nasal cavity once a week (3 times in total). The dose of antigen (fluid volume) was adjusted to 15 ⁇ l in all experimental groups, and 7.5 ⁇ l per nose was administered. At this time, PBS was administered as a control.
- Blood was collected before immunization and one week after each immunization, and systemic immune responses were evaluated by measuring IgG antibody titers against botulinum toxin, TT, or gag p24 in serum. Further, one week after the final immunization, the nasal cavity was washed with 200 ⁇ l of PBS, and the immune response in the mucosal system was evaluated by measuring the IgA antibody titer in the nasal cavity washing solution. The antibody titer was evaluated by ELISA.
- the antibody titers of IgG1, IgG2a, IgG2b, and IgG3 subclasses were measured, and the antibody production pattern at the subclass level was also evaluated, so that the immune balance of Th1 / Th2 after immunization was estimated. Furthermore, the number of antigen-specific IgA producing cells (plasma cells) in the nasal cavity tissue one week after the final immunization was evaluated by the ELISPOT method.
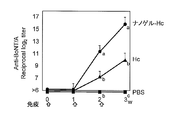
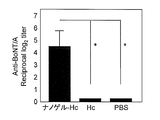
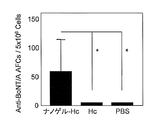
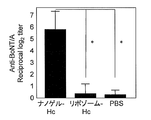
- FIG. 1 shows the total IgG antibody titer against botulinum toxin in serum.
- FIG. 2 shows antibody titers of IgG1, IgG2a, IgG2b and Ig3 against botulinum toxin in serum collected after 3 immunizations.
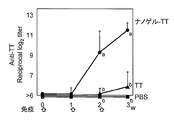
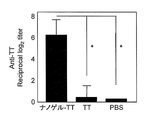
- FIG. 3 shows the total IgG antibody titer against TT in serum
- FIG. 4 shows the antibody titers of IgG1, IgG2a, IgG2b and Ig3 against TT in serum collected after 3 immunizations.
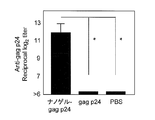
- FIG. 5 shows the gag p24-specific IgG antibody titer after the third immunization.
- FIG. 6 shows the IgA antibody titer against botulinum toxin in the nasal lavage fluid after the third immunization
- FIG. 7 shows the IgA antibody titer against TT in the nasal lavage fluid after the third immunization.
- the total IgG antibody titer against gag p24 was remarkably high. This causes a stronger systemic immune response when Hc, TT, or gag p24 is administered as a complex with a cationic nanogel compared to when Hc, TT, or gag p24 is administered alone. It shows that.
- most of the antigen-specific IgG was an IgG1 type subclass, and the IgG2a level was remarkably low. From the above results, it was speculated that Th2 type humoral immune response was effectively induced by nasal administration of vaccine antigen as a complex with cationic nanogel.
- IgA antibody titers were hardly observed when Hc or TT was administered alone, but botulinum toxin was observed when Hc or TT was administered as a complex with cationic nanogel.
- a high IgA antibody titer against TT was observed. This indicates that the mucosal immune reaction was induced in the nasal mucosa only when the mucosal vaccine of the present invention comprising a complex of an antigen and a cationic nanogel was administered nasally.
- FIG. 8 shows a comparison of the number of botulinum toxin antigen-specific IgA producing cells in the nasal mucosal tissue. As shown in FIG. 8, no IgA-producing cells were produced when administered with Hc alone, but IgA-producing cells were produced when a complex of Hc and cationic nanogel was administered.
- Example 3 Neutralizing effect after nasal immunization using nanogel mucosal vaccine Cationic nanogel vaccine using heavy chain C-terminal non-toxic region (Hc, molecular weight 45000) of botulinum toxin prepared in Example 1 as an antigen or Hc alone
- Hc heavy chain C-terminal non-toxic region
- mice were immunized intranasally in the same manner as in Example 2.
- PBS was administered as a negative control.
- 25,000 times (500 ng) botulinum toxin obtained by Prof. Toshiji Kosaki, graduate School of Life and Environmental Sciences, Osaka Prefecture University
- the intraperitoneal administration of the intraperitoneal dose was analyzed.
- botulinum progenitor toxin obtained from Wako Pure Chemical Industries
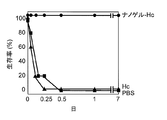
- FIG. 9 shows the survival rate of the mice over time after intraperitoneal administration of botulinum toxin. As shown in FIG. 9, all mice immunized with Hc alone died within one day, but all mice survived even one week after administration of Hc as a complex with cationic nanogel. This result indicates that powerful neutralization immunity in the systemic system can be induced by nasal administration of a complex of Hc and cationic nanogel.
- FIG. 10 shows the survival rate over time when botulinum progenitor toxin is administered intranasally. As shown in FIG. 10, all mice immunized with Hc alone died within one day, but all mice survived even one week after administration of Hc as a complex with cationic nanogel. This result shows that botulinum mucosal infection was effectively blocked by botulinum toxin-specific mucosal IgA induced by nasal administration of a complex of Hc and cationic nanogel.
- Example 4 Immunity-inducing effect of cationic nanogel vaccine compared with cationic liposome vaccine Cationic nanogel vaccine with heavy chain C-terminal non-toxic region (Hc, molecular weight 45000) of botulinum toxin prepared in Example 1 or the same
- Hc heavy chain C-terminal non-toxic region
- FIG. 11 shows the total IgG antibody titer against botulinum toxin after three immunizations.
- FIG. 12 shows the total IgA antibody titer against botulinum toxin after three immunizations.
- the total IgA antibody titer against botulinum toxin was significantly higher when Hc was administered as a complex with cationic nanogel than when Hc was administered as a complex with cationic liposomes.
- Example 5 Antigen retention effect of cation nanogel vaccine in nasal cavity tissue and presence / absence of transfer to cranial nervous system 111In (indium) using DTPA anhydride for heavy chain C-terminal non-toxic region (Hc, molecular weight 45000) of botulinum toxin was labeled by known methods. The labeling efficiency was 728.3233 ⁇ 115.3543 CPM / ng. Thereafter, labeled Hc was conjugated to the nanogel. The mouse was administered nasally with a nanogel mucosal vaccine combined with 1,000,000 CPM of labeled Hc or labeled Hc alone.
- Figure 13 shows the results of gamma ray measurements in the brain (A), olfactory bulb (B), nasal cavity tissue (C), nasal cavity related lymphoid tissue (NALT) (D), cervical lymph node (E) and spleen (F). Show.
- the nanogel mucosal vaccine was retained for a long time, particularly in the nasal cavity tissue (C), but the transition to the brain and olfactory bulb was not confirmed.
- This result shows that when the mucosal vaccine containing the cationic nanogel of the present invention is administered nasally, the antigen storage effect in the nasal cavity is higher than when the mucosal vaccine is administered alone. It can be applied as a safe and effective nasal administration agent that does not migrate to the central nervous system.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Immunology (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Virology (AREA)
- Botany (AREA)
- Optics & Photonics (AREA)
- Nanotechnology (AREA)
- Physics & Mathematics (AREA)
- Inorganic Chemistry (AREA)
- Biomedical Technology (AREA)
- AIDS & HIV (AREA)
- Molecular Biology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicinal Preparation (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP09823688.8A EP2345419B8 (en) | 2008-10-31 | 2009-10-30 | Mucosal vaccine using cationic nanogel |
| US13/126,357 US20110206729A1 (en) | 2008-10-31 | 2009-10-30 | Mucosal vaccine using cationic nanogel |
| ES09823688.8T ES2624722T3 (es) | 2008-10-31 | 2009-10-30 | Vacuna mucosa que emplea nanogel catiónico |
| US14/478,127 US8961983B2 (en) | 2008-10-31 | 2014-09-05 | Mucosal vaccine using cationic nanogel |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008-281065 | 2008-10-31 | ||
| JP2008281065A JP5344558B2 (ja) | 2008-10-31 | 2008-10-31 | カチオン性ナノゲルを用いる粘膜ワクチン |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/126,357 A-371-Of-International US20110206729A1 (en) | 2008-10-31 | 2009-10-30 | Mucosal vaccine using cationic nanogel |
| US14/478,127 Division US8961983B2 (en) | 2008-10-31 | 2014-09-05 | Mucosal vaccine using cationic nanogel |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2010050578A1 true WO2010050578A1 (ja) | 2010-05-06 |
Family
ID=42128937
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2009/068647 Ceased WO2010050578A1 (ja) | 2008-10-31 | 2009-10-30 | カチオン性ナノゲルを用いる粘膜ワクチン |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US20110206729A1 (enExample) |
| EP (1) | EP2345419B8 (enExample) |
| JP (1) | JP5344558B2 (enExample) |
| ES (1) | ES2624722T3 (enExample) |
| WO (1) | WO2010050578A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013085021A1 (ja) * | 2011-12-09 | 2013-06-13 | 株式会社林原 | 抗体産生増強用の組成物 |
| WO2022210465A1 (ja) * | 2021-03-30 | 2022-10-06 | 国立大学法人東京大学 | ナノゲル被覆型ワクチン |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2153820A4 (en) * | 2007-05-14 | 2013-12-25 | Konica Minolta Holdings Inc | LIPOSOME AND METHOD FOR PREPARING THE SAME |
| JP5344558B2 (ja) | 2008-10-31 | 2013-11-20 | 国立大学法人 東京医科歯科大学 | カチオン性ナノゲルを用いる粘膜ワクチン |
| MX365496B (es) | 2009-10-21 | 2019-06-05 | Revance Therapeutics Inc | Metodos y sistemas para purificar la neurotoxina botulinica no acomplejada. |
| JP5860480B2 (ja) | 2011-01-11 | 2016-02-16 | キャプシュゲル・ベルジウム・エヌ・ヴィ | プルランを含む新しい硬カプセル |
| ES2673957T5 (es) | 2011-10-03 | 2021-12-15 | Croda Int Plc | Nanopartículas, procedimiento para la preparación y utilización de las mismas como portadoras de moléculas anfipáticas o hidrofóbicas en el campo de la medicina, incluyendo el tratamiento del cáncer, y compuestos de tipo alimentario |
| JP6310720B2 (ja) * | 2014-02-17 | 2018-04-11 | 知的財産戦略ネットワーク株式会社 | 肺炎球菌経鼻ワクチン |
| WO2015186678A1 (ja) * | 2014-06-04 | 2015-12-10 | 第一三共株式会社 | 粘膜ワクチン用アジュバント |
| US20170319706A1 (en) * | 2014-11-18 | 2017-11-09 | Dow Global Technologies Llc | Delivering a drug to a mucosal surface |
| EP3548076A2 (en) | 2016-12-03 | 2019-10-09 | The UAB Research Foundation | Pneumococcal vaccine combining selected alpha helical domains and proline rich domains of pneumococcal surface protein a |
| EP3609476B1 (en) | 2017-04-14 | 2025-06-04 | Capsugel Belgium NV | Pullulan capsules |
| AU2018253392B2 (en) | 2017-04-14 | 2023-11-02 | Capsugel Belgium Nv | Process for making pullulan |
| CN107583059B (zh) * | 2017-10-31 | 2021-03-30 | 宁夏医科大学 | 一种可包载量子点的阳离子脂质体流感疫苗及其制备方法 |
| CN112566656A (zh) * | 2018-08-03 | 2021-03-26 | 国立大学法人东京大学 | 诱导细胞性免疫的经鼻疫苗 |
| JP2020019754A (ja) * | 2018-08-03 | 2020-02-06 | 国立研究開発法人農業・食品産業技術総合研究機構 | ウシ乳房炎に対する粘膜ワクチン組成物 |
| JP2024545092A (ja) | 2021-12-08 | 2024-12-05 | イミュニティバイオ,インコーポレーテッド | ネオエピトープワクチン送達賦形剤及びそれを製造する方法 |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0433497B2 (enExample) | 1987-04-28 | 1992-06-03 | Nittetsu Mining Co Ltd | |
| JPH05339169A (ja) | 1992-03-03 | 1993-12-21 | Dai Ichi Seiyaku Co Ltd | 経口ワクチン |
| WO1998009650A1 (en) * | 1996-09-06 | 1998-03-12 | Mitsubishi Chemical Corporation | Vaccinal preparations |
| WO2000012564A1 (en) | 1998-08-31 | 2000-03-09 | Nof Corporation | High-purity polysaccharide containing hydrophobic groups and process for producing the same |
| JP2005298644A (ja) | 2004-04-09 | 2005-10-27 | Kazunari Akiyoshi | ナノゲル工学によるハイブリッドゲルの調製とバイオマテリアル応用 |
| WO2006049032A1 (ja) | 2004-11-01 | 2006-05-11 | Tokyo Medical And Dental University | ナノゲル-アパタイト複合体の調製 |
| JP2006143808A (ja) | 2004-11-17 | 2006-06-08 | Tokyo Medical & Dental Univ | 量子ドット(Qdot)−ナノゲル複合体の調製 |
| WO2007083643A1 (ja) | 2006-01-18 | 2007-07-26 | National University Corporation Tokyo Medical And Dental University | 骨形成促進物質とナノゲルを含有する骨形成用生体材料 |
| JP2007252304A (ja) | 2006-03-24 | 2007-10-04 | Tokyo Medical & Dental Univ | ナノゲルを用いたタンパク質の細胞内導入法 |
| JP2008231343A (ja) * | 2007-03-23 | 2008-10-02 | Kumamoto Univ | ワクチン剤 |
| JP2008281065A (ja) | 2007-05-09 | 2008-11-20 | Nsk Ltd | 玉軸受 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5866135A (en) * | 1994-04-21 | 1999-02-02 | North American Vaccine, Inc. | Group A streptococcal polysaccharide immunogenic compositions and methods |
| US6967088B1 (en) * | 1995-03-16 | 2005-11-22 | Allergan, Inc. | Soluble recombinant botulinum toxin proteins |
| GB0717864D0 (en) * | 2007-09-13 | 2007-10-24 | Peptcell Ltd | Peptide sequences and compositions |
| JP5344558B2 (ja) | 2008-10-31 | 2013-11-20 | 国立大学法人 東京医科歯科大学 | カチオン性ナノゲルを用いる粘膜ワクチン |
-
2008
- 2008-10-31 JP JP2008281065A patent/JP5344558B2/ja active Active
-
2009
- 2009-10-30 US US13/126,357 patent/US20110206729A1/en not_active Abandoned
- 2009-10-30 WO PCT/JP2009/068647 patent/WO2010050578A1/ja not_active Ceased
- 2009-10-30 EP EP09823688.8A patent/EP2345419B8/en active Active
- 2009-10-30 ES ES09823688.8T patent/ES2624722T3/es active Active
-
2014
- 2014-09-05 US US14/478,127 patent/US8961983B2/en active Active
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0433497B2 (enExample) | 1987-04-28 | 1992-06-03 | Nittetsu Mining Co Ltd | |
| JPH05339169A (ja) | 1992-03-03 | 1993-12-21 | Dai Ichi Seiyaku Co Ltd | 経口ワクチン |
| WO1998009650A1 (en) * | 1996-09-06 | 1998-03-12 | Mitsubishi Chemical Corporation | Vaccinal preparations |
| WO2000012564A1 (en) | 1998-08-31 | 2000-03-09 | Nof Corporation | High-purity polysaccharide containing hydrophobic groups and process for producing the same |
| JP2005298644A (ja) | 2004-04-09 | 2005-10-27 | Kazunari Akiyoshi | ナノゲル工学によるハイブリッドゲルの調製とバイオマテリアル応用 |
| WO2006049032A1 (ja) | 2004-11-01 | 2006-05-11 | Tokyo Medical And Dental University | ナノゲル-アパタイト複合体の調製 |
| JP2006143808A (ja) | 2004-11-17 | 2006-06-08 | Tokyo Medical & Dental Univ | 量子ドット(Qdot)−ナノゲル複合体の調製 |
| WO2007083643A1 (ja) | 2006-01-18 | 2007-07-26 | National University Corporation Tokyo Medical And Dental University | 骨形成促進物質とナノゲルを含有する骨形成用生体材料 |
| JP2007252304A (ja) | 2006-03-24 | 2007-10-04 | Tokyo Medical & Dental Univ | ナノゲルを用いたタンパク質の細胞内導入法 |
| JP2008231343A (ja) * | 2007-03-23 | 2008-10-02 | Kumamoto Univ | ワクチン剤 |
| JP2008281065A (ja) | 2007-05-09 | 2008-11-20 | Nsk Ltd | 玉軸受 |
Non-Patent Citations (3)
| Title |
|---|
| HASEGAWA ET AL., SAIBOU KOUGAKU, vol. 26, no. 6, 2007, pages 679 - 685 |
| See also references of EP2345419A4 |
| SHUNJI KOZAKI: "Division of Veterinary Science, School of Life and Environmental Sciences", OSAKA PREFECTURE UNIVERSITY |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013085021A1 (ja) * | 2011-12-09 | 2013-06-13 | 株式会社林原 | 抗体産生増強用の組成物 |
| WO2022210465A1 (ja) * | 2021-03-30 | 2022-10-06 | 国立大学法人東京大学 | ナノゲル被覆型ワクチン |
| EP4316511A4 (en) * | 2021-03-30 | 2025-05-21 | The University of Tokyo | NANOGEL-COATED VACCINE |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2010105968A (ja) | 2010-05-13 |
| EP2345419B1 (en) | 2017-02-22 |
| US20110206729A1 (en) | 2011-08-25 |
| ES2624722T3 (es) | 2017-07-17 |
| US20140370056A1 (en) | 2014-12-18 |
| EP2345419B8 (en) | 2017-04-26 |
| JP5344558B2 (ja) | 2013-11-20 |
| EP2345419A4 (en) | 2013-07-10 |
| EP2345419A1 (en) | 2011-07-20 |
| US8961983B2 (en) | 2015-02-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5344558B2 (ja) | カチオン性ナノゲルを用いる粘膜ワクチン | |
| JP6407208B2 (ja) | 合成ナノキャリア混合ワクチン | |
| JP6382936B2 (ja) | ナノ粒子ベースの組成物 | |
| KR20180043352A (ko) | 다가 vlp 접합체 | |
| Lahiri et al. | Nanoparticles based antibacterial vaccines: Novel strategy to combat antimicrobial resistance | |
| Mat Rani et al. | Outer membrane vesicles as biomimetic vaccine carriers against infections and cancers | |
| US9540420B2 (en) | Mucosal vaccines | |
| ES2374648T3 (es) | Composiciones de vacunas liposómicas que comprenden un antígeno polisacárido y un adyuvante proteico. | |
| JP2023091085A (ja) | 粘膜アジュバント | |
| WO2013085021A1 (ja) | 抗体産生増強用の組成物 | |
| Yılmaz Çolak | Bacterial Membrane Vesicles as a Novel Vaccine Platform against SARS-CoV-2 | |
| Rosales-Mendoza et al. | Silica-based mucosal nanovaccines | |
| US20250144203A1 (en) | Nanostructure-based vector system for bacterial and viral antigens | |
| Ohta | Cholesteryl Pullulan Nanoparticles-Encapsulated TNF-a: An Effective Mucosal Vaccine Adjuvant Against | |
| Nayerhoda | Development of Liposomal Encapsulation of Polysaccharides (Leps) Vaccine Design and Protection in Aged Hosts against Streptococcus pneumoniae Infections | |
| CA3212267A1 (en) | Nanogel-coated vaccine | |
| HK40043455A (en) | Nanoparticle-based compositions | |
| HK1192462A (en) | Novel vaccine containing adjuvant capable of inducing mucosal immunity | |
| BRPI0700860B1 (pt) | Forma farmacêutica na forma de uma microesfera de pss recoberta com dodab e biomolécula com carga oposta à camada de dodab, composição farmacêutica, medicamento, e uso da forma farmacêutica |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 09823688 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 13126357 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2009823688 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2009823688 Country of ref document: EP |