KR20160086818A - 요법에 대한 반응의 결정 방법 - Google Patents
요법에 대한 반응의 결정 방법 Download PDFInfo
- Publication number
- KR20160086818A KR20160086818A KR1020167009042A KR20167009042A KR20160086818A KR 20160086818 A KR20160086818 A KR 20160086818A KR 1020167009042 A KR1020167009042 A KR 1020167009042A KR 20167009042 A KR20167009042 A KR 20167009042A KR 20160086818 A KR20160086818 A KR 20160086818A
- Authority
- KR
- South Korea
- Prior art keywords
- mice
- erk
- ratio
- subject
- fmr1
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 51
- 238000002560 therapeutic procedure Methods 0.000 title claims abstract description 18
- 230000004044 response Effects 0.000 title abstract description 10
- 238000011282 treatment Methods 0.000 claims abstract description 89
- 208000001914 Fragile X syndrome Diseases 0.000 claims abstract description 54
- 208000010877 cognitive disease Diseases 0.000 claims abstract description 27
- 238000009226 cognitive therapy Methods 0.000 claims abstract description 16
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 29
- 201000010099 disease Diseases 0.000 claims description 24
- 230000008901 benefit Effects 0.000 claims description 11
- 210000004698 lymphocyte Anatomy 0.000 claims description 11
- 210000004369 blood Anatomy 0.000 claims description 6
- 239000008280 blood Substances 0.000 claims description 6
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 claims description 5
- 238000003018 immunoassay Methods 0.000 claims description 3
- 210000005087 mononuclear cell Anatomy 0.000 claims 1
- 241000699670 Mus sp. Species 0.000 description 198
- 101150082209 Fmr1 gene Proteins 0.000 description 143
- RYKKQQUKJJGFMN-HVDRVSQOSA-N 4,5-bis(hydroxymethyl)-2-methylpyridin-3-ol;(2s)-5-oxopyrrolidine-2-carboxylic acid Chemical compound OC(=O)[C@@H]1CCC(=O)N1.CC1=NC=C(CO)C(CO)=C1O RYKKQQUKJJGFMN-HVDRVSQOSA-N 0.000 description 102
- 238000012360 testing method Methods 0.000 description 87
- 230000000694 effects Effects 0.000 description 76
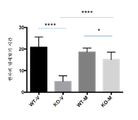
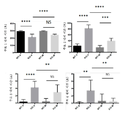
- 102000007665 Extracellular Signal-Regulated MAP Kinases Human genes 0.000 description 68
- 239000000523 sample Substances 0.000 description 33
- 241000699666 Mus <mouse, genus> Species 0.000 description 26
- 239000000090 biomarker Substances 0.000 description 24
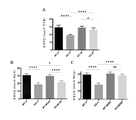
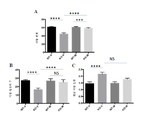
- 230000006399 behavior Effects 0.000 description 17
- 230000036541 health Effects 0.000 description 17
- 210000004556 brain Anatomy 0.000 description 15
- 230000003750 conditioning effect Effects 0.000 description 15
- 238000011813 knockout mouse model Methods 0.000 description 15
- 230000026731 phosphorylation Effects 0.000 description 14
- 238000006366 phosphorylation reaction Methods 0.000 description 14
- 230000004036 social memory Effects 0.000 description 13
- 208000019901 Anxiety disease Diseases 0.000 description 12
- 230000006735 deficit Effects 0.000 description 12
- 230000007613 environmental effect Effects 0.000 description 12
- 208000013403 hyperactivity Diseases 0.000 description 12
- 206010003805 Autism Diseases 0.000 description 11
- 208000020706 Autistic disease Diseases 0.000 description 11
- 238000004458 analytical method Methods 0.000 description 11
- 230000036506 anxiety Effects 0.000 description 11
- 210000001320 hippocampus Anatomy 0.000 description 11
- 238000005259 measurement Methods 0.000 description 11
- 210000002569 neuron Anatomy 0.000 description 11
- 230000008859 change Effects 0.000 description 10
- 238000002474 experimental method Methods 0.000 description 10
- 230000006870 function Effects 0.000 description 10
- 230000015654 memory Effects 0.000 description 10
- 101150086096 Eif2ak3 gene Proteins 0.000 description 9
- 210000004027 cell Anatomy 0.000 description 9
- 230000035772 mutation Effects 0.000 description 9
- 108090000623 proteins and genes Proteins 0.000 description 9
- 230000014616 translation Effects 0.000 description 9
- 208000028698 Cognitive impairment Diseases 0.000 description 8
- 238000001243 protein synthesis Methods 0.000 description 8
- 230000035945 sensitivity Effects 0.000 description 8
- 208000024891 symptom Diseases 0.000 description 8
- 238000004422 calculation algorithm Methods 0.000 description 7
- 230000000971 hippocampal effect Effects 0.000 description 7
- 238000007912 intraperitoneal administration Methods 0.000 description 7
- 238000012706 support-vector machine Methods 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 230000003542 behavioural effect Effects 0.000 description 6
- 230000008014 freezing Effects 0.000 description 6
- 238000007710 freezing Methods 0.000 description 6
- 230000002269 spontaneous effect Effects 0.000 description 6
- 230000002159 abnormal effect Effects 0.000 description 5
- 238000000540 analysis of variance Methods 0.000 description 5
- 208000029560 autism spectrum disease Diseases 0.000 description 5
- 208000035475 disorder Diseases 0.000 description 5
- 239000003205 fragrance Substances 0.000 description 5
- 210000001577 neostriatum Anatomy 0.000 description 5
- 210000002442 prefrontal cortex Anatomy 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 238000007637 random forest analysis Methods 0.000 description 5
- 238000012549 training Methods 0.000 description 5
- 108010032606 Fragile X Mental Retardation Protein Proteins 0.000 description 4
- 102000007338 Fragile X Mental Retardation Protein Human genes 0.000 description 4
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 4
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 4
- 208000026139 Memory disease Diseases 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 239000012491 analyte Substances 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- 238000004364 calculation method Methods 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 238000011156 evaluation Methods 0.000 description 4
- 239000005090 green fluorescent protein Substances 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- 238000012544 monitoring process Methods 0.000 description 4
- 235000019645 odor Nutrition 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 238000012502 risk assessment Methods 0.000 description 4
- 229960001534 risperidone Drugs 0.000 description 4
- RAPZEAPATHNIPO-UHFFFAOYSA-N risperidone Chemical compound FC1=CC=C2C(C3CCN(CC3)CCC=3C(=O)N4CCCCC4=NC=3C)=NOC2=C1 RAPZEAPATHNIPO-UHFFFAOYSA-N 0.000 description 4
- 206010010904 Convulsion Diseases 0.000 description 3
- 101000828537 Homo sapiens Synaptic functional regulator FMR1 Proteins 0.000 description 3
- 206010022998 Irritability Diseases 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 3
- 208000036626 Mental retardation Diseases 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 230000006978 adaptation Effects 0.000 description 3
- 239000012472 biological sample Substances 0.000 description 3
- 230000001149 cognitive effect Effects 0.000 description 3
- 238000010276 construction Methods 0.000 description 3
- 230000007812 deficiency Effects 0.000 description 3
- 238000002405 diagnostic procedure Methods 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 238000000684 flow cytometry Methods 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 239000013074 reference sample Substances 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 230000000007 visual effect Effects 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 208000027534 Emotional disease Diseases 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 201000006347 Intellectual Disability Diseases 0.000 description 2
- 229920005439 Perspex® Polymers 0.000 description 2
- 238000000692 Student's t-test Methods 0.000 description 2
- 102100023532 Synaptic functional regulator FMR1 Human genes 0.000 description 2
- 206010044565 Tremor Diseases 0.000 description 2
- 210000001766 X chromosome Anatomy 0.000 description 2
- 210000000683 abdominal cavity Anatomy 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 230000016571 aggressive behavior Effects 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 238000013528 artificial neural network Methods 0.000 description 2
- 210000004958 brain cell Anatomy 0.000 description 2
- 230000005754 cellular signaling Effects 0.000 description 2
- 230000002490 cerebral effect Effects 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 238000007635 classification algorithm Methods 0.000 description 2
- 238000004891 communication Methods 0.000 description 2
- 238000003066 decision tree Methods 0.000 description 2
- 210000001787 dendrite Anatomy 0.000 description 2
- 238000004332 deodorization Methods 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 210000004295 hippocampal neuron Anatomy 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000013383 initial experiment Methods 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 239000002207 metabolite Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 238000010172 mouse model Methods 0.000 description 2
- 230000001537 neural effect Effects 0.000 description 2
- 238000010606 normalization Methods 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 239000004926 polymethyl methacrylate Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000004043 responsiveness Effects 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 230000003997 social interaction Effects 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 230000001360 synchronised effect Effects 0.000 description 2
- 239000002023 wood Substances 0.000 description 2
- USSIQXCVUWKGNF-UHFFFAOYSA-N 6-(dimethylamino)-4,4-diphenylheptan-3-one Chemical compound C=1C=CC=CC=1C(CC(C)N(C)C)(C(=O)CC)C1=CC=CC=C1 USSIQXCVUWKGNF-UHFFFAOYSA-N 0.000 description 1
- 240000005020 Acaciella glauca Species 0.000 description 1
- 206010001488 Aggression Diseases 0.000 description 1
- 208000024827 Alzheimer disease Diseases 0.000 description 1
- 208000000044 Amnesia Diseases 0.000 description 1
- 102000007592 Apolipoproteins Human genes 0.000 description 1
- 108010071619 Apolipoproteins Proteins 0.000 description 1
- 206010003591 Ataxia Diseases 0.000 description 1
- 208000006096 Attention Deficit Disorder with Hyperactivity Diseases 0.000 description 1
- 206010063659 Aversion Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 206010008025 Cerebellar ataxia Diseases 0.000 description 1
- 206010012289 Dementia Diseases 0.000 description 1
- 208000012239 Developmental disease Diseases 0.000 description 1
- 208000027776 Extrapyramidal disease Diseases 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 208000018522 Gastrointestinal disease Diseases 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 208000030979 Language Development disease Diseases 0.000 description 1
- 108091026898 Leader sequence (mRNA) Proteins 0.000 description 1
- 102000019149 MAP kinase activity proteins Human genes 0.000 description 1
- 108040008097 MAP kinase activity proteins Proteins 0.000 description 1
- 208000016285 Movement disease Diseases 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 206010039424 Salivary hypersecretion Diseases 0.000 description 1
- 208000033712 Self injurious behaviour Diseases 0.000 description 1
- 206010041243 Social avoidant behaviour Diseases 0.000 description 1
- 206010041349 Somnolence Diseases 0.000 description 1
- 238000003639 Student–Newman–Keuls (SNK) method Methods 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- 208000021017 Weight Gain Diseases 0.000 description 1
- 101150003160 X gene Proteins 0.000 description 1
- 230000001594 aberrant effect Effects 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 208000012761 aggressive behavior Diseases 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 239000000935 antidepressant agent Substances 0.000 description 1
- 229940005513 antidepressants Drugs 0.000 description 1
- 239000000164 antipsychotic agent Substances 0.000 description 1
- 229940005529 antipsychotics Drugs 0.000 description 1
- 230000007529 anxiety like behavior Effects 0.000 description 1
- 101150010487 are gene Proteins 0.000 description 1
- 239000003693 atypical antipsychotic agent Substances 0.000 description 1
- 231100000871 behavioral problem Toxicity 0.000 description 1
- 238000013542 behavioral therapy Methods 0.000 description 1
- 238000009227 behaviour therapy Methods 0.000 description 1
- 238000012742 biochemical analysis Methods 0.000 description 1
- 238000010876 biochemical test Methods 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- 230000004641 brain development Effects 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 210000005056 cell body Anatomy 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 208000026106 cerebrovascular disease Diseases 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 238000013145 classification model Methods 0.000 description 1
- 231100000870 cognitive problem Toxicity 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 231100000867 compulsive behavior Toxicity 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 230000000875 corresponding effect Effects 0.000 description 1
- 238000002790 cross-validation Methods 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 238000013211 curve analysis Methods 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 208000010643 digestive system disease Diseases 0.000 description 1
- 208000002173 dizziness Diseases 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 230000002996 emotional effect Effects 0.000 description 1
- DNJIEGIFACGWOD-UHFFFAOYSA-N ethanethiol Chemical compound CCS DNJIEGIFACGWOD-UHFFFAOYSA-N 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 230000007661 gastrointestinal function Effects 0.000 description 1
- 208000018685 gastrointestinal system disease Diseases 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 230000003370 grooming effect Effects 0.000 description 1
- 208000035474 group of disease Diseases 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 230000009610 hypersensitivity Effects 0.000 description 1
- 238000005286 illumination Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 206010021654 increased appetite Diseases 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000013101 initial test Methods 0.000 description 1
- 238000011221 initial treatment Methods 0.000 description 1
- 230000003933 intellectual function Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 230000000366 juvenile effect Effects 0.000 description 1
- 208000011977 language disease Diseases 0.000 description 1
- 201000003723 learning disability Diseases 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 238000002595 magnetic resonance imaging Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 238000010339 medical test Methods 0.000 description 1
- 230000006984 memory degeneration Effects 0.000 description 1
- 206010027175 memory impairment Diseases 0.000 description 1
- 208000023060 memory loss Diseases 0.000 description 1
- 230000003340 mental effect Effects 0.000 description 1
- 229960001797 methadone Drugs 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 230000037125 natural defense Effects 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 238000003909 pattern recognition Methods 0.000 description 1
- 208000020861 perceptual disease Diseases 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 239000000902 placebo Substances 0.000 description 1
- 229940068196 placebo Drugs 0.000 description 1
- 210000002381 plasma Anatomy 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 102000054765 polymorphisms of proteins Human genes 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 238000007828 protein synthesis assay Methods 0.000 description 1
- -1 protein- Substances 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 235000003499 redwood Nutrition 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 208000026451 salivation Diseases 0.000 description 1
- 201000000980 schizophrenia Diseases 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 230000011273 social behavior Effects 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 230000003238 somatosensory effect Effects 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 230000003956 synaptic plasticity Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 230000002123 temporal effect Effects 0.000 description 1
- 210000003478 temporal lobe Anatomy 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000002747 voluntary effect Effects 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 210000004885 white matter Anatomy 0.000 description 1
- 230000003936 working memory Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4415—Pyridoxine, i.e. Vitamin B6
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/4015—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil having oxo groups directly attached to the heterocyclic ring, e.g. piracetam, ethosuximide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6893—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to diseases not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Urology & Nephrology (AREA)
- Molecular Biology (AREA)
- Hematology (AREA)
- Food Science & Technology (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Biotechnology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Physics & Mathematics (AREA)
- Pathology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Medicines Containing Plant Substances (AREA)
- Steroid Compounds (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361875384P | 2013-09-09 | 2013-09-09 | |
| US61/875,384 | 2013-09-09 | ||
| US14/038,258 US20150073023A1 (en) | 2013-09-09 | 2013-09-26 | Method Of Treating Fragile X Syndrome And Related Disorders |
| US14/038,258 | 2013-09-26 | ||
| US201461991351P | 2014-05-09 | 2014-05-09 | |
| US61/991,351 | 2014-05-09 | ||
| PCT/US2014/054816 WO2015035402A1 (en) | 2013-09-09 | 2014-09-09 | Methods of determining response to therapy |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160086818A true KR20160086818A (ko) | 2016-07-20 |
Family
ID=52629036
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167009042A Withdrawn KR20160086818A (ko) | 2013-09-09 | 2014-09-09 | 요법에 대한 반응의 결정 방법 |
| KR1020167009040A Withdrawn KR20160078956A (ko) | 2013-09-09 | 2014-09-09 | 취약 x 증후군 및 관련 장애의 치료 방법 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167009040A Withdrawn KR20160078956A (ko) | 2013-09-09 | 2014-09-09 | 취약 x 증후군 및 관련 장애의 치료 방법 |
Country Status (12)
| Country | Link |
|---|---|
| EP (2) | EP3043792A2 (enExample) |
| JP (2) | JP2016530291A (enExample) |
| KR (2) | KR20160086818A (enExample) |
| CN (2) | CN105917225A (enExample) |
| AU (2) | AU2014315026A1 (enExample) |
| CA (2) | CA2923421A1 (enExample) |
| EA (2) | EA201690559A1 (enExample) |
| IL (2) | IL244343A0 (enExample) |
| MX (2) | MX2016003002A (enExample) |
| SG (2) | SG11201601830PA (enExample) |
| TW (2) | TW201605443A (enExample) |
| WO (2) | WO2015033224A2 (enExample) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109021022B (zh) * | 2013-07-31 | 2021-04-16 | Udc 爱尔兰有限责任公司 | 发光的二氮杂苯并咪唑碳烯金属配合物 |
| US20150073023A1 (en) | 2013-09-09 | 2015-03-12 | Alcobra Ltd. | Method Of Treating Fragile X Syndrome And Related Disorders |
| CN108538365A (zh) * | 2017-03-24 | 2018-09-14 | 华东师范大学 | 一种基于数据分析技术的自闭症社交能力评估系统 |
| WO2019197632A1 (en) * | 2018-04-13 | 2019-10-17 | Healx Limited | Treatment of fragile x syndrome |
| KR20190121569A (ko) * | 2018-04-18 | 2019-10-28 | 건국대학교 글로컬산학협력단 | 아그마틴 및 이의 약학적으로 허용 가능한 염을 포함하는 취약 x 증후군 예방 또는 치료용 약학조성물 |
| PL3813816T3 (pl) * | 2018-06-07 | 2023-07-31 | Ovid Therapeutics Inc. | Zastosowanie kwasu (s)-3-amino-4-(difluorometylenylo)cyklopentano-1-eno-1-karboksylowego i związków pokrewnych, kwasu (1s,3s)-3-amino-4-(difluorometylideno)cyklopentano-1-karboksylowego w leczeniu zespołu łamliwego chromosomu x lub zespołu drżenia/ataksji związanego z zespołem łamliwego chromosomu x |
| CN115335053B (zh) * | 2020-01-08 | 2024-08-09 | 株式会社纽若梵提 | 包含麦角乙脲化合物作为有效成分用于治疗脆性x染色体综合征或相关发育障碍的组合物 |
| CN115397414A (zh) * | 2020-02-07 | 2022-11-25 | 株式会社纽若梵提 | 包含利美尼定的用于治疗脆性x染色体综合征的组合物 |
| EP4171528A1 (en) * | 2020-06-29 | 2023-05-03 | Zynerba Pharmaceuticals, Inc. | Treatment of fragile x syndrome with cannabidiol |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IT1131856B (it) | 1980-06-30 | 1986-06-25 | Baldacci Lab Spa | Composizione farmaceutica per il trattamento di intossicazioni alcooliche |
| US20020155170A1 (en) * | 2000-11-30 | 2002-10-24 | Walsh William John | Nutrient supplements and methods for treating autism and for preventing the onset of autism |
| IL187159A0 (en) | 2007-07-03 | 2009-02-11 | Gur Megiddo | Use of metadoxine in relief of alcohol intoxication |
| WO2010150261A1 (en) * | 2009-06-25 | 2010-12-29 | Alcobra Ltd. | A method for the treatment, alleviation of symptoms of, relieving, improving and preventing a cognitive disease, disorder or condition |
-
2014
- 2014-09-05 TW TW103130856A patent/TW201605443A/zh unknown
- 2014-09-05 TW TW103130859A patent/TW201606304A/zh unknown
- 2014-09-09 AU AU2014315026A patent/AU2014315026A1/en not_active Abandoned
- 2014-09-09 MX MX2016003002A patent/MX2016003002A/es unknown
- 2014-09-09 WO PCT/IB2014/002398 patent/WO2015033224A2/en not_active Ceased
- 2014-09-09 EA EA201690559A patent/EA201690559A1/ru unknown
- 2014-09-09 WO PCT/US2014/054816 patent/WO2015035402A1/en not_active Ceased
- 2014-09-09 CA CA2923421A patent/CA2923421A1/en not_active Abandoned
- 2014-09-09 KR KR1020167009042A patent/KR20160086818A/ko not_active Withdrawn
- 2014-09-09 CA CA2922901A patent/CA2922901A1/en not_active Abandoned
- 2014-09-09 KR KR1020167009040A patent/KR20160078956A/ko not_active Withdrawn
- 2014-09-09 EP EP14830858.8A patent/EP3043792A2/en not_active Withdrawn
- 2014-09-09 SG SG11201601830PA patent/SG11201601830PA/en unknown
- 2014-09-09 JP JP2016539645A patent/JP2016530291A/ja active Pending
- 2014-09-09 EA EA201690557A patent/EA201690557A1/ru unknown
- 2014-09-09 CN CN201480060722.4A patent/CN105917225A/zh active Pending
- 2014-09-09 AU AU2014316779A patent/AU2014316779A1/en not_active Abandoned
- 2014-09-09 CN CN201480049671.5A patent/CN105517546A/zh active Pending
- 2014-09-09 JP JP2016540930A patent/JP2016530536A/ja active Pending
- 2014-09-09 MX MX2016003006A patent/MX2016003006A/es unknown
- 2014-09-09 SG SG11201601605YA patent/SG11201601605YA/en unknown
- 2014-09-09 EP EP14776939.2A patent/EP3044589A1/en not_active Withdrawn
-
2016
- 2016-02-29 IL IL244343A patent/IL244343A0/en unknown
- 2016-03-06 IL IL244453A patent/IL244453A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| WO2015033224A2 (en) | 2015-03-12 |
| EA201690557A1 (ru) | 2016-07-29 |
| CN105917225A (zh) | 2016-08-31 |
| TW201606304A (zh) | 2016-02-16 |
| SG11201601605YA (en) | 2016-04-28 |
| EA201690559A1 (ru) | 2016-08-31 |
| EP3044589A1 (en) | 2016-07-20 |
| IL244453A0 (en) | 2016-04-21 |
| KR20160078956A (ko) | 2016-07-05 |
| CN105517546A (zh) | 2016-04-20 |
| JP2016530291A (ja) | 2016-09-29 |
| AU2014315026A1 (en) | 2016-03-24 |
| WO2015035402A1 (en) | 2015-03-12 |
| CA2923421A1 (en) | 2015-03-12 |
| MX2016003006A (es) | 2016-06-10 |
| JP2016530536A (ja) | 2016-09-29 |
| WO2015033224A3 (en) | 2015-07-02 |
| MX2016003002A (es) | 2016-09-08 |
| IL244343A0 (en) | 2016-04-21 |
| CA2922901A1 (en) | 2015-03-12 |
| EP3043792A2 (en) | 2016-07-20 |
| TW201605443A (zh) | 2016-02-16 |
| AU2014316779A1 (en) | 2016-03-17 |
| SG11201601830PA (en) | 2016-04-28 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20160086818A (ko) | 요법에 대한 반응의 결정 방법 | |
| Stessman et al. | Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases | |
| Fromer et al. | Gene expression elucidates functional impact of polygenic risk for schizophrenia | |
| Labonté et al. | Sex-specific transcriptional signatures in human depression | |
| Ozkul et al. | A heritable profile of six miRNAs in autistic patients and mouse models | |
| Lucariello et al. | Whole exome sequencing of Rett syndrome-like patients reveals the mutational diversity of the clinical phenotype | |
| Pramparo et al. | Cell cycle networks link gene expression dysregulation, mutation, and brain maldevelopment in autistic toddlers | |
| Hu et al. | Mutations in PTRH2 cause novel infantile‐onset multisystem disease with intellectual disability, microcephaly, progressive ataxia, and muscle weakness | |
| Frick et al. | Serum proteomics reveal APOE-ε4-dependent and APOE-ε4-independent protein signatures in Alzheimer’s disease | |
| Stradomska et al. | Serum very long-chain fatty acids (VLCFA) levels as predictive biomarkers of diseases severity and probability of survival in peroxisomal disorders | |
| Benedetti et al. | Social behavior in 16p11. 2 and 22q11. 2 copy number variations: Insights from mice and humans | |
| Tan et al. | Mouse models as a tool for discovering new neurological diseases | |
| Kamath et al. | A molecular census of midbrain dopaminergic neurons in Parkinson’s disease | |
| Kaare et al. | Depression-associated Negr1 gene-deficiency induces alterations in the monoaminergic neurotransmission enhancing time-dependent sensitization to amphetamine in male mice | |
| Logan et al. | Cognitive heterogeneity reveals molecular signatures of age-related impairment | |
| Corvin et al. | The cognitive genetics of neuropsychiatric disorders | |
| Lu et al. | Eighteen-year-old man with autism, obsessive compulsive disorder and a SHANK2 variant presents with severe anorexia that responds to high-dose fluoxetine | |
| Reiling et al. | The association of mitochondrial content with prevalent and incident type 2 diabetes | |
| US9851355B2 (en) | Methods of determining response to therapy | |
| Zhuang et al. | Mega-analysis of gene expression in mouse models of Alzheimer’s Disease | |
| Hengel et al. | Heterozygous RAB3A variants cause cerebellar ataxia by a partial loss-of-function mechanism | |
| Rasmussen et al. | Aging, longevity and health | |
| Wang et al. | Trajectory of plasma lipidomes associated with the risk of late-onset Alzheimer’s disease pathogenesis: a longitudinal study in the ADNI cohort | |
| Tansley et al. | Single-cell RNA sequencing reveals time-and sex-specific responses of spinal cord microglia to peripheral nerve injury and links ApoE to neuropathic pain | |
| JP2023506637A (ja) | 双極性障害の診断及び治療におけるSynaptotagmin-7の使用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20160406 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |