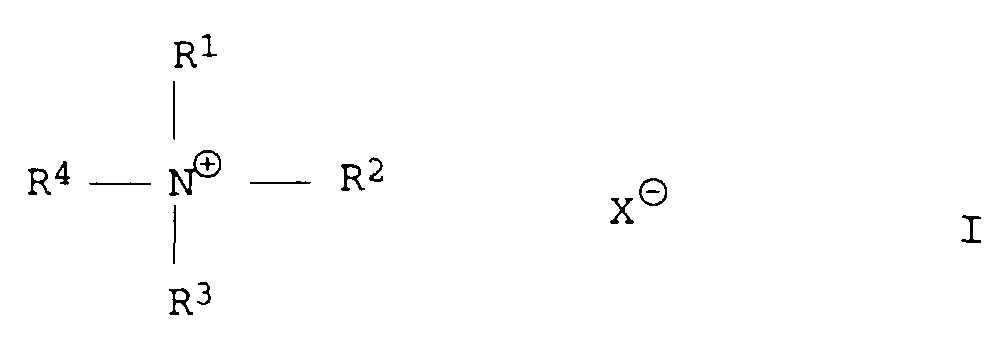EP0807704B1 - Recovery of fibres from bonded nonwovens - Google Patents
Recovery of fibres from bonded nonwovens Download PDFInfo
- Publication number
- EP0807704B1 EP0807704B1 EP97107491A EP97107491A EP0807704B1 EP 0807704 B1 EP0807704 B1 EP 0807704B1 EP 97107491 A EP97107491 A EP 97107491A EP 97107491 A EP97107491 A EP 97107491A EP 0807704 B1 EP0807704 B1 EP 0807704B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- alkaline earth
- binder
- earth metal
- aqueous solution
- salt
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000004745 nonwoven fabric Substances 0.000 title abstract description 23
- 238000011084 recovery Methods 0.000 title description 4
- -1 alkali salt anions Chemical class 0.000 claims abstract description 26
- 239000011230 binding agent Substances 0.000 claims abstract description 26
- 150000003839 salts Chemical class 0.000 claims abstract description 23
- 238000000034 method Methods 0.000 claims abstract description 16
- 150000007942 carboxylates Chemical group 0.000 claims abstract description 14
- 229910052784 alkaline earth metal Inorganic materials 0.000 claims abstract description 13
- 229920000642 polymer Polymers 0.000 claims abstract description 12
- 239000003444 phase transfer catalyst Substances 0.000 claims abstract description 10
- 239000004435 Oxo alcohol Substances 0.000 claims abstract description 4
- 150000003863 ammonium salts Chemical class 0.000 claims abstract description 4
- 150000002191 fatty alcohols Chemical class 0.000 claims abstract description 4
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 claims abstract description 3
- JSPLKZUTYZBBKA-UHFFFAOYSA-N trioxidane Chemical compound OOO JSPLKZUTYZBBKA-UHFFFAOYSA-N 0.000 claims abstract description 3
- 229910001424 calcium ion Inorganic materials 0.000 claims abstract 2
- 239000000835 fiber Substances 0.000 claims description 29
- 239000007864 aqueous solution Substances 0.000 claims description 15
- 150000001450 anions Chemical class 0.000 claims description 7
- 229910052783 alkali metal Inorganic materials 0.000 claims description 5
- 150000001447 alkali salts Chemical class 0.000 abstract description 6
- 150000001768 cations Chemical class 0.000 abstract description 4
- 229910001420 alkaline earth metal ion Inorganic materials 0.000 abstract description 2
- 229920005596 polymer binder Polymers 0.000 abstract description 2
- 239000002491 polymer binding agent Substances 0.000 abstract description 2
- 239000012266 salt solution Substances 0.000 abstract 4
- PUAQLLVFLMYYJJ-UHFFFAOYSA-N 2-aminopropiophenone Chemical compound CC(N)C(=O)C1=CC=CC=C1 PUAQLLVFLMYYJJ-UHFFFAOYSA-N 0.000 abstract 1
- 239000013522 chelant Substances 0.000 abstract 1
- 239000000178 monomer Substances 0.000 description 16
- 239000000243 solution Substances 0.000 description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 12
- 125000004432 carbon atom Chemical group C* 0.000 description 9
- 239000000203 mixture Substances 0.000 description 7
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 239000011575 calcium Substances 0.000 description 5
- 238000006116 polymerization reaction Methods 0.000 description 5
- 238000004064 recycling Methods 0.000 description 5
- 229920002554 vinyl polymer Polymers 0.000 description 5
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 4
- 229920006037 cross link polymer Polymers 0.000 description 4
- 239000006185 dispersion Substances 0.000 description 4
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 229920003043 Cellulose fiber Polymers 0.000 description 3
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical class C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 description 3
- 150000001298 alcohols Chemical class 0.000 description 3
- 125000003545 alkoxy group Chemical group 0.000 description 3
- 239000001913 cellulose Substances 0.000 description 3
- 238000004132 cross linking Methods 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- 238000007720 emulsion polymerization reaction Methods 0.000 description 3
- 150000002334 glycols Chemical class 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 3
- 229920005789 ACRONAL® acrylic binder Polymers 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- KAKZBPTYRLMSJV-UHFFFAOYSA-N Butadiene Chemical compound C=CC=C KAKZBPTYRLMSJV-UHFFFAOYSA-N 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- YNQLUTRBYVCPMQ-UHFFFAOYSA-N Ethylbenzene Chemical compound CCC1=CC=CC=C1 YNQLUTRBYVCPMQ-UHFFFAOYSA-N 0.000 description 2
- RRHGJUQNOFWUDK-UHFFFAOYSA-N Isoprene Chemical compound CC(=C)C=C RRHGJUQNOFWUDK-UHFFFAOYSA-N 0.000 description 2
- BAPJBEWLBFYGME-UHFFFAOYSA-N Methyl acrylate Chemical compound COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 description 2
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical group CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 2
- URLKBWYHVLBVBO-UHFFFAOYSA-N Para-Xylene Chemical group CC1=CC=C(C)C=C1 URLKBWYHVLBVBO-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical compound ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 2
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 2
- 239000008186 active pharmaceutical agent Substances 0.000 description 2
- 150000001342 alkaline earth metals Chemical class 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 150000001244 carboxylic acid anhydrides Chemical group 0.000 description 2
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 2
- 150000001735 carboxylic acids Chemical class 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 2
- RWGFKTVRMDUZSP-UHFFFAOYSA-N cumene Chemical compound CC(C)C1=CC=CC=C1 RWGFKTVRMDUZSP-UHFFFAOYSA-N 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 238000010528 free radical solution polymerization reaction Methods 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 238000005470 impregnation Methods 0.000 description 2
- 150000002825 nitriles Chemical class 0.000 description 2
- 229920001515 polyalkylene glycol Polymers 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 238000012673 precipitation polymerization Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 238000010557 suspension polymerization reaction Methods 0.000 description 2
- 229920001567 vinyl ester resin Polymers 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 1
- QOVCUELHTLHMEN-UHFFFAOYSA-N 1-butyl-4-ethenylbenzene Chemical compound CCCCC1=CC=C(C=C)C=C1 QOVCUELHTLHMEN-UHFFFAOYSA-N 0.000 description 1
- DMADTXMQLFQQII-UHFFFAOYSA-N 1-decyl-4-ethenylbenzene Chemical compound CCCCCCCCCCC1=CC=C(C=C)C=C1 DMADTXMQLFQQII-UHFFFAOYSA-N 0.000 description 1
- OZCMOJQQLBXBKI-UHFFFAOYSA-N 1-ethenoxy-2-methylpropane Chemical compound CC(C)COC=C OZCMOJQQLBXBKI-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- JAHNSTQSQJOJLO-UHFFFAOYSA-N 2-(3-fluorophenyl)-1h-imidazole Chemical compound FC1=CC=CC(C=2NC=CN=2)=C1 JAHNSTQSQJOJLO-UHFFFAOYSA-N 0.000 description 1
- OEPOKWHJYJXUGD-UHFFFAOYSA-N 2-(3-phenylmethoxyphenyl)-1,3-thiazole-4-carbaldehyde Chemical compound O=CC1=CSC(C=2C=C(OCC=3C=CC=CC=3)C=CC=2)=N1 OEPOKWHJYJXUGD-UHFFFAOYSA-N 0.000 description 1
- GOXQRTZXKQZDDN-UHFFFAOYSA-N 2-Ethylhexyl acrylate Chemical compound CCCCC(CC)COC(=O)C=C GOXQRTZXKQZDDN-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- JLBJTVDPSNHSKJ-UHFFFAOYSA-N 4-Methylstyrene Chemical compound CC1=CC=C(C=C)C=C1 JLBJTVDPSNHSKJ-UHFFFAOYSA-N 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 1
- NLHHRLWOUZZQLW-UHFFFAOYSA-N Acrylonitrile Chemical compound C=CC#N NLHHRLWOUZZQLW-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical class OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical group [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- 229920000049 Carbon (fiber) Polymers 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- ZGTMUACCHSMWAC-UHFFFAOYSA-L EDTA disodium salt (anhydrous) Chemical compound [Na+].[Na+].OC(=O)CN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O ZGTMUACCHSMWAC-UHFFFAOYSA-L 0.000 description 1
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical compound CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical group FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 1
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 1
- GYCMBHHDWRMZGG-UHFFFAOYSA-N Methylacrylonitrile Chemical compound CC(=C)C#N GYCMBHHDWRMZGG-UHFFFAOYSA-N 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 229920002266 Pluriol® Polymers 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 229920000297 Rayon Polymers 0.000 description 1
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 1
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 1
- 229920002522 Wood fibre Polymers 0.000 description 1
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- XYLMUPLGERFSHI-UHFFFAOYSA-N alpha-Methylstyrene Chemical compound CC(=C)C1=CC=CC=C1 XYLMUPLGERFSHI-UHFFFAOYSA-N 0.000 description 1
