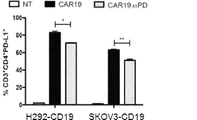
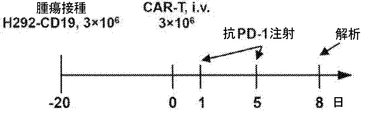
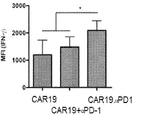
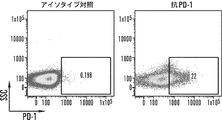
JP2020517263A - がんを治療するための組成物及び方法 - Google Patents
がんを治療するための組成物及び方法 Download PDFInfo
- Publication number
- JP2020517263A JP2020517263A JP2019556956A JP2019556956A JP2020517263A JP 2020517263 A JP2020517263 A JP 2020517263A JP 2019556956 A JP2019556956 A JP 2019556956A JP 2019556956 A JP2019556956 A JP 2019556956A JP 2020517263 A JP2020517263 A JP 2020517263A
- Authority
- JP
- Japan
- Prior art keywords
- cells
- cell
- receptor
- antigen
- car
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
- A61K38/1774—Immunoglobulin superfamily (e.g. CD2, CD4, CD8, ICAM molecules, B7 molecules, Fc-receptors, MHC-molecules)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39541—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against normal tissues, cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/30—Cellular immunotherapy characterised by the recombinant expression of specific molecules in the cells of the immune system
- A61K40/31—Chimeric antigen receptors [CAR]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
- A61K40/421—Immunoglobulin superfamily
- A61K40/4211—CD19 or B4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/7051—T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70517—CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70521—CD28, CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
- A61K2039/507—Comprising a combination of two or more separate antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/31—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterized by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/38—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/55—Lung
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/59—Reproductive system, e.g. uterus, ovaries, cervix or testes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/33—Fusion polypeptide fusions for targeting to specific cell types, e.g. tissue specific targeting, targeting of a bacterial subspecies
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Pharmacology & Pharmacy (AREA)
- Cell Biology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Biomedical Technology (AREA)
- Endocrinology (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Hematology (AREA)
- Biotechnology (AREA)
- Developmental Biology & Embryology (AREA)
- Virology (AREA)
- Peptides Or Proteins (AREA)
- Enzymes And Modification Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762487358P | 2017-04-19 | 2017-04-19 | |
| US62/487,358 | 2017-04-19 | ||
| PCT/US2018/028427 WO2018195348A1 (en) | 2017-04-19 | 2018-04-19 | Compositions and methods for treating cancer |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2020517263A true JP2020517263A (ja) | 2020-06-18 |
| JP2020517263A5 JP2020517263A5 (enExample) | 2020-07-30 |
Family
ID=63856183
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019556956A Pending JP2020517263A (ja) | 2017-04-19 | 2018-04-19 | がんを治療するための組成物及び方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20210095029A1 (enExample) |
| EP (1) | EP3612222A4 (enExample) |
| JP (1) | JP2020517263A (enExample) |
| CN (1) | CN110536700A (enExample) |
| AU (1) | AU2018254517A1 (enExample) |
| WO (1) | WO2018195348A1 (enExample) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20210112310A (ko) * | 2018-12-06 | 2021-09-14 | 광동 티씨알큐어 바이오파마 테크놀로지 씨오., 엘티디. | 종양 항원, tgf-베타 및 면역체크포인트를 표적으로 하는 tcr-t 세포 복합 치료법 |
| US12419913B2 (en) | 2019-02-08 | 2025-09-23 | Dna Twopointo, Inc. | Modification of CAR-T cells |
| WO2020214957A1 (en) | 2019-04-19 | 2020-10-22 | Tcrcure Biopharma Corp. | Anti-pd-1 antibodies and uses thereof |
| EP3999550A4 (en) | 2019-07-17 | 2023-09-06 | Legend Biotech USA Inc. | ANTI-DLL3 CHIMERIC ANTIGEN RECEPTORS AND THEIR USES |
| WO2021155518A1 (en) * | 2020-02-05 | 2021-08-12 | Tcrcure Biopharma Corp | Anti-hpv t cell receptors and engineered cells |
| TW202216768A (zh) | 2020-06-22 | 2022-05-01 | 美商恩格姆生物製藥公司 | Lair-1結合劑及其使用方法 |
| CN111704675B (zh) * | 2020-07-08 | 2022-05-10 | 武汉波睿达生物科技有限公司 | 一种治疗hiv感染的双特异性嵌合抗原受体、基因、构建方法及其应用 |
| CN111826400A (zh) * | 2020-07-21 | 2020-10-27 | 中科宝承生物医学科技有限公司 | 一种双特异性抗体nk细胞制备方法及其细胞和应用 |
| CN114437229B (zh) * | 2020-11-01 | 2024-03-12 | 复旦大学 | 携带pd-1単链抗体且靶向egfr抗原的car t免疫细胞的制备及其用途 |
| TW202330909A (zh) * | 2021-09-09 | 2023-08-01 | 大陸商深圳市菲鵬生物治療股份有限公司 | 轉基因免疫效應細胞、其製備方法、慢病毒、構建體、癌症治療藥物及其應用 |
| CN115025217B (zh) * | 2022-05-13 | 2023-05-05 | 广东齐美医药生物科技集团有限公司 | 干细胞裂解物联合活性多糖以及酪氨酸酶抑制剂在制备药物或化妆品中的用途 |
| CN119286780B (zh) * | 2024-12-12 | 2025-03-21 | 山东省成体细胞产业技术研究院有限公司 | 一种t细胞的体外培养方法 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016508728A (ja) * | 2013-02-26 | 2016-03-24 | メモリアル スローン−ケタリング キャンサー センター | 免疫療法のための組成物および方法 |
| CN105796597A (zh) * | 2016-03-11 | 2016-07-27 | 江苏三特生物科技有限公司 | 携带pd-l1和ctla-4抗体基因的car-t细胞在肿瘤免疫上的应用 |
| WO2016126608A1 (en) * | 2015-02-02 | 2016-08-11 | Novartis Ag | Car-expressing cells against multiple tumor antigens and uses thereof |
| WO2016172583A1 (en) * | 2015-04-23 | 2016-10-27 | Novartis Ag | Treatment of cancer using chimeric antigen receptor and protein kinase a blocker |
| WO2016210129A1 (en) * | 2015-06-23 | 2016-12-29 | Memorial Sloan-Kettering Cancer Center | Novel pd-1 immune modulating agents |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017133175A1 (en) * | 2016-02-04 | 2017-08-10 | Nanjing Legend Biotech Co., Ltd. | Engineered mammalian cells for cancer therapy |
| EP3432924A1 (en) * | 2016-03-23 | 2019-01-30 | Novartis AG | Cell secreted minibodies and uses thereof |
| CN107523547A (zh) * | 2016-06-20 | 2017-12-29 | 上海细胞治疗研究院 | 一种高效稳定表达抑制性抗体的car‑t细胞及其用途 |
-
2018
- 2018-04-19 EP EP18787412.8A patent/EP3612222A4/en not_active Withdrawn
- 2018-04-19 JP JP2019556956A patent/JP2020517263A/ja active Pending
- 2018-04-19 AU AU2018254517A patent/AU2018254517A1/en not_active Abandoned
- 2018-04-19 US US16/603,792 patent/US20210095029A1/en not_active Abandoned
- 2018-04-19 CN CN201880025945.5A patent/CN110536700A/zh active Pending
- 2018-04-19 WO PCT/US2018/028427 patent/WO2018195348A1/en not_active Ceased
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016508728A (ja) * | 2013-02-26 | 2016-03-24 | メモリアル スローン−ケタリング キャンサー センター | 免疫療法のための組成物および方法 |
| WO2016126608A1 (en) * | 2015-02-02 | 2016-08-11 | Novartis Ag | Car-expressing cells against multiple tumor antigens and uses thereof |
| WO2016172583A1 (en) * | 2015-04-23 | 2016-10-27 | Novartis Ag | Treatment of cancer using chimeric antigen receptor and protein kinase a blocker |
| WO2016210129A1 (en) * | 2015-06-23 | 2016-12-29 | Memorial Sloan-Kettering Cancer Center | Novel pd-1 immune modulating agents |
| CN105796597A (zh) * | 2016-03-11 | 2016-07-27 | 江苏三特生物科技有限公司 | 携带pd-l1和ctla-4抗体基因的car-t细胞在肿瘤免疫上的应用 |
Non-Patent Citations (1)
| Title |
|---|
| CLINICAL CANCER RESEARCH, vol. 19, no. 20, JPN6021017927, 2013, pages 5636 - 5646, ISSN: 0004507491 * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3612222A4 (en) | 2020-12-23 |
| US20210095029A1 (en) | 2021-04-01 |
| EP3612222A1 (en) | 2020-02-26 |
| CN110536700A (zh) | 2019-12-03 |
| WO2018195348A9 (en) | 2019-10-03 |
| AU2018254517A1 (en) | 2019-12-05 |
| WO2018195348A1 (en) | 2018-10-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2020517263A (ja) | がんを治療するための組成物及び方法 | |
| US20240083968A1 (en) | Treatment of cancer using chimeric cd3 receptor proteins | |
| US20190375815A1 (en) | Treatment of cancer using chimeric t cell receptor proteins having multiple specificities | |
| JP7379803B2 (ja) | Nkg2dドメインを含む多重特異性キメラ受容体およびその使用法 | |
| JP2024518011A (ja) | 多様な免疫細胞のための単鎖および多鎖合成抗原受容体 | |
| JP7595583B2 (ja) | 抗原特異的cd19標的化car-t細胞 | |
| CN118546959A (zh) | Cd19定向性嵌合抗原受体及其在免疫疗法中的用途 | |
| CN111629734A (zh) | 用于共刺激的新型平台、新型car设计以及过继性细胞疗法的其他增强 | |
| JP2018518972A (ja) | 腫瘍特異的活性化のためのマスキングキメラ抗原受容体t細胞 | |
| KR20190101979A (ko) | 합성 면역 수용체 및 이의 사용 방법 | |
| WO2017219936A1 (zh) | 一种高效稳定表达激活型抗体的car-t细胞及其用途 | |
| WO2019129177A1 (zh) | 抗体修饰的嵌合抗原受体修饰t细胞及其用途 | |
| CA3189677A1 (en) | Chimeric molecules providing targeted costimulation for adoptive cell therapy | |
| CN112969470A (zh) | 用于扩增抗原特异性car-t细胞、组合物的方法及其相关用途 | |
| WO2022037562A1 (en) | Engineered immunoresponsive cells and uses thereof | |
| CN113728007A (zh) | 人源化抗叶酸受体1嵌合抗原受体及其用途 | |
| WO2021244626A1 (zh) | 靶向cldn18.2的嵌合抗原受体及其用途 | |
| WO2019149279A1 (zh) | 细胞免疫治疗的组合 | |
| WO2020227595A1 (en) | Clec4-targeted car-t-cells | |
| US20250345429A1 (en) | Compositions and methods comprising chimeric adaptor polypeptides | |
| US20210230224A1 (en) | Car-t cells targeting glioma stem cells for the treatment of glioblastoma multiforme | |
| US20210079111A1 (en) | Cd19-cd20 bispecific and dual passway car-t and methods for use thereof | |
| HK40015393A (en) | Compositions and methods for treating cancer | |
| RU2826298C2 (ru) | Car-t-клетки, специфически нацеленные на антиген cd19 | |
| WO2025140480A1 (zh) | 经改造的iNKT细胞 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20191018 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200512 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20200512 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200518 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20210309 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210517 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20211208 |