JP2019031663A - 表面修飾ナノ粒子 - Google Patents
表面修飾ナノ粒子 Download PDFInfo
- Publication number
- JP2019031663A JP2019031663A JP2018143494A JP2018143494A JP2019031663A JP 2019031663 A JP2019031663 A JP 2019031663A JP 2018143494 A JP2018143494 A JP 2018143494A JP 2018143494 A JP2018143494 A JP 2018143494A JP 2019031663 A JP2019031663 A JP 2019031663A
- Authority
- JP
- Japan
- Prior art keywords
- nanoparticles
- ligand
- nanoparticle
- agent
- binding
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 0 CC(CC*)CC(CCC*)*[N+]([O-])=O Chemical compound CC(CC*)CC(CCC*)*[N+]([O-])=O 0.000 description 3
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/02—Use of particular materials as binders, particle coatings or suspension media therefor
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82B—NANOSTRUCTURES FORMED BY MANIPULATION OF INDIVIDUAL ATOMS, MOLECULES, OR LIMITED COLLECTIONS OF ATOMS OR MOLECULES AS DISCRETE UNITS; MANUFACTURE OR TREATMENT THEREOF
- B82B3/00—Manufacture or treatment of nanostructures by manipulation of individual atoms or molecules, or limited collections of atoms or molecules as discrete units
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y20/00—Nanooptics, e.g. quantum optics or photonic crystals
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y40/00—Manufacture or treatment of nanostructures
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D251/00—Heterocyclic compounds containing 1,3,5-triazine rings
- C07D251/02—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings
- C07D251/12—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members
- C07D251/26—Heterocyclic compounds containing 1,3,5-triazine rings not condensed with other rings having three double bonds between ring members or between ring members and non-ring members with only hetero atoms directly attached to ring carbon atoms
- C07D251/40—Nitrogen atoms
- C07D251/54—Three nitrogen atoms
- C07D251/70—Other substituted melamines
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F7/00—Compounds containing elements of Groups 4 or 14 of the Periodic Table
- C07F7/02—Silicon compounds
- C07F7/08—Compounds having one or more C—Si linkages
- C07F7/0834—Compounds having one or more O-Si linkage
- C07F7/0838—Compounds with one or more Si-O-Si sequences
- C07F7/087—Compounds of unknown structure containing a Si-O-Si sequence
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/50—Sympathetic, colour changing or similar inks
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/02—Use of particular materials as binders, particle coatings or suspension media therefor
- C09K11/025—Use of particular materials as binders, particle coatings or suspension media therefor non-luminescent particle coatings or suspension media
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/08—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials
- C09K11/56—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials containing sulfur
- C09K11/562—Chalcogenides
- C09K11/565—Chalcogenides with zinc cadmium
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/08—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials
- C09K11/62—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials containing gallium, indium or thallium
- C09K11/621—Chalcogenides
- C09K11/623—Chalcogenides with zinc or cadmium
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/08—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials
- C09K11/70—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials containing phosphorus
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/08—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials
- C09K11/70—Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic luminescent materials containing phosphorus
- C09K11/701—Chalcogenides
- C09K11/703—Chalcogenides with zinc or cadmium
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10H—INORGANIC LIGHT-EMITTING SEMICONDUCTOR DEVICES HAVING POTENTIAL BARRIERS
- H10H20/00—Individual inorganic light-emitting semiconductor devices having potential barriers, e.g. light-emitting diodes [LED]
- H10H20/80—Constructional details
- H10H20/85—Packages
- H10H20/851—Wavelength conversion means
- H10H20/8511—Wavelength conversion means characterised by their material, e.g. binder
- H10H20/8512—Wavelength conversion materials
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10H—INORGANIC LIGHT-EMITTING SEMICONDUCTOR DEVICES HAVING POTENTIAL BARRIERS
- H10H20/00—Individual inorganic light-emitting semiconductor devices having potential barriers, e.g. light-emitting diodes [LED]
- H10H20/80—Constructional details
- H10H20/85—Packages
- H10H20/851—Wavelength conversion means
- H10H20/8515—Wavelength conversion means not being in contact with the bodies
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1059—Heterocyclic compounds characterised by ligands containing three nitrogen atoms as heteroatoms
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1088—Heterocyclic compounds characterised by ligands containing oxygen as the only heteroatom
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/70—Nanostructure
- Y10S977/773—Nanoparticle, i.e. structure having three dimensions of 100 nm or less
- Y10S977/774—Exhibiting three-dimensional carrier confinement, e.g. quantum dots
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Nanotechnology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Manufacturing & Machinery (AREA)
- Composite Materials (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Optics & Photonics (AREA)
- Biophysics (AREA)
- Luminescent Compositions (AREA)
- Physical Or Chemical Processes And Apparatus (AREA)
- Compounds Of Alkaline-Earth Elements, Aluminum Or Rare-Earth Metals (AREA)
- Pigments, Carbon Blacks, Or Wood Stains (AREA)
- Led Device Packages (AREA)
- Inks, Pencil-Leads, Or Crayons (AREA)
- Treatments Of Macromolecular Shaped Articles (AREA)
Abstract
Description
大きさが2〜100nmのオーダーであって、多くの場合、量子ドット及び/又はナノ粒子と称される粒子からなる化合物半導体の調製及びキャラクタリゼーション(characterization)に大きな関心が持たれてきた。これらの研究は、ナノ粒子におけるサイズ調整可能な電子、光学及び化学特性に主に焦点を当ててきた。生物学的ラベリング(biological labeling)、太陽電池、触媒作用、生物学的イメージング、発光ダイオードのような多様な商業的用途への適用可能性から、半導体ナノ粒子は、大きな関心を集めている。
ナノ粒子の多くの用途は、半導体ナノ粒子が、特定の媒体と相溶性を有する(compatible)ことを要求している。例えば、蛍光標識、インビボ(in vivo)イメージングや治療学などの幾つかの生物学的用途は、ナノ粒子が水性環境と相溶性を有することを要求している。その他の用途では、ナノ粒子は、芳香族化合物、アルコール、エステルやケトンなどの有機媒体中に分散可能であることが望ましい。例えば、有機系分散剤中に分散した半導体ナノ粒子を含有するインク配合物は、光起電(PV)デバイス用の半導体材料の薄膜を製造するために重要である。
608nmで赤色発光する、カドミウムフリーの量子ドットナノ粒子(CFQD)(InP/ZnS)(200mg)を、イソプロピルミリステート(100マイクロリットル)と共にトルエン(1mL)中に分散させた。混合物を約1乃至2分間50℃で加熱し、その後、室温で15時間ゆっくりと振とうさせた。HMMM(Cymel 303) (400mg)、モノヒドロキシポリジメチルシロキサン(MW 5kD) (200mg)、及びp−トルエンスルホン酸(70mg)のトルエン溶液(4mL)を、ナノ粒子分散液に加えた。混合液を脱気し、電磁撹拌機を用いて300rpmで撹拌しつつ、140℃で4時間還流させた。窒素のストリームを1時間、フラスコに通して、求核基とHMMMの反応で生じる揮発性の副産物を確実に除去した。混合物を室温まで冷めるままにし、不活性ガス下で保存した。表面修飾ナノ粒子は、未修飾のナノ粒子と比べて、蛍光量子収率の損失が小さく又は無く、発光ピーク又は半値全幅(FWHM)値に変化はなかった。表面修飾ナノ粒子は、様々な分子量の(10乃至1000kD)PDMSポリマーに良好に分散し、残留トルエンを除去した後でさえ、分散したままであった。反対に、PDMSに分散させた同じ濃度の未修飾ナノ粒子は、凝集して、ホストシリコーンから分離した。
525nmで緑色発光する、カドミウムフリーの量子ドットナノ粒子(CFQD)(InP/ZnS)(200mg)を、イソプロピルミリステート(100マイクロリットル)と共にトルエン(1mL)中に分散させた。混合物を約1乃至2分間50℃で加熱し、その後、室温で15時間ゆっくりと振とうさせた。HMMM(Cymel 303) (400mg)、トリメチロールプロパントリグリシジルエーテル(200mg)、サリチル酸(70mg)のトルエン溶液(4mL)を、ナノ粒子分散液に加えた。混合液を脱気し、電磁撹拌機を用いて300rpmで撹拌しつつ、140℃で4時間還流させた。窒素のストリームを1時間、フラスコに通して、求核基とHMMMの反応で生じる揮発性の副産物を確実に除去した。混合物を室温まで冷めるままにし、不活性ガス下で保存した。表面修飾ナノ粒子は、未修飾のナノ粒子と比べて、蛍光量子収率の損失が小さく又は無く、発光ピーク又は半値全幅(FWHM)値に変化はなかった。表面修飾ナノ粒子は、様々な分子量のエポキシポリマーに良好に分散し、残留トルエンを除去した後でさえ、分散したままであった。反対に、同じ濃度の未修飾ナノ粒子は、凝集して、ホストマトリクスから分離した。
608nmで赤色発光する、カドミウムフリーの量子ドットナノ粒子(CFQD)(InP/ZnS)(200mg)を、イソプロピルミリステート(100マイクロリットル)と共にトルエン(1mL)中に分散させた。混合物を約1乃至2分間50℃で加熱し、その後、室温で15時間ゆっくりと振とうさせた。HMMM(Cymel 303) (400mg)、モノメトキシポリエチレンオキシド(CH3O−PEG2000−OH)(400mg)、サリチル酸(50mg)のトルエン溶液(4mL)を、ナノ粒子分散液に加えた。混合液を脱気し、電磁撹拌機を用いて300rpmで撹拌しつつ、130℃で2時間還流させた。窒素のストリームを1時間、フラスコに通して、求核基とHMMMの反応で生じる揮発性の副産物を確実に除去した。混合物を室温まで冷めるままにし、不活性ガス下で保存した。表面修飾ナノ粒子は、未修飾のナノ粒子と比べて、蛍光量子収率の損失が小さく又は無く、発光ピーク又は半値全幅(FWHM)値に変化はなかった。修飾されたドットのアリコートをポリスチレン樹脂又はポリスチレンコポリマー樹脂(トルエン中5%固形分、例えば、スチレン−エチレン/ブチレン−スチレン又はスチレン−エチレン/プロピレン−スチレン(SEPS、SEBS、Kraton))と混合し、修飾されたナノ粒子は、ホストポリスチレン樹脂に非常に良く分散し、残留トルエンを除去した後でも分散したままであった。同じナノ粒子濃度にて、未修飾でそのままのナノ粒子は、凝集して、ホスト樹脂から分離した。表面修飾ナノ粒子のフィルムは均一であるが、未修飾のナノ粒子のフィルムでは、ナノ粒子は顕著に凝集した。
608nmで赤色発光する、カドミウムフリーの量子ドットナノ粒子(CFQD)(InP/ZnS/ZnO)(200mg)を、イソプロピルミリステート(100マイクロリットル)と共にトルエン(1mL)中に分散させた。混合物を約1乃至2分間50℃で加熱し、その後、室温で15時間ゆっくりと振とうさせた。HMMM(Cymel 303) (400mg)、モノメトキシポリエチレンオキシド(CH3O−PEG2000−OH)(400mg)、サリチル酸(50mg)のトルエン溶液(4mL)を、ナノ粒子分散液に加えた。混合液を脱気し、電磁撹拌機を用いて300rpmで撹拌しつつ、140℃で4時間還流させた。窒素のストリームを1時間、フラスコに通して、求核基とHMMMの反応で生じる揮発性の副産物を確実に除去した。混合物を室温まで冷めるままにし、不活性ガス下で保存した。表面修飾ナノ粒子は、未修飾のナノ粒子と比べて、蛍光量子収率の損失が小さく又は無く、発光ピーク又は半値全幅(FWHM)値に変化はなかった。
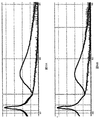
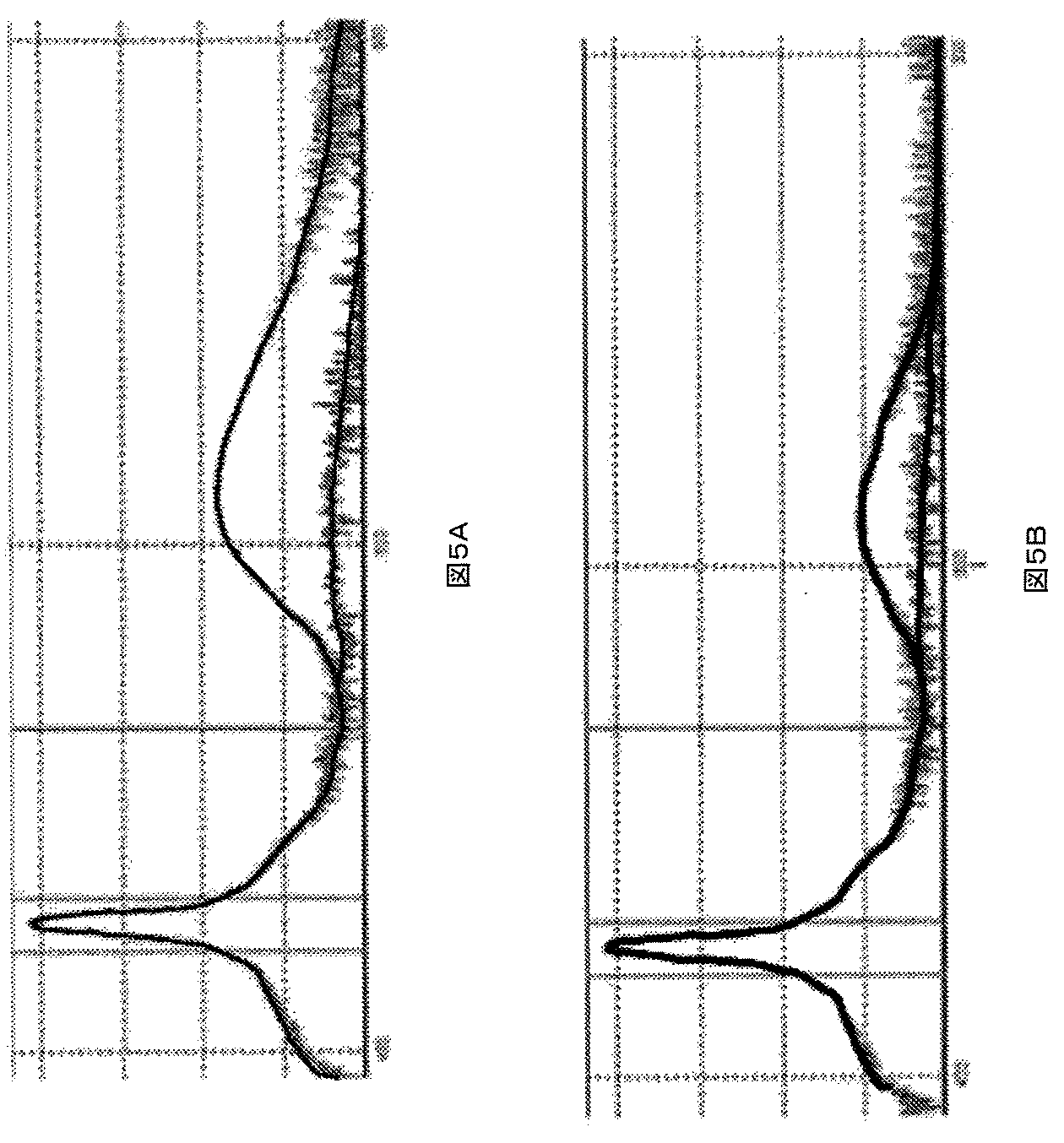
エポキシ相溶性ナノ粒子を、実施例2について説明したように調整した。エポキシ相溶性ナノ粒子を、LEDエポキシ封止剤(EX135)に加えた。封止剤と青色発光LEDチップとを用いて、LEDを調整した。図8Aは、表面修飾ナノ粒子を組み込んでいるLEDの発光曲線を示している。発光測定を、一週間毎日、その後、毎週行った。図8Bは、未修飾のナノ粒子を組み込んでいるLEDの発光曲線を示している。未修飾のナノ粒子を、最初にアクリルビーズに組み込み、その後、ビーズをエポキシに封入した。予想したように、両方のLEDの発光強度は、LEDが劣化するにつれて、時間と共に減少した。しかしながら、表面修飾ナノ粒子を組み込んでいるLEDの絶対発光強度は、未修飾ナノ粒子を組み込んでいるLEDの強度の約2倍となっている。
Claims (10)
- ナノ粒子とそれに結合するグアイフェネシンとを含む組成物。
- ナノ粒子は、当該ナノ粒子の表面と会合する第1の部分と、結合/架橋剤と結合する第2の部分を含むリガンド相互作用剤を更に含んでおり、グアイフェネシンは結合/架橋剤と結合する、請求項1に記載の組成物。
- 架橋/架橋剤はメラミン化合物である、請求項2に記載の組成物。
- メラミン化合物は、ヘキサメトキシメチルメラミンである、請求項3に記載の組成物。
- リガンド相互作用剤は、C8−C20脂肪酸及びC8−C20脂肪酸エステルの何れかである、請求項2に記載の組成物。
- ナノ粒子の表面にあるキャッピングリガンドを更に含んでおり、
リガンド相互作用剤の第1の部分は、キャッピングリガンドとインターカレーションする、請求項2に記載の組成物。 - 極性溶媒を更に含む、請求項1に記載の組成物。
- アクリレートを更に含む、請求項1に記載の組成物。
- ナノ粒子は半導体ナノ粒子である、請求項1に記載の組成物。
- インクである、請求項1に記載の組成物。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161579440P | 2011-12-22 | 2011-12-22 | |
| US61/579,440 | 2011-12-22 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017045600A Division JP6381713B2 (ja) | 2011-12-22 | 2017-03-10 | 表面修飾ナノ粒子 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2019031663A true JP2019031663A (ja) | 2019-02-28 |
| JP6827447B2 JP6827447B2 (ja) | 2021-02-10 |
Family
ID=47901229
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014548244A Active JP6111264B2 (ja) | 2011-12-22 | 2012-12-21 | 表面修飾ナノ粒子 |
| JP2017045600A Active JP6381713B2 (ja) | 2011-12-22 | 2017-03-10 | 表面修飾ナノ粒子 |
| JP2018143494A Active JP6827447B2 (ja) | 2011-12-22 | 2018-07-31 | 表面修飾ナノ粒子 |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014548244A Active JP6111264B2 (ja) | 2011-12-22 | 2012-12-21 | 表面修飾ナノ粒子 |
| JP2017045600A Active JP6381713B2 (ja) | 2011-12-22 | 2017-03-10 | 表面修飾ナノ粒子 |
Country Status (7)
| Country | Link |
|---|---|
| US (5) | US9115097B2 (ja) |
| EP (1) | EP2794464B1 (ja) |
| JP (3) | JP6111264B2 (ja) |
| KR (2) | KR102086729B1 (ja) |
| CN (2) | CN107474019A (ja) |
| HK (1) | HK1203474A1 (ja) |
| WO (1) | WO2013093631A2 (ja) |
Families Citing this family (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6111264B2 (ja) * | 2011-12-22 | 2017-04-05 | ナノコ テクノロジーズ リミテッド | 表面修飾ナノ粒子 |
| EP3083878B1 (en) * | 2013-12-17 | 2018-10-03 | 3M Innovative Properties Company | Composite nanoparticles including a phthalic acid derivative |
| PL2886126T3 (pl) | 2013-12-23 | 2017-11-30 | Exchange Imaging Technologies Gmbh | Nanocząstka sprzężona z peptydami wiążącymi CD44 |
| EP4223433B1 (en) * | 2014-01-06 | 2025-12-17 | Nanoco Technologies Ltd | Cadmium-free quantum dot nanoparticles |
| KR20160102292A (ko) * | 2014-01-06 | 2016-08-29 | 나노코 테크놀로지스 리미티드 | 표면 변형된 나노입자 |
| CN106103645B (zh) * | 2014-03-10 | 2019-05-28 | 3M创新有限公司 | 包括硫醇取代的有机硅的复合纳米粒子 |
| WO2015140642A2 (en) * | 2014-03-18 | 2015-09-24 | Nanoco Technologies, Ltd | Quantum dot compositions |
| US9466771B2 (en) | 2014-07-23 | 2016-10-11 | Osram Sylvania Inc. | Wavelength converters and methods for making the same |
| WO2016072806A2 (ko) * | 2014-11-06 | 2016-05-12 | 포항공과대학교 산학협력단 | 코어-쉘 구조의 페로브스카이트 나노결정입자 발광체, 이의 제조방법 및 이를 이용한 발광소자 |
| JP6606820B2 (ja) * | 2014-11-12 | 2019-11-20 | コニカミノルタ株式会社 | 半導体ナノ粒子内包樹脂粒子、該半導体ナノ粒子内包樹脂粒子からなる組織染色用染色剤、および組織染色法 |
| CN107250912B (zh) * | 2015-02-27 | 2021-06-22 | 默克专利有限公司 | 光敏组合物和色彩转换膜 |
| JP6729554B2 (ja) * | 2015-03-23 | 2020-07-22 | コニカミノルタ株式会社 | 組成物及びそれを含有する光学機能性膜 |
| WO2017086362A1 (ja) * | 2015-11-20 | 2017-05-26 | Jsr株式会社 | ナノ粒子集合体及びその製造方法、ナノ粒子集合体組成物、波長変換層、並びにリガンド |
| CN105870346B (zh) * | 2016-04-15 | 2018-07-03 | 深圳市华星光电技术有限公司 | Led显示屏的制造方法和led显示屏 |
| US10059585B2 (en) | 2016-06-28 | 2018-08-28 | Nanoco Technologies Ltd. | Formation of 2D flakes from chemical cutting of prefabricated nanoparticles and van der Waals heterostructure devices made using the same |
| US20180011346A1 (en) * | 2016-07-05 | 2018-01-11 | Nanoco Technologies Ltd. | Probe for targeting and manipulating mitochondrial function using quantum dots |
| US20180009659A1 (en) * | 2016-07-05 | 2018-01-11 | Nanoco Technologies Ltd. | Ligand conjugated quantum dot nanoparticles and methods of detecting dna methylation using same |
| US20180067121A1 (en) | 2016-09-06 | 2018-03-08 | Nanoco Technologies Ltd. | Exosome-conjugated quantum dot nanoparticles and methods of detecting exosomes and cancer using same |
| WO2018065860A1 (en) * | 2016-10-04 | 2018-04-12 | Nanoco Technologies Ltd. | Polymerizable quantum dot nanoparticles and their use as therapeutic, ablation and tattooing agents |
| US20180133345A1 (en) * | 2016-11-15 | 2018-05-17 | Nanoco Technologies Ltd. | Nano-Devices for Detection and Treatment of Cancer |
| CN106622333A (zh) * | 2016-12-16 | 2017-05-10 | 池州方达科技有限公司 | 一种甲基丙烯酸羟丙酯合成用沸石分子筛的预处理方法 |
| CN106669650A (zh) * | 2016-12-16 | 2017-05-17 | 池州方达科技有限公司 | 一种催化剂载体陶瓷微球的预处理方法 |
| US10610591B2 (en) * | 2017-03-15 | 2020-04-07 | Nanoco Technologies Ltd. | Light responsive quantum dot drug delivery system |
| WO2018226925A1 (en) * | 2017-06-07 | 2018-12-13 | Nanosys, Inc. | Thiolated hydrophilic ligands for improved quantum dot reliability in resin films |
| KR20190126105A (ko) * | 2017-07-11 | 2019-11-08 | 티씨엘 코포레이션 | 양자점 및 그 제조방법 |
| WO2019035957A1 (en) * | 2017-08-16 | 2019-02-21 | Nanosys, Inc. | PEG-BASED LIGANDS HAVING IMPROVED DISPERSION CAPABILITY AND IMPROVED PERFORMANCE |
| WO2019038731A1 (en) * | 2017-08-25 | 2019-02-28 | Sabic Global Technologies B.V. | COMPOSITION COMPRISING RETICULATED QUANTIC POINTS |
| JP2021500329A (ja) | 2017-10-18 | 2021-01-07 | ナノコ テクノロジーズ リミテッド | 5−アミノレブリン酸を用いて医用画像処理及び光線療法を向上させる方法 |
| KR102087299B1 (ko) * | 2018-04-09 | 2020-03-10 | 숭실대학교산학협력단 | 양자점 박막, 이의 패터닝 방법 및 이를 적용한 양자점 발광소자 |
| JP7434181B2 (ja) * | 2018-05-03 | 2024-02-20 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | 架橋された配位子 |
| WO2020120970A1 (en) | 2018-12-13 | 2020-06-18 | Nanoco Technologies Ltd | Methods for enhancing indocyanine green medical imaging and phototherapy |
| KR20220020280A (ko) * | 2019-06-13 | 2022-02-18 | 쇼에이 가가쿠 가부시키가이샤 | 반도체 나노 입자 복합체, 반도체 나노 입자 복합체 분산액, 반도체 나노 입자 복합체 조성물, 반도체 나노 입자 복합체 경화막 및 반도체 나노 입자 복합체의 정제 방법 |
| CN112111042B (zh) * | 2019-06-21 | 2024-04-05 | 康码(上海)生物科技有限公司 | 一种生物磁性微球及其制备方法和使用方法 |
| KR102746318B1 (ko) * | 2019-10-01 | 2024-12-24 | 삼성디스플레이 주식회사 | 양자점, 이를 포함하는 조성물과 복합체, 및 이를 포함하는 전자 소자 |
| US20210190775A1 (en) | 2019-12-18 | 2021-06-24 | Nanoco Technologies Ltd. | Compositions and methods for tagging and detecting nucleic acids |
| KR102813209B1 (ko) * | 2020-05-18 | 2025-05-27 | 삼성디스플레이 주식회사 | 표면 개질용 리간드, 이를 포함하는 발광 소자, 및 표시 장치의 제조 방법 |
| KR20210149971A (ko) | 2020-06-02 | 2021-12-10 | 삼성디스플레이 주식회사 | 양자점 조성물, 발광 소자 및 이를 포함하는 표시 장치 |
| US20220018837A1 (en) | 2020-07-17 | 2022-01-20 | Nanoco Technologies Ltd. | Method for the Detection of Surface-Mounted Biological Materials and Pathogens |
| KR102742152B1 (ko) | 2020-12-04 | 2024-12-12 | 재단법인대구경북과학기술원 | 전 무기기반 표면 리간드 치환에 기반하여 형성된 광활성인 나노입자 및 이를 이용한 패터닝 방법 |
| CN114806540A (zh) * | 2021-01-20 | 2022-07-29 | 三星显示有限公司 | 量子点及包含量子点的油墨组合物、光学构件和电子器件 |
| CN117510440A (zh) * | 2023-10-31 | 2024-02-06 | 苏州世华新材料科技股份有限公司 | 一种适用于丙烯酸系胶粘剂的环氧类交联剂及其制备方法 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62116507A (ja) * | 1985-10-04 | 1987-05-28 | ワ−ナ−−ランバ−ト・コンパニ− | 医薬吸着物およびその製法 |
| JP2010533198A (ja) * | 2007-07-10 | 2010-10-21 | ザ レジェンツ オブ ザ ユニヴァースティ オブ カリフォルニア | 選択した組織へ組成物を送達するための材料と方法 |
Family Cites Families (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5750664A (en) * | 1995-06-05 | 1998-05-12 | Eisai Co., Ltd. | Substituted liposaccharides useful in the treatment and prevention of endotoxemia |
| GB9518910D0 (en) | 1995-09-15 | 1995-11-15 | Imperial College | Process |
| CA2403620C (en) * | 2000-03-20 | 2012-01-10 | Massachusetts Institute Of Technology | Inorganic chromophore bioconjugates |
| US6649138B2 (en) * | 2000-10-13 | 2003-11-18 | Quantum Dot Corporation | Surface-modified semiconductive and metallic nanoparticles having enhanced dispersibility in aqueous media |
| US7273904B2 (en) * | 2002-10-03 | 2007-09-25 | The Board Of Trustees Of The University Of Arkansas | Nanocrystals in ligand boxes exhibiting enhanced chemical, photochemical, and thermal stability, and methods of making the same |
| GB0409877D0 (en) | 2004-04-30 | 2004-06-09 | Univ Manchester | Preparation of nanoparticle materials |
| US7588828B2 (en) | 2004-04-30 | 2009-09-15 | Nanoco Technologies Limited | Preparation of nanoparticle materials |
| EP1809720B1 (en) * | 2004-10-29 | 2012-05-02 | Life Technologies Corporation | Functionalized fluorescent nanocrystals, and methods for their preparation and use |
| EP1883820A4 (en) | 2005-05-04 | 2010-06-16 | Agency Science Tech & Res | INNOVATIVE WATER-SOLUBLE NANOCRYSTALS COMPRISING A LOW MOLECULAR WEIGHT COATING REAGENT AND METHODS FOR PREPARING THE SAME |
| GB2472542B (en) | 2005-08-12 | 2011-03-23 | Nanoco Technologies Ltd | Nanoparticles |
| GB0522027D0 (en) | 2005-10-28 | 2005-12-07 | Nanoco Technologies Ltd | Controlled preparation of nanoparticle materials |
| DE102006012467A1 (de) | 2006-03-17 | 2007-09-20 | Merck Patent Gmbh | Redispergierbare Nanopartikel |
| EP2027224A2 (en) * | 2006-06-14 | 2009-02-25 | E.I. Du Pont De Nemours And Company | Coated substrate having enhanced scratch and mar resistance |
| JP5459896B2 (ja) | 2007-03-05 | 2014-04-02 | 株式会社半導体エネルギー研究所 | 配線及び記憶素子の作製方法 |
| US8568888B2 (en) * | 2007-03-15 | 2013-10-29 | Nanovere Technologies, Inc. | Dendritic polyurethane coating |
| KR100845403B1 (ko) * | 2007-04-16 | 2008-07-10 | 유창국 | 유/무기 하이브리드 코팅제 및 그 제조방법과 열경화방법 |
| WO2009054464A1 (ja) * | 2007-10-26 | 2009-04-30 | Teijin Limited | 透明導電性積層体及び透明タッチパネル |
| DE102007056342A1 (de) * | 2007-11-22 | 2009-05-28 | Merck Patent Gmbh | Oberflächenmodifizierte Konversionsleuchtstoffe |
| US20110189102A1 (en) * | 2008-02-08 | 2011-08-04 | Kairdolf Brad A | Coated quantum dots and methods of making and using thereof |
| GB0813273D0 (en) * | 2008-07-19 | 2008-08-27 | Nanoco Technologies Ltd | Method for producing aqueous compatible nanoparticles |
| EP2323947B1 (en) * | 2008-08-06 | 2019-07-03 | Life Technologies Corporation | Water-dispersable nanoparticles |
| GB0814458D0 (en) * | 2008-08-07 | 2008-09-10 | Nanoco Technologies Ltd | Surface functionalised nanoparticles |
| US20110226995A1 (en) * | 2008-10-03 | 2011-09-22 | Life Technologies Corporation | Compositions and methods for functionalizing or crosslinking ligands on nanoparticle surfaces |
| KR101018111B1 (ko) * | 2008-10-07 | 2011-02-25 | 삼성엘이디 주식회사 | 양자점-금속산화물 복합체, 양자점-금속산화물 복합체의 제조방법 및 양자점-금속산화물 복합체를 포함하는 발광장치 |
| GB0901857D0 (en) * | 2009-02-05 | 2009-03-11 | Nanoco Technologies Ltd | Encapsulated nanoparticles |
| CN101857766A (zh) * | 2009-04-09 | 2010-10-13 | 赢创德固赛(中国)投资有限公司 | 钛系纳米粒子/聚酯复合涂料的制备方法及由此制得的复合涂料 |
| KR101711085B1 (ko) * | 2009-10-09 | 2017-03-14 | 삼성전자 주식회사 | 나노 복합 입자, 그 제조방법 및 상기 나노 복합 입자를 포함하는 소자 |
| JP2012246470A (ja) * | 2011-05-31 | 2012-12-13 | Sharp Corp | 半導体ナノ粒子の製造方法、半導体ナノ粒子、ならびにこれを用いた蛍光体 |
| JP6111264B2 (ja) * | 2011-12-22 | 2017-04-05 | ナノコ テクノロジーズ リミテッド | 表面修飾ナノ粒子 |
-
2012
- 2012-12-21 JP JP2014548244A patent/JP6111264B2/ja active Active
- 2012-12-21 KR KR1020147019554A patent/KR102086729B1/ko active Active
- 2012-12-21 US US13/723,418 patent/US9115097B2/en active Active
- 2012-12-21 WO PCT/IB2012/003015 patent/WO2013093631A2/en not_active Ceased
- 2012-12-21 CN CN201710878049.0A patent/CN107474019A/zh active Pending
- 2012-12-21 HK HK15104081.5A patent/HK1203474A1/xx unknown
- 2012-12-21 EP EP12832767.3A patent/EP2794464B1/en active Active
- 2012-12-21 CN CN201280063722.0A patent/CN104245568B/zh active Active
- 2012-12-21 KR KR1020207006264A patent/KR102184715B1/ko active Active
-
2015
- 2015-08-21 US US14/832,631 patent/US9840664B2/en active Active
-
2017
- 2017-03-10 JP JP2017045600A patent/JP6381713B2/ja active Active
- 2017-12-04 US US15/831,019 patent/US10377944B2/en active Active
-
2018
- 2018-07-31 JP JP2018143494A patent/JP6827447B2/ja active Active
-
2019
- 2019-06-14 US US16/442,060 patent/US10669475B2/en active Active
-
2020
- 2020-05-08 US US16/870,146 patent/US11053436B2/en active Active
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62116507A (ja) * | 1985-10-04 | 1987-05-28 | ワ−ナ−−ランバ−ト・コンパニ− | 医薬吸着物およびその製法 |
| JP2010533198A (ja) * | 2007-07-10 | 2010-10-21 | ザ レジェンツ オブ ザ ユニヴァースティ オブ カリフォルニア | 選択した組織へ組成物を送達するための材料と方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN104245568B (zh) | 2017-10-03 |
| CN104245568A (zh) | 2014-12-24 |
| US11053436B2 (en) | 2021-07-06 |
| EP2794464A2 (en) | 2014-10-29 |
| WO2013093631A3 (en) | 2014-01-23 |
| US9115097B2 (en) | 2015-08-25 |
| US20190359884A1 (en) | 2019-11-28 |
| JP2017164740A (ja) | 2017-09-21 |
| WO2013093631A2 (en) | 2013-06-27 |
| US10377944B2 (en) | 2019-08-13 |
| US20200263086A1 (en) | 2020-08-20 |
| KR102184715B1 (ko) | 2020-11-30 |
| US20180142146A1 (en) | 2018-05-24 |
| US10669475B2 (en) | 2020-06-02 |
| JP6381713B2 (ja) | 2018-08-29 |
| KR102086729B1 (ko) | 2020-03-10 |
| US20160046859A1 (en) | 2016-02-18 |
| JP6827447B2 (ja) | 2021-02-10 |
| HK1203474A1 (en) | 2015-10-30 |
| KR20200027052A (ko) | 2020-03-11 |
| US20130190493A1 (en) | 2013-07-25 |
| JP6111264B2 (ja) | 2017-04-05 |
| CN107474019A (zh) | 2017-12-15 |
| JP2015511167A (ja) | 2015-04-16 |
| KR20140108684A (ko) | 2014-09-12 |
| US9840664B2 (en) | 2017-12-12 |
| EP2794464B1 (en) | 2023-11-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6381713B2 (ja) | 表面修飾ナノ粒子 | |
| JP6537667B2 (ja) | 表面修飾ナノ粒子 | |
| JP5519899B2 (ja) | キャップ分子が表面に結合しているナノ粒子用分散剤、これを用いたナノ粒子を分散させる方法、及びこれを含むナノ粒子含有薄膜 | |
| TWI608076B (zh) | 以金屬硫醇聚合物穩定化的量子點 | |
| JP2017512245A (ja) | カドミウムフリー量子ドットナノ粒子 | |
| TW201035036A (en) | Encapsulated nanoparticles |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20180807 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20190820 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20191113 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200428 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20200727 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200916 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20210112 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210119 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6827447 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: R3D03 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |