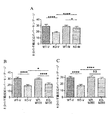
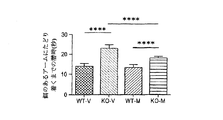
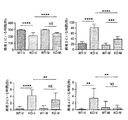
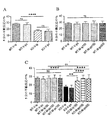
JP2016530291A - 脆弱x症候群および関連障害の処置方法 - Google Patents
脆弱x症候群および関連障害の処置方法 Download PDFInfo
- Publication number
- JP2016530291A JP2016530291A JP2016539645A JP2016539645A JP2016530291A JP 2016530291 A JP2016530291 A JP 2016530291A JP 2016539645 A JP2016539645 A JP 2016539645A JP 2016539645 A JP2016539645 A JP 2016539645A JP 2016530291 A JP2016530291 A JP 2016530291A
- Authority
- JP
- Japan
- Prior art keywords
- metadoxine
- mice
- treatment
- administration
- treated
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4415—Pyridoxine, i.e. Vitamin B6
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/4015—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil having oxo groups directly attached to the heterocyclic ring, e.g. piracetam, ethosuximide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Urology & Nephrology (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Food Science & Technology (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Biotechnology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Medicines Containing Plant Substances (AREA)
- Steroid Compounds (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361875384P | 2013-09-09 | 2013-09-09 | |
| US61/875,384 | 2013-09-09 | ||
| US14/038,258 US20150073023A1 (en) | 2013-09-09 | 2013-09-26 | Method Of Treating Fragile X Syndrome And Related Disorders |
| US14/038,258 | 2013-09-26 | ||
| US201461991351P | 2014-05-09 | 2014-05-09 | |
| US61/991,351 | 2014-05-09 | ||
| PCT/IB2014/002398 WO2015033224A2 (en) | 2013-09-09 | 2014-09-09 | Methods of treating fragile x syndrome and related disorders |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2016530291A true JP2016530291A (ja) | 2016-09-29 |
| JP2016530291A5 JP2016530291A5 (enExample) | 2017-10-19 |
Family
ID=52629036
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016539645A Pending JP2016530291A (ja) | 2013-09-09 | 2014-09-09 | 脆弱x症候群および関連障害の処置方法 |
| JP2016540930A Pending JP2016530536A (ja) | 2013-09-09 | 2014-09-09 | 療法に対する奏効性の決定方法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016540930A Pending JP2016530536A (ja) | 2013-09-09 | 2014-09-09 | 療法に対する奏効性の決定方法 |
Country Status (12)
| Country | Link |
|---|---|
| EP (2) | EP3043792A2 (enExample) |
| JP (2) | JP2016530291A (enExample) |
| KR (2) | KR20160086818A (enExample) |
| CN (2) | CN105917225A (enExample) |
| AU (2) | AU2014315026A1 (enExample) |
| CA (2) | CA2923421A1 (enExample) |
| EA (2) | EA201690559A1 (enExample) |
| IL (2) | IL244343A0 (enExample) |
| MX (2) | MX2016003002A (enExample) |
| SG (2) | SG11201601830PA (enExample) |
| TW (2) | TW201605443A (enExample) |
| WO (2) | WO2015033224A2 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023521274A (ja) * | 2020-02-07 | 2023-05-24 | ニューロヴェンティ カンパニー リミテッド | リルメニジン化合物を有効成分として含む脆弱x症候群または関連発達障害を治療するための組成物 |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109021022B (zh) * | 2013-07-31 | 2021-04-16 | Udc 爱尔兰有限责任公司 | 发光的二氮杂苯并咪唑碳烯金属配合物 |
| US20150073023A1 (en) | 2013-09-09 | 2015-03-12 | Alcobra Ltd. | Method Of Treating Fragile X Syndrome And Related Disorders |
| CN108538365A (zh) * | 2017-03-24 | 2018-09-14 | 华东师范大学 | 一种基于数据分析技术的自闭症社交能力评估系统 |
| WO2019197632A1 (en) * | 2018-04-13 | 2019-10-17 | Healx Limited | Treatment of fragile x syndrome |
| KR20190121569A (ko) * | 2018-04-18 | 2019-10-28 | 건국대학교 글로컬산학협력단 | 아그마틴 및 이의 약학적으로 허용 가능한 염을 포함하는 취약 x 증후군 예방 또는 치료용 약학조성물 |
| PL3813816T3 (pl) * | 2018-06-07 | 2023-07-31 | Ovid Therapeutics Inc. | Zastosowanie kwasu (s)-3-amino-4-(difluorometylenylo)cyklopentano-1-eno-1-karboksylowego i związków pokrewnych, kwasu (1s,3s)-3-amino-4-(difluorometylideno)cyklopentano-1-karboksylowego w leczeniu zespołu łamliwego chromosomu x lub zespołu drżenia/ataksji związanego z zespołem łamliwego chromosomu x |
| CN115335053B (zh) * | 2020-01-08 | 2024-08-09 | 株式会社纽若梵提 | 包含麦角乙脲化合物作为有效成分用于治疗脆性x染色体综合征或相关发育障碍的组合物 |
| EP4171528A1 (en) * | 2020-06-29 | 2023-05-03 | Zynerba Pharmaceuticals, Inc. | Treatment of fragile x syndrome with cannabidiol |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002043507A2 (en) * | 2000-11-30 | 2002-06-06 | The Health Research Institute | Nutrient supplements and methods for treating autism and for preventing the onset of autism |
| JP2012531402A (ja) * | 2009-06-25 | 2012-12-10 | アルコブラ、リミテッド | 認知疾患、障害または病態の治療、症状緩和、軽減、改善および予防方法 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IT1131856B (it) | 1980-06-30 | 1986-06-25 | Baldacci Lab Spa | Composizione farmaceutica per il trattamento di intossicazioni alcooliche |
| IL187159A0 (en) | 2007-07-03 | 2009-02-11 | Gur Megiddo | Use of metadoxine in relief of alcohol intoxication |
-
2014
- 2014-09-05 TW TW103130856A patent/TW201605443A/zh unknown
- 2014-09-05 TW TW103130859A patent/TW201606304A/zh unknown
- 2014-09-09 AU AU2014315026A patent/AU2014315026A1/en not_active Abandoned
- 2014-09-09 MX MX2016003002A patent/MX2016003002A/es unknown
- 2014-09-09 WO PCT/IB2014/002398 patent/WO2015033224A2/en not_active Ceased
- 2014-09-09 EA EA201690559A patent/EA201690559A1/ru unknown
- 2014-09-09 WO PCT/US2014/054816 patent/WO2015035402A1/en not_active Ceased
- 2014-09-09 CA CA2923421A patent/CA2923421A1/en not_active Abandoned
- 2014-09-09 KR KR1020167009042A patent/KR20160086818A/ko not_active Withdrawn
- 2014-09-09 CA CA2922901A patent/CA2922901A1/en not_active Abandoned
- 2014-09-09 KR KR1020167009040A patent/KR20160078956A/ko not_active Withdrawn
- 2014-09-09 EP EP14830858.8A patent/EP3043792A2/en not_active Withdrawn
- 2014-09-09 SG SG11201601830PA patent/SG11201601830PA/en unknown
- 2014-09-09 JP JP2016539645A patent/JP2016530291A/ja active Pending
- 2014-09-09 EA EA201690557A patent/EA201690557A1/ru unknown
- 2014-09-09 CN CN201480060722.4A patent/CN105917225A/zh active Pending
- 2014-09-09 AU AU2014316779A patent/AU2014316779A1/en not_active Abandoned
- 2014-09-09 CN CN201480049671.5A patent/CN105517546A/zh active Pending
- 2014-09-09 JP JP2016540930A patent/JP2016530536A/ja active Pending
- 2014-09-09 MX MX2016003006A patent/MX2016003006A/es unknown
- 2014-09-09 SG SG11201601605YA patent/SG11201601605YA/en unknown
- 2014-09-09 EP EP14776939.2A patent/EP3044589A1/en not_active Withdrawn
-
2016
- 2016-02-29 IL IL244343A patent/IL244343A0/en unknown
- 2016-03-06 IL IL244453A patent/IL244453A0/en unknown
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002043507A2 (en) * | 2000-11-30 | 2002-06-06 | The Health Research Institute | Nutrient supplements and methods for treating autism and for preventing the onset of autism |
| JP2012531402A (ja) * | 2009-06-25 | 2012-12-10 | アルコブラ、リミテッド | 認知疾患、障害または病態の治療、症状緩和、軽減、改善および予防方法 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2023521274A (ja) * | 2020-02-07 | 2023-05-24 | ニューロヴェンティ カンパニー リミテッド | リルメニジン化合物を有効成分として含む脆弱x症候群または関連発達障害を治療するための組成物 |
| JP7412583B2 (ja) | 2020-02-07 | 2024-01-12 | ニューロヴェンティ カンパニー リミテッド | リルメニジン化合物を有効成分として含む脆弱x症候群または関連発達障害を治療するための組成物 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2015033224A2 (en) | 2015-03-12 |
| EA201690557A1 (ru) | 2016-07-29 |
| KR20160086818A (ko) | 2016-07-20 |
| CN105917225A (zh) | 2016-08-31 |
| TW201606304A (zh) | 2016-02-16 |
| SG11201601605YA (en) | 2016-04-28 |
| EA201690559A1 (ru) | 2016-08-31 |
| EP3044589A1 (en) | 2016-07-20 |
| IL244453A0 (en) | 2016-04-21 |
| KR20160078956A (ko) | 2016-07-05 |
| CN105517546A (zh) | 2016-04-20 |
| AU2014315026A1 (en) | 2016-03-24 |
| WO2015035402A1 (en) | 2015-03-12 |
| CA2923421A1 (en) | 2015-03-12 |
| MX2016003006A (es) | 2016-06-10 |
| JP2016530536A (ja) | 2016-09-29 |
| WO2015033224A3 (en) | 2015-07-02 |
| MX2016003002A (es) | 2016-09-08 |
| IL244343A0 (en) | 2016-04-21 |
| CA2922901A1 (en) | 2015-03-12 |
| EP3043792A2 (en) | 2016-07-20 |
| TW201605443A (zh) | 2016-02-16 |
| AU2014316779A1 (en) | 2016-03-17 |
| SG11201601830PA (en) | 2016-04-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2016530291A (ja) | 脆弱x症候群および関連障害の処置方法 | |
| ES2750728T3 (es) | Uso de cannabinoides en combinación con Aripriprazol | |
| CA2580619C (en) | Use of memantine (namenda) to treat autism, compulsivity, and impulsivity | |
| KR102014883B1 (ko) | 근위축성 측삭 경화증 치료용 신규 조성물 | |
| ES2690061T3 (es) | Composiciones para tratar la enfermedad de Parkinson | |
| JP2010504338A (ja) | 筋萎縮性側索硬化症(ALS)の治療のための水素化ピリド[4,3−b]インドール | |
| JP2021521122A (ja) | 筋萎縮性側索硬化症および関連障害の治療のための併用療法 | |
| WO2001068136A1 (en) | Drugs for complications of diabetes and neuropathy and utilization thereof | |
| JP2012528818A (ja) | Cmt及び関連障害を処置するための新たな組成物 | |
| AU2016268096B2 (en) | Extended release pharmaceutical compositions of levetiracetam | |
| JP2019527216A (ja) | ジアミノフェノチアジンの投与及び投与量 | |
| JP7296472B2 (ja) | プリドピジンを使用したミトコンドリア関連疾患および障害(それらの症状を含む)の治療 | |
| JP2024133735A (ja) | Iv型コラーゲン疾患の処置のためのビフェニルスルホンアミド化合物 | |
| CN109069450A (zh) | 神经障碍的新的组合疗法 | |
| WO2020165802A1 (en) | Compositions and methods relating to use of agonists of alpha5-containing gabaa receptors | |
| US9851354B2 (en) | Methods of treating fragile X syndrome and related disorders | |
| JP2023546924A (ja) | 神経系障害を処置するための抗プリン作動性組成物の投与 | |
| US20110065674A1 (en) | Methods and compositions for improving cognitive function | |
| AU2018309710A1 (en) | Methods and compositions for treating neurological conditions | |
| AU2011235981B2 (en) | Use of Memantine (Namenda) to Treat Autism, Compulsivity, and Impulsivity | |
| JPWO2001068136A1 (ja) | 糖尿病性合併症及び神経障害用の薬剤とその使用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170908 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20170908 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20180425 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20180508 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20181204 |