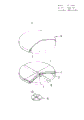CN85106361B - 培养细菌的培养皿及其用途 - Google Patents
培养细菌的培养皿及其用途 Download PDFInfo
- Publication number
- CN85106361B CN85106361B CN85106361A CN85106361A CN85106361B CN 85106361 B CN85106361 B CN 85106361B CN 85106361 A CN85106361 A CN 85106361A CN 85106361 A CN85106361 A CN 85106361A CN 85106361 B CN85106361 B CN 85106361B
- Authority
- CN
- China
- Prior art keywords
- culture dish
- substratum
- sheet
- aperture
- culture
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 241000894006 Bacteria Species 0.000 title claims abstract description 56
- 238000012258 culturing Methods 0.000 title claims abstract description 13
- 229920003002 synthetic resin Polymers 0.000 claims description 9
- 239000000057 synthetic resin Substances 0.000 claims description 9
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 8
- 229910052760 oxygen Inorganic materials 0.000 claims description 8
- 239000001301 oxygen Substances 0.000 claims description 8
- -1 vinyl-vinyl alkoxide Chemical class 0.000 claims description 8
- 238000007789 sealing Methods 0.000 claims description 7
- 238000000926 separation method Methods 0.000 claims description 7
- 230000004888 barrier function Effects 0.000 claims description 4
- 229920002981 polyvinylidene fluoride Polymers 0.000 claims description 3
- 239000000126 substance Substances 0.000 claims description 3
- 239000004677 Nylon Substances 0.000 claims description 2
- 229920001328 Polyvinylidene chloride Polymers 0.000 claims description 2
- 150000004945 aromatic hydrocarbons Chemical class 0.000 claims description 2
- 229920002678 cellulose Polymers 0.000 claims description 2
- 229920001778 nylon Polymers 0.000 claims description 2
- 229920003214 poly(methacrylonitrile) Polymers 0.000 claims description 2
- 229920002239 polyacrylonitrile Polymers 0.000 claims description 2
- 239000004417 polycarbonate Substances 0.000 claims description 2
- 229920000515 polycarbonate Polymers 0.000 claims description 2
- 239000005020 polyethylene terephthalate Substances 0.000 claims description 2
- 229920000139 polyethylene terephthalate Polymers 0.000 claims description 2
- 229920000642 polymer Polymers 0.000 claims description 2
- 229920000915 polyvinyl chloride Polymers 0.000 claims description 2
- 239000004800 polyvinyl chloride Substances 0.000 claims description 2
- 230000002093 peripheral effect Effects 0.000 claims 1
- 229920003051 synthetic elastomer Polymers 0.000 claims 1
- 239000005061 synthetic rubber Substances 0.000 claims 1
- 239000003814 drug Substances 0.000 abstract description 38
- 238000000034 method Methods 0.000 abstract description 33
- 229940079593 drug Drugs 0.000 abstract description 23
- 239000001963 growth medium Substances 0.000 abstract description 5
- 238000002955 isolation Methods 0.000 abstract description 3
- 230000035945 sensitivity Effects 0.000 abstract description 2
- 230000003204 osmotic effect Effects 0.000 description 51
- 239000000463 material Substances 0.000 description 14
- 230000001580 bacterial effect Effects 0.000 description 11
- 238000011081 inoculation Methods 0.000 description 9
- 239000002609 medium Substances 0.000 description 9
- 229920001817 Agar Polymers 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 239000008272 agar Substances 0.000 description 6
- 239000006916 nutrient agar Substances 0.000 description 6
- 238000007747 plating Methods 0.000 description 6
- 238000003113 dilution method Methods 0.000 description 5
- 238000007689 inspection Methods 0.000 description 5
- 235000015097 nutrients Nutrition 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- QYQDKDWGWDOFFU-IUODEOHRSA-N Cefotiam Chemical compound CN(C)CCN1N=NN=C1SCC1=C(C(O)=O)N2C(=O)[C@@H](NC(=O)CC=3N=C(N)SC=3)[C@H]2SC1 QYQDKDWGWDOFFU-IUODEOHRSA-N 0.000 description 4
- 235000010419 agar Nutrition 0.000 description 4
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 238000011109 contamination Methods 0.000 description 4
- 238000009792 diffusion process Methods 0.000 description 4
- 230000012010 growth Effects 0.000 description 4
- 230000005764 inhibitory process Effects 0.000 description 4
- 239000012092 media component Substances 0.000 description 4
- OGJPXUAPXNRGGI-UHFFFAOYSA-N norfloxacin Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 OGJPXUAPXNRGGI-UHFFFAOYSA-N 0.000 description 4
- 241000607142 Salmonella Species 0.000 description 3
- 241000191940 Staphylococcus Species 0.000 description 3
- 244000052616 bacterial pathogen Species 0.000 description 3
- NNULBSISHYWZJU-LLKWHZGFSA-N ceftizoxime Chemical compound N([C@@H]1C(N2C(=CCS[C@@H]21)C(O)=O)=O)C(=O)\C(=N/OC)C1=CSC(N)=N1 NNULBSISHYWZJU-LLKWHZGFSA-N 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 238000009629 microbiological culture Methods 0.000 description 3
- 230000001717 pathogenic effect Effects 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 241000193830 Bacillus <bacterium> Species 0.000 description 2
- 241000589562 Brucella Species 0.000 description 2
- 208000035473 Communicable disease Diseases 0.000 description 2
- 206010059866 Drug resistance Diseases 0.000 description 2
- 241000194033 Enterococcus Species 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 2
- 229920002125 Sokalan® Polymers 0.000 description 2
- 241000191967 Staphylococcus aureus Species 0.000 description 2
- 241000607598 Vibrio Species 0.000 description 2
- 238000010981 drying operation Methods 0.000 description 2
- 208000001848 dysentery Diseases 0.000 description 2
- 238000007429 general method Methods 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 239000004584 polyacrylic acid Substances 0.000 description 2
- 230000003014 reinforcing effect Effects 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 238000011282 treatment Methods 0.000 description 2
- 238000002255 vaccination Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 241000193755 Bacillus cereus Species 0.000 description 1
- 241000589876 Campylobacter Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- 241000606790 Haemophilus Species 0.000 description 1
- 241000588749 Klebsiella oxytoca Species 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 241000194017 Streptococcus Species 0.000 description 1
- 241000607734 Yersinia <bacteria> Species 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 239000005030 aluminium foil Substances 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000006161 blood agar Substances 0.000 description 1
- 238000002815 broth microdilution Methods 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229960001242 cefotiam Drugs 0.000 description 1
- 229960001991 ceftizoxime Drugs 0.000 description 1
- 230000000973 chemotherapeutic effect Effects 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 239000007330 chocolate agar Substances 0.000 description 1
- 238000003759 clinical diagnosis Methods 0.000 description 1
- 230000001332 colony forming effect Effects 0.000 description 1
- 230000006837 decompression Effects 0.000 description 1
- 238000007872 degassing Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 239000000645 desinfectant Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 229960001180 norfloxacin Drugs 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 238000012549 training Methods 0.000 description 1
- 238000002525 ultrasonication Methods 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/02—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving viable microorganisms
- C12Q1/04—Determining presence or kind of microorganism; Use of selective media for testing antibiotics or bacteriocides; Compositions containing a chemical indicator therefor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12M—APPARATUS FOR ENZYMOLOGY OR MICROBIOLOGY; APPARATUS FOR CULTURING MICROORGANISMS FOR PRODUCING BIOMASS, FOR GROWING CELLS OR FOR OBTAINING FERMENTATION OR METABOLIC PRODUCTS, i.e. BIOREACTORS OR FERMENTERS
- C12M23/00—Constructional details, e.g. recesses, hinges
- C12M23/02—Form or structure of the vessel
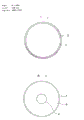
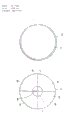
- C12M23/10—Petri dish
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- General Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biomedical Technology (AREA)
- Clinical Laboratory Science (AREA)
- Sustainable Development (AREA)
- Toxicology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Biophysics (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP59212163A JPS6192561A (ja) | 1984-10-09 | 1984-10-09 | 細菌培養用シヤ−レ |
| JP59-212163 | 1984-10-09 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN85106361A CN85106361A (zh) | 1986-04-10 |
| CN85106361B true CN85106361B (zh) | 1988-01-20 |
Family
ID=16617946
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN85106361A Expired CN85106361B (zh) | 1984-10-09 | 1985-08-24 | 培养细菌的培养皿及其用途 |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US4775628A (enExample) |
| EP (1) | EP0181075B1 (enExample) |
| JP (1) | JPS6192561A (enExample) |
| KR (1) | KR890003945B1 (enExample) |
| CN (1) | CN85106361B (enExample) |
| AU (1) | AU567225B2 (enExample) |
| CA (1) | CA1252375A (enExample) |
| DE (1) | DE3565997D1 (enExample) |
Families Citing this family (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5202262A (en) * | 1984-01-31 | 1993-04-13 | Millipore Corporation | Apparatus for microbiological testing of liquids |
| FI72741C (fi) * | 1986-02-21 | 1987-07-10 | Valio Meijerien | Foerfarande foer bestaemning mikrobkoncentrationer medelst en smaeltagarmetod samt daeri anvaenda skaolar. |
| JPH0640813B2 (ja) * | 1988-02-17 | 1994-06-01 | ダイキン工業株式会社 | 培養器 |
| GB8905001D0 (en) * | 1989-03-04 | 1989-04-19 | Univ Leicester | Screening for natural products of microbial metabolism |
| US5190879A (en) * | 1990-05-08 | 1993-03-02 | Bowolfe, Inc. | Controlled environment animal isolation systems |
| EP0553182A1 (de) * | 1990-10-17 | 1993-08-04 | REULECKE, Fritz | Behälter für kulturmedien und verwendung des behälters |
| CH686521A5 (fr) * | 1991-05-23 | 1996-04-15 | Eduard Dr Engelbrecht | Support pour milieux de culture compartimente et milieux de culture électifs complémentaires. |
| US5463223A (en) * | 1994-01-24 | 1995-10-31 | Patwong Technologies, Inc. | Disposable all purpose micro sample holder |
| US5593891A (en) * | 1994-11-10 | 1997-01-14 | Banes; Albert J. | Culture plate with splash guard |
| US6001586A (en) * | 1996-03-29 | 1999-12-14 | Genencor International, Inc. | Compartmentalization method for screening microorganisms |
| JP2003225083A (ja) * | 2002-02-05 | 2003-08-12 | Sony Corp | 円盤状培養媒体 |
| US7384599B2 (en) * | 2003-01-30 | 2008-06-10 | Randy Brewer | Apparatus for drug testing |
| US20050239200A1 (en) * | 2004-04-23 | 2005-10-27 | Beckwith Scott W | Devices for culturing anaerobic microorganisms and methods of using the same |
| JP4740584B2 (ja) * | 2004-12-14 | 2011-08-03 | オリンパス株式会社 | 観察装置 |
| JP4810093B2 (ja) * | 2004-12-28 | 2011-11-09 | オリンパス株式会社 | 培養観察装置および標本トレー保温装置および蓋 |
| WO2007149525A2 (en) * | 2006-06-22 | 2007-12-27 | Manhattan Diagnostics Corp. | Petri dish with filter |
| US8921283B2 (en) * | 2006-10-30 | 2014-12-30 | Washington University | Method for generating microscopic patterns of protein and other macromolecules |
| EP2148921B1 (en) * | 2007-05-30 | 2017-07-19 | Nikon Corporation | Incubation container |
| US7968062B1 (en) | 2007-07-06 | 2011-06-28 | Richard Carle Putnam | Drug disposal and verification device |
| DE102007059199A1 (de) * | 2007-12-08 | 2009-06-10 | Cognis Ip Management Gmbh | Hautmodell |
| US8163540B2 (en) * | 2008-07-18 | 2012-04-24 | Carlo Acosta | Filtered petri dish |
| MX368123B (es) | 2011-11-07 | 2019-09-19 | Rapid Micro Biosystems Inc | Cassette para pruebas de esterilidad. |
| US10407707B2 (en) | 2012-04-16 | 2019-09-10 | Rapid Micro Biosystems, Inc. | Cell culturing device |
| US9695458B2 (en) | 2013-08-27 | 2017-07-04 | Parker-Hannifin Corporation | Sample dish and compressed gas microbial test unit |
| EP3180416A1 (de) * | 2014-08-14 | 2017-06-21 | Merck Patent GmbH | Petrischale und verfahren zur mikrobiologischen untersuchung von flüssigkeiten mittels membranfiltration |
| CN107090401A (zh) * | 2017-06-09 | 2017-08-25 | 天津施特雷生物科技股份有限公司 | 一种干制一次性无菌微生物培养皿 |
| CN109679833B (zh) * | 2019-01-10 | 2021-10-26 | 航天神舟生物科技集团有限公司 | 多因素一体化筛选平板及其制作工具和制作方法 |
| CN109679828B (zh) * | 2019-02-22 | 2020-06-09 | 胥振国 | 一种细菌培养用培养皿及使用方法 |
| WO2024080882A1 (en) * | 2022-10-10 | 2024-04-18 | Farm Medix Limited | Improved microbiological media container |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE839245C (de) * | 1949-04-15 | 1952-05-19 | Ewald Dr Med Kanz | Verfahren und Einrichtung zur Zuechtung von Kleinlebewesen |
| US2677646A (en) * | 1952-03-22 | 1954-05-04 | Lovell Chemical Company | Unit for bacterial analysis |
| US2954327A (en) * | 1955-08-02 | 1960-09-27 | Kanz Ewald | Container for nutrient media |
| US2874091A (en) * | 1956-07-23 | 1959-02-17 | Hyland Lab | Disposable culturing device |
| US3073750A (en) * | 1959-05-07 | 1963-01-15 | Talb Ind Inc | Culture dish |
| US3055808A (en) * | 1959-07-23 | 1962-09-25 | Baltimore Biolog Lab Inc | Hermetically sealed petri dish |
| DE1210129B (de) * | 1964-12-03 | 1966-02-03 | St Luke S Hospital Res Foundat | Zum Zuechten anaerober Kulturen dienende abdeckbare Flachschale |
| US3630849A (en) * | 1969-04-24 | 1971-12-28 | David B Land | Surface micro-organism contamination assays |
| US3729382A (en) * | 1970-12-09 | 1973-04-24 | Becton Dickinson Co | Microorganism sampling dish |
| US3809617A (en) * | 1972-11-15 | 1974-05-07 | American Cyanamid Co | Device for detecting anticholinesterase materials |
| SE403382B (sv) * | 1974-03-12 | 1978-08-14 | Orion Yhtyme Oy Orion Diagnost | Sett vid undersokning av effekten av ett biologiskt aktivt emne pa tillvexten av mikroorganismer som odlas pa ett fast eller gelformigt odlingsmedium |
| US4030980A (en) * | 1975-05-15 | 1977-06-21 | Corning Glass Works | Apparatus and method for identification of selected clinical yeast |
| US4271270A (en) * | 1977-10-27 | 1981-06-02 | Lukacsek Patricia A | Apparatus for culturing and examining fungi |
| DE3102571A1 (de) * | 1981-01-27 | 1982-09-16 | C.A. Greiner und Söhne GmbH & Co KG, 7440 Nürtingen | Petrischale |
| JPS5863382A (ja) * | 1981-10-13 | 1983-04-15 | Terumo Corp | 多層微生物培養検査器 |
| US4435508A (en) * | 1981-11-20 | 1984-03-06 | Gabridge Michael G | Tissue culture vessel |
| DE3218532A1 (de) * | 1982-05-17 | 1983-11-17 | C.A. Greiner und Söhne GmbH & Co KG, 7440 Nürtingen | Mit einem naehrboden gefuellte schale zur zuechtung von bakterien und kleinpilzen |
-
1984
- 1984-10-09 JP JP59212163A patent/JPS6192561A/ja active Granted
-
1985
- 1985-07-23 KR KR1019850005241A patent/KR890003945B1/ko not_active Expired
- 1985-08-08 AU AU45918/85A patent/AU567225B2/en not_active Ceased
- 1985-08-20 CA CA000489085A patent/CA1252375A/en not_active Expired
- 1985-08-24 CN CN85106361A patent/CN85106361B/zh not_active Expired
- 1985-09-11 US US06/774,757 patent/US4775628A/en not_active Expired - Lifetime
- 1985-09-23 EP EP85306742A patent/EP0181075B1/en not_active Expired
- 1985-09-23 DE DE8585306742T patent/DE3565997D1/de not_active Expired
-
1987
- 1987-11-20 US US07/123,141 patent/US4801548A/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| AU4591885A (en) | 1986-04-17 |
| KR860003345A (ko) | 1986-05-23 |
| US4801548A (en) | 1989-01-31 |
| EP0181075B1 (en) | 1988-11-02 |
| DE3565997D1 (en) | 1988-12-08 |
| AU567225B2 (en) | 1987-11-12 |
| JPS6192561A (ja) | 1986-05-10 |
| CN85106361A (zh) | 1986-04-10 |
| CA1252375A (en) | 1989-04-11 |
| JPH0558705B2 (enExample) | 1993-08-27 |
| US4775628A (en) | 1988-10-04 |
| EP0181075A1 (en) | 1986-05-14 |
| KR890003945B1 (ko) | 1989-10-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN85106361B (zh) | 培养细菌的培养皿及其用途 | |
| Martí et al. | Antimicrobial characterization of advanced materials for bioengineering applications | |
| CA1077814A (en) | Storage stable antibiotic susceptibility test kit | |
| Athar et al. | The development of resistance by Candida species to polyene antibiotics in vitro | |
| US3992265A (en) | Antibiotic susceptibility testing | |
| US5817510A (en) | Device and method for evaluating microorganisms | |
| US4090920A (en) | Disposable antibiotic susceptability test package | |
| Lambden et al. | Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae | |
| US3107204A (en) | Microbiological testing method and structure therefor | |
| US3787290A (en) | Method and means for assaying biological factors demonstrating quantal response | |
| Deighton et al. | [17] Methods for studying biofilms produced by staphylococcus epidermidis | |
| Heimdahl et al. | Beta-lactamase-producing Bacteroides species in the oral cavity in relation to penicillin therapy | |
| Marrie et al. | Influence of mucoidy on antibody coating of Pseudomonas aeruginosa | |
| Eagle | THE BINDING OF PENICILLIN IN RELATION TO ITS CYTOTOXIC ACTION: III. THE BINDING OF PENICILLIN BY MAMMALIAN CELLS IN TISSUE CULTURE (HELA AND L STRAINS) | |
| EP0346732A1 (en) | Biological sample collection and transport device | |
| Skusa et al. | Proof-of-Concept standardized approach using a single-disk method analogous to antibiotic disk diffusion assays for routine phage susceptibility testing in diagnostic laboratories | |
| Woodford et al. | The effect of media on antimicrobial susceptibility testing of Neisseria gonorrhoeae | |
| Kennedy et al. | Virulence and adhesive properties of serotypes A and B of Candida albicans isolated from paediatric burn patients | |
| Mowjood et al. | Small-colony forms of enteric bacteria after exposure to aminoglycosides | |
| Blair | Laboratory diagnosis of staphylococcal infections | |
| Lacey et al. | Loss of antibiotic resistance in Staphylococcus aureus in vivo probably resulting from cloxacillin therapy | |
| CN112831539A (zh) | 一种需氧微生物的体外检测试剂盒 | |
| CN105255718A (zh) | 一种检验棉拭子及其制备方法和检验方法 | |
| Flowers | Use of sensitivity discs as primary antibiotic standards in MIC determination. | |
| Khan et al. | IN VITRO COMPARATIVE EFFICACY OF SELECTED ANTIBIOTICS AGAINST ESCHERICHIA COLI ISOLATED FROM BROILERS |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C13 | Decision | ||
| GR02 | Examined patent application | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| C19 | Lapse of patent right due to non-payment of the annual fee | ||
| CF01 | Termination of patent right due to non-payment of annual fee |