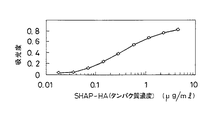
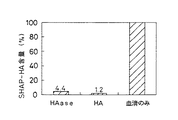
JP3976848B2 - 血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット - Google Patents
血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット Download PDFInfo
- Publication number
- JP3976848B2 JP3976848B2 JP20545897A JP20545897A JP3976848B2 JP 3976848 B2 JP3976848 B2 JP 3976848B2 JP 20545897 A JP20545897 A JP 20545897A JP 20545897 A JP20545897 A JP 20545897A JP 3976848 B2 JP3976848 B2 JP 3976848B2
- Authority
- JP
- Japan
- Prior art keywords
- hyaluronic acid
- protein
- conjugate
- antibody
- shap
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 229920002674 hyaluronan Polymers 0.000 claims description 91
- 229960003160 hyaluronic acid Drugs 0.000 claims description 86
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 claims description 81
- 238000000034 method Methods 0.000 claims description 71
- 102100032912 CD44 antigen Human genes 0.000 claims description 58
- 101000868273 Homo sapiens CD44 antigen Proteins 0.000 claims description 57
- 230000027455 binding Effects 0.000 claims description 57
- 102000004169 proteins and genes Human genes 0.000 claims description 56
- 108090000623 proteins and genes Proteins 0.000 claims description 56
- 108010013214 Hyaluronan Receptors Proteins 0.000 claims description 36
- 102000018866 Hyaluronan Receptors Human genes 0.000 claims description 36
- 210000002966 serum Anatomy 0.000 claims description 32
- 238000000691 measurement method Methods 0.000 claims description 24
- 102000037865 fusion proteins Human genes 0.000 claims description 21
- 108020001507 fusion proteins Proteins 0.000 claims description 21
- 238000005259 measurement Methods 0.000 claims description 21
- 208000019423 liver disease Diseases 0.000 claims description 19
- 206010003246 arthritis Diseases 0.000 claims description 15
- 230000036961 partial effect Effects 0.000 claims description 15
- 102000016611 Proteoglycans Human genes 0.000 claims description 13
- 108010067787 Proteoglycans Proteins 0.000 claims description 13
- 101001054921 Homo sapiens Lymphatic vessel endothelial hyaluronic acid receptor 1 Proteins 0.000 claims description 11
- 102100026849 Lymphatic vessel endothelial hyaluronic acid receptor 1 Human genes 0.000 claims description 11
- 101710132601 Capsid protein Proteins 0.000 claims description 5
- 102100032859 Protein AMBP Human genes 0.000 claims description 5
- 229940099552 hyaluronan Drugs 0.000 claims description 5
- KIUKXJAPPMFGSW-MNSSHETKSA-N hyaluronan Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)C1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H](C(O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-MNSSHETKSA-N 0.000 claims description 5
- 108700041430 link Proteins 0.000 claims description 4
- 229940122618 Trypsin inhibitor Drugs 0.000 claims description 3
- 239000002753 trypsin inhibitor Substances 0.000 claims description 3
- 235000018102 proteins Nutrition 0.000 description 45
- 239000000126 substance Substances 0.000 description 41
- 238000002372 labelling Methods 0.000 description 29
- 239000007790 solid phase Substances 0.000 description 25
- 239000000243 solution Substances 0.000 description 19
- 101000976697 Homo sapiens Inter-alpha-trypsin inhibitor heavy chain H1 Proteins 0.000 description 18
- 101000609396 Homo sapiens Inter-alpha-trypsin inhibitor heavy chain H2 Proteins 0.000 description 18
- 101000609406 Homo sapiens Inter-alpha-trypsin inhibitor heavy chain H3 Proteins 0.000 description 18
- 102100023490 Inter-alpha-trypsin inhibitor heavy chain H1 Human genes 0.000 description 18
- 108010093564 inter-alpha-inhibitor Proteins 0.000 description 17
- 210000004369 blood Anatomy 0.000 description 12
- 239000008280 blood Substances 0.000 description 12
- 210000004027 cell Anatomy 0.000 description 12
- 238000002360 preparation method Methods 0.000 description 12
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 11
- 239000002953 phosphate buffered saline Substances 0.000 description 11
- 239000012530 fluid Substances 0.000 description 9
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 8
- 102000004190 Enzymes Human genes 0.000 description 7
- 108090000790 Enzymes Proteins 0.000 description 7
- 238000011088 calibration curve Methods 0.000 description 7
- 210000000845 cartilage Anatomy 0.000 description 7
- 238000005406 washing Methods 0.000 description 7
- 102100027735 Hyaluronan mediated motility receptor Human genes 0.000 description 6
- 108010003272 Hyaluronate lyase Proteins 0.000 description 6
- 102000001974 Hyaluronidases Human genes 0.000 description 6
- 108060003951 Immunoglobulin Proteins 0.000 description 6
- 230000000903 blocking effect Effects 0.000 description 6
- 239000007853 buffer solution Substances 0.000 description 6
- 238000001514 detection method Methods 0.000 description 6
- 229940088598 enzyme Drugs 0.000 description 6
- 108010003425 hyaluronan-mediated motility receptor Proteins 0.000 description 6
- 229960002773 hyaluronidase Drugs 0.000 description 6
- 102000018358 immunoglobulin Human genes 0.000 description 6
- 102000013415 peroxidase activity proteins Human genes 0.000 description 6
- 108040007629 peroxidase activity proteins Proteins 0.000 description 6
- 210000001179 synovial fluid Anatomy 0.000 description 6
- 238000009007 Diagnostic Kit Methods 0.000 description 5
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 5
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 5
- 101800001691 Inter-alpha-trypsin inhibitor light chain Proteins 0.000 description 5
- 239000000427 antigen Substances 0.000 description 5
- 102000036639 antigens Human genes 0.000 description 5
- 108091007433 antigens Proteins 0.000 description 5
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 5
- 239000002299 complementary DNA Substances 0.000 description 5
- 238000003745 diagnosis Methods 0.000 description 5
- 238000011002 quantification Methods 0.000 description 5
- 208000003947 Knee Osteoarthritis Diseases 0.000 description 4
- 108091005804 Peptidases Proteins 0.000 description 4
- 102000035195 Peptidases Human genes 0.000 description 4
- 108090000631 Trypsin Proteins 0.000 description 4
- 102000004142 Trypsin Human genes 0.000 description 4
- 238000002835 absorbance Methods 0.000 description 4
- 229960002685 biotin Drugs 0.000 description 4
- 235000020958 biotin Nutrition 0.000 description 4
- 239000011616 biotin Substances 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 108090000765 processed proteins & peptides Proteins 0.000 description 4
- 206010039073 rheumatoid arthritis Diseases 0.000 description 4
- 239000012588 trypsin Substances 0.000 description 4
- 229960001322 trypsin Drugs 0.000 description 4
- -1 ECMRIII Proteins 0.000 description 3
- 102400001240 Inter-alpha-trypsin inhibitor light chain Human genes 0.000 description 3
- 206010027476 Metastases Diseases 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 108010090804 Streptavidin Proteins 0.000 description 3
- 238000001042 affinity chromatography Methods 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 229960000789 guanidine hydrochloride Drugs 0.000 description 3
- PJJJBBJSCAKJQF-UHFFFAOYSA-N guanidinium chloride Chemical compound [Cl-].NC(N)=[NH2+] PJJJBBJSCAKJQF-UHFFFAOYSA-N 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 230000009401 metastasis Effects 0.000 description 3
- 201000008482 osteoarthritis Diseases 0.000 description 3
- 239000008055 phosphate buffer solution Substances 0.000 description 3
- 210000002381 plasma Anatomy 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 210000002700 urine Anatomy 0.000 description 3
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 2
- YRNWIFYIFSBPAU-UHFFFAOYSA-N 4-[4-(dimethylamino)phenyl]-n,n-dimethylaniline Chemical compound C1=CC(N(C)C)=CC=C1C1=CC=C(N(C)C)C=C1 YRNWIFYIFSBPAU-UHFFFAOYSA-N 0.000 description 2
- 108090001008 Avidin Proteins 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 102000012422 Collagen Type I Human genes 0.000 description 2
- 108010022452 Collagen Type I Proteins 0.000 description 2
- 102000004266 Collagen Type IV Human genes 0.000 description 2
- 108010042086 Collagen Type IV Proteins 0.000 description 2
- 108010067306 Fibronectins Proteins 0.000 description 2
- 102000016359 Fibronectins Human genes 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 108010093096 Immobilized Enzymes Proteins 0.000 description 2
- 239000004365 Protease Substances 0.000 description 2
- 108010071390 Serum Albumin Proteins 0.000 description 2
- 102000007562 Serum Albumin Human genes 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 150000001413 amino acids Chemical group 0.000 description 2
- 230000002917 arthritic effect Effects 0.000 description 2
- 229940096422 collagen type i Drugs 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- 239000000287 crude extract Substances 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 238000002405 diagnostic procedure Methods 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000006911 enzymatic reaction Methods 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 2
- 238000001502 gel electrophoresis Methods 0.000 description 2
- 238000010353 genetic engineering Methods 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 230000003053 immunization Effects 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 239000012086 standard solution Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000005199 ultracentrifugation Methods 0.000 description 2
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 1
- 102000012440 Acetylcholinesterase Human genes 0.000 description 1
- 108010022752 Acetylcholinesterase Proteins 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 102100036601 Aggrecan core protein Human genes 0.000 description 1
- 108010067219 Aggrecans Proteins 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 108091016585 CD44 antigen Proteins 0.000 description 1
- 101100351961 Caenorhabditis elegans pgp-1 gene Proteins 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 102000011632 Caseins Human genes 0.000 description 1
- 108010076119 Caseins Proteins 0.000 description 1
- 229920001287 Chondroitin sulfate Polymers 0.000 description 1
- 108090000819 Chondroitin-sulfate-ABC endolyases Proteins 0.000 description 1
- 102000037716 Chondroitin-sulfate-ABC endolyases Human genes 0.000 description 1
- 108010023736 Chondroitinases and Chondroitin Lyases Proteins 0.000 description 1
- 102000011413 Chondroitinases and Chondroitin Lyases Human genes 0.000 description 1
- 108090000317 Chymotrypsin Proteins 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- SNRUBQQJIBEYMU-UHFFFAOYSA-N Dodecane Natural products CCCCCCCCCCCC SNRUBQQJIBEYMU-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 102000006395 Globulins Human genes 0.000 description 1
- 108010044091 Globulins Proteins 0.000 description 1
- 108010051815 Glutamyl endopeptidase Proteins 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- 108010070675 Glutathione transferase Proteins 0.000 description 1
- 229920002683 Glycosaminoglycan Polymers 0.000 description 1
- 102100029100 Hematopoietic prostaglandin D synthase Human genes 0.000 description 1
- 229920002971 Heparan sulfate Polymers 0.000 description 1
- 208000012659 Joint disease Diseases 0.000 description 1
- 102100038609 Lactoperoxidase Human genes 0.000 description 1
- 108010023244 Lactoperoxidase Proteins 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 108010011756 Milk Proteins Proteins 0.000 description 1
- 102000014171 Milk Proteins Human genes 0.000 description 1
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical group CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 description 1
- 108010043296 Neurocan Proteins 0.000 description 1
- 102100030466 Neurocan core protein Human genes 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 241001504519 Papio ursinus Species 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 101710162629 Trypsin inhibitor Proteins 0.000 description 1
- 229940022698 acetylcholinesterase Drugs 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 238000003277 amino acid sequence analysis Methods 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 229940127219 anticoagulant drug Drugs 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N aspartic acid group Chemical group N[C@@H](CC(=O)O)C(=O)O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 239000012888 bovine serum Substances 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- AIYUHDOJVYHVIT-UHFFFAOYSA-M caesium chloride Chemical compound [Cl-].[Cs+] AIYUHDOJVYHVIT-UHFFFAOYSA-M 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- VDQQXEISLMTGAB-UHFFFAOYSA-N chloramine T Chemical compound [Na+].CC1=CC=C(S(=O)(=O)[N-]Cl)C=C1 VDQQXEISLMTGAB-UHFFFAOYSA-N 0.000 description 1
- 229940094517 chondroitin 4-sulfate Drugs 0.000 description 1
- KXKPYJOVDUMHGS-OSRGNVMNSA-N chondroitin sulfate Chemical group CC(=O)N[C@H]1[C@H](O)O[C@H](OS(O)(=O)=O)[C@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](C(O)=O)O1 KXKPYJOVDUMHGS-OSRGNVMNSA-N 0.000 description 1
- 229940059329 chondroitin sulfate Drugs 0.000 description 1
- 229960002376 chymotrypsin Drugs 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 230000006334 disulfide bridging Effects 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 230000002414 glycolytic effect Effects 0.000 description 1
- 108010083213 heparitinsulfate lyase Proteins 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 210000000629 knee joint Anatomy 0.000 description 1
- 229940057428 lactoperoxidase Drugs 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- HWYHZTIRURJOHG-UHFFFAOYSA-N luminol Chemical compound O=C1NNC(=O)C2=C1C(N)=CC=C2 HWYHZTIRURJOHG-UHFFFAOYSA-N 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 208000037819 metastatic cancer Diseases 0.000 description 1
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 235000021239 milk protein Nutrition 0.000 description 1
- 230000004899 motility Effects 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 238000007500 overflow downdraw method Methods 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- KHIWWQKSHDUIBK-UHFFFAOYSA-N periodic acid Chemical compound OI(=O)(=O)=O KHIWWQKSHDUIBK-UHFFFAOYSA-N 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 238000001814 protein method Methods 0.000 description 1
- 239000012460 protein solution Substances 0.000 description 1
- 229940024999 proteolytic enzymes for treatment of wounds and ulcers Drugs 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000012898 sample dilution Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 239000012679 serum free medium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 239000012089 stop solution Substances 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 108091005703 transmembrane proteins Proteins 0.000 description 1
- 102000035160 transmembrane proteins Human genes 0.000 description 1
- 210000003954 umbilical cord Anatomy 0.000 description 1
- 239000013598 vector Substances 0.000 description 1
- 210000004127 vitreous body Anatomy 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Landscapes
- Peptides Or Proteins (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP20545897A JP3976848B2 (ja) | 1996-07-16 | 1997-07-15 | 血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP20528296 | 1996-07-16 | ||
| JP8-205282 | 1996-07-16 | ||
| JP20545897A JP3976848B2 (ja) | 1996-07-16 | 1997-07-15 | 血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006010713A Division JP4217716B2 (ja) | 1996-07-16 | 2006-01-19 | 血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット |
| JP2006010714A Division JP4217717B2 (ja) | 1996-07-16 | 2006-01-19 | 血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JPH1082784A JPH1082784A (ja) | 1998-03-31 |
| JPH1082784A5 JPH1082784A5 (enExample) | 2005-02-10 |
| JP3976848B2 true JP3976848B2 (ja) | 2007-09-19 |
Family
ID=26514978
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP20545897A Expired - Fee Related JP3976848B2 (ja) | 1996-07-16 | 1997-07-15 | 血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP3976848B2 (enExample) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1059087A1 (de) * | 1999-06-08 | 2000-12-13 | Peter Prof. Dr. Prehm | Antigene von rheumatischen Autoimmunerkrankungen |
| WO2000074705A1 (de) * | 1999-06-08 | 2000-12-14 | Peter Prehm | Antigene von rheumatischen autoimmunkrankheiten |
| WO2005114186A1 (ja) * | 2004-05-20 | 2005-12-01 | Wako Pure Chemical Industries, Ltd. | ヒアルロン酸バインディングプロテインを用いたヒアルロン酸の測定方法 |
| JP2006250862A (ja) * | 2005-03-14 | 2006-09-21 | Seikagaku Kogyo Co Ltd | 妊娠中毒症の検知方法及び検知キット |
| US7812125B2 (en) * | 2007-03-27 | 2010-10-12 | Anamar Medical Ab | Cartilage intermediate layer protein 2 C1 and its use to differentiate osteoarthritis from rheumatoid arthritis and non-disease conditions |
| TWI651535B (zh) * | 2017-10-05 | 2019-02-21 | 高雄醫學大學 | 以血液玻尿酸數値評估登革熱病毒感染者之疾病嚴重度之方法 |
| US11730811B2 (en) | 2017-10-05 | 2023-08-22 | Kaohsiung Medical University | Method for treating subject suffering from flavivirus infection |
-
1997
- 1997-07-15 JP JP20545897A patent/JP3976848B2/ja not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JPH1082784A (ja) | 1998-03-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU594651B2 (en) | Immunoassays for protein analytes, particularly HB A1c, involving sample denaturation | |
| JP5197535B2 (ja) | リソソーム病の検出方法 | |
| JP3694894B2 (ja) | 正常アグリカンの測定法および測定用キット | |
| JP2003202338A (ja) | 体液中のコラーゲン断片を測定する方法、該方法を実施するためのテストキット及び手段、並びにコラ−ゲンの代謝に関連する疾患の存在を診断するために該方法を使用する方法・用途 | |
| Baeckström et al. | Discrimination of MUC1 mucins from other sialyl-Lea-carrying glycoproteins produced by colon carcinoma cells using a novel monoclonal antibody | |
| US6210902B1 (en) | Estimation of the fragmentation pattern of collagen in body fluids and the diagnosis of disorders associated with the metabolism of collagen | |
| JPH0684971B2 (ja) | 軟骨組織異常の診断法 | |
| JPS60502167A (ja) | 軟骨に生じる変化の測定法 | |
| JP3224356B2 (ja) | ヒトおよび動物における骨および結合組織の疾患を検出する方法 | |
| JP3976848B2 (ja) | 血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット | |
| AU653536B2 (en) | Measuring non-dystrophin proteins and diagnosing muscular dystrophy | |
| CA2496984C (en) | Thrombospondin fragments and uses thereof in clinical assays for cancer and generation of antibodies and other binding agents | |
| US6372442B1 (en) | Method of characterizing the degradation of type II collagen | |
| JP4217717B2 (ja) | 血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット | |
| JP4217716B2 (ja) | 血清由来ヒアルロナン結合性タンパク質−ヒアルロン酸結合体の測定法および測定キット | |
| US20030166031A1 (en) | Assay for anti-INGAP antibodies | |
| JPH083486B2 (ja) | 体液中の基底膜コラーゲンの測定法及び基底膜コラーゲン―ドメインnc1に対する抗体の検出法 | |
| JP4124526B2 (ja) | 正常アグリカン測定法とその応用 | |
| EP0398292B1 (en) | Diagnostic composition for rheumatoid arthritis | |
| JPWO2002042769A1 (ja) | 抗ラミニン−1抗体の測定法およびその応用 | |
| JPH09229930A (ja) | ヒアルロン酸の測定方法及び測定用キット | |
| US20040259172A1 (en) | Assay of isomerised and/or optically inverted proteins and protein fragments | |
| JP2915530B2 (ja) | ラミニン フラグメント | |
| JP3023103B2 (ja) | ラミニンフラグメント測定方法 | |
| JPH09311132A (ja) | IgA腎症の診断法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20040304 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20040304 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20050914 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20051129 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20060119 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20060119 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20070612 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20070620 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20100629 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110629 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110629 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120629 Year of fee payment: 5 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130629 Year of fee payment: 6 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |