EP3334848B1 - Verfahren zur rückgewinnung von metallhaltigem material aus einem verbundwerkstoff - Google Patents
Verfahren zur rückgewinnung von metallhaltigem material aus einem verbundwerkstoff Download PDFInfo
- Publication number
- EP3334848B1 EP3334848B1 EP16836271.3A EP16836271A EP3334848B1 EP 3334848 B1 EP3334848 B1 EP 3334848B1 EP 16836271 A EP16836271 A EP 16836271A EP 3334848 B1 EP3334848 B1 EP 3334848B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- metal
- reductant
- product
- composite material
- matrix
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B5/00—General methods of reducing to metals
- C22B5/02—Dry methods smelting of sulfides or formation of mattes
- C22B5/04—Dry methods smelting of sulfides or formation of mattes by aluminium, other metals or silicon
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/05—Metallic powder characterised by the size or surface area of the particles
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B21/00—Obtaining aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B21/00—Obtaining aluminium
- C22B21/02—Obtaining aluminium with reducing
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B34/00—Obtaining refractory metals
- C22B34/10—Obtaining titanium, zirconium or hafnium
- C22B34/12—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B34/00—Obtaining refractory metals
- C22B34/10—Obtaining titanium, zirconium or hafnium
- C22B34/12—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08
- C22B34/1263—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08 obtaining metallic titanium from titanium compounds, e.g. by reduction
- C22B34/1268—Obtaining titanium or titanium compounds from ores or scrap by metallurgical processing; preparation of titanium compounds from other titanium compounds see C01G23/00 - C01G23/08 obtaining metallic titanium from titanium compounds, e.g. by reduction using alkali or alkaline-earth metals or amalgams
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B34/00—Obtaining refractory metals
- C22B34/20—Obtaining niobium, tantalum or vanadium
- C22B34/22—Obtaining vanadium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B9/00—General processes of refining or remelting of metals; Apparatus for electroslag or arc remelting of metals
- C22B9/02—Refining by liquating, filtering, centrifuging, distilling, or supersonic wave action including acoustic waves
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B9/00—General processes of refining or remelting of metals; Apparatus for electroslag or arc remelting of metals
- C22B9/04—Refining by applying a vacuum
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2301/00—Metallic composition of the powder or its coating
- B22F2301/20—Refractory metals
- B22F2301/205—Titanium, zirconium or hafnium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2304/00—Physical aspects of the powder
- B22F2304/10—Micron size particles, i.e. above 1 micrometer up to 500 micrometer
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P10/00—Technologies related to metal processing
- Y02P10/20—Recycling
Definitions
- the present invention relates to a method for the recovery of a metal-containing product (M Prod ) from a composite material .
- WO 2006/042360 provides a method for producing titanium by reaction of titanium tetrachloride with magnesium in a reactor, which may comprise a fluidised bed.
- the temperature in the reactor is above the melting point of magnesium, but below the melting point of magnesium chloride.
- the method produces particles comprising titanium which are removed from the reactor and processed in order to recover titanium particles generally having a particle size of greater than 500 ⁇ m.
- the method of WO 2006/042360 is operated under an excess of magnesium with unreacted magnesium optionally collected and recycled to the reactor. This is understood to achieve complete conversion of TiCl 4 to titanium metal, while avoiding the formation of sub-chlorides, TiCl 2 and TiCl 3 .
- US 5 078 789 A discloses a process of vacuum distillation of sponge refractory metals, such as zirconium and/or hafnium and titanium that were produced by reduction of the metal tetrachloride with magnesium, so as to remove both unreacted magnesium and the magnesium chloride produced during the reaction.
- WO 2008/067614 A1 discloses a method for recovering a metal from composite particles comprising particles of the metal to be recovered dispersed in a matrix of another metal salt.
- the method includes mixing the composite particles in a molten salt which has a melt temperature that is (i) at least equal to the melting point of the another metal salt, (ii) below the boiling point of the another metal salt and (iii) below the melting point of the metal to be recovered.
- the particles of the metal to be recovered are consolidated and allowed to settle under gravity.
- KR 2012 0074132 A appears to disclose a method and an apparatus for reacting magnesium and titanium tetrachloride to continuously produce titanium sponge and magnesium chloride.
- the applicant has identified methods for producing composite materials from at least one metal compound in which an excess of oxidant is fed to the reactor during processing.
- the composite material will generally be in finely divided form and the method, generally, does not place significant weight on the exclusion of by-products in the composite material.
- the methods for the production of the composite material are described in detail in a co-pending international patent application with the title "METHOD FOR THE PRODUCTION OF A COMPOSITE MATERIAL USING EXCESS OXIDANT", filed on the same date as the present application. The contents of the co-pending application are incorporated herein in their entirety.
- M Prod metal-containing product
- product metal will be used to describe a product that is a metal, an alloy or an inter-metallic. That is, the term “product metal” as used herein is intended to include within its scope product comprising (i) one metallic element, (ii) two or more metallic elements, or (iii) one or more metallic elements together with one or more non-metallic elements.
- the one or more metal compounds (M P C R ) of the product metal (M P ) in one or more oxidation states may comprise at least two metal halides. If so, the metal halides may be preferably selected from the group consisting of halides of titanium, aluminium and vanadium.
- the composite material is formed by contacting Mg with an excess of TiCl 4 in a fluidised bed reactor to form Ti metal dispersed in a MgCl 2 matrix
- TiCl 4 may react with one atom of Mg and produce MgCl 2 and TiCl 2 .
- Mg reacts with TiCl 2 and forms a second MgCl 2 and a single Ti atom. Therefore, at its limit, it is envisaged that the finely divided metal component (M P ) may be present in the protective matrix of MgCl 2 on an atomic scale. Such examples would represent true "primary particles" of the metal component (M P ).
- the metal component (M P ) of these preferred embodiments of the invention is the lack of a protective oxide layer.
- the metal component (M P ) particles of these embodiments do not have an activation barrier, which correlates with a lower activation energy (increase in reactivity) of the metal component (M P ).
- generally small particles are highly pyrophoric.
- the composite material of the preferred embodiments of the invention is, comparatively, not. For conventional metal powders of approximately ⁇ 10um, pyrophoricity becomes a major issue, but can be serious even at much larger sizes (>100um) under some conditions.
- the protective matrix of the composite material of the invention advantageously overcomes this issue.
- the composite material may be combined with any one or more of the groups consisting of beryllium, boron, carbon, nitrogen, oxygen, aluminium, silicon, phosphorous, sulphur, scandium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc, gallium, germanium, arsenic, selenium, yttrium, zirconium, niobium, molybdenum, ruthenium, rhodium, palladium, silver, cadmium, indium, tin, antimony, tellurium, hafnium, tantalum, tungsten, rhenium, osmium, iridium, platinum, gold, lead, bismuth, the Rare Earths and compounds thereof.
- the groups consisting of beryllium, boron, carbon, nitrogen, oxygen, aluminium, silicon, phosphorous, sulphur, scandium, vanadium, chromium, manganese, iron, cobalt, nickel
- the metal-containing product (M Prod ) may consist of the product metal (M P ) and the method may comprise recovering the product metal (M P ) from the composite material.
- the method may further comprise a post-treatment of the recovered product metal (M P ).
- the post-treatment comprises, milling, grinding, coating, pressing, heat treating (e.g. aging, annealing, quenching, tempering), rolling, forming, casting, hot or cold isostatic pressing (HIPing or CIP), moulding, melting, sintering, blending, extruding, drawing, forging, turning, welding, atomising and/or spraying.
- the method may further comprise pre-treating the composite material prior to the treatment step.
- the pre-treatment may comprise at least one of compacting, milling and grinding the composite material.
- the metal-containing product (M Prod ) formed according to the invention may comprise particulate metal having a particle size of less than 500 ⁇ m, preferably from 20-300 ⁇ m, or may comprise ingots of the product metal (M P ).
- the product metal (M P ) is an alloy, for example an alloy of two or more of titanium, vanadium and aluminium.
- the alloy may approximate Ti64.
- Ti64 alloy generally refers to an alloy having a chemical composition of 6% aluminium, 4% vanadium, 0.25% (maximum) iron, 0.2% (maximum) oxygen, and the remainder titanium. Ti64 is also commonly referred to as Grade 5 titanium.
- palladium may be incorporated into the composite material to facilitate recovery of Grade 7 titanium.
- Grade 7 titanium contains 0.12 to 0.25% palladium. The small quantity of palladium provides enhanced crevice corrosion resistance at low temperatures and high pH.
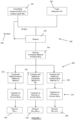
- metal compound (M P C) 110 of a product metal (M P ) and a reductant (R) 120 capable of reducing the metal compound (M P C) 110 of the product metal (M P ) are supplied to a reactor 130.
- the amount of metal compound (M P C) 110 supplied to the reactor 130, including any recycled metal compound (M P C) 140, is in excess relative to the amount of reductant 120 supplied to the reactor 130.
- Composite material 150 is recovered from the reactor 130.
- the composite material comprises a matrix of oxidised reductant (R o ), product metal (M P ) dispersed in the matrix of oxidised reductant (R o ), and one or more metal compounds (M P C R ) of the product metal (M P ) in one or more oxidation states dispersed in the matrix of oxidised reductant (R o ).
- the composite material may further comprise reductant (R).
- the prevailing conditions in the reactor 130 ensure, with sufficient time, the melting of the reductant 120.
- the time required for melting of solid reductant 120 depends upon numerous factors, including the feed mechanism, whether the reductant 120 is fed with other materials, the temperature of the reactor 130, the reaction intensity of the reactor 130 per unit volume, the particulate density of the reductant 120 feed at any single location and, if other reductant or reagent or inert streams are in or are entering into the reactor, the proximity to these components and their respective temperatures when impinging on particles of the reductant 120.
- the interaction of the reductant (R) 120 upon contacting other surfaces in the reactor 130 will depend on its phase at that time. If the reductant 120 particle is solid, it is possible the reductant 120 particle will collide and rebound. It will then continue to interact with other surfaces and environments in the reactor 130.
- the reductant 120 particle is molten when it interacts with other surfaces, it may wet the surface. Depending upon the nature of the solid-liquid interaction the thickness of the layer formed will vary. It is considered that this may be manipulated through varying intensity of interactions, density of reductant 120 feed, temperature and time, etc.
- molten reductant in the reactor 130 is as a standalone mass, wetted on a surface or combined with other surfaces, at some point it will interact with oxidant and react. At this point the thickness or the wetted layer or size of the molten mass or particle is considered of some importance in determining the extent of reaction of the reductant (R) 120 and the morphology of the final composite material 150.
- the amount of oxidant in the reactor relative to reductant (R) will be an important factor in determining the probability of the above mentioned interactions.
- the present invention relates to a method 200 for the recovery of a metal-containing product (M Prod ) from a composite material 150.
- the method comprises the recovery of the product metal (M P ) directly from the composite material 210, or may comprise recovery after combining the composite material with composite material of other product metal (M P' ) 220, and/or other compounding material (C M ) 230.
- various products may be recovered, including without limitation a metal-containing product (M Prod ) 240, an alloy or mixture of metal-containing product (M Prod /M Prod' ) 250, and a mixture or composite metal-containing product (M Prod /C M ) 260.
- reductant (R) Once a desired composite material is formulated (i.e. with or without additional material), it is treated 270 to recover the metal-containing product 240, 250, 260.
- Treatment 270 of the composite material aim to at least partially remove the one or more metal compounds (M P C R ) from the matrix of oxidised reductant (R o ) to form the metal-containing product (M Prod ).
- the aim of the treatment step 270 is to substantially completely remove the one or more metal compounds (M P C R ) from the matrix of oxidised reductant (R o ).
- two methods of treatment 270 are particularly useful for the removal of the one or more metal compounds (M P C R ) from the matrix of oxidised reductant (R o ). These include distillation, particularly vacuum distillation, and reduction of the one or more metal compounds (M P C R ) to product metal (M P ) in the presence of a reductant. These two options may be performed independently, or in combination. It will be appreciated, though, that other suitable options may be employed.
- the treatment step 270 may suitably comprise melting the composite material and subsequent separation of the one or more metal compounds (M P C R ).
- the treatment step 270 may also result in the removal of the matrix of oxidised reductant (R o ) from the composite material.
- the one or more metal compounds (M P C R ) and the oxidised reductant (R o ) may be removed from the composite material by vacuum distillation.
- the treatment 270 may further result in the removal of reductant (R), if present, which may be recycled to the reductant feed 120, or be otherwise recovered.
- the metal-containing product 240, 250, 260 may consist of product metal (M P ) 240, an alloy or mixture of product metals (M P /M P' ) 250, and a mixture or composite (M P /C M ) 260.
- the finely divided nature of the product metal (M P ) in the composite material 150 is encased in materials chemically inert to itself by the nature of their generation.
- Product metal of similar compositional properties but without the same physical characteristics engendered by the encasement process will not respond to the same recovery process with analogous results. That is, the surface is free from any protective or passivation layer meaning that it will respond differently to physical interactions than its macroscopic bulk counterpart.
- the individual components of product metal encased in inert material provide for building blocks that may or may not combine to various extents and by different driving forces through the process of recovery to yield the product metal (M P ).
- the conditions under which the liberation of the product metal (M P ) from the composite matrix proceeds and the conditions throughout the recovery process have a significantly determinative impact on the way in which each divided product metal building block interacts with others, and ultimately on the manner in which they may or may not combine and the morphology and microstructure of the recovered product metal.
- Example 1 - CP2 Titanium recovery from a predominantly MgCl 2 matrix
- the vessel was purged with air and the remnant material was recovered from the vessel, comprising approximately 5g of titanium metal.
- the metal was in the form of loosely sintered spheres with size approximately one half of the particulate size of the composite material fed into the vessel.
- the recovery process produced commercially pure grade 2 titanium.
- 15g of composite material, black in colour, in spherical particulate form comprising a matrix of magnesium chloride, titanium metal, aluminium metal, magnesium and quantities of titanium sub-halides (TiCl 2 and TiCl 3 ) was combined with 15g of composite material, black in colour, in spherical particulate form comprising a matrix of magnesium chloride, titanium metal, vanadium metal, magnesium and quantities of titanium sub-halides (TiCl 2 and TiCl 3 ) and possibly vanadium sub-halides.
- the total of 30g of composite material was ground under inert conditions to form a homogeneous composition and then placed in a vessel made from stainless steel. The degree of milling could be altered for differing levels of uniformity.
- the vessel was placed under vacuum at a pressure of approximately 0.01kPa.
- An argon purge was supplied at a rate of 10 mg/min.
- the vessel was then heated externally to a temperature of 900 °C at a heating rate of 31 °C per minute.
- the vessel was then left at a temperature of 900 °C for one hour before being cooled to room temperature.
- the vessel was purged with air and the remnant material was recovered from the vessel, comprising approximately 5g of titanium, aluminium and vanadium containing metal in proportion with the sum of the product metal in the input composite material.
- the metal was in the form of closely packed sintered particles with irregular shape.
- Example 3 Liberation of product metal from predominantly MgCl 2 matrix at atmospheric conditions
- the contents of the vessel was recovered and found to be a white and silver coloured mass comprised of titanium metal and magnesium chloride. There was no appearance of green or violet colouring to indicate presence of titanium sub-halides in the salt phase.
- the mass was broken up and milled to a powder and returned to the stainless steel vessel.
- the vessel was placed under vacuum at a pressure of approximately 0.01kPa.
- An argon purge was supplied at a rate of 10 mg/min.
- the vessel was then heated externally to a temperature of 900 °C at a heating rate of 31 °C per minute.
- the vessel was then left at a temperature of 900 °C for one hour before being cooled to room temperature.
- the vessel was purged with air and the remnant material was recovered from the vessel, comprising approximately 5g of titanium metal.
- the product metal was liberated from the protective matrix and enabled to consolidate to a degree by sintering.
- melting of the matrix provides the opportunity for partially reduced or oxidised compounds to be liberated from the matrix structure and a significantly enhanced opportunity to interact and react with other compounds in the matrix, or be removed by boiling.
- composite material black in colour, in angular particulate form comprising a matrix of magnesium chloride, titanium metal, magnesium and quantities of titanium sub-halides (TiCl 2 and TiCl 3 ) was placed in an open alumina cup.
- the cup was placed in a vacuum furnace at a pressure of approximately 0.01kPa.
- An argon purge was supplied at a rate of 2 mg/min.
- the furnace was then heated to a temperature of 500 °C at a heating rate of 100 °C per minute, which under vacuum is sufficient for removing titanium and promoting the disproportionation of titanium sub-halides.
- the vessel was then left at a temperature of 500 °C for one hour before being cooled to room temperature.
- Figure 2 shows the loss of weight of the material over time in the process. It can be seen the weight stabilises after a short period.
- the remnant material was recovered from the vessel, comprising approximately 30mg of metal containing composite material with a substantially reduced sub-halide content.
- This composite could be put through further recovery processes, where the impact of the significant volatile sub-halide content would be reduced or eliminated. Such impacts could include significant increase in total volatile content, difficulty in controlling sub-halide conversion to metal or removal.
- a titanium composite containing significant sub-halide content was passed through vacuum at 600°C with a residence time of 2 hours.
- the feed composite comprised uniform black spheres with particle size of ⁇ 2mm.
- Figure 3 shows the total content of titanium and magnesium as determined by XRF of the feed material and several subsequent samples of the recovered material.
- Figure 4 shows a similar plot but separately shows the individual content of titanium and magnesium.
- the process reduces the mass ration of titanium to magnesium from 1.12 substantially towards the theoretical ratio for a two-phase mixture of titanium metal and magnesium chloride of 0.985. As such the stability and predictability of the recovered composite to subsequent processing will be increased.
- the cup was then placed in a vacuum furnace at a pressure of approximately 0.01kPa.
- An argon purge was supplied at a rate of 2 mg/min.
- the furnace was then heated to a temperature of 1100 °C at a heating rate of 10 °C per minute.
- the sample was shown to melt again at approximately 350°C. Surprisingly all volatiles were removed below 700°C, leaving titanium metal behind. This represents a significant reduction in the temperature required to recover the metal component based on the original composite composition.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Manufacturing & Machinery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Environmental & Geological Engineering (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Physics & Mathematics (AREA)
- Acoustics & Sound (AREA)
- Manufacture And Refinement Of Metals (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
Claims (13)
- Verfahren zur Rückgewinnung eines metallhaltigen Produkts (MProd), umfassendBereitstellen eines Verbundstoffs, der eine Matrix aus oxidiertem Reduktionsmittel (R0) eines Reduktionsmittels (R), ein Produktmetall (MP), das in der Matrix aus oxidiertem Reduktionsmittel (R0) dispergiert ist, eine oder mehrere Metallverbindungen (MPCR) des Produktmetalls (MP) in einem oder mehreren Oxidationszuständen, die in der Matrix aus oxidiertem Reduktionsmittel (R0) dispergiert ist bzw. sind, und das Reduktionsmittel (R) umfasst, wobei das Reduktionsmittel (R) bis zu 3 Gew.-% des Verbundstoffs umfasst; undBehandeln des Verbundstoffs, um die eine oder mehreren Metallverbindungen (MPCR) zumindest teilweise von der Matrix aus oxidiertem Reduktionsmittel (R0) zu entfernen, um das metallhaltige Produkt (MProd) zu bilden, wobei das Behandeln ausgewählt wird aus Destillieren, Reduzieren oder Schmelzen,wobei das Reduktionsmittel (R) ausgewählt wird aus der Gruppe, die besteht aus Mg, Na, K, Li, Ba, Ca, Be, Al und einer beliebigen Kombination davon und die eine oder mehreren Metallverbindungen (MPCR) des Produktmetalls (MP) in einem oder mehreren Oxidationszuständen ein Metallhalogenid ist bzw. sind, das bzw. die ausgewählt wird aus der Gruppe, die besteht aus Halogeniden von Titan, Aluminium, Vanadium, Chrom, Niob, Molybdän, Zirkon, Zinn, Hafnium, Eisen, Kupfer, Nickel, Wismut, Mangan, Palladium, Wolfram, Cadmium, Zink, Silber, Cobalt, Tantal, Ruthenium und den seltenen Erden oder einer Kombination von beliebigen zwei oder mehr davon.
- Verfahren nach Anspruch 1, wobei der Behandlungsschritt das Destillieren der einen oder mehreren Metallverbindungen (MPCR) von der Matrix aus oxidiertem Reduktionsmittel (R0) umfasst.
- Verfahren nach Anspruch 2, wobei die Destillation zu mindestens einem von (i) Verflüchtigung der einen oder mehreren Metallverbindungen (MPCR) und (ii) Reduktion der einen oder mehreren Metallverbindungen (MPCR) beim Vorhandensein eines Reduktionsmittels (R) zu dem Produktmetall (MP) führt.
- Verfahren nach Anspruch 2 oder Anspruch 3, wobei die eine oder mehreren Metallverbindungen (MPCR) und wahlweise das oxidierte Reduktionsmittel (R0) von dem Verbundstoff durch Vakuumdestillation entfernt werden.
- Verfahren nach Anspruch 4, wobei das oxidierte Reduktionsmittel (R0) Magnesiumchlorid umfasst und die Vakuumdestillation bei einer Temperatur von 700°C bis 950°C durchgeführt wird, und wobei das Produktmetall (MP) wahlweise mindestens Titan umfasst.
- Verfahren nach Anspruch 1, wobei der Behandlungsschritt das Reduzieren der einen oder mehreren Metallverbindungen (MPCR) des Produktmetalls (MP) in einem oder mehreren Oxidationszuständen zu dem Produktmetall (MP) beim Vorhandensein eines Reduktionsmittels (R) umfasst.
- Verfahren nach Anspruch 1, wobei der Behandlungsschritt das Schmelzen des Verbundstoffs und das Rückgewinnen des metallhaltigen Produkts (MProd) von der Schmelze umfasst.
- Verfahren nach Anspruch 7, wobei das Schmelzen bei einer Temperatur unterhalb der einzelnen Schmelztemperaturen jeder Komponente der Matrix durch Bildung einer untereutektischen oder übereutektischen Zusammensetzung, vorzugsweise einer eutektischen Zusammensetzung, durchgeführt wird.
- Verfahren nach Anspruch 8, wobei das Rückgewinnen des metallhaltigen Produkts (MProd) von der Schmelze das Unterwerfen der Schmelze Bedingungen, unter denen das Produktmetall (MP), die eine oder mehreren Metallverbindungen (MPCR) und das oxidierte Reduktionsmittel (R0) getrennte Schichten in der Schmelze bilden, und das Rückgewinnen der Schicht aus Produktmetall (MP) umfasst.
- Verfahren nach Anspruch 7, wobei das Rückgewinnen des metallhaltigen Produkts (MProd) von der Schmelze die Auflösung von Komponenten des Verbundstoffs, wie beispielsweise der einen oder mehreren Metallverbindungen (MPCR) und des oxidierten Reduktionsmittels (R0), umfasst.
- Verfahren nach einem der vorhergehenden Ansprüche, wobei die eine oder mehreren Metallverbindungen (MPCR) des Produktmetalls (MP) in einem oder mehreren Oxidationszuständen ein oder mehrere Metallhalogenide (MPX) des Produktmetalls (MP) umfassen.
- Verfahren nach einem der vorhergehenden Ansprüche, wobei der Verbundstoff in der Form von Partikeln vorliegt, die typischerweise eine durchschnittliche Partikelgröße von bis zu 500 µm, vorzugsweise von 20 bis 300 µm, aufweisen.
- Verfahren nach einem der vorhergehenden Ansprüche, wobei das metallhaltige Produkt (MProd) aus dem Produktmetall (MP) besteht und das Verfahren das Rückgewinnen des Produktmetalls (MP) von dem Verbundstoff und eine Nachbehandlung des rückgewonnenen Produktmetalls (MP) umfasst, wobei die Nachbehandlung Fräsen, Schleifen, Beschichten, Pressen, Wärmebehandlung (z. B. Altern, Glühen, Härten, Anlassen), Walzen, Umformen, Gießen, heißes oder kaltes isostatisches Pressen (HIP oder CIP), Formen, Schmelzen, Sintern, Mischen, Extrudieren, Ziehen, Schmieden, Drehen, Schweißen, Zerstäuben und/oder Sprühen umfasst.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2015903278A AU2015903278A0 (en) | 2015-08-14 | Method for recovery of metal-containing material from a composite material | |
| PCT/AU2016/050745 WO2017027914A1 (en) | 2015-08-14 | 2016-08-12 | Method for recovery of metal-containing material from a composite material |
Publications (4)
| Publication Number | Publication Date |
|---|---|
| EP3334848A1 EP3334848A1 (de) | 2018-06-20 |
| EP3334848A4 EP3334848A4 (de) | 2018-06-27 |
| EP3334848C0 EP3334848C0 (de) | 2025-07-09 |
| EP3334848B1 true EP3334848B1 (de) | 2025-07-09 |
Family
ID=58050397
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16836271.3A Active EP3334848B1 (de) | 2015-08-14 | 2016-08-12 | Verfahren zur rückgewinnung von metallhaltigem material aus einem verbundwerkstoff |
Country Status (9)
| Country | Link |
|---|---|
| US (3) | US11162157B2 (de) |
| EP (1) | EP3334848B1 (de) |
| JP (1) | JP6815388B2 (de) |
| CN (2) | CN108350526A (de) |
| AU (1) | AU2016309952B2 (de) |
| EA (1) | EA038189B1 (de) |
| SA (1) | SA518390930B1 (de) |
| WO (1) | WO2017027914A1 (de) |
| ZA (1) | ZA201801273B (de) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108350526A (zh) * | 2015-08-14 | 2018-07-31 | 库吉钛私人有限公司 | 从复合材料中回收含金属材料的方法 |
| CN111876617B (zh) * | 2020-08-03 | 2022-02-22 | 国家地质实验测试中心 | 一种提取钼、铼和放射性成因187Os的方法 |
| CN111876597B (zh) * | 2020-08-03 | 2022-02-22 | 国家地质实验测试中心 | 一种从辉钼矿中提取放射性成因187Os的方法 |
| US20220411894A1 (en) * | 2021-06-24 | 2022-12-29 | Iowa State University Research Foundation, Inc. | Method of separating one or more elements from an alloy |
| EP4522362A4 (de) * | 2022-05-12 | 2025-10-08 | Coogee Titanium Pty Ltd | Pyrophore metalle und metalllegierungen |
| CN116477584B (zh) * | 2023-04-20 | 2024-12-17 | 中国科学院化学研究所 | 一种近空间废旧硒光伏电池回收利用硒的方法 |
Family Cites Families (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB694921A (en) * | 1950-08-10 | 1953-07-29 | Titan Co Inc | A method for the production of titanium metal or a fused salt mixture from titanium tetrachloride |
| GB827470A (en) | 1956-04-03 | 1960-02-03 | Walter M Weil | Treatment of titanium dioxide ores |
| LU61733A1 (de) | 1969-12-04 | 1971-01-18 | ||
| CA950204A (en) | 1970-06-08 | 1974-07-02 | Hans G. Brandstatter | Direct reduction process for making titanium |
| US4279641A (en) | 1978-08-25 | 1981-07-21 | The Dow Chemical Company | Salt-coated magnesium granules |
| CN85100812A (zh) | 1984-10-05 | 1986-09-03 | 通用汽车公司 | 用钙热还原法还原稀土氧化物 |
| US4738389A (en) | 1984-10-19 | 1988-04-19 | Martin Marietta Corporation | Welding using metal-ceramic composites |
| JPH0747787B2 (ja) | 1989-05-24 | 1995-05-24 | 株式会社エヌ・ケイ・アール | チタン粉末またはチタン複合粉末の製造方法 |
| US5098471A (en) | 1989-12-06 | 1992-03-24 | Westinghouse Electric Corp. | Separation of magnesium from magnesium chloride and zirconium and/or hafnium subchlorides in the production of zirconium and/or hafnium sponge metal |
| US5078789A (en) | 1990-10-31 | 1992-01-07 | Westinghouse Electric Corp. | Continuous vacuum distillation and furnace therefor |
| DE69128692T2 (de) * | 1990-11-09 | 1998-06-18 | Toyoda Chuo Kenkyusho Kk | Titanlegierung aus Sinterpulver und Verfahren zu deren Herstellung |
| JPH0681051A (ja) * | 1991-10-30 | 1994-03-22 | Toho Titanium Co Ltd | ハロゲン化金属の還元反応による金属の製造方法 |
| US5589274A (en) | 1994-05-13 | 1996-12-31 | Hughes Electronics | Thermal control coating |
| US5498446A (en) | 1994-05-25 | 1996-03-12 | Washington University | Method and apparatus for producing high purity and unagglomerated submicron particles |
| US6409797B2 (en) | 1994-08-01 | 2002-06-25 | International Titanium Powder Llc | Method of making metals and other elements from the halide vapor of the metal |
| US5958106A (en) | 1994-08-01 | 1999-09-28 | International Titanium Powder, L.L.C. | Method of making metals and other elements from the halide vapor of the metal |
| JPH0873906A (ja) | 1994-08-31 | 1996-03-19 | Toho Titanium Co Ltd | チタン粉末の製造方法 |
| US5641424A (en) | 1995-07-10 | 1997-06-24 | Xerox Corporation | Magnetic refrigerant compositions and processes for making and using |
| US6194083B1 (en) | 1997-07-28 | 2001-02-27 | Kabushiki Kaisha Toshiba | Ceramic composite material and its manufacturing method, and heat resistant member using thereof |
| GB9812169D0 (en) * | 1998-06-05 | 1998-08-05 | Univ Cambridge Tech | Purification method |
| JP2000096160A (ja) | 1998-09-25 | 2000-04-04 | Taiyo Koko Co Ltd | バナジウム系水素吸蔵合金用材料及びその製造方法 |
| JP4132526B2 (ja) * | 1999-12-28 | 2008-08-13 | 東邦チタニウム株式会社 | 粉末状チタンの製造方法 |
| JP2002129250A (ja) | 2000-10-30 | 2002-05-09 | Katsutoshi Ono | 金属チタンの製造方法 |
| US7175721B2 (en) | 2001-04-27 | 2007-02-13 | Santoku Corporation | Method for preparing Cr-Ti-V type hydrogen occlusion alloy |
| JP2002339006A (ja) * | 2001-05-15 | 2002-11-27 | Sumitomo Titanium Corp | チタン粉末及びチタン合金粉末の製造方法 |
| DE10140089A1 (de) | 2001-08-16 | 2003-02-27 | Degussa | Superparamagnetische oxidische Partikel, Verfahren zu deren Herstellung und ihre Verwendung |
| UA79310C2 (en) | 2002-09-07 | 2007-06-11 | Int Titanium Powder Llc | Methods for production of alloys or ceramics with the use of armstrong method and device for their realization |
| US6955703B2 (en) | 2002-12-26 | 2005-10-18 | Millennium Inorganic Chemicals, Inc. | Process for the production of elemental material and alloys |
| JP2004283694A (ja) | 2003-03-20 | 2004-10-14 | Honda Motor Co Ltd | 水素貯蔵材粉末およびその製造方法 |
| KR101148573B1 (ko) | 2003-07-04 | 2012-05-21 | 커먼웰쓰 사이언티픽 앤드 인더스트리얼 리서치 오가니제이션 | 금속 화합물의 제조를 위한 방법 및 장치 |
| WO2005028145A2 (en) * | 2003-09-15 | 2005-03-31 | International Titanium Powder, Llc. | Method, apparatus and system for segregating salt from metal powder |
| US20090120239A1 (en) | 2004-07-30 | 2009-05-14 | Commonwealth Scientific And Industrial Research Organisation | Industrial process |
| JP2006045602A (ja) | 2004-08-03 | 2006-02-16 | Akio Fuwa | 樹枝状チタン粉の製造方法 |
| WO2006083326A2 (en) | 2004-08-07 | 2006-08-10 | Cabot Corporation | Gas dispersion manufacture of nanoparticulates and nanoparticulate-containing products and processing thereof |
| WO2006042360A1 (en) | 2004-10-20 | 2006-04-27 | Commonwealth Scientific And Industrial Research Organisation | Low temperature industrial process |
| JP5479886B2 (ja) * | 2006-03-27 | 2014-04-23 | コモンウェルス サイエンティフィック アンドインダストリアル リサーチ オーガナイゼーション | 金属化合物の製造のための装置および方法 |
| NZ548675A (en) * | 2006-07-20 | 2008-12-24 | Titanox Dev Ltd | A process for producing titanium metal alloy powder from titanium dioxide and aluminium |
| WO2008067614A1 (en) * | 2006-12-08 | 2008-06-12 | Commonwealth Scientific And Industrial Research Organisation | Separation method for metal recovery |
| TR200707197A1 (tr) * | 2007-10-22 | 2009-04-21 | Karakaya İshak | Tungsten içeren bileşiklerden elektrokimyasal metotlarla tungsten ve tungsten alaşımları kazanımı. |
| JP2009132970A (ja) | 2007-11-30 | 2009-06-18 | Osaka Titanium Technologies Co Ltd | 金属粒子の造粒方法および溶融塩中の金属粒子の分離方法 |
| JP6129556B2 (ja) | 2009-12-18 | 2017-05-17 | コモンウェルス サイエンティフィック アンド インダストリアル リサーチ オーガナイゼーション | 低アルミニウムチタン−アルミニウム合金を製造する方法 |
| EP2569068B1 (de) | 2010-05-04 | 2020-11-18 | Coogee Titanium Pty Ltd | Trennverfahren |
| KR101220545B1 (ko) | 2010-12-27 | 2013-01-10 | 재단법인 포항산업과학연구원 | 티타늄 스폰지의 연속 제조장치 |
| KR20150029624A (ko) | 2012-06-06 | 2015-03-18 | 씨에스아이알 | 결정질 티타늄 분말의 생산 공정 |
| CN102921953A (zh) | 2012-10-31 | 2013-02-13 | 昆明理工大学 | 一种由TiO2制备金属钛粉的方法 |
| KR102676361B1 (ko) * | 2015-08-14 | 2024-06-19 | 쿠지 타이타늄 피티와이 엘티디 | 과량의 산화제를 사용하는 복합재의 제조 방법 |
| CN108350526A (zh) * | 2015-08-14 | 2018-07-31 | 库吉钛私人有限公司 | 从复合材料中回收含金属材料的方法 |
-
2016
- 2016-08-12 CN CN201680058477.2A patent/CN108350526A/zh active Pending
- 2016-08-12 AU AU2016309952A patent/AU2016309952B2/en active Active
- 2016-08-12 EA EA201890484A patent/EA038189B1/ru not_active IP Right Cessation
- 2016-08-12 EP EP16836271.3A patent/EP3334848B1/de active Active
- 2016-08-12 US US15/540,725 patent/US11162157B2/en active Active
- 2016-08-12 JP JP2018509595A patent/JP6815388B2/ja active Active
- 2016-08-12 WO PCT/AU2016/050745 patent/WO2017027914A1/en not_active Ceased
- 2016-08-12 CN CN202410244490.3A patent/CN119932341A/zh active Pending
-
2018
- 2018-02-14 SA SA518390930A patent/SA518390930B1/ar unknown
- 2018-02-23 ZA ZA2018/01273A patent/ZA201801273B/en unknown
-
2021
- 2021-11-01 US US17/516,323 patent/US20220056557A1/en not_active Abandoned
-
2023
- 2023-12-29 US US18/400,413 patent/US20240229189A9/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| WO2017027914A1 (en) | 2017-02-23 |
| US20180274059A1 (en) | 2018-09-27 |
| EP3334848A4 (de) | 2018-06-27 |
| AU2016309952B2 (en) | 2022-01-27 |
| JP6815388B2 (ja) | 2021-01-20 |
| EA038189B1 (ru) | 2021-07-21 |
| EP3334848C0 (de) | 2025-07-09 |
| US20240229189A9 (en) | 2024-07-11 |
| US20220056557A1 (en) | 2022-02-24 |
| EA201890484A1 (ru) | 2018-08-31 |
| AU2016309952A1 (en) | 2018-04-05 |
| CN108350526A (zh) | 2018-07-31 |
| SA518390930B1 (ar) | 2022-10-18 |
| EP3334848A1 (de) | 2018-06-20 |
| JP2018526538A (ja) | 2018-09-13 |
| WO2017027914A9 (en) | 2017-07-13 |
| US20240132994A1 (en) | 2024-04-25 |
| US11162157B2 (en) | 2021-11-02 |
| ZA201801273B (en) | 2019-06-26 |
| CN119932341A (zh) | 2025-05-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20240229189A9 (en) | Method for recovery of metal-containing material from a composite material | |
| EP3142816B1 (de) | Herstellung von im wesentlichen kugelförmigen metallgranulaten | |
| CA2784196C (en) | Method for producing low aluminium titanium-aluminium alloys | |
| WO2014187867A1 (en) | Process for manufacturing metal containing powder | |
| EP3334847B1 (de) | Verfahren zur herstellung eines verbundstoffes unter verwendung von überschüssigem oxidationsmittel | |
| WO2003033750A1 (en) | Grain refining agent for cast aluminum products | |
| EP3334849B1 (de) | Verfahren welche reaktive partikeln mit hohem oberflächenbereich pro volumen verwenden | |
| AU7645287A (en) | Metal powder and sponge and processes for the production thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20180313 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| A4 | Supplementary search report drawn up and despatched |
Effective date: 20180529 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C22B 21/00 20060101ALI20180518BHEP Ipc: B01D 3/10 20060101ALI20180518BHEP Ipc: C22B 9/02 20060101ALI20180518BHEP Ipc: C22B 9/04 20060101ALI20180518BHEP Ipc: C22B 34/22 20060101ALI20180518BHEP Ipc: C22B 5/04 20060101ALI20180518BHEP Ipc: C22B 34/12 20060101AFI20180518BHEP |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20200303 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: B22F 1/05 20220101ALI20250306BHEP Ipc: C22B 9/02 20060101ALI20250306BHEP Ipc: B01D 3/10 20060101ALI20250306BHEP Ipc: C22B 9/04 20060101ALI20250306BHEP Ipc: C22B 5/04 20060101ALI20250306BHEP Ipc: C22B 34/22 20060101ALI20250306BHEP Ipc: C22B 21/00 20060101ALI20250306BHEP Ipc: C22B 34/12 20060101AFI20250306BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20250319 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602016092861 Country of ref document: DE |
|
| U01 | Request for unitary effect filed |
Effective date: 20250729 |
|
| U07 | Unitary effect registered |
Designated state(s): AT BE BG DE DK EE FI FR IT LT LU LV MT NL PT RO SE SI Effective date: 20250804 |
|
| U20 | Renewal fee for the european patent with unitary effect paid |
Year of fee payment: 10 Effective date: 20250827 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250814 Year of fee payment: 10 |