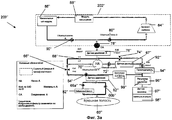
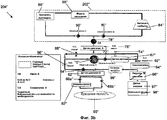
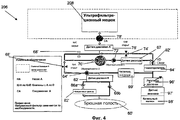
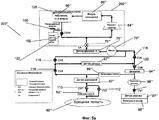
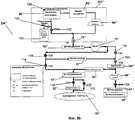
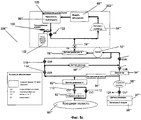
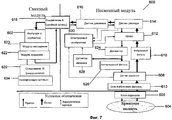
RU2525205C2 - Проточная система устройства диализа и переносное устройство диализа - Google Patents
Проточная система устройства диализа и переносное устройство диализа Download PDFInfo
- Publication number
- RU2525205C2 RU2525205C2 RU2011102163/14A RU2011102163A RU2525205C2 RU 2525205 C2 RU2525205 C2 RU 2525205C2 RU 2011102163/14 A RU2011102163/14 A RU 2011102163/14A RU 2011102163 A RU2011102163 A RU 2011102163A RU 2525205 C2 RU2525205 C2 RU 2525205C2
- Authority
- RU
- Russia
- Prior art keywords
- specified
- dialysate
- flow line
- pump
- sorbent
- Prior art date
Links
- 238000000502 dialysis Methods 0.000 title claims abstract description 128
- 239000002594 sorbent Substances 0.000 claims abstract description 166
- 210000000683 abdominal cavity Anatomy 0.000 claims abstract description 72
- 239000000126 substance Substances 0.000 claims abstract description 38
- 238000012546 transfer Methods 0.000 claims abstract description 34
- 239000007788 liquid Substances 0.000 claims abstract description 27
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 claims description 84
- 239000007789 gas Substances 0.000 claims description 49
- 229910021529 ammonia Inorganic materials 0.000 claims description 42
- 102000009123 Fibrin Human genes 0.000 claims description 38
- 108010073385 Fibrin Proteins 0.000 claims description 38
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 claims description 38
- 229950003499 fibrin Drugs 0.000 claims description 38
- 238000003860 storage Methods 0.000 claims description 32
- 239000000356 contaminant Substances 0.000 claims description 27
- 244000005700 microbiome Species 0.000 claims description 27
- 239000012530 fluid Substances 0.000 claims description 23
- 238000000746 purification Methods 0.000 claims description 10
- 239000003344 environmental pollutant Substances 0.000 claims description 7
- 231100000719 pollutant Toxicity 0.000 claims description 7
- 238000009825 accumulation Methods 0.000 claims description 6
- 238000001914 filtration Methods 0.000 claims description 6
- 230000005540 biological transmission Effects 0.000 claims description 5
- 238000004140 cleaning Methods 0.000 claims description 5
- 230000002572 peristaltic effect Effects 0.000 claims description 5
- 238000005086 pumping Methods 0.000 claims description 5
- 206010062237 Renal impairment Diseases 0.000 claims description 4
- 230000033001 locomotion Effects 0.000 claims description 4
- 231100000857 poor renal function Toxicity 0.000 claims description 4
- 238000007599 discharging Methods 0.000 claims description 3
- 238000012544 monitoring process Methods 0.000 claims description 3
- 230000000694 effects Effects 0.000 abstract description 4
- 239000003814 drug Substances 0.000 abstract description 3
- 230000005611 electricity Effects 0.000 abstract 1
- 208000017169 kidney disease Diseases 0.000 abstract 1
- 230000008901 benefit Effects 0.000 description 32
- 238000000034 method Methods 0.000 description 17
- 238000010586 diagram Methods 0.000 description 16
- 238000000108 ultra-filtration Methods 0.000 description 14
- 230000000844 anti-bacterial effect Effects 0.000 description 13
- 238000001631 haemodialysis Methods 0.000 description 9
- 230000000322 hemodialysis Effects 0.000 description 9
- 230000002159 abnormal effect Effects 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 239000012528 membrane Substances 0.000 description 7
- 239000003053 toxin Substances 0.000 description 7
- 231100000765 toxin Toxicity 0.000 description 7
- 108700012359 toxins Proteins 0.000 description 7
- 230000008569 process Effects 0.000 description 6
- 230000001960 triggered effect Effects 0.000 description 6
- 241000894006 Bacteria Species 0.000 description 5
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 5
- 230000002411 adverse Effects 0.000 description 5
- 230000002209 hydrophobic effect Effects 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 239000000546 pharmaceutical excipient Substances 0.000 description 5
- 239000004202 carbamide Substances 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 239000003792 electrolyte Substances 0.000 description 4
- 238000005265 energy consumption Methods 0.000 description 4
- 239000004973 liquid crystal related substance Substances 0.000 description 4
- 230000002503 metabolic effect Effects 0.000 description 4
- 235000015097 nutrients Nutrition 0.000 description 4
- 208000001647 Renal Insufficiency Diseases 0.000 description 3
- 230000003187 abdominal effect Effects 0.000 description 3
- 230000009931 harmful effect Effects 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 210000003734 kidney Anatomy 0.000 description 3
- 201000006370 kidney failure Diseases 0.000 description 3
- 238000007726 management method Methods 0.000 description 3
- 230000000813 microbial effect Effects 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 239000002699 waste material Substances 0.000 description 3
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 229940126062 Compound A Drugs 0.000 description 2
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 2
- 239000004677 Nylon Substances 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 238000004422 calculation algorithm Methods 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 238000011109 contamination Methods 0.000 description 2
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 229920001778 nylon Polymers 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- -1 polypropylene Polymers 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 230000001172 regenerating effect Effects 0.000 description 2
- 230000008929 regeneration Effects 0.000 description 2
- 238000011069 regeneration method Methods 0.000 description 2
- 230000035939 shock Effects 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 239000002441 uremic toxin Substances 0.000 description 2
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 206010067484 Adverse reaction Diseases 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 208000032953 Device battery issue Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 125000002066 L-histidyl group Chemical group [H]N1C([H])=NC(C([H])([H])[C@](C(=O)[*])([H])N([H])[H])=C1[H] 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- LEHOTFFKMJEONL-UHFFFAOYSA-N Uric Acid Chemical compound N1C(=O)NC(=O)C2=C1NC(=O)N2 LEHOTFFKMJEONL-UHFFFAOYSA-N 0.000 description 1
- TVWHNULVHGKJHS-UHFFFAOYSA-N Uric acid Natural products N1C(=O)NC(=O)C2NC(=O)NC21 TVWHNULVHGKJHS-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 229940127219 anticoagulant drug Drugs 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000002457 bidirectional effect Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 208000020832 chronic kidney disease Diseases 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000007485 conventional hemodialysis Methods 0.000 description 1
- 229940109239 creatinine Drugs 0.000 description 1
- 238000013500 data storage Methods 0.000 description 1
- 238000001784 detoxification Methods 0.000 description 1
- 239000000385 dialysis solution Substances 0.000 description 1
- 235000015872 dietary supplement Nutrition 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 208000028208 end stage renal disease Diseases 0.000 description 1
- 201000000523 end stage renal failure Diseases 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000037023 motor activity Effects 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 230000037081 physical activity Effects 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 229920002492 poly(sulfone) Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 239000002516 radical scavenger Substances 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 239000003440 toxic substance Substances 0.000 description 1
- 239000010891 toxic waste Substances 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 229940045136 urea Drugs 0.000 description 1
- 229940116269 uric acid Drugs 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/14—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis
- A61M1/16—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis with membranes
- A61M1/1694—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis with membranes with recirculating dialysing liquid
- A61M1/1696—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis with membranes with recirculating dialysing liquid with dialysate regeneration
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/22—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising organic material
- B01J20/24—Naturally occurring macromolecular compounds, e.g. humic acids or their derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/14—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis
- A61M1/16—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis with membranes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/14—Dialysis systems; Artificial kidneys; Blood oxygenators ; Reciprocating systems for treatment of body fluids, e.g. single needle systems for hemofiltration or pheresis
- A61M1/28—Peritoneal dialysis ; Other peritoneal treatment, e.g. oxygenation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/36—Other treatment of blood in a by-pass of the natural circulatory system, e.g. temperature adaptation, irradiation ; Extra-corporeal blood circuits
- A61M1/3679—Other treatment of blood in a by-pass of the natural circulatory system, e.g. temperature adaptation, irradiation ; Extra-corporeal blood circuits by absorption
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M1/00—Suction or pumping devices for medical purposes; Devices for carrying-off, for treatment of, or for carrying-over, body-liquids; Drainage systems
- A61M1/36—Other treatment of blood in a by-pass of the natural circulatory system, e.g. temperature adaptation, irradiation ; Extra-corporeal blood circuits
- A61M1/3687—Chemical treatment
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/10—Selective adsorption, e.g. chromatography characterised by constructional or operational features
- B01D15/20—Selective adsorption, e.g. chromatography characterised by constructional or operational features relating to the conditioning of the sorbent material
- B01D15/206—Packing or coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/26—Selective adsorption, e.g. chromatography characterised by the separation mechanism
- B01D15/36—Selective adsorption, e.g. chromatography characterised by the separation mechanism involving ionic interaction, e.g. ion-exchange, ion-pair, ion-suppression or ion-exclusion
- B01D15/361—Ion-exchange
- B01D15/362—Cation-exchange
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/26—Selective adsorption, e.g. chromatography characterised by the separation mechanism
- B01D15/36—Selective adsorption, e.g. chromatography characterised by the separation mechanism involving ionic interaction, e.g. ion-exchange, ion-pair, ion-suppression or ion-exclusion
- B01D15/361—Ion-exchange
- B01D15/363—Anion-exchange
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/0203—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising compounds of metals not provided for in B01J20/04
- B01J20/0211—Compounds of Ti, Zr, Hf
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/06—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising oxides or hydroxides of metals not provided for in group B01J20/04
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/02—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material
- B01J20/20—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising inorganic material comprising free carbon; comprising carbon obtained by carbonising processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/22—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof comprising organic material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28014—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their form
- B01J20/28033—Membrane, sheet, cloth, pad, lamellar or mat
- B01J20/28035—Membrane, sheet, cloth, pad, lamellar or mat with more than one layer, e.g. laminates, separated sheets
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28014—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their form
- B01J20/28052—Several layers of identical or different sorbents stacked in a housing, e.g. in a column
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J39/00—Cation exchange; Use of material as cation exchangers; Treatment of material for improving the cation exchange properties
- B01J39/08—Use of material as cation exchangers; Treatment of material for improving the cation exchange properties
- B01J39/12—Compounds containing phosphorus
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J41/00—Anion exchange; Use of material as anion exchangers; Treatment of material for improving the anion exchange properties
- B01J41/08—Use of material as anion exchangers; Treatment of material for improving the anion exchange properties
- B01J41/10—Inorganic material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2220/00—Aspects relating to sorbent materials
- B01J2220/40—Aspects relating to the composition of sorbent or filter aid materials
- B01J2220/46—Materials comprising a mixture of inorganic and organic materials
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Heart & Thoracic Surgery (AREA)
- Analytical Chemistry (AREA)
- Organic Chemistry (AREA)
- Vascular Medicine (AREA)
- Urology & Nephrology (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Inorganic Chemistry (AREA)
- Cardiology (AREA)
- General Chemical & Material Sciences (AREA)
- External Artificial Organs (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US7499708P | 2008-06-23 | 2008-06-23 | |
| US61/074,997 | 2008-06-23 | ||
| PCT/SG2009/000230 WO2009157878A1 (en) | 2008-06-23 | 2009-06-22 | A flow system of a dialysis device and a portable dialysis device |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2011102163A RU2011102163A (ru) | 2012-07-27 |
| RU2525205C2 true RU2525205C2 (ru) | 2014-08-10 |
Family
ID=41444786
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2011102163/14A RU2525205C2 (ru) | 2008-06-23 | 2009-06-22 | Проточная система устройства диализа и переносное устройство диализа |
| RU2011102164/05A RU2514956C2 (ru) | 2008-06-23 | 2009-06-22 | Сорбент для диализа |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2011102164/05A RU2514956C2 (ru) | 2008-06-23 | 2009-06-22 | Сорбент для диализа |
Country Status (11)
| Country | Link |
|---|---|
| US (4) | US9242036B2 (enExample) |
| EP (2) | EP2303354B1 (enExample) |
| JP (4) | JP5439482B2 (enExample) |
| CN (3) | CN102123749A (enExample) |
| AU (2) | AU2009263046B2 (enExample) |
| BR (2) | BRPI0915397B8 (enExample) |
| ES (2) | ES2823159T3 (enExample) |
| NZ (2) | NZ590471A (enExample) |
| RU (2) | RU2525205C2 (enExample) |
| TW (2) | TWI482659B (enExample) |
| WO (2) | WO2009157877A1 (enExample) |
Families Citing this family (165)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8202248B2 (en) | 2004-08-18 | 2012-06-19 | Sequana Medical Ag | Dialysis implant and methods of use |
| WO2006086490A1 (en) | 2005-02-07 | 2006-08-17 | Medtronic, Inc. | Ion imbalance detector |
| KR20140021725A (ko) | 2005-12-13 | 2014-02-20 | 엑스테라 메디컬 코퍼레이션 | 혈액으로부터 병원성 미생물, 염증 세포 또는 염증 단백질의 체외 제거를 위한 방법 |
| US8240636B2 (en) | 2009-01-12 | 2012-08-14 | Fresenius Medical Care Holdings, Inc. | Valve system |
| US8535522B2 (en) | 2009-02-12 | 2013-09-17 | Fresenius Medical Care Holdings, Inc. | System and method for detection of disconnection in an extracorporeal blood circuit |
| US9358331B2 (en) | 2007-09-13 | 2016-06-07 | Fresenius Medical Care Holdings, Inc. | Portable dialysis machine with improved reservoir heating system |
| US8597505B2 (en) | 2007-09-13 | 2013-12-03 | Fresenius Medical Care Holdings, Inc. | Portable dialysis machine |
| US9308307B2 (en) | 2007-09-13 | 2016-04-12 | Fresenius Medical Care Holdings, Inc. | Manifold diaphragms |
| US8137553B2 (en) | 2007-11-29 | 2012-03-20 | Fresenius Medical Care Holdings, Inc. | Priming system and method for dialysis systems |
| US9199022B2 (en) | 2008-09-12 | 2015-12-01 | Fresenius Medical Care Holdings, Inc. | Modular reservoir assembly for a hemodialysis and hemofiltration system |
| US8040493B2 (en) | 2007-10-11 | 2011-10-18 | Fresenius Medical Care Holdings, Inc. | Thermal flow meter |
| US8105487B2 (en) | 2007-09-25 | 2012-01-31 | Fresenius Medical Care Holdings, Inc. | Manifolds for use in conducting dialysis |
| CA3057807C (en) | 2007-11-29 | 2021-04-20 | Thomas P. Robinson | System and method for conducting hemodialysis and hemofiltration |
| DE102008011827A1 (de) | 2008-02-29 | 2009-09-10 | Fresenius Medical Care Deutschland Gmbh | Verfahren zur Ansteuerung von Ventilen zur Flusswegsteuerung und Maschinen, insbesondere medizinische Behandlungsmaschinen |
| EP2303354B1 (en) | 2008-06-23 | 2020-08-12 | Temasek Polytechnic | A sorbent for a dialysis device |
| KR101508483B1 (ko) | 2008-10-30 | 2015-04-06 | 프레제니우스 메디칼 케어 홀딩스 인코퍼레이티드 | 모듈화된 휴대용 투석장치 |
| US9399091B2 (en) | 2009-09-30 | 2016-07-26 | Medtronic, Inc. | System and method to regulate ultrafiltration |
| WO2011068897A1 (en) | 2009-12-01 | 2011-06-09 | Exthera Medical, Llc | Method for removing cytokines from blood with surface immobilized polysaccharides |
| GB201002824D0 (en) * | 2010-02-19 | 2010-04-07 | Temasek Polytechnic | A method of preparing a substrate for immobilization of functional substances thereon and the substrate obtained therefrom |
| PL2590696T3 (pl) * | 2010-07-05 | 2017-08-31 | Gambro Lundia Ab | Ambulatoryjne urządzenie do ultrafiltracji, powiązane sposoby i produkt w postaci programu komputerowego |
| GB201019228D0 (en) * | 2010-11-15 | 2010-12-29 | Corp | Dialysis device and method of dialysis |
| WO2012112724A1 (en) | 2011-02-15 | 2012-08-23 | Exthera Medical, Llc | Device and method for removal of blood-borne pathogens, toxins and inflammatory cytokines |
| EP2675518B1 (en) | 2011-02-16 | 2019-05-15 | Sequana Medical AG | Apparatus for treating intracorporeal fluid accumulation |
| CN103619372A (zh) | 2011-03-23 | 2014-03-05 | 纳科斯达格医药股份有限公司 | 腹膜透析系统、装置和方法 |
| US9861733B2 (en) | 2012-03-23 | 2018-01-09 | Nxstage Medical Inc. | Peritoneal dialysis systems, devices, and methods |
| US9848778B2 (en) | 2011-04-29 | 2017-12-26 | Medtronic, Inc. | Method and device to monitor patients with kidney disease |
| US9302036B2 (en) | 2011-04-29 | 2016-04-05 | Medtronic, Inc. | Blood fluid removal system performance monitoring |
| US9456755B2 (en) | 2011-04-29 | 2016-10-04 | Medtronic, Inc. | Method and device to monitor patients with kidney disease |
| US9017277B2 (en) | 2011-05-02 | 2015-04-28 | Medtronic, Inc. | System and implantable device for treating chronic kidney disease |
| BR112014001614A2 (pt) * | 2011-07-29 | 2017-02-21 | Baxter Healthcare Sa | aparelho para o tratamento de dialisado gasto, aparelho para a execução de uma terapia de diálise e cartucho de regeneração de dialisado |
| CN105288763B (zh) | 2011-08-02 | 2018-01-02 | 美敦力公司 | 带有具有可控的顺应性容积的流动路径的血液透析系统 |
| WO2013025844A2 (en) | 2011-08-16 | 2013-02-21 | Medtronic, Inc. | Modular hemodialysis system |
| CN103842004B (zh) | 2011-08-22 | 2016-11-23 | 美敦力公司 | 双流吸附剂盒 |
| US9463267B2 (en) | 2011-10-21 | 2016-10-11 | National University Of Singapore | Array of elements and a human-computer interface device |
| AU2012336423B2 (en) * | 2011-11-08 | 2017-08-03 | Temasek Polytechnic | Sensing system for detecting a substance in a dialysate |
| US10018607B2 (en) * | 2011-11-08 | 2018-07-10 | Temasek Polytechnic | Sensing system for detecting a substance in a dialysate |
| WO2013101888A1 (en) * | 2011-12-29 | 2013-07-04 | Fresenius Medical Care Holdings, Inc. | Materials for removal of toxins in sorbent dialysis and methods and systems using same |
| US9713668B2 (en) | 2012-01-04 | 2017-07-25 | Medtronic, Inc. | Multi-staged filtration system for blood fluid removal |
| MX355633B (es) * | 2012-01-19 | 2018-04-25 | Baxter Int | Método y composición para eliminar las toxinas urémicas en los procesos de diálisis. |
| US8585635B2 (en) | 2012-02-15 | 2013-11-19 | Sequana Medical Ag | Systems and methods for treating chronic liver failure based on peritoneal dialysis |
| EP2861273B1 (en) | 2012-06-13 | 2017-08-23 | ExThera Medical Corporation | Use of heparin and carbohydrates to treat cancer |
| SE537060C2 (sv) * | 2012-11-23 | 2014-12-23 | Triomed Ab | Regenereringskrets för regenerering av en dialysvätska innefattande en adsorbentpatron |
| US20140158588A1 (en) | 2012-12-10 | 2014-06-12 | Medtronic, Inc. | pH AND BUFFER MANAGEMENT SYSTEM FOR HEMODIALYSIS SYSTEMS |
| US9399090B2 (en) | 2012-12-10 | 2016-07-26 | Medtronic, Inc. | Potassium loaded ion-exchange material for use in a dialysate regeneration system |
| US10905816B2 (en) | 2012-12-10 | 2021-02-02 | Medtronic, Inc. | Sodium management system for hemodialysis |
| US9201036B2 (en) | 2012-12-21 | 2015-12-01 | Fresenius Medical Care Holdings, Inc. | Method and system of monitoring electrolyte levels and composition using capacitance or induction |
| US9157786B2 (en) | 2012-12-24 | 2015-10-13 | Fresenius Medical Care Holdings, Inc. | Load suspension and weighing system for a dialysis machine reservoir |
| US9713666B2 (en) | 2013-01-09 | 2017-07-25 | Medtronic, Inc. | Recirculating dialysate fluid circuit for blood measurement |
| US11565029B2 (en) | 2013-01-09 | 2023-01-31 | Medtronic, Inc. | Sorbent cartridge with electrodes |
| US9707328B2 (en) | 2013-01-09 | 2017-07-18 | Medtronic, Inc. | Sorbent cartridge to measure solute concentrations |
| US11154648B2 (en) | 2013-01-09 | 2021-10-26 | Medtronic, Inc. | Fluid circuits for sorbent cartridge with sensors |
| US9579443B2 (en) | 2013-01-10 | 2017-02-28 | Fresenius Medical Care Holdings, Inc. | Peritoneal dialysis systems and related devices and methods |
| US10543052B2 (en) | 2013-02-01 | 2020-01-28 | Medtronic, Inc. | Portable dialysis cabinet |
| US9526822B2 (en) | 2013-02-01 | 2016-12-27 | Medtronic, Inc. | Sodium and buffer source cartridges for use in a modular controlled compliant flow path |
| US9173987B2 (en) * | 2013-02-01 | 2015-11-03 | Medtronic, Inc. | Degassing module for a controlled compliant flow path |
| US10010663B2 (en) | 2013-02-01 | 2018-07-03 | Medtronic, Inc. | Fluid circuit for delivery of renal replacement therapies |
| US10850016B2 (en) | 2013-02-01 | 2020-12-01 | Medtronic, Inc. | Modular fluid therapy system having jumpered flow paths and systems and methods for cleaning and disinfection |
| WO2014121159A1 (en) | 2013-02-01 | 2014-08-07 | Medtronic, Inc. | Fluid circuits for sorbent cartridges with sensors |
| EP2950838B1 (en) | 2013-02-01 | 2020-04-01 | Medtronic, Inc. | Sorbent cartridge with electrodes |
| US9623164B2 (en) | 2013-02-01 | 2017-04-18 | Medtronic, Inc. | Systems and methods for multifunctional volumetric fluid control |
| US9144640B2 (en) | 2013-02-02 | 2015-09-29 | Medtronic, Inc. | Sorbent cartridge configurations for improved dialysate regeneration |
| US9827361B2 (en) | 2013-02-02 | 2017-11-28 | Medtronic, Inc. | pH buffer measurement system for hemodialysis systems |
| US20160022897A1 (en) | 2013-03-13 | 2016-01-28 | Nxstage Medical, Inc. | Single pass dialysis combined with multiple pass albumin dialysis |
| US20140263062A1 (en) | 2013-03-14 | 2014-09-18 | Fresenius Medical Care Holdings, Inc. | Universal portable machine for online hemodiafiltration using regenerated dialysate |
| US9433720B2 (en) | 2013-03-14 | 2016-09-06 | Fresenius Medical Care Holdings, Inc. | Universal portable artificial kidney for hemodialysis and peritoneal dialysis |
| EP3013445B1 (en) | 2013-06-24 | 2019-11-06 | ExThera Medical Corporation | Blood filtration system containing mannose coated substrate |
| EP3102107A4 (en) | 2013-11-04 | 2018-02-07 | Medtronic, Inc. | Method and device to manage fluid volumes in the body |
| CN105705177B (zh) | 2013-11-08 | 2019-10-22 | 艾克塞拉医疗公司 | 使用吸附介质诊断感染性疾病的方法 |
| US9354640B2 (en) | 2013-11-11 | 2016-05-31 | Fresenius Medical Care Holdings, Inc. | Smart actuator for valve |
| US10099215B2 (en) | 2013-11-26 | 2018-10-16 | Medtronic, Inc. | Management of recharger effluent pH |
| US9895477B2 (en) | 2013-11-26 | 2018-02-20 | Medtronic, Inc. | Detachable module for recharging sorbent materials with optional bypass |
| US10052612B2 (en) | 2013-11-26 | 2018-08-21 | Medtronic, Inc. | Zirconium phosphate recharging method and apparatus |
| US10052624B2 (en) | 2013-11-26 | 2018-08-21 | Medtronic, Inc. | Zirconium phosphate and zirconium oxide recharging flow paths |
| US10537875B2 (en) | 2013-11-26 | 2020-01-21 | Medtronic, Inc. | Precision recharging of sorbent materials using patient and session data |
| US10004839B2 (en) | 2013-11-26 | 2018-06-26 | Medtronic, Inc. | Multi-use sorbent cartridge |
| US9943780B2 (en) | 2013-11-26 | 2018-04-17 | Medtronic, Inc. | Module for in-line recharging of sorbent materials with optional bypass |
| US9981245B2 (en) | 2013-11-26 | 2018-05-29 | Medtronic, Inc. | Method and apparatus for zirconium oxide recharging |
| US10099214B2 (en) | 2013-11-26 | 2018-10-16 | Medtronic, Inc. | Zirconium phosphate and zirconium oxide recharger control logic and operational process algorithms |
| US9884145B2 (en) | 2013-11-26 | 2018-02-06 | Medtronic, Inc. | Parallel modules for in-line recharging of sorbents using alternate duty cycles |
| US10159957B2 (en) | 2013-11-26 | 2018-12-25 | Medtronic, Inc. | Zirconium phosphate recharging customization |
| US10064986B2 (en) | 2013-11-26 | 2018-09-04 | Medtronic, Inc. | Recharger for recharging zirconium phosphate and zirconium oxide modules |
| US9974896B2 (en) * | 2014-06-24 | 2018-05-22 | Medtronic, Inc. | Method of zirconium phosphate recharging |
| EP3073911B8 (en) | 2013-11-27 | 2025-09-03 | Mozarc Medical US LLC | Precision dialysis monitoring and synchonization system |
| DE102013021957A1 (de) | 2013-12-20 | 2015-06-25 | Fresenius Medical Care Deutschland Gmbh | Verfahren zur Entfernung proteingebundener Urämietoxine durch Adsorption an dialysierbare Hilfsstoffe |
| JP6259108B2 (ja) * | 2014-03-17 | 2018-01-10 | フレセニウス メディカル ケア ホールディングス インコーポレーテッド | 透析溶液の浄化に有用なカートリッジ |
| CA2946294C (en) | 2014-04-24 | 2023-03-21 | Exthera Medical Corporation | Method for removing bacteria from blood using high flow rate |
| US10596309B2 (en) | 2014-04-25 | 2020-03-24 | Nextkidney Sa | Hemodialysis system |
| WO2015173833A2 (en) * | 2014-05-13 | 2015-11-19 | Gowrishankar W | Mobile continuous ambulatory peritoneal dialysis system |
| EP3160533B1 (en) | 2014-06-24 | 2020-08-12 | Medtronic Inc. | Sorbent pouch |
| CN106659827B (zh) | 2014-06-24 | 2019-07-09 | 美敦力公司 | 使用脲酶袋补充透析系统中的脲酶 |
| US10272363B2 (en) | 2014-06-24 | 2019-04-30 | Medtronic, Inc. | Urease introduction system for replenishing urease in a sorbent cartridge |
| WO2015199766A1 (en) | 2014-06-24 | 2015-12-30 | Medtronic, Inc. | Modular dialysate regeneration assembly |
| EP3160531B1 (en) * | 2014-06-24 | 2019-08-14 | Medtronic Inc. | Replenisihing urease in dialysis systems using a urease introducer |
| EP3160534A4 (en) | 2014-06-24 | 2018-03-07 | Medtronic Inc. | Stacked sorbent assembly |
| US10016550B2 (en) | 2014-09-12 | 2018-07-10 | Easydial, Inc. | Portable hemodialysis assembly with ammonia sensor |
| AU2015321601B2 (en) | 2014-09-22 | 2020-04-23 | Exthera Medical Corporation | Wearable hemoperfusion device |
| AU2015328547B2 (en) | 2014-10-06 | 2020-05-14 | Kci Licensing, Inc. | Multi-function dressing structure for negative-pressure therapy |
| US10874787B2 (en) | 2014-12-10 | 2020-12-29 | Medtronic, Inc. | Degassing system for dialysis |
| US10098993B2 (en) | 2014-12-10 | 2018-10-16 | Medtronic, Inc. | Sensing and storage system for fluid balance |
| US9713665B2 (en) | 2014-12-10 | 2017-07-25 | Medtronic, Inc. | Degassing system for dialysis |
| US9895479B2 (en) | 2014-12-10 | 2018-02-20 | Medtronic, Inc. | Water management system for use in dialysis |
| WO2016190853A1 (en) * | 2015-05-26 | 2016-12-01 | Medtronic, Inc. | Multi-use sorbent cartridge |
| WO2016191041A1 (en) * | 2015-05-26 | 2016-12-01 | Medtronic, Inc. | Zirconium phosphate and zirconium oxide recharging flow paths |
| US20160375190A1 (en) * | 2015-05-28 | 2016-12-29 | Cook Medical Technologies Llc | Peritoneal dialysis systems and methods |
| US11044984B2 (en) * | 2015-06-29 | 2021-06-29 | Novalung Gmbh | Carrying device for a gas exchange device |
| US10603421B2 (en) | 2015-09-16 | 2020-03-31 | Fresenius Medical Care Holdings, Inc. | Cartridges useful in cleaning dialysis solutions |
| CN105214624B (zh) * | 2015-11-02 | 2017-08-04 | 李建中 | 一种透析液吸附填料、其制备方法和应用 |
| US10335534B2 (en) | 2015-11-06 | 2019-07-02 | Medtronic, Inc. | Dialysis prescription optimization for decreased arrhythmias |
| US10786615B2 (en) | 2016-03-02 | 2020-09-29 | Exthera Medical Corporation | Method for treating drug intoxication |
| US11911551B2 (en) | 2016-03-02 | 2024-02-27 | Exthera Medical Corporation | Method for treating drug intoxication |
| US10874790B2 (en) | 2016-08-10 | 2020-12-29 | Medtronic, Inc. | Peritoneal dialysis intracycle osmotic agent adjustment |
| US12329892B2 (en) | 2016-04-04 | 2025-06-17 | Mozarc Medical Us Llc | Dextrose concentration sensor for a peritoneal dialysis system |
| US10994064B2 (en) | 2016-08-10 | 2021-05-04 | Medtronic, Inc. | Peritoneal dialysate flow path sensing |
| WO2017197493A1 (en) * | 2016-05-20 | 2017-11-23 | MCCALLUM, Angela Jane | Multi-purpose equipment stand |
| US10716922B2 (en) | 2016-08-26 | 2020-07-21 | Sequana Medical Nv | Implantable fluid management system having clog resistant catheters, and methods of using same |
| JP7071338B2 (ja) | 2016-08-26 | 2022-05-18 | セクアナ メディカル エヌブイ | 埋め込み型装置によって生成されたデータを管理及び分析するためのシステム及び方法 |
| US11013843B2 (en) | 2016-09-09 | 2021-05-25 | Medtronic, Inc. | Peritoneal dialysis fluid testing system |
| US10981148B2 (en) | 2016-11-29 | 2021-04-20 | Medtronic, Inc. | Zirconium oxide module conditioning |
| CA3044805A1 (en) | 2016-12-05 | 2018-06-14 | Temasek Polytechnic | Sorbent for a dialysis device and dialysis system |
| US11167070B2 (en) | 2017-01-30 | 2021-11-09 | Medtronic, Inc. | Ganged modular recharging system |
| WO2018215917A1 (en) | 2017-05-24 | 2018-11-29 | Sequana Medical Ag | Direct sodium removal method, solution and apparatus to reduce fluid overload in heart failure patients |
| US11559618B2 (en) | 2017-05-24 | 2023-01-24 | Sequana Medical Nv | Formulations and methods for direct sodium removal in patients having severe renal dysfunction |
| US10960381B2 (en) | 2017-06-15 | 2021-03-30 | Medtronic, Inc. | Zirconium phosphate disinfection recharging and conditioning |
| DE102017113853A1 (de) | 2017-06-22 | 2018-12-27 | B. Braun Avitum Ag | Hämokompatibler Adsorber zur Dialyse proteingebundener Urämietoxine |
| EP3641850B1 (en) | 2017-06-24 | 2024-10-09 | NxStage Medical Inc. | Peritoneal dialysis fluid preparation systems |
| US10607590B2 (en) * | 2017-09-05 | 2020-03-31 | Fresenius Medical Care Holdings, Inc. | Masking noises from medical devices, including dialysis machines |
| US11278654B2 (en) | 2017-12-07 | 2022-03-22 | Medtronic, Inc. | Pneumatic manifold for a dialysis system |
| CN108245725A (zh) * | 2018-01-15 | 2018-07-06 | 常洁 | 腹膜透析装置 |
| US11033667B2 (en) | 2018-02-02 | 2021-06-15 | Medtronic, Inc. | Sorbent manifold for a dialysis system |
| US11110215B2 (en) | 2018-02-23 | 2021-09-07 | Medtronic, Inc. | Degasser and vent manifolds for dialysis |
| WO2019169081A2 (en) | 2018-02-28 | 2019-09-06 | Nxstage Medical, Inc. | Fluid preparation and treatment devices, methods, and systems |
| CN108479110A (zh) * | 2018-04-10 | 2018-09-04 | 深圳市菲鹏生物制药股份有限公司 | 一种细胞培养液脱色剂及脱色方法 |
| US20200040323A1 (en) * | 2018-07-31 | 2020-02-06 | Fresenius Medical Care Holdings, Inc. | Urease Purification And Purified Urease Products Thereof And Sorbent Cartridges, Systems And Methods Using The Same |
| US12285552B2 (en) | 2018-08-14 | 2025-04-29 | Mozarc Medical Us Llc | Precision dialysis therapy based on sorbent effluent analysis |
| US11213616B2 (en) | 2018-08-24 | 2022-01-04 | Medtronic, Inc. | Recharge solution for zirconium phosphate |
| US11806457B2 (en) | 2018-11-16 | 2023-11-07 | Mozarc Medical Us Llc | Peritoneal dialysis adequacy meaurements |
| US11090421B2 (en) | 2018-11-28 | 2021-08-17 | Baxter International Inc. | Systems and methods for batch sorbent material reuse |
| US11253849B2 (en) | 2018-11-28 | 2022-02-22 | Baxter International Inc. | Systems and methods for onsite sorbent material reuse |
| US11925916B2 (en) | 2018-11-28 | 2024-03-12 | Baxter International Inc. | Method and composition for removing uremic toxins |
| US11806456B2 (en) | 2018-12-10 | 2023-11-07 | Mozarc Medical Us Llc | Precision peritoneal dialysis therapy based on dialysis adequacy measurements |
| US12433976B2 (en) | 2019-01-08 | 2025-10-07 | Mozarc Medical Us Llc | Bicarbonate sensor for dialysis |
| CN114269406A (zh) * | 2019-04-05 | 2022-04-01 | 奇德尼实验室公司 | 用于肾脏治疗的吸着剂 |
| MX2021013515A (es) | 2019-05-16 | 2022-01-04 | Exthera Medical Corp | Metodo para modular la estructura del glucocaliz endotelial. |
| US11484875B2 (en) * | 2019-07-09 | 2022-11-01 | Uop Llc | Process for removing mercury ions from bodily fluids using titanium metallate ion exchange compositions |
| WO2021050695A1 (en) * | 2019-09-13 | 2021-03-18 | The Trustees Of Indiana University | Devices and methods for modulating adma in blood |
| WO2021155203A1 (en) * | 2020-01-31 | 2021-08-05 | Qidni Labs Inc. | System for controlling a renal therapy device |
| US20230147619A1 (en) | 2020-02-18 | 2023-05-11 | Icinnovation Bv | Wearable and portable device for recirculating flow dialysis |
| EP3868417A1 (en) | 2020-02-18 | 2021-08-25 | ICinnovation BV | Wearable and portable device for recirculating flow dialysis |
| US12485212B2 (en) | 2020-04-13 | 2025-12-02 | Temasek Polytechnic | Dialysate regenerator and system comprising the same |
| US12128165B2 (en) | 2020-04-27 | 2024-10-29 | Mozarc Medical Us Llc | Dual stage degasser |
| EP4157391A4 (en) * | 2020-05-29 | 2023-11-15 | Victor Gura | PORTABLE DIALYSIS SYSTEM WITH ULTRAFILTRATE MODULE |
| US11759560B2 (en) * | 2020-09-15 | 2023-09-19 | Fresenius Medical Care Holdings, Inc. | Safety mechanism for a dialysis system |
| US20220096962A1 (en) * | 2020-09-30 | 2022-03-31 | Uop Llc | Process for removing lead, mercury, potassium, and ammonium ions from bodily fluids using rare-earth silicate ion exchange compositions |
| US12318528B2 (en) | 2020-10-30 | 2025-06-03 | Mozarc Medical Us Llc | Variable orifice fistula graft |
| EP3991770B1 (en) | 2020-10-30 | 2024-10-30 | Bellco S.r.l. | Dialysis cassette with pump features |
| CA3200034A1 (en) * | 2020-12-02 | 2022-06-09 | James R. Hanrahan | Dialysate filter |
| US12397093B2 (en) | 2021-05-18 | 2025-08-26 | Mozarc Medical Us Llc | Sorbent cartridge designs |
| CN113499664B (zh) * | 2021-06-28 | 2023-07-21 | 中国神华煤制油化工有限公司 | 脱汞剂及其制备方法和脱除烟气中单质汞的方法 |
| US12154673B2 (en) | 2021-08-02 | 2024-11-26 | Mozarc Medical Us Llc | Artificial intelligence assisted home therapy settings for dialysis |
| US11850344B2 (en) | 2021-08-11 | 2023-12-26 | Mozarc Medical Us Llc | Gas bubble sensor |
| US11965763B2 (en) | 2021-11-12 | 2024-04-23 | Mozarc Medical Us Llc | Determining fluid flow across rotary pump |
| US11944733B2 (en) | 2021-11-18 | 2024-04-02 | Mozarc Medical Us Llc | Sodium and bicarbonate control |
| WO2023126577A1 (en) * | 2021-12-30 | 2023-07-06 | Weeefiner Oy | An equipment and a method for scavenging of ions and molecules from fluid |
| WO2025095860A1 (en) * | 2023-11-01 | 2025-05-08 | Vivance Pte Ltd | Particulate porous materials |
| CN117718029B (zh) * | 2023-12-18 | 2024-06-21 | 江苏杰瑞医疗技术有限公司 | 一种高效的尿毒症毒素吸附剂及其制备方法与应用 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5141493A (en) * | 1990-01-26 | 1992-08-25 | Sarcos Group | Peritoneal dialysis system |
| US5350357A (en) * | 1993-03-03 | 1994-09-27 | Deka Products Limited Partnership | Peritoneal dialysis systems employing a liquid distribution and pumping cassette that emulates gravity flow |
| US6168578B1 (en) * | 1999-02-18 | 2001-01-02 | Melvin Diamond | Portable kidney dialysis system |
| US6595948B2 (en) * | 2000-10-04 | 2003-07-22 | Terumo Kabushiki Kaisha | Peritoneal dialysis apparatus |
| RU2290209C2 (ru) * | 2005-03-09 | 2006-12-27 | Федеральное государственное унитарное предприятие "Российский федеральный ядерный центр-Всероссийский научно-исследовательский институт экспериментальной физики" Федерального агентства по атомной энергии(ФГУП "РФЯЦ-ВНИИЭФ") | Способ очистки диализирующего раствора в аппаратах "искусственная почка" и устройство для его осуществления |
Family Cites Families (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2755214C3 (de) * | 1977-12-10 | 1980-12-11 | Dr. Eduard Fresenius Chemisch-Pharmazeutische Industrie Kg Apparatebau Kg, 6380 Bad Homburg | Vorrichtung zur periodischen Spülung der Bauchhöhle |
| PT69258A (en) | 1978-02-27 | 1979-03-01 | Lilly Co Eli | Process for preparing a dialysis composition |
| US4581141A (en) * | 1978-02-27 | 1986-04-08 | Purdue Research Foundation | Dialysis material and method for removing uremic substances |
| DE3101159C2 (de) | 1981-01-16 | 1985-08-22 | Udipi Ramakrishna Dr. 5100 Aachen Shettigar | Verfahren zur Reinigung von Blut und künstliche Niere zur Durchführung des Verfahrens |
| RU2064429C1 (ru) * | 1992-04-09 | 1996-07-27 | Владимир Васильевич Стрелко | Углеродный сорбент и способ его получения |
| EP0714312B1 (en) * | 1994-06-17 | 2001-08-29 | Baxter International Inc. | Apparatus for purified pulse peritoneal dialysis |
| JPH09327511A (ja) * | 1996-06-12 | 1997-12-22 | A S A Sangyo Kk | 腹膜透析液の回収・再生方法並びにそのための処理装置及び付属器具 |
| JP2000084070A (ja) | 1998-09-09 | 2000-03-28 | Nissho Corp | 腹膜透析装置 |
| JP4837842B2 (ja) * | 2001-06-19 | 2011-12-14 | テルモ株式会社 | 腹膜透析装置 |
| US7033498B2 (en) * | 2000-11-28 | 2006-04-25 | Renal Solutions, Inc. | Cartridges useful in cleaning dialysis solutions |
| US6627164B1 (en) * | 2000-11-28 | 2003-09-30 | Renal Solutions, Inc. | Sodium zirconium carbonate and zirconium basic carbonate and methods of making the same |
| US6579460B1 (en) * | 2001-03-13 | 2003-06-17 | Uop Llc | Process and composition for removing toxins from bodily fluids |
| US7241272B2 (en) * | 2001-11-13 | 2007-07-10 | Baxter International Inc. | Method and composition for removing uremic toxins in dialysis processes |
| US20030103888A1 (en) * | 2001-11-13 | 2003-06-05 | Ton That Hai | Granular zirconium phosphate and methods for synthesis of same |
| TWI239859B (en) * | 2001-11-28 | 2005-09-21 | Renal Solutions Inc | Cartridges useful in cleaning dialysis solutions |
| US20030114787A1 (en) * | 2001-12-13 | 2003-06-19 | Victor Gura | Wearable peritoneal dialysis system |
| WO2004009158A2 (en) * | 2002-07-19 | 2004-01-29 | Baxter International Inc. | Systems and methods for performing peritoneal dialysis |
| ES2339239T3 (es) | 2002-07-19 | 2010-05-18 | Baxter International Inc. | Sistema para dialisis peritoneal. |
| US20040099593A1 (en) * | 2002-11-25 | 2004-05-27 | Potito De Paolis | Concurrent dialysate purification cartridge |
| WO2004105589A2 (en) * | 2003-05-28 | 2004-12-09 | Hemocleanse Technologies, Llc | Sorbent reactor for extracorporeal blood treatment systems, peritoneal dialysis systems, and other body fluid treatment systems |
| US8029454B2 (en) * | 2003-11-05 | 2011-10-04 | Baxter International Inc. | High convection home hemodialysis/hemofiltration and sorbent system |
| CN1897993B (zh) * | 2003-12-24 | 2012-02-08 | 凯米卡技术有限公司 | 用于便携式人类透析的透析液再生系统 |
| AU2005322322B2 (en) * | 2004-12-28 | 2010-04-01 | Renal Solutions, Inc. | Method of synthesizing zirconium phosphate particles |
| US20070017943A1 (en) | 2005-07-21 | 2007-01-25 | Foy Noel P | Towel tote |
| MX2008008892A (es) * | 2006-01-30 | 2008-11-27 | Univ California | Metodos y aparatos de dialisis peritoneal. |
| US8012118B2 (en) * | 2006-03-08 | 2011-09-06 | Fresenius Medical Care Holdings, Inc. | Artificial kidney dialysis system |
| US8715221B2 (en) * | 2006-03-08 | 2014-05-06 | Fresenius Medical Care Holdings, Inc. | Wearable kidney |
| JP5027591B2 (ja) * | 2006-08-18 | 2012-09-19 | 株式会社カネカ | 架橋ポリマー粒子およびその製法 |
| US9415150B2 (en) * | 2007-11-09 | 2016-08-16 | Baxter Healthcare S.A. | Balanced flow dialysis machine |
| EP2303354B1 (en) | 2008-06-23 | 2020-08-12 | Temasek Polytechnic | A sorbent for a dialysis device |
| US9821105B2 (en) * | 2008-07-01 | 2017-11-21 | Baxter International Inc. | Nanoclay sorbents for dialysis |
-
2009
- 2009-06-22 EP EP09770498.5A patent/EP2303354B1/en active Active
- 2009-06-22 US US13/000,811 patent/US9242036B2/en active Active
- 2009-06-22 WO PCT/SG2009/000229 patent/WO2009157877A1/en not_active Ceased
- 2009-06-22 US US13/000,830 patent/US9067017B2/en active Active
- 2009-06-22 BR BRPI0915397A patent/BRPI0915397B8/pt active IP Right Grant
- 2009-06-22 ES ES09770499T patent/ES2823159T3/es active Active
- 2009-06-22 RU RU2011102163/14A patent/RU2525205C2/ru active
- 2009-06-22 CN CN2009801323271A patent/CN102123749A/zh active Pending
- 2009-06-22 ES ES09770498T patent/ES2821448T3/es active Active
- 2009-06-22 NZ NZ590471A patent/NZ590471A/xx not_active IP Right Cessation
- 2009-06-22 CN CN200980131854.0A patent/CN102176934B/zh not_active Expired - Fee Related
- 2009-06-22 JP JP2011516228A patent/JP5439482B2/ja active Active
- 2009-06-22 JP JP2011516227A patent/JP2011525403A/ja active Pending
- 2009-06-22 RU RU2011102164/05A patent/RU2514956C2/ru active
- 2009-06-22 AU AU2009263046A patent/AU2009263046B2/en not_active Ceased
- 2009-06-22 NZ NZ590467A patent/NZ590467A/xx unknown
- 2009-06-22 CN CN201610512305.XA patent/CN106111079A/zh active Pending
- 2009-06-22 BR BRPI0915400A patent/BRPI0915400A2/pt not_active IP Right Cessation
- 2009-06-22 AU AU2009263045A patent/AU2009263045B2/en active Active
- 2009-06-22 EP EP09770499.3A patent/EP2310069B1/en active Active
- 2009-06-22 WO PCT/SG2009/000230 patent/WO2009157878A1/en not_active Ceased
- 2009-06-23 TW TW098120993A patent/TWI482659B/zh active
- 2009-06-23 TW TW098120991A patent/TW201014622A/zh unknown
-
2014
- 2014-07-17 JP JP2014146372A patent/JP2015013129A/ja active Pending
-
2015
- 2015-12-29 US US14/982,482 patent/US20160151555A1/en not_active Abandoned
-
2016
- 2016-07-29 JP JP2016150024A patent/JP6588873B2/ja active Active
-
2020
- 2020-02-17 US US16/792,774 patent/US11590272B2/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5141493A (en) * | 1990-01-26 | 1992-08-25 | Sarcos Group | Peritoneal dialysis system |
| US5350357A (en) * | 1993-03-03 | 1994-09-27 | Deka Products Limited Partnership | Peritoneal dialysis systems employing a liquid distribution and pumping cassette that emulates gravity flow |
| US6168578B1 (en) * | 1999-02-18 | 2001-01-02 | Melvin Diamond | Portable kidney dialysis system |
| US6595948B2 (en) * | 2000-10-04 | 2003-07-22 | Terumo Kabushiki Kaisha | Peritoneal dialysis apparatus |
| RU2290209C2 (ru) * | 2005-03-09 | 2006-12-27 | Федеральное государственное унитарное предприятие "Российский федеральный ядерный центр-Всероссийский научно-исследовательский институт экспериментальной физики" Федерального агентства по атомной энергии(ФГУП "РФЯЦ-ВНИИЭФ") | Способ очистки диализирующего раствора в аппаратах "искусственная почка" и устройство для его осуществления |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2525205C2 (ru) | Проточная система устройства диализа и переносное устройство диализа | |
| US11135347B2 (en) | Dialysis device and method of dialysis | |
| US8096969B2 (en) | Peritoneal dialysis methods and apparatus | |
| US9138524B2 (en) | Sensing system for detecting a substance in a dialysate | |
| US20050101901A1 (en) | Wearable continuous renal replacement therapy device | |
| US20060241543A1 (en) | Method for installing and servicing a wearable continuous renal replacement therapy device | |
| US11992590B2 (en) | Combination wearable and stationary dialysis system with ultrafiltrate module | |
| HK1150197A (en) | A flow system of a dialysis device and a portable dialysis device | |
| HK1150197B (en) | A flow system of a dialysis device and a portable dialysis device | |
| HK1187560A (en) | Dialysis device and method of dialysis | |
| HK1187560B (en) | Dialysis device and method of dialysis |