KR20160031510A - 전자 장치용 전극 표면 변성 층 - Google Patents
전자 장치용 전극 표면 변성 층 Download PDFInfo
- Publication number
- KR20160031510A KR20160031510A KR1020167003360A KR20167003360A KR20160031510A KR 20160031510 A KR20160031510 A KR 20160031510A KR 1020167003360 A KR1020167003360 A KR 1020167003360A KR 20167003360 A KR20167003360 A KR 20167003360A KR 20160031510 A KR20160031510 A KR 20160031510A
- Authority
- KR
- South Korea
- Prior art keywords
- group
- electrode
- carbon atoms
- layer
- tfd
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000012986 modification Methods 0.000 title claims description 33
- 230000004048 modification Effects 0.000 title claims description 33
- 239000002904 solvent Substances 0.000 claims abstract description 56
- 229910052750 molybdenum Inorganic materials 0.000 claims abstract description 39
- 229910052804 chromium Inorganic materials 0.000 claims abstract description 36
- 238000000151 deposition Methods 0.000 claims abstract description 28
- 229910052721 tungsten Inorganic materials 0.000 claims abstract description 27
- 239000004065 semiconductor Substances 0.000 claims description 83
- 125000000217 alkyl group Chemical group 0.000 claims description 65
- 125000004432 carbon atom Chemical group C* 0.000 claims description 56
- 238000000034 method Methods 0.000 claims description 55
- 239000000758 substrate Substances 0.000 claims description 37
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 24
- 125000003118 aryl group Chemical group 0.000 claims description 23
- 238000004519 manufacturing process Methods 0.000 claims description 22
- 150000001875 compounds Chemical class 0.000 claims description 19
- 239000010409 thin film Substances 0.000 claims description 18
- 229920000642 polymer Polymers 0.000 claims description 17
- -1 4-butylphenyl Chemical group 0.000 claims description 16
- YTPLMLYBLZKORZ-UHFFFAOYSA-N Thiophene Chemical group C=1C=CSC=1 YTPLMLYBLZKORZ-UHFFFAOYSA-N 0.000 claims description 15
- 125000003545 alkoxy group Chemical group 0.000 claims description 15
- 229910052737 gold Inorganic materials 0.000 claims description 15
- 125000001072 heteroaryl group Chemical group 0.000 claims description 14
- FCEHBMOGCRZNNI-UHFFFAOYSA-N 1-benzothiophene Chemical group C1=CC=C2SC=CC2=C1 FCEHBMOGCRZNNI-UHFFFAOYSA-N 0.000 claims description 10
- 125000001424 substituent group Chemical group 0.000 claims description 10
- 125000003342 alkenyl group Chemical group 0.000 claims description 8
- 229910052739 hydrogen Inorganic materials 0.000 claims description 6
- 229910052760 oxygen Inorganic materials 0.000 claims description 6
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 claims description 6
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 6
- 125000003368 amide group Chemical group 0.000 claims description 5
- 125000003277 amino group Chemical group 0.000 claims description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 5
- 125000006165 cyclic alkyl group Chemical group 0.000 claims description 5
- 229910052736 halogen Inorganic materials 0.000 claims description 5
- 150000002367 halogens Chemical class 0.000 claims description 5
- 239000001301 oxygen Substances 0.000 claims description 5
- 229910052717 sulfur Inorganic materials 0.000 claims description 5
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 4
- ZADPBFCGQRWHPN-UHFFFAOYSA-N boronic acid Chemical compound OBO ZADPBFCGQRWHPN-UHFFFAOYSA-N 0.000 claims description 4
- VBXDEEVJTYBRJJ-UHFFFAOYSA-N diboronic acid Chemical compound OBOBO VBXDEEVJTYBRJJ-UHFFFAOYSA-N 0.000 claims description 4
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 4
- 229910052709 silver Inorganic materials 0.000 claims description 4
- 239000011593 sulfur Substances 0.000 claims description 4
- 229910052802 copper Inorganic materials 0.000 claims description 3
- 150000002148 esters Chemical class 0.000 claims description 3
- 239000001257 hydrogen Substances 0.000 claims description 3
- 229910052711 selenium Inorganic materials 0.000 claims description 3
- 229930192474 thiophene Natural products 0.000 claims description 3
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical group [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 claims description 2
- 239000010410 layer Substances 0.000 description 110
- 239000000243 solution Substances 0.000 description 46
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 37
- 239000011651 chromium Substances 0.000 description 35
- 229910052751 metal Inorganic materials 0.000 description 24
- 239000002184 metal Substances 0.000 description 24
- 239000003849 aromatic solvent Substances 0.000 description 18
- 239000010931 gold Substances 0.000 description 16
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical group CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 14
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 14
- RDOXTESZEPMUJZ-UHFFFAOYSA-N anisole Chemical compound COC1=CC=CC=C1 RDOXTESZEPMUJZ-UHFFFAOYSA-N 0.000 description 12
- 239000000463 material Substances 0.000 description 11
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 10
- 238000002347 injection Methods 0.000 description 9
- 239000007924 injection Substances 0.000 description 9
- URLKBWYHVLBVBO-UHFFFAOYSA-N Para-Xylene Chemical group CC1=CC=C(C)C=C1 URLKBWYHVLBVBO-UHFFFAOYSA-N 0.000 description 8
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 8
- 238000010438 heat treatment Methods 0.000 description 8
- IVSZLXZYQVIEFR-UHFFFAOYSA-N m-xylene Chemical group CC1=CC=CC(C)=C1 IVSZLXZYQVIEFR-UHFFFAOYSA-N 0.000 description 8
- 239000000203 mixture Substances 0.000 description 8
- 238000004528 spin coating Methods 0.000 description 8
- OFIYHXOOOISSDN-UHFFFAOYSA-N tellanylidenegallium Chemical compound [Te]=[Ga] OFIYHXOOOISSDN-UHFFFAOYSA-N 0.000 description 8
- 238000004140 cleaning Methods 0.000 description 7
- 230000008021 deposition Effects 0.000 description 7
- 239000002019 doping agent Substances 0.000 description 7
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 7
- 229940078552 o-xylene Drugs 0.000 description 7
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 6
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 6
- 239000002800 charge carrier Substances 0.000 description 6
- UZKWTJUDCOPSNM-UHFFFAOYSA-N methoxybenzene Substances CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 6
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 6
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 6
- 239000004810 polytetrafluoroethylene Substances 0.000 description 6
- CXWXQJXEFPUFDZ-UHFFFAOYSA-N tetralin Chemical compound C1=CC=C2CCCCC2=C1 CXWXQJXEFPUFDZ-UHFFFAOYSA-N 0.000 description 6
- 238000012546 transfer Methods 0.000 description 6
- XMWRBQBLMFGWIX-UHFFFAOYSA-N C60 fullerene Chemical compound C12=C3C(C4=C56)=C7C8=C5C5=C9C%10=C6C6=C4C1=C1C4=C6C6=C%10C%10=C9C9=C%11C5=C8C5=C8C7=C3C3=C7C2=C1C1=C2C4=C6C4=C%10C6=C9C9=C%11C5=C5C8=C3C3=C7C1=C1C2=C4C6=C2C9=C5C3=C12 XMWRBQBLMFGWIX-UHFFFAOYSA-N 0.000 description 5
- 239000010408 film Substances 0.000 description 5
- 239000011521 glass Substances 0.000 description 5
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 5
- 229910052782 aluminium Inorganic materials 0.000 description 4
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical group [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 229910003472 fullerene Inorganic materials 0.000 description 4
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 4
- 238000013508 migration Methods 0.000 description 4
- 230000005012 migration Effects 0.000 description 4
- UHOVQNZJYSORNB-UHFFFAOYSA-N monobenzene Natural products C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 4
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- 238000007639 printing Methods 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 238000010129 solution processing Methods 0.000 description 4
- HQALDKFFRYFTKP-UHFFFAOYSA-N 2-[4-[4-(2-benzyl-1-benzothiophen-3-yl)phenyl]-2-bromo-6-(3-methoxyphenyl)phenoxy]acetic acid Chemical group COC1=CC=CC(C=2C(=C(Br)C=C(C=2)C=2C=CC(=CC=2)C=2C3=CC=CC=C3SC=2CC=2C=CC=CC=2)OCC(O)=O)=C1 HQALDKFFRYFTKP-UHFFFAOYSA-N 0.000 description 3
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 125000004106 butoxy group Chemical group [*]OC([H])([H])C([H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 125000004122 cyclic group Chemical group 0.000 description 3
- 125000000753 cycloalkyl group Chemical group 0.000 description 3
- 238000004925 denaturation Methods 0.000 description 3
- 230000036425 denaturation Effects 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 238000001704 evaporation Methods 0.000 description 3
- 238000004770 highest occupied molecular orbital Methods 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- AUHZEENZYGFFBQ-UHFFFAOYSA-N mesitylene Substances CC1=CC(C)=CC(C)=C1 AUHZEENZYGFFBQ-UHFFFAOYSA-N 0.000 description 3
- 125000001827 mesitylenyl group Chemical group [H]C1=C(C(*)=C(C([H])=C1C([H])([H])[H])C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- 239000011733 molybdenum Substances 0.000 description 3
- 238000003672 processing method Methods 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 239000004332 silver Substances 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 125000004665 trialkylsilyl group Chemical group 0.000 description 3
- DPZNOMCNRMUKPS-UHFFFAOYSA-N 1,3-Dimethoxybenzene Chemical compound COC1=CC=CC(OC)=C1 DPZNOMCNRMUKPS-UHFFFAOYSA-N 0.000 description 2
- OHBQPCCCRFSCAX-UHFFFAOYSA-N 1,4-Dimethoxybenzene Chemical compound COC1=CC=C(OC)C=C1 OHBQPCCCRFSCAX-UHFFFAOYSA-N 0.000 description 2
- QPUYECUOLPXSFR-UHFFFAOYSA-N 1-methylnaphthalene Chemical compound C1=CC=C2C(C)=CC=CC2=C1 QPUYECUOLPXSFR-UHFFFAOYSA-N 0.000 description 2
- YBYIRNPNPLQARY-UHFFFAOYSA-N 1H-indene Chemical group C1=CC=C2CC=CC2=C1 YBYIRNPNPLQARY-UHFFFAOYSA-N 0.000 description 2
- GNKZMNRKLCTJAY-UHFFFAOYSA-N 4'-Methylacetophenone Chemical compound CC(=O)C1=CC=C(C)C=C1 GNKZMNRKLCTJAY-UHFFFAOYSA-N 0.000 description 2
- NTPLXRHDUXRPNE-UHFFFAOYSA-N 4-methoxyacetophenone Chemical compound COC1=CC=C(C(C)=O)C=C1 NTPLXRHDUXRPNE-UHFFFAOYSA-N 0.000 description 2
- KWOLFJPFCHCOCG-UHFFFAOYSA-N Acetophenone Chemical compound CC(=O)C1=CC=CC=C1 KWOLFJPFCHCOCG-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- 239000004812 Fluorinated ethylene propylene Substances 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 229920003189 Nylon 4,6 Polymers 0.000 description 2
- 239000002033 PVDF binder Substances 0.000 description 2
- 229920006169 Perfluoroelastomer Polymers 0.000 description 2
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 2
- 230000001133 acceleration Effects 0.000 description 2
- 150000004996 alkyl benzenes Chemical class 0.000 description 2
- 125000002178 anthracenyl group Chemical group C1(=CC=CC2=CC3=CC=CC=C3C=C12)* 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 125000002785 azepinyl group Chemical group 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 239000004020 conductor Substances 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 239000003989 dielectric material Substances 0.000 description 2
- 238000003618 dip coating Methods 0.000 description 2
- 238000007606 doctor blade method Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 125000001301 ethoxy group Chemical group [H]C([H])([H])C([H])([H])O* 0.000 description 2
- MTZQAGJQAFMTAQ-UHFFFAOYSA-N ethyl benzoate Chemical compound CCOC(=O)C1=CC=CC=C1 MTZQAGJQAFMTAQ-UHFFFAOYSA-N 0.000 description 2
- 125000002541 furyl group Chemical group 0.000 description 2
- 150000002343 gold Chemical class 0.000 description 2
- 238000007646 gravure printing Methods 0.000 description 2
- 125000002883 imidazolyl group Chemical group 0.000 description 2
- 238000005470 impregnation Methods 0.000 description 2
- 125000003454 indenyl group Chemical group C1(C=CC2=CC=CC=C12)* 0.000 description 2
- AMGQUBHHOARCQH-UHFFFAOYSA-N indium;oxotin Chemical compound [In].[Sn]=O AMGQUBHHOARCQH-UHFFFAOYSA-N 0.000 description 2
- 238000007641 inkjet printing Methods 0.000 description 2
- 125000001786 isothiazolyl group Chemical group 0.000 description 2
- 125000000842 isoxazolyl group Chemical group 0.000 description 2
- 238000004768 lowest unoccupied molecular orbital Methods 0.000 description 2
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 125000002971 oxazolyl group Chemical group 0.000 description 2
- SLIUAWYAILUBJU-UHFFFAOYSA-N pentacene Chemical compound C1=CC=CC2=CC3=CC4=CC5=CC=CC=C5C=C4C=C3C=C21 SLIUAWYAILUBJU-UHFFFAOYSA-N 0.000 description 2
- 229920009441 perflouroethylene propylene Polymers 0.000 description 2
- 239000010702 perfluoropolyether Substances 0.000 description 2
- 125000001792 phenanthrenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3C=CC12)* 0.000 description 2
- 238000009832 plasma treatment Methods 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 229920002493 poly(chlorotrifluoroethylene) Polymers 0.000 description 2
- 239000005023 polychlorotrifluoroethylene (PCTFE) polymer Substances 0.000 description 2
- 229920002620 polyvinyl fluoride Polymers 0.000 description 2
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 2
- 125000004309 pyranyl group Chemical group O1C(C=CC=C1)* 0.000 description 2
- 125000003373 pyrazinyl group Chemical group 0.000 description 2
- 125000003226 pyrazolyl group Chemical group 0.000 description 2
- 125000002098 pyridazinyl group Chemical group 0.000 description 2
- 125000004076 pyridyl group Chemical group 0.000 description 2
- 125000000714 pyrimidinyl group Chemical group 0.000 description 2
- 125000000168 pyrrolyl group Chemical group 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 238000007764 slot die coating Methods 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 125000003831 tetrazolyl group Chemical group 0.000 description 2
- 238000002207 thermal evaporation Methods 0.000 description 2
- 125000001113 thiadiazolyl group Chemical group 0.000 description 2
- 125000000335 thiazolyl group Chemical group 0.000 description 2
- 125000001544 thienyl group Chemical group 0.000 description 2
- 125000003944 tolyl group Chemical group 0.000 description 2
- 125000001425 triazolyl group Chemical group 0.000 description 2
- 125000004642 (C1-C12) alkoxy group Chemical group 0.000 description 1
- 125000004400 (C1-C12) alkyl group Chemical group 0.000 description 1
- BQCIDUSAKPWEOX-UHFFFAOYSA-N 1,1-Difluoroethene Chemical compound FC(F)=C BQCIDUSAKPWEOX-UHFFFAOYSA-N 0.000 description 1
- QZYDOKBVZJLQCK-UHFFFAOYSA-N 1,2-diethoxybenzene Chemical compound CCOC1=CC=CC=C1OCC QZYDOKBVZJLQCK-UHFFFAOYSA-N 0.000 description 1
- MKGFYMKFBCWNCP-UHFFFAOYSA-N 1,3-diethoxybenzene Chemical compound CCOC1=CC=CC(OCC)=C1 MKGFYMKFBCWNCP-UHFFFAOYSA-N 0.000 description 1
- VWGNFIQXBYRDCH-UHFFFAOYSA-N 1,4-diethoxybenzene Chemical compound CCOC1=CC=C(OCC)C=C1 VWGNFIQXBYRDCH-UHFFFAOYSA-N 0.000 description 1
- VLILFBZIVHDKIJ-UHFFFAOYSA-N 1-(2-ethylphenyl)ethanone Chemical compound CCC1=CC=CC=C1C(C)=O VLILFBZIVHDKIJ-UHFFFAOYSA-N 0.000 description 1
- ZRYRILAFFDKOPB-UHFFFAOYSA-N 1-(3-ethylphenyl)ethanone Chemical compound CCC1=CC=CC(C(C)=O)=C1 ZRYRILAFFDKOPB-UHFFFAOYSA-N 0.000 description 1
- BAYUSCHCCGXLAY-UHFFFAOYSA-N 1-(3-methoxyphenyl)ethanone Chemical compound COC1=CC=CC(C(C)=O)=C1 BAYUSCHCCGXLAY-UHFFFAOYSA-N 0.000 description 1
- OMONCKYJLBVWOQ-UHFFFAOYSA-N 1-ethoxy-2-methoxybenzene Chemical compound CCOC1=CC=CC=C1OC OMONCKYJLBVWOQ-UHFFFAOYSA-N 0.000 description 1
- JWMKUKRBNODZFA-UHFFFAOYSA-N 1-ethoxy-2-methylbenzene Chemical compound CCOC1=CC=CC=C1C JWMKUKRBNODZFA-UHFFFAOYSA-N 0.000 description 1
- UALKQROXOHJHFG-UHFFFAOYSA-N 1-ethoxy-3-methylbenzene Chemical compound CCOC1=CC=CC(C)=C1 UALKQROXOHJHFG-UHFFFAOYSA-N 0.000 description 1
- DSUIDSZRTNMKTA-UHFFFAOYSA-N 1-methoxyethoxybenzene Chemical compound COC(C)OC1=CC=CC=C1 DSUIDSZRTNMKTA-UHFFFAOYSA-N 0.000 description 1
- YXWWHNCQZBVZPV-UHFFFAOYSA-N 2'-methylacetophenone Chemical compound CC(=O)C1=CC=CC=C1C YXWWHNCQZBVZPV-UHFFFAOYSA-N 0.000 description 1
- IXHWGNYCZPISET-UHFFFAOYSA-N 2-[4-(dicyanomethylidene)-2,3,5,6-tetrafluorocyclohexa-2,5-dien-1-ylidene]propanedinitrile Chemical compound FC1=C(F)C(=C(C#N)C#N)C(F)=C(F)C1=C(C#N)C#N IXHWGNYCZPISET-UHFFFAOYSA-N 0.000 description 1
- YRNDGUSDBCARGC-UHFFFAOYSA-N 2-methoxyacetophenone Chemical compound COCC(=O)C1=CC=CC=C1 YRNDGUSDBCARGC-UHFFFAOYSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 238000012935 Averaging Methods 0.000 description 1
- NXCJGZDOQBRBCM-UHFFFAOYSA-N C[Si](C)(C)SC(N)=S Chemical compound C[Si](C)(C)SC(N)=S NXCJGZDOQBRBCM-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- 229920001780 ECTFE Polymers 0.000 description 1
- 229920002449 FKM Polymers 0.000 description 1
- 229920001774 Perfluoroether Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 125000004442 acylamino group Chemical group 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 125000004450 alkenylene group Chemical group 0.000 description 1
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- 125000004419 alkynylene group Chemical group 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N alpha-acetylene Natural products C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 238000005452 bending Methods 0.000 description 1
- 150000001555 benzenes Chemical class 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 229920001940 conductive polymer Polymers 0.000 description 1
- 229920000547 conjugated polymer Polymers 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- HHNHBFLGXIUXCM-GFCCVEGCSA-N cyclohexylbenzene Chemical compound [CH]1CCCC[C@@H]1C1=CC=CC=C1 HHNHBFLGXIUXCM-GFCCVEGCSA-N 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 239000000412 dendrimer Substances 0.000 description 1
- 229920000736 dendritic polymer Polymers 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 239000007772 electrode material Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- XSXVXSCMWUJXOS-UHFFFAOYSA-N ethyl 2-ethylbenzoate Chemical compound CCOC(=O)C1=CC=CC=C1CC XSXVXSCMWUJXOS-UHFFFAOYSA-N 0.000 description 1
- SOUAXOGPALPTTC-UHFFFAOYSA-N ethyl 2-methylbenzoate Chemical compound CCOC(=O)C1=CC=CC=C1C SOUAXOGPALPTTC-UHFFFAOYSA-N 0.000 description 1
- KRBVDDXLFALXDC-UHFFFAOYSA-N ethyl 3-ethylbenzoate Chemical compound CCOC(=O)C1=CC=CC(CC)=C1 KRBVDDXLFALXDC-UHFFFAOYSA-N 0.000 description 1
- WSJNYOVBJSOQST-UHFFFAOYSA-N ethyl 3-methylbenzoate Chemical compound CCOC(=O)C1=CC=CC(C)=C1 WSJNYOVBJSOQST-UHFFFAOYSA-N 0.000 description 1
- ZPUKPAPWEWUPTC-UHFFFAOYSA-N ethyl 4-ethylbenzoate Chemical compound CCOC(=O)C1=CC=C(CC)C=C1 ZPUKPAPWEWUPTC-UHFFFAOYSA-N 0.000 description 1
- NWPWRAWAUYIELB-UHFFFAOYSA-N ethyl 4-methylbenzoate Chemical compound CCOC(=O)C1=CC=C(C)C=C1 NWPWRAWAUYIELB-UHFFFAOYSA-N 0.000 description 1
- 125000002534 ethynyl group Chemical group [H]C#C* 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000013213 extrapolation Methods 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 229920001973 fluoroelastomer Polymers 0.000 description 1
- 229920002313 fluoropolymer Polymers 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- HCDGVLDPFQMKDK-UHFFFAOYSA-N hexafluoropropylene Chemical group FC(F)=C(F)C(F)(F)F HCDGVLDPFQMKDK-UHFFFAOYSA-N 0.000 description 1
- 239000008240 homogeneous mixture Substances 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- FSPSELPMWGWDRY-UHFFFAOYSA-N m-Methylacetophenone Chemical compound CC(=O)C1=CC=CC(C)=C1 FSPSELPMWGWDRY-UHFFFAOYSA-N 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 150000002736 metal compounds Chemical class 0.000 description 1
- 229910001092 metal group alloy Inorganic materials 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- UFWIBTONFRDIAS-UHFFFAOYSA-N naphthalene-acid Natural products C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 1
- 150000002790 naphthalenes Chemical class 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 125000001181 organosilyl group Chemical group [SiH3]* 0.000 description 1
- UUEVFMOUBSLVJW-UHFFFAOYSA-N oxo-[[1-[2-[2-[2-[4-(oxoazaniumylmethylidene)pyridin-1-yl]ethoxy]ethoxy]ethyl]pyridin-4-ylidene]methyl]azanium;dibromide Chemical compound [Br-].[Br-].C1=CC(=C[NH+]=O)C=CN1CCOCCOCCN1C=CC(=C[NH+]=O)C=C1 UUEVFMOUBSLVJW-UHFFFAOYSA-N 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- DLRJIFUOBPOJNS-UHFFFAOYSA-N phenetole Chemical compound CCOC1=CC=CC=C1 DLRJIFUOBPOJNS-UHFFFAOYSA-N 0.000 description 1
- 229920002120 photoresistant polymer Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 239000002952 polymeric resin Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 125000003748 selenium group Chemical group *[Se]* 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- 239000002335 surface treatment layer Substances 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- 125000001302 tertiary amino group Chemical group 0.000 description 1
- BFKJFAAPBSQJPD-UHFFFAOYSA-N tetrafluoroethene Chemical group FC(F)=C(F)F BFKJFAAPBSQJPD-UHFFFAOYSA-N 0.000 description 1
- QHGNHLZPVBIIPX-UHFFFAOYSA-N tin(ii) oxide Chemical class [Sn]=O QHGNHLZPVBIIPX-UHFFFAOYSA-N 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 239000013638 trimer Substances 0.000 description 1
- 150000003657 tungsten Chemical class 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- ABDKAPXRBAPSQN-UHFFFAOYSA-N veratrole Chemical compound COC1=CC=CC=C1OC ABDKAPXRBAPSQN-UHFFFAOYSA-N 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 229920003249 vinylidene fluoride hexafluoropropylene elastomer Polymers 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- H01L51/105—
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D495/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms
- C07D495/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D495/04—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D495/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms
- C07D495/12—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms in which the condensed system contains three hetero rings
- C07D495/14—Ortho-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D495/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms
- C07D495/22—Heterocyclic compounds containing in the condensed system at least one hetero ring having sulfur atoms as the only ring hetero atoms in which the condensed system contains four or more hetero rings
-
- H01L51/0074—
-
- H01L51/0541—
-
- H01L51/0545—
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
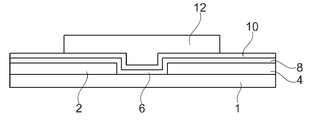
- H10K10/00—Organic devices specially adapted for rectifying, amplifying, oscillating or switching; Organic capacitors or resistors having potential barriers
- H10K10/40—Organic transistors
- H10K10/46—Field-effect transistors, e.g. organic thin-film transistors [OTFT]
- H10K10/462—Insulated gate field-effect transistors [IGFETs]
- H10K10/464—Lateral top-gate IGFETs comprising only a single gate
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K10/00—Organic devices specially adapted for rectifying, amplifying, oscillating or switching; Organic capacitors or resistors having potential barriers
- H10K10/40—Organic transistors
- H10K10/46—Field-effect transistors, e.g. organic thin-film transistors [OTFT]
- H10K10/462—Insulated gate field-effect transistors [IGFETs]
- H10K10/466—Lateral bottom-gate IGFETs comprising only a single gate
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K10/00—Organic devices specially adapted for rectifying, amplifying, oscillating or switching; Organic capacitors or resistors having potential barriers
- H10K10/80—Constructional details
- H10K10/82—Electrodes
- H10K10/84—Ohmic electrodes, e.g. source or drain electrodes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K71/00—Manufacture or treatment specially adapted for the organic devices covered by this subclass
- H10K71/10—Deposition of organic active material
- H10K71/12—Deposition of organic active material using liquid deposition, e.g. spin coating
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/341—Transition metal complexes, e.g. Ru(II)polypyridine complexes
-
- H01L2031/0344—
-
- H01L2251/10—
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/111—Organic polymers or oligomers comprising aromatic, heteroaromatic, or aryl chains, e.g. polyaniline, polyphenylene or polyphenylene vinylene
- H10K85/115—Polyfluorene; Derivatives thereof
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6576—Polycyclic condensed heteroaromatic hydrocarbons comprising only sulfur in the heteroaromatic polycondensed ring system, e.g. benzothiophene
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Crystallography & Structural Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Materials Engineering (AREA)
- Thin Film Transistor (AREA)
- Organic Chemistry (AREA)
- Electrodes Of Semiconductors (AREA)
- Polyoxymethylene Polymers And Polymers With Carbon-To-Carbon Bonds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB1312609.9A GB201312609D0 (en) | 2013-07-15 | 2013-07-15 | Method |
| GB1312609.9 | 2013-07-15 | ||
| PCT/GB2014/000283 WO2015008015A1 (en) | 2013-07-15 | 2014-07-11 | Electrode surface modification layer for electronic devices |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160031510A true KR20160031510A (ko) | 2016-03-22 |
Family
ID=49081267
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167003360A Withdrawn KR20160031510A (ko) | 2013-07-15 | 2014-07-11 | 전자 장치용 전극 표면 변성 층 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US9793504B2 (enExample) |
| JP (1) | JP6356240B2 (enExample) |
| KR (1) | KR20160031510A (enExample) |
| CN (1) | CN105378960B (enExample) |
| DE (1) | DE112014003272T5 (enExample) |
| GB (2) | GB201312609D0 (enExample) |
| TW (1) | TW201517339A (enExample) |
| WO (1) | WO2015008015A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10115903B2 (en) * | 2012-12-18 | 2018-10-30 | Merck Patent Gmbh | Emitter having a condensed ring system |
| JP7387685B2 (ja) * | 2021-09-17 | 2023-11-28 | 株式会社Kokusai Electric | 半導体装置の製造方法、基板処理方法、プログラム、および基板処理装置 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7045285B1 (en) * | 1996-11-05 | 2006-05-16 | Clinical Micro Sensors, Inc. | Electronic transfer moieties attached to peptide nucleic acids |
| US6501217B2 (en) * | 1998-02-02 | 2002-12-31 | International Business Machines Corporation | Anode modification for organic light emitting diodes |
| JP4364586B2 (ja) * | 2003-08-27 | 2009-11-18 | Tdk株式会社 | 有機電界効果トランジスタ及びその製造方法 |
| US7642038B2 (en) * | 2004-03-24 | 2010-01-05 | Semiconductor Energy Laboratory Co., Ltd. | Method for forming pattern, thin film transistor, display device, method for manufacturing thereof, and television apparatus |
| US8187746B2 (en) * | 2008-05-16 | 2012-05-29 | Uchicago Argonne, Llc | Surface modification agents for lithium batteries |
| KR20100091663A (ko) * | 2009-02-11 | 2010-08-19 | 삼성전자주식회사 | 표면개질제, 이를 사용하여 제조된 적층 구조, 그 구조의 제조방법 및 이를 포함하는 트랜지스터 |
| US8637857B2 (en) * | 2010-04-06 | 2014-01-28 | Basf Se | Substituted carbazole derivatives and use thereof in organic electronics |
| GB201013820D0 (en) * | 2010-08-18 | 2010-09-29 | Cambridge Display Tech Ltd | Low contact resistance organic thin film transistors |
| GB201021277D0 (en) * | 2010-12-15 | 2011-01-26 | Cambridge Display Tech Ltd | Semiconductor blend |
| US9315724B2 (en) * | 2011-06-14 | 2016-04-19 | Basf Se | Metal complexes comprising azabenzimidazole carbene ligands and the use thereof in OLEDs |
| EP2736911A1 (en) * | 2011-07-27 | 2014-06-04 | Merck Patent GmbH | Small molecules and their use as organic semiconductors |
| GB201118997D0 (en) * | 2011-11-03 | 2011-12-14 | Cambridge Display Tech Ltd | Electronic device and method |
| TW201348241A (zh) * | 2011-12-30 | 2013-12-01 | Imp Innovations Ltd | 有機半導體材料之非習用性化學摻雜 |
| GB201203159D0 (en) * | 2012-02-23 | 2012-04-11 | Smartkem Ltd | Organic semiconductor compositions |
| KR101952706B1 (ko) * | 2012-07-24 | 2019-02-28 | 삼성디스플레이 주식회사 | 유기 발광 소자 및 이를 포함하는 유기 발광 표시 장치 |
| US20140116509A1 (en) * | 2012-10-30 | 2014-05-01 | Sean Andrew Vail | Solid-State Dye-Sensitized Solar Cell Using Oxidative Dopant |
| GB201502113D0 (en) * | 2015-02-09 | 2015-03-25 | Cambridge Display Tech Ltd | Solution for a semiconducting layer of an organic electronic device |
-
2013
- 2013-07-15 GB GBGB1312609.9A patent/GB201312609D0/en not_active Ceased
-
2014
- 2014-07-11 US US14/905,091 patent/US9793504B2/en not_active Expired - Fee Related
- 2014-07-11 CN CN201480040221.XA patent/CN105378960B/zh not_active Expired - Fee Related
- 2014-07-11 GB GB1522168.2A patent/GB2529600A/en not_active Withdrawn
- 2014-07-11 KR KR1020167003360A patent/KR20160031510A/ko not_active Withdrawn
- 2014-07-11 JP JP2016526687A patent/JP6356240B2/ja not_active Expired - Fee Related
- 2014-07-11 DE DE112014003272.7T patent/DE112014003272T5/de not_active Withdrawn
- 2014-07-11 WO PCT/GB2014/000283 patent/WO2015008015A1/en not_active Ceased
- 2014-07-15 TW TW103124308A patent/TW201517339A/zh unknown
Also Published As
| Publication number | Publication date |
|---|---|
| GB2529600A (en) | 2016-02-24 |
| JP2016531423A (ja) | 2016-10-06 |
| CN105378960A (zh) | 2016-03-02 |
| WO2015008015A1 (en) | 2015-01-22 |
| US9793504B2 (en) | 2017-10-17 |
| TW201517339A (zh) | 2015-05-01 |
| CN105378960B (zh) | 2018-09-18 |
| GB201312609D0 (en) | 2013-08-28 |
| US20160149147A1 (en) | 2016-05-26 |
| DE112014003272T5 (de) | 2016-04-14 |
| JP6356240B2 (ja) | 2018-07-11 |
| GB201522168D0 (en) | 2016-01-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN103155196B (zh) | 低接触电阻的有机薄膜晶体管 | |
| JP6247636B2 (ja) | 有機半導体組成物および有機トランジスタ | |
| JP6368303B2 (ja) | 半導体層を調製する方法 | |
| US20140353647A1 (en) | Organic Thin Film Transistors And Method of Making Them | |
| CN103262277A (zh) | 半导体共混物 | |
| US20170141332A1 (en) | Organic transistor | |
| CN105190924B (zh) | 有机半导体共混物 | |
| KR20160031510A (ko) | 전자 장치용 전극 표면 변성 층 | |
| Liu et al. | Small molecule: polymer blends for n‐type organic thin film transistors via bar‐coating in air | |
| Paulus | N-heteroacenes in Organic Field-effect Transistors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20160205 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |