EP0451437A2 - Tôle galvanisée en acier ayant des caractéristiques améliorées de déformabilité par pression et sa méthode de fabrication - Google Patents
Tôle galvanisée en acier ayant des caractéristiques améliorées de déformabilité par pression et sa méthode de fabrication Download PDFInfo
- Publication number
- EP0451437A2 EP0451437A2 EP91100089A EP91100089A EP0451437A2 EP 0451437 A2 EP0451437 A2 EP 0451437A2 EP 91100089 A EP91100089 A EP 91100089A EP 91100089 A EP91100089 A EP 91100089A EP 0451437 A2 EP0451437 A2 EP 0451437A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- steel sheet
- galvanized steel
- inorganic compound
- press formability
- high press
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C22/00—Chemical surface treatment of metallic material by reaction of the surface with a reactive liquid, leaving reaction products of surface material in the coating, e.g. conversion coatings, passivation of metals
- C23C22/78—Pretreatment of the material to be coated
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M103/00—Lubricating compositions characterised by the base-material being an inorganic material
- C10M103/06—Metal compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M173/00—Lubricating compositions containing more than 10% water
- C10M173/02—Lubricating compositions containing more than 10% water not containing mineral or fatty oils
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/02—Water
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/0603—Metal compounds used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/061—Carbides; Hydrides; Nitrides
- C10M2201/0613—Carbides; Hydrides; Nitrides used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/062—Oxides; Hydroxides; Carbonates or bicarbonates
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/062—Oxides; Hydroxides; Carbonates or bicarbonates
- C10M2201/0623—Oxides; Hydroxides; Carbonates or bicarbonates used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/063—Peroxides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/065—Sulfides; Selenides; Tellurides
- C10M2201/0653—Sulfides; Selenides; Tellurides used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/06—Metal compounds
- C10M2201/065—Sulfides; Selenides; Tellurides
- C10M2201/066—Molybdenum sulfide
- C10M2201/0663—Molybdenum sulfide used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
- C10M2201/0803—Inorganic acids or salts thereof used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
- C10M2201/081—Inorganic acids or salts thereof containing halogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
- C10M2201/082—Inorganic acids or salts thereof containing nitrogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/08—Inorganic acids or salts thereof
- C10M2201/084—Inorganic acids or salts thereof containing sulfur, selenium or tellurium
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/085—Phosphorus oxides, acids or salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/085—Phosphorus oxides, acids or salts
- C10M2201/0853—Phosphorus oxides, acids or salts used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/086—Chromium oxides, acids or salts
- C10M2201/0863—Chromium oxides, acids or salts used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/087—Boron oxides, acids or salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/087—Boron oxides, acids or salts
- C10M2201/0873—Boron oxides, acids or salts used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/1006—Compounds containing silicon used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/102—Silicates
- C10M2201/1023—Silicates used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/102—Silicates
- C10M2201/103—Clays; Mica; Zeolites
- C10M2201/1033—Clays; Mica; Zeolites used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/105—Silica
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/10—Compounds containing silicon
- C10M2201/105—Silica
- C10M2201/1053—Silica used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2201/00—Inorganic compounds or elements as ingredients in lubricant compositions
- C10M2201/12—Glass
- C10M2201/123—Glass used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/02—Groups 1 or 11
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/04—Groups 2 or 12
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2050/00—Form in which the lubricant is applied to the material being lubricated
- C10N2050/015—Dispersions of solid lubricants
- C10N2050/02—Dispersions of solid lubricants dissolved or suspended in a carrier which subsequently evaporates to leave a lubricant coating
Definitions
- the present invention relates to a galvanized steel sheet with a high press formability characteristic adapted for use as an automotive non-corrosion steel sheet and a method for manufacturing the same.
- alloy electroplated steel sheets such as hot dip galvanized steel sheets, galvannealed steel sheets, electrogalvanized steel sheets, steel sheets electrogalvanized with Zn-Ni, etc., have been developed and placed into use.
- the inventors hereof examined the press formability of those galvanized steel sheets. Thereupon, it was revealed that the galvanized steel sheets, in contrast with the conventionally used cold-rolled steel sheets, are subjected to so great a frictional resistance against a mold during press forming operation that their press formability is relatively low.
- the steel sheet is prevented from being smoothly introduced into those portions which are subjected to hard sliding motion, such as a bead portion of a press mold for fixing the steel sheet at the time of press forming.
- the steel sheet may be broken. Since the proper cushion pressure range (range of cushion pressure within which the steel sheet cannot be wrinkled or broken) of the galvanized steel sheets for the press forming is much narrower than that of the cold-rolled steel sheets, the galvanized sheets are poorer in productivity. Therefore, an improvement of the frictional characteristics of the galvanized steel sheets is urgently needed.
- the frictional characteristics experienced during a press forming operation are greatly influenced by the properties of the plating surface which is directly in contact with the mold. Accordingly, the frictional characteristics are tentatively improved by coating the plating surface with some material other than a zinc plating, e.g. an organic high molecular film, so that the plating surface is kept from direct contact with the mold and is lubricated.
- some material other than a zinc plating e.g. an organic high molecular film
- a method for improving the formability of a steel sheet by improving the frictional characteristics of the sheet surface is disclosed in Published Examined Japanese Patent Application No. 61-26600, for example. According to this method, a specific organic high molecular film is formed on the steel sheet surface. Further, a lubricated-surface steel sheet is proposed which is coated with a film composed mainly of metallic soap or higher fatty acid wax, for example.
- galvanized steel sheets When galvanized steel sheets are used for automotive purposes, they are usually phosphated before being coated after the press forming operation. In doing this, however, the organic film partially remains on the steel sheet without being thoroughly removed by alkali degreasing as a pretreatment for the phosphating process. Therefore, a normal crystal of a phosphate on galvanized layer cannot be produced by the phosphating process. As a result, the adhesion of the coating film is lowered, so that the corrosion resistance of the coated steel sheet is deteriorated.
- the object of the present invention is to provide a galvanized steel sheet having better frictional characteristics and considerably improved in press formability without lowering the phosphatability thereof.
- a galvanized steel sheet with high press formability comprising an inorganic compound on a metallic deposit, the friction coefficient of the galvanized steel sheet being 80% or less of the friction coefficient obtained without the existence of the inorganic compound, and the amount of insoluble slimes of the inorganic compound left after water washing or alkali degreasing being less than 1 mg/m2.
- a method for manufacturing a galvanized steel sheet with high press formability comprising steps of bringing the plating surface of the galvanized steel sheet into contact with a water solution containing 0.1 wt% or more of a borate of alkaline metal, and drying the steel sheet by heating within a temperature range of 60 to 400°C.
- the inventors hereof examined the relationship between the coefficient of friction and press formability of a galvanized steel sheet. Thereupon, it was observed that the friction coefficient of a galvanized steel sheet, subjected to galvanizing such as electrogalvanizing, galvannealing, etc., is 0.15 or more, compared with about 0.10 for that of a conventional cold-rolled steel sheet with high press formability.
- the friction coefficient is a value which is obtained by oiled-state measurement in a draw-bead extraction test.
- the galvanized steel sheet has such a high friction coefficient because zinc and zinc alloy have so low a melting point and so great an affinity for other metals, especially for cast iron and the like frequently used for press molds, that they can easily stick to a mold. Since the frictional characteristics during press forming are influenced by the properties of the plating surface which is brought directly into contact with the mold, it was supposed that the frictional characteristics can be improved by coating the plating surface with some other material than a zinc plating so that the un-coated plating surface is kept from direct contact with the mold and is lubricated.
- the inventors continued the examination to find that the aforementioned problem on the press forming can be solved by providing a specific inorganic compound on the plating surface so that the resulting friction coefficient is 80% or less of the friction coefficient obtained without any specific substance on the plating surface, preferably 0.15 or less.
- a galvanized steel sheet according to the invention has an inorganic compound on its metallic galvanized layer.
- Such galvanized steel sheets include galvannealed steel sheets, hot dip galvanized steel sheets, electrogalvanized steel sheets, steel sheets electrogalvanized with alloyed zinc, such as Zn-Ni, Zn-Fe, etc., and steel sheets flashed with Fe-P, Fe-Zn, etc., for example.
- the inorganic compound should be one whose friction coefficient can be lowered when it exists on the metallic galvanized layer of the galvanized steel sheet, and the greater part of which can be removed by dissolution in water or by alkali degreasing. Any inorganic compounds may be used for the purpose provided they fulfill these requirements.
- Inorganic compounds suitably used according to the present invention include borates, carbonates, phosphates, sulfates, nitrates, chlorides, hydroxides, and oxides of alkaline metals such as Na and K, alkaline earth metals such as Ca and Mg, and metals or metalloids such as Fe, Ni, Co, Al, Ti, Si, etc., for example.
- the galvanized steel sheet according to the present invention can be easily manufactured by bringing the steel sheet into contact with a water solution of the inorganic compound and then drying the resulting sheet, as mentioned later.
- the inorganic compound is expected to be water-soluble.
- the inorganic compound should preferably be low-priced, in view of costs, and be highly soluble in water or basic aqueous solutions, since it is to be dissolved and removed by water washing or alkali degreasing.
- salts of alkaline metals are particularly suited for the purpose.
- hydrous borates of alkaline metals are very effective for the improvement of the frictional characteristics, and they typically include borax (sodium tetraborate: Na2B4O7 ⁇ 10H2O) which can be industrially mass-produced and is low-priced.
- hydrous borates of alkaline metals greatly surpasses an anhydrous one in solubility in water for washing or alkali for alkali degreasing.
- film is thoroughly dissolved by the alkali degreasing preceding the phosphating, and never remains on the steel sheet at all. This is the reason why hydrous borates of alkaline metals are preferably used.
- the inorganic compound on the zinc-based plating is not limited in form.
- a filmy or particulate compound is used for the purpose.
- the coating weight of the inorganic compound on the surface of the galvanized steel sheet has to be adjusted so that the friction coefficient of the galvanized steel sheet with the inorganic compound on plated layer is 80% or less of that of a galvanized steel sheet with no inorganic compound thereon, preferably 0.15 or less at the absolute value of coefficient of friction.
- the amount of insoluble slimes of the inorganic compound should be less than 1 mg/m2 at the end of the degreasing process before the phosphating process, lest the production of a satisfactory phosphate crystal be hindered during the phosphating process.
- the coating weight of a hydrous borate of alkaline metal is restricted to a range of 10 to 1,000 mg/m2 for the following reasons. If the coating weight is less than 10 mg/m2, a satisfactory improvement of the frictional characteristics cannot be achieved. If the coating weight exceeds 1,000 mg/m2, on the other hand, the effect of the improvement of the frictional characteristics is saturated, and besides, the film partially remains on the plated layer without being thoroughly removed in the degreasing process before the phosphating process, thus exerting a bad influence on the subsequent phosphating process.
- the coating weight ranges from 100 to 1,000 mg/m2.
- Fig. 1 shows the relationships between the coating weight of borax (sodium tetraborate: Na2B4O7 ⁇ 5H2O) on a galvannealed steel sheet, the coefficient of friction obtained in a frictional characteristic test, and the water-resistant secondary adhesion. These individual tests are conducted in the manner described later in connection with various examples. As shown in Fig. 1, the frictional characteristics can be greatly improved without affecting satisfactory phosphatability in the case where the coating weight of borax ranges from 10 to 1,000 mg/m2.
- a galvanized steel sheet having the aforesaid film of the hydrous borate of alkaline metal thereon may be manufactured in the following manner.
- the galvanized steel sheet is brought into contact with a water solution containing 0.1 wt% or more of the borate of alkaline metal by dipping, spraying, or coating by means of a roll coater or the like, and is then dried by heating within a temperature range of 60 to 400°C.
- the borate content of the alkaline metal is adjusted to 0.1 wt% or more for the following reason.
- the present method in which the galvanized steel sheet is dried by heating after being brought into contact with the aqueous solution, entails some operational disadvantages, such as lowering of line speed.
- the lower limit of the heating temperature range for the drying is adjusted to 60°C because if the heating temperature is lower than 60°C, a solid film effective for the improvement of the frictional characteristics as aforesaid cannot be formed on the plating.
- the upper limit is set at 400°C because if the sheet is heated to a temperature exceeding 400°C, the hydrous crystal of the borate of alkaline metal turns into an anhydrous crystal, so that borate film turns into one, which is difficult to dissolve.
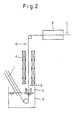
- Fig. 2 shows a process for manufacturing the galvanized steel sheet.
- numeral 1 denotes the steel sheet; 2, a hot zinc pot; 3, coating weight control means; 4, a galvannealing furnace; and 5, a cooling zone.
- a aqueous solution containing the aforesaid borate of alkaline metal is sprayed so that the steel sheet is brought into contact with a mist of the solution.
- the solution containing the borate of alkaline metal may be sprayed at any positions kept at a temperature within the temperature range for the steel sheet.
- the spraying can be effected at positions A, B and C of Fig. 2, which will be described later in connection with the examples.
- a aqueous solution containing 5 wt% of potassium tetraborate (K2B4O7 ⁇ 4H2O) was sprayed on the steel sheet at a position (position A of Fig. 2) reached immediately after the steel sheet is wiped over the melt plating pot.
- the sheet temperature immediately before the spraying was 380°C.
- a aqueous solution containing 0.7 wt% of sodium metaborate (NaBO2 ⁇ 4H2O) was sprayed on the steel sheet at a position (position B of Fig. 2) reached immediately after the steel sheet gets out of the alloying furnace.
- the sheet temperature immediately before the spraying was 250°C.
- An electrogalvanized steel sheet with the coating weight of 60/60 g/m2 (equivalent to the green material of Example 1).
- a hot dip galvanized steel sheet with the coating weight of 90/90 g/m2 (equivalent to the green material of Example 4).
- a galvannealed steel sheet with the coating weight of 45/45 g/m2 and alloy content of 9/9% (equivalent to the green material of Example 5).
- Rust preventing oil containing 10 wt% of molybdenum sulfide (MoS2) was applied to a steel sheet electroplated with Zn-Ni alloy having the coating weight of 30/30 g/m2 and Ni content of 11/11%.
- a force D F required for the extraction of a sample 7 when a roll 6 was fixed and a force D R required when the roll 6 was allowed to rotate were obtained, as shown in Fig. 3, and a friction coefficient ⁇ of the sample 7 was calculated according to the following equation based on these two values, whereby the frictional characteristics were evaluated.
- the friction coefficient ratio compared with 100% for the friction coefficient of a similar galvanized steel sheet (green material) was also calculated.
- ⁇ is the friction coefficient between the roll and the sample
- p force applied in the diametrical direction of the roll
- R roll radius
- ⁇ center angle
- P F thrust load of a center punch.
- test conditions are given as follows: Sample size: 20 400 mm Sliding speed: 500 mm/sec. Distance of slide: 100 mm Thrust load of center punch: 100 kgf Cleaning oil: 0.5 g/m2 (applied)
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Coating With Molten Metal (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP93348/90 | 1990-04-09 | ||
| JP9334890 | 1990-04-09 | ||
| JP33429690 | 1990-11-30 | ||
| JP334296/90 | 1990-11-30 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0451437A2 true EP0451437A2 (fr) | 1991-10-16 |
| EP0451437A3 EP0451437A3 (en) | 1992-01-22 |
| EP0451437B1 EP0451437B1 (fr) | 1995-04-05 |
Family
ID=26434742
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP91100089A Expired - Lifetime EP0451437B1 (fr) | 1990-04-09 | 1991-01-02 | Tôle galvanisée en acier ayant des caractéristiques améliorées de déformabilité par pression et sa méthode de fabrication |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP0451437B1 (fr) |
| CA (1) | CA2033862C (fr) |
| DE (1) | DE69108594T2 (fr) |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1347427A (fr) * | 1962-02-02 | 1963-12-27 | Paroxite London Ltd | Perfectionnements apportés aux produits d'enrobage utilisés pour le formage à froid des métaux |
| GB1083474A (en) * | 1965-01-15 | 1967-09-13 | Lubrizol Corp | Aqueous phosphating solutions |
| AT348308B (de) * | 1976-01-28 | 1979-02-12 | Servimetal | Verfahren zum sehr schnellen fliesspressen von leichtmetallen und leichtmetall-legierungen |
| DE2751222A1 (de) * | 1977-10-26 | 1979-05-03 | Bbc Brown Boveri & Cie | Hochtemperatur-schmiermittel |
| JPH01172578A (ja) * | 1987-12-25 | 1989-07-07 | Kawasaki Steel Corp | プレス加工性に優れた亜鉛系めっき鋼板及びその製造方法 |
| FR2629103A1 (fr) * | 1988-03-23 | 1989-09-29 | Lorraine Laminage | Tole metallique pour emboutissage; procede et dispositif de traitement de surface pour sa fabrication |
-
1991
- 1991-01-02 DE DE69108594T patent/DE69108594T2/de not_active Expired - Fee Related
- 1991-01-02 EP EP91100089A patent/EP0451437B1/fr not_active Expired - Lifetime
- 1991-01-09 CA CA002033862A patent/CA2033862C/fr not_active Expired - Fee Related
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1347427A (fr) * | 1962-02-02 | 1963-12-27 | Paroxite London Ltd | Perfectionnements apportés aux produits d'enrobage utilisés pour le formage à froid des métaux |
| GB1083474A (en) * | 1965-01-15 | 1967-09-13 | Lubrizol Corp | Aqueous phosphating solutions |
| AT348308B (de) * | 1976-01-28 | 1979-02-12 | Servimetal | Verfahren zum sehr schnellen fliesspressen von leichtmetallen und leichtmetall-legierungen |
| DE2751222A1 (de) * | 1977-10-26 | 1979-05-03 | Bbc Brown Boveri & Cie | Hochtemperatur-schmiermittel |
| JPH01172578A (ja) * | 1987-12-25 | 1989-07-07 | Kawasaki Steel Corp | プレス加工性に優れた亜鉛系めっき鋼板及びその製造方法 |
| FR2629103A1 (fr) * | 1988-03-23 | 1989-09-29 | Lorraine Laminage | Tole metallique pour emboutissage; procede et dispositif de traitement de surface pour sa fabrication |
Non-Patent Citations (1)
| Title |
|---|
| WORLD PATENTS INDEX LATEST, Week 8933, Derwent Publications Ltd., London, GB; AN 89-237899; & JP-A-01 172 578 (KAWASAKI STEEL KK) 07 July 1989 * |
Also Published As
| Publication number | Publication date |
|---|---|
| DE69108594D1 (de) | 1995-05-11 |
| EP0451437A3 (en) | 1992-01-22 |
| EP0451437B1 (fr) | 1995-04-05 |
| DE69108594T2 (de) | 1995-08-17 |
| CA2033862A1 (fr) | 1991-10-10 |
| CA2033862C (fr) | 1999-05-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6555249B1 (en) | Surface treated steel sheet and method for production thereof | |
| JP3346338B2 (ja) | 亜鉛系めっき鋼板およびその製造方法 | |
| US5853850A (en) | Lubricant film coated steel sheet with excellent phosphatability and method for producing same | |
| KR100234452B1 (ko) | 아연계 도금 강판 및 그 제조방법 | |
| US6537674B1 (en) | Surface treated steel sheet | |
| US5487919A (en) | Method of manufacturing of galvanized steel sheet having high press formability | |
| KR20020035775A (ko) | 아연 함유 도금된 고장력 강판 | |
| EP1080246B1 (fr) | Acier traite en surface et procede de fabrication correspondant | |
| EP0451437B1 (fr) | Tôle galvanisée en acier ayant des caractéristiques améliorées de déformabilité par pression et sa méthode de fabrication | |
| JP3445683B2 (ja) | プレス性、化成処理性、接着剤適合性に優れた亜鉛系めっき鋼板の製造方法 | |
| JP2007119872A (ja) | 合金化溶融亜鉛めっき鋼板の製造方法および合金化溶融亜鉛めっき鋼板 | |
| JP3132979B2 (ja) | 潤滑性、化成処理性、接着剤適合性に優れた亜鉛系めっき鋼板 | |
| JPH0713306B2 (ja) | プレス加工性に優れた亜鉛系めっき鋼板及びその製造方法 | |
| JP2713002B2 (ja) | 亜鉛系めっき鋼板の製造方法 | |
| JP3199980B2 (ja) | 潤滑性、化成処理性、接着剤適合性に優れた亜鉛系めっき鋼板 | |
| JP2965090B2 (ja) | プレス加工性およびリン酸塩処理性に優れた亜鉛系めっき鋼板の製造方法 | |
| JP3555604B2 (ja) | 耐食性、成形性に優れた表面処理鋼板およびその製造方法 | |
| US5322741A (en) | Aluminum alloy sheet with improved formability and method of production | |
| JP2819429B2 (ja) | プレス成形性、化成処理性に優れた亜鉛系めっき鋼板 | |
| JP2003253458A (ja) | 亜鉛系めっき鋼板及びその製造方法 | |
| JP3858706B2 (ja) | プレス成形性に優れた亜鉛めっき鋼板 | |
| JP3153097B2 (ja) | 潤滑性、化成処理性、接着剤適合性、溶接性に優れた亜鉛系めっき鋼板 | |
| CA2010898C (fr) | Tole d'acier galvanisee et recuite resistant au farinage et a l'ecaillage; procede de fabrication | |
| JP3111888B2 (ja) | 亜鉛系メッキ鋼板の製造方法 | |
| JP3111886B2 (ja) | 高潤滑合金化溶融亜鉛めっき鋼板の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): BE DE ES FR GB IT NL |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): BE DE ES FR GB IT NL |
|
| 17P | Request for examination filed |
Effective date: 19920227 |
|
| 17Q | First examination report despatched |
Effective date: 19920921 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE DE ES FR GB IT NL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRE;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED.SCRIBED TIME-LIMIT Effective date: 19950405 Ref country code: BE Effective date: 19950405 Ref country code: ES Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19950405 |
|
| REF | Corresponds to: |
Ref document number: 69108594 Country of ref document: DE Date of ref document: 19950511 |
|
| ET | Fr: translation filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20001227 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20001228 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20010125 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20010131 Year of fee payment: 11 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020801 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020801 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20020102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20020930 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20020801 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |