WO2023243718A1 - リチウムイオン伝導材料及び二次電池 - Google Patents
リチウムイオン伝導材料及び二次電池 Download PDFInfo
- Publication number
- WO2023243718A1 WO2023243718A1 PCT/JP2023/022430 JP2023022430W WO2023243718A1 WO 2023243718 A1 WO2023243718 A1 WO 2023243718A1 JP 2023022430 W JP2023022430 W JP 2023022430W WO 2023243718 A1 WO2023243718 A1 WO 2023243718A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- formula
- ocf
- independently
- integer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/628—Inhibitors, e.g. gassing inhibitors, corrosion inhibitors
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/06—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors mainly consisting of other non-metallic substances
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/06—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors mainly consisting of other non-metallic substances
- H01B1/08—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors mainly consisting of other non-metallic substances oxides
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/06—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors mainly consisting of other non-metallic substances
- H01B1/12—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors mainly consisting of other non-metallic substances organic substances
- H01B1/122—Ionic conductors
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0561—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of inorganic materials only
- H01M10/0562—Solid materials
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/42—Methods or arrangements for servicing or maintenance of secondary cells or secondary half-cells
- H01M10/4235—Safety or regulating additives or arrangements in electrodes, separators or electrolyte
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- This application discloses a lithium ion conductive material and a secondary battery.
- perfluoropolyethers are employed as various battery materials.
- perfluoropolyether itself does not have lithium ion conductivity
- the resistance of the battery tends to increase.
- a new technology is needed that can make perfluoropolyether exhibit lithium ion conductivity.
- R F is represented by the formula (2): -(OC 6 F 12 ) a -(OC 5 F 10 ) b -(OC 4 F 8 ) c -(OC 3 R Fa 6 ) d -(OC 2 F 4 ) e -(OCF 2 ) f - (2 )
- R Fa is independently a hydrogen atom, a fluorine atom, or a chlorine atom at each occurrence
- a, b, c, d, e and f are each independently an integer from 0 to 200
- the sum of a, b, c, d, e and f is 1 or more
- the order of existence of each repeating unit enclosed in parentheses with a, b, c, d, e or f is arbitrary in the formula, However, when all R Fa are hydrogen atoms or chlorine atoms, at least one of a, b