- 150000001449 anionic compounds Chemical class 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Chemical group BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Chemical group 0.000 description 1
- CQEYYJKEWSMYFG-UHFFFAOYSA-N butyl acrylate Chemical compound CCCCOC(=O)C=C CQEYYJKEWSMYFG-UHFFFAOYSA-N 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000004917 carbon fiber Substances 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- YACLQRRMGMJLJV-UHFFFAOYSA-N chloroprene Chemical compound ClC(=C)C=C YACLQRRMGMJLJV-UHFFFAOYSA-N 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 238000012674 dispersion polymerization Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- GLVVKKSPKXTQRB-UHFFFAOYSA-N ethenyl dodecanoate Chemical compound CCCCCCCCCCCC(=O)OC=C GLVVKKSPKXTQRB-UHFFFAOYSA-N 0.000 description 1
- UIWXSTHGICQLQT-UHFFFAOYSA-N ethenyl propanoate Chemical compound CCC(=O)OC=C UIWXSTHGICQLQT-UHFFFAOYSA-N 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000011737 fluorine Chemical group 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- YJSSCAJSFIGKSN-UHFFFAOYSA-N hex-1-en-2-ylbenzene Chemical compound CCCCC(=C)C1=CC=CC=C1 YJSSCAJSFIGKSN-UHFFFAOYSA-N 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 229910001412 inorganic anion Inorganic materials 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- XJRBAMWJDBPFIM-UHFFFAOYSA-N methyl vinyl ether Chemical compound COC=C XJRBAMWJDBPFIM-UHFFFAOYSA-N 0.000 description 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N methylenebutanedioic acid Natural products OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 description 1
- 239000002557 mineral fiber Substances 0.000 description 1
- 230000006855 networking Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 229940078552 o-xylene Drugs 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- 150000002891 organic anions Chemical class 0.000 description 1
- 229940039748 oxalate Drugs 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- PNJWIWWMYCMZRO-UHFFFAOYSA-N pent‐4‐en‐2‐one Natural products CC(=O)CC=C PNJWIWWMYCMZRO-UHFFFAOYSA-N 0.000 description 1
- 229920002239 polyacrylonitrile Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 238000007639 printing Methods 0.000 description 1
- HJWLCRVIBGQPNF-UHFFFAOYSA-N prop-2-enylbenzene Chemical compound C=CCC1=CC=CC=C1 HJWLCRVIBGQPNF-UHFFFAOYSA-N 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- ZNCPFRVNHGOPAG-UHFFFAOYSA-L sodium oxalate Chemical class [Na+].[Na+].[O-]C(=O)C([O-])=O ZNCPFRVNHGOPAG-UHFFFAOYSA-L 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 239000012209 synthetic fiber Substances 0.000 description 1
- BFKJFAAPBSQJPD-UHFFFAOYSA-N tetrafluoroethene Chemical group FC(F)=C(F)F BFKJFAAPBSQJPD-UHFFFAOYSA-N 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 239000002025 wood fiber Substances 0.000 description 1
Classifications
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H1/00—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres
- D04H1/40—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties
- D04H1/58—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties by applying, incorporating or activating chemical or thermoplastic bonding agents, e.g. adhesives
- D04H1/587—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties by applying, incorporating or activating chemical or thermoplastic bonding agents, e.g. adhesives characterised by the bonding agents used
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H1/00—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres
- D04H1/40—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties
- D04H1/42—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties characterised by the use of certain kinds of fibres insofar as this use has no preponderant influence on the consolidation of the fleece
- D04H1/4326—Condensation or reaction polymers
- D04H1/435—Polyesters
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H1/00—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres
- D04H1/40—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties
- D04H1/58—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties by applying, incorporating or activating chemical or thermoplastic bonding agents, e.g. adhesives
- D04H1/64—Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibres from fleeces or layers composed of fibres without existing or potential cohesive properties by applying, incorporating or activating chemical or thermoplastic bonding agents, e.g. adhesives the bonding agent being applied in wet state, e.g. chemical agents in dispersions or solutions
- D04H1/645—Impregnation followed by a solidification process
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H3/00—Non-woven fabrics formed wholly or mainly of yarns or like filamentary material of substantial length
- D04H3/005—Synthetic yarns or filaments
- D04H3/009—Condensation or reaction polymers
- D04H3/011—Polyesters
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04H—MAKING TEXTILE FABRICS, e.g. FROM FIBRES OR FILAMENTARY MATERIAL; FABRICS MADE BY SUCH PROCESSES OR APPARATUS, e.g. FELTS, NON-WOVEN FABRICS; COTTON-WOOL; WADDING ; NON-WOVEN FABRICS FROM STAPLE FIBRES, FILAMENTS OR YARNS, BONDED WITH AT LEAST ONE WEB-LIKE MATERIAL DURING THEIR CONSOLIDATION
- D04H3/00—Non-woven fabrics formed wholly or mainly of yarns or like filamentary material of substantial length
- D04H3/08—Non-woven fabrics formed wholly or mainly of yarns or like filamentary material of substantial length characterised by the method of strengthening or consolidating
- D04H3/12—Non-woven fabrics formed wholly or mainly of yarns or like filamentary material of substantial length characterised by the method of strengthening or consolidating with filaments or yarns secured together by chemical or thermo-activatable bonding agents, e.g. adhesives, applied or incorporated in liquid or solid form
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S260/00—Chemistry of carbon compounds
- Y10S260/31—Ionic cross-link
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S260/00—Chemistry of carbon compounds
- Y10S260/43—Promoting degradability of polymers
Definitions
- the invention relates to a method for the recovery of Nonwoven fibers.
- Nonwoven fabrics can be coated or impregnation with a binder.
- binders are used that crosslink after application to the nonwoven.
- the binders can contain several monomers ethylenically unsaturated groups or monomers with other reactive Groups, e.g. Methylol groups.
- a networking over metal salt groups, e.g. crosslinking via Ca carboxylate groups is e.g. known from EP 442 370. It is also desirable to have fibers to be recovered from nonwovens bound with crosslinked binders.
- the object of the present invention was therefore a method for Recovery of fibers from crosslinked binders or consolidated nonwovens.
- nonwovens can be made from the most varied Fibers are used.
- synthetic fibers such as viscose, polyester, Polyamide, polypropylene, polyacrylonitrile, carbon fibers or fibers of homo- and copolymers of vinyl chloride or Tetrafluoroethylene or fibers of natural origin such as Cellulose, cellulose, cellulose, cotton or wood fibers or also glass, ceramic, or mineral fibers or mixtures of this.
- the fibers are folded into non-woven fabrics and then with solidified a binder, for which purpose the binder in known Way e.g. by impregnation, spraying, cracking, dipping or printing on the fibers. Then follows generally drying to remove the solvent, in general water. In this way, known to those skilled in the art a bound, i.e. get consolidated nonwoven.
- the nonwoven fabrics thus produced find e.g. Application as Base materials for roofing membranes or floor coverings.
- a binder are generally used polymers, which build up from ethylenically unsaturated monomers.
- the polymers contain carboxylate groups which are alkaline earth metal are networked, i.e. they are cross-linked with metal salts.
- the polymers preferably contain 0.1 to 30% by weight, particularly preferably 0.5 to 25% by weight and very particularly preferably 5 to 20 % By weight of carboxylate groups, based on the weight of the polymer (weight of alkaline earth cations not included).
- the carboxylate groups are preferably 50 to 100, especially preferably 80 to 100% as a salt with alkaline earth metal ions.
- Preferred alkaline earth metal cations are Ca 2+ and Ba 2+ , Mg 2+ .
- Ca 2+ is particularly preferred.
- the metal salt crosslinked polymers are e.g. based on polymers available with carboxylic acid or carboxylic anhydride groups by adding an alkaline earth salt, e.g. an oxide, hydroxide, Carbonates or bicarbonates e.g. for aqueous dispersion or solution of the polymer as described in EP-A-442 370 is.
- an alkaline earth salt e.g. an oxide, hydroxide, Carbonates or bicarbonates e.g. for aqueous dispersion or solution of the polymer as described in EP-A-442 370 is.
