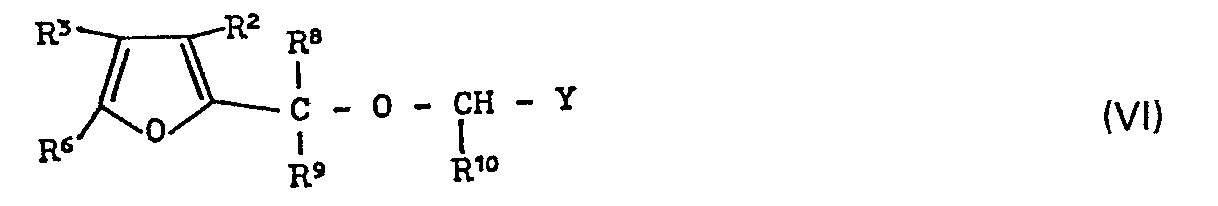EP0000002B1 - Dérivés du tétrahydrofuranne, leurs procédés de préparation et leur utilisation comme herbicides - Google Patents
Dérivés du tétrahydrofuranne, leurs procédés de préparation et leur utilisation comme herbicides Download PDFInfo
- Publication number
- EP0000002B1 EP0000002B1 EP78100007A EP78100007A EP0000002B1 EP 0000002 B1 EP0000002 B1 EP 0000002B1 EP 78100007 A EP78100007 A EP 78100007A EP 78100007 A EP78100007 A EP 78100007A EP 0000002 B1 EP0000002 B1 EP 0000002B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- tetrahydrofuran
- atoms
- alkyl
- phenyl
- formula
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 238000000034 method Methods 0.000 title claims description 37
- 239000004009 herbicide Substances 0.000 title claims description 10
- 238000002360 preparation method Methods 0.000 title claims description 9
- 150000007984 tetrahydrofuranes Chemical class 0.000 title claims 6
- 150000001875 compounds Chemical class 0.000 claims description 37
- 125000000217 alkyl group Chemical group 0.000 claims description 29
- 229910052736 halogen Inorganic materials 0.000 claims description 24
- 150000002367 halogens Chemical group 0.000 claims description 24
- 125000004432 carbon atom Chemical group C* 0.000 claims description 22
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 21
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims description 21
- 239000001257 hydrogen Substances 0.000 claims description 21
- 229910052739 hydrogen Inorganic materials 0.000 claims description 21
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 20
- 125000001188 haloalkyl group Chemical group 0.000 claims description 19
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 17
- 125000005843 halogen group Chemical group 0.000 claims description 17
- 125000003545 alkoxy group Chemical group 0.000 claims description 16
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 claims description 13
- 239000003054 catalyst Substances 0.000 claims description 12
- 239000003085 diluting agent Substances 0.000 claims description 12
- 239000000460 chlorine Substances 0.000 claims description 11
- 229910052801 chlorine Inorganic materials 0.000 claims description 11
- 229910052731 fluorine Inorganic materials 0.000 claims description 11
- 239000011737 fluorine Substances 0.000 claims description 11
- 150000002431 hydrogen Chemical class 0.000 claims description 11
- BSYVTEYKTMYBMK-UHFFFAOYSA-N tetrahydrofurfuryl alcohol Chemical class OCC1CCCO1 BSYVTEYKTMYBMK-UHFFFAOYSA-N 0.000 claims description 10
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 claims description 7
- 229910052794 bromium Inorganic materials 0.000 claims description 7
- 125000005913 (C3-C6) cycloalkyl group Chemical group 0.000 claims description 6
- 125000004183 alkoxy alkyl group Chemical group 0.000 claims description 6
- 125000006515 benzyloxy alkyl group Chemical group 0.000 claims description 6
- 125000006656 (C2-C4) alkenyl group Chemical group 0.000 claims description 4
- 239000004606 Fillers/Extenders Substances 0.000 claims description 4
- 239000003377 acid catalyst Substances 0.000 claims description 4
- 229910052784 alkaline earth metal Inorganic materials 0.000 claims description 4
- 125000003342 alkenyl group Chemical group 0.000 claims description 4
- 125000004852 dihydrofuranyl group Chemical group O1C(CC=C1)* 0.000 claims description 4
- 150000002009 diols Chemical class 0.000 claims description 4
- 150000002240 furans Chemical class 0.000 claims description 4
- 229910052783 alkali metal Inorganic materials 0.000 claims description 3
- 150000001340 alkali metals Chemical class 0.000 claims description 3
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 3
- 125000000051 benzyloxy group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])O* 0.000 claims description 3
- 125000004438 haloalkoxy group Chemical group 0.000 claims description 3
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 claims description 3
- 125000006678 phenoxycarbonyl group Chemical group 0.000 claims description 3
- PMPVIKIVABFJJI-UHFFFAOYSA-N Cyclobutane Chemical compound C1CCC1 PMPVIKIVABFJJI-UHFFFAOYSA-N 0.000 claims description 2
- LVZWSLJZHVFIQJ-UHFFFAOYSA-N Cyclopropane Chemical compound C1CC1 LVZWSLJZHVFIQJ-UHFFFAOYSA-N 0.000 claims description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 claims description 2
- 150000001342 alkaline earth metals Chemical class 0.000 claims description 2
- RGSFGYAAUTVSQA-UHFFFAOYSA-N pentamethylene Natural products C1CCCC1 RGSFGYAAUTVSQA-UHFFFAOYSA-N 0.000 claims description 2
- 230000008635 plant growth Effects 0.000 claims description 2
- 125000001424 substituent group Chemical group 0.000 claims description 2
- 125000003107 substituted aryl group Chemical group 0.000 claims description 2
- 239000004094 surface-active agent Substances 0.000 claims description 2
- 125000006193 alkinyl group Chemical group 0.000 claims 3
- 125000004817 pentamethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 claims 1
- 229920006395 saturated elastomer Polymers 0.000 claims 1
- -1 for example Chemical class 0.000 description 40
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical class C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 32
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 15
- 241000196324 Embryophyta Species 0.000 description 14
- 238000006243 chemical reaction Methods 0.000 description 14
- 239000002904 solvent Substances 0.000 description 13
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- 239000000203 mixture Substances 0.000 description 12
- 239000007858 starting material Substances 0.000 description 10
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 9
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 9
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 8
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 7
- 244000062793 Sorghum vulgare Species 0.000 description 7
- 230000002363 herbicidal effect Effects 0.000 description 7
- DHKBLXWNJOEMPY-UHFFFAOYSA-N 2-[1-[(2-chlorophenyl)methoxy]propyl]furan Chemical compound C=1C=COC=1C(CC)OCC1=CC=CC=C1Cl DHKBLXWNJOEMPY-UHFFFAOYSA-N 0.000 description 6
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 6
- 244000025254 Cannabis sativa Species 0.000 description 6
- 229910000104 sodium hydride Inorganic materials 0.000 description 6
- 239000012312 sodium hydride Substances 0.000 description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 5
- 239000007795 chemical reaction product Substances 0.000 description 5
- 239000003995 emulsifying agent Substances 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 235000019713 millet Nutrition 0.000 description 5
- FFWQLZFIMNTUCZ-UHFFFAOYSA-N 1-(bromomethyl)-2-fluorobenzene Chemical compound FC1=CC=CC=C1CBr FFWQLZFIMNTUCZ-UHFFFAOYSA-N 0.000 description 4
- BSIIGUGKOPPTPZ-UHFFFAOYSA-N 1-bromo-4-(chloromethyl)benzene Chemical compound ClCC1=CC=C(Br)C=C1 BSIIGUGKOPPTPZ-UHFFFAOYSA-N 0.000 description 4
- 244000075850 Avena orientalis Species 0.000 description 4
- 241000132536 Cirsium Species 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 150000002170 ethers Chemical class 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 4
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 3
- BASMANVIUSSIIM-UHFFFAOYSA-N 1-chloro-2-(chloromethyl)benzene Chemical compound ClCC1=CC=CC=C1Cl BASMANVIUSSIIM-UHFFFAOYSA-N 0.000 description 3
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 description 3
- HZHNTVRRDQAENF-UHFFFAOYSA-N 5-[(2-fluorophenyl)methoxy]pentane-1,4-diol Chemical compound OCCCC(O)COCC1=CC=CC=C1F HZHNTVRRDQAENF-UHFFFAOYSA-N 0.000 description 3
- 244000105624 Arachis hypogaea Species 0.000 description 3
- 0 C*(C)C(C)(C)N(C)N Chemical compound C*(C)C(C)(C)N(C)N 0.000 description 3
- 241000219146 Gossypium Species 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- 244000042664 Matricaria chamomilla Species 0.000 description 3
- 235000007232 Matricaria chamomilla Nutrition 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- 241000207763 Solanum Species 0.000 description 3
- 238000009835 boiling Methods 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 239000012442 inert solvent Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 229910000510 noble metal Inorganic materials 0.000 description 3
- 239000012074 organic phase Substances 0.000 description 3
- 239000003960 organic solvent Substances 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000011541 reaction mixture Substances 0.000 description 3
- 238000010992 reflux Methods 0.000 description 3
- 229910052703 rhodium Inorganic materials 0.000 description 3
- 239000010948 rhodium Substances 0.000 description 3
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 description 3
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 2
- YZIFVWOCPGPNHB-UHFFFAOYSA-N 1,2-dichloro-4-(chloromethyl)benzene Chemical compound ClCC1=CC=C(Cl)C(Cl)=C1 YZIFVWOCPGPNHB-UHFFFAOYSA-N 0.000 description 2
- LBOBESSDSGODDD-UHFFFAOYSA-N 1,3-dichloro-2-(chloromethyl)benzene Chemical compound ClCC1=C(Cl)C=CC=C1Cl LBOBESSDSGODDD-UHFFFAOYSA-N 0.000 description 2
- OMZINLIPPVNUOG-UHFFFAOYSA-N 1,4-dichloro-2-(chloromethyl)benzene Chemical compound ClCC1=CC(Cl)=CC=C1Cl OMZINLIPPVNUOG-UHFFFAOYSA-N 0.000 description 2
- PHDKMRIKFBWJGE-UHFFFAOYSA-N 1-(bromomethyl)-2,4,5-trichlorobenzene Chemical compound ClC1=CC(Cl)=C(CBr)C=C1Cl PHDKMRIKFBWJGE-UHFFFAOYSA-N 0.000 description 2
- WGLUZJWOTTXZIC-UHFFFAOYSA-N 1-(bromomethyl)-2,4-dimethylbenzene Chemical compound CC1=CC=C(CBr)C(C)=C1 WGLUZJWOTTXZIC-UHFFFAOYSA-N 0.000 description 2
- SCBZBMXPJYMXRC-UHFFFAOYSA-N 1-(bromomethyl)-3-fluorobenzene Chemical compound FC1=CC=CC(CBr)=C1 SCBZBMXPJYMXRC-UHFFFAOYSA-N 0.000 description 2
- UFCSSWZQROEFBZ-UHFFFAOYSA-N 1-(bromomethyl)-4-chloro-2-fluorobenzene Chemical compound FC1=CC(Cl)=CC=C1CBr UFCSSWZQROEFBZ-UHFFFAOYSA-N 0.000 description 2
- NVNPLEPBDPJYRZ-UHFFFAOYSA-N 1-(bromomethyl)-4-fluorobenzene Chemical compound FC1=CC=C(CBr)C=C1 NVNPLEPBDPJYRZ-UHFFFAOYSA-N 0.000 description 2
- CHMJJIHXWABUHA-UHFFFAOYSA-N 1-(chloromethyl)-2,3-dimethylbenzene Chemical compound CC1=CC=CC(CCl)=C1C CHMJJIHXWABUHA-UHFFFAOYSA-N 0.000 description 2
- BBXDMCQDLOCXRA-UHFFFAOYSA-N 1-(chloromethyl)-2-(trifluoromethyl)benzene Chemical compound FC(F)(F)C1=CC=CC=C1CCl BBXDMCQDLOCXRA-UHFFFAOYSA-N 0.000 description 2
