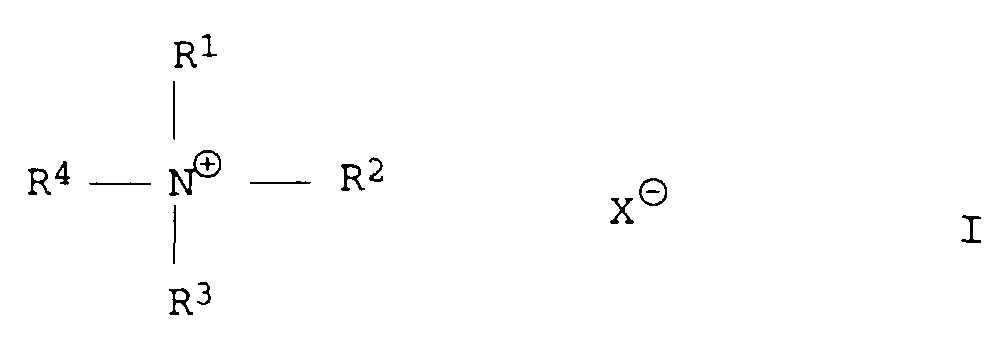EP0807704B1 - Wiedergewinnung von Fasern aus gebundenen Faservliesen - Google Patents
Wiedergewinnung von Fasern aus gebundenen Faservliesen Download PDFInfo
- Publication number
- EP0807704B1 EP0807704B1 EP97107491A EP97107491A EP0807704B1 EP 0807704 B1 EP0807704 B1 EP 0807704B1 EP 97107491 A EP97107491 A EP 97107491A EP 97107491 A EP97107491 A EP 97107491A EP 0807704 B1 EP0807704 B1 EP 0807704B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- alkaline earth
- binder
- earth metal
- aqueous solution
- salt
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Classifications
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H1/00—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres
- D04H1/40—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties
- D04H1/58—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties by applying, incorporating or activating chemical or thermoplastic bonding agents, e.g. adhesives
- D04H1/587—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties by applying, incorporating or activating chemical or thermoplastic bonding agents, e.g. adhesives characterised by the bonding agents used
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H1/00—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres
- D04H1/40—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties
- D04H1/42—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties characterised by the use of certain kinds of fibres insofar as this use has no preponderant influence on the consolidation of the fleece
- D04H1/4326—Condensation or reaction polymers
- D04H1/435—Polyesters
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H1/00—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres
- D04H1/40—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties
- D04H1/58—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties by applying, incorporating or activating chemical or thermoplastic bonding agents, e.g. adhesives
- D04H1/64—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties by applying, incorporating or activating chemical or thermoplastic bonding agents, e.g. adhesives the bonding agent being applied in wet state, e.g. chemical agents in dispersions or solutions
- D04H1/645—Impregnation followed by a solidification process
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H3/00—Non-woven fabrics formed wholly or mainly of yarns or like filamentary material of substantial length
- D04H3/005—Synthetic yarns or filaments
- D04H3/009—Condensation or reaction polymers
- D04H3/011—Polyesters
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H3/00—Non-woven fabrics formed wholly or mainly of yarns or like filamentary material of substantial length
- D04H3/08—Non-woven fabrics formed wholly or mainly of yarns or like filamentary material of substantial length characterised by the method of strengthening or consolidating
- D04H3/12—Non-woven fabrics formed wholly or mainly of yarns or like filamentary material of substantial length characterised by the method of strengthening or consolidating with filaments or yarns secured together by chemical or thermo-activatable bonding agents, e.g. adhesives, applied or incorporated in liquid or solid form
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S260/00—Chemistry of carbon compounds
- Y10S260/31—Ionic cross-link
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S260/00—Chemistry of carbon compounds
- Y10S260/43—Promoting degradability of polymers
Definitions
- the invention relates to a method for the recovery of Nonwoven fibers.
- Nonwoven fabrics can be coated or impregnation with a binder.
- binders are used that crosslink after application to the nonwoven.
- the binders can contain several monomers ethylenically unsaturated groups or monomers with other reactive Groups, e.g. Methylol groups.
- a networking over metal salt groups, e.g. crosslinking via Ca carboxylate groups is e.g. known from EP 442 370. It is also desirable to have fibers to be recovered from nonwovens bound with crosslinked binders.
- the object of the present invention was therefore a method for Recovery of fibers from crosslinked binders or consolidated nonwovens.
- nonwovens can be made from the most varied Fibers are used.
- synthetic fibers such as viscose, polyester, Polyamide, polypropylene, polyacrylonitrile, carbon fibers or fibers of homo- and copolymers of vinyl chloride or Tetrafluoroethylene or fibers of natural origin such as Cellulose, cellulose, cellulose, cotton or wood fibers or also glass, ceramic, or mineral fibers or mixtures of this.
- the fibers are folded into non-woven fabrics and then with solidified a binder, for which purpose the binder in known Way e.g. by impregnation, spraying, cracking, dipping or printing on the fibers. Then follows generally drying to remove the solvent, in general water. In this way, known to those skilled in the art a bound, i.e. get consolidated nonwoven.
- the nonwoven fabrics thus produced find e.g. Application as Base materials for roofing membranes or floor coverings.
- a binder are generally used polymers, which build up from ethylenically unsaturated monomers.
- the polymers contain carboxylate groups which are alkaline earth metal are networked, i.e. they are cross-linked with metal salts.
- the polymers preferably contain 0.1 to 30% by weight, particularly preferably 0.5 to 25% by weight and very particularly preferably 5 to 20 % By weight of carboxylate groups, based on the weight of the polymer (weight of alkaline earth cations not included).
- the carboxylate groups are preferably 50 to 100, especially preferably 80 to 100% as a salt with alkaline earth metal ions.
- Preferred alkaline earth metal cations are Ca 2+ and Ba 2+ , Mg 2+ .
- Ca 2+ is particularly preferred.
- the metal salt crosslinked polymers are e.g. based on polymers available with carboxylic acid or carboxylic anhydride groups by adding an alkaline earth salt, e.g. an oxide, hydroxide, Carbonates or bicarbonates e.g. for aqueous dispersion or solution of the polymer as described in EP-A-442 370 is.
- an alkaline earth salt e.g. an oxide, hydroxide, Carbonates or bicarbonates e.g. for aqueous dispersion or solution of the polymer as described in EP-A-442 370 is.
- the metal salt crosslinked polymer is preferably from the following Monomers A), B) and C) built up:
- Monomers A) are monomers with at least one carboxylic acid or Carboxylic anhydride group, which converts to the metal salt groups can be. Particularly noteworthy are acrylic acid, Methacrylic acid, itaconic acid, maleic acid, maleic anhydride.
- the amount of these monomers is determined by the desired one Content of carboxylate groups cross-linked with alkaline earth metal cations.
- Main monomers B) selected from C 1 -C 20 alkyl (meth) acrylates, vinyl esters of carboxylic acids containing up to 20 C atoms, vinyl aromatics with up to 20 C atoms, ethylenically unsaturated nitriles, vinyl halides are of particular technical importance.
- Examples include (meth) acrylic acid alkyl esters with a C 1 -C 10 alkyl radical, such as methyl methacrylate, methyl acrylate, n-butyl acrylate, ethyl acrylate and 2-ethylhexyl acrylate.
- mixtures of the (meth) acrylic acid alkyl esters suitable.
- Vinyl esters of carboxylic acids with 1 to 20 C atoms are e.g. Vinyl laurate, stearate, vinyl propionate, vinyl versatic acid and vinyl acetate.
- Suitable vinyl aromatic compounds are vinyl toluene, ⁇ - and p-methylstyrene, ⁇ -butylstyrene, 4-n-butylstyrene, 4-n-decylstyrene and preferably styrene.
- nitriles are Acrylonitrile and methacrylonitrile.
- the vinyl halides are substituted with chlorine, fluorine or bromine ethylenically unsaturated compounds, preferably vinyl chloride and Vinylidene chloride.
- vinyl ethers examples include Vinyl methyl ether or vinyl isobutyl ether. Vinyl ether of 1 to 4 carbon atoms is preferred containing alcohols.
- hydrocarbons with 2 to 8 carbon atoms and two olefinic Double bonds are butadiene, isoprene and chloroprene.
- monomers C for example monomers containing hydroxyl groups, in particular C 1 -C 10 -hydroxyalkyl (meth) acrylates or (meth) acrylamide, can be used in the polymer.
- Customary polymers generally consist of at least 40, preferably at least 60, particularly preferably at least 80% by weight of the above main monomers B).
- the polymerization can be carried out by customary polymerization processes take place, e.g. by substance, emulsion, suspension, Dispersion, precipitation and solution polymerization. With the above Polymerization is preferred to exclude worked by oxygen, preferably in a stream of nitrogen.
- the usual equipment for all polymerization methods used e.g. Stirred tanks, stirred tank cascades, autoclaves, tubular reactors and kneader. Preference is given to the Solution, emulsion, precipitation or suspension polymerization worked. The methods of solution are particularly preferred and especially emulsion polymerization.
- the polymerization can in solvents or diluents, e.g.
- Solvent or diluent water if necessary, with proportions up to 60 wt .-% of alcohols or glycols used. Especially water is preferably used.
- the metal-crosslinked polymers are generally in the form their aqueous solution or dispersion applied to the nonwovens. After drying, the nonwovens are bound, i.e. solidified.
- the non-woven fabrics then generally contain 1 to 40 parts by weight, preferably 5 to 30 parts by weight, of the metal-crosslinked Binder, based on 100 parts by weight of fibers.
- the fibers are recovered according to the method of the invention by removing the binder from the nonwoven, i.e. of the Fibers is separated.
- the nonwoven fabric is washed with an aqueous solution of a salt, (hereinafter referred to as "soluble salt), whose anion with the Alkaline earth formation forms a salt that is sparingly soluble in water.
- soluble salt a salt that is sparingly soluble in water.
- the soluble salt can be inorganic or organic Trade salt.
- it is an alkali metal salt.
- the anion of the soluble salt can e.g. around oxalate or carbonate, which e.g. with the calcium cation form sparingly soluble salt.
- the anion of the soluble salt can also be, for example, an organic anion which forms a poorly soluble complex with the alkaline earth metal, for example Ca 2+ .
- EDTA is particularly worth mentioning here.
- the soluble salt contained in the aqueous solution has preferably a solubility of at least 10 g / l water 23 ° C.
- the aqueous solution preferably contains the salt in amounts of 0.02 to 15 parts by weight, particularly preferably in amounts of 0.1 to 10 parts by weight, very particularly preferably from 0.5 to 2.5 parts by weight, based on 100 parts by weight of water.
- the solubility of the formed during treatment of the nonwoven sparingly soluble alkaline earth metal salt is preferably smaller than 0.5 g / l water at 23 ° C.
- the aqueous solution preferably contains another phase transfer catalyst.
- phase transfer catalysts are e.g. in Chimia 34 (1980) No. 1, pages 12 to 20.
- phase transfer catalysts may be mentioned e.g. Polyalkylene glycols which e.g. is commercially available under the name Pluriol® or quaternary, organic Ammonium salts, e.g. commercially available under the name Lutensit are.
- Quaternary organic ammonium salts include, in particular, those of the formula I. mentioned, wherein R 1 -R 4 independently of one another are an organic radical, preferably a hydrocarbon radical having 1 to 12 carbon atoms, preferably 1 to 6 carbon atoms and X ⁇ is an anion, preferably an inorganic anion, for example Cl - , Br ⁇ stands.
- phase transfer catalysts are those with Alkoxy groups, preferably with 2 to 20 alkoxy groups, e.g. Alkylphenol ethoxylates (e.g. Lutensole® AP, emulsifier 825 fatty alcohol ethoxylates (e.g. Lutensol A8) and oxo alcohol ethoxylates (e.g. Lutensol AO7, Lutensol ON80).
- Alkylphenol ethoxylates e.g. Lutensole® AP
- emulsifier 825 fatty alcohol ethoxylates e.g. Lutensol A8
- oxo alcohol ethoxylates e.g. Lutensol AO7, Lutensol ON80.
- phase transfer catalyst alkoxylation of alkylphenols, fatty alcohols or Contain oxo alcohols with alkylene oxides, preferably ethylene oxide these phase transfer catalysts alkoxy groups.
- the content of the phase transfer catalyst in the aqueous solution is preferably 0.01 to 1 part by weight, particularly preferably 0.08 to 0.5 part by weight, based on 100 parts by weight of water.
- the temperature of the aqueous solution can e.g. 10 to 100 ° C, in particular 15 to 80 ° C and particularly preferably 20 to 50 ° C. Temperatures above 30, in particular above, are advantageous 40 ° C, for an even better and, above all, faster replacement of the To reach binder from the nonwoven.
- the nonwoven fabric can be used as a whole or in shredded form method according to the invention are supplied.
- the nonwoven fabric is preferably divided into parts with an edge length of Crushed 1 to 10 cm.
- the nonwoven fabric is preferred added to the aqueous solution.
- the amount of nonwoven is preferably 1 to 200 g, particularly preferably 5 up to 150 g and very particularly preferably 10 to 80 g per liter Solution.
- the duration of treatment can be shortened by intensive stirring become. Generally, the time required is 5 minutes up to 1 hour. With strong stirring, however, are less sufficient than 30 minutes, in particular less than 20 minutes to detach at least 80% by weight of the binder from the fiber and recover the fibers.
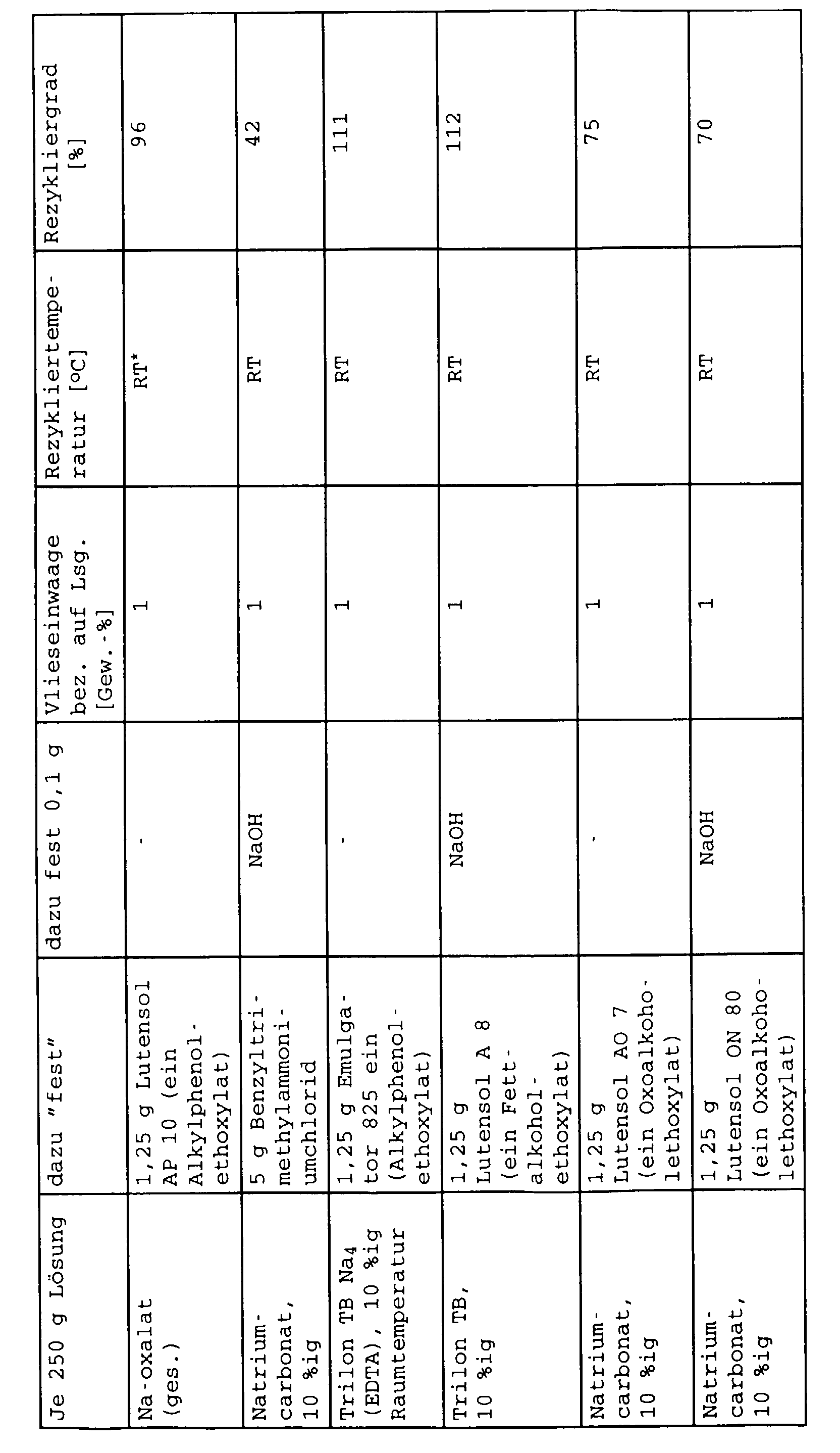
- Acronal® DS 2324x Binder application of 20% by weight ⁇ 2 (solid / solid).
- Acronal DS 2324X is an aqueous dispersion of a metal salt-crosslinked polymer (crosslinking of the carboxylate groups of the polymer with Ca 2+ cations) on an acrylate basis, the drying was carried out at 5 minutes at 200 ° C. in a Mathis laboratory dryer, after which the approximately A-4 sized fleece sheets were cut into pieces of about 1 cm 2 .
- a cold saturated sodium oxalate solution (approx. 3% by weight) and in each case a 10% by weight solution of soda or Na 4 EDTA (Trilon B) in water were prepared.
- the amounts of the phase transfer catalyst indicated in the table and optionally sodium hydroxide solution were added.
- the fleece pieces were cleaned after about 24 hours using a laboratory stirrer Stirred at 2000 rpm for 15 min.
- the fibers were then filtered through a 60 ⁇ sieve, 2 h dried at 130 ° C and weighed.
- the degree of recycling is 100%. Values above 100% in the table can be explained by the fact that when the fibers were filtered off, very fine fibers were possibly not retained by the sieve and the weight of the fibers after recycling was too low.
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Chemical Or Physical Treatment Of Fibers (AREA)
- Nonwoven Fabrics (AREA)
- Treatments For Attaching Organic Compounds To Fibrous Goods (AREA)
Description
- die Faservliese mit einem Polymerbindemittel mit Carboxylatgruppen gebunden sind, wobei die Carboxylatgruppen über Erdalkalikationen vernetzt sind
- die Faservliese mit einer wäßrigen Lösung eines Alkalisalzes behandelt werden, wobei das Anion des Alkalisalzes mit den Erdalkalikationen ein schwerlösliches Salz oder Komplex bildet und anschließend
- die vom Bindemittel befreiten Fasern abgetrennt werden.
Claims (7)
- Verfahren zum Ablösen von Bindemitteln von mit diesen Bindemitteln gebundenen Faservliesen, dadurch gekennzeichnet, daßdie Faservliese mit einem Polymerbindemittel mit Carboxylatgruppen gebunden sind, wobei die Carboxylatgruppen über Erdalkalikationen vernetzt sinddie Faservliese mit einer wäßrigen Lösung eines Alkalisalzes behandelt werden, wobei das Anion des Alkalisalzes mit den Erdalkalikationen ein schwerlösliches Salz oder Komplex bildet und anschließenddie vom Bindemittel befreiten Fasern abgetrennt werden.
- Verfahren gemäß Anspruch 1, dadurch gekennzeichnet, daß die wäßrige Lösung zusätzlich einen Phasentransferkatalysator enthält.
- Verfahren gemäß Anspruch 1 oder 2, dadurch gekennzeichnet, daß der Phasentransferkatalysator ausgewählt ist aus der Gruppe der Alkylphenolethoxylate, Fettalkoholethoxylate, Oxo-alkoholethoxylate und quaternären, organischen Ammoniumsalze.
- Verfahren gemäß einen der Ansprüche 1 bis 3, dadurch gekennzeichnet, daß es sich bei den Erdalkalikationen um Ca2+ handelt.
- Verfahren gemäß einen der Ansprüche 1 bis 4, dadurch gekennzeichnet, daß es sich bei den Polymerbindemittel, mit dem das Faservlies gebunden ist, um ein radikalisch polymerisiertes Polymerisat mit einem Carboxylatgruppengehalt von 0,1 bis 30 Gew.-%, bezogen auf das Polymerisat, handelt und 50 bis 100 Gew.-% der Carboxylatgruppen als Salz mit einem Erdalkalikation vorliegen.
- Verfahren gemäß einen der Ansprüche 1 bis 5, dadurch gekennzeichnet, daß die wäßrige Lösung des Alkalisalzes eine Temperatur von 10 bis 100°C hat.
- Verfahren gemäß einem der Ansprüche 1 bis 6, dadurch gekennzeichnet, daß das Faservlies in die wäßrige Lösung des Alkalisalzes gegeben wird, die wäßrige Lösung gegebenenfalls gerührt wird und nach 1 bis 60 Minuten die vom Bindemittel befreiten Fasern abgetrennt werden.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19619639 | 1996-05-15 | ||
| DE19619639A DE19619639A1 (de) | 1996-05-15 | 1996-05-15 | Wiedergewinnung von Fasern aus gebundenen Faservliesen |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0807704A1 EP0807704A1 (de) | 1997-11-19 |
| EP0807704B1 true EP0807704B1 (de) | 2000-11-02 |
Family
ID=7794430
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP97107491A Expired - Lifetime EP0807704B1 (de) | 1996-05-15 | 1997-05-07 | Wiedergewinnung von Fasern aus gebundenen Faservliesen |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US6004428A (de) |
| EP (1) | EP0807704B1 (de) |
| AT (1) | ATE197323T1 (de) |
| DE (2) | DE19619639A1 (de) |
| ES (1) | ES2152593T3 (de) |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6429261B1 (en) | 2000-05-04 | 2002-08-06 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6444214B1 (en) | 2000-05-04 | 2002-09-03 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6495080B1 (en) | 1997-10-03 | 2002-12-17 | Kimberly-Clark Worldwide, Inc. | Methods for making water-sensitive compositions for improved processability and fibers including same |
| US6548592B1 (en) | 2000-05-04 | 2003-04-15 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6579570B1 (en) | 2000-05-04 | 2003-06-17 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6586529B2 (en) | 2001-02-01 | 2003-07-01 | Kimberly-Clark Worldwide, Inc. | Water-dispersible polymers, a method of making same and items using same |
| US6599848B1 (en) | 2000-05-04 | 2003-07-29 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6630558B2 (en) | 1998-12-31 | 2003-10-07 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive hard water dispersible polymers and applications therefor |
| US6653406B1 (en) | 2000-05-04 | 2003-11-25 | Kimberly Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6683143B1 (en) | 2000-05-04 | 2004-01-27 | Kimberly Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6713414B1 (en) | 2000-05-04 | 2004-03-30 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6815502B1 (en) | 2000-05-04 | 2004-11-09 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersable polymers, a method of making same and items using same |
| US7276459B1 (en) | 2000-05-04 | 2007-10-02 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US7456117B2 (en) | 2002-09-20 | 2008-11-25 | Kimberly-Clark Worldwide, Inc. | Ion triggerable, cationic polymers, a method of making same and items using same |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5969052A (en) | 1996-12-31 | 1999-10-19 | Kimberly Clark Worldwide, Inc. | Temperature sensitive polymers and water-dispersible products containing the polymers |
| US5986004A (en) | 1997-03-17 | 1999-11-16 | Kimberly-Clark Worldwide, Inc. | Ion sensitive polymeric materials |
| US5935880A (en) * | 1997-03-31 | 1999-08-10 | Wang; Kenneth Y. | Dispersible nonwoven fabric and method of making same |
| US6043317A (en) | 1997-05-23 | 2000-03-28 | Kimberly-Clark Worldwide, Inc. | Ion sensitive binder for fibrous materials |
| US6835678B2 (en) | 2000-05-04 | 2004-12-28 | Kimberly-Clark Worldwide, Inc. | Ion sensitive, water-dispersible fabrics, a method of making same and items using same |
| US7101612B2 (en) | 2000-05-04 | 2006-09-05 | Kimberly Clark Worldwide, Inc. | Pre-moistened wipe product |
| US7255816B2 (en) * | 2000-11-10 | 2007-08-14 | Kimberly-Clark Worldwide, Inc. | Method of recycling bonded fibrous materials and synthetic fibers and fiber-like materials produced thereof |
| DE10103213A1 (de) | 2001-01-25 | 2002-08-14 | Wacker Polymer Systems Gmbh | Verfahren zur Herstellung von wiederverwertbaren Formkörpern |
| US6897168B2 (en) | 2001-03-22 | 2005-05-24 | Kimberly-Clark Worldwide, Inc. | Water-dispersible, cationic polymers, a method of making same and items using same |
| US6828014B2 (en) | 2001-03-22 | 2004-12-07 | Kimberly-Clark Worldwide, Inc. | Water-dispersible, cationic polymers, a method of making same and items using same |
| US6908966B2 (en) | 2001-03-22 | 2005-06-21 | Kimberly-Clark Worldwide, Inc. | Water-dispersible, cationic polymers, a method of making same and items using same |
| US7070854B2 (en) | 2001-03-22 | 2006-07-04 | Kimberly-Clark Worldwide, Inc. | Water-dispersible, cationic polymers, a method of making same and items using same |
| US7772138B2 (en) | 2002-05-21 | 2010-08-10 | Kimberly-Clark Worldwide, Inc. | Ion sensitive, water-dispersible polymers, a method of making same and items using same |
| US7101456B2 (en) | 2002-09-20 | 2006-09-05 | Kimberly-Clark Worldwide, Inc. | Ion triggerable, cationic polymers, a method of making same and items using same |
| US6994865B2 (en) | 2002-09-20 | 2006-02-07 | Kimberly-Clark Worldwide, Inc. | Ion triggerable, cationic polymers, a method of making same and items using same |
| US6960371B2 (en) | 2002-09-20 | 2005-11-01 | Kimberly-Clark Worldwide, Inc. | Water-dispersible, cationic polymers, a method of making same and items using same |
| US7141519B2 (en) | 2002-09-20 | 2006-11-28 | Kimberly-Clark Worldwide, Inc. | Ion triggerable, cationic polymers, a method of making same and items using same |
| DE102005037113A1 (de) * | 2005-08-03 | 2007-02-08 | Basf Ag | Verwendung einer thermisch härtbaren wässrigen Zusammensetzung als Bindemittel für Substrate |
| DE102009010938A1 (de) * | 2009-02-27 | 2010-09-09 | Celanese Emulsions Gmbh | Mineralwollfasermatten, Verfahren zu deren Herstellung und Verwendung |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4009313A (en) * | 1972-08-30 | 1977-02-22 | Minnesota Mining And Manufacturing Company | Enzymatically dispersible non-woven webs |
| DE2747182C2 (de) * | 1977-10-20 | 1985-08-14 | Wacker-Chemie GmbH, 8000 München | Bindemittel für Faservliese |
| US5082697A (en) * | 1988-02-17 | 1992-01-21 | The Dow Chemical Company | Polymer salt complex for fiber or fabric treatment |
| US5270376A (en) * | 1990-02-16 | 1993-12-14 | Basf Aktiengesellschaft | Aqueous polymer dispersion having divalent metal salt(s) incorporated therein |
| DE4004915A1 (de) * | 1990-02-16 | 1991-08-22 | Basf Ag | Waessrige polymerisatdispersionen |
| US5149543A (en) * | 1990-10-05 | 1992-09-22 | Massachusetts Institute Of Technology | Ionically cross-linked polymeric microcapsules |
| DE19535792A1 (de) * | 1995-09-26 | 1997-03-27 | Basf Ag | Verfahren zur Herstellung wiederverwertbarer Faserverbundwerkstoffe |
-
1996
- 1996-05-15 DE DE19619639A patent/DE19619639A1/de not_active Withdrawn
-
1997
- 1997-05-06 US US08/852,064 patent/US6004428A/en not_active Expired - Fee Related
- 1997-05-07 EP EP97107491A patent/EP0807704B1/de not_active Expired - Lifetime
- 1997-05-07 ES ES97107491T patent/ES2152593T3/es not_active Expired - Lifetime
- 1997-05-07 DE DE59702552T patent/DE59702552D1/de not_active Expired - Fee Related
- 1997-05-07 AT AT97107491T patent/ATE197323T1/de not_active IP Right Cessation
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6495080B1 (en) | 1997-10-03 | 2002-12-17 | Kimberly-Clark Worldwide, Inc. | Methods for making water-sensitive compositions for improved processability and fibers including same |
| US6630558B2 (en) | 1998-12-31 | 2003-10-07 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive hard water dispersible polymers and applications therefor |
| US6599848B1 (en) | 2000-05-04 | 2003-07-29 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6548592B1 (en) | 2000-05-04 | 2003-04-15 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6579570B1 (en) | 2000-05-04 | 2003-06-17 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6429261B1 (en) | 2000-05-04 | 2002-08-06 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6444214B1 (en) | 2000-05-04 | 2002-09-03 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6653406B1 (en) | 2000-05-04 | 2003-11-25 | Kimberly Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6683143B1 (en) | 2000-05-04 | 2004-01-27 | Kimberly Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6713414B1 (en) | 2000-05-04 | 2004-03-30 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6815502B1 (en) | 2000-05-04 | 2004-11-09 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersable polymers, a method of making same and items using same |
| US7276459B1 (en) | 2000-05-04 | 2007-10-02 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6586529B2 (en) | 2001-02-01 | 2003-07-01 | Kimberly-Clark Worldwide, Inc. | Water-dispersible polymers, a method of making same and items using same |
| US7456117B2 (en) | 2002-09-20 | 2008-11-25 | Kimberly-Clark Worldwide, Inc. | Ion triggerable, cationic polymers, a method of making same and items using same |
Also Published As
| Publication number | Publication date |
|---|---|
| DE59702552D1 (de) | 2000-12-07 |
| ES2152593T3 (es) | 2001-02-01 |
| US6004428A (en) | 1999-12-21 |
| EP0807704A1 (de) | 1997-11-19 |
| ATE197323T1 (de) | 2000-11-15 |
| DE19619639A1 (de) | 1997-11-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0807704B1 (de) | Wiedergewinnung von Fasern aus gebundenen Faservliesen | |
| DE69809639T2 (de) | (hydroxyalkyl)harnstoff vernetzer | |
| DE2836319C2 (de) | ||
| EP0084809B1 (de) | Acrylkunststoffdispersion | |
| DE2749386C2 (de) | ||
| DE2009218B2 (de) | Verfahren zur Perlpolymerisation von äthylenisch ungesättigten Monomeren | |
| EP0143175B1 (de) | Formaldehydfreie wässrige Kunststoffdispersionen auf Basis eines vernetzbaren Polymerisats, Verfahren zu ihrer Herstellung und ihre Verwendung | |
| EP0384125A1 (de) | Filmbildende, selbstvernetzende wässrige Kunststoffdispersion | |
| EP0019169A1 (de) | Verwendung von wässrigen Dispersionen von amidgruppenhaltigen Emulsions-Copolymerisaten zum Verfestigen von Faservliesen | |
| DE1086208B (de) | Verfahren zur Herstellung von gebundenen Faservliesen | |
| DE2308996A1 (de) | Fasern aus polyolefinischen materialien und verfahren zu ihrer herstellung und verwendung | |
| DE1420618A1 (de) | Verfahren zur Herstellung von Cellulosematerial | |
| EP2199333B1 (de) | Binderverfestigtes, textiles Flächengebilde, Verfahren zu dessen Herstellung und dessen Verwendung | |
| DE4040959C1 (de) | ||
| DE951300C (de) | Verfahren zur Herstellung einer gut faerbbaren Acrylnitrilkunststoffmasse | |
| DE1232103B (de) | Verfahren zur Herstellung von Beschichtungen, Impraegnierungen und Verklebungen von Faser-substraten wie Geweben, Vliessen sowie zum Kaschieren von Schaumstoffen mit Geweben unter Verwendung von Pfropfpolymerisaten | |
| EP0735061A1 (de) | Verwendung kationisch stabilisierter wässriger Polymerisatdispersionen als Bindemittel für Formkörper auf der Basis von einer negativen Oberflächenladung aufweisenden feinteiligen Materialien | |
| WO1998011156A1 (de) | Verfahren zur minderung der geruchsemission wässriger polymerisatdispersionen | |
| DE2228862B2 (de) | ||
| EP0193107A2 (de) | Gebundenes textiles Flächengebilde und Verfahren zu seiner Herstellung | |
| DE2429399A1 (de) | Fluorierte acrylsaeureester und ihre polymerisationsprodukte | |
| DE2326053A1 (de) | Feuerhemmende polymere und verfahren zu ihrer herstellung | |
| DE1444068C (de) | Verfahren zur Herstellung von Vliesstoffen | |
| DE10103028A1 (de) | Wasserlösliche Polymerisate auf Basis von Acryl-bzw. Methacrylderivaten | |
| DE1545098C3 (de) | Verfahren zur Polymerisation von Äthylen |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19970904 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE ES FR GB IT LI NL SE |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| 17Q | First examination report despatched |
Effective date: 20000229 |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE ES FR GB IT LI NL SE |
|
| REF | Corresponds to: |
Ref document number: 197323 Country of ref document: AT Date of ref document: 20001115 Kind code of ref document: T |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20001109 |
|
| REF | Corresponds to: |
Ref document number: 59702552 Country of ref document: DE Date of ref document: 20001207 |
|
| ITF | It: translation for a ep patent filed | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2152593 Country of ref document: ES Kind code of ref document: T3 |
|
| ET | Fr: translation filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010507 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010606 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010606 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20020412 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20020415 Year of fee payment: 6 Ref country code: NL Payment date: 20020415 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20020501 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20020507 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20020521 Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030507 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030508 Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030531 |
|
| BERE | Be: lapsed |
Owner name: *BASF A.G. Effective date: 20030531 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20031201 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20030507 |
|
| EUG | Se: european patent has lapsed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040130 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20031201 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20030508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050507 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20080515 Year of fee payment: 12 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20091201 |