- the metal salt crosslinked polymer is preferably from the following Monomers A), B) and C) built up:
- Monomers A) are monomers with at least one carboxylic acid or Carboxylic anhydride group, which converts to the metal salt groups can be. Particularly noteworthy are acrylic acid, Methacrylic acid, itaconic acid, maleic acid, maleic anhydride.
- the amount of these monomers is determined by the desired one Content of carboxylate groups cross-linked with alkaline earth metal cations.
- Main monomers B) selected from C 1 -C 20 alkyl (meth) acrylates, vinyl esters of carboxylic acids containing up to 20 C atoms, vinyl aromatics with up to 20 C atoms, ethylenically unsaturated nitriles, vinyl halides are of particular technical importance.
- Examples include (meth) acrylic acid alkyl esters with a C 1 -C 10 alkyl radical, such as methyl methacrylate, methyl acrylate, n-butyl acrylate, ethyl acrylate and 2-ethylhexyl acrylate.
- mixtures of the (meth) acrylic acid alkyl esters suitable.
- Vinyl esters of carboxylic acids with 1 to 20 C atoms are e.g. Vinyl laurate, stearate, vinyl propionate, vinyl versatic acid and vinyl acetate.
- Suitable vinyl aromatic compounds are vinyl toluene, ⁇ - and p-methylstyrene, ⁇ -butylstyrene, 4-n-butylstyrene, 4-n-decylstyrene and preferably styrene.
- nitriles are Acrylonitrile and methacrylonitrile.
- the vinyl halides are substituted with chlorine, fluorine or bromine ethylenically unsaturated compounds, preferably vinyl chloride and Vinylidene chloride.
- vinyl ethers examples include Vinyl methyl ether or vinyl isobutyl ether. Vinyl ether of 1 to 4 carbon atoms is preferred containing alcohols.
- hydrocarbons with 2 to 8 carbon atoms and two olefinic Double bonds are butadiene, isoprene and chloroprene.
- monomers C for example monomers containing hydroxyl groups, in particular C 1 -C 10 -hydroxyalkyl (meth) acrylates or (meth) acrylamide, can be used in the polymer.
- Customary polymers generally consist of at least 40, preferably at least 60, particularly preferably at least 80% by weight of the above main monomers B).
- the polymerization can be carried out by customary polymerization processes take place, e.g. by substance, emulsion, suspension, Dispersion, precipitation and solution polymerization. With the above Polymerization is preferred to exclude worked by oxygen, preferably in a stream of nitrogen.
- the usual equipment for all polymerization methods used e.g. Stirred tanks, stirred tank cascades, autoclaves, tubular reactors and kneader. Preference is given to the Solution, emulsion, precipitation or suspension polymerization worked. The methods of solution are particularly preferred and especially emulsion polymerization.
- the polymerization can in solvents or diluents, e.g.
- Solvent or diluent water if necessary, with proportions up to 60 wt .-% of alcohols or glycols used. Especially water is preferably used.
- the metal-crosslinked polymers are generally in the form their aqueous solution or dispersion applied to the nonwovens. After drying, the nonwovens are bound, i.e. solidified.
- the non-woven fabrics then generally contain 1 to 40 parts by weight, preferably 5 to 30 parts by weight, of the metal-crosslinked Binder, based on 100 parts by weight of fibers.
- the fibers are recovered according to the method of the invention by removing the binder from the nonwoven, i.e. of the Fibers is separated.
- the nonwoven fabric is washed with an aqueous solution of a salt, (hereinafter referred to as "soluble salt), whose anion with the Alkaline earth formation forms a salt that is sparingly soluble in water.
- soluble salt a salt that is sparingly soluble in water.
- the soluble salt can be inorganic or organic Trade salt.
- it is an alkali metal salt.
- the anion of the soluble salt can e.g. around oxalate or carbonate, which e.g. with the calcium cation form sparingly soluble salt.
- the anion of the soluble salt can also be, for example, an organic anion which forms a poorly soluble complex with the alkaline earth metal, for example Ca 2+ .
- EDTA is particularly worth mentioning here.
- the soluble salt contained in the aqueous solution has preferably a solubility of at least 10 g / l water 23 ° C.
- the aqueous solution preferably contains the salt in amounts of 0.02 to 15 parts by weight, particularly preferably in amounts of 0.1 to 10 parts by weight, very particularly preferably from 0.5 to 2.5 parts by weight, based on 100 parts by weight of water.
- the solubility of the formed during treatment of the nonwoven sparingly soluble alkaline earth metal salt is preferably smaller than 0.5 g / l water at 23 ° C.
- the aqueous solution preferably contains another phase transfer catalyst.
- phase transfer catalysts are e.g. in Chimia 34 (1980) No. 1, pages 12 to 20.
- phase transfer catalysts may be mentioned e.g. Polyalkylene glycols which e.g. is commercially available under the name Pluriol® or quaternary, organic Ammonium salts, e.g. commercially available under the name Lutensit are.
- Quaternary organic ammonium salts include, in particular, those of the formula I. mentioned, wherein R 1 -R 4 independently of one another are an organic radical, preferably a hydrocarbon radical having 1 to 12 carbon atoms, preferably 1 to 6 carbon atoms and X ⁇ is an anion, preferably an inorganic anion, for example Cl - , Br ⁇ stands.
- phase transfer catalysts are those with Alkoxy groups, preferably with 2 to 20 alkoxy groups, e.g. Alkylphenol ethoxylates (e.g. Lutensole® AP, emulsifier 825 fatty alcohol ethoxylates (e.g. Lutensol A8) and oxo alcohol ethoxylates (e.g. Lutensol AO7, Lutensol ON80).
- Alkylphenol ethoxylates e.g. Lutensole® AP
- emulsifier 825 fatty alcohol ethoxylates e.g. Lutensol A8
- oxo alcohol ethoxylates e.g. Lutensol AO7, Lutensol ON80.
- phase transfer catalyst alkoxylation of alkylphenols, fatty alcohols or Contain oxo alcohols with alkylene oxides, preferably ethylene oxide these phase transfer catalysts alkoxy groups.
- the content of the phase transfer catalyst in the aqueous solution is preferably 0.01 to 1 part by weight, particularly preferably 0.08 to 0.5 part by weight, based on 100 parts by weight of water.
- the temperature of the aqueous solution can e.g. 10 to 100 ° C, in particular 15 to 80 ° C and particularly preferably 20 to 50 ° C. Temperatures above 30, in particular above, are advantageous 40 ° C, for an even better and, above all, faster replacement of the To reach binder from the nonwoven.
- the nonwoven fabric can be used as a whole or in shredded form method according to the invention are supplied.
- the nonwoven fabric is preferably divided into parts with an edge length of Crushed 1 to 10 cm.
- the nonwoven fabric is preferred added to the aqueous solution.
- the amount of nonwoven is preferably 1 to 200 g, particularly preferably 5 up to 150 g and very particularly preferably 10 to 80 g per liter Solution.
- the duration of treatment can be shortened by intensive stirring become. Generally, the time required is 5 minutes up to 1 hour. With strong stirring, however, are less sufficient than 30 minutes, in particular less than 20 minutes to detach at least 80% by weight of the binder from the fiber and recover the fibers.
- Acronal® DS 2324x Binder application of 20% by weight ⁇ 2 (solid / solid).
- Acronal DS 2324X is an aqueous dispersion of a metal salt-crosslinked polymer (crosslinking of the carboxylate groups of the polymer with Ca 2+ cations) on an acrylate basis, the drying was carried out at 5 minutes at 200 ° C. in a Mathis laboratory dryer, after which the approximately A-4 sized fleece sheets were cut into pieces of about 1 cm 2 .
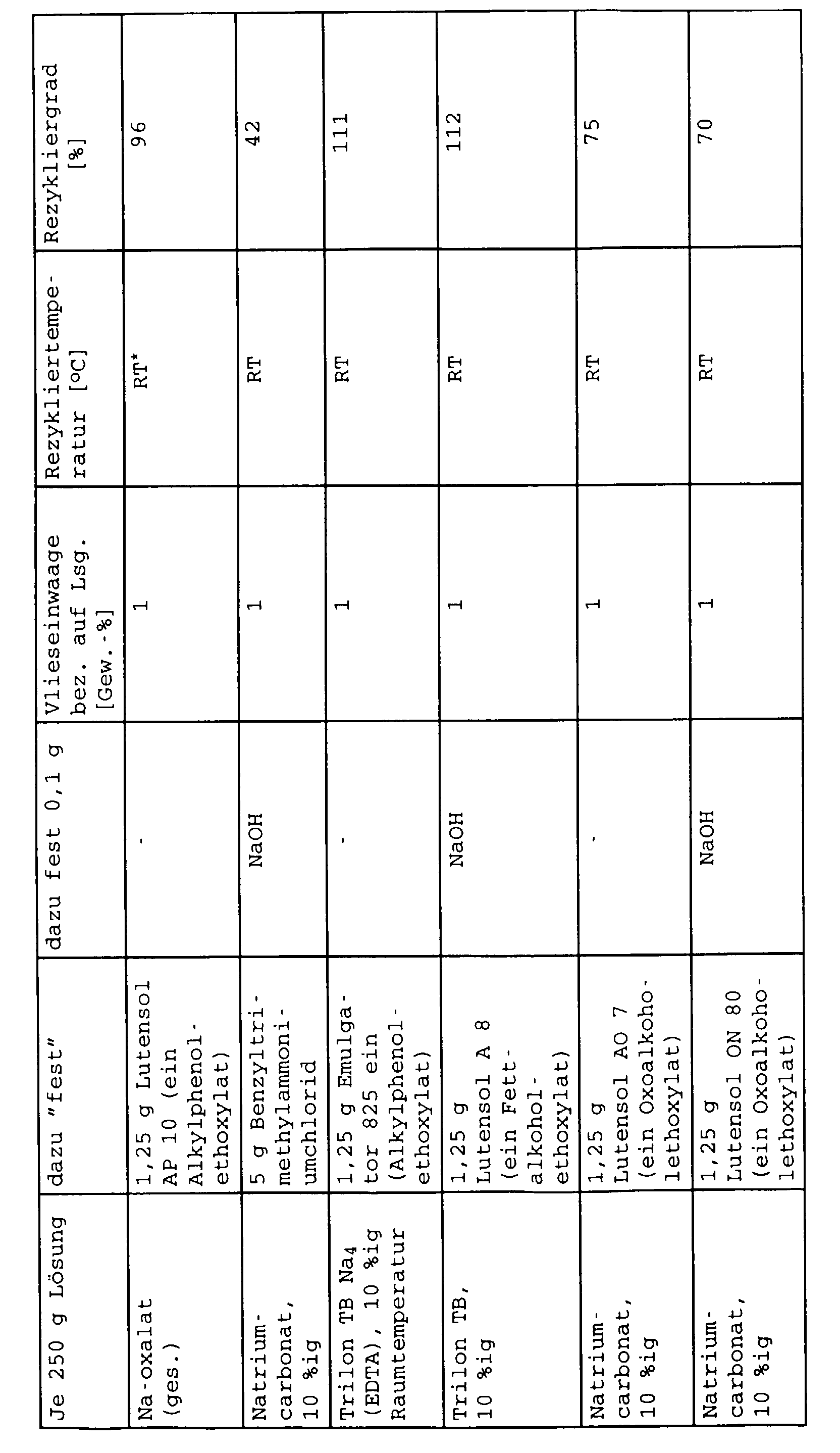
- a cold saturated sodium oxalate solution (approx. 3% by weight) and in each case a 10% by weight solution of soda or Na 4 EDTA (Trilon B) in water were prepared.
- the amounts of the phase transfer catalyst indicated in the table and optionally sodium hydroxide solution were added.
- the fleece pieces were cleaned after about 24 hours using a laboratory stirrer Stirred at 2000 rpm for 15 min.
- the fibers were then filtered through a 60 ⁇ sieve, 2 h dried at 130 ° C and weighed.
- the degree of recycling is 100%. Values above 100% in the table can be explained by the fact that when the fibers were filtered off, very fine fibers were possibly not retained by the sieve and the weight of the fibers after recycling was too low.
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Chemical Or Physical Treatment Of Fibers (AREA)
- Nonwoven Fabrics (AREA)
- Treatments For Attaching Organic Compounds To Fibrous Goods (AREA)
Abstract
Description
Gegenstand der Erfindung ist ein Verfahren zur Rückgewinnung von Fasern aus Faservliesen. Faservliese können durch Beschichten bzw. Imprägnieren mit einem Bindemittel verfestigt werden.The invention relates to a method for the recovery of Nonwoven fibers. Nonwoven fabrics can be coated or impregnation with a binder.
Grundsätzlich besteht der Wunsch nach recycelfähigen Faservliesen, bei denen nach Gebrauch die Fasern wieder zurückgewonnen werden können. Dazu muß das Bindemittel von dem verfestigten Faservlies abgelöst werden. Entsprechend wurden gemäß JP 5 384 189 und der unveröffentlichten deutschen Patentanmeldung P 19 535 792.3 (0050/46241) zur Verfestigung von Faservliesen unvernetzte Bindemittel verwendet, welche durch Überführung von Carboxylgruppen in Carboxylatgruppen in Wasser löslich sind und so von den Fasern wieder abgetrennt werden können.Basically there is a desire for recyclable nonwovens, where the fibers are recovered after use can be. To do this, the binder must be solidified Nonwoven can be detached. Accordingly, according to JP 5 384 189 and the unpublished German patent application P 19 535 792.3 (0050/46241) for strengthening nonwovens uncrosslinked binders, which are obtained by transferring Carboxyl groups in carboxylate groups are soluble in water and so can be separated from the fibers.
Um Faservliese mit guten Festigkeiten zu erhalten, werden Bindemittel verwendet, die nach Aufbringen auf das Faservlies vernetzen. Zur Vernetzung können die Bindemittel Monomere mit mehreren ethylenisch ungesättigten Gruppen oder Monomere mit sonstigen reaktiven Gruppen, z.B. Methylolgruppen, enthalten. Eine Vernetzung über Metallsalzgruppen, z.B. eine Vernetzung über Ca-carboxylatgruppen ist z.B. aus EP 442 370 bekannt. Gewünscht ist, auch Fasern aus mit vernetzten Bindemitteln gebundenen Faservliesen zurückzugewinnen.In order to obtain nonwovens with good strength, binders are used used that crosslink after application to the nonwoven. For crosslinking, the binders can contain several monomers ethylenically unsaturated groups or monomers with other reactive Groups, e.g. Methylol groups. A networking over metal salt groups, e.g. crosslinking via Ca carboxylate groups is e.g. known from EP 442 370. It is also desirable to have fibers to be recovered from nonwovens bound with crosslinked binders.
Aufgabe der vorliegenden Erfindung war daher ein Verfahren zur Rückgewinnung von Fasern aus mit vernetzten Bindemitteln gebundenen bzw. verfestigten Faservliesen.The object of the present invention was therefore a method for Recovery of fibers from crosslinked binders or consolidated nonwovens.
Die Aufgabe wurde gelöst durch ein Verfahren zum Ablösen von Bindemitteln von mit diesen Bindemitteln gebunden Faservliesen, dadurch gekennzeichnet, daß
- die Faservliese mit einem Polymerbindemittel mit Carboxylatgruppen gebunden sind, wobei die Carboxylatgruppen über Erdalkalikationen vernetzt sind
- die Faservliese mit einer wäßrigen Lösung eines Alkalisalzes behandelt werden, wobei das Anion des Alkalisalzes mit den Erdalkalikationen ein schwerlösliches Salz oder Komplex bildet und anschließend
- die vom Bindemittel befreiten Fasern abgetrennt werden.
- the nonwoven fabrics are bound with a polymer binder with carboxylate groups, the carboxylate groups being crosslinked via alkaline earth metal cations
- the nonwoven fabrics are treated with an aqueous solution of an alkali salt, the anion of the alkali salt forming a poorly soluble salt or complex with the alkaline earth metal cations and then
- the fibers freed from the binder are separated.
Für das erfindungsgemäße Verfahren können Faservliese aus unterschiedlichsten Fasern Verwendung finden.For the method according to the invention, nonwovens can be made from the most varied Fibers are used.
In Betracht kommen z.B. synthetische Fasern wie Viskose-, Polyester-, Polyamid-, Polypropylen-, Polyacrylnitril-, Carbonfasern oder Fasern von Homo- und Copolymerisaten des Vinylchlorides oder Tetrafluorethylens oder auch Fasern natürlichen Ursprungs wie Zellstoff-, Zellwolle-, Cellulose-, Baumwolle- oder Holzfasern oder auch Glas-, Keramik-, oder Mineralfasern oder Mischungen hiervon.For example, synthetic fibers such as viscose, polyester, Polyamide, polypropylene, polyacrylonitrile, carbon fibers or fibers of homo- and copolymers of vinyl chloride or Tetrafluoroethylene or fibers of natural origin such as Cellulose, cellulose, cellulose, cotton or wood fibers or also glass, ceramic, or mineral fibers or mixtures of this.
Die Fasern werden zu Faservliesen zusammengelegt und dann mit einem Bindemittel verfestigt, wozu das Bindemittel in bekannter Weise z.B. durch Imprägnieren, Besprühen, Aufrackeln, Tauchen oder Bedrucken auf die Fasern gebracht wird. Anschließend erfolgt im allgemeinen eine Trocknung zur Entfernung des Lösemittels, im allgemeinen Wasser. Auf diese, dem Fachmann bekannte Weise wird ein gebundenes, d.h. verfestigtes Faservlies erhalten.The fibers are folded into non-woven fabrics and then with solidified a binder, for which purpose the binder in known Way e.g. by impregnation, spraying, cracking, dipping or printing on the fibers. Then follows generally drying to remove the solvent, in general water. In this way, known to those skilled in the art a bound, i.e. get consolidated nonwoven.
Die so hergestellten Faservliese finden z.B. Anwendung als Trägermaterialien für Dachbahnen bzw. Fußbodenbeläge. Als Bindemittel werden im allgemeinen Polymerisate verwendet, welche sich aus ethylenisch ungesättigten Monomeren aufbauen.The nonwoven fabrics thus produced find e.g. Application as Base materials for roofing membranes or floor coverings. As a binder are generally used polymers, which build up from ethylenically unsaturated monomers.
Die Polymerisate enthalten Carboxylatgruppen, welche über Erdalkalikationen vernetzt sind, d.h. sie sind metallsalzvernetzt.The polymers contain carboxylate groups which are alkaline earth metal are networked, i.e. they are cross-linked with metal salts.
Bevorzugt enthalten die Polymerisate 0,1 bis 30 Gew.-%, besonders bevorzugt 0,5 bis 25 Gew.-% und ganz besonders bevorzugt 5 bis 20 Gew.-% Carboxylatgruppen, bezogen auf das Polymerisatgewicht (Gewicht der Erdalkalikationen nicht mitberechnet).The polymers preferably contain 0.1 to 30% by weight, particularly preferably 0.5 to 25% by weight and very particularly preferably 5 to 20 % By weight of carboxylate groups, based on the weight of the polymer (weight of alkaline earth cations not included).
Die Carboxylatgruppen liegen bevorzugt zu 50 bis 100, besonders bevorzugt zu 80 bis 100 % als Salz mit Erdalkalikationen vor.The carboxylate groups are preferably 50 to 100, especially preferably 80 to 100% as a salt with alkaline earth metal ions.
Bevorzugte Erdalkalikationen sind Ca2+ und Ba2+, Mg2+. Besonders bevorzugt ist Ca2+.Preferred alkaline earth metal cations are Ca 2+ and Ba 2+ , Mg 2+ . Ca 2+ is particularly preferred.
Die metallsalzvernetzten Polymerisate sind z.B. ausgehend von Polymerisaten mit Carbonsäure- oder Carbonsäureanhydridgruppen erhältlich durch Zugabe eines Erdalkalisalzes, z.B. eines Oxids,Hydroxids, Carbonats oder Hydrogencarbonate z.B. zur wäßrigen Dispersion oder Lösung des Polymerisats, wie es in EP-A-442 370 beschrieben ist. The metal salt crosslinked polymers are e.g. based on polymers available with carboxylic acid or carboxylic anhydride groups by adding an alkaline earth salt, e.g. an oxide, hydroxide, Carbonates or bicarbonates e.g. for aqueous dispersion or solution of the polymer as described in EP-A-442 370 is.
Das metallsalzvernetzte Polymerisat ist bevorzugt aus folgenden Monomeren A), B) und C) aufgebaut:The metal salt crosslinked polymer is preferably from the following Monomers A), B) and C) built up:
Monomere A) sind Monomere mit mindestens einer Carbonsäure- oder Carbonsäureanhydridgruppe, welche in die Metallsalzgruppen überführt werden können. Zu nennen sind insbesondere Acrylsäure, Methacrylsäure, Itaconsäure, Maleinsäure, Maleinsäureanhydrid.Monomers A) are monomers with at least one carboxylic acid or Carboxylic anhydride group, which converts to the metal salt groups can be. Particularly noteworthy are acrylic acid, Methacrylic acid, itaconic acid, maleic acid, maleic anhydride.
Die Menge dieser Monomeren ergibt sich durch den gewünschten Gehalt an mit Erdalkalikationen vernetzten Carboxylatgruppen.The amount of these monomers is determined by the desired one Content of carboxylate groups cross-linked with alkaline earth metal cations.
Von technischer Bedeutung sind insbesondere sogenannte Hauptmonomere B), ausgewählt aus C1-C20-Alkyl(meth)acrylaten, Vinylestern von bis zu 20 C-Atome enthaltenden Carbonsäuren, Vinylaromaten mit bis zu 20 C-Atomen, ethylenisch ungesättigten Nitrilen, Vinylhalogeniden, Vinylethern von 1 bis 10 C-Atome enthaltenden Alkoholen, aliphatischen Kohlenwasserstoffen mit 2 bis 8 C-Atomen und 1 oder 2 Doppelbindungen oder Mischungen dieser Monomeren.Main monomers B) selected from C 1 -C 20 alkyl (meth) acrylates, vinyl esters of carboxylic acids containing up to 20 C atoms, vinyl aromatics with up to 20 C atoms, ethylenically unsaturated nitriles, vinyl halides are of particular technical importance. Vinyl ethers of alcohols containing 1 to 10 carbon atoms, aliphatic hydrocarbons with 2 to 8 carbon atoms and 1 or 2 double bonds or mixtures of these monomers.
Zu nennen sind z.B. (Meth)acrylsäurealkylester mit einem C1-C10-Alkylrest, wie Methylmethacrylat, Methylacrylat, n-Butylacrylat, Ethylacrylat und 2-Ethylhexylacrylat.Examples include (meth) acrylic acid alkyl esters with a C 1 -C 10 alkyl radical, such as methyl methacrylate, methyl acrylate, n-butyl acrylate, ethyl acrylate and 2-ethylhexyl acrylate.
Insbesondere sind auch Mischungen der (Meth)acrylsäurealkylester geeignet.In particular, mixtures of the (meth) acrylic acid alkyl esters suitable.
Vinylester von Carbonsäuren mit 1 bis 20 C-Atomen sind z.B. Vinyllaurat, -stearat, Vinylpropionat, Versaticsäurevinylester und Vinylacetat.Vinyl esters of carboxylic acids with 1 to 20 C atoms are e.g. Vinyl laurate, stearate, vinyl propionate, vinyl versatic acid and vinyl acetate.
Als vinylaromatische Verbindungen kommen Vinyltoluol, α- und p-Methylstyrol, α-Butylstyrol, 4-n-Butylstyrol, 4-n-Decylstyrol und vorzugsweise Styrol in Betracht. Beispiele für Nitrile sind Acrylnitril und Methacrylnitril.Suitable vinyl aromatic compounds are vinyl toluene, α- and p-methylstyrene, α-butylstyrene, 4-n-butylstyrene, 4-n-decylstyrene and preferably styrene. Examples of nitriles are Acrylonitrile and methacrylonitrile.
Die Vinylhalogenide sind mit Chlor, Fluor oder Brom substituierte ethylenisch ungesättigte Verbindungen, bevorzugt Vinylchlorid und Vinylidenchlorid. The vinyl halides are substituted with chlorine, fluorine or bromine ethylenically unsaturated compounds, preferably vinyl chloride and Vinylidene chloride.
Als Vinylether zu nennen sind z.B. Vinylmethylether oder Vinylisobutylether. Bevorzugt wird Vinylether von 1 bis 4 C-Atome enthaltenden Alkoholen.Examples of vinyl ethers include Vinyl methyl ether or vinyl isobutyl ether. Vinyl ether of 1 to 4 carbon atoms is preferred containing alcohols.
Als Kohlenwasserstoffe mit 2 bis 8 C-Atomen und zwei olefinischen Doppelbindungen seien Butadien, Isopren und Chloropren genannt.As hydrocarbons with 2 to 8 carbon atoms and two olefinic Double bonds are butadiene, isoprene and chloroprene.
Neben diesen Hauptmonomeren können weitere Monomere C), z.B. Hydroxylgruppen enthaltende Monomere, insbesondere C1-C10-Hydroxyalkyl(meth)acrylate oder (Meth)acrylamid, im Polymerisat Verwendung finden.In addition to these main monomers, other monomers C), for example monomers containing hydroxyl groups, in particular C 1 -C 10 -hydroxyalkyl (meth) acrylates or (meth) acrylamide, can be used in the polymer.
Übliche Polymerisate bestehen im allgemeinen zu mindestens 40, vorzugsweise zu mindestens 60, besonders bevorzugt zu mindestens 80 Gew.-% aus den obigen Hauptmonomeren B).Customary polymers generally consist of at least 40, preferably at least 60, particularly preferably at least 80% by weight of the above main monomers B).
Die Polymerisation kann nach üblichen Polymerisationsverfahren erfolgen, z.B. durch Substanz-, Emulsions-, Suspensions-, Dispersions-, Fällungs- und Lösungspolymerisation. Bei den genannten Polymerisationsverfahren wird bevorzugt unter Ausschluß von Sauerstoff gearbeitet, vorzugsweise in einem Stickstoffstrom. Für alle Polymerisationsmethoden werden die üblichen Apparaturen verwendet, z.B. Rührkessel, Rührkesselkaskaden, Autoklaven, Rohrreaktoren und Kneter. Bevorzugt wird nach der Methode der Lösungs-, Emulsions-, Fällungs- oder Suspensionspolymerisation gearbeitet. Besonders bevorzugt sind die Methoden der Lösungs- und insbesondere Emulsionspolymerisation. Die Polymerisation kann in Lösungs- oder Verdünnungsmitteln, wie z.B. Toluol, o-Xylol, p-Xylol, Cumol, Chlorbenzol, Ethylbenzol, technischen Mischungen von Alkylaromaten, Cyclohexan, technischen Aliphatenmischungen, Aceton, Cyclohexanon, Tetrahydrofuran, Dioxan, Glykolen und Glykolderivaten, Polyalkylenglykolen und deren Derivate, Diethylether, tert.-Butylmethylether, Essigsäuremethylester, Isopropanol, Ethanol, Wasser oder Mischungen wie z.B. Isopropanol/Wasser-Mischungen ausgeführt werden. Vorzugsweise wird als Lösungs- oder Verdünnungsmittel Wasser gegebenenfalls mit Anteilen bis zu 60 Gew.-% an Alkoholen oder Glykolen verwendet. Besonders bevorzugt wird Wasser eingesetzt.The polymerization can be carried out by customary polymerization processes take place, e.g. by substance, emulsion, suspension, Dispersion, precipitation and solution polymerization. With the above Polymerization is preferred to exclude worked by oxygen, preferably in a stream of nitrogen. The usual equipment for all polymerization methods used, e.g. Stirred tanks, stirred tank cascades, autoclaves, tubular reactors and kneader. Preference is given to the Solution, emulsion, precipitation or suspension polymerization worked. The methods of solution are particularly preferred and especially emulsion polymerization. The polymerization can in solvents or diluents, e.g. Toluene, o-xylene, p-xylene, Cumene, chlorobenzene, ethylbenzene, technical mixtures of alkyl aromatics, cyclohexane, technical aliphatic mixtures, Acetone, cyclohexanone, tetrahydrofuran, dioxane, glycols and glycol derivatives, Polyalkylene glycols and their derivatives, diethyl ether, tert-butyl methyl ether, methyl acetate, isopropanol, Ethanol, water or mixtures such as Isopropanol / water mixtures be carried out. Preferably, as Solvent or diluent water, if necessary, with proportions up to 60 wt .-% of alcohols or glycols used. Especially water is preferably used.
Die metallvernetzten Polymerisate werden im allgemeinen in Form ihrer wäßrigen Lösung oder Dispersion auf die Faservliese aufgebracht. Nach der Trocknung sind die Faservliese gebunden, d.h. verfestigt. Die Faservliese enthalten dann im allgemeinen 1 bis 40 Gew.-Teile, bevorzugt 5 bis 30 Gew.-Teile, der metallvernetzten Bindemittels, bezogen auf 100 Gew.-Teile Fasern.The metal-crosslinked polymers are generally in the form their aqueous solution or dispersion applied to the nonwovens. After drying, the nonwovens are bound, i.e. solidified. The non-woven fabrics then generally contain 1 to 40 parts by weight, preferably 5 to 30 parts by weight, of the metal-crosslinked Binder, based on 100 parts by weight of fibers.
Nach der späteren Verwendung der gebundenen Faservliese können die Fasern gemäß dem erfindungsgemäßen Verfahren zurückgewonnen werden, indem das BIndemittel aus dem Faservlies, d.h. von den Fasern abgetrennt wird.After the later use of the bonded non-woven fabrics can the fibers are recovered according to the method of the invention by removing the binder from the nonwoven, i.e. of the Fibers is separated.
Dazu wird das Faservlies mit einer wäßrigen Lösung eines Salzes, (im nachfolgenden "lösliches Salz genannt), dessen Anion mit dem Erdalkalikation ein in Wasser schwerlösliches Salz bildet, behandelt.For this, the nonwoven fabric is washed with an aqueous solution of a salt, (hereinafter referred to as "soluble salt), whose anion with the Alkaline earth formation forms a salt that is sparingly soluble in water.
Bei den löslichem Salz kann es sich um ein anorganisches oder organisches Salz handeln.The soluble salt can be inorganic or organic Trade salt.
Insbesondere handelt es sich um ein Alkalimetallsalz.In particular, it is an alkali metal salt.
Bei dem Anion des löslichen Salzes kann es sich z.B. um Oxalat oder Carbonat handeln, welche z.B. mit dem Calziumkation ein schwerlösliches Salz bilden.The anion of the soluble salt can e.g. around oxalate or carbonate, which e.g. with the calcium cation form sparingly soluble salt.
Bei dem Anion des löslichen Salzes kann es sich z.B. auch um ein organisches Anion handeln, welches mit den Erdalkalikation, z.B. Ca2+, einem schwerlöslichen Komplex bildet. Als Anion zu nennen ist hier insbesondere EDTA.The anion of the soluble salt can also be, for example, an organic anion which forms a poorly soluble complex with the alkaline earth metal, for example Ca 2+ . EDTA is particularly worth mentioning here.
Das lösliche Salz, das in der wäßrigen Lösung enthalten ist, hat vorzugsweise eine Löslichkeit von mindestens 10 g/·l Wasser bei 23°C.The soluble salt contained in the aqueous solution has preferably a solubility of at least 10 g / l water 23 ° C.
Die wäßrige Lösung enthält das Salz vorzugsweise in Mengen von 0,02 bis 15 Gew.-Teile, besonders bevorzugt in Mengen von 0,1 bis 10 Gew.-Teilen, ganz besonders bevorzugt von 0,5 bis 2,5 Gew.-Teilen, bezogen auf 100 Gew.-Teile Wasser.The aqueous solution preferably contains the salt in amounts of 0.02 to 15 parts by weight, particularly preferably in amounts of 0.1 to 10 parts by weight, very particularly preferably from 0.5 to 2.5 parts by weight, based on 100 parts by weight of water.
Die Löslichkeit des bei Behandlung des Faservlieses gebildeten, schwerlöslichen Erdalkalisalzes ist dagegen vorzugsweise kleiner als 0,5 g/l Wasser bei 23°C.The solubility of the formed during treatment of the nonwoven sparingly soluble alkaline earth metal salt, however, is preferably smaller than 0.5 g / l water at 23 ° C.
Die wäßrige Lösung enthält neben dem löslichen Salz vorzugsweise noch einen Phasentransferkatalysator.In addition to the soluble salt, the aqueous solution preferably contains another phase transfer catalyst.
Geeignete Phasentransferkatalysatoren sind z.B. in Chimia 34 (1980) Nr. 1, Seite 12 bis 20. Als Phasentransferkatalysatoren genannt seien z.B. Polyalkylenglykole, welcher z.B. unter dem Namen Pluriol® im Handel ist oder quaternäre, organische Ammoniumsalze, welche z.B. unter dem Namen Lutensit im Handel sind.Suitable phase transfer catalysts are e.g. in Chimia 34 (1980) No. 1, pages 12 to 20. As phase transfer catalysts may be mentioned e.g. Polyalkylene glycols which e.g. is commercially available under the name Pluriol® or quaternary, organic Ammonium salts, e.g. commercially available under the name Lutensit are.
Als quaternäre, organische Ammoniumsalze seien insbesondere solche der Formel I genannt, worin R1-R4 unabhängig voneinander für einen organischen Rest, bevorzugt einen Kohlenwasserstoffrest mit 1 bis 12 C-Atomen, bevorzugt 1 bis 6 C-Atomen stehen und X⊖ für ein Anion, bevorzugt ein anorganisches Anion z.B. Cl-, Br⊖ steht.Quaternary organic ammonium salts include, in particular, those of the formula I. mentioned, wherein R 1 -R 4 independently of one another are an organic radical, preferably a hydrocarbon radical having 1 to 12 carbon atoms, preferably 1 to 6 carbon atoms and X ⊖ is an anion, preferably an inorganic anion, for example Cl - , Br ⊖ stands.
Besonders bevorzugte Phasentransferkatalysatoren sind solche mit Alkoxygruppen, bevorzugt mit 2 bis 20 Alkoxygruppen, z.B. Alkylphenolethoxylate (z.B. Lutensole® AP, Emulgator 825 Fettalkoholethoxylate (z.B. Lutensol A8) und Oxo-alkoholethoxylate (z.B. Lutensol AO7, Lutensol ON80).Particularly preferred phase transfer catalysts are those with Alkoxy groups, preferably with 2 to 20 alkoxy groups, e.g. Alkylphenol ethoxylates (e.g. Lutensole® AP, emulsifier 825 fatty alcohol ethoxylates (e.g. Lutensol A8) and oxo alcohol ethoxylates (e.g. Lutensol AO7, Lutensol ON80).
Durch Alkoxylierung von Alkylphenolen, Fettalkoholen bzw. von Oxoalkoholen mit Alkylenoxiden, vorzugsweise Ethylenoxid enthalten diese Phasentransferkatalysatoren Alkoxygruppen.By alkoxylation of alkylphenols, fatty alcohols or Contain oxo alcohols with alkylene oxides, preferably ethylene oxide these phase transfer catalysts alkoxy groups.
Der Gehalt des Phasentransferkatalysators in der wäßrigen Lösung beträgt vorzugsweise 0,01 bis 1 Gew.-Teil, besonders bevorzugt 0,08 bis 0,5 Gew.-Teile, bezogen auf 100 Gew.-Teile Wasser.The content of the phase transfer catalyst in the aqueous solution is preferably 0.01 to 1 part by weight, particularly preferably 0.08 to 0.5 part by weight, based on 100 parts by weight of water.
Zusätzlich können der wäßrigen Lösung z.B. noch Basen, insbesondere Natronlauge zugesetzt werden, um den Anteil von Alkalikationen zu erhöhen.In addition, e.g. still bases, especially Sodium hydroxide solution is added to the proportion of alkali cations to increase.
Die Temperatur der wäßrigen Lösung kann z.B. 10 bis 100°C, insbesondere 15 bis 80°C und besonders bevorzugt 20 bis 50°C betragen. Vorteilhaft sind Temperaturen über 30, insbesondere über 40°C, um eine noch bessere und vor allem schnellere Ablösung des Bindemittels von dem Faservlies zu erreichen.The temperature of the aqueous solution can e.g. 10 to 100 ° C, in particular 15 to 80 ° C and particularly preferably 20 to 50 ° C. Temperatures above 30, in particular above, are advantageous 40 ° C, for an even better and, above all, faster replacement of the To reach binder from the nonwoven.
Das Faservlies kann als ganzes oder in zerkleinerter Form den erfindungsgemäßen Verfahren zugeführt werden. The nonwoven fabric can be used as a whole or in shredded form method according to the invention are supplied.
Bevorzugt wird das Faservlies in Teile mit einer Kantenlänge von 1 bis 10 cm zerkleinert.The nonwoven fabric is preferably divided into parts with an edge length of Crushed 1 to 10 cm.
Zur Behandlung mit der wäßrigen Lösung wird das Faservlies vorzugsweise in die wäßrige Lösung gegeben. Die Menge des Faservlieses beträgt dabei vorzugsweise 1 bis 200 g, besonders bevorzugt 5 bis 150 g und ganz besonders bevorzugt 10 bis 80 g pro Liter Lösung. Die Behandlungsdauer kann durch intensives Rühren verkürzt werden. Im allgemeinen liegt die notwendige Dauer bei 5 Minuten bis 1 Stunde. Bei starkem Rühren sind jedoch schon weniger als 30 Minuten, insbesondere weniger als 20 Minuten ausreichend um mindestens 80 Gew.-% des Bindemittels von der Faser zu lösen und die Fasern zurückzugewinnen.For treatment with the aqueous solution, the nonwoven fabric is preferred added to the aqueous solution. The amount of nonwoven is preferably 1 to 200 g, particularly preferably 5 up to 150 g and very particularly preferably 10 to 80 g per liter Solution. The duration of treatment can be shortened by intensive stirring become. Generally, the time required is 5 minutes up to 1 hour. With strong stirring, however, are less sufficient than 30 minutes, in particular less than 20 minutes to detach at least 80% by weight of the binder from the fiber and recover the fibers.
Polyester-Spinnvliese der Fa. Hoechst, Bobingen wurden imprägniert mit Acronal® DS 2324x (Bindemittelauftrag von 20 Gew.-% ±2 (fest/fest). Bei Acronal DS 2324X handelt es sich um eine wäßrige Dispersion eines metallsalzvernetzten Polymerisats (Vernetzung der Carboxylatgruppen des Polymerisats mit Ca2+ Kationen) auf Acrylatbasis. Die Trocknung erfolgte bei 5 Min. bei 200°C im Mathis Labortrockner. Anschließend wurden die in etwa DIN A-4 großen Vliesbögen in Stücke von ca. 1 cm2 geschnitten.Polyester spunbonded fabrics from Hoechst, Bobingen, were impregnated with Acronal® DS 2324x (binder application of 20% by weight ± 2 (solid / solid). Acronal DS 2324X is an aqueous dispersion of a metal salt-crosslinked polymer (crosslinking of the carboxylate groups of the polymer with Ca 2+ cations) on an acrylate basis, the drying was carried out at 5 minutes at 200 ° C. in a Mathis laboratory dryer, after which the approximately A-4 sized fleece sheets were cut into pieces of about 1 cm 2 .
Es wurde eine kaltgesättigte Natriumoxalatlösung (ca. 3 Gew.-%) und jeweils eine 10 gew.-%ige Lösung von Soda bzw. Na4 EDTA (Trilon B) in Wasser hergestellt. In je 250 g der erhaltenen Lösung wurden die in der Tabelle angegebenen Mengen des Phasentransferkatalysators und gegebenenfalls noch Natronlauge gegeben.A cold saturated sodium oxalate solution (approx. 3% by weight) and in each case a 10% by weight solution of soda or Na 4 EDTA (Trilon B) in water were prepared. In each 250 g of the solution obtained, the amounts of the phase transfer catalyst indicated in the table and optionally sodium hydroxide solution were added.
Zur Rückgewinnung der bindemittelfreien Fasern aus den Faservliesen wurden Stücke des zerkleinerten Faservlieses (s.o.) in 250 g der Lösung gegeben. Die Temperatur der Lösung und die Menge des Faservlieses ist in der Tabelle angegeben.For the recovery of binder-free fibers from the nonwovens pieces of the shredded nonwoven (see above) were in Given 250 g of the solution. The temperature of the solution and the amount of the nonwoven is shown in the table.
Die Vliesstücke wurden nach ca. 24 h Standzeit mittels einem Laborrührer 15 Min mit 2000 U/min aufgerührt.The fleece pieces were cleaned after about 24 hours using a laboratory stirrer Stirred at 2000 rpm for 15 min.
Anschließend wurden die Fasern über ein 60 µ-Sieb abfiltriert, 2 h bei 130°C getrocknet und zurückgewogen.The fibers were then filtered through a 60 μ sieve, 2 h dried at 130 ° C and weighed.
Der in der Tabelle angegebene Rezykliergrad R berechnet sich gemäß
Im Falle, daß die zurückgewogenen Fasern völlig bindemittelfrei sind, ergibt sich ein Recyclierungsgrad von 100 %. Werte über 100 % in der Tabelle erklären sich dadurch, daß bei Abfiltrierung der Fasern gegebenenfalls sehr feine Fasern vom Sieb nicht zurückgehalten wurden und so das Gewicht der Fasern nach Recyclierung zu gering war. In the event that the weighed fibers are completely free of binders, the degree of recycling is 100%. Values above 100% in the table can be explained by the fact that when the fibers were filtered off, very fine fibers were possibly not retained by the sieve and the weight of the fibers after recycling was too low.
Claims (7)
- A process for dissolving a binder off a fiber web bonded therewith, which comprisestreating the fiber web, which is bonded with a polymeric binder having carboxylate groups crosslinked via alkaline earth metal cations, with an aqueous solution of an alkali metal salt to form a sparingly soluble salt or complex between the anion of the alkali metal salt and the alkaline earth metal cations, and thenremoving the fiber freed of the binder.
- A process as claimed in claim 1, wherein the aqueous solution further comprises a phase transfer catalyst.
- A process as claimed in claim 1 or 2, wherein the phase transfer catalyst is selected from the group consisting of the alkylphenol ethoxylates, fatty alcohol ethoxylates, oxo alcohol ethoxylates and quaternary organic ammonium salts.
- A process as claimed in any of claims 1 to 3, wherein the alkaline earth metal cations are Ca2+.
- A process as claimed in any of claims 1 to 4, wherein the polymeric binder bonding the fiber web is a free-radically polymerized polymer having a carboxylate group content of from 0.1 to 30% by weight, based on the polymer, and from 50 to 100% by weight of the carboxylate groups are present as a salt with an alkaline earth metal cation.
- A process as claimed in any of claims 1 to 5, wherein the aqueous solution of the alkali metal salt has a temperature of from 10 to 100°C.
- A process as claimed in any of claims 1 to 6, wherein the fiber web is introduced into the aqueous solution of the alkali metal salt, the aqueous solution is stirred if necessary, and the fiber freed of the binder is separated off after from 1 to 60 minutes.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19619639A DE19619639A1 (en) | 1996-05-15 | 1996-05-15 | Recovery of fibers from bonded nonwovens |
| DE19619639 | 1996-05-15 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0807704A1 EP0807704A1 (en) | 1997-11-19 |
| EP0807704B1 true EP0807704B1 (en) | 2000-11-02 |
Family
ID=7794430
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP97107491A Expired - Lifetime EP0807704B1 (en) | 1996-05-15 | 1997-05-07 | Recovery of fibres from bonded nonwovens |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US6004428A (en) |
| EP (1) | EP0807704B1 (en) |
| AT (1) | ATE197323T1 (en) |
| DE (2) | DE19619639A1 (en) |
| ES (1) | ES2152593T3 (en) |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6429261B1 (en) | 2000-05-04 | 2002-08-06 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6444214B1 (en) | 2000-05-04 | 2002-09-03 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6495080B1 (en) | 1997-10-03 | 2002-12-17 | Kimberly-Clark Worldwide, Inc. | Methods for making water-sensitive compositions for improved processability and fibers including same |
| US6548592B1 (en) | 2000-05-04 | 2003-04-15 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6579570B1 (en) | 2000-05-04 | 2003-06-17 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6586529B2 (en) | 2001-02-01 | 2003-07-01 | Kimberly-Clark Worldwide, Inc. | Water-dispersible polymers, a method of making same and items using same |
| US6599848B1 (en) | 2000-05-04 | 2003-07-29 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6630558B2 (en) | 1998-12-31 | 2003-10-07 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive hard water dispersible polymers and applications therefor |
| US6653406B1 (en) | 2000-05-04 | 2003-11-25 | Kimberly Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6683143B1 (en) | 2000-05-04 | 2004-01-27 | Kimberly Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6713414B1 (en) | 2000-05-04 | 2004-03-30 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6815502B1 (en) | 2000-05-04 | 2004-11-09 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersable polymers, a method of making same and items using same |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5969052A (en) | 1996-12-31 | 1999-10-19 | Kimberly Clark Worldwide, Inc. | Temperature sensitive polymers and water-dispersible products containing the polymers |
| US5986004A (en) | 1997-03-17 | 1999-11-16 | Kimberly-Clark Worldwide, Inc. | Ion sensitive polymeric materials |
| US5935880A (en) * | 1997-03-31 | 1999-08-10 | Wang; Kenneth Y. | Dispersible nonwoven fabric and method of making same |
| US6043317A (en) | 1997-05-23 | 2000-03-28 | Kimberly-Clark Worldwide, Inc. | Ion sensitive binder for fibrous materials |
| US6835678B2 (en) | 2000-05-04 | 2004-12-28 | Kimberly-Clark Worldwide, Inc. | Ion sensitive, water-dispersible fabrics, a method of making same and items using same |
| US7255816B2 (en) * | 2000-11-10 | 2007-08-14 | Kimberly-Clark Worldwide, Inc. | Method of recycling bonded fibrous materials and synthetic fibers and fiber-like materials produced thereof |
| DE10103213A1 (en) | 2001-01-25 | 2002-08-14 | Wacker Polymer Systems Gmbh | Process for the production of recyclable moldings |
| US6828014B2 (en) | 2001-03-22 | 2004-12-07 | Kimberly-Clark Worldwide, Inc. | Water-dispersible, cationic polymers, a method of making same and items using same |
| US7772138B2 (en) | 2002-05-21 | 2010-08-10 | Kimberly-Clark Worldwide, Inc. | Ion sensitive, water-dispersible polymers, a method of making same and items using same |
| DE102005037113A1 (en) * | 2005-08-03 | 2007-02-08 | Basf Ag | Use of a thermally curable aqueous composition as a binder for substrates |
| DE102009010938A1 (en) * | 2009-02-27 | 2010-09-09 | Celanese Emulsions Gmbh | Mineral wool fiber mats, process for their preparation and use |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4009313A (en) * | 1972-08-30 | 1977-02-22 | Minnesota Mining And Manufacturing Company | Enzymatically dispersible non-woven webs |
| DE2747182C2 (en) * | 1977-10-20 | 1985-08-14 | Wacker-Chemie GmbH, 8000 München | Binders for nonwovens |
| US5082697A (en) * | 1988-02-17 | 1992-01-21 | The Dow Chemical Company | Polymer salt complex for fiber or fabric treatment |
| DE4004915A1 (en) * | 1990-02-16 | 1991-08-22 | Basf Ag | WAFER POLYMERISATE DISPERSIONS |
| US5270376A (en) * | 1990-02-16 | 1993-12-14 | Basf Aktiengesellschaft | Aqueous polymer dispersion having divalent metal salt(s) incorporated therein |
| US5149543A (en) * | 1990-10-05 | 1992-09-22 | Massachusetts Institute Of Technology | Ionically cross-linked polymeric microcapsules |
| DE19535792A1 (en) * | 1995-09-26 | 1997-03-27 | Basf Ag | Process for the production of recyclable fiber composites |
-
1996
- 1996-05-15 DE DE19619639A patent/DE19619639A1/en not_active Withdrawn
-
1997
- 1997-05-06 US US08/852,064 patent/US6004428A/en not_active Expired - Fee Related
- 1997-05-07 ES ES97107491T patent/ES2152593T3/en not_active Expired - Lifetime
- 1997-05-07 AT AT97107491T patent/ATE197323T1/en not_active IP Right Cessation
- 1997-05-07 EP EP97107491A patent/EP0807704B1/en not_active Expired - Lifetime
- 1997-05-07 DE DE59702552T patent/DE59702552D1/en not_active Expired - Fee Related
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6495080B1 (en) | 1997-10-03 | 2002-12-17 | Kimberly-Clark Worldwide, Inc. | Methods for making water-sensitive compositions for improved processability and fibers including same |
| US6630558B2 (en) | 1998-12-31 | 2003-10-07 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive hard water dispersible polymers and applications therefor |
| US6429261B1 (en) | 2000-05-04 | 2002-08-06 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6444214B1 (en) | 2000-05-04 | 2002-09-03 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6548592B1 (en) | 2000-05-04 | 2003-04-15 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6579570B1 (en) | 2000-05-04 | 2003-06-17 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6599848B1 (en) | 2000-05-04 | 2003-07-29 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6653406B1 (en) | 2000-05-04 | 2003-11-25 | Kimberly Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6683143B1 (en) | 2000-05-04 | 2004-01-27 | Kimberly Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6713414B1 (en) | 2000-05-04 | 2004-03-30 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersible polymers, a method of making same and items using same |
| US6815502B1 (en) | 2000-05-04 | 2004-11-09 | Kimberly-Clark Worldwide, Inc. | Ion-sensitive, water-dispersable polymers, a method of making same and items using same |
| US6586529B2 (en) | 2001-02-01 | 2003-07-01 | Kimberly-Clark Worldwide, Inc. | Water-dispersible polymers, a method of making same and items using same |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2152593T3 (en) | 2001-02-01 |
| DE59702552D1 (en) | 2000-12-07 |
| DE19619639A1 (en) | 1997-11-20 |
| ATE197323T1 (en) | 2000-11-15 |
| EP0807704A1 (en) | 1997-11-19 |
| US6004428A (en) | 1999-12-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0807704B1 (en) | Recovery of fibres from bonded nonwovens | |
| DE69809639T2 (en) | (HYDROXYALKYL) UREA CROSSLINKER | |
| DE19721347C2 (en) | Process for the production of ester plasticizers | |
| EP0384125B1 (en) | Film-forming self-curing aqueous plastic dispersions | |
| EP0084809B1 (en) | Dispersion of acryl plastics | |
| DE2009218B2 (en) | Process for the bead polymerization of ethylenically unsaturated monomers | |
| EP0143175B1 (en) | Cross-linkable resinous aqueous dispersions not containing formaldehyde, method for their preparation and their application | |
| DE60108157T2 (en) | PRECIOUS METAL SEPARATION FROM LIQUIDS USING FUNCTIONALIZED POLYMER FIBERS | |
| EP0019169A1 (en) | Use of an aqueous dispersion of an emulsion copolymer that contains amide groups for bonding nonwovens | |
| EP2199333B1 (en) | Binder-strengthened textile sheet, method for its production and application | |
| DE2504514A1 (en) | FLUORINE CARBONIC ACID AMIDES AND THEIR POLYMERIZATION PRODUCTS | |
| DE4040959C1 (en) | ||
| DE60027507T2 (en) | Fire-retardant for textiles and method for refractory of textiles | |
| DE951300C (en) | Process for the production of an acrylonitrile plastic mass which is easy to color | |
| EP0033115A2 (en) | Process for finishing textiles | |
| DE1469378B2 (en) | MAKING FIBERS AND FIBER BUILDINGS DIRT-REPELLENT | |
| DE69203556T2 (en) | N-allyl-N-dialkoxyethylamide or amine monomers. | |
| DE1232103B (en) | Process for the production of coatings, impregnations and gluing of fiber substrates such as fabrics, fleeces as well as for the lamination of foams with fabrics using graft polymers | |
| DE2326053C3 (en) | Process for the production of a fire retardant polymer and its use | |
| DE2250972A1 (en) | USE OF ION EXCHANGERS IN THE WASHING PROCESS | |
| DE1105176B (en) | Process for the isolation of solid chlorinated or sulfochlorinated homopolymers or copolymers of olefins from their solutions | |
| DE1300823C2 (en) | PROCESS FOR REMOVING PRINTED PAPER PRODUCTS | |
| DE1444068C (en) | Process for the production of nonwovens | |
| DE1769699A1 (en) | Process for the production of bonded nonwovens by the wet process | |
| DE4135499A1 (en) | FLEECE EQUIPMENT |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19970904 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE ES FR GB IT LI NL SE |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| 17Q | First examination report despatched |
Effective date: 20000229 |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE ES FR GB IT LI NL SE |
|
| REF | Corresponds to: |
Ref document number: 197323 Country of ref document: AT Date of ref document: 20001115 Kind code of ref document: T |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20001109 |
|
| REF | Corresponds to: |
Ref document number: 59702552 Country of ref document: DE Date of ref document: 20001207 |
|
| ITF | It: translation for a ep patent filed | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2152593 Country of ref document: ES Kind code of ref document: T3 |
|
| ET | Fr: translation filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010507 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010606 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010606 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20020412 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20020415 Year of fee payment: 6 Ref country code: NL Payment date: 20020415 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20020501 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20020507 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20020521 Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030507 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030508 Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20030531 |
|
| BERE | Be: lapsed |
Owner name: *BASF A.G. Effective date: 20030531 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20031201 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20030507 |
|
| EUG | Se: european patent has lapsed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20040130 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20031201 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20030508 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050507 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20080515 Year of fee payment: 12 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20091201 |