DE102012005978A1 - Chirurgisches Implantat - Google Patents
Chirurgisches Implantat Download PDFInfo
- Publication number
- DE102012005978A1 DE102012005978A1 DE102012005978A DE102012005978A DE102012005978A1 DE 102012005978 A1 DE102012005978 A1 DE 102012005978A1 DE 102012005978 A DE102012005978 A DE 102012005978A DE 102012005978 A DE102012005978 A DE 102012005978A DE 102012005978 A1 DE102012005978 A1 DE 102012005978A1
- Authority
- DE
- Germany
- Prior art keywords
- implant
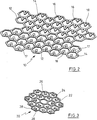
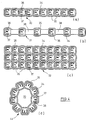
- projection
- basic structure
- implant according
- pores
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000007943 implant Substances 0.000 title claims abstract description 146
- 239000011148 porous material Substances 0.000 claims abstract description 88
- 239000000463 material Substances 0.000 claims abstract description 20
- -1 polypropylene Polymers 0.000 claims description 11
- 239000004744 fabric Substances 0.000 claims description 9
- 229920000642 polymer Polymers 0.000 claims description 9
- 229920001308 poly(aminoacid) Polymers 0.000 claims description 8
- 229920000098 polyolefin Polymers 0.000 claims description 8
- 108010035532 Collagen Proteins 0.000 claims description 5
- 102000008186 Collagen Human genes 0.000 claims description 5
- 239000004743 Polypropylene Substances 0.000 claims description 5
- 229920001436 collagen Polymers 0.000 claims description 5
- 229920001155 polypropylene Polymers 0.000 claims description 5
- 206010019909 Hernia Diseases 0.000 claims description 4
- 229920000388 Polyphosphate Polymers 0.000 claims description 4
- 229920001577 copolymer Polymers 0.000 claims description 4
- 150000002148 esters Chemical class 0.000 claims description 4
- 229920006254 polymer film Polymers 0.000 claims description 4
- 239000001205 polyphosphate Substances 0.000 claims description 4
- 235000011176 polyphosphates Nutrition 0.000 claims description 4
- 210000004872 soft tissue Anatomy 0.000 claims description 4
- 239000004952 Polyamide Substances 0.000 claims description 3
- 239000011248 coating agent Substances 0.000 claims description 3
- 238000000576 coating method Methods 0.000 claims description 3
- 239000006260 foam Substances 0.000 claims description 3
- 210000003205 muscle Anatomy 0.000 claims description 3
- 229920002647 polyamide Polymers 0.000 claims description 3
- 239000000126 substance Substances 0.000 claims description 3
- 238000003856 thermoforming Methods 0.000 claims description 3
- 239000002033 PVDF binder Substances 0.000 claims description 2
- 229920002732 Polyanhydride Polymers 0.000 claims description 2
- 239000004698 Polyethylene Substances 0.000 claims description 2
- 229920000954 Polyglycolide Polymers 0.000 claims description 2
- 229920001273 Polyhydroxy acid Polymers 0.000 claims description 2
- 229920000331 Polyhydroxybutyrate Polymers 0.000 claims description 2
- 229920001710 Polyorthoester Polymers 0.000 claims description 2
- 239000004793 Polystyrene Substances 0.000 claims description 2
- 239000002253 acid Substances 0.000 claims description 2
- 150000007513 acids Chemical class 0.000 claims description 2
- 229920003232 aliphatic polyester Polymers 0.000 claims description 2
- 125000003118 aryl group Chemical group 0.000 claims description 2
- 239000001913 cellulose Substances 0.000 claims description 2
- 229920002678 cellulose Polymers 0.000 claims description 2
- 229920006037 cross link polymer Polymers 0.000 claims description 2
- 239000011521 glass Substances 0.000 claims description 2
- 239000004745 nonwoven fabric Substances 0.000 claims description 2
- 230000002093 peripheral effect Effects 0.000 claims description 2
- 239000005015 poly(hydroxybutyrate) Substances 0.000 claims description 2
- 229920000218 poly(hydroxyvalerate) Polymers 0.000 claims description 2
- 229920000747 poly(lactic acid) Polymers 0.000 claims description 2
- 229920002463 poly(p-dioxanone) polymer Polymers 0.000 claims description 2
- 229920002627 poly(phosphazenes) Polymers 0.000 claims description 2
- 229920006260 polyaryletherketone Polymers 0.000 claims description 2
- 229920001610 polycaprolactone Polymers 0.000 claims description 2
- 229920000515 polycarbonate Polymers 0.000 claims description 2
- 239000004417 polycarbonate Substances 0.000 claims description 2
- 229920002721 polycyanoacrylate Polymers 0.000 claims description 2
- 239000000622 polydioxanone Substances 0.000 claims description 2
- 229920000728 polyester Polymers 0.000 claims description 2
- 229920000570 polyether Polymers 0.000 claims description 2
- 229920000573 polyethylene Polymers 0.000 claims description 2
- 229920001223 polyethylene glycol Polymers 0.000 claims description 2
- 229920001195 polyisoprene Polymers 0.000 claims description 2
- 229920005591 polysilicon Polymers 0.000 claims description 2
- 229920001296 polysiloxane Polymers 0.000 claims description 2
- 229920002223 polystyrene Polymers 0.000 claims description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 claims description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 claims description 2
- 229920002635 polyurethane Polymers 0.000 claims description 2
- 239000004814 polyurethane Substances 0.000 claims description 2
- 229920002451 polyvinyl alcohol Polymers 0.000 claims description 2
- 235000019422 polyvinyl alcohol Nutrition 0.000 claims description 2
- 229920002981 polyvinylidene fluoride Polymers 0.000 claims description 2
- 150000005846 sugar alcohols Polymers 0.000 claims description 2
- 150000008163 sugars Chemical class 0.000 claims description 2
- 229920002845 Poly(methacrylic acid) Polymers 0.000 claims 1
- 229920002125 Sokalan® Chemical class 0.000 claims 1
- 239000004584 polyacrylic acid Chemical class 0.000 claims 1
- 239000002759 woven fabric Substances 0.000 claims 1
- 210000001519 tissue Anatomy 0.000 description 12
- 238000005520 cutting process Methods 0.000 description 11
- 238000000034 method Methods 0.000 description 9
- 230000000694 effects Effects 0.000 description 4
- 239000004753 textile Substances 0.000 description 4
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- 238000003698 laser cutting Methods 0.000 description 3
- 238000010309 melting process Methods 0.000 description 3
- 230000008719 thickening Effects 0.000 description 3
- LCSKNASZPVZHEG-UHFFFAOYSA-N 3,6-dimethyl-1,4-dioxane-2,5-dione;1,4-dioxane-2,5-dione Chemical group O=C1COC(=O)CO1.CC1OC(=O)C(C)OC1=O LCSKNASZPVZHEG-UHFFFAOYSA-N 0.000 description 2
- 238000005266 casting Methods 0.000 description 2
- 239000000834 fixative Substances 0.000 description 2
- 239000011888 foil Substances 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 229920000117 poly(dioxanone) Polymers 0.000 description 2
- 238000004080 punching Methods 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 238000003466 welding Methods 0.000 description 2
- BUHVIAUBTBOHAG-FOYDDCNASA-N (2r,3r,4s,5r)-2-[6-[[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]amino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound COC1=CC(OC)=CC(C(CNC=2C=3N=CN(C=3N=CN=2)[C@H]2[C@@H]([C@H](O)[C@@H](CO)O2)O)C=2C(=CC=CC=2)C)=C1 BUHVIAUBTBOHAG-FOYDDCNASA-N 0.000 description 1
- QNRATNLHPGXHMA-XZHTYLCXSA-N (r)-(6-ethoxyquinolin-4-yl)-[(2s,4s,5r)-5-ethyl-1-azabicyclo[2.2.2]octan-2-yl]methanol;hydrochloride Chemical compound Cl.C([C@H]([C@H](C1)CC)C2)CN1[C@@H]2[C@H](O)C1=CC=NC2=CC=C(OCC)C=C21 QNRATNLHPGXHMA-XZHTYLCXSA-N 0.000 description 1
- RKDVKSZUMVYZHH-UHFFFAOYSA-N 1,4-dioxane-2,5-dione Chemical compound O=C1COC(=O)CO1 RKDVKSZUMVYZHH-UHFFFAOYSA-N 0.000 description 1
- 239000004831 Hot glue Substances 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 238000004026 adhesive bonding Methods 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000009956 embroidering Methods 0.000 description 1
- 229920000295 expanded polytetrafluoroethylene Polymers 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000003733 fiber-reinforced composite Substances 0.000 description 1
- 238000005755 formation reaction Methods 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- JJTUDXZGHPGLLC-UHFFFAOYSA-N lactide Chemical compound CC1OC(=O)C(C)OC1=O JJTUDXZGHPGLLC-UHFFFAOYSA-N 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920000193 polymethacrylate Polymers 0.000 description 1
- 235000015277 pork Nutrition 0.000 description 1
- 230000003014 reinforcing effect Effects 0.000 description 1
- 238000009958 sewing Methods 0.000 description 1
- 239000007779 soft material Substances 0.000 description 1
- 108010068483 strattice Proteins 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 108010070228 surgisis Proteins 0.000 description 1
- 230000002123 temporal effect Effects 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/0063—Implantable repair or support meshes, e.g. hernia meshes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/0063—Implantable repair or support meshes, e.g. hernia meshes
- A61F2002/0068—Implantable repair or support meshes, e.g. hernia meshes having a special mesh pattern
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0008—Fixation appliances for connecting prostheses to the body
- A61F2220/0016—Fixation appliances for connecting prostheses to the body with sharp anchoring protrusions, e.g. barbs, pins, spikes
Landscapes
- Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
Priority Applications (14)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102012005978A DE102012005978A1 (de) | 2012-03-23 | 2012-03-23 | Chirurgisches Implantat |
| US14/385,300 US9949815B2 (en) | 2012-03-23 | 2013-03-21 | Surgical implant |
| BR112014023567-8A BR112014023567B1 (pt) | 2012-03-23 | 2013-03-21 | Implante cirúrgico e uso do mesmo |
| JP2015500797A JP6158290B2 (ja) | 2012-03-23 | 2013-03-21 | 外科用インプラント |
| MX2014011445A MX359283B (es) | 2012-03-23 | 2013-03-21 | Implante quirúrgico. |
| EP13716955.3A EP2827798A1 (en) | 2012-03-23 | 2013-03-21 | Surgical implant |
| CA2868152A CA2868152C (en) | 2012-03-23 | 2013-03-21 | Surgical implant |
| RU2014142637A RU2647188C2 (ru) | 2012-03-23 | 2013-03-21 | Хирургический имплантат |
| PCT/EP2013/000868 WO2013139482A1 (en) | 2012-03-23 | 2013-03-21 | Surgical implant |
| AU2013234678A AU2013234678B2 (en) | 2012-03-23 | 2013-03-21 | Surgical implant |
| CN201380016022.0A CN104302244B (zh) | 2012-03-23 | 2013-03-21 | 外科植入物 |
| NZ630130A NZ630130A (en) | 2012-03-23 | 2013-03-21 | Surgical implant |
| US14/069,863 US20140066958A1 (en) | 2012-03-23 | 2013-11-01 | Surgical Implant |
| IN7517DEN2014 IN2014DN07517A (enExample) | 2012-03-23 | 2014-09-09 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102012005978A DE102012005978A1 (de) | 2012-03-23 | 2012-03-23 | Chirurgisches Implantat |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| DE102012005978A1 true DE102012005978A1 (de) | 2013-09-26 |
Family
ID=48139872
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| DE102012005978A Ceased DE102012005978A1 (de) | 2012-03-23 | 2012-03-23 | Chirurgisches Implantat |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US9949815B2 (enExample) |
| EP (1) | EP2827798A1 (enExample) |
| JP (1) | JP6158290B2 (enExample) |
| CN (1) | CN104302244B (enExample) |
| AU (1) | AU2013234678B2 (enExample) |
| BR (1) | BR112014023567B1 (enExample) |
| CA (1) | CA2868152C (enExample) |
| DE (1) | DE102012005978A1 (enExample) |
| IN (1) | IN2014DN07517A (enExample) |
| MX (1) | MX359283B (enExample) |
| NZ (1) | NZ630130A (enExample) |
| RU (1) | RU2647188C2 (enExample) |
| WO (1) | WO2013139482A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102013004573A1 (de) | 2013-03-11 | 2014-09-11 | Johnson & Johnson Medical Gmbh | Chirurgisches Implantat |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8969473B2 (en) | 2009-02-21 | 2015-03-03 | Sofradim Production | Compounds and medical devices activated with solvophobic linkers |
| US9475709B2 (en) | 2010-08-25 | 2016-10-25 | Lockheed Martin Corporation | Perforated graphene deionization or desalination |
| US9870895B2 (en) | 2014-01-31 | 2018-01-16 | Lockheed Martin Corporation | Methods for perforating two-dimensional materials using a broad ion field |
| US10980919B2 (en) | 2016-04-14 | 2021-04-20 | Lockheed Martin Corporation | Methods for in vivo and in vitro use of graphene and other two-dimensional materials |
| US9834809B2 (en) | 2014-02-28 | 2017-12-05 | Lockheed Martin Corporation | Syringe for obtaining nano-sized materials for selective assays and related methods of use |
| US10653824B2 (en) | 2012-05-25 | 2020-05-19 | Lockheed Martin Corporation | Two-dimensional materials and uses thereof |
| US9744617B2 (en) | 2014-01-31 | 2017-08-29 | Lockheed Martin Corporation | Methods for perforating multi-layer graphene through ion bombardment |
| US9610546B2 (en) | 2014-03-12 | 2017-04-04 | Lockheed Martin Corporation | Separation membranes formed from perforated graphene and methods for use thereof |
| CN104582748A (zh) * | 2012-08-13 | 2015-04-29 | 柯惠Lp公司 | 包括膜的可植入的多孔装置 |
| US9592475B2 (en) | 2013-03-12 | 2017-03-14 | Lockheed Martin Corporation | Method for forming perforated graphene with uniform aperture size |
| US9572918B2 (en) | 2013-06-21 | 2017-02-21 | Lockheed Martin Corporation | Graphene-based filter for isolating a substance from blood |
| SG11201606287VA (en) | 2014-01-31 | 2016-08-30 | Lockheed Corp | Processes for forming composite structures with a two-dimensional material using a porous, non-sacrificial supporting layer |
| CA2942496A1 (en) | 2014-03-12 | 2015-09-17 | Lockheed Martin Corporation | Separation membranes formed from perforated graphene |
| AU2015311978A1 (en) | 2014-09-02 | 2017-05-11 | Lockheed Martin Corporation | Hemodialysis and hemofiltration membranes based upon a two-dimensional membrane material and methods employing same |
| WO2017023376A1 (en) | 2015-08-05 | 2017-02-09 | Lockheed Martin Corporation | Perforatable sheets of graphene-based material |
| WO2017023377A1 (en) | 2015-08-06 | 2017-02-09 | Lockheed Martin Corporation | Nanoparticle modification and perforation of graphene |
| HK1258127A1 (zh) * | 2015-09-11 | 2019-11-08 | Lifecell Corporation | 穿孔組織基質 |
| EP3147216B1 (de) | 2015-09-23 | 2019-08-07 | Airbus Defence and Space GmbH | Elektrische enteisung für luftfahrzeuge |
| WO2017180135A1 (en) | 2016-04-14 | 2017-10-19 | Lockheed Martin Corporation | Membranes with tunable selectivity |
| KR20180133430A (ko) | 2016-04-14 | 2018-12-14 | 록히드 마틴 코포레이션 | 결함 형성 또는 힐링의 인 시츄 모니터링 및 제어를 위한 방법 |
| JP2019511451A (ja) | 2016-04-14 | 2019-04-25 | ロッキード・マーチン・コーポレーション | 浮遊法を用いてグラフェンシートを大判転写用に処理する方法 |
| WO2017180139A1 (en) | 2016-04-14 | 2017-10-19 | Lockheed Martin Corporation | Two-dimensional membrane structures having flow passages |
| CA3020880A1 (en) | 2016-04-14 | 2017-10-19 | Lockheed Martin Corporation | Selective interfacial mitigation of graphene defects |
| ES3037878T3 (en) | 2016-09-06 | 2025-10-07 | Biocircuit Tech Inc | Devices for repairing damage to a nerve |
| CN111432749B (zh) | 2017-10-19 | 2023-04-04 | C.R.巴德公司 | 自抓握式疝假体 |
| EP3628763B1 (en) * | 2018-09-27 | 2021-06-02 | Sofradim Production | Bioresorbable knit for hernia repair and method for manufacturing the same |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003099160A1 (en) | 2002-05-23 | 2003-12-04 | Ethicon Gmbh | Medical implant |
| WO2006092236A1 (en) | 2005-03-01 | 2006-09-08 | Ethicon Gmbh | Surgical implant |
| US7331199B2 (en) | 2000-04-20 | 2008-02-19 | Sofradim Production | Adhering prosthetic knitting fabric, method for making same and reinforcement implant for treating parietal deficiencies |
| WO2010086515A1 (fr) | 2009-01-30 | 2010-08-05 | Textile Hi -Tec (T.H.T.) | Plaque implantable pour la refection de parois |
| EP2368524A2 (en) | 2010-03-25 | 2011-09-28 | Tyco Healthcare Group LP | Hernia patch |
| WO2011159700A1 (en) | 2010-06-14 | 2011-12-22 | Ethicon, Inc. | Composite anisotropic tissue reinforcing implants having alignment markers and methods of manufacturing same |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5397355A (en) * | 1994-07-19 | 1995-03-14 | Stentco, Inc. | Intraluminal stent |
| ATE420599T1 (de) * | 2000-05-19 | 2009-01-15 | Coapt Systems Inc | Angleichvorrichtung für gewebe |
| US20050182477A1 (en) * | 2001-12-20 | 2005-08-18 | White Geoffrey H. | Intraluminal stent and graft |
| US20050096731A1 (en) * | 2002-07-11 | 2005-05-05 | Kareen Looi | Cell seeded expandable body |
| US20080249597A1 (en) * | 2007-04-05 | 2008-10-09 | Russell Scott M | Thin film tissue repair matrix |
| US20090112236A1 (en) * | 2007-10-29 | 2009-04-30 | Tyco Healthcare Group Lp | Filament-Reinforced Composite Fiber |
| ITTO20080329A1 (it) * | 2008-05-02 | 2009-11-03 | Ermanno Trabucco | Protesi chirurgica a doppio strato per la riparazione dei tessuti molli |
| RU2389449C2 (ru) * | 2008-06-04 | 2010-05-20 | Закрытое акционерное общество "Научно-производственный комплекс "Экофлон" | Грыжевой имплант из пористого политетрафторэтилена |
| WO2010093333A1 (en) * | 2009-02-11 | 2010-08-19 | Nanyang Technological University | Multi-layered surgical prosthesis |
| US8858577B2 (en) * | 2010-05-19 | 2014-10-14 | University Of Utah Research Foundation | Tissue stabilization system |
-
2012
- 2012-03-23 DE DE102012005978A patent/DE102012005978A1/de not_active Ceased
-
2013
- 2013-03-21 CA CA2868152A patent/CA2868152C/en not_active Expired - Fee Related
- 2013-03-21 EP EP13716955.3A patent/EP2827798A1/en not_active Ceased
- 2013-03-21 BR BR112014023567-8A patent/BR112014023567B1/pt not_active IP Right Cessation
- 2013-03-21 US US14/385,300 patent/US9949815B2/en active Active
- 2013-03-21 RU RU2014142637A patent/RU2647188C2/ru not_active IP Right Cessation
- 2013-03-21 NZ NZ630130A patent/NZ630130A/en not_active IP Right Cessation
- 2013-03-21 CN CN201380016022.0A patent/CN104302244B/zh not_active Expired - Fee Related
- 2013-03-21 MX MX2014011445A patent/MX359283B/es active IP Right Grant
- 2013-03-21 WO PCT/EP2013/000868 patent/WO2013139482A1/en not_active Ceased
- 2013-03-21 AU AU2013234678A patent/AU2013234678B2/en not_active Ceased
- 2013-03-21 JP JP2015500797A patent/JP6158290B2/ja not_active Expired - Fee Related
- 2013-11-01 US US14/069,863 patent/US20140066958A1/en not_active Abandoned
-
2014
- 2014-09-09 IN IN7517DEN2014 patent/IN2014DN07517A/en unknown
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7331199B2 (en) | 2000-04-20 | 2008-02-19 | Sofradim Production | Adhering prosthetic knitting fabric, method for making same and reinforcement implant for treating parietal deficiencies |
| WO2003099160A1 (en) | 2002-05-23 | 2003-12-04 | Ethicon Gmbh | Medical implant |
| WO2006092236A1 (en) | 2005-03-01 | 2006-09-08 | Ethicon Gmbh | Surgical implant |
| WO2010086515A1 (fr) | 2009-01-30 | 2010-08-05 | Textile Hi -Tec (T.H.T.) | Plaque implantable pour la refection de parois |
| EP2368524A2 (en) | 2010-03-25 | 2011-09-28 | Tyco Healthcare Group LP | Hernia patch |
| WO2011159700A1 (en) | 2010-06-14 | 2011-12-22 | Ethicon, Inc. | Composite anisotropic tissue reinforcing implants having alignment markers and methods of manufacturing same |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102013004573A1 (de) | 2013-03-11 | 2014-09-11 | Johnson & Johnson Medical Gmbh | Chirurgisches Implantat |
| WO2014139633A1 (en) | 2013-03-11 | 2014-09-18 | Johnson & Johnson Medical Gmbh | Surgical implant |
| US10052184B2 (en) | 2013-03-11 | 2018-08-21 | Johnson & Johnson Medical Gmbh | Surgical implant |
Also Published As
| Publication number | Publication date |
|---|---|
| US9949815B2 (en) | 2018-04-24 |
| CA2868152C (en) | 2020-07-14 |
| US20140066958A1 (en) | 2014-03-06 |
| IN2014DN07517A (enExample) | 2015-04-24 |
| RU2647188C2 (ru) | 2018-03-14 |
| BR112014023567A2 (enExample) | 2017-06-20 |
| CA2868152A1 (en) | 2013-09-26 |
| CN104302244B (zh) | 2019-01-01 |
| MX2014011445A (es) | 2015-04-10 |
| RU2014142637A (ru) | 2016-05-20 |
| JP2015513934A (ja) | 2015-05-18 |
| MX359283B (es) | 2018-09-21 |
| AU2013234678B2 (en) | 2017-02-02 |
| BR112014023567B1 (pt) | 2022-01-04 |
| AU2013234678A1 (en) | 2014-10-30 |
| NZ630130A (en) | 2015-09-25 |
| CN104302244A (zh) | 2015-01-21 |
| JP6158290B2 (ja) | 2017-07-05 |
| EP2827798A1 (en) | 2015-01-28 |
| US20150066063A1 (en) | 2015-03-05 |
| WO2013139482A8 (en) | 2014-10-02 |
| WO2013139482A1 (en) | 2013-09-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE102012005978A1 (de) | Chirurgisches Implantat | |
| DE10153334B4 (de) | Flächiges Implantat | |
| DE102013014295A1 (de) | Chirurgisches Implantat | |
| EP1982655B2 (de) | Occluder zum Verschliessen eines Herzohres und Herstellungsverfahren dafür | |
| DE60108818T2 (de) | Verstärkte Submucosaschicht des Dünndarms | |
| DE102013208924A1 (de) | Chirurgisches Implantat umfassend einer Lage mit Öffnungen | |
| DE102013004574A1 (de) | Chirurgisches Implantat | |
| DE102013004573A1 (de) | Chirurgisches Implantat | |
| EP1411997B1 (de) | Textiles implantat mit monofilen polyvinylfluorid-fäden | |
| EP1099421B1 (de) | Flächiges Implantat und Verfahren zu seiner Herstellung | |
| DE19613730C2 (de) | Flächiges Implantat zum Verstärken oder Verschließen von Körpergewebe | |
| DE60209787T2 (de) | Flächiges implantat | |
| EP1038508B1 (de) | Flächiges Implantat | |
| EP1991160B1 (de) | Flächiges implantat, insbesondere herniennetz | |
| DE102011007844A1 (de) | Medizinisches Produkt und Verfahren zu seiner Herstellung | |
| DE10019604A1 (de) | Implantat | |
| HK1222787A1 (zh) | 圖案化植入體及方法 | |
| DE10221320A1 (de) | Flächiges Implantat aus textilem Fadenmaterial, insbesondere Herniennetz | |
| DE102004047974A1 (de) | Chirurgischer Haken | |
| DE102008057213A1 (de) | Medizintechnisches Produkt, ein chirurgisches Kit sowie ein Herstellungsverfahren für das medizintechnische Produkt | |
| DE102009020901A1 (de) | Beschichteter Faden mit Verankerungsstrukturen zur Verankerung in biologischen Geweben | |
| DE102014012717A1 (de) | Chirurgisches Implantat | |
| DE102015013992A1 (de) | Chirgurgisches Implantat und Verfahren zu dessen Herstellung | |
| DE102014015179A1 (de) | Verfahren zur Herstellung eines chirurgischen Implantats mit einer Markierung | |
| DE102007063214B4 (de) | Flächiges Implantat, insbesondere zur Hernienversorgung |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| R012 | Request for examination validly filed | ||
| R002 | Refusal decision in examination/registration proceedings | ||
| R003 | Refusal decision now final |