JP2020517263A - がんを治療するための組成物及び方法 - Google Patents
がんを治療するための組成物及び方法 Download PDFInfo
- Publication number
- JP2020517263A JP2020517263A JP2019556956A JP2019556956A JP2020517263A JP 2020517263 A JP2020517263 A JP 2020517263A JP 2019556956 A JP2019556956 A JP 2019556956A JP 2019556956 A JP2019556956 A JP 2019556956A JP 2020517263 A JP2020517263 A JP 2020517263A
- Authority
- JP
- Japan
- Prior art keywords
- cells
- cell
- receptor
- antigen
- car
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2818—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against CD28 or CD152
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
- A61K38/1774—Immunoglobulin superfamily (e.g. CD2, CD4, CD8, ICAM molecules, B7 molecules, Fc-receptors, MHC-molecules)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39541—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against normal tissues, cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/30—Cellular immunotherapy characterised by the recombinant expression of specific molecules in the cells of the immune system
- A61K40/31—Chimeric antigen receptors [CAR]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4202—Receptors, cell surface antigens or cell surface determinants
- A61K40/421—Immunoglobulin superfamily
- A61K40/4211—CD19 or B4
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/7051—T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70517—CD8
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70521—CD28, CD152
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
- A61K2039/507—Comprising a combination of two or more separate antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/31—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterized by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/38—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/55—Lung
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/59—Reproductive system, e.g. uterus, ovaries, cervix or testes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/33—Fusion polypeptide fusions for targeting to specific cell types, e.g. tissue specific targeting, targeting of a bacterial subspecies
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Cell Biology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Mycology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Microbiology (AREA)
- Biomedical Technology (AREA)
- Endocrinology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Enzymes And Modification Thereof (AREA)
- Peptides Or Proteins (AREA)
- Hematology (AREA)
- Biotechnology (AREA)
- Developmental Biology & Embryology (AREA)
- Virology (AREA)
Abstract
Description
この出願には、2017年4月19日に出願された米国仮特許出願第62/487,358号に対する米国特許法第35条§119(e)に基づく優先権の主張が含まれ、その全体は参照により本明細書に組み込まれる。
本発明は、国立衛生研究所により授与された助成金番号AI068978及びEB017206の下での政府支援によりなされた。政府は本発明に一定の権利を有する。
本明細書には、キメラ抗原受容体(CAR)及びチェックポイント阻害剤(CPI)を含むT細胞を含む組成物、ならびにがんを治療するために組成物を使用する方法が記載されている。
本明細書のすべての刊行物は、各個々の刊行物または特許出願が参照により組み込まれることが具体的かつ個別に示された場合と同じ程度に参照により組み込まれる。以下の説明には、本発明を理解するのに有用な情報が含まれる。本明細書で提供される情報のいずれかが先行技術であるか、または現在請求されている発明に関連すること、または具体的または暗黙的に参照される刊行物が先行技術であることを認めるものではない。
以下の実施形態及びその態様は、範囲を限定するものではなく、模範的かつ例示的であることを意味するシステム、組成物、及び方法と併せて説明及び図示される。
本明細書に引用されるすべての参考文献は、完全に記載されているかのように、その全体が参照により組み込まれる。別に定義されない限り、本明細書で使用される技術用語及び科学用語は、本発明が属する分野の当業者によって一般に理解されるのと同じ意味を有する。Allen et al., Remington: The Science and Practice of Pharmacy 22nd ed., Pharmaceutical Press (September 15, 2012); Hornyak et al., Introduction to Nanoscience and Nanotechnology, CRC Press (2008); Singleton and Sainsbury, Dictionary of Microbiology and Molecular Biology 3rd ed., revised ed., J. Wiley & Sons (New York, NY 2006); Smith, March’s Advanced Organic Chemistry Reactions, Mechanisms and Structure 7th ed., J. Wiley & Sons (New York, NY 2013); Singleton, Dictionary of DNA and Genome Technology 3rd ed., Wiley−Blackwell (November 28, 2012); 及びGreen and Sambrook, Molecular Cloning: A Laboratory Manual 4th ed., Cold Spring Harbor Laboratory Press (Cold Spring Harbor, NY 2012)は、本出願で使用される用語の多くに対する一般的なガイドを当業者に提供する。抗体の調製方法については、Greenfield, Antibodies A Laboratory Manual 2nd ed., Cold Spring Harbor Press (Cold Spring Harbor NY, 2013); Kohler and Milstein, Derivation of specific antibody−producing tissue culture and tumor lines by cell fusion, Eur. J. Immunol. 1976 Jul, 6(7):511−9; Queen and Selick, Humanized immunoglobulins, U. S. Patent No. 5,585,089 (1996 Dec);及びRiechmann et al., Reshaping human antibodies for therapy, Nature 1988 Mar 24, 332(6162):323−7を参照されたい。
本明細書で使用される場合、用語「含む(comprising)」または「含む(comprises)」は、実施形態に有用であるか有用でないかどうかにかかわらず、不特定の要素の包含に対して開かれている組成物、方法、及びそれぞれの構成要素(複数可)に関して使用される。一般に、本明細書で使用される用語は一般に「オープン」用語として意図されていることを当業者は理解するであろう(例えば、用語「含む(including)」は「含むが、これに限定されない」と解釈されるべきであり、用語「持っている」は「少なくとも持っている」と解釈されるべきであり、用語「含む(includes)」は「含むがそれに限定されない」などと解釈されるべきである。)。
様々な実施形態において、本発明は医薬組成物を提供する。医薬組成物は、本明細書に記載されるように、CAR及びチェックポイント阻害剤をコードする核酸を含む細胞を含む。本発明の医薬組成物は、任意の薬学的に許容される賦形剤を含み得る。「薬学的に許容される賦形剤」とは、一般に安全、非毒性、及び望ましい医薬組成物の調製に有用な賦形剤を意味し、獣医学的使用及びヒトの薬学的使用に許容される賦形剤を含む。そのような賦形剤は、固体、液体、半固体、またはエアロゾル組成物の場合は気体であってもよい。賦形剤の例には、デンプン、糖、微結晶セルロース、希釈剤、造粒剤、潤滑剤、結合剤、崩壊剤、湿潤剤、乳化剤、着色剤、離型剤、コーティング剤、甘味料、香料(flavoring agents)、香料(perfuming agents)、防腐剤、酸化防止剤、可塑剤、ゲル化剤、増粘剤、硬化剤、硬化剤、懸濁剤、界面活性剤、保湿剤、担体、安定剤、及びそれらの組み合わせが含まれるが、これらに限定されない。
様々な実施形態において、本発明は、本明細書に記載の1つ以上のCAR及び1つ以上のCPIをコードする核酸を含む細胞を含む組成物を含む、がんを治療するためのキットを提供する。
実験方法
マウス
6〜8週齢の雌NOD.Cg−PrkdcscidIL2Rgtm1Wj1.Sz(NSG)マウスは、Jackson Laboratory(Farmington, CT)から購入した。すべての動物実験は、NIHの動物管理及び使用委員会のガイドラインに従って実施され、NCIの動物管理及び使用委員会によって承認されたプロトコルの下で実施された。
細胞株SKOV3及び293TはATCCから入手した。肺がん株NCI−H292は、Dr. Ite Laird−Offringa(University of Southern California, Los Angeles, CA)から提供された。H292−CD19及びSKOV3−CD19細胞株は、親NCI−H292及びSKOV3細胞に、ヒトCD19のcDNAをコードするレンチウイルスベクターで形質導入することにより生成された。形質導入されたH292及びSKOV3細胞を抗ヒトCD19抗体(BioLegend, San Diego, CA)で染色し、選別してCD19過剰発現細胞の比較的純粋な集団を得た。SKOV3、SKOV3−CD19、NCI−H292、及びH292−CD19細胞は、10%ウシ胎児血清(FBS)、2mM L−グルタミン、10mM HEPES、100U/mlペニシリン及び100μg/mlストレプトマイシンを補充したRPMI−1640培地からなるR10培地で維持された。293T細胞は、10%FBS、2mM L−グルタミン、10mM HEPES、100U/mlペニシリン及び100μg/mlストレプトマイシンを補充したDMEM培地からなるD10培地で培養した。上記のすべての細胞培養培地及び補充物は、Hyclone(Logan、UT)から購入した。ヒト末梢血単核細胞(PBMC)は、5%ヒトAB血清(GemCell, West Sacramento, CA)、1%HEPES(Gibco,Grand Island,NY)、1%Pen−Strep(Gibco)、1%GlutaMax(Gibco)、及び0.2%N−アセチルシステイン(Sigma−Aldrich、St.Louis,MO)を補充したX−Vivo 15培地(Lonza, Walkersville, MD)で構成されるT細胞培地(TCM)で培養した。
抗CD19 CAR(CAR)をコードするレトロウイルスベクターは、前述のようにWolfgang Uckert教授から頂いたMP71レトロウイルスベクターに基づいて構築された(Engels B, et al. 2003. Retroviral vectors for high−level transgene expression in T lymphocytes. Hum Gene Ther 14: 1155−68)。次いで、抗PD−1 scFv(CAR.αPD1)を含む抗CD19 CARをコードするベクターを、抗CD19 CARに基づいて生成した。CAR.αPD1ベクターの挿入物は、インフレームの5’末端から3’末端において以下の成分:抗CD19 CAR、EcoRI部位、ヒトIL−2由来のリーダー配列、抗PD−1 scFv軽鎖可変領域、GSリンカー、抗PD−1 scFv重鎖可変領域、HAタグ配列、及びNotI部位、から構成された。
標準的なリン酸カルシウム沈殿プロトコルを用いて、293T細胞の一過性トランスフェクションによりレトロウイルスベクターを調製した。15cm組織培養皿で培養した293T細胞に、37.5μgのレトロウイルスバックボーンプラスミド、18.75μgのエンベローププラスミドpGALV、及び30μgの、gag−polをコードするパッケージングプラスミドをトランスフェクトした。ウイルス上清をトランスフェクションの48時間後に回収し、使用前に0.45μmフィルター(Corning, Corning, NY)で濾過した。
凍結ヒトPBMCは、AllCells(Alameda, CA)から入手した。PBMCをTCMで解凍し、一晩休ませた。レトロウイルス形質導入の前に、50ng/ml OKT3、50ng/ml抗CD28抗体、及び10ng/ml組換えヒトIL−2(PeproTech,Rocky Hill,NJ)と培養することにより、PBMCを2日間活性化させた。ウェルあたり15μgのレトロネクチン(Clontech Laboratories,Mountain View,CA)でコーティングした、組織培養処理が無い12ウェルプレートに、新鮮に回収したレトロウイルスの上清を、形質導入のために32℃で2000×gで2時間遠心分離することによってスピンロードした。新鮮なウイルス上清を用いて、ベクターのスピンローディングを1回繰り返した。活性化されたPBMCを5×105細胞/mlの濃度で、10ng/mlの組換えヒトIL−2を補充した新しいTCMで再懸濁し、ベクターをロードしたプレートに加えた。プレートを1000×g、32℃で10分間回転させ、37℃、5%CO2で一晩インキュベートした。同じ形質導入手順が翌日に繰り返された。ex vivoでの増殖中、培地を補充し、細胞密度を2日ごとに5×105/mlに調整した。
細胞表面の抗CD19 CAR発現を検出するために、細胞をプロテインLで染色した。FACS染色の前に、5×105個の細胞を回収し、FACSバッファー(5%ウシ血清アルブミン画分Vを含むPBS)で3回洗浄した。次いで、細胞を1μgのビオチン化プロテインLで4℃で30分間染色した。細胞をFACSバッファーで3回洗浄した後、FACSバッファー中の0.1μgのFITC結合ストレプトアビジンとともに4℃で10分間インキュベートした。細胞を洗浄し、TransFix細胞抗原安定化試薬(Thermo Scientific, Waltham, MA)で4℃で10分間固定した。次いで、細胞を2回洗浄し、4℃で10分間、抗CD3、抗CD4、及び抗CD8で染色した。細胞を洗浄し、PBSに再懸濁した。MACSquantサイトメーター(Miltenyi Biotec, San Diego, CA)を用いて蛍光を評価し、FlowJoソフトウェア(Tree Star, Ashland, OR)を用いてすべてのFACSデータを解析した。
T細胞(1×106)を、96ウェル丸底プレートで、GolgiPlug(BD Biosciences, San Jose, CA)を用いて、37℃、5%CO2で6時間、1:1の比率で標的細胞とともに培養した。細胞内染色には、PE−Cy5.5−抗CD3、FITC−抗CD4、パシフィックブルー−CD8、PE−抗IFN−γ及びPE−抗Ki67抗体を用いた。Cytofix/Cytoperm Fixation及びPermeabilizationキット(BD Biosciences)を用いて、細胞膜を透過性にし、製造元の指示に従って細胞内染色を行った。
細胞培養上清を回収し、製造元の指示に従って、Pierce(商標)抗HA磁気ビーズ(Thermo Scientific, Waltham, MA)で抗PD−1 scFvを精製した。次いで、精製された抗体をSDS−PAGEに供し、ウエスタンブロット解析のためにニトロセルロース膜(Thermo Scientific, Waltham, MA)に移した。前述のように、抗HAタグ抗体(Abcam, Cambridge, MA)でウエスタンブロットを解析した(Xu S et al.2012. Discovery of an orally active small−molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc Natl Acad Sci U S A 109: 16348−53)。
IFN−γは、製造元の指示に従って、ヒトIFN−γELISAキット(BD Biosciences, San Jose, CA)を用いて測定した。手短に言えば、96ウェルELISAプレート(Thermo Scientific, Waltham, MA)を4℃で一晩、示されたタンパク質に対する捕捉抗体200ng/ウェルでコーティングした。翌日、プレートを洗浄バッファー(0.05%Tween 20を含むPBS)で洗浄し、室温で2時間、アッセイバッファー(10%FBSを含むPBS)でブロックした。等量の血清または細胞培養上清をプレートに加え、室温で2時間インキュベートした。次いで、プレートを洗浄し、検出抗体とともに室温で1時間インキュベートした。抗PD−1抗体及び分泌された抗PD−1 scFvを測定するために、組換えヒトPD−1(rhPD−1)を用いてプレートをプレコートした。ヤギ抗マウスIgG1−HRP及び抗HAタグ抗体を、それぞれ検出抗体として用いた。
96ウェルアッセイプレート(Thermo Scientific, Waltham, MA)に3μg/mlの抗ヒトCD3抗体を4℃で一晩コーティングした。2日目に、ウェルの上清を吸引し、ウェルあたり100μlのPBSで、ウェルを1回洗浄した。100μlのPBS中の10μg/mlのrhPD−L1/Fc(R&D Systems, Minneapolis, MN)を添加した。次いで、各ウェルに、10μlのPBS中の100μg/mlのヤギ抗ヒトIgG Fc抗体を加えた。アッセイプレートを37℃で4時間インキュベートした。ヒトT細胞を採取し、1回洗浄し、次いで、TCMで1×106細胞/mlに再懸濁した。アッセイプレートのウェルを吸引した。次いで、100μlのヒトT細胞懸濁液(1×105)と、GolgiPlug(BD Biosciences)を補充した、形質導入3日後のCARまたはCAR.αPD1 T細胞培養物の上清100μlを各ウェルに加えた。プレートを覆い、37℃、5%CO2で一晩インキュベートした。インキュベーション後、T細胞を回収し、細胞内でIFN−γで染色した。
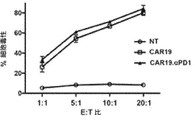
標的細胞の溶解(H292−CD19)は、標的細胞の生存を陰性対照細胞(NCI−H292)の生存と比較することにより測定された。この方法は,以前に記載されている(Kochenderfer JN, et al 2009. Construction and preclinical evaluation of an anti−CD19 chimeric antigen receptor. J Immunother 32: 689−702)。NCI−H292細胞は、濃度1.5×106細胞/mLで、細胞運動をモニターする(Invitrogen, Carlsbad, CA)ために、蛍光色素である5μM CellTracker Orange(5−(及び−6)−(((4−クロロメチル)ベンゾイル)アミノ)テトラメチルローダミン)(CMTMR)を有するR10培地に懸濁することにより標識された。細胞を37℃で30分間インキュベートし、次いで、2回洗浄し、新鮮なR10培地に懸濁した。H292−CD19細胞は、0.1%BSAと5μM カルボキシフルオレセインスクシンイミジルエステル(CFSE)蛍光色素を伴うPBSに、1×106細胞/mLの濃度で懸濁することにより標識された。細胞を37℃で30分間インキュベートした。インキュベーション後、同量のFBSを細胞懸濁液に添加し、室温で2分間インキュベートした。次いで、細胞を2回洗浄し、新鮮なR10培地に懸濁した。等量のNCI−H292及びH292−CD19細胞(各5×104個)を、エフェクターCAR−T細胞を有する各培養の同じウェルで組み合わせた。共培養は、次のエフェクター対標的比、1:1及び5:1で、丸底96ウェルプレートに3連でセットアップされた。培養物を37℃で4時間インキュベートした後、製造元の指示(BD Biosciences)に従って、7−AAD標識を行った。フローサイトメトリー解析を行って、残りの生(7−AAD陰性)標的細胞を定量化した。それぞれの共培養について、H292−CD19生細胞の割合をNCI−H292生細胞の割合で割ることにより、H292−CD19細胞のパーセント生存率を決定した。エフェクター細胞を含まない標的細胞と陰性対照細胞のみを含むウェルでは、H292−CD19細胞の割合とNCI−H292細胞の割合の比を計算し、開始細胞数と自発的な細胞死の変動を補正するために使用した。細胞毒性を3回測定し、平均±SEMで示した。
3×105個のH292−CD19細胞をD10培地に懸濁し、6ウェルプレートに播種した。標的細胞が付着したら、非形質導入T細胞、CAR及びCAR.αPD1 T細胞を回収し、PBSで2回洗浄した。次いで、細胞を、10μM CFSEを含むPBSに1×106細胞/mLの濃度で懸濁することにより標識し、37℃で60分間インキュベートした。インキュベーション後、細胞を2回洗浄し、新鮮なTCMに懸濁した。共培養のために、同数のT細胞を標的細胞に加えた。共培養は、エフェクター対標的比、1:1で、3連で設定された。培養物を37℃で96時間インキュベートした。フローサイトメトリー解析を実施して、T細胞上のCFSEの強度を定量化した。増殖率を3回測定し、平均±SEMで示した。
6〜8週齢で、マウスに3×106のH292−CD19細胞を皮下接種し、10〜13日後、平均腫瘍サイズが100〜120mm3に達したら、マウスを100μl PBS中の1×106または3×106 CAR形質導入T細胞のi.v.による養子移入で治療した。CAR発現は、ドナーに適合した非形質導入T細胞の添加により、両方のCAR群において20%に正規化された。腫瘍の成長を週に2回モニターした。腫瘍サイズをキャリパーで測定し、次の式で計算した:W2×L/2。明らかな体重減少、腫瘍の潰瘍形成、または腫瘍サイズが1000mm3を超えた場合に、マウスを安楽死させた。
GraphPad Prismバージョン5.01で統計解析を実行した。Tukeyの多重比較による一元配置分散分析を実行して、in vitroアッセイで異なる群間の違いを評価した。腫瘍成長曲線は、反復測定による一元配置分散分析を用いて解析した(Tukeyの多重比較法)。マウス生存曲線は、カプラン・マイヤー解析(ボンフェローニ補正によるログランク検定)によって評価した。P値が0.05未満の場合、統計的に有意と見なされた。調査結果の有意性は次のように定義された。ns=有意ではない、P> 0.05;*、P<0.05;**、P<0.01;***、P<0.001。
この研究で使用したレトロウイルスベクター構築物の概略図を図1Aに示す。抗CD19 scFv、CD8ヒンジ、CD28膜貫通及び細胞内共刺激ドメイン、ならびに細胞内CD3ζドメインから構成される抗CD19 CARをコードするレトロウイルスベクターをCAR19と命名した。抗CD19 CAR及び分泌抗PD−1 scFvの両方をコードするレトロウイルスベクターをCAR19.αPD1と命名した。ヒトPBMCに各構築物を形質導入して、初代リンパ球におけるCARの発現をテストした。図1Bに見られるように、CAR発現はヒトT細胞の両方の構築物で観察されたが、抗PD−1分泌CAR19 T細胞は細胞表面でわずかに低いレベルのCARを発現した。抗PD−1の発現及び分泌を、形質導入の3日後に細胞上清でウエスタンブロット解析及びELISAを行うことにより評価した。CAR19.αPD1で形質導入されたT細胞により、抗PD−1が正常に発現及び分泌されることが観察された(図1C及び図1D)。
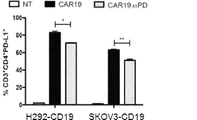
抗原特異的刺激を通じて抗PD−1分泌CAR19 T細胞のエフェクター機能をさらに評価するために、CAR19及びCAR19.αPD1 T細胞の両方を、H292−CD19またはSKOV3−CD19標的細胞と異なる期間共培養し、どちらもPD−L1の表面発現が高いことが示された(図8)。次いで、異なる時点のT細胞を採取し、上清中の細胞機能マーカーIFN−γをELISAで測定した。24時間抗原刺激すると、抗PD−1の分泌の有無にかかわらず、CAR19及びCAR19.αPD1 T細胞の両方に同程度のIFN−γ分泌が見られた(図2A及び図9A)。しかしながら、72時間後、CAR19.αPD1 T細胞は、H292−CD19細胞による刺激後、親のCAR19 T細胞と比較して、有意に高いIFN−γを分泌した(図2A)。同様に、96時間の抗原刺激後、抗PD−1を分泌するCAR19 T細胞は、親のCAR19 T細胞によって発現されるものよりも有意に多くのIFN−γを発現した(図2A及び図9A)。
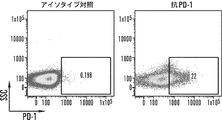
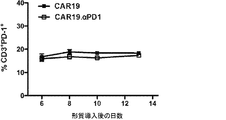
ヒトGD2及びマウスHER2 CAR T細胞でのPD−1の発現は、抗原特異的活性化後に増加することが示されており、PD−1の遮断はT細胞でPD−1の発現をダウンレギュレートすることがわかった。分泌された抗PD−1 scFvがヒトT細胞を消耗から保護する効果を評価するために、操作されたCAR T細胞を、H292−CD19またはSKOV3−CD19標的細胞と24時間共培養し、T細胞消耗マーカーのPD−1について染色した。PD−1の発現は、抗原特異的刺激後、CAR19及びCAR19.αPD1 T細胞の両方で有意にアップレギュレートされることがわかった。比較すると、CAR19.αPD1 T細胞でのアップレギュレートされたPD−1の発現は、親のCAR19 T細胞での発現よりも有意に低かった(図3A、図3B、及び図13A−13C)。しかしながら、抗原特異的刺激なしでは、CAR19及びCAR19.αPD1 T細胞の両方でのPD−1の発現は、T細胞増殖の過程で同様の安定したレベルを維持した(図13D及び13E)。
CAR19.αPD1 T細胞の抗腫瘍効果を評価するために、確立されたH292−CD19皮下腫瘍(約100mm3)を有するNSGマウスに1×106個のCAR操作T細胞を養子移入した。動物実験の実験手順を図4Aに示す。図4Bのデータは、3つの抗CD19 CAR T細胞群すべてが、実験中に、非形質導入T細胞、または抗PD−1抗体治療と併用した非形質導入T細胞と比較して、腫瘍サイズの減少を示したことを示す。しかしながら、親のCAR19 T細胞、または抗PD−1抗体治療と組み合わせたCAR19 T細胞と比較して、CAR19.αPD1 T細胞治療は抗腫瘍効果を有意に増強し、それはT細胞注入後1週間で明らかになった(図4B)。特に、養子細胞移植の17日後、CAR19.αPD1 T細胞で処理したマウスの腫瘍がほぼ消失することが観察された。親のCAR19 T細胞群または組み合わせ群では、6匹中4匹のマウス(約70%)が依然として進行性または安定性の病状を示し、30%未満の腫瘍サイズの減少のみを経験した(図4C)。担がんマウスの全生存率も評価された。親のCAR19 T細胞治療単独(17%)、または抗PD−1抗体とCAR19 T細胞治療の組み合わせ(17%)と比較して、CAR19.αPD1 T細胞治療が長期生存(100%)を有意に延長したことが示された(図4D)。
次に、CAR T細胞の生着及び拡大をin vivoで評価した。T細胞注入の2日後、マウスを安楽死させ、腫瘍、血液、脾臓、骨髄を含む様々な臓器や組織をヒトT細胞染色のために採取した。すべての群のT細胞がほとんど増殖せず、検査したすべての組織で2%未満のT細胞しか観察されないことがわかった。ほとんどのT細胞(1〜2%)が脾臓に帰巣し、特定の割合のT細胞(0.1〜0.5%)が血中を循環していた。転移したT細胞の浸潤レベルは、腫瘍及び骨髄では低かった。さらに、非形質導入T細胞とCAR形質導入T細胞との間のT細胞の割合は、検査したすべての組織でほとんど差がないことを示した(図5A)。しかしながら、T細胞注入の1週間後の10日目に、検査したすべての組織でCAR T細胞の有意な拡大が観察されたが、非形質導入T細胞はほとんど存在しなかった。特に、in vitroデータと一致して、CAR19.αPD1 T細胞は、特に腫瘍、脾臓、血液において、親のCAR19 T細胞と比較して有意に高い増殖率を示した(図5B及び図5C)。
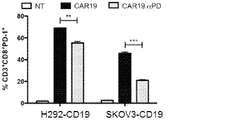
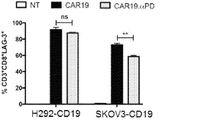
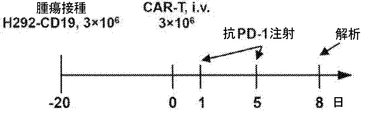
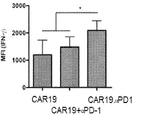
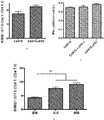
CAR19.αPD1 T細胞療法後に観察される抗腫瘍効果の増強が腫瘍部位でのCAR T細胞の機能の増加と相関するかどうかをさらに決定するために、3×106個のCAR T細胞を受ける前に、マウスにH292−CD19腫瘍を負荷した。実験計画を図6Aに示す。T細胞注入の8日後、マウスを安楽死させ、フローサイトメトリーを用いて、腫瘍、血液、脾臓、骨髄のT細胞を解析した。CAR T細胞治療と比較して、注入された抗PD−1抗体はin vivoでT細胞の拡大を促進する効果がほとんどないことが観察された。しかしながら、以前の観察結果と一致して(図5B)、CAR19.αPD1レジメンで処理したマウスのT細胞は、腫瘍、血液、脾臓でより高い割合で増殖した(図6B)。腫瘍浸潤リンパ球(TIL)の中の細胞傷害性CD8+T細胞の集団は、抗腫瘍免疫と自発的な腫瘍制御の誘発に重要であることが示されている。従って、CD8+T細胞とCD4+T細胞の比率をTIL間で解析した。親のCAR19 T細胞と比較して、CAR19.αPD1 T細胞はCD8+T細胞とCD4+T細胞の比が有意に高いことが示されたが、併用療法ではCAR T細胞単剤療法と比較してCD8+T細胞とCD4+T細胞の比は同様であった(図6C)。同様に、血液及び脾臓では、CAR19.αPD1 T細胞治療におけるCD8+対CD4+の比率も、親のCAR19 T細胞単剤療法及び併用療法群よりも有意に高かったが(図6C)、T細胞注入前のCAR19とCAR19.αPD1 T細胞との間のCD8+T細胞比とCD4+T細胞比との間でほとんど違いはなかった(図14A)。さらに、腫瘍浸潤CD8+T細胞でのPD−1の発現を評価し、注入及び分泌された抗PD−1抗体の両方がPD−1の発現を有意に減少させることができることがわかった(図6D)。また、ex vivo培養を行い、抗CD3/CD28抗体または標的細胞H292−CD19でTILを活性化した。親CAR19 T細胞または抗PD−1抗体の全身治療と組み合わせたCAR19 T細胞のいずれかと比較して、養子移入されたCAR19.αPD1 T細胞でIFN−γの有意に高い発現が観察された。CAR T細胞単独療法と併用療法との間でIFN−γの発現にほとんど差は認められなかった(図6E及び図6F)。さらに、血清中のIFN−γ及び抗PD−1抗体の発現を測定したところ、すべての群でIFN−γの発現にほとんど差が見られなかった(図14C)。特に、CAR19 T細胞治療と比較して、CAR19.αPD1 T細胞療法は血清中の抗PD−1濃度が有意に高かったが、その濃度は抗PD−1抗体の全身注射の場合よりも15倍以上低かった(図6G)。
Claims (17)
- キメラ抗原受容体(CAR)及びチェックポイント阻害剤(CPI)をコードする核酸、またはCAR及びCPIをコードする複数の核酸を含む、細胞。
- 前記CARが、分化クラスター(CD)19、CD22、CD23、骨髄増殖性白血病タンパク質(MPL)、CD30、CD32、CD20、CD70、CD79b、CD99、CD123、CD138、CD179b、CD200R、CD276、CD324、Fc受容体様5(FcRH5)、CD171、CS−1(シグナル伝達リンパ球活性化分子ファミリー7、SLAMF7)、C型レクチン様分子1(CLL−1)、CD33、カドヘリン1、カドヘリン6、カドヘリン16、カドヘリン17、カドヘリン19、上皮成長因子受容体変異体III(EGFRviii)、ガングリオシドGD2、ガングリオシドGD3、ヒト白血球抗原A2(HLA−A2)、B細胞成熟抗原(BCMA)、Tn抗原、前立腺特異的膜抗原(PSMA)、オーファン受容体1のような受容体チロシンキナーゼ(ROR1)、FMS様チロシンキナーゼ3(FLT3)、線維芽細胞活性化タンパク質(FAP)、腫瘍関連糖タンパク質(TAG)−72、CD38、CD44v6、がん胎児性抗原(CEA)、上皮細胞接着分子(EpCAM)、B7−H3(CD276)、KIT、インターロイキン−13受容体サブユニットアルファ−2(IL−13Ra2)、インターロイキン−11受容体サブユニットアルファ(IL11Ra)、メソセリン、前立腺幹細胞抗原(PSCA)、血管上皮成長因子受容体2(VEGFR2)、ルイスY、CD24、血小板由来成長因子受容体ベータ(PDGFR−ベータ)、プロテアーゼセリン21(PRSS21)、シアリル糖脂質段階特異的胚抗原4(SSEA−4)、CD20、免疫グロブリンのFc領域、組織因子、葉酸受容体アルファ、上皮成長因子受容体2(ERBB2)、ムチン1(MUC1)、上皮成長因子受容体(EGFR)、神経小付着分子(NCAM)、プロスターゼ、前立腺酸性ホスファターゼ(PAP)、伸長因子2変異(ELF2M)、エフリンB2、インスリン様成長因子I受容体(IGF−I受容体)、炭酸脱水酵素IX(CAIX)、潜伏膜タンパク質2(LMP2)、メラニン細胞タンパク質gpl00、bcr−abl、チロシナーゼ、エリスロポエチン産生肝細胞がんA2(EphA2)、フコシル化モノシアロガングリオシド(フコシルGM1)、シアリルルイスa(sLea)、ガングリオシドGM3、トランスグルタミナーゼ5(TGS5)、高分子量メラノーマ関連抗原(HMWMAA)、o−アセチル−GD2ガングリオシド、葉酸受容体ベータ、TEM1/CD248、腫瘍内皮マーカー7関連(TEM7R)、クローディン6(CLDN6)、甲状腺刺激ホルモン受容体(TSHR)、T細胞受容体(TCR)−ベータ1定常鎖、TCRベータ2定常鎖、TCRガンマ−デルタ、Gタンパク質共役受容体クラスCグループ5メンバーD(GPRC5D)、CXORF61タンパク質、CD97、CD179a、未分化リンパ腫キナーゼ(ALK)、ポリシアル酸、胎盤特異的1(PLAC1)、炭水化物抗原GloboH、乳房分化抗原NY−BR−1、ウロプラキン−2(UPK2)、A型肝炎ウイルス細胞受容体1(HAVCR1)、アドレナリン受容体ベータ3(ADRB3)、パネキシン3(PANX3)、Gタンパク質共役受容体20(GPR20)、リンパ球抗原6ファミリーメンバーK(LY6K)、嗅覚受容体ファミリー51サブファミリーEメンバー2(OR51E2)、T細胞受容体γ鎖代替リーディングフレームタンパク質(TARP)、ウィルムス腫瘍抗原1タンパク質(WT1)、がん精巣抗原NY−ESO−1、がん精巣抗原LAGE−la、レグマイン、ヒトパピローマウイルス(HPV)E6、HPV E7、ヒトTリンパ球向性ウイルス(HTLV1)−Tax、カポジ肉腫関連ヘルペスウイルス糖タンパク質(KSHV)K8.1タンパク質、エプスタイン−バーウイルス(EBV)にコードされた糖タンパク質350(EBB gp350)、HIV1−エンベロープ糖タンパク質gp120、マルチプレックス自動ゲノムエンジニアリング(MAGE)−Al、転座−Ets−白血病ウイルス(ETV)タンパク質6−AML、精子タンパク質17、X抗原ファミリーメンバー(XAGE)1、膜貫通型チロシンタンパク質キナーゼ受容体Tie2、黒色腫がん精巣抗原MAD−CT−1、黒色腫がん精巣抗原MAD−CT−2、Fos関連抗原1、p53、p53変異体、プロスタイン、サバイビン及びテロメラーゼ、前立腺がん腫瘍抗原−1(PCTA−1)/ガレクチン8、メランA/MART1、Ras変異体、ヒトテロメラーゼ逆転写酵素(hTERT)、デルタ様3(DLL3)、トロホブラスト細胞表面抗原2(TROP2)、タンパク質チロシンキナーゼ−7(PTK7)、グアニル酸シクラーゼC(GCC)、アルファフェトプロテイン(AFP)、肉腫転座ブレークポイント、メラノーマ−アポトーシス阻害剤(ML−IAP)、ERG(TMPRSS2 ETS融合遺伝子)、N−アセチルグルコサミニルトランスフェラーゼV(NA17)、ペアボックスタンパク質Pax−3(PAX3)、アンドロゲン受容体、サイクリンBl、v−myc鳥類骨髄細胞腫ウイルスがん遺伝子神経芽細胞腫由来ホモログ(MYCN)、RasホモログファミリーメンバーC(RhoC)、チロシナーゼ関連タンパク質2(TRP−2)、チトクロームP4501B1(CYP1B1)、CCCTC結合因子(亜鉛フィンガータンパク質)様(BORISまたはブラザー・オブ・ザ・レギュレーター・オブ・インプリンテッド・サイト)、T細胞3によって認識される扁平上皮がん抗原(SART3)、PAX5、プロアクロシン結合タンパク質sp32(OY−TES1)、リンパ球特異的タンパク質チロシンキナーゼ(LCK)、キナーゼアンカータンパク質4(AKAP−4)、滑膜肉腫、Xブレークポイント2(SSX2)、高度糖化最終産物受容体(RAGE−1)、腎遍在1(RUl)、RU2、腸内カルボキシルエステラーゼ、熱ショックタンパク質70−2変異体(mut hsp70−2)、CD79a、CD79b、CD72、白血球関連免疫グロブリン様受容体1(LAIR1)、IgA受容体のFcフラグメント(FCAR)、白血球免疫グロブリン様受容体サブファミリーAメンバー2(LILRA2)、CD300分子様ファミリーメンバーf(CD300LF)、C型レクチンドメインファミリー12メンバーA(CLEC12A)、骨髄間質細胞抗原2(BST2)、EGF様モジュール含有ムチン様ホルモン受容体様2(EMR2)、リンパ球抗原75(LY75)、グリピカン3(GPC3)、Fc受容体様5(FCRL5)、免疫グロブリンラムダ様ポリペプチド1(IGLL1)、FITC、黄体形成ホルモン受容体(LHR)、卵胞刺激ホルモン受容体(FSHR)、絨毛性ゴナドトロピンホルモン受容体(CGHR)、CCケモカイン受容体4(CCR4)、ガングリオシドGD3、シグナリングリンパ球活性化分子(SLAM)ファミリーメンバー6(SLAMF6)、SLAMF4、黄体形成ホルモン受容体(LHR)、卵胞刺激ホルモン受容体(FSHR)、絨毛性ゴナドトロピンホルモン受容体(CGHR)、またはそれらの組み合わせを標的とする、請求項1に記載の細胞。
- 前記チェックポイント阻害剤がPD−1を標的とする、請求項1に記載の細胞。
- 前記チェックポイント阻害剤が抗PD−1 scFvである、請求項4に記載の細胞。
- 前記チェックポイント阻害剤が、PD−1、LAG−3、TIM3、B7−H1、CD160、P1H、2B4、CEACAM−1、CEACAM−3、CEACAM−5、TIGIT、CTLA−4、BTLA、及びLAIR1のいずれか1つ以上を標的とする、請求項1に記載の細胞。
- 前記細胞がTリンパ球細胞(T細胞)である、請求項1に記載の細胞。
- 前記細胞がナチュラルキラー(NK)細胞である、請求項1に記載の細胞。
- 前記CPIが構成的に発現される、請求項1に記載の細胞。
- 前記抗PD−1 scFvが構成的に発現される、請求項5に記載の細胞。
- キメラ抗原受容体(CAR)をコードする第1のポリヌクレオチドと、チェックポイント阻害剤(CPI)をコードする第2のポリヌクレオチドとを含む、核酸。
- 請求項10に記載の核酸によりコードされる、ポリペプチド。
- 請求項10に記載の核酸を含む、ベクター。
- 請求項1〜9のいずれか一項に記載の細胞を含む、医薬組成物。
- それを必要とする対象に、治療有効量の、請求項1〜9のいずれか一項に記載の細胞を投与することを含む、がんを治療する方法。
- 前記がんが肺がんである、請求項15に記載の方法。
- 治療有効量の、化学療法または放射線照射を含む既存の療法を前記対象に施すことをさらに含む、請求項15に記載の方法。
- 前記細胞及び前記既存の療法が連続的または同時に施される、請求項17に記載の方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762487358P | 2017-04-19 | 2017-04-19 | |
| US62/487,358 | 2017-04-19 | ||
| PCT/US2018/028427 WO2018195348A1 (en) | 2017-04-19 | 2018-04-19 | Compositions and methods for treating cancer |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2020517263A true JP2020517263A (ja) | 2020-06-18 |
| JP2020517263A5 JP2020517263A5 (ja) | 2020-07-30 |
Family
ID=63856183
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019556956A Pending JP2020517263A (ja) | 2017-04-19 | 2018-04-19 | がんを治療するための組成物及び方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20210095029A1 (ja) |
| EP (1) | EP3612222A4 (ja) |
| JP (1) | JP2020517263A (ja) |
| CN (1) | CN110536700A (ja) |
| AU (1) | AU2018254517A1 (ja) |
| WO (1) | WO2018195348A1 (ja) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SG11202105975SA (en) * | 2018-12-06 | 2021-07-29 | Guangdong Tcrcure Biopharma Technology Co Ltd | Combinational tcr-t cell therapy targeting tumor antigens, tgf-beta, and immune checkpoints |
| CN114072427B (zh) * | 2019-07-17 | 2024-04-26 | 南京传奇生物科技有限公司 | 抗dll3嵌合抗原受体及其用途 |
| WO2021155518A1 (en) * | 2020-02-05 | 2021-08-12 | Tcrcure Biopharma Corp | Anti-hpv t cell receptors and engineered cells |
| CN116209676A (zh) | 2020-06-22 | 2023-06-02 | 恩格姆生物制药公司 | Lair-1结合剂及其使用方法 |
| CN111704675B (zh) * | 2020-07-08 | 2022-05-10 | 武汉波睿达生物科技有限公司 | 一种治疗hiv感染的双特异性嵌合抗原受体、基因、构建方法及其应用 |
| CN111826400A (zh) * | 2020-07-21 | 2020-10-27 | 中科宝承生物医学科技有限公司 | 一种双特异性抗体nk细胞制备方法及其细胞和应用 |
| CN114437229B (zh) * | 2020-11-01 | 2024-03-12 | 复旦大学 | 携带pd-1単链抗体且靶向egfr抗原的car t免疫细胞的制备及其用途 |
| TW202330909A (zh) * | 2021-09-09 | 2023-08-01 | 大陸商深圳市菲鵬生物治療股份有限公司 | 轉基因免疫效應細胞、其製備方法、慢病毒、構建體、癌症治療藥物及其應用 |
| CN115025217B (zh) * | 2022-05-13 | 2023-05-05 | 广东齐美医药生物科技集团有限公司 | 干细胞裂解物联合活性多糖以及酪氨酸酶抑制剂在制备药物或化妆品中的用途 |
| CN119286780B (zh) * | 2024-12-12 | 2025-03-21 | 山东省成体细胞产业技术研究院有限公司 | 一种t细胞的体外培养方法 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016508728A (ja) * | 2013-02-26 | 2016-03-24 | メモリアル スローン−ケタリング キャンサー センター | 免疫療法のための組成物および方法 |
| CN105796597A (zh) * | 2016-03-11 | 2016-07-27 | 江苏三特生物科技有限公司 | 携带pd-l1和ctla-4抗体基因的car-t细胞在肿瘤免疫上的应用 |
| WO2016126608A1 (en) * | 2015-02-02 | 2016-08-11 | Novartis Ag | Car-expressing cells against multiple tumor antigens and uses thereof |
| WO2016172583A1 (en) * | 2015-04-23 | 2016-10-27 | Novartis Ag | Treatment of cancer using chimeric antigen receptor and protein kinase a blocker |
| WO2016210129A1 (en) * | 2015-06-23 | 2016-12-29 | Memorial Sloan-Kettering Cancer Center | Novel pd-1 immune modulating agents |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017133175A1 (en) * | 2016-02-04 | 2017-08-10 | Nanjing Legend Biotech Co., Ltd. | Engineered mammalian cells for cancer therapy |
| EP3432924A1 (en) * | 2016-03-23 | 2019-01-30 | Novartis AG | Cell secreted minibodies and uses thereof |
| CN107523547A (zh) * | 2016-06-20 | 2017-12-29 | 上海细胞治疗研究院 | 一种高效稳定表达抑制性抗体的car‑t细胞及其用途 |
-
2018
- 2018-04-19 US US16/603,792 patent/US20210095029A1/en not_active Abandoned
- 2018-04-19 WO PCT/US2018/028427 patent/WO2018195348A1/en unknown
- 2018-04-19 AU AU2018254517A patent/AU2018254517A1/en not_active Abandoned
- 2018-04-19 JP JP2019556956A patent/JP2020517263A/ja active Pending
- 2018-04-19 CN CN201880025945.5A patent/CN110536700A/zh active Pending
- 2018-04-19 EP EP18787412.8A patent/EP3612222A4/en not_active Withdrawn
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016508728A (ja) * | 2013-02-26 | 2016-03-24 | メモリアル スローン−ケタリング キャンサー センター | 免疫療法のための組成物および方法 |
| WO2016126608A1 (en) * | 2015-02-02 | 2016-08-11 | Novartis Ag | Car-expressing cells against multiple tumor antigens and uses thereof |
| WO2016172583A1 (en) * | 2015-04-23 | 2016-10-27 | Novartis Ag | Treatment of cancer using chimeric antigen receptor and protein kinase a blocker |
| WO2016210129A1 (en) * | 2015-06-23 | 2016-12-29 | Memorial Sloan-Kettering Cancer Center | Novel pd-1 immune modulating agents |
| CN105796597A (zh) * | 2016-03-11 | 2016-07-27 | 江苏三特生物科技有限公司 | 携带pd-l1和ctla-4抗体基因的car-t细胞在肿瘤免疫上的应用 |
Non-Patent Citations (1)
| Title |
|---|
| CLINICAL CANCER RESEARCH, vol. 19, no. 20, JPN6021017927, 2013, pages 5636 - 5646, ISSN: 0004507491 * |
Also Published As
| Publication number | Publication date |
|---|---|
| CN110536700A (zh) | 2019-12-03 |
| WO2018195348A9 (en) | 2019-10-03 |
| US20210095029A1 (en) | 2021-04-01 |
| WO2018195348A1 (en) | 2018-10-25 |
| EP3612222A1 (en) | 2020-02-26 |
| AU2018254517A1 (en) | 2019-12-05 |
| EP3612222A4 (en) | 2020-12-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2020517263A (ja) | がんを治療するための組成物及び方法 | |
| US20240083968A1 (en) | Treatment of cancer using chimeric cd3 receptor proteins | |
| US20190375815A1 (en) | Treatment of cancer using chimeric t cell receptor proteins having multiple specificities | |
| JP7379803B2 (ja) | Nkg2dドメインを含む多重特異性キメラ受容体およびその使用法 | |
| JP2024518011A (ja) | 多様な免疫細胞のための単鎖および多鎖合成抗原受容体 | |
| JP2018518972A (ja) | 腫瘍特異的活性化のためのマスキングキメラ抗原受容体t細胞 | |
| CN110352068A (zh) | 合成的免疫受体及其使用方法 | |
| JP7595583B2 (ja) | 抗原特異的cd19標的化car-t細胞 | |
| WO2017219936A1 (zh) | 一种高效稳定表达激活型抗体的car-t细胞及其用途 | |
| WO2019129177A1 (zh) | 抗体修饰的嵌合抗原受体修饰t细胞及其用途 | |
| CA3189677A1 (en) | Chimeric molecules providing targeted costimulation for adoptive cell therapy | |
| CN113728007A (zh) | 人源化抗叶酸受体1嵌合抗原受体及其用途 | |
| WO2019149279A1 (zh) | 细胞免疫治疗的组合 | |
| WO2021244626A1 (zh) | 靶向cldn18.2的嵌合抗原受体及其用途 | |
| WO2020227595A1 (en) | Clec4-targeted car-t-cells | |
| WO2022037562A1 (en) | Engineered immunoresponsive cells and uses thereof | |
| CN112969470A (zh) | 用于扩增抗原特异性car-t细胞、组合物的方法及其相关用途 | |
| CN115485369A (zh) | γδT细胞及其用途 | |
| US20210230224A1 (en) | Car-t cells targeting glioma stem cells for the treatment of glioblastoma multiforme | |
| US20210079111A1 (en) | Cd19-cd20 bispecific and dual passway car-t and methods for use thereof | |
| HK40015393A (en) | Compositions and methods for treating cancer | |
| RU2826298C2 (ru) | Car-t-клетки, специфически нацеленные на антиген cd19 | |
| WO2025140480A1 (zh) | 经改造的iNKT细胞 | |
| HK40052654A (en) | Methods for expanding antigen-specific car-t cells, compositions and uses related thereto |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20191018 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200512 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20200512 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200518 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20210309 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210517 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20211208 |