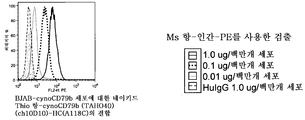
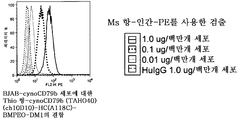
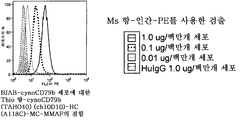
KR20100128286A - 조혈계 기원의 종양 치료용 조성물 및 방법 - Google Patents
조혈계 기원의 종양 치료용 조성물 및 방법 Download PDFInfo
- Publication number
- KR20100128286A KR20100128286A KR1020107019272A KR20107019272A KR20100128286A KR 20100128286 A KR20100128286 A KR 20100128286A KR 1020107019272 A KR1020107019272 A KR 1020107019272A KR 20107019272 A KR20107019272 A KR 20107019272A KR 20100128286 A KR20100128286 A KR 20100128286A
- Authority
- KR
- South Korea
- Prior art keywords
- seq
- antibody
- amino acid
- acid sequence
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 0 C*C(N(C)[C@](C)C(O[C@@](CC(N(C)c(cc(CC(C)=CC=C[C@]([C@@](C1)(N2)O)OC)cc3OC)c3Cl)=O)[C@]3(C)O[C@]3[C@](C)[C@]1OC2=O)=O)=O Chemical compound C*C(N(C)[C@](C)C(O[C@@](CC(N(C)c(cc(CC(C)=CC=C[C@]([C@@](C1)(N2)O)OC)cc3OC)c3Cl)=O)[C@]3(C)O[C@]3[C@](C)[C@]1OC2=O)=O)=O 0.000 description 1
- HVSWNFDRECNCOE-UHFFFAOYSA-N CC(C)(C)OC(NNCC(ON(C(CC1)=O)C1=O)=O)=O Chemical compound CC(C)(C)OC(NNCC(ON(C(CC1)=O)C1=O)=O)=O HVSWNFDRECNCOE-UHFFFAOYSA-N 0.000 description 1
- ZTMBXTMPSQKDCW-UHFFFAOYSA-N CC(C)(C)OC(NNc(cc1)ccc1C(ON(C(CC1)=O)C1=O)=O)=O Chemical compound CC(C)(C)OC(NNc(cc1)ccc1C(ON(C(CC1)=O)C1=O)=O)=O ZTMBXTMPSQKDCW-UHFFFAOYSA-N 0.000 description 1
- QYEAAMBIUQLHFQ-UHFFFAOYSA-N O=C(CCSSc1ncccc1)NCCCCCC(ON(C(CC1)=O)C1=O)=O Chemical compound O=C(CCSSc1ncccc1)NCCCCCC(ON(C(CC1)=O)C1=O)=O QYEAAMBIUQLHFQ-UHFFFAOYSA-N 0.000 description 1
- JWDFQMWEFLOOED-UHFFFAOYSA-N O=C(CCSSc1ncccc1)ON(C(CC1)=O)C1=O Chemical compound O=C(CCSSc1ncccc1)ON(C(CC1)=O)C1=O JWDFQMWEFLOOED-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
- A61K47/6817—Toxins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6851—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a determinant of a tumour cell
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
-
- G01N33/575—
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/624—Disulfide-stabilized antibody (dsFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/77—Internalization into the cell
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Cell Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Epidemiology (AREA)
- Toxicology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Hematology (AREA)
- Oncology (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Food Science & Technology (AREA)
- Physics & Mathematics (AREA)
- Hospice & Palliative Care (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/023,811 US20090068178A1 (en) | 2002-05-08 | 2008-01-31 | Compositions and Methods for the Treatment of Tumor of Hematopoietic Origin |
| US12/023,811 | 2008-01-31 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20100128286A true KR20100128286A (ko) | 2010-12-07 |
Family
ID=40578249
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020107019272A Withdrawn KR20100128286A (ko) | 2008-01-31 | 2009-01-13 | 조혈계 기원의 종양 치료용 조성물 및 방법 |
Country Status (16)
| Country | Link |
|---|---|
| US (4) | US20090068178A1 (enExample) |
| EP (1) | EP2247312A2 (enExample) |
| JP (1) | JP2011515069A (enExample) |
| KR (1) | KR20100128286A (enExample) |
| CN (1) | CN102014964A (enExample) |
| AR (1) | AR071829A1 (enExample) |
| AU (1) | AU2009210627A1 (enExample) |
| BR (1) | BRPI0908854A2 (enExample) |
| CA (1) | CA2712518A1 (enExample) |
| CL (1) | CL2009000082A1 (enExample) |
| IL (1) | IL206970A0 (enExample) |
| MX (1) | MX2010008199A (enExample) |
| NZ (1) | NZ587652A (enExample) |
| PE (1) | PE20091404A1 (enExample) |
| RU (1) | RU2010136303A (enExample) |
| WO (1) | WO2009099719A2 (enExample) |
Families Citing this family (60)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8709412B2 (en) | 2001-06-29 | 2014-04-29 | The Board Of Trustees Of The Leland Stanford Junior University | Modulation of TIM receptor activity in combination with cytoreductive therapy |
| US20110045005A1 (en) | 2001-10-19 | 2011-02-24 | Craig Crowley | Compositions and methods for the treatment of tumor of hematopoietic origin |
| US20090068178A1 (en) * | 2002-05-08 | 2009-03-12 | Genentech, Inc. | Compositions and Methods for the Treatment of Tumor of Hematopoietic Origin |
| DK1725249T3 (en) * | 2003-11-06 | 2014-03-17 | Seattle Genetics Inc | Monomethylvaline compounds capable of conjugating to ligands. |
| US20110166319A1 (en) * | 2005-02-11 | 2011-07-07 | Immunogen, Inc. | Process for preparing purified drug conjugates |
| AU2006283726C1 (en) | 2005-08-24 | 2015-05-07 | Immunogen, Inc. | Process for preparing maytansinoid antibody conjugates |
| RU2557319C2 (ru) | 2007-07-16 | 2015-07-20 | Дженентек, Инк. | ГУМАНИЗИРОВАННЫЕ АНТИТЕЛА ПРОТИВ CD79b И ИММУНОКОНЪЮГАТЫ И СПОСОБЫ ПРИМЕНЕНИЯ |
| ES2381788T3 (es) * | 2007-07-16 | 2012-05-31 | Genentech, Inc. | Anticuerpos anti-CD79b e inmunoconjugados y métodos de uso |
| HUE029869T2 (en) | 2008-01-31 | 2017-04-28 | Genentech Inc | Anti-cd79b antibodies and immunoconjugates and methods of use |
| KR102444399B1 (ko) | 2009-06-03 | 2022-09-16 | 이뮤노젠 아이엔씨 | 메이탄시노이드의 제조방법 |
| EP2533764A4 (en) | 2010-02-08 | 2016-08-31 | Univ Nebraska | BIOMINERAL AND METAL BINDING LIPOSOME, THEIR SYNTHESIS AND METHOD FOR THE PRODUCTION THEREOF |
| TW201129383A (en) * | 2010-02-10 | 2011-09-01 | Immunogen Inc | CD20 antibodies and uses thereof |
| JP5797210B2 (ja) * | 2010-03-02 | 2015-10-21 | シアトル ジェネティックス, インコーポレイテッド | 抗体をスクリーニングするための方法 |
| CA2815363C (en) | 2010-10-22 | 2020-07-14 | Seattle Genetics, Inc. | Synergistic effects between auristatin-based antibody drug conjugates and inhibitors of the pi3k-akt mtor pathway |
| ME03353B (me) | 2011-03-29 | 2019-10-20 | Immunogen Inc | Priprema konjugata antitela i majtanzinoida jednostepenim postupkom |
| MY170719A (en) * | 2011-04-01 | 2019-08-27 | Wyeth Llc | Antibody-drug conjugates |
| ES2887208T3 (es) | 2012-05-15 | 2021-12-22 | Concortis Biosystems Corp | Conjugados de fármacos y usos de los mismos |
| JP2015524821A (ja) * | 2012-08-02 | 2015-08-27 | ジェイエヌ バイオサイエンシーズ エルエルシー | システイン変異及びμ尾部を介して多量体化した抗体又は融合タンパク質 |
| AU2013326897A1 (en) * | 2012-10-04 | 2015-05-07 | Immunogen, Inc. | Use of an ion exchange membrane to remove impurities from cell-binding agent cytotoxic agent conjugates |
| US10035817B2 (en) | 2012-10-04 | 2018-07-31 | Immunogen, Inc. | Method of purifying cell-binding agent-cytotoxic agent conjugates with a PVDF membrane |
| US10260089B2 (en) | 2012-10-29 | 2019-04-16 | The Research Foundation Of The State University Of New York | Compositions and methods for recognition of RNA using triple helical peptide nucleic acids |
| WO2014085527A1 (en) | 2012-11-29 | 2014-06-05 | Bayer Healthcare Llc | Humanized monoclonal antibodies against activated protein c and uses thereof |
| NZ630601A (en) | 2012-12-21 | 2017-05-26 | Biolliance C V | Hydrophilic self-immolative linkers and conjugates thereof |
| WO2014124316A2 (en) | 2013-02-08 | 2014-08-14 | Irm Llc | Specific sites for modifying antibodies to make immunoconjugates |
| AU2014228172B2 (en) * | 2013-03-15 | 2018-12-06 | Abbvie Deutschland Gmbh & Co.Kg | Anti-EGFR antibody drug conjugate formulations |
| US10570151B2 (en) | 2013-03-15 | 2020-02-25 | Regeneron Pharmaceuticals, Inc. | Biologically active molecules, conjugates thereof, and therapeutic uses |
| MA38567A1 (fr) | 2013-04-16 | 2017-09-29 | Genentech Inc | Variantes de pertuzumab et leur évaluation |
| EP3027220A1 (en) * | 2013-08-01 | 2016-06-08 | Agensys, Inc. | Antibody drug conjugates (adc) that bind to cd37 proteins |
| AU2014307080B2 (en) | 2013-08-12 | 2018-06-07 | Genentech, Inc. | 1-(chloromethyl)-2,3-dihydro-1H-benzo(E)indole dimer antibody-drug conjugate compounds, and methods of use and treatment |
| EA038192B1 (ru) * | 2013-08-26 | 2021-07-21 | Регенерон Фармасьютикалз, Инк. | Фармацевтические композиции, содержащие диастереомеры макролида, способы их синтезирования и терапевтическое применение |
| CA3187392A1 (en) | 2013-10-15 | 2015-04-23 | Seagen Inc. | Pegylated drug-linkers for improved ligand-drug conjugate pharmacokinetics |
| US10836821B2 (en) | 2013-10-15 | 2020-11-17 | Sorrento Therapeutics, Inc. | Drug-conjugates with a targeting molecule and two different drugs |
| CN105828840B (zh) | 2013-12-16 | 2020-08-04 | 基因泰克公司 | 1-(氯甲基)-2,3-二氢-1H-苯并[e]吲哚二聚体抗体-药物缀合物化合物及使用和治疗方法 |
| JP6636925B2 (ja) | 2013-12-17 | 2020-01-29 | ノバルティス アーゲー | 細胞障害性ペプチドおよびその抱合体 |
| WO2015189791A1 (en) | 2014-06-13 | 2015-12-17 | Novartis Ag | Auristatin derivatives and conjugates thereof |
| RU2017101662A (ru) | 2014-06-20 | 2018-07-23 | Биоэллаенс К.В. | Конъюгаты антитела против фолатного рецептора альфа (fra) c лекарственным средством и способы их применения |
| UA121866C2 (uk) | 2014-08-06 | 2020-08-10 | Астеллас Фарма Інк. | АНТИТІЛО ДО Ig<font face="Symbol">b </font>ЛЮДИНИ |
| EP3188761A4 (en) * | 2014-09-02 | 2018-04-18 | ImmunoGen, Inc. | Methods for formulating antibody drug conjugate compositions |
| US20160082120A1 (en) | 2014-09-23 | 2016-03-24 | Genentech, Inc. | METHODS OF USING ANTI-CD79b IMMUNOCONJUGATES |
| AU2015358325A1 (en) | 2014-12-05 | 2017-05-25 | Genentech, Inc. | Anti-CD79b antibodies and methods of use |
| CA2975383C (en) | 2015-01-28 | 2023-09-12 | Sorrento Therapeutics, Inc. | Antibody drug conjugates comprising dolastatin derivatives |
| CN106153935B (zh) * | 2015-03-26 | 2018-05-08 | 广州瑞博奥生物科技有限公司 | 一种定量检测CD79α的酶联免疫试剂盒 |
| BR112017020149A8 (pt) | 2015-03-27 | 2023-05-02 | Regeneron Pharma | Derivados de maitansinoide, conjugados dos mesmos e métodos de uso |
| MA44334A (fr) | 2015-10-29 | 2018-09-05 | Novartis Ag | Conjugués d'anticorps comprenant un agoniste du récepteur de type toll |
| US11077187B2 (en) | 2015-11-17 | 2021-08-03 | Oklahoma Medical Research Foundation | Epitope of optimized humanized monoclonal antibodies against activated protein C and uses thereof |
| SG10202010590UA (en) | 2015-12-04 | 2020-12-30 | Seattle Genetics Inc | Conjugates of quaternized tubulysin compounds |
| US11793880B2 (en) | 2015-12-04 | 2023-10-24 | Seagen Inc. | Conjugates of quaternized tubulysin compounds |
| CN108713026B (zh) | 2016-01-08 | 2023-01-06 | 美国全心医药生技股份有限公司 | 四价抗psgl-1抗体及其用途 |
| MA43094B1 (fr) | 2016-01-25 | 2020-10-28 | Regeneron Pharma | Dérivés de maytansinoïde, leurs conjugués, et procédés d'utilisation |
| US11844839B2 (en) | 2016-03-25 | 2023-12-19 | Seagen Inc. | Process for the preparation of pegylated drug-linkers and intermediates thereof |
| KR102697394B1 (ko) | 2017-02-28 | 2024-08-20 | 씨젠 인크. | 컨쥬게이션을 위한 시스테인 변이된 항체 |
| JP7527787B2 (ja) | 2017-03-24 | 2024-08-05 | シージェン インコーポレイテッド | グルクロニド薬物-リンカーの調製のためのプロセスおよびその中間体 |
| CN109893538B (zh) * | 2017-12-07 | 2021-05-07 | 苏州凯祥生物科技有限公司 | 聚炔类在降尿酸中的新用途 |
| AU2020213565A1 (en) | 2019-01-28 | 2021-08-05 | Tuojie Biotech (Shanghai) Co., Ltd. | Anti-CD79B antibody, antigen-binding fragment thereof, and pharmaceutical use thereof |
| WO2020181846A1 (zh) * | 2019-03-11 | 2020-09-17 | 凯惠科技发展(上海)有限公司 | 一种含半胱氨酸的抗体、药物偶联物及其应用 |
| EP4190812A4 (en) | 2020-07-27 | 2024-09-04 | Tuojie Biotech (Shanghai) Co., Ltd. | ANTI-CD79B ANTIBODY-DRUG CONJUGATE, ITS PREPARATION METHOD AND ITS PHARMACEUTICAL USE |
| TW202302648A (zh) * | 2021-03-12 | 2023-01-16 | 美商健生生物科技公司 | Cd79b抗體於自體免疫治療應用之用途 |
| WO2023287925A2 (en) * | 2021-07-15 | 2023-01-19 | Nx Prenatal Inc. | Longitudinal predictive model for predicting adverse gestational outcomes |
| WO2023037608A1 (ja) * | 2021-09-10 | 2023-03-16 | ソニーグループ株式会社 | 自律移動体、情報処理方法、及び、プログラム |
| EP4634227A1 (en) * | 2022-12-13 | 2025-10-22 | Seagen Inc. | Site-specific engineered cysteine antibody drug conjugates |
Family Cites Families (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4137230A (en) * | 1977-11-14 | 1979-01-30 | Takeda Chemical Industries, Ltd. | Method for the production of maytansinoids |
| US4307016A (en) * | 1978-03-24 | 1981-12-22 | Takeda Chemical Industries, Ltd. | Demethyl maytansinoids |
| US4265814A (en) * | 1978-03-24 | 1981-05-05 | Takeda Chemical Industries | Matansinol 3-n-hexadecanoate |
| JPS5562090A (en) * | 1978-10-27 | 1980-05-10 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| JPS55164687A (en) * | 1979-06-11 | 1980-12-22 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| JPS5566585A (en) * | 1978-11-14 | 1980-05-20 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| US4256746A (en) * | 1978-11-14 | 1981-03-17 | Takeda Chemical Industries | Dechloromaytansinoids, their pharmaceutical compositions and method of use |
| JPS55102583A (en) * | 1979-01-31 | 1980-08-05 | Takeda Chem Ind Ltd | 20-acyloxy-20-demethylmaytansinoid compound |
| JPS55162791A (en) * | 1979-06-05 | 1980-12-18 | Takeda Chem Ind Ltd | Antibiotic c-15003pnd and its preparation |
| JPS55164685A (en) * | 1979-06-08 | 1980-12-22 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| JPS55164686A (en) * | 1979-06-11 | 1980-12-22 | Takeda Chem Ind Ltd | Novel maytansinoid compound and its preparation |
| US4309428A (en) * | 1979-07-30 | 1982-01-05 | Takeda Chemical Industries, Ltd. | Maytansinoids |
| JPS5645483A (en) * | 1979-09-19 | 1981-04-25 | Takeda Chem Ind Ltd | C-15003phm and its preparation |
| JPS5645485A (en) * | 1979-09-21 | 1981-04-25 | Takeda Chem Ind Ltd | Production of c-15003pnd |
| EP0028683A1 (en) * | 1979-09-21 | 1981-05-20 | Takeda Chemical Industries, Ltd. | Antibiotic C-15003 PHO and production thereof |
| WO1982001188A1 (en) * | 1980-10-08 | 1982-04-15 | Takeda Chemical Industries Ltd | 4,5-deoxymaytansinoide compounds and process for preparing same |
| US4450254A (en) * | 1980-11-03 | 1984-05-22 | Standard Oil Company | Impact improvement of high nitrile resins |
| US4315929A (en) * | 1981-01-27 | 1982-02-16 | The United States Of America As Represented By The Secretary Of Agriculture | Method of controlling the European corn borer with trewiasine |
| US4313946A (en) * | 1981-01-27 | 1982-02-02 | The United States Of America As Represented By The Secretary Of Agriculture | Chemotherapeutically active maytansinoids from Trewia nudiflora |
| JPS57192389A (en) * | 1981-05-20 | 1982-11-26 | Takeda Chem Ind Ltd | Novel maytansinoid |
| US4816567A (en) * | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US5618920A (en) * | 1985-11-01 | 1997-04-08 | Xoma Corporation | Modular assembly of antibody genes, antibodies prepared thereby and use |
| US4975278A (en) * | 1988-02-26 | 1990-12-04 | Bristol-Myers Company | Antibody-enzyme conjugates in combination with prodrugs for the delivery of cytotoxic agents to tumor cells |
| US5208020A (en) * | 1989-10-25 | 1993-05-04 | Immunogen Inc. | Cytotoxic agents comprising maytansinoids and their therapeutic use |
| US6075181A (en) * | 1990-01-12 | 2000-06-13 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US6150584A (en) * | 1990-01-12 | 2000-11-21 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US5644033A (en) * | 1992-12-22 | 1997-07-01 | Health Research, Inc. | Monoclonal antibodies that define a unique antigen of human B cell antigen receptor complex and methods of using same for diagnosis and treatment |
| JPH0719832A (ja) * | 1993-06-21 | 1995-01-20 | Canon Inc | 複数画像の対応点抽出方法 |
| ATE282630T1 (de) * | 1993-10-01 | 2004-12-15 | Teikoku Hormone Mfg Co Ltd | Dolastatin-derivate |
| US6172213B1 (en) * | 1997-07-02 | 2001-01-09 | Genentech, Inc. | Anti-IgE antibodies and method of improving polypeptides |
| US6248564B1 (en) * | 1997-08-29 | 2001-06-19 | Harvard University | Mutant MHC class I molecules |
| EP2180054A1 (en) * | 1999-12-24 | 2010-04-28 | Genentech, Inc. | Methods and compositions for prolonging elimination half-times of bioactive compounds |
| US20040001827A1 (en) * | 2002-06-28 | 2004-01-01 | Dennis Mark S. | Serum albumin binding peptides for tumor targeting |
| US20020150573A1 (en) * | 2000-11-10 | 2002-10-17 | The Rockefeller University | Anti-Igalpha-Igbeta antibody for lymphoma therapy |
| US20040018194A1 (en) * | 2000-11-28 | 2004-01-29 | Francisco Joseph A. | Recombinant anti-CD30 antibodies and uses thereof |
| US20110045005A1 (en) * | 2001-10-19 | 2011-02-24 | Craig Crowley | Compositions and methods for the treatment of tumor of hematopoietic origin |
| US20050238650A1 (en) * | 2002-04-17 | 2005-10-27 | Genentech, Inc. | Compositions and methods for the treatment of tumor of hematopoietic origin |
| US20090068178A1 (en) * | 2002-05-08 | 2009-03-12 | Genentech, Inc. | Compositions and Methods for the Treatment of Tumor of Hematopoietic Origin |
| US20070207142A1 (en) * | 2002-05-08 | 2007-09-06 | Genentech, Inc. | Compositions and methods for the treatment of tumor of hematopoietic origin |
| US20110042260A1 (en) * | 2003-04-10 | 2011-02-24 | Craig Crowley | Compositions and methods for the treatment of tumor of hematopoietic origin |
| US8088387B2 (en) * | 2003-10-10 | 2012-01-03 | Immunogen Inc. | Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates |
| DK1725249T3 (en) * | 2003-11-06 | 2014-03-17 | Seattle Genetics Inc | Monomethylvaline compounds capable of conjugating to ligands. |
| ES2472690T3 (es) * | 2003-11-17 | 2014-07-02 | Genentech, Inc. | Anticuerpo contra CD22 para el tratamiento de tumores de origen hematopoy�tico |
| JP5064037B2 (ja) * | 2004-02-23 | 2012-10-31 | ジェネンテック, インコーポレイテッド | 複素環式自壊的リンカーおよび結合体 |
| CN114053429A (zh) * | 2004-06-01 | 2022-02-18 | 健泰科生物技术公司 | 抗体-药物偶联物和方法 |
| ES2579805T3 (es) * | 2004-09-23 | 2016-08-16 | Genentech, Inc. | Anticuerpos y conjugados modificados por ingeniería genética con cisteína |
| ES2381788T3 (es) * | 2007-07-16 | 2012-05-31 | Genentech, Inc. | Anticuerpos anti-CD79b e inmunoconjugados y métodos de uso |
| RU2557319C2 (ru) * | 2007-07-16 | 2015-07-20 | Дженентек, Инк. | ГУМАНИЗИРОВАННЫЕ АНТИТЕЛА ПРОТИВ CD79b И ИММУНОКОНЪЮГАТЫ И СПОСОБЫ ПРИМЕНЕНИЯ |
| HUE029869T2 (en) * | 2008-01-31 | 2017-04-28 | Genentech Inc | Anti-cd79b antibodies and immunoconjugates and methods of use |
-
2008
- 2008-01-31 US US12/023,811 patent/US20090068178A1/en not_active Abandoned
-
2009
- 2009-01-13 EP EP09707533A patent/EP2247312A2/en not_active Withdrawn
- 2009-01-13 CN CN2009801110932A patent/CN102014964A/zh active Pending
- 2009-01-13 CA CA2712518A patent/CA2712518A1/en not_active Abandoned
- 2009-01-13 NZ NZ587652A patent/NZ587652A/xx not_active IP Right Cessation
- 2009-01-13 WO PCT/US2009/030851 patent/WO2009099719A2/en not_active Ceased
- 2009-01-13 RU RU2010136303/10A patent/RU2010136303A/ru not_active Application Discontinuation
- 2009-01-13 MX MX2010008199A patent/MX2010008199A/es active IP Right Grant
- 2009-01-13 JP JP2010545049A patent/JP2011515069A/ja not_active Withdrawn
- 2009-01-13 AU AU2009210627A patent/AU2009210627A1/en not_active Abandoned
- 2009-01-13 BR BRPI0908854-7A patent/BRPI0908854A2/pt not_active IP Right Cessation
- 2009-01-13 KR KR1020107019272A patent/KR20100128286A/ko not_active Withdrawn
- 2009-01-16 PE PE2009000055A patent/PE20091404A1/es not_active Application Discontinuation
- 2009-01-16 AR ARP090100153A patent/AR071829A1/es not_active Application Discontinuation
- 2009-01-16 CL CL2009000082A patent/CL2009000082A1/es unknown
-
2010
- 2010-07-13 IL IL206970A patent/IL206970A0/en unknown
- 2010-09-09 US US12/878,920 patent/US20110070243A1/en not_active Abandoned
- 2010-10-12 US US12/902,434 patent/US20110206658A1/en not_active Abandoned
-
2017
- 2017-01-13 US US15/406,583 patent/US20170362318A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US20090068178A1 (en) | 2009-03-12 |
| EP2247312A2 (en) | 2010-11-10 |
| CA2712518A1 (en) | 2009-08-13 |
| NZ587652A (en) | 2012-12-21 |
| US20110206658A1 (en) | 2011-08-25 |
| IL206970A0 (en) | 2010-12-30 |
| US20170362318A1 (en) | 2017-12-21 |
| CN102014964A (zh) | 2011-04-13 |
| AR071829A1 (es) | 2010-07-21 |
| BRPI0908854A2 (pt) | 2019-09-24 |
| PE20091404A1 (es) | 2009-09-23 |
| RU2010136303A (ru) | 2012-03-10 |
| US20110070243A1 (en) | 2011-03-24 |
| CL2009000082A1 (es) | 2012-03-02 |
| MX2010008199A (es) | 2010-11-30 |
| WO2009099719A2 (en) | 2009-08-13 |
| JP2011515069A (ja) | 2011-05-19 |
| AU2009210627A1 (en) | 2009-08-13 |
| WO2009099719A3 (en) | 2009-10-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20100128286A (ko) | 조혈계 기원의 종양 치료용 조성물 및 방법 | |
| AU2018223053B2 (en) | Novel modulators and methods of use | |
| US10836831B2 (en) | Anti-PTK7 antibodies and methods of use | |
| KR101960004B1 (ko) | Cldn6 생체내 표적-지향된 항체를 이용하는 암 치료법 | |
| KR101297467B1 (ko) | Dr5 항체 및 그의 용도 | |
| RU2568066C2 (ru) | Антитела против fgfr3 и способы их применения | |
| KR101245983B1 (ko) | 인간화 항-tgf-베타 항체 | |
| KR20100057009A (ko) | 인간화 항-cd79b 항체 및 면역접합체 및 사용 방법 | |
| TW201235049A (en) | Anti-CD79b antibodies and immunoconjugates and methods of use | |
| EP2817338A2 (en) | Dll3 modulators and methods of use | |
| KR101432474B1 (ko) | 항-연장된 ⅰ형 글라이코스핑고지질 항체, 이의 유도체 및 용도 | |
| HK40044653A (en) | Novel modulators and methods of use | |
| NZ615285B2 (en) | Novel modulators and methods of use | |
| NZ708615B2 (en) | Novel modulators and methods of use | |
| HK1191031B (en) | Novel modulators and methods of use | |
| HK1191031A (en) | Novel modulators and methods of use | |
| HK1167666B (en) | Anti-fgfr3 antibodies and methods using same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20100830 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |