CN101066559A - 氢存储粉末和制备该粉末的方法 - Google Patents
氢存储粉末和制备该粉末的方法 Download PDFInfo
- Publication number
- CN101066559A CN101066559A CNA2007101051710A CN200710105171A CN101066559A CN 101066559 A CN101066559 A CN 101066559A CN A2007101051710 A CNA2007101051710 A CN A2007101051710A CN 200710105171 A CN200710105171 A CN 200710105171A CN 101066559 A CN101066559 A CN 101066559A
- Authority
- CN
- China
- Prior art keywords
- hydrogen storage
- powder
- alloy
- hydrogen
- reactor
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000001257 hydrogen Substances 0.000 title claims abstract description 153
- 229910052739 hydrogen Inorganic materials 0.000 title claims abstract description 153
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 title claims abstract description 148
- 239000000843 powder Substances 0.000 title claims abstract description 70
- 238000003860 storage Methods 0.000 title claims abstract description 47
- 238000004519 manufacturing process Methods 0.000 title description 7
- 239000000463 material Substances 0.000 claims abstract description 95
- 238000005984 hydrogenation reaction Methods 0.000 claims description 28
- 238000006356 dehydrogenation reaction Methods 0.000 claims description 21
- 238000001816 cooling Methods 0.000 claims description 17
- 238000010438 heat treatment Methods 0.000 claims description 12
- 150000004678 hydrides Chemical class 0.000 claims description 9
- 238000010298 pulverizing process Methods 0.000 claims description 7
- 239000002826 coolant Substances 0.000 claims 1
- 239000000956 alloy Substances 0.000 abstract description 67
- 238000000034 method Methods 0.000 abstract description 67
- 229910045601 alloy Inorganic materials 0.000 abstract description 64
- 239000011232 storage material Substances 0.000 abstract description 62
- 238000007254 oxidation reaction Methods 0.000 abstract description 52
- 230000004913 activation Effects 0.000 abstract description 41
- 230000001590 oxidative effect Effects 0.000 abstract description 15
- 239000003607 modifier Substances 0.000 abstract description 14
- 238000004845 hydriding Methods 0.000 abstract 1
- 230000003647 oxidation Effects 0.000 description 31
- 238000006253 efflorescence Methods 0.000 description 28
- 206010037844 rash Diseases 0.000 description 28
- 239000007789 gas Substances 0.000 description 23
- 238000006243 chemical reaction Methods 0.000 description 21
- 239000000203 mixture Substances 0.000 description 20
- 239000002245 particle Substances 0.000 description 19
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 18
- 238000005516 engineering process Methods 0.000 description 13
- 238000010010 raising Methods 0.000 description 13
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 12
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 12
- 229910052751 metal Inorganic materials 0.000 description 11
- 230000008569 process Effects 0.000 description 11
- 229910052786 argon Inorganic materials 0.000 description 10
- 239000002184 metal Substances 0.000 description 10
- 229910052782 aluminium Inorganic materials 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 239000000523 sample Substances 0.000 description 9
- 230000005518 electrochemistry Effects 0.000 description 8
- 239000007772 electrode material Substances 0.000 description 8
- 229910052759 nickel Inorganic materials 0.000 description 8
- 229910052719 titanium Inorganic materials 0.000 description 8
- 229910052720 vanadium Inorganic materials 0.000 description 8
- 238000002485 combustion reaction Methods 0.000 description 7
- 238000005530 etching Methods 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- 229910052726 zirconium Inorganic materials 0.000 description 7
- 229910052804 chromium Inorganic materials 0.000 description 6
- 239000010410 layer Substances 0.000 description 6
- 229910052749 magnesium Inorganic materials 0.000 description 6
- 238000006386 neutralization reaction Methods 0.000 description 6
- 239000007800 oxidant agent Substances 0.000 description 6
- 229910052760 oxygen Inorganic materials 0.000 description 6
- 238000003756 stirring Methods 0.000 description 6
- 229910052718 tin Inorganic materials 0.000 description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 238000005266 casting Methods 0.000 description 5
- 229910052742 iron Inorganic materials 0.000 description 5
- 229910052748 manganese Inorganic materials 0.000 description 5
- 229910052987 metal hydride Inorganic materials 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 239000001301 oxygen Substances 0.000 description 5
- 239000002994 raw material Substances 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 238000010521 absorption reaction Methods 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 229910052802 copper Inorganic materials 0.000 description 4
- 239000010949 copper Substances 0.000 description 4
- 238000011049 filling Methods 0.000 description 4
- 238000000227 grinding Methods 0.000 description 4
- 150000004681 metal hydrides Chemical class 0.000 description 4
- 229910052750 molybdenum Inorganic materials 0.000 description 4
- -1 nickel metal hydride Chemical class 0.000 description 4
- 229910052758 niobium Inorganic materials 0.000 description 4
- 230000003068 static effect Effects 0.000 description 4
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 230000000996 additive effect Effects 0.000 description 3
- 239000002585 base Substances 0.000 description 3
- 238000010276 construction Methods 0.000 description 3
- 238000003795 desorption Methods 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- 150000002431 hydrogen Chemical class 0.000 description 3
- 229910052746 lanthanum Inorganic materials 0.000 description 3
- 238000002203 pretreatment Methods 0.000 description 3
- 238000004080 punching Methods 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 238000010301 surface-oxidation reaction Methods 0.000 description 3
- 229910052721 tungsten Inorganic materials 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 229910017961 MgNi Inorganic materials 0.000 description 2
- 229910001122 Mischmetal Inorganic materials 0.000 description 2
- 229910005813 NiMH Inorganic materials 0.000 description 2
- GQPLMRYTRLFLPF-UHFFFAOYSA-N Nitrous Oxide Chemical compound [O-][N+]#N GQPLMRYTRLFLPF-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 125000004429 atom Chemical group 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000003197 catalytic effect Effects 0.000 description 2
- 238000007600 charging Methods 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 239000013068 control sample Substances 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 238000009689 gas atomisation Methods 0.000 description 2
- 238000009413 insulation Methods 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 239000012254 powdered material Substances 0.000 description 2
- 229910052761 rare earth metal Inorganic materials 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 230000002269 spontaneous effect Effects 0.000 description 2
- 238000009718 spray deposition Methods 0.000 description 2
- 238000013517 stratification Methods 0.000 description 2
- 239000002344 surface layer Substances 0.000 description 2
- 229910052715 tantalum Inorganic materials 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 229910052727 yttrium Inorganic materials 0.000 description 2
- HMUNWXXNJPVALC-UHFFFAOYSA-N 1-[4-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]piperazin-1-yl]-2-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethanone Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)N1CCN(CC1)C(CN1CC2=C(CC1)NN=N2)=O HMUNWXXNJPVALC-UHFFFAOYSA-N 0.000 description 1
- WZFUQSJFWNHZHM-UHFFFAOYSA-N 2-[4-[2-(2,3-dihydro-1H-inden-2-ylamino)pyrimidin-5-yl]piperazin-1-yl]-1-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)ethanone Chemical compound C1C(CC2=CC=CC=C12)NC1=NC=C(C=N1)N1CCN(CC1)CC(=O)N1CC2=C(CC1)NN=N2 WZFUQSJFWNHZHM-UHFFFAOYSA-N 0.000 description 1
- 229910004247 CaCu Inorganic materials 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- 229910000975 Carbon steel Inorganic materials 0.000 description 1
- 229910052684 Cerium Inorganic materials 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- 229910010340 TiFe Inorganic materials 0.000 description 1
- 229910001093 Zr alloy Inorganic materials 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- UBAZGMLMVVQSCD-UHFFFAOYSA-N carbon dioxide;molecular oxygen Chemical compound O=O.O=C=O UBAZGMLMVVQSCD-UHFFFAOYSA-N 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 239000010962 carbon steel Substances 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 238000009388 chemical precipitation Methods 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000007599 discharging Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 239000002659 electrodeposit Substances 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 239000011261 inert gas Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 229910000765 intermetallic Inorganic materials 0.000 description 1
- 239000001995 intermetallic alloy Substances 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000013081 microcrystal Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 239000007773 negative electrode material Substances 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 239000001272 nitrous oxide Substances 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 238000002161 passivation Methods 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000003672 processing method Methods 0.000 description 1
- 230000036632 reaction speed Effects 0.000 description 1
- 229910052702 rhenium Inorganic materials 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 238000007790 scraping Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 238000010008 shearing Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 238000006557 surface reaction Methods 0.000 description 1
- 238000002834 transmittance Methods 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/24—Electrodes for alkaline accumulators
- H01M4/242—Hydrogen storage electrodes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
- B22F1/14—Treatment of metallic powder
- B22F1/145—Chemical treatment, e.g. passivation or decarburisation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/02—Making metallic powder or suspensions thereof using physical processes
- B22F9/023—Hydrogen absorption
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/0005—Reversible uptake of hydrogen by an appropriate medium, i.e. based on physical or chemical sorption phenomena or on reversible chemical reactions, e.g. for hydrogen storage purposes ; Reversible gettering of hydrogen; Reversible uptake of hydrogen by electrodes
- C01B3/001—Reversible uptake of hydrogen by an appropriate medium, i.e. based on physical or chemical sorption phenomena or on reversible chemical reactions, e.g. for hydrogen storage purposes ; Reversible gettering of hydrogen; Reversible uptake of hydrogen by electrodes characterised by the uptaking medium; Treatment thereof
- C01B3/0031—Intermetallic compounds; Metal alloys; Treatment thereof
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/0005—Reversible uptake of hydrogen by an appropriate medium, i.e. based on physical or chemical sorption phenomena or on reversible chemical reactions, e.g. for hydrogen storage purposes ; Reversible gettering of hydrogen; Reversible uptake of hydrogen by electrodes
- C01B3/001—Reversible uptake of hydrogen by an appropriate medium, i.e. based on physical or chemical sorption phenomena or on reversible chemical reactions, e.g. for hydrogen storage purposes ; Reversible gettering of hydrogen; Reversible uptake of hydrogen by electrodes characterised by the uptaking medium; Treatment thereof
- C01B3/0084—Solid storage mediums characterised by their shape, e.g. pellets, sintered shaped bodies, sheets, porous compacts, spongy metals, hollow particles, solids with cavities, layered solids
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/04—Processes of manufacture in general
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/38—Selection of substances as active materials, active masses, active liquids of elements or alloys
- H01M4/383—Hydrogen absorbing alloys
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2998/00—Supplementary information concerning processes or compositions relating to powder metallurgy
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/32—Hydrogen storage
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/10—Process efficiency
- Y02P20/129—Energy recovery, e.g. by cogeneration, H2recovery or pressure recovery turbines
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Electrochemistry (AREA)
- Engineering & Computer Science (AREA)
- Combustion & Propulsion (AREA)
- Inorganic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Powder Metallurgy (AREA)
- Hydrogen, Water And Hydrids (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/575313 | 2000-05-19 | ||
| US09/575,313 US6461766B1 (en) | 1998-08-27 | 2000-05-19 | Hydrogen storage powder and process for preparing the same |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB018130720A Division CN1327549C (zh) | 2000-05-19 | 2001-05-18 | 氢存储粉末和制备该粉末的方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN101066559A true CN101066559A (zh) | 2007-11-07 |
Family
ID=24299803
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNA2007101051710A Pending CN101066559A (zh) | 2000-05-19 | 2001-05-18 | 氢存储粉末和制备该粉末的方法 |
| CNB018130720A Expired - Fee Related CN1327549C (zh) | 2000-05-19 | 2001-05-18 | 氢存储粉末和制备该粉末的方法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB018130720A Expired - Fee Related CN1327549C (zh) | 2000-05-19 | 2001-05-18 | 氢存储粉末和制备该粉末的方法 |
Country Status (10)
| Country | Link |
|---|---|
| US (3) | US6461766B1 (enExample) |
| EP (1) | EP1293003A4 (enExample) |
| JP (1) | JP5250170B2 (enExample) |
| CN (2) | CN101066559A (enExample) |
| AU (1) | AU2001264737A1 (enExample) |
| BR (1) | BR0110983A (enExample) |
| CA (1) | CA2409218A1 (enExample) |
| MX (1) | MXPA02011364A (enExample) |
| TW (1) | TW531919B (enExample) |
| WO (1) | WO2001091210A1 (enExample) |
Families Citing this family (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6461766B1 (en) * | 1998-08-27 | 2002-10-08 | Ovonic Battery Company, Inc. | Hydrogen storage powder and process for preparing the same |
| US6841512B1 (en) * | 1999-04-12 | 2005-01-11 | Ovonic Battery Company, Inc. | Finely divided metal catalyst and method for making same |
| US6270719B1 (en) * | 1999-04-12 | 2001-08-07 | Ovonic Battery Company, Inc. | Modified electrochemical hydrogen storage alloy having increased capacity, rate capability and catalytic activity |
| US6589686B2 (en) * | 2001-02-28 | 2003-07-08 | Ovonic Battery Company, Inc. | Method of fuel cell activation |
| US6864002B1 (en) * | 2001-10-19 | 2005-03-08 | Christopher K. Dyer | Fuel cell system and method for producing electrical energy |
| US20040016769A1 (en) * | 2002-03-15 | 2004-01-29 | Redmond Scott D. | Hydrogen storage, distribution, and recovery system |
| US7169489B2 (en) | 2002-03-15 | 2007-01-30 | Fuelsell Technologies, Inc. | Hydrogen storage, distribution, and recovery system |
| US7399325B1 (en) | 2002-03-15 | 2008-07-15 | Fuelsell Technologies, Inc. | Method and apparatus for a hydrogen fuel cassette distribution and recovery system |
| JP4183959B2 (ja) * | 2002-03-22 | 2008-11-19 | 株式会社日本製鋼所 | 水素吸蔵合金の製造方法 |
| US7011768B2 (en) | 2002-07-10 | 2006-03-14 | Fuelsell Technologies, Inc. | Methods for hydrogen storage using doped alanate compositions |
| US20040065171A1 (en) | 2002-10-02 | 2004-04-08 | Hearley Andrew K. | Soild-state hydrogen storage systems |
| US7365366B2 (en) * | 2003-01-06 | 2008-04-29 | Showa Denka K.K. | Boron phosphide-based semiconductor light-emitting device and production method thereof |
| US6830725B2 (en) * | 2003-04-01 | 2004-12-14 | Texaco Ovonic Battery Systems, Llc | Hydrogen storage alloys having a high porosity surface layer |
| US7250386B2 (en) * | 2003-07-18 | 2007-07-31 | Energy Conversion Devices, Inc. | Quantum limit catalysts and hydrogen storage materials |
| US7175826B2 (en) * | 2003-12-29 | 2007-02-13 | General Electric Company | Compositions and methods for hydrogen storage and recovery |
| NO325620B1 (no) * | 2003-10-21 | 2008-06-30 | Revolt Technology Ltd | Elektrode, fremgangsmate for fremstilling derav, metall/luft-brenselcelle og metallhydrid-battericelle |
| JP4908778B2 (ja) * | 2004-06-30 | 2012-04-04 | キヤノン株式会社 | 固体高分子型燃料電池の触媒層の製造方法および固体高分子型燃料電池の製造方法 |
| US20060057019A1 (en) | 2004-09-16 | 2006-03-16 | Kwo Young | Hydrogen storage alloys having reduced PCT hysteresis |
| RU2301723C1 (ru) * | 2005-11-14 | 2007-06-27 | Российская Федерация, от имени которой выступает государственный заказчик Федеральное агентство по атомной энергии | Способ получения мелкодисперсного порошка титана |
| US20070141464A1 (en) * | 2005-12-21 | 2007-06-21 | Qunjian Huang | Porous metal hydride electrode |
| WO2010016946A2 (en) * | 2008-08-08 | 2010-02-11 | Henkel Corporation | Low temperature curing compositions |
| US8246903B2 (en) * | 2008-09-09 | 2012-08-21 | H.C. Starck Inc. | Dynamic dehydriding of refractory metal powders |
| DE102009018874A1 (de) * | 2009-04-24 | 2010-11-04 | Systec System- Und Anlagentechnik Gmbh & Co.Kg | Nickelhaltiges Elektrodenmaterial |
| FR2950876B1 (fr) * | 2009-10-07 | 2012-02-10 | Commissariat Energie Atomique | Procede de traitement d'un materiau getter et procede d'encapsulation d'un tel materiau getter |
| WO2012166808A2 (en) * | 2011-06-01 | 2012-12-06 | Target Technology International, Ltd. | Nickel alloys for hydrogen storage and the generation of energy therefrom |
| CN103894602B (zh) * | 2012-12-27 | 2017-02-08 | 北京有色金属研究总院 | 一种提高稀土镁基储氢合金循环寿命的表面处理方法 |
| CN104919069B (zh) | 2013-01-07 | 2019-05-07 | 奥佛电池公司 | 金属氢化物合金 |
| US8877378B2 (en) | 2013-01-07 | 2014-11-04 | Ovonic Battery Company, Inc. | Metal hydride alloy with catalyst particles and channels |
| US9350014B2 (en) | 2013-01-07 | 2016-05-24 | Ovonic Battery Company, Inc. | Nanoscale nickel-based catalytic material |
| US20140194282A1 (en) * | 2013-01-07 | 2014-07-10 | Ovonic Battery Company, Inc. | Metal hydride alloy with catalytic particles |
| CN110820031A (zh) * | 2019-11-19 | 2020-02-21 | 有研工程技术研究院有限公司 | 一种微型吸气剂的制备方法 |
| US11266965B1 (en) | 2021-01-14 | 2022-03-08 | Shandong University | Ultra-low-speed rotating low-strain high-filling-rate hydrogen storage alloy reaction device and technology |
| KR20240039147A (ko) * | 2021-07-23 | 2024-03-26 | 하니스 아이피, 엘엘씨 | 비발화성 수소 저장 합금 및 당해 합금을 사용하는 수소 저장 시스템 |
| CN113735057A (zh) * | 2021-08-31 | 2021-12-03 | 苏州睿分电子科技有限公司 | 一种免活化储氢材料及其制备方法和装置 |
| CN114381644B (zh) * | 2021-12-10 | 2022-10-21 | 厚普清洁能源(集团)股份有限公司 | 一种钒钛基储氢合金粉末及其制备方法 |
| CN114619026B (zh) * | 2022-03-15 | 2024-01-12 | 厦门厦钨氢能科技有限公司 | 一种复合固态贮氢材料及其制备方法 |
| CN115519119A (zh) * | 2022-09-23 | 2022-12-27 | 江苏智仁景行新材料研究院有限公司 | 一种含内生氢化物的铝合金粉及其制备方法 |
Family Cites Families (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
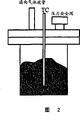
| US4457136A (en) * | 1981-03-23 | 1984-07-03 | Sekisui Kagaku Kogyo Kabushiki Kaisha | Metal hydride reactor |
| US4402915A (en) * | 1981-05-06 | 1983-09-06 | Sekisui Kagaku Kogyo Kabushiki Kaisha | Metal hydride reactor |
| US4623597A (en) * | 1982-04-28 | 1986-11-18 | Energy Conversion Devices, Inc. | Rechargeable battery and electrode used therein |
| US4551400A (en) * | 1984-04-18 | 1985-11-05 | Energy Conversion Devices, Inc. | Hydrogen storage materials and methods of sizing and preparing the same for electrochemical applications |
| US4923770A (en) * | 1985-03-29 | 1990-05-08 | The Standard Oil Company | Amorphous metal alloy compositions for reversible hydrogen storage and electrodes made therefrom |
| JPS62167201A (ja) * | 1986-01-17 | 1987-07-23 | Daido Steel Co Ltd | 水素貯蔵合金の活性化処理方法 |
| US4716088A (en) * | 1986-12-29 | 1987-12-29 | Energy Conversion Devices, Inc. | Activated rechargeable hydrogen storage electrode and method |
| US4728586A (en) * | 1986-12-29 | 1988-03-01 | Energy Conversion Devices, Inc. | Enhanced charge retention electrochemical hydrogen storage alloys and an enhanced charge retention electrochemical cell |
| US4893756A (en) | 1988-09-22 | 1990-01-16 | Energy Conversion Devices, Inc. | Hydride reactor apparatus for hydrogen comminution of metal hydride hydrogen storage material |
| US5536591A (en) * | 1990-04-26 | 1996-07-16 | Ovonic Battery Company, Inc. | Electrochemical hydrogen storage alloys for nickel metal hydride batteries |
| US5185221A (en) * | 1992-02-06 | 1993-02-09 | Gates Energy Products, Inc. | Metal hydride electrode and electrochemical cell |
| US5248510A (en) * | 1992-02-18 | 1993-09-28 | Hughes Aircraft Company | Cobalt oxide passivation of nickel battery electrode substrates |
| DE4343321A1 (de) * | 1993-12-18 | 1995-06-22 | Varta Batterie | Elektrischer Akkumulator |
| US5695530A (en) * | 1994-03-14 | 1997-12-09 | Hong; Kuochih | Method for making high charging efficiency and fast oxygen recombination rechargeable hydride batteries |
| CA2184377C (en) * | 1994-03-31 | 2000-12-12 | Han Wu | Improved metal hydride hydrogen storage electrodes |
| CN1145694A (zh) * | 1994-03-31 | 1997-03-19 | 摩托罗拉公司 | 改进的金属氢化物储氢电极 |
| US5451474A (en) * | 1994-04-04 | 1995-09-19 | Motorola, Inc. | Metal hydride hydrogen storage electrodes |
| US5616432A (en) * | 1994-06-14 | 1997-04-01 | Ovonic Battery Company, Inc. | Electrochemical hydrogen storage alloys and batteries fabricated from Mg containing base alloys |
| AU4469196A (en) * | 1994-12-22 | 1996-07-10 | Energy Conversion Devices Inc. | Magnesium mechanical alloys for thermal hydrogen storage |
| JP3016064B2 (ja) * | 1995-09-27 | 2000-03-06 | 古河電池株式会社 | 電池用水素吸蔵合金粉末の製造方法 |
| US5840440A (en) * | 1995-11-20 | 1998-11-24 | Ovonic Battery Company, Inc. | Hydrogen storage materials having a high density of non-conventional useable hydrogen storing sites |
| US5682592A (en) * | 1996-07-16 | 1997-10-28 | Korea Institute Of Science And Technology | Fabrication method for paste-type metal hydride electrode |
| JP3223858B2 (ja) * | 1996-12-24 | 2001-10-29 | 松下電器産業株式会社 | アルカリ蓄電池とその正極活物質およびその製造方法 |
| EP0851515A3 (en) * | 1996-12-27 | 2004-10-27 | Canon Kabushiki Kaisha | Powdery material, electrode member, method for manufacturing same and secondary cell |
| US5932372A (en) * | 1997-01-02 | 1999-08-03 | Lightyear Technologies Inc. | Composite materials, processes for manufacturing the composites, composite electrode, hydrogen occluding composite, and electrochemical cell utilizing the composite |
| JPH10195575A (ja) * | 1997-01-10 | 1998-07-28 | Mitsubishi Materials Corp | 初期活性の良好な水素貯蔵合金 |
| JPH11130401A (ja) * | 1997-10-29 | 1999-05-18 | Toyota Central Res & Dev Lab Inc | 水素吸蔵合金の高活性化処理方法 |
| EP0940865A3 (en) * | 1998-03-05 | 2004-11-03 | Matsushita Electric Industrial Co., Ltd | Active materials for the positive electrode in alkaline storage battery and the manufacturing method of them |
| JPH11339789A (ja) * | 1998-05-26 | 1999-12-10 | Toshiba Battery Co Ltd | 金属酸化物・水素蓄電池 |
| JP2000073101A (ja) * | 1998-08-27 | 2000-03-07 | Daido Steel Co Ltd | 水素吸蔵合金粉末の製造方法 |
| US6461766B1 (en) * | 1998-08-27 | 2002-10-08 | Ovonic Battery Company, Inc. | Hydrogen storage powder and process for preparing the same |
| US6120936A (en) * | 1998-08-27 | 2000-09-19 | Ovonic Battery Company, Inc. | Method for powder formation of a hydrogen storage alloy |
| US6270719B1 (en) * | 1999-04-12 | 2001-08-07 | Ovonic Battery Company, Inc. | Modified electrochemical hydrogen storage alloy having increased capacity, rate capability and catalytic activity |
-
2000
- 2000-05-19 US US09/575,313 patent/US6461766B1/en not_active Expired - Lifetime
-
2001
- 2001-05-16 US US09/859,164 patent/US6740448B2/en not_active Expired - Lifetime
- 2001-05-18 MX MXPA02011364A patent/MXPA02011364A/es not_active Application Discontinuation
- 2001-05-18 EP EP01939193A patent/EP1293003A4/en not_active Withdrawn
- 2001-05-18 BR BR0110983-9A patent/BR0110983A/pt not_active Application Discontinuation
- 2001-05-18 CA CA002409218A patent/CA2409218A1/en not_active Abandoned
- 2001-05-18 WO PCT/US2001/016344 patent/WO2001091210A1/en not_active Ceased
- 2001-05-18 JP JP2001587503A patent/JP5250170B2/ja not_active Expired - Fee Related
- 2001-05-18 CN CNA2007101051710A patent/CN101066559A/zh active Pending
- 2001-05-18 CN CNB018130720A patent/CN1327549C/zh not_active Expired - Fee Related
- 2001-05-18 TW TW090111880A patent/TW531919B/zh not_active IP Right Cessation
- 2001-05-18 AU AU2001264737A patent/AU2001264737A1/en not_active Abandoned
-
2002
- 2002-10-07 US US10/266,193 patent/US6789757B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| JP2003534637A (ja) | 2003-11-18 |
| US20030148180A1 (en) | 2003-08-07 |
| AU2001264737A1 (en) | 2001-12-03 |
| US6789757B2 (en) | 2004-09-14 |
| EP1293003A4 (en) | 2006-10-25 |
| TW531919B (en) | 2003-05-11 |
| CA2409218A1 (en) | 2001-11-29 |
| BR0110983A (pt) | 2003-12-30 |
| WO2001091210A1 (en) | 2001-11-29 |
| EP1293003A1 (en) | 2003-03-19 |
| MXPA02011364A (es) | 2004-01-26 |
| US6461766B1 (en) | 2002-10-08 |
| US20030038197A1 (en) | 2003-02-27 |
| JP5250170B2 (ja) | 2013-07-31 |
| CN1443377A (zh) | 2003-09-17 |
| US6740448B2 (en) | 2004-05-25 |
| CN1327549C (zh) | 2007-07-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1327549C (zh) | 氢存储粉末和制备该粉末的方法 | |
| JP2003534637A5 (enExample) | ||
| JP3231230B2 (ja) | 電極用水素蓄積活物質の形成方法 | |
| WO2001048837A2 (en) | Method for making hydrogen storage alloy | |
| US6120936A (en) | Method for powder formation of a hydrogen storage alloy | |
| Zhao et al. | Ti2Ni alloy: a potential candidate for hydrogen storage in nickel/metal hydride secondary batteries | |
| CN101402115A (zh) | 一种金属间化合物纳米颗粒的原位合成方法 | |
| Cui et al. | Synthesis and electrode characteristics of the new composite alloys Mg2Ni-xwt.% Ti2Ni | |
| Yu et al. | A Ti-V-based bcc phase alloy for use as metal hydride electrode with high discharge capacity | |
| CN1272460C (zh) | RE-Mg-Ni三元或三元以上体系储氢合金及其非晶合金的制备方法 | |
| CN108097947B (zh) | 一种高容量Mg-Zn-Ni三元贮氢合金及其制备方法 | |
| Yuexiang et al. | Characteristics of a low-cobalt AB5-type hydrogen storage alloy obtained by a gas-atomization processing | |
| CN1272461C (zh) | 一种非晶态储氢复合材料及其制造方法 | |
| JP3755841B2 (ja) | マグネシウム系水素吸蔵材料及びその製造方法 | |
| CN101436665B (zh) | 一种非晶态钛-铜-镍基储氢复合材料 | |
| JP3984668B2 (ja) | 水素吸蔵材料の活性化方法 | |
| CA1269083A (en) | Hydrogen storage materials and methods of sizing and preparing the same for electrochemical applications | |
| JPH06248306A (ja) | 水素吸蔵合金粉末の製造方法 | |
| CN106756355A (zh) | 燃料电池用Mg‑Sn‑Ni三元贮氢中间合金、贮氢材料和制备方法 | |
| JP2002540575A (ja) | 金属含有電極材料とその製造方法 | |
| JPH07296810A (ja) | 水素吸蔵合金粉末とニッケル−水素電池 | |
| CN120464926A (zh) | 一种稀土储氢高熵合金制备方法 | |
| Chen et al. | Development of high energy nickel-metal hydride cell | |
| Zhang | Synthesis, characterization and electrochemical hydrogen storage properties of mechanicalyl alloyed Ti-Mg-Ni: application as negative electrode for Ni-MH battery | |
| JP2000087172A (ja) | 水素吸蔵材料およびその製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| EE01 | Entry into force of recordation of patent licensing contract |
Assignee: Yuasa (Tianjin) Industrial Co., Ltd. Assignor: GS Yuasa International Corporation Contract record no.: 2011990000074 Denomination of invention: Hydrogen storage powder and process for preparing the same License type: Common License Open date: 20071107 Record date: 20110201 |
|
| EC01 | Cancellation of recordation of patent licensing contract |
Assignee: Yuasa (Tianjin) Industrial Co., Ltd. Assignor: GS Yuasa International Corporation Contract record no.: 2011990000074 Date of cancellation: 20130217 |
|
| LICC | Enforcement, change and cancellation of record of contracts on the licence for exploitation of a patent or utility model | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication |
Application publication date: 20071107 |