- KIXKPLUFGGYZPN-UHFFFAOYSA-N 1-(chloromethyl)-2-fluoro-3-methylbenzene Chemical compound CC1=CC=CC(CCl)=C1F KIXKPLUFGGYZPN-UHFFFAOYSA-N 0.000 description 2
- FFJVADDDXFORRX-UHFFFAOYSA-N 1-(chloromethyl)-2-fluoro-4-methylbenzene Chemical compound CC1=CC=C(CCl)C(F)=C1 FFJVADDDXFORRX-UHFFFAOYSA-N 0.000 description 2
- UAWVMPOAIVZWFQ-UHFFFAOYSA-N 1-(chloromethyl)-2-methoxybenzene Chemical compound COC1=CC=CC=C1CCl UAWVMPOAIVZWFQ-UHFFFAOYSA-N 0.000 description 2
- XGASTRVQNVVYIZ-UHFFFAOYSA-N 1-(chloromethyl)-3-(trifluoromethyl)benzene Chemical compound FC(F)(F)C1=CC=CC(CCl)=C1 XGASTRVQNVVYIZ-UHFFFAOYSA-N 0.000 description 2
- VGISFWWEOGVMED-UHFFFAOYSA-N 1-(chloromethyl)-3-methoxybenzene Chemical compound COC1=CC=CC(CCl)=C1 VGISFWWEOGVMED-UHFFFAOYSA-N 0.000 description 2
- MCHDHQVROPEJJT-UHFFFAOYSA-N 1-(chloromethyl)-4-(trifluoromethyl)benzene Chemical compound FC(F)(F)C1=CC=C(CCl)C=C1 MCHDHQVROPEJJT-UHFFFAOYSA-N 0.000 description 2
- MOHYOXXOKFQHDC-UHFFFAOYSA-N 1-(chloromethyl)-4-methoxybenzene Chemical compound COC1=CC=C(CCl)C=C1 MOHYOXXOKFQHDC-UHFFFAOYSA-N 0.000 description 2
- DMHZDOTYAVHSEH-UHFFFAOYSA-N 1-(chloromethyl)-4-methylbenzene Chemical compound CC1=CC=C(CCl)C=C1 DMHZDOTYAVHSEH-UHFFFAOYSA-N 0.000 description 2
- HLQZCRVEEQKNMS-UHFFFAOYSA-N 1-(chloromethyl)-4-phenylbenzene Chemical compound C1=CC(CCl)=CC=C1C1=CC=CC=C1 HLQZCRVEEQKNMS-UHFFFAOYSA-N 0.000 description 2
- NQEGTLSSOYPESM-UHFFFAOYSA-N 1-[(2,6-dichlorophenyl)methoxy]-2,5-dimethylhexane-2,5-diol Chemical compound CC(C)(O)CCC(C)(O)COCC1=C(Cl)C=CC=C1Cl NQEGTLSSOYPESM-UHFFFAOYSA-N 0.000 description 2
- XGAJEVLUOOYYCU-UHFFFAOYSA-N 1-[(2,6-dichlorophenyl)methoxy]-5-methylhexane-2,5-diol Chemical compound CC(C)(O)CCC(O)COCC1=C(Cl)C=CC=C1Cl XGAJEVLUOOYYCU-UHFFFAOYSA-N 0.000 description 2
- NEGGYLKKUWLXRG-UHFFFAOYSA-N 1-[(2-bromophenyl)methoxy]-2,5-dimethylhexane-2,5-diol Chemical compound CC(C)(O)CCC(C)(O)COCC1=CC=CC=C1Br NEGGYLKKUWLXRG-UHFFFAOYSA-N 0.000 description 2
- WDYOGRQEGCAYDP-UHFFFAOYSA-N 1-[(2-chlorophenyl)methoxy]-2,5-dimethylhexane-2,5-diol Chemical compound CC(C)(O)CCC(C)(O)COCC1=CC=CC=C1Cl WDYOGRQEGCAYDP-UHFFFAOYSA-N 0.000 description 2
- PWRLCIXEJRDDLE-UHFFFAOYSA-N 1-[(2-fluorophenyl)methoxy]-2,5-dimethylhexane-2,5-diol Chemical compound CC(C)(O)CCC(C)(O)COCC1=CC=CC=C1F PWRLCIXEJRDDLE-UHFFFAOYSA-N 0.000 description 2
- AFHHDBUDORELKI-UHFFFAOYSA-N 1-[(2-fluorophenyl)methoxy]-5-methylhexane-2,5-diol Chemical compound CC(C)(O)CCC(O)COCC1=CC=CC=C1F AFHHDBUDORELKI-UHFFFAOYSA-N 0.000 description 2
- AIFVTPIMVJMQAZ-UHFFFAOYSA-N 1-[(4-fluorophenyl)methoxy]-2,5-dimethylhexane-2,5-diol Chemical compound CC(C)(O)CCC(C)(O)COCC1=CC=C(F)C=C1 AIFVTPIMVJMQAZ-UHFFFAOYSA-N 0.000 description 2
- SOKHOHIUXYIFBO-UHFFFAOYSA-N 1-[(4-fluorophenyl)methoxy]-5-methylhexane-2,5-diol Chemical compound CC(C)(O)CCC(O)COCC1=CC=C(F)C=C1 SOKHOHIUXYIFBO-UHFFFAOYSA-N 0.000 description 2
- UDKGXKYEWBGQCG-UHFFFAOYSA-N 1-bromo-3-(chloromethyl)benzene Chemical compound ClCC1=CC=CC(Br)=C1 UDKGXKYEWBGQCG-UHFFFAOYSA-N 0.000 description 2
- QBFRQIVHIHPAPQ-UHFFFAOYSA-N 1-chloro-2-(chloromethyl)-3-methylbenzene Chemical compound CC1=CC=CC(Cl)=C1CCl QBFRQIVHIHPAPQ-UHFFFAOYSA-N 0.000 description 2
- HXTMQWIYPHKZSY-UHFFFAOYSA-N 1-chloro-3-(chloromethyl)-2-methylbenzene Chemical compound CC1=C(Cl)C=CC=C1CCl HXTMQWIYPHKZSY-UHFFFAOYSA-N 0.000 description 2
- DDGRAFHHXYIQQR-UHFFFAOYSA-N 1-chloro-3-(chloromethyl)benzene Chemical compound ClCC1=CC=CC(Cl)=C1 DDGRAFHHXYIQQR-UHFFFAOYSA-N 0.000 description 2
- JQZAEUFPPSRDOP-UHFFFAOYSA-N 1-chloro-4-(chloromethyl)benzene Chemical compound ClCC1=CC=C(Cl)C=C1 JQZAEUFPPSRDOP-UHFFFAOYSA-N 0.000 description 2
- QYAMWWFYJKOQCS-UHFFFAOYSA-N 2,2,5-trimethyl-5-(phenylmethoxymethyl)oxolane Chemical compound O1C(C)(C)CCC1(C)COCC1=CC=CC=C1 QYAMWWFYJKOQCS-UHFFFAOYSA-N 0.000 description 2
- CPZWBYFMHKTVMA-UHFFFAOYSA-N 2,2,5-trimethyl-5-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound CC1=CC=CC=C1COCC1(C)OC(C)(C)CC1 CPZWBYFMHKTVMA-UHFFFAOYSA-N 0.000 description 2
- MZDNGZIZPXRHCV-UHFFFAOYSA-N 2,2-dimethyl-5-(phenylmethoxymethyl)-5-propyloxolane Chemical compound C=1C=CC=CC=1COCC1(CCC)CCC(C)(C)O1 MZDNGZIZPXRHCV-UHFFFAOYSA-N 0.000 description 2
- BKBFJWZULVDAPS-UHFFFAOYSA-N 2,2-dimethyl-5-[(2-methylphenyl)methoxymethyl]-5-propyloxolane Chemical compound C=1C=CC=C(C)C=1COCC1(CCC)CCC(C)(C)O1 BKBFJWZULVDAPS-UHFFFAOYSA-N 0.000 description 2
- PIYKGXDXJLMPRB-UHFFFAOYSA-N 2,3-dihydrofuran-2-ylmethanol Chemical class OCC1CC=CO1 PIYKGXDXJLMPRB-UHFFFAOYSA-N 0.000 description 2
- QUTRUAMGUBVPCU-UHFFFAOYSA-N 2,5-dimethyl-1-[(2-methylphenyl)methoxy]hexane-2,5-diol Chemical compound CC1=CC=CC=C1COCC(C)(O)CCC(C)(C)O QUTRUAMGUBVPCU-UHFFFAOYSA-N 0.000 description 2
- NQTLJRTZZFPBQH-UHFFFAOYSA-N 2,5-dimethyl-1-phenylmethoxyhexane-2,5-diol Chemical compound CC(C)(O)CCC(C)(O)COCC1=CC=CC=C1 NQTLJRTZZFPBQH-UHFFFAOYSA-N 0.000 description 2
- HOXSDYSZXAATIK-UHFFFAOYSA-N 2-(bromomethyl)-1,3,5-trichlorobenzene Chemical compound ClC1=CC(Cl)=C(CBr)C(Cl)=C1 HOXSDYSZXAATIK-UHFFFAOYSA-N 0.000 description 2
- DJXBUSVMEONVRS-UHFFFAOYSA-N 2-(bromomethyl)-4-chloro-1-fluorobenzene Chemical compound FC1=CC=C(Cl)C=C1CBr DJXBUSVMEONVRS-UHFFFAOYSA-N 0.000 description 2
- MJXRENZUAQXZGJ-UHFFFAOYSA-N 2-(chloromethyl)-1,3-difluorobenzene Chemical compound FC1=CC=CC(F)=C1CCl MJXRENZUAQXZGJ-UHFFFAOYSA-N 0.000 description 2
- HPVRFWQMBYLJRL-UHFFFAOYSA-N 2-(chloromethyl)-1,3-dimethylbenzene Chemical compound CC1=CC=CC(C)=C1CCl HPVRFWQMBYLJRL-UHFFFAOYSA-N 0.000 description 2
- GGJVGJOFFKFBTQ-UHFFFAOYSA-N 2-(chloromethyl)-1-fluoro-3-methylbenzene Chemical compound CC1=CC=CC(F)=C1CCl GGJVGJOFFKFBTQ-UHFFFAOYSA-N 0.000 description 2
- NRDFOZDHNQKLOO-UHFFFAOYSA-N 2-(chloromethyl)-1-fluoro-4-methylbenzene Chemical compound CC1=CC=C(F)C(CCl)=C1 NRDFOZDHNQKLOO-UHFFFAOYSA-N 0.000 description 2
- ZVVKMKQFBNCQFY-UHFFFAOYSA-N 2-(chloromethyl)-5-(phenylmethoxymethyl)oxolane Chemical compound O1C(CCl)CCC1COCC1=CC=CC=C1 ZVVKMKQFBNCQFY-UHFFFAOYSA-N 0.000 description 2
- UJFNFVXKBDGWQE-UHFFFAOYSA-N 2-(chloromethyl)-5-[(2,6-dichlorophenyl)methoxymethyl]oxolane Chemical compound O1C(CCl)CCC1COCC1=C(Cl)C=CC=C1Cl UJFNFVXKBDGWQE-UHFFFAOYSA-N 0.000 description 2
- JEBVEJKYHMBLGK-UHFFFAOYSA-N 2-(chloromethyl)-5-[(2-fluorophenyl)methoxymethyl]oxolane Chemical compound FC1=CC=CC=C1COCC1OC(CCl)CC1 JEBVEJKYHMBLGK-UHFFFAOYSA-N 0.000 description 2
- ZXEWGRKSTWPKGZ-UHFFFAOYSA-N 2-(chloromethyl)-5-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound CC1=CC=CC=C1COCC1OC(CCl)CC1 ZXEWGRKSTWPKGZ-UHFFFAOYSA-N 0.000 description 2
- VYLTVJVZYLEEPT-UHFFFAOYSA-N 2-(chloromethyl)-5-[(4-fluorophenyl)methoxymethyl]oxolane Chemical compound C1=CC(F)=CC=C1COCC1OC(CCl)CC1 VYLTVJVZYLEEPT-UHFFFAOYSA-N 0.000 description 2
- BPCILMOBJZBKQE-UHFFFAOYSA-N 2-(methoxymethyl)-5-(phenylmethoxymethyl)oxolane Chemical compound O1C(COC)CCC1COCC1=CC=CC=C1 BPCILMOBJZBKQE-UHFFFAOYSA-N 0.000 description 2
- FMPUQUAPZLECEH-UHFFFAOYSA-N 2-(methoxymethyl)-5-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound O1C(COC)CCC1COCC1=CC=CC=C1C FMPUQUAPZLECEH-UHFFFAOYSA-N 0.000 description 2
- PJMFAZRMYNNOIH-UHFFFAOYSA-N 2-(oxolan-2-yl)propan-2-ol Chemical compound CC(C)(O)C1CCCO1 PJMFAZRMYNNOIH-UHFFFAOYSA-N 0.000 description 2
- NPWOUOLTGAZJLW-UHFFFAOYSA-N 2-(phenylmethoxymethyl)-2-propyloxolane Chemical compound C=1C=CC=CC=1COCC1(CCC)CCCO1 NPWOUOLTGAZJLW-UHFFFAOYSA-N 0.000 description 2
- CSQPGXGOGFVQOF-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-2,5,5-trimethyloxolane Chemical compound O1C(C)(C)CCC1(C)COCC1=C(Cl)C=CC=C1Cl CSQPGXGOGFVQOF-UHFFFAOYSA-N 0.000 description 2
- FAPFFBYXLHFENH-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-2-methyl-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(C)COCC1=C(Cl)C=CC=C1Cl FAPFFBYXLHFENH-UHFFFAOYSA-N 0.000 description 2
- NLNDFHQWRJEVPK-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-2-methyloxolane Chemical compound ClC=1C=CC=C(Cl)C=1COCC1(C)CCCO1 NLNDFHQWRJEVPK-UHFFFAOYSA-N 0.000 description 2
- GOGBAFOLIZSRCM-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-2-phenyloxolane Chemical compound ClC1=CC=CC(Cl)=C1COCC1(C=2C=CC=CC=2)OCCC1 GOGBAFOLIZSRCM-UHFFFAOYSA-N 0.000 description 2
- JJGHUWBBFBSTFA-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-5,5-dimethyl-2-propyloxolane Chemical compound ClC=1C=CC=C(Cl)C=1COCC1(CCC)CCC(C)(C)O1 JJGHUWBBFBSTFA-UHFFFAOYSA-N 0.000 description 2
- YXTLDMQNDRDCRQ-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-5-ethenyl-2,5-dimethyloxolane Chemical compound ClC=1C=CC=C(Cl)C=1COCC1(C)CCC(C)(C=C)O1 YXTLDMQNDRDCRQ-UHFFFAOYSA-N 0.000 description 2
- IJLRIXJHZHKIPI-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-2,5,5-trimethyloxolane Chemical compound O1C(C)(C)CCC1(C)COCC1=CC=CC=C1Br IJLRIXJHZHKIPI-UHFFFAOYSA-N 0.000 description 2
- LZYVHXRJFVMPTM-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-2-methyl-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(C)COCC1=CC=CC=C1Br LZYVHXRJFVMPTM-UHFFFAOYSA-N 0.000 description 2
- ZIHJMWQOLOEUPF-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-2-methyloxolane Chemical compound C=1C=CC=C(Br)C=1COCC1(C)CCCO1 ZIHJMWQOLOEUPF-UHFFFAOYSA-N 0.000 description 2
- UCJMYKGHHMBXGA-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-2-phenyloxolane Chemical compound BrC1=CC=CC=C1COCC1(C=2C=CC=CC=2)OCCC1 UCJMYKGHHMBXGA-UHFFFAOYSA-N 0.000 description 2
- TURHWTNACVTZJL-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-2-propyloxolane Chemical compound C=1C=CC=C(Br)C=1COCC1(CCC)CCCO1 TURHWTNACVTZJL-UHFFFAOYSA-N 0.000 description 2
- QHCBQZZSSKDZFH-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-5,5-dimethyl-2-propyloxolane Chemical compound C=1C=CC=C(Br)C=1COCC1(CCC)CCC(C)(C)O1 QHCBQZZSSKDZFH-UHFFFAOYSA-N 0.000 description 2
- VPJQDQJPMBIWDO-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-5-(chloromethyl)oxolane Chemical compound O1C(CCl)CCC1COCC1=CC=CC=C1Br VPJQDQJPMBIWDO-UHFFFAOYSA-N 0.000 description 2
- PRXHYJVMUAKZDC-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-5-ethenyl-2,5-dimethyloxolane Chemical compound C=1C=CC=C(Br)C=1COCC1(C)CCC(C)(C=C)O1 PRXHYJVMUAKZDC-UHFFFAOYSA-N 0.000 description 2
- VJACJCDMOGWNOD-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-5-methyloxolane Chemical compound O1C(C)CCC1COCC1=CC=CC=C1Br VJACJCDMOGWNOD-UHFFFAOYSA-N 0.000 description 2
- NXKPYGVMKNQTTO-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-2,5,5-trimethyloxolane Chemical compound O1C(C)(C)CCC1(C)COCC1=CC=CC=C1Cl NXKPYGVMKNQTTO-UHFFFAOYSA-N 0.000 description 2
- PCHJSZORQJTRLV-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-2-methyl-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(C)COCC1=CC=CC=C1Cl PCHJSZORQJTRLV-UHFFFAOYSA-N 0.000 description 2
- GUYGXZXVUJAFHP-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-2-methyloxolane Chemical compound C=1C=CC=C(Cl)C=1COCC1(C)CCCO1 GUYGXZXVUJAFHP-UHFFFAOYSA-N 0.000 description 2
- HEAFCTMAGQTDBF-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-2-phenyloxolane Chemical compound ClC1=CC=CC=C1COCC1(C=2C=CC=CC=2)OCCC1 HEAFCTMAGQTDBF-UHFFFAOYSA-N 0.000 description 2
- LFQYHNMZRWLQTM-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-2-propyloxolane Chemical compound C=1C=CC=C(Cl)C=1COCC1(CCC)CCCO1 LFQYHNMZRWLQTM-UHFFFAOYSA-N 0.000 description 2
- CVRNBMQKNGBNMD-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-5,5-dimethyl-2-propyloxolane Chemical compound C=1C=CC=C(Cl)C=1COCC1(CCC)CCC(C)(C)O1 CVRNBMQKNGBNMD-UHFFFAOYSA-N 0.000 description 2
- XGEPMGSLLZNBEY-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-5-(methoxymethyl)oxolane Chemical compound O1C(COC)CCC1COCC1=CC=CC=C1Cl XGEPMGSLLZNBEY-UHFFFAOYSA-N 0.000 description 2
- WXFQLXKCZMKKRA-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-5-ethenyl-2,5-dimethyloxolane Chemical compound C=1C=CC=C(Cl)C=1COCC1(C)CCC(C)(C=C)O1 WXFQLXKCZMKKRA-UHFFFAOYSA-N 0.000 description 2
- VHCQTZUHCIIYST-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-5-methyloxolane Chemical compound O1C(C)CCC1COCC1=CC=CC=C1Cl VHCQTZUHCIIYST-UHFFFAOYSA-N 0.000 description 2
- SURLUVQNFGUFTB-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxy-phenylmethyl]furan Chemical compound FC1=CC=CC=C1COC(C=1C=CC=CC=1)C1=CC=CO1 SURLUVQNFGUFTB-UHFFFAOYSA-N 0.000 description 2
- TUNABBXKJAVEIB-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]-2,5,5-trimethyloxolane Chemical compound O1C(C)(C)CCC1(C)COCC1=CC=CC=C1F TUNABBXKJAVEIB-UHFFFAOYSA-N 0.000 description 2
- JJVFOAXTMHHMOE-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]-2-methyl-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(C)COCC1=CC=CC=C1F JJVFOAXTMHHMOE-UHFFFAOYSA-N 0.000 description 2
- IYCGKGPKQMVFGS-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]-2-methyloxolane Chemical compound C=1C=CC=C(F)C=1COCC1(C)CCCO1 IYCGKGPKQMVFGS-UHFFFAOYSA-N 0.000 description 2
- SOTWTNXZKNLSGL-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]-2-phenyloxolane Chemical compound FC1=CC=CC=C1COCC1(C=2C=CC=CC=2)OCCC1 SOTWTNXZKNLSGL-UHFFFAOYSA-N 0.000 description 2
- BYWUKBASPYORKK-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]-2-propyloxolane Chemical compound C=1C=CC=C(F)C=1COCC1(CCC)CCCO1 BYWUKBASPYORKK-UHFFFAOYSA-N 0.000 description 2
- URRROFBFYSHTPB-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]-5,5-dimethyl-2-propyloxolane Chemical compound C=1C=CC=C(F)C=1COCC1(CCC)CCC(C)(C)O1 URRROFBFYSHTPB-UHFFFAOYSA-N 0.000 description 2
- ABNAFSPBZCVCGK-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]-5-(methoxymethyl)oxolane Chemical compound O1C(COC)CCC1COCC1=CC=CC=C1F ABNAFSPBZCVCGK-UHFFFAOYSA-N 0.000 description 2
- UZSWBRJAAZNFPW-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]-5-methyloxolane Chemical compound O1C(C)CCC1COCC1=CC=CC=C1F UZSWBRJAAZNFPW-UHFFFAOYSA-N 0.000 description 2
- HKTZJHJPVUAAKO-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]furan Chemical compound FC1=CC=CC=C1COCC1=CC=CO1 HKTZJHJPVUAAKO-UHFFFAOYSA-N 0.000 description 2
- POZNELRRNMDROF-UHFFFAOYSA-N 2-[(2-methylphenyl)methoxymethyl]-2-phenyloxolane Chemical compound CC1=CC=CC=C1COCC1(C=2C=CC=CC=2)OCCC1 POZNELRRNMDROF-UHFFFAOYSA-N 0.000 description 2
- SLAGQUPXLGRRGB-UHFFFAOYSA-N 2-[(2-methylphenyl)methoxymethyl]-2-propyloxolane Chemical compound C=1C=CC=C(C)C=1COCC1(CCC)CCCO1 SLAGQUPXLGRRGB-UHFFFAOYSA-N 0.000 description 2
- SLLCNWGGLWAEQN-UHFFFAOYSA-N 2-[(4-fluorophenyl)methoxymethyl]-2-methyl-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(C)COCC1=CC=C(F)C=C1 SLLCNWGGLWAEQN-UHFFFAOYSA-N 0.000 description 2
- RDLZGMBTRBAMRM-UHFFFAOYSA-N 2-[(4-fluorophenyl)methoxymethyl]-2-methyloxolane Chemical compound C=1C=C(F)C=CC=1COCC1(C)CCCO1 RDLZGMBTRBAMRM-UHFFFAOYSA-N 0.000 description 2
- ULXIXYMWORNZLM-UHFFFAOYSA-N 2-[(4-fluorophenyl)methoxymethyl]-2-phenyloxolane Chemical compound C1=CC(F)=CC=C1COCC1(C=2C=CC=CC=2)OCCC1 ULXIXYMWORNZLM-UHFFFAOYSA-N 0.000 description 2
- BMQKVQNDFPSZID-UHFFFAOYSA-N 2-[(4-fluorophenyl)methoxymethyl]-2-propyloxolane Chemical compound C=1C=C(F)C=CC=1COCC1(CCC)CCCO1 BMQKVQNDFPSZID-UHFFFAOYSA-N 0.000 description 2
- JPLQTLSMWPHGTH-UHFFFAOYSA-N 2-[(4-fluorophenyl)methoxymethyl]-5-methyloxolane Chemical compound O1C(C)CCC1COCC1=CC=C(F)C=C1 JPLQTLSMWPHGTH-UHFFFAOYSA-N 0.000 description 2
- LLJZZQHCUWTRLB-UHFFFAOYSA-N 2-[1-[(2-fluorophenyl)methoxy]propyl]furan Chemical compound C=1C=COC=1C(CC)OCC1=CC=CC=C1F LLJZZQHCUWTRLB-UHFFFAOYSA-N 0.000 description 2
- SYYYSBYJAFDKAX-UHFFFAOYSA-N 2-[2-[(2-fluorophenyl)methoxy]propan-2-yl]furan Chemical compound C=1C=COC=1C(C)(C)OCC1=CC=CC=C1F SYYYSBYJAFDKAX-UHFFFAOYSA-N 0.000 description 2
- LOAIHMKHUWHCRR-UHFFFAOYSA-N 2-[3-[(2-chlorophenyl)methoxy]pentan-3-yl]furan Chemical compound C=1C=COC=1C(CC)(CC)OCC1=CC=CC=C1Cl LOAIHMKHUWHCRR-UHFFFAOYSA-N 0.000 description 2
- VMMNAUGXBDMFIB-UHFFFAOYSA-N 2-ethenyl-2,5-dimethyl-5-(phenylmethoxymethyl)oxolane Chemical compound C=1C=CC=CC=1COCC1(C)CCC(C)(C=C)O1 VMMNAUGXBDMFIB-UHFFFAOYSA-N 0.000 description 2
- NYMUDNPGODQPJR-UHFFFAOYSA-N 2-ethenyl-2,5-dimethyl-5-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound CC1=CC=CC=C1COCC1(C)OC(C)(C=C)CC1 NYMUDNPGODQPJR-UHFFFAOYSA-N 0.000 description 2
- ZYCZGTUHTOKBTD-UHFFFAOYSA-N 2-ethenyl-5-[(2-fluorophenyl)methoxymethyl]-2,5-dimethyloxolane Chemical compound C=1C=CC=C(F)C=1COCC1(C)CCC(C)(C=C)O1 ZYCZGTUHTOKBTD-UHFFFAOYSA-N 0.000 description 2
- HONSGXIITVDAMV-UHFFFAOYSA-N 2-ethenyl-5-[(4-fluorophenyl)methoxymethyl]-2,5-dimethyloxolane Chemical compound C=1C=C(F)C=CC=1COCC1(C)CCC(C)(C=C)O1 HONSGXIITVDAMV-UHFFFAOYSA-N 0.000 description 2
- WGKNKTPCVRVORQ-UHFFFAOYSA-N 2-methyl-2-(phenylmethoxymethyl)oxolane Chemical compound C=1C=CC=CC=1COCC1(C)CCCO1 WGKNKTPCVRVORQ-UHFFFAOYSA-N 0.000 description 2
- QOSLFCLSXAFWMM-UHFFFAOYSA-N 2-methyl-2-[(2-methylphenyl)methoxymethyl]-5-phenyloxolane Chemical compound CC1=CC=CC=C1COCC1(C)OC(C=2C=CC=CC=2)CC1 QOSLFCLSXAFWMM-UHFFFAOYSA-N 0.000 description 2
- SJJPSZFPIWHLSU-UHFFFAOYSA-N 2-methyl-2-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound CC1=CC=CC=C1COCC1(C)OCCC1 SJJPSZFPIWHLSU-UHFFFAOYSA-N 0.000 description 2
- SZHAXWSBZCKKTC-UHFFFAOYSA-N 2-methyl-5-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound O1C(C)CCC1COCC1=CC=CC=C1C SZHAXWSBZCKKTC-UHFFFAOYSA-N 0.000 description 2
- JBZKTPCQRLZFQA-UHFFFAOYSA-N 2-methyl-5-phenyl-2-(phenylmethoxymethyl)oxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(C)COCC1=CC=CC=C1 JBZKTPCQRLZFQA-UHFFFAOYSA-N 0.000 description 2
- LZHXDSVPDOWKQD-UHFFFAOYSA-N 2-phenyl-2-(phenylmethoxymethyl)oxolane Chemical compound C=1C=CC=CC=1COCC1(C=2C=CC=CC=2)CCCO1 LZHXDSVPDOWKQD-UHFFFAOYSA-N 0.000 description 2
- GGTQWWTYUKXFPP-UHFFFAOYSA-N 4-(bromomethyl)-2-chloro-1-fluorobenzene Chemical compound FC1=CC=C(CBr)C=C1Cl GGTQWWTYUKXFPP-UHFFFAOYSA-N 0.000 description 2
- UBQRAAXAHIKWRI-UHFFFAOYSA-N 4-(chloromethyl)-1,2-dimethylbenzene Chemical compound CC1=CC=C(CCl)C=C1C UBQRAAXAHIKWRI-UHFFFAOYSA-N 0.000 description 2
- QDXAXXIPBMVWOH-UHFFFAOYSA-N 4-chloro-1-(chloromethyl)-2-methylbenzene Chemical compound CC1=CC(Cl)=CC=C1CCl QDXAXXIPBMVWOH-UHFFFAOYSA-N 0.000 description 2
- JMGAFTBKTDKOHT-UHFFFAOYSA-N 4-chloro-2-(chloromethyl)-1-methylbenzene Chemical compound CC1=CC=C(Cl)C=C1CCl JMGAFTBKTDKOHT-UHFFFAOYSA-N 0.000 description 2
- VCXSNHNCTQWGLW-UHFFFAOYSA-N 5-[(2,6-dichlorophenyl)methoxy]pentane-1,4-diol Chemical compound OCCCC(O)COCC1=C(Cl)C=CC=C1Cl VCXSNHNCTQWGLW-UHFFFAOYSA-N 0.000 description 2
- LLROBJYWIOSQKL-UHFFFAOYSA-N 5-[(2-bromophenyl)methoxy]pentane-1,4-diol Chemical compound OCCCC(O)COCC1=CC=CC=C1Br LLROBJYWIOSQKL-UHFFFAOYSA-N 0.000 description 2
- GDIIQQMAKWSSMU-UHFFFAOYSA-N 5-[(2-chlorophenyl)methoxy]pentane-1,4-diol Chemical compound OCCCC(O)COCC1=CC=CC=C1Cl GDIIQQMAKWSSMU-UHFFFAOYSA-N 0.000 description 2
- BYVUVWFMEGTFEV-UHFFFAOYSA-N 5-[(2-methylphenyl)methoxy]pentane-1,4-diol Chemical compound CC1=CC=CC=C1COCC(O)CCCO BYVUVWFMEGTFEV-UHFFFAOYSA-N 0.000 description 2
- BNIBUAREJQEOEH-UHFFFAOYSA-N 5-[(4-fluorophenyl)methoxy]pentane-1,4-diol Chemical compound OCCCC(O)COCC1=CC=C(F)C=C1 BNIBUAREJQEOEH-UHFFFAOYSA-N 0.000 description 2
- CCRKBOTZLVPXCE-UHFFFAOYSA-N 5-methyl-1-[(2-methylphenyl)methoxy]hexane-2,5-diol Chemical compound CC1=CC=CC=C1COCC(O)CCC(C)(C)O CCRKBOTZLVPXCE-UHFFFAOYSA-N 0.000 description 2
- SUIYLBMZDWHUKP-UHFFFAOYSA-N 5-methyl-1-phenylmethoxyhexane-2,5-diol Chemical compound CC(C)(O)CCC(O)COCC1=CC=CC=C1 SUIYLBMZDWHUKP-UHFFFAOYSA-N 0.000 description 2
- JBTLFIIYVNDDEK-UHFFFAOYSA-N 5-phenylmethoxypentane-1,4-diol Chemical compound OCCCC(O)COCC1=CC=CC=C1 JBTLFIIYVNDDEK-UHFFFAOYSA-N 0.000 description 2
- 240000001592 Amaranthus caudatus Species 0.000 description 2
- 235000009328 Amaranthus caudatus Nutrition 0.000 description 2
- 244000099147 Ananas comosus Species 0.000 description 2
- 235000007119 Ananas comosus Nutrition 0.000 description 2
- 244000003416 Asparagus officinalis Species 0.000 description 2
- 235000005340 Asparagus officinalis Nutrition 0.000 description 2
- 235000005781 Avena Nutrition 0.000 description 2
- 235000007319 Avena orientalis Nutrition 0.000 description 2
- 241000219198 Brassica Species 0.000 description 2
- 240000002791 Brassica napus Species 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- 241000132570 Centaurea Species 0.000 description 2
- 235000007866 Chamaemelum nobile Nutrition 0.000 description 2
- 235000000509 Chenopodium ambrosioides Nutrition 0.000 description 2
- 244000098897 Chenopodium botrys Species 0.000 description 2
- 235000005490 Chenopodium botrys Nutrition 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 229920000742 Cotton Polymers 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- DOBFVRYBSRAZFI-UHFFFAOYSA-N FC1=C(COC(C)C=2OC=CC2)C=CC=C1 Chemical compound FC1=C(COC(C)C=2OC=CC2)C=CC=C1 DOBFVRYBSRAZFI-UHFFFAOYSA-N 0.000 description 2
- 241000234642 Festuca Species 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 235000010469 Glycine max Nutrition 0.000 description 2
- 244000068988 Glycine max Species 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 235000021506 Ipomoea Nutrition 0.000 description 2
- 241000207783 Ipomoea Species 0.000 description 2
- 240000001549 Ipomoea eriocarpa Species 0.000 description 2
- 235000005146 Ipomoea eriocarpa Nutrition 0.000 description 2
- 241000520028 Lamium Species 0.000 description 2
- 241000209510 Liliopsida Species 0.000 description 2
- 241000227653 Lycopersicon Species 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- JNZISVCMXDLMRQ-UHFFFAOYSA-N O(C=C1C=C(CCl)C=CC1=COCl)Cl Chemical compound O(C=C1C=C(CCl)C=CC1=COCl)Cl JNZISVCMXDLMRQ-UHFFFAOYSA-N 0.000 description 2
- 240000007594 Oryza sativa Species 0.000 description 2
- 235000007164 Oryza sativa Nutrition 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- 241000209117 Panicum Species 0.000 description 2
- 235000006443 Panicum miliaceum subsp. miliaceum Nutrition 0.000 description 2
- 235000009037 Panicum miliaceum subsp. ruderale Nutrition 0.000 description 2
- 240000001090 Papaver somniferum Species 0.000 description 2
- 239000005662 Paraffin oil Substances 0.000 description 2
- 235000010627 Phaseolus vulgaris Nutrition 0.000 description 2
- 244000046052 Phaseolus vulgaris Species 0.000 description 2
- 241000219843 Pisum Species 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 241000209056 Secale Species 0.000 description 2
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 2
- 235000002634 Solanum Nutrition 0.000 description 2
- 235000011684 Sorghum saccharatum Nutrition 0.000 description 2
- 240000006694 Stellaria media Species 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 241000209140 Triticum Species 0.000 description 2
- 235000021307 Triticum Nutrition 0.000 description 2
- 241000219422 Urtica Species 0.000 description 2
- 240000008042 Zea mays Species 0.000 description 2
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 2
- 239000001089 [(2R)-oxolan-2-yl]methanol Substances 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 2
- 125000002877 alkyl aryl group Chemical group 0.000 description 2
- 125000000304 alkynyl group Chemical group 0.000 description 2
- VSCWAEJMTAWNJL-UHFFFAOYSA-K aluminium trichloride Chemical compound Cl[Al](Cl)Cl VSCWAEJMTAWNJL-UHFFFAOYSA-K 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- OVHDZBAFUMEXCX-UHFFFAOYSA-N benzyl 4-methylbenzenesulfonate Chemical compound C1=CC(C)=CC=C1S(=O)(=O)OCC1=CC=CC=C1 OVHDZBAFUMEXCX-UHFFFAOYSA-N 0.000 description 2
- AGEZXYOZHKGVCM-UHFFFAOYSA-N benzyl bromide Chemical compound BrCC1=CC=CC=C1 AGEZXYOZHKGVCM-UHFFFAOYSA-N 0.000 description 2
- KCXMKQUNVWSEMD-UHFFFAOYSA-N benzyl chloride Chemical compound ClCC1=CC=CC=C1 KCXMKQUNVWSEMD-UHFFFAOYSA-N 0.000 description 2
- 229940073608 benzyl chloride Drugs 0.000 description 2
- OQROAIRCEOBYJA-UHFFFAOYSA-N bromodiphenylmethane Chemical compound C=1C=CC=CC=1C(Br)C1=CC=CC=C1 OQROAIRCEOBYJA-UHFFFAOYSA-N 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 125000001309 chloro group Chemical group Cl* 0.000 description 2
- JHIVVAPYMSGYDF-UHFFFAOYSA-N cyclohexanone Chemical compound O=C1CCCCC1 JHIVVAPYMSGYDF-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 235000013399 edible fruits Nutrition 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- XPFVYQJUAUNWIW-UHFFFAOYSA-N furfuryl alcohol Chemical class OCC1=CC=CO1 XPFVYQJUAUNWIW-UHFFFAOYSA-N 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 150000004678 hydrides Chemical class 0.000 description 2
- 150000004679 hydroxides Chemical class 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 150000007522 mineralic acids Chemical class 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 2
- SBPZYRHUASAHAV-UHFFFAOYSA-N oxolan-2-yl(phenyl)methanol Chemical compound C=1C=CC=CC=1C(O)C1CCCO1 SBPZYRHUASAHAV-UHFFFAOYSA-N 0.000 description 2
- 239000006072 paste Substances 0.000 description 2
- 235000020232 peanut Nutrition 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920000151 polyglycol Polymers 0.000 description 2
- 239000010695 polyglycol Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 235000009566 rice Nutrition 0.000 description 2
- 239000011435 rock Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- HEMHJVSKTPXQMS-UHFFFAOYSA-M sodium hydroxide Inorganic materials [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- 229910052938 sodium sulfate Inorganic materials 0.000 description 2
- 235000011152 sodium sulphate Nutrition 0.000 description 2
- 239000002689 soil Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 238000005507 spraying Methods 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- VZGDMQKNWNREIO-UHFFFAOYSA-N tetrachloromethane Chemical compound ClC(Cl)(Cl)Cl VZGDMQKNWNREIO-UHFFFAOYSA-N 0.000 description 2
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 description 1
- AIPJZPPOFWCJRC-UHFFFAOYSA-N 1,2-dichloro-3-(chloromethyl)benzene Chemical compound ClCC1=CC=CC(Cl)=C1Cl AIPJZPPOFWCJRC-UHFFFAOYSA-N 0.000 description 1
- VYBHHNJUTAAMCG-UHFFFAOYSA-N 1-(1-bromoethyl)-2-fluorobenzene Chemical compound CC(Br)C1=CC=CC=C1F VYBHHNJUTAAMCG-UHFFFAOYSA-N 0.000 description 1
- LKNZNIHNBFZDQM-UHFFFAOYSA-N 1-(1-bromoethyl)-2-methylbenzene Chemical compound CC(Br)C1=CC=CC=C1C LKNZNIHNBFZDQM-UHFFFAOYSA-N 0.000 description 1
- VQRBXYBBGHOGFT-UHFFFAOYSA-N 1-(chloromethyl)-2-methylbenzene Chemical compound CC1=CC=CC=C1CCl VQRBXYBBGHOGFT-UHFFFAOYSA-N 0.000 description 1
- LZBOHNCMCCSTJX-UHFFFAOYSA-N 1-(chloromethyl)-3-methylbenzene Chemical compound CC1=CC=CC(CCl)=C1 LZBOHNCMCCSTJX-UHFFFAOYSA-N 0.000 description 1
- MWXWHUXLVXOXBZ-UHFFFAOYSA-N 1-(furan-2-yl)propan-1-ol Chemical compound CCC(O)C1=CC=CO1 MWXWHUXLVXOXBZ-UHFFFAOYSA-N 0.000 description 1
- FGNVEEOZAACRKW-UHFFFAOYSA-N 1-(oxolan-2-yl)ethanol Chemical compound CC(O)C1CCCO1 FGNVEEOZAACRKW-UHFFFAOYSA-N 0.000 description 1
- DDVSFIUKWUTKES-UHFFFAOYSA-N 1-bromo-2-(chloromethyl)benzene Chemical compound ClCC1=CC=CC=C1Br DDVSFIUKWUTKES-UHFFFAOYSA-N 0.000 description 1
- CRRUGYDDEMGVDY-UHFFFAOYSA-N 1-bromoethylbenzene Chemical compound CC(Br)C1=CC=CC=C1 CRRUGYDDEMGVDY-UHFFFAOYSA-N 0.000 description 1
- IRSVDHPYXFLLDS-UHFFFAOYSA-N 2,4-dichloro-1-(chloromethyl)benzene Chemical compound ClCC1=CC=C(Cl)C=C1Cl IRSVDHPYXFLLDS-UHFFFAOYSA-N 0.000 description 1
- GUUPQNFQKIUOOE-UHFFFAOYSA-N 2,5-diethyl-2-[(2-fluorophenyl)methoxymethyl]-5-methyloxolane Chemical compound O1C(CC)(C)CCC1(CC)COCC1=CC=CC=C1F GUUPQNFQKIUOOE-UHFFFAOYSA-N 0.000 description 1
- ZCUYSRPVZUQGBB-UHFFFAOYSA-N 2,5-diethyl-2-[(4-fluorophenyl)methoxymethyl]-5-methyloxolane Chemical compound O1C(CC)(C)CCC1(CC)COCC1=CC=C(F)C=C1 ZCUYSRPVZUQGBB-UHFFFAOYSA-N 0.000 description 1
- ZJLSVACLFKSPKA-UHFFFAOYSA-N 2,5-diethyl-2-methyl-5-(phenylmethoxymethyl)oxolane Chemical compound O1C(CC)(C)CCC1(CC)COCC1=CC=CC=C1 ZJLSVACLFKSPKA-UHFFFAOYSA-N 0.000 description 1
- JJAVLBYJDFANJH-UHFFFAOYSA-N 2,5-diethyl-2-methyl-5-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound O1C(CC)(C)CCC1(CC)COCC1=CC=CC=C1C JJAVLBYJDFANJH-UHFFFAOYSA-N 0.000 description 1
- XAUDAGHTNAOGTC-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-2,5-diethyl-5-methyloxolane Chemical compound O1C(CC)(C)CCC1(CC)COCC1=C(Cl)C=CC=C1Cl XAUDAGHTNAOGTC-UHFFFAOYSA-N 0.000 description 1
- FBHJTZISRBINGH-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-2-ethyl-5,5-dimethyloxolane Chemical compound ClC=1C=CC=C(Cl)C=1COCC1(CC)CCC(C)(C)O1 FBHJTZISRBINGH-UHFFFAOYSA-N 0.000 description 1
- QFICOBQQVKRNPW-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-2-ethyl-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(CC)COCC1=C(Cl)C=CC=C1Cl QFICOBQQVKRNPW-UHFFFAOYSA-N 0.000 description 1
- ZOMAVKGEJZZKMV-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-5-ethyl-2,5-dimethyloxolane Chemical compound O1C(CC)(C)CCC1(C)COCC1=C(Cl)C=CC=C1Cl ZOMAVKGEJZZKMV-UHFFFAOYSA-N 0.000 description 1
- KYZMQEDEDPZHGS-UHFFFAOYSA-N 2-[(2,6-dichlorophenyl)methoxymethyl]-5-ethyloxolane Chemical compound O1C(CC)CCC1COCC1=C(Cl)C=CC=C1Cl KYZMQEDEDPZHGS-UHFFFAOYSA-N 0.000 description 1
- CKKIXEWLSHEWGH-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-2,5-diethyl-5-methyloxolane Chemical compound O1C(CC)(C)CCC1(CC)COCC1=CC=CC=C1Br CKKIXEWLSHEWGH-UHFFFAOYSA-N 0.000 description 1
- LCVKTNKQPSBRDT-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-2-ethyl-5,5-dimethyloxolane Chemical compound C=1C=CC=C(Br)C=1COCC1(CC)CCC(C)(C)O1 LCVKTNKQPSBRDT-UHFFFAOYSA-N 0.000 description 1
- FQLMTPVBUSFJTC-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-2-ethyl-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(CC)COCC1=CC=CC=C1Br FQLMTPVBUSFJTC-UHFFFAOYSA-N 0.000 description 1
- NRUAMQNFTYTPAA-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-2-ethyloxolane Chemical compound C=1C=CC=C(Br)C=1COCC1(CC)CCCO1 NRUAMQNFTYTPAA-UHFFFAOYSA-N 0.000 description 1
- NNCLELSQTXEHQN-UHFFFAOYSA-N 2-[(2-bromophenyl)methoxymethyl]-5-ethyl-2,5-dimethyloxolane Chemical compound O1C(CC)(C)CCC1(C)COCC1=CC=CC=C1Br NNCLELSQTXEHQN-UHFFFAOYSA-N 0.000 description 1
- TZMNZYVYARAVCP-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-2,5-diethyl-5-methyloxolane Chemical compound O1C(CC)(C)CCC1(CC)COCC1=CC=CC=C1Cl TZMNZYVYARAVCP-UHFFFAOYSA-N 0.000 description 1
- WEHDXTNMAIWVIA-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-2-ethyl-5,5-dimethyloxolane Chemical compound C=1C=CC=C(Cl)C=1COCC1(CC)CCC(C)(C)O1 WEHDXTNMAIWVIA-UHFFFAOYSA-N 0.000 description 1
- POJALDLXTKDWDL-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-2-ethyl-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(CC)COCC1=CC=CC=C1Cl POJALDLXTKDWDL-UHFFFAOYSA-N 0.000 description 1
- JUJDMVCVRAPOCT-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-2-ethyloxolane Chemical compound C=1C=CC=C(Cl)C=1COCC1(CC)CCCO1 JUJDMVCVRAPOCT-UHFFFAOYSA-N 0.000 description 1
- VHDJCFBTPLSRGY-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-5-ethyl-2,5-dimethyloxolane Chemical compound O1C(CC)(C)CCC1(C)COCC1=CC=CC=C1Cl VHDJCFBTPLSRGY-UHFFFAOYSA-N 0.000 description 1
- ZBZMYRLKYBLMDO-UHFFFAOYSA-N 2-[(2-chlorophenyl)methoxymethyl]-5-ethyloxolane Chemical compound O1C(CC)CCC1COCC1=CC=CC=C1Cl ZBZMYRLKYBLMDO-UHFFFAOYSA-N 0.000 description 1
- JVJSQLSWWMSFOP-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]-2,3-dihydrofuran Chemical compound FC1=CC=CC=C1COCC1OC=CC1 JVJSQLSWWMSFOP-UHFFFAOYSA-N 0.000 description 1
- UCTVVMDPKQUHRG-UHFFFAOYSA-N 2-[(2-fluorophenyl)methoxymethyl]oxolane Chemical compound FC1=CC=CC=C1COCC1OCCC1 UCTVVMDPKQUHRG-UHFFFAOYSA-N 0.000 description 1
- UFGLVLIICPGVOR-UHFFFAOYSA-N 2-[1-[(2-chlorophenyl)methoxy]propyl]oxolane Chemical compound C1CCOC1C(CC)OCC1=CC=CC=C1Cl UFGLVLIICPGVOR-UHFFFAOYSA-N 0.000 description 1
- VONWPEXRCLHKRJ-UHFFFAOYSA-N 2-chloro-n-phenylacetamide Chemical class ClCC(=O)NC1=CC=CC=C1 VONWPEXRCLHKRJ-UHFFFAOYSA-N 0.000 description 1
- JXMIHOFLSSHALX-UHFFFAOYSA-N 2-ethyl-2,5-dimethyl-5-(phenylmethoxymethyl)oxolane Chemical compound O1C(CC)(C)CCC1(C)COCC1=CC=CC=C1 JXMIHOFLSSHALX-UHFFFAOYSA-N 0.000 description 1
- PNIBHJJXONNZHH-UHFFFAOYSA-N 2-ethyl-2,5-dimethyl-5-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound O1C(CC)(C)CCC1(C)COCC1=CC=CC=C1C PNIBHJJXONNZHH-UHFFFAOYSA-N 0.000 description 1
- RYDSUZGECMYFRJ-UHFFFAOYSA-N 2-ethyl-2-(phenylmethoxymethyl)oxolane Chemical compound C=1C=CC=CC=1COCC1(CC)CCCO1 RYDSUZGECMYFRJ-UHFFFAOYSA-N 0.000 description 1
- WUHXBAXZWLXJPI-UHFFFAOYSA-N 2-ethyl-2-[(2-fluorophenyl)methoxymethyl]-5,5-dimethyloxolane Chemical compound C=1C=CC=C(F)C=1COCC1(CC)CCC(C)(C)O1 WUHXBAXZWLXJPI-UHFFFAOYSA-N 0.000 description 1
- ZZZGTMCPVUUURZ-UHFFFAOYSA-N 2-ethyl-2-[(2-fluorophenyl)methoxymethyl]-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(CC)COCC1=CC=CC=C1F ZZZGTMCPVUUURZ-UHFFFAOYSA-N 0.000 description 1
- JGJBRGVDXJVFBP-UHFFFAOYSA-N 2-ethyl-2-[(2-fluorophenyl)methoxymethyl]oxolane Chemical compound C=1C=CC=C(F)C=1COCC1(CC)CCCO1 JGJBRGVDXJVFBP-UHFFFAOYSA-N 0.000 description 1
- VLKQYUBIHZGEJK-UHFFFAOYSA-N 2-ethyl-2-[(2-methylphenyl)methoxymethyl]-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(CC)COCC1=CC=CC=C1C VLKQYUBIHZGEJK-UHFFFAOYSA-N 0.000 description 1
- STZPHCRDVGBKCV-UHFFFAOYSA-N 2-ethyl-2-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound C=1C=CC=C(C)C=1COCC1(CC)CCCO1 STZPHCRDVGBKCV-UHFFFAOYSA-N 0.000 description 1
- JMWACBQIZOBULK-UHFFFAOYSA-N 2-ethyl-2-[(4-fluorophenyl)methoxymethyl]-5,5-dimethyloxolane Chemical compound C=1C=C(F)C=CC=1COCC1(CC)CCC(C)(C)O1 JMWACBQIZOBULK-UHFFFAOYSA-N 0.000 description 1
- SXPZDXFPKWIPAI-UHFFFAOYSA-N 2-ethyl-2-[(4-fluorophenyl)methoxymethyl]-5-phenyloxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(CC)COCC1=CC=C(F)C=C1 SXPZDXFPKWIPAI-UHFFFAOYSA-N 0.000 description 1
- QIMIDFZJVRPXQH-UHFFFAOYSA-N 2-ethyl-2-[(4-fluorophenyl)methoxymethyl]oxolane Chemical compound C=1C=C(F)C=CC=1COCC1(CC)CCCO1 QIMIDFZJVRPXQH-UHFFFAOYSA-N 0.000 description 1
- BLTMSPVTTVFDDJ-UHFFFAOYSA-N 2-ethyl-5,5-dimethyl-2-(phenylmethoxymethyl)oxolane Chemical compound C=1C=CC=CC=1COCC1(CC)CCC(C)(C)O1 BLTMSPVTTVFDDJ-UHFFFAOYSA-N 0.000 description 1
- TYQHEQKWNAMBOF-UHFFFAOYSA-N 2-ethyl-5,5-dimethyl-2-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound C=1C=CC=C(C)C=1COCC1(CC)CCC(C)(C)O1 TYQHEQKWNAMBOF-UHFFFAOYSA-N 0.000 description 1
- PAUXTAMXWQYCCW-UHFFFAOYSA-N 2-ethyl-5-(phenylmethoxymethyl)oxolane Chemical compound O1C(CC)CCC1COCC1=CC=CC=C1 PAUXTAMXWQYCCW-UHFFFAOYSA-N 0.000 description 1
- MLGQEXSNOOKKET-UHFFFAOYSA-N 2-ethyl-5-[(2-fluorophenyl)methoxymethyl]-2,5-dimethyloxolane Chemical compound O1C(CC)(C)CCC1(C)COCC1=CC=CC=C1F MLGQEXSNOOKKET-UHFFFAOYSA-N 0.000 description 1
- WGGPSCIGWWMAPT-UHFFFAOYSA-N 2-ethyl-5-[(2-fluorophenyl)methoxymethyl]oxolane Chemical compound O1C(CC)CCC1COCC1=CC=CC=C1F WGGPSCIGWWMAPT-UHFFFAOYSA-N 0.000 description 1
- PADPZQJBJWTGNW-UHFFFAOYSA-N 2-ethyl-5-[(2-methylphenyl)methoxymethyl]oxolane Chemical compound O1C(CC)CCC1COCC1=CC=CC=C1C PADPZQJBJWTGNW-UHFFFAOYSA-N 0.000 description 1
- KYHFLAONQXTXIN-UHFFFAOYSA-N 2-ethyl-5-[(4-fluorophenyl)methoxymethyl]-2,5-dimethyloxolane Chemical compound O1C(CC)(C)CCC1(C)COCC1=CC=C(F)C=C1 KYHFLAONQXTXIN-UHFFFAOYSA-N 0.000 description 1
- VQOKAWUKGACKQM-UHFFFAOYSA-N 2-ethyl-5-phenyl-2-(phenylmethoxymethyl)oxolane Chemical compound C1CC(C=2C=CC=CC=2)OC1(CC)COCC1=CC=CC=C1 VQOKAWUKGACKQM-UHFFFAOYSA-N 0.000 description 1
- ZAXYIJDAHSKPMF-UHFFFAOYSA-N 2-methyl-5-(phenylmethoxymethyl)heptane-2,5-diol Chemical compound CC(O)(C)CCC(O)(CC)COCC1=CC=CC=C1 ZAXYIJDAHSKPMF-UHFFFAOYSA-N 0.000 description 1
- ILYQZYHNIZOEST-UHFFFAOYSA-N 2-methyl-5-[(2-methylphenyl)methoxymethyl]heptane-2,5-diol Chemical compound CC(O)(C)CCC(O)(CC)COCC1=CC=CC=C1C ILYQZYHNIZOEST-UHFFFAOYSA-N 0.000 description 1
- ZTJLYUVAFAMUKO-UHFFFAOYSA-N 2-phenylethanesulfonic acid Chemical compound OS(=O)(=O)CCC1=CC=CC=C1 ZTJLYUVAFAMUKO-UHFFFAOYSA-N 0.000 description 1
- SNEVRMALOYTKTQ-UHFFFAOYSA-N 5-[(2,6-dichlorophenyl)methoxymethyl]-2-methylheptane-2,5-diol Chemical compound CC(O)(C)CCC(O)(CC)COCC1=C(Cl)C=CC=C1Cl SNEVRMALOYTKTQ-UHFFFAOYSA-N 0.000 description 1
- DHXDIPOKQHSERV-UHFFFAOYSA-N 5-[(2-chlorophenyl)methoxymethyl]-2-methylheptane-2,5-diol Chemical compound CC(O)(C)CCC(O)(CC)COCC1=CC=CC=C1Cl DHXDIPOKQHSERV-UHFFFAOYSA-N 0.000 description 1
- KGQWYCRLDGFFDU-UHFFFAOYSA-N 5-[(2-fluorophenyl)methoxymethyl]-2-methylheptane-2,5-diol Chemical compound CC(O)(C)CCC(O)(CC)COCC1=CC=CC=C1F KGQWYCRLDGFFDU-UHFFFAOYSA-N 0.000 description 1
- XQRTVMXGNHAKMF-UHFFFAOYSA-N 5-[(4-fluorophenyl)methoxymethyl]-2-methylheptane-2,5-diol Chemical compound CC(O)(C)CCC(O)(CC)COCC1=CC=C(F)C=C1 XQRTVMXGNHAKMF-UHFFFAOYSA-N 0.000 description 1
- 241000219144 Abutilon Species 0.000 description 1
- 235000006760 Acer pensylvanicum Nutrition 0.000 description 1
- 240000002245 Acer pensylvanicum Species 0.000 description 1
- 241000254032 Acrididae Species 0.000 description 1
- 241000209136 Agropyron Species 0.000 description 1
- 241000743339 Agrostis Species 0.000 description 1
- 241000234282 Allium Species 0.000 description 1
- 240000006108 Allium ampeloprasum Species 0.000 description 1
- 235000005254 Allium ampeloprasum Nutrition 0.000 description 1
- 241000743985 Alopecurus Species 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 241000219318 Amaranthus Species 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 1
- 241000272814 Anser sp. Species 0.000 description 1
- 241000404028 Anthemis Species 0.000 description 1
- 241001666377 Apera Species 0.000 description 1
- 235000003911 Arachis Nutrition 0.000 description 1
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- 240000005528 Arctium lappa Species 0.000 description 1
- 235000003130 Arctium lappa Nutrition 0.000 description 1
- 235000008078 Arctium minus Nutrition 0.000 description 1
- 235000000832 Ayote Nutrition 0.000 description 1
- 235000016068 Berberis vulgaris Nutrition 0.000 description 1
- 241000335053 Beta vulgaris Species 0.000 description 1
- 241000611157 Brachiaria Species 0.000 description 1
- 241000339490 Brachyachne Species 0.000 description 1
- 235000011331 Brassica Nutrition 0.000 description 1
- 235000003351 Brassica cretica Nutrition 0.000 description 1
- 235000011293 Brassica napus Nutrition 0.000 description 1
- 240000007124 Brassica oleracea Species 0.000 description 1
- 235000003899 Brassica oleracea var acephala Nutrition 0.000 description 1
- 235000011301 Brassica oleracea var capitata Nutrition 0.000 description 1
- 235000001169 Brassica oleracea var oleracea Nutrition 0.000 description 1
- 235000000540 Brassica rapa subsp rapa Nutrition 0.000 description 1
- 235000003343 Brassica rupestris Nutrition 0.000 description 1
- 235000004977 Brassica sinapistrum Nutrition 0.000 description 1
- 241000209200 Bromus Species 0.000 description 1
- GXQSGZSVGDGQPW-UHFFFAOYSA-N C(C1=CC=CC=C1)OC(C)C=1OC=CC1 Chemical compound C(C1=CC=CC=C1)OC(C)C=1OC=CC1 GXQSGZSVGDGQPW-UHFFFAOYSA-N 0.000 description 1
- HQIVQWROFCAJPD-UHFFFAOYSA-N CC(C1=CC=CC=C1)Cl.BrC1=CC=C(CCl)C=C1 Chemical compound CC(C1=CC=CC=C1)Cl.BrC1=CC=C(CCl)C=C1 HQIVQWROFCAJPD-UHFFFAOYSA-N 0.000 description 1
- PVYLTLOATXEAJK-UHFFFAOYSA-N CC1=C(COC(C)C=2OC=CC2)C=CC=C1 Chemical compound CC1=C(COC(C)C=2OC=CC2)C=CC=C1 PVYLTLOATXEAJK-UHFFFAOYSA-N 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 241000320316 Carduus Species 0.000 description 1
- 241000219312 Chenopodium Species 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 241000207199 Citrus Species 0.000 description 1
- 240000007154 Coffea arabica Species 0.000 description 1
- 241000207892 Convolvulus Species 0.000 description 1
- 235000010071 Cucumis prophetarum Nutrition 0.000 description 1
- 244000024469 Cucumis prophetarum Species 0.000 description 1
- 240000008067 Cucumis sativus Species 0.000 description 1
- 235000010799 Cucumis sativus var sativus Nutrition 0.000 description 1
- 235000009854 Cucurbita moschata Nutrition 0.000 description 1
- 240000001980 Cucurbita pepo Species 0.000 description 1
- 235000009804 Cucurbita pepo subsp pepo Nutrition 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- 241000234653 Cyperus Species 0.000 description 1
- 241000320605 Dactyloctenium Species 0.000 description 1
- 241000208296 Datura Species 0.000 description 1
- 241000208175 Daucus Species 0.000 description 1
- 244000000626 Daucus carota Species 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- 235000017896 Digitaria Nutrition 0.000 description 1
- 241001303487 Digitaria <clam> Species 0.000 description 1
- 241000192043 Echinochloa Species 0.000 description 1
- 235000001950 Elaeis guineensis Nutrition 0.000 description 1
- 244000127993 Elaeis melanococca Species 0.000 description 1
- 241000202829 Eleocharis Species 0.000 description 1
- 235000007351 Eleusine Nutrition 0.000 description 1
- 241000209215 Eleusine Species 0.000 description 1
- 244000078127 Eleusine coracana Species 0.000 description 1
- 235000013499 Eleusine coracana subsp coracana Nutrition 0.000 description 1
- 235000006369 Emex spinosa Nutrition 0.000 description 1
- 244000294661 Emex spinosa Species 0.000 description 1
- 241001290564 Fimbristylis Species 0.000 description 1
- 241000816457 Galeopsis Species 0.000 description 1
- 241000748465 Galinsoga Species 0.000 description 1
- 241001101998 Galium Species 0.000 description 1
- 240000005702 Galium aparine Species 0.000 description 1
- 235000014820 Galium aparine Nutrition 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 235000009438 Gossypium Nutrition 0.000 description 1
- 241000209219 Hordeum Species 0.000 description 1
- 235000007340 Hordeum vulgare Nutrition 0.000 description 1
- 240000005979 Hordeum vulgare Species 0.000 description 1
- 241001327265 Ischaemum Species 0.000 description 1
- 239000005909 Kieselgur Substances 0.000 description 1
- 241000208822 Lactuca Species 0.000 description 1
- 241000801118 Lepidium Species 0.000 description 1
- 235000007849 Lepidium sativum Nutrition 0.000 description 1
- 244000211187 Lepidium sativum Species 0.000 description 1
- 241000208202 Linaceae Species 0.000 description 1
- 241000064140 Lindernia Species 0.000 description 1
- 241000208204 Linum Species 0.000 description 1
- 235000004431 Linum usitatissimum Nutrition 0.000 description 1
- 235000002262 Lycopersicon Nutrition 0.000 description 1
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 1
- 235000017945 Matricaria Nutrition 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- 240000005561 Musa balbisiana Species 0.000 description 1
- 235000018290 Musa x paradisiaca Nutrition 0.000 description 1
- 241000208125 Nicotiana Species 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 235000011096 Papaver Nutrition 0.000 description 1
- 235000008753 Papaver somniferum Nutrition 0.000 description 1
- 241001268782 Paspalum dilatatum Species 0.000 description 1
- 241000219833 Phaseolus Species 0.000 description 1
- 241000746981 Phleum Species 0.000 description 1
- 241000209504 Poaceae Species 0.000 description 1
- 241000205407 Polygonum Species 0.000 description 1
- 244000292697 Polygonum aviculare Species 0.000 description 1
- 235000006386 Polygonum aviculare Nutrition 0.000 description 1
- 241000219295 Portulaca Species 0.000 description 1
- 244000234609 Portulaca oleracea Species 0.000 description 1
- 235000001855 Portulaca oleracea Nutrition 0.000 description 1
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 1
- 239000007868 Raney catalyst Substances 0.000 description 1
- 241000490453 Rorippa Species 0.000 description 1
- 241000341978 Rotala Species 0.000 description 1
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 description 1
- 241000209051 Saccharum Species 0.000 description 1
- 240000009132 Sagittaria sagittifolia Species 0.000 description 1
- 235000008406 SarachaNachtschatten Nutrition 0.000 description 1
- 241000202758 Scirpus Species 0.000 description 1
- 241000780602 Senecio Species 0.000 description 1
- 240000003705 Senecio vulgaris Species 0.000 description 1
- 244000275012 Sesbania cannabina Species 0.000 description 1
- 235000005775 Setaria Nutrition 0.000 description 1
- 241000232088 Setaria <nematode> Species 0.000 description 1
- 235000004790 Solanum aculeatissimum Nutrition 0.000 description 1
- 235000008424 Solanum demissum Nutrition 0.000 description 1
- 235000018253 Solanum ferox Nutrition 0.000 description 1
- 235000000208 Solanum incanum Nutrition 0.000 description 1
- 235000013131 Solanum macrocarpon Nutrition 0.000 description 1
- 235000009869 Solanum phureja Nutrition 0.000 description 1
- 235000000341 Solanum ptychanthum Nutrition 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000017622 Solanum xanthocarpum Nutrition 0.000 description 1
- 241000488874 Sonchus Species 0.000 description 1
- 235000017967 Sphenoclea zeylanica Nutrition 0.000 description 1
- 244000273618 Sphenoclea zeylanica Species 0.000 description 1
- 241000272534 Struthio camelus Species 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 1
- 244000269722 Thea sinensis Species 0.000 description 1
- 244000299461 Theobroma cacao Species 0.000 description 1
- 235000009470 Theobroma cacao Nutrition 0.000 description 1
- 241000159750 Urtica cannabina Species 0.000 description 1
- 235000009108 Urtica dioica Nutrition 0.000 description 1
- 241000219873 Vicia Species 0.000 description 1
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical group ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 1
- 241000405217 Viola <butterfly> Species 0.000 description 1
- 241001506766 Xanthium Species 0.000 description 1
- 241000209149 Zea Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000016383 Zea mays subsp huehuetenangensis Nutrition 0.000 description 1
- 229910000102 alkali metal hydride Inorganic materials 0.000 description 1
- 150000008046 alkali metal hydrides Chemical class 0.000 description 1
- 150000008055 alkyl aryl sulfonates Chemical class 0.000 description 1
- 150000008051 alkyl sulfates Chemical class 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 229960000892 attapulgite Drugs 0.000 description 1
- ZDKRMIJRCHPKLW-UHFFFAOYSA-N benzyl methanesulfonate Chemical compound CS(=O)(=O)OCC1=CC=CC=C1 ZDKRMIJRCHPKLW-UHFFFAOYSA-N 0.000 description 1
- 235000021028 berry Nutrition 0.000 description 1
- QKSKPIVNLNLAAV-UHFFFAOYSA-N bis(2-chloroethyl) sulfide Chemical compound ClCCSCCCl QKSKPIVNLNLAAV-UHFFFAOYSA-N 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 150000008280 chlorinated hydrocarbons Chemical class 0.000 description 1
- 150000008422 chlorobenzenes Chemical class 0.000 description 1
- 235000020971 citrus fruits Nutrition 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 235000016213 coffee Nutrition 0.000 description 1
- 235000013353 coffee beverage Nutrition 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 244000038559 crop plants Species 0.000 description 1
- 239000002837 defoliant Substances 0.000 description 1
- 239000002274 desiccant Substances 0.000 description 1
- GUJOJGAPFQRJSV-UHFFFAOYSA-N dialuminum;dioxosilane;oxygen(2-);hydrate Chemical compound O.[O-2].[O-2].[O-2].[Al+3].[Al+3].O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O GUJOJGAPFQRJSV-UHFFFAOYSA-N 0.000 description 1
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 238000010410 dusting Methods 0.000 description 1
- 235000005489 dwarf bean Nutrition 0.000 description 1
- 244000013123 dwarf bean Species 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 241001233957 eudicotyledons Species 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 235000013312 flour Nutrition 0.000 description 1
- 230000000855 fungicidal effect Effects 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 125000004995 haloalkylthio group Chemical group 0.000 description 1
- 238000005984 hydrogenation reaction Methods 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000000749 insecticidal effect Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 229920005610 lignin Polymers 0.000 description 1
- 235000009973 maize Nutrition 0.000 description 1
- 229910001507 metal halide Inorganic materials 0.000 description 1
- 150000005309 metal halides Chemical class 0.000 description 1
- 150000004692 metal hydroxides Chemical class 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 229910052901 montmorillonite Inorganic materials 0.000 description 1
- 235000010460 mustard Nutrition 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 235000014571 nuts Nutrition 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 229910052762 osmium Inorganic materials 0.000 description 1
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 1
- MUMZUERVLWJKNR-UHFFFAOYSA-N oxoplatinum Chemical compound [Pt]=O MUMZUERVLWJKNR-UHFFFAOYSA-N 0.000 description 1
- SJLOMQIUPFZJAN-UHFFFAOYSA-N oxorhodium Chemical compound [Rh]=O SJLOMQIUPFZJAN-UHFFFAOYSA-N 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 229910052625 palygorskite Inorganic materials 0.000 description 1
- 125000003538 pentan-3-yl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 239000003444 phase transfer catalyst Substances 0.000 description 1
- 150000004714 phosphonium salts Chemical class 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229910003446 platinum oxide Inorganic materials 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 239000003531 protein hydrolysate Substances 0.000 description 1
- 235000015136 pumpkin Nutrition 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 229910003450 rhodium oxide Inorganic materials 0.000 description 1
- 229910052707 ruthenium Inorganic materials 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 238000009331 sowing Methods 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000010902 straw Substances 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000013616 tea Nutrition 0.000 description 1
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 1
- 229960000278 theophylline Drugs 0.000 description 1
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 235000014101 wine Nutrition 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/34—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members
- C07D307/38—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having two or three double bonds between ring members or between ring members and non-ring members with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D307/40—Radicals substituted by oxygen atoms
- C07D307/42—Singly bound oxygen atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/08—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings with oxygen as the ring hetero atom
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/02—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms
- A01N43/04—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom
- A01N43/06—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings
- A01N43/12—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one or more oxygen or sulfur atoms as the only ring hetero atoms with one hetero atom five-membered rings condensed with a carbocyclic ring
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/04—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
- C07D307/10—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having no double bonds between ring members or between ring members and non-ring members with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D307/12—Radicals substituted by oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D407/00—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00
- C07D407/02—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00 containing two hetero rings
- C07D407/12—Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms as the only ring hetero atoms, not provided for by group C07D405/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F01—MACHINES OR ENGINES IN GENERAL; ENGINE PLANTS IN GENERAL; STEAM ENGINES
- F01D—NON-POSITIVE DISPLACEMENT MACHINES OR ENGINES, e.g. STEAM TURBINES
- F01D1/00—Non-positive-displacement machines or engines, e.g. steam turbines
Definitions
- the invention relates to tetrahydrofuran derivatives, several processes for their preparation and their use as herbicides, in particular as selective herbicides.
- chloroacetanilides such as, for example, 2-ethyl-6-methyl-N- (1'-methyl-2'-methoxyethyl) chloroacetanilide
- grass-like weeds cf. DE-A-2 328 340.
- selectivity of these compounds is not always satisfactory.
- tetrahydrofuran derivatives have strong herbicidal, in particular selective herbicidal properties.
- the tetrahydrofuran derivatives according to the invention are superior to the known herbicides, such as, for example, 2-ethyl-6-methyl-N - (1'-methyl-2'-methoxyethyl) -chloroacetanilide, in their herbicidal action and also show a markedly better selectivity important kulfur plants.
- the active compounds according to the invention thus represent a significant enrichment of herbicides, especially grass herbicides.
- the alcoholates of the formula II are known or can be prepared by known methods. They are obtained, for example, by reacting the corresponding 2-hydroxymethyltetrahydrofuran derivatives with suitable strong bases, such as, for example, alkali or alkaline earth amides, hydrides or hydroxides, in an inert solvent.
- suitable strong bases such as, for example, alkali or alkaline earth amides, hydrides or hydroxides.
- suitable strong bases such as, for example, alkali or alkaline earth amides, hydrides or hydroxides
- Formula III generally defines the compounds to be used as starting materials for process variant (a).
- Formula IV generally defines the diols to be used as starting materials for process variant (b).
- the formulas Va, Vb and Vc generally define the dihydrofuran derivatives to be used as starting materials for process variant (c).
- Formula VI generally defines the furan derivatives to be used as starting materials for process variant (d).
- the alcoholates of the formulas VIII, VIIIb, VIIIc and VIII are known or can be prepared by known methods. They are obtained, for example, by reacting the corresponding 2-hydroxymethyldihydrofuran derivatives or 2-hydroxymethyl furan derivatives with suitable strong bases, such as, for example, alkali metal or alkaline earth metal amides, hydrides or hydroxides, in an inert solvent.
- suitable strong bases such as, for example, alkali metal or alkaline earth metal amides, hydrides or hydroxides
- the 2-hydroxymethyl derivatives mentioned are likewise known or can be prepared by known methods (cf., inter alia, H. Kröper in Houben-Weyl, 'Methods of Organic Chemistry', Volume 6/3, pp. 519ff [1965] and those therein literature cited).
- Inert organic solvents are preferably suitable as diluents for the reaction according to process variant (a) according to the invention.
- These preferably include ethers such as diethyl ether, tetrahydrofuran or dioxane, aromatic hydrocarbons such as benzene or toluene, and in individual cases also chlorinated hydrocarbons such as chloroform, methylene chloride or carbon tetrachloride.
- reaction temperatures can be varied within a substantial range. Generally one works between 0 and 120 ° C, preferably at 20 to 100 ° C.
- the process variant (a) according to the invention is preferably carried out in molar amounts. However, it is also possible to use the alcoholates of the formula II or the compounds of the formula III in excess of up to 1 mol.
- water is added to the reaction mixture, the organic phase is separated off and worked up and purified in the customary manner. In individual cases, the end product can also be distilled off from the reaction product directly after the solvent.
- the procedure is expediently started from a 2-hydroxymethyl-tetrahydrofuran derivative, the latter converted into the alkali metal alcoholate of the formula 11 in a suitable inert solvent using alkali metal hydride or amide, and the latter immediately without isolation reacted with a compound of the formula III and thus obtained the compounds of the formula according to the invention in one operation.
- the compound of formula III can also be added to the reaction mixture before the alcoholate is prepared.
- the preparation of the alcoholate of the formula II and the reaction according to the invention by process (a) are advantageously carried out in one Two-phase system, such as aqueous sodium or potassium hydroxide solution / toluene or methylene chloride, carried out with the addition of a phase transfer catalyst, such as ammonium or phosphonium compounds.
- a phase transfer catalyst such as ammonium or phosphonium compounds.
- reaction according to process variant (b) according to the invention is preferably carried out without a solvent.
- the reaction according to the invention in process variant (b) is carried out in the presence of an acid catalyst.
- an acid catalyst can be used. These preferably include organic acids, such as p-toluenesulfonic acid, inorganic acids, such as hydrochloric acid and sulfuric acid, and metal halides, such as aluminum chloride.
- reaction temperatures in process variant (b) can be varied within a substantial range. Generally one works between 80 and 250 ° C, preferably between about 100 and 220 ° C.
- reaction mixture is distilled in vacuo and the water is then separated off in the customary manner.
- inert organic solvents are preferably suitable when using a diluent.
- a diluent preferably include alcohols, such as methanol and ethanol, and ethers, such as diethyl ether and tetrahydrofuran.
- the reactions according to the invention in process variants (c) and (d) are carried out in the presence of a catalyst.
- a catalyst You can use all commonly used hydrogenation catalysts. These preferably include noble metal, noble metal oxide (or noble metal hydroxide) catalysts or so-called 'Raney catalysts', such as in particular platinum, platinum oxide, nickel, rhodium, rhodium oxide, ruthenium, palladium and osmium.
- reaction temperatures can be varied over a wide range in process variants (c) and (d). Generally one works between 20 and 200 ° C, preferably at 80 to 150 ° C.
- the reactions according to process variants (c) and (d) can be carried out under normal pressure, but also under elevated pressure, preferably at 1 to 200 atm.
- the compounds of the formula according to the invention can optionally be present in different geometric isomers which can be obtained in different proportions. They are also available as optical isomers. In this case, it can occur that certain isomers have a greater activity than others, so that it may be expedient to prepare or isolate the more active components. All the isomers are claimed according to the invention.
- the active compounds according to the invention influence plant growth and can therefore be used as defoliants, desiccants, haulm killers, germ inhibitors and in particular as weed killers. Weeds in the broadest sense are all plants that grow up in places where they are undesirable. Whether the substances according to the invention act as total or selective herbicides depends essentially on the amount used.
- the active compounds according to the invention can be used, for example, in the following plants: dicotyledon weeds of the genera: mustard (Sinapsis), cress (Lepidium), bedstraw (Galium), starwort (Stellaria), chamomile (Matricaria), dog chamomile (Anthemis), button herb (Galinsoga ), Goose foot (Chenopodium), nettle (Urtica), ragwort (Senecio), foxtail (Amaranthus), purslane (Portulaca), Norway burdock (Xanthium), winch (Convolvulus), morning glory (Ipomoea), knotweed (Polygonum), sesbanie (Sesbania ), Ambrosia (Ambrosia), Thistle (Cirsium), Thistle (Carduus), Goose Thistle (Sonchus), Nightshade (Solanum), Marsh Cress (Rorippa), Rotala, Ciderweed (Li
- Monocot crops of the genera Rice (Orzya), Maize (Zea), Wheat (Triticum), Barley (Hordeum), Oats (Avena), Rye (Secale), Black Millet (Sorghum), Millet (Panicum), Sugar Cane (Saccharum), Pineapple (pineapple), asparagus (asparagus), leek (allium).
- the compounds are suitable for total weed control, e.g. on industrial and track systems and on paths and squares with and without tree cover.
- the compounds for weed control in permanent crops can e.g. Forestry, ornamental trees, fruit, wine, citrus, nut, banana, coffee, tea, rubber, oil palm, cocoa, berry fruit and hop plants and for selective weed control in annual crops.
- the active compounds according to the invention have, in particular, strong herbicidal effects against grasses, without damaging various crop plants. They can therefore preferably be used for selective weed control.
- the following crops are particularly suitable: beets, soybeans, beans, cotton, rapeseed, peanuts, vegetables, corn and rice.
- the active compounds according to the invention can be converted into the customary formulations such as solutions, emulsions, suspensions, powders, pastes and granules. These are prepared in a known manner, for example by mixing the active ingredients with extenders, that is to say liquid solvents, pressurized liquefied gases and / or solid carriers, if appropriate using surface-active agents, that is to say emulsifiers and / or dispersants and / or foam-generating agents. If water is used as an extender, organic solvents can, for example, also be used as auxiliary solvents.
- extenders that is to say liquid solvents, pressurized liquefied gases and / or solid carriers, if appropriate using surface-active agents, that is to say emulsifiers and / or dispersants and / or foam-generating agents.
- organic solvents can, for example, also be used as auxiliary solvents.
- liquid solvents aromatics, such as xylene, toluene, benzene or alkylnaphthalenes, chlorinated aromatics or chlorinated aliphatic hydrocarbons, such as chlorobenzenes, chloroethylene or methylene chloride, aliphatic hydrocarbons, such as cyclohexane or paraffins, for example petroleum fractions, alcohols, such as butanol or Glycol and their ethers and esters, ketones, such as acetone, methyl ethyl ketone, methyl isobutyl ketone or cyclohexanone, strongly polar solvents, such as dimethylformamide and dimethyl sulfoxide, and water;
- Liquefied gaseous extenders or carriers mean liquids which are gaseous at normal temperature and under normal pressure, for example aerosol propellants, such as dichlorodifluoromethane or trichlorofluoromethane; as solid carriers: natural
- the active compounds according to the invention as such or in their formulations for strengthening and supplementing their spectrum of action, can be combined with other herbicidal active compounds, depending on the intended use, finished formulations or tank mixes being possible.
- the formulations generally contain between 0.1 and 95 percent by weight of active compound, preferably between 0.5 and 90 percent by weight.
- the active compounds can be used as such, in the form of their formulations or the use forms prepared therefrom, such as ready-to-use solutions, emulsions, suspensions, powders, pastes and granules. They are used in the usual way, e.g. by spraying, spraying, dusting, scattering and pouring.
- the active compounds according to the invention can be applied both after and in particular before the plants emerge. They can also be worked into the soil before sowing.
- the amount of active ingredient used can fluctuate in larger areas. It essentially depends on the type of effect you want. In general, the application rates are between 0.1 and 10 kg of active ingredient per ha, preferably between 0.2 and 6 kg / ha.
- the active compounds according to the invention not only have herbicidal properties, but also a fungicidal and insecticidal activity.
- active compound 1 part by weight of active compound is mixed with the stated amount of solvent, the stated amount of emulsifier is added and the concentrate is diluted with water to the desired concentration.
- the mixture is then heated under reflux for 30 minutes, cooled to 50 ° C. and 32 g (0.2 mol) of 2-chlorobenzyl chloride are then added dropwise to the sodium salt thus obtained.
- the mixture is then heated under reflux for a further 3 hours, 20 ml of methanol are added to destroy excess sodium hydride and the mixture is concentrated by distilling off the solvent in vacuo.
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Health & Medical Sciences (AREA)
- Pest Control & Pesticides (AREA)
- Dentistry (AREA)
- General Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Environmental Sciences (AREA)
- Plant Pathology (AREA)
- Agronomy & Crop Science (AREA)
- Mechanical Engineering (AREA)
- General Engineering & Computer Science (AREA)
- Furan Compounds (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Claims (5)
de manière connue en soi, en présence d'un catalyseur acide et éventuellement en présence d'un diluant; ou bien
de manière connue en soi, en présence d'un catalyseur et le cas échéant en présence d'un diluant, ou bien
de manière connue en soi, en présence d'un catalyseur et le cas échéant en présence d'un diluant.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19772724675 DE2724675A1 (de) | 1977-06-01 | 1977-06-01 | Tetrahydrofuran-derivate, verfahren zu ihrer herstellung sowie ihre verwendung als herbizide |
| DE2724675 | 1977-06-01 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0000002A1 EP0000002A1 (fr) | 1978-12-20 |
| EP0000002B1 true EP0000002B1 (fr) | 1981-08-26 |
Family
ID=6010385
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP78100007A Expired EP0000002B1 (fr) | 1977-06-01 | 1978-06-01 | Dérivés du tétrahydrofuranne, leurs procédés de préparation et leur utilisation comme herbicides |
Country Status (10)
| Country | Link |
|---|---|
| EP (1) | EP0000002B1 (fr) |
| JP (1) | JPS543057A (fr) |
| BR (1) | BR7803476A (fr) |
| DD (1) | DD137320A5 (fr) |
| DE (2) | DE2724675A1 (fr) |
| DK (1) | DK242578A (fr) |
| IL (1) | IL54802A0 (fr) |
| IT (1) | IT7823999A0 (fr) |
| PT (1) | PT68098A (fr) |
| ZA (1) | ZA783090B (fr) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3982114A1 (fr) | 2020-10-06 | 2022-04-13 | Eitel, Veronika | Support pour un objet à examiner |
| EP4063016A1 (fr) | 2021-03-25 | 2022-09-28 | Verein zur Förderung von Innovationen durch Forschung, Entwicklung und Technologietransfer e.V. (Verein INNOVENT e.V.) | Buse d'atomiseur |
Families Citing this family (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4289884A (en) * | 1979-01-08 | 1981-09-15 | Shell Oil Company | Herbicidal tetrahydrofuran derivatives |
| US4400198A (en) * | 1980-01-21 | 1983-08-23 | Shell Oil Company | Herbicidal tetrahydrofuran derivatives |
| CA1152510A (fr) * | 1980-01-21 | 1983-08-23 | Shell Canada Limited | Alcools insatures et utilisation dans la preparation d'oxolanes |
| US4356023A (en) | 1980-06-30 | 1982-10-26 | Shell Oil Company | Certain herbicidal tetrahydrofurans |
| EP0064306B1 (fr) * | 1981-05-01 | 1986-05-21 | Shell Internationale Researchmaatschappij B.V. | Tétrahydrofurannes substitués herbicides |
| US4670041A (en) * | 1981-12-16 | 1987-06-02 | E. I. Du Pont De Nemours And Company | Oxabicycloalkane herbicides |
| US4439225A (en) * | 1982-06-14 | 1984-03-27 | Shell Oil Company | Herbicidal cyano-tetrahydrofuranylmethyl ether and cyano-tetrahydropyranylmethyl ether derivatives |
| US4493936A (en) * | 1982-06-14 | 1985-01-15 | Shell Oil Company | 2-Cyano-tetrahydrofuran-5-methanols |
| US4579582A (en) * | 1982-08-18 | 1986-04-01 | Chevron Research Company | 5-deoxy-3-O-arylmethyl or substituted arylmethyl-1,2-O-alkylidene-α-D-xylofuranose herbicide derivatives |
| US4594094A (en) * | 1983-04-04 | 1986-06-10 | Shell Oil Company | Oxacycloalkane-alpha-(thio)carboxylic acid derivatives and use as plant growth regulators and herbicides |
| US4497649A (en) * | 1983-08-18 | 1985-02-05 | Chevron Research Company | 5-O-Acyl-5-C-alkyl-3-O-arylmethyl or substituted arylmethyl-1,2-O-alkylidene-αD-gluco-pentofuranose and β-L-ido-pentofuranose herbicides |
| US4725302A (en) * | 1984-11-27 | 1988-02-16 | Ciba-Geigy Corporation | Substituted phenylhydrazines and phenyloxadiazolinones and pesticidal usage thereof |
| JPS6234626A (ja) * | 1985-08-06 | 1987-02-14 | Kawasaki Steel Corp | 電縫管の製造方法 |
| DE3628287A1 (de) * | 1986-08-20 | 1988-02-25 | Erlau Ag Eisen Drahtwerk | Reifenkette |
| IT1197873B (it) * | 1986-10-15 | 1988-12-21 | Erba Farmitalia | Procedimento per la preparazione di azetidinoni |
| AT392238B (de) * | 1987-04-16 | 1991-02-25 | Jenbacher Werke Ag | Einrichtung zur steuerung von druckluftgesteuerten bremsen an schienenfahrzeugen |
| AT387931B (de) * | 1987-08-13 | 1989-04-10 | Iag Ind Automatisierungsges M | Heisspresse zum herstellen von scheibenbremsbelaegen |
| AT397333B (de) * | 1988-06-20 | 1994-03-25 | Elin Union Ag | Einrichtung zur befestigung von bauteilen |
| AT390413B (de) * | 1988-07-20 | 1990-05-10 | Steinbach Gerhard Ludwig | Selbstklebende folie fuer kraftwagen-karosserien |
| AT399414B (de) * | 1989-03-21 | 1995-05-26 | Rudolf Dipl Ing Winkler | Vorrichtung zur abstandsmessung für kraftfahrzeuge |
| AT396897B (de) * | 1990-12-21 | 1993-12-27 | Haltmeier Georg | Mittel zum imprägnieren von holz |
| AT399074B (de) * | 1991-12-23 | 1995-03-27 | Lenhard Ges M B H | Vorrichtung zum halten von leiterplatten |
| AT398158B (de) * | 1992-07-16 | 1994-10-25 | Attrezzature Meccanismi Minute | Spannvorrichtung |
| AT398199B (de) * | 1992-11-27 | 1994-10-25 | Chemie Linz Gmbh | Verfahren zur herstellung von arylhydantoinen |
| US7295509B2 (en) | 2000-09-13 | 2007-11-13 | Qualcomm, Incorporated | Signaling method in an OFDM multiple access system |
| FR2873555B1 (fr) * | 2004-07-28 | 2008-04-18 | Compin Sa | Siege pour vehicule de transport en commun |
| SI2254549T2 (sl) | 2008-03-17 | 2019-08-30 | Alcon Research, Ltd. | Vodni farmacevtski sestavki, ki vsebujejo komplekse borata/poliola |
| US10268277B2 (en) | 2014-09-30 | 2019-04-23 | Hewlett-Packard Development Company, L.P. | Gesture based manipulation of three-dimensional images |
| KR20250106304A (ko) | 2022-11-17 | 2025-07-09 | 사노피 | Ceacam5 항체-약물 접합체 및 이의 사용 방법 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL7712227A (nl) * | 1976-11-08 | 1978-05-10 | Inco Europ Ltd | Werkwijze voor het opslaan van waterstof waarbij het later weer kan worden vrijgemaakt. |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1370678A (fr) * | 1962-06-29 | 1964-08-28 | Shell Res Ltd | Dérivés molluscicides de l'éther diméthylique |
| GB1591093A (en) * | 1976-11-10 | 1981-06-17 | Shell Int Research | 2-benzyloxymethylfuran derivatives and their use as herbicides |
-
1977
- 1977-06-01 DE DE19772724675 patent/DE2724675A1/de not_active Withdrawn
-
1978
- 1978-05-22 DD DD78205504A patent/DD137320A5/xx unknown
- 1978-05-29 IL IL54802A patent/IL54802A0/xx unknown
- 1978-05-29 PT PT68098A patent/PT68098A/pt unknown
- 1978-05-30 JP JP6394878A patent/JPS543057A/ja active Pending
- 1978-05-30 ZA ZA00783090A patent/ZA783090B/xx unknown
- 1978-05-30 IT IT7823999A patent/IT7823999A0/it unknown
- 1978-05-31 DK DK242578A patent/DK242578A/da unknown
- 1978-05-31 BR BR787803476A patent/BR7803476A/pt unknown
- 1978-06-01 DE DE7878100007T patent/DE2860975D1/de not_active Expired
- 1978-06-01 EP EP78100007A patent/EP0000002B1/fr not_active Expired
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL7712227A (nl) * | 1976-11-08 | 1978-05-10 | Inco Europ Ltd | Werkwijze voor het opslaan van waterstof waarbij het later weer kan worden vrijgemaakt. |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3982114A1 (fr) | 2020-10-06 | 2022-04-13 | Eitel, Veronika | Support pour un objet à examiner |
| EP4063016A1 (fr) | 2021-03-25 | 2022-09-28 | Verein zur Förderung von Innovationen durch Forschung, Entwicklung und Technologietransfer e.V. (Verein INNOVENT e.V.) | Buse d'atomiseur |
Also Published As
| Publication number | Publication date |
|---|---|
| DK242578A (da) | 1978-12-02 |
| IT7823999A0 (it) | 1978-05-30 |
| PT68098A (de) | 1978-06-01 |
| EP0000002A1 (fr) | 1978-12-20 |
| DE2724675A1 (de) | 1978-12-14 |
| ZA783090B (en) | 1979-06-27 |
| DE2860975D1 (en) | 1981-11-19 |
| IL54802A0 (en) | 1978-07-31 |
| DD137320A5 (de) | 1979-08-29 |
| JPS543057A (en) | 1979-01-11 |
| BR7803476A (pt) | 1979-02-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0000002B1 (fr) | Dérivés du tétrahydrofuranne, leurs procédés de préparation et leur utilisation comme herbicides | |
| EP0423482B1 (fr) | Dérivés de 5H-furan-2-one | |
| CH633548A5 (de) | Tetrahydrofuranaether-derivate, verfahren zu ihrer herstellung sowie ihre verwendung als herbizide. | |
| EP0030676B1 (fr) | Dérivés d'acide phénoxybenzoique, procédé pour leur préparation et leur utilisation comme herbicides et comme agents de régulation de la croissance des plantes | |
| EP0000051B1 (fr) | N-acylméthyl-chloroacétanilides, procédé pour leur préparation et leur utilisation comme herbicides. | |
| EP0011179B1 (fr) | N-phénylurées, procédé pour leur préparation, leur utilisation comme herbicides, ainsi que les intermédiaires et leur préparation | |
| DE2538178A1 (de) | Trifluormethylphenoxy-phenylharnstoffe, verfahren zu ihrer herstellung und ihre verwendung als herbizide | |
| DE2937645A1 (de) | Tetrahydropyranether-derivate, verfahren zu ihrer herstellung sowie ihre verwendung als herbizide | |
| EP0002214B1 (fr) | Ethers benzyliques de 1,2-diols cycliques, leurs méthodes de préparation et leur utilisation comme herbicides | |
| EP0024017A2 (fr) | N-pyrimidinylméthyl-halogénoacétanilides, procédé pour leur préparation et leur utilisation comme herbicides; intermédiaires et leur préparation | |
| EP0010163A1 (fr) | N-Diazolylalcoyl-chloroacétanilides, procédé pour leur préparation et leur utilisation comme herbicides | |
| DE2703477A1 (de) | Chlormethansulfonsaeureanilide, verfahren zu ihrer herstellung sowie ihre verwendung als herbizide | |
| EP0300307A2 (fr) | Hétérocycles azotés N-arylés | |
| EP0010715A1 (fr) | N-alcoylene-halogenoacetanilides substitués par un groupe oxime, procédés pour leur préparation, herbicides les contenant, procédé pour la lutte contre les mauvaises herbes, procédé pour la préparation de ces herbicides | |
| EP0008091A1 (fr) | N-Pyrazolylméthyl-halogénoacétanilides substituées, procédé pour leur préparation ainsi que leur utilisation comme herbicides | |
| EP0009693B1 (fr) | N-(1,3-Thiazolyl)-alcoyl-chloroacétanilides, procédé pour leur préparation et leur utilisation comme herbicides | |
| EP0351676A2 (fr) | Benzothiazolones | |
| DE2426651C3 (de) | (-)-Antipode des 3-(p-Chlorphenyl)-2-chlorpropionsäuremethylesters, Verfahren zu dessen Herstellung sowie dessen Verwendung als Herbizid | |
| EP0089538B1 (fr) | Dérivés optiquement actifs de l'acide phénoxybenzoique, procédé de préparation et utilisation comme herbicides | |
| EP0010166A1 (fr) | N-(1,2-Azolyl)alcoyl chloroacétanilides, procédé pour leur préparation et leur application comme herbicides | |
| DE3513488A1 (de) | Herbizide mittel | |
| DE2647460A1 (de) | Heterocyclisch substituierte 4-amino-1,2,4-triazin-5-one, verfahren zu ihrer herstellung sowie ihre verwendung als herbizide | |
| DE2520815A1 (de) | 4-trifluormethyl-4'-sulfonsaeureamid- diphenylaether, verfahren zu ihrer herstellung und ihre verwendung als herbizide | |
| EP0094538A2 (fr) | 4,6-Diamino-s-triazines contenant du fluor, procédé et intermédiaires pour leur préparation et leur utilisation comme herbicide | |
| EP0247486A1 (fr) | Dérivés d'oxabicyclo[2.2.1]heptène |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE CH DE FR GB NL SE |
|
| 17P | Request for examination filed | ||
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Designated state(s): BE CH DE FR GB NL SE Kind code of ref document: B1 Designated state(s): BE CH DE FR GB NL SE |
|
| REF | Corresponds to: |
Ref document number: 2860975 Country of ref document: DE Date of ref document: 19811119 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 19820331 Year of fee payment: 5 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Effective date: 19820601 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Effective date: 19820630 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19820630 Year of fee payment: 5 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Effective date: 19830101 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Effective date: 19830301 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19830331 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Effective date: 19830602 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19881117 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GR Payment date: 19910827 Year of fee payment: 5 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: AUVA Free format text: DAS OBENGENANNTE PATENTGESUCH IST ZURUECKGEWIESEN WORDEN. |
|
| EUG | Se: european patent has lapsed |
Ref document number: 78100007.0 Effective date: 19850610 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |