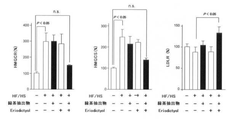
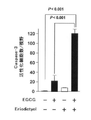
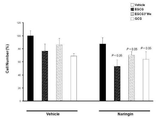
JP6912763B2 - カテキンの機能性増強法 - Google Patents
カテキンの機能性増強法 Download PDFInfo
- Publication number
- JP6912763B2 JP6912763B2 JP2016529649A JP2016529649A JP6912763B2 JP 6912763 B2 JP6912763 B2 JP 6912763B2 JP 2016529649 A JP2016529649 A JP 2016529649A JP 2016529649 A JP2016529649 A JP 2016529649A JP 6912763 B2 JP6912763 B2 JP 6912763B2
- Authority
- JP
- Japan
- Prior art keywords
- eriodictyol
- effect
- egcg
- action
- component
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23F—COFFEE; TEA; THEIR SUBSTITUTES; MANUFACTURE, PREPARATION, OR INFUSION THEREOF
- A23F3/00—Tea; Tea substitutes; Preparations thereof
- A23F3/16—Tea extraction; Tea extracts; Treating tea extract; Making instant tea
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/105—Plant extracts, their artificial duplicates or their derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
- A61K31/353—3,4-Dihydrobenzopyrans, e.g. chroman, catechin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7048—Compounds having saccharide radicals and heterocyclic rings having oxygen as a ring hetero atom, e.g. leucoglucosan, hesperidin, erythromycin, nystatin, digitoxin or digoxin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/75—Rutaceae (Rue family)
- A61K36/752—Citrus, e.g. lime, orange or lemon
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/82—Theaceae (Tea family), e.g. camellia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Botany (AREA)
- Mycology (AREA)
- Medical Informatics (AREA)
- Alternative & Traditional Medicine (AREA)
- Polymers & Plastics (AREA)
- Microbiology (AREA)
- Food Science & Technology (AREA)
- Biotechnology (AREA)
- Immunology (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Molecular Biology (AREA)
- Nutrition Science (AREA)
- Cardiology (AREA)
- Obesity (AREA)
- Heart & Thoracic Surgery (AREA)
- Neurology (AREA)
- Rheumatology (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Pain & Pain Management (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Child & Adolescent Psychology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014132891 | 2014-06-27 | ||
| JP2014132891 | 2014-06-27 | ||
| PCT/JP2015/068302 WO2015199169A1 (ja) | 2014-06-27 | 2015-06-25 | カテキンの機能性増強法 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020068670A Division JP2020121990A (ja) | 2014-06-27 | 2020-04-06 | カテキンの機能性増強法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JPWO2015199169A1 JPWO2015199169A1 (ja) | 2017-05-25 |
| JP6912763B2 true JP6912763B2 (ja) | 2021-08-04 |
Family
ID=54938246
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016529649A Active JP6912763B2 (ja) | 2014-06-27 | 2015-06-25 | カテキンの機能性増強法 |
| JP2020068670A Pending JP2020121990A (ja) | 2014-06-27 | 2020-04-06 | カテキンの機能性増強法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020068670A Pending JP2020121990A (ja) | 2014-06-27 | 2020-04-06 | カテキンの機能性増強法 |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US10251408B2 (enExample) |
| JP (2) | JP6912763B2 (enExample) |
| WO (1) | WO2015199169A1 (enExample) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6912763B2 (ja) * | 2014-06-27 | 2021-08-04 | 国立大学法人九州大学 | カテキンの機能性増強法 |
| JP6881890B2 (ja) * | 2015-05-14 | 2021-06-02 | 花王株式会社 | カテキン類吸収促進剤 |
| CN109152931A (zh) * | 2016-03-28 | 2019-01-04 | 可口可乐公司 | 具有类黄酮的甜菊醇糖苷或罗汉果苷甜味剂的甜度和味道改善 |
| JP7061303B2 (ja) | 2016-08-10 | 2022-04-28 | 国立大学法人九州大学 | 67kDaラミニンレセプター活性化剤及びその使用 |
| FR3061021B1 (fr) * | 2016-12-23 | 2020-04-03 | Etablissement Francais Du Sang | Antagoniste specifique de tlr4 dans le traitement du myelome multiple |
| KR102428987B1 (ko) * | 2017-05-16 | 2022-08-04 | (주)아모레퍼시픽 | 차나무의 추출물을 함유하는 조성물 |
| KR20190047873A (ko) | 2017-10-30 | 2019-05-09 | (주)아모레퍼시픽 | 특정 성분 함량이 증가된 차 추출물을 포함하는 지방대사 이상의 예방 및 개선용 조성물 |
| JP2019151568A (ja) * | 2018-03-01 | 2019-09-12 | アサヒ飲料株式会社 | 酸性スフィンゴミエリナーゼ活性向上剤、酸性スフィンゴミエリナーゼ活性向上用組成物、及び抗アレルギー用組成物 |
| WO2020096299A1 (ko) * | 2018-11-05 | 2020-05-14 | (주)아모레퍼시픽 | 성분 함량이 변화된 녹차 추출물, 및 이를 포함하는 조성물 |
| JP7364497B2 (ja) | 2020-03-04 | 2023-10-18 | トヨタ自動車株式会社 | 緑茶抽出物含有組成物、機能性食品 |
| JP2022092966A (ja) * | 2020-12-11 | 2022-06-23 | トヨタ自動車株式会社 | ジペプチジルペプチダーゼiv阻害剤、機能性表示食品 |
| EP4284355A1 (en) * | 2021-01-28 | 2023-12-06 | Abbott Laboratories | Methods and nutritional compositions for improving muscle energy production and/or strength |
| JP2023008374A (ja) * | 2021-07-06 | 2023-01-19 | トヨタ自動車株式会社 | 抗肥満剤用組成物、機能性食品 |
| CN114990169B (zh) * | 2022-06-16 | 2023-07-28 | 中国农业科学院茶叶研究所 | 一种制备EGCG-3”-Me的方法 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08283154A (ja) * | 1995-04-12 | 1996-10-29 | Nippon Shinyaku Co Ltd | 脂質代謝改善剤 |
| US6379714B1 (en) * | 1995-04-14 | 2002-04-30 | Pharmaprint, Inc. | Pharmaceutical grade botanical drugs |
| DE69924240T2 (de) | 1999-06-23 | 2006-02-09 | ICT Integrated Circuit Testing Gesellschaft für Halbleiterprüftechnik mbH | Ladungsträgerteilchenstrahlvorrichtung |
| EP1123304B1 (en) * | 1999-08-19 | 2003-10-08 | Korea Research Institute of Bioscience and Biotechnology | Flavonoids derived from citrus peels as collagen-induced platelet aggregation inhibitor |
| JP4246980B2 (ja) * | 2001-09-11 | 2009-04-02 | 花王株式会社 | 皮膚外用剤 |
| JP2004075619A (ja) * | 2002-08-20 | 2004-03-11 | Naris Cosmetics Co Ltd | タイプ2ヘルパーt細胞型サイトカイン抑制剤 |
| JP2005075805A (ja) * | 2003-09-03 | 2005-03-24 | Kyoto Univ | 酵素阻害剤 |
| US20110274680A1 (en) * | 2009-10-02 | 2011-11-10 | Mazed Mohammad A | Chemical composition and its delivery for lowering the risks of alzheimer's, cardiov ascular and type-2 diabetes diseases |
| JP5517421B2 (ja) * | 2007-08-03 | 2014-06-11 | 花王株式会社 | 容器詰飲料 |
| JP6912763B2 (ja) * | 2014-06-27 | 2021-08-04 | 国立大学法人九州大学 | カテキンの機能性増強法 |
-
2015
- 2015-06-25 JP JP2016529649A patent/JP6912763B2/ja active Active
- 2015-06-25 WO PCT/JP2015/068302 patent/WO2015199169A1/ja not_active Ceased
- 2015-06-25 US US15/321,134 patent/US10251408B2/en active Active
-
2020
- 2020-04-06 JP JP2020068670A patent/JP2020121990A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2015199169A1 (ja) | 2017-05-25 |
| JP2020121990A (ja) | 2020-08-13 |
| WO2015199169A1 (ja) | 2015-12-30 |
| US20170156361A1 (en) | 2017-06-08 |
| US10251408B2 (en) | 2019-04-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6912763B2 (ja) | カテキンの機能性増強法 | |
| Kim et al. | Apigetrin induces extrinsic apoptosis, autophagy and G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS human gastric cancer cell | |
| Wang et al. | Naringenin prevents ischaemic stroke damage via anti‐apoptotic and anti‐oxidant effects | |
| Yo et al. | Licorice and licochalcone-A induce autophagy in LNCaP prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway | |
| Li et al. | Piperlongumine analogue L50377 induces pyroptosis via ROS mediated NF-κB suppression in non-small-cell lung cancer | |
| Demir et al. | Selective cytotoxic effect of Rhododendron luteum extract on human colon and liver cancer cells | |
| Yang et al. | Inhibitory effects of cocoa tea (Camellia ptilophylla) in human hepatocellular carcinoma HepG2 in vitro and in vivo through apoptosis | |
| Rao et al. | Strychnos nux-vomica root extract induces apoptosis in the human multiple myeloma cell line—U266B1 | |
| Kang et al. | Sappanone A protects mice against cisplatin-induced kidney injury | |
| EP3824898A1 (en) | Dehydrated extracts based phyto complex comprising citrus bergamia and its pharmaceutical use | |
| Parisotto et al. | The antitumor activity of extracts from Cordia verbenacea DC obtained by supercritical fluid extraction | |
| Kou et al. | Ampelopsin attenuates 6-OHDA-induced neurotoxicity by regulating GSK-3β/NRF2/ARE signalling | |
| Kim et al. | The extract of the immature fruit of Poncirus trifoliata induces apoptosis in colorectal cancer cells via mitochondrial autophagy | |
| Gorlach et al. | Polyphenols from evening primrose (Oenothera paradoxa) defatted seeds induce apoptosis in human colon cancer Caco-2 cells | |
| Khatibi et al. | In vitro evaluation of cytotoxic and antiproliferative effects of Portulaca oleracea ethanolic extracton on hela cell line | |
| Nagai et al. | Anti-obesity effects of Asian dayflower, Commelina communis, in mice with high-fat diet-induced obesity and in 3T3-L1 cells | |
| KR101959731B1 (ko) | 새싹보리 추출물을 포함하는 갱년기 장애의 예방 또는 치료용 조성물 | |
| Murugaiyah et al. | Lipid-lowering effect of hydroalcoholic extracts of Gynura procumbens in Chemical-and High-fat diet-induced hyperlipidemic rats | |
| KR101917363B1 (ko) | 콩 발아배아 추출물을 포함하는 여성 갱년기 질환의 예방 또는 치료용 약학조성물 | |
| JP7364497B2 (ja) | 緑茶抽出物含有組成物、機能性食品 | |
| KR101387970B1 (ko) | 암 예방 및 치료용 조성물 및 건강기능식품 | |
| KR102040119B1 (ko) | 신선초 추출물로부터 분리된 화합물을 함유하는 근육관련 질환의 예방 및 치료용 조성물 | |
| KR101655554B1 (ko) | 택사 추출물, 이의 분획물 또는 이로부터 분리된 화합물을 포함하는 골질환의 예방 또는 치료용 약학적 조성물 | |
| ÖNER et al. | Novel Natural Compounds for Hepatocellular Carcinoma Treatment | |
| Li et al. | Study on the influence of curcumin on chemosensitivity of nephroblastoma cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180612 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180612 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20190604 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190718 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20200107 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200406 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20200406 |
|
| C11 | Written invitation by the commissioner to file amendments |
Free format text: JAPANESE INTERMEDIATE CODE: C11 Effective date: 20200421 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20200521 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20200526 |
|
| A912 | Re-examination (zenchi) completed and case transferred to appeal board |
Free format text: JAPANESE INTERMEDIATE CODE: A912 Effective date: 20200821 |
|
| C211 | Notice of termination of reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C211 Effective date: 20200825 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20201222 |
|
| C13 | Notice of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: C13 Effective date: 20210224 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20210413 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20210420 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210423 |
|
| C302 | Record of communication |
Free format text: JAPANESE INTERMEDIATE CODE: C302 Effective date: 20210423 |
|
| C23 | Notice of termination of proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C23 Effective date: 20210518 |
|
| C03 | Trial/appeal decision taken |
Free format text: JAPANESE INTERMEDIATE CODE: C03 Effective date: 20210615 |
|
| C30A | Notification sent |
Free format text: JAPANESE INTERMEDIATE CODE: C3012 Effective date: 20210615 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210629 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6912763 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R157 | Certificate of patent or utility model (correction) |
Free format text: JAPANESE INTERMEDIATE CODE: R157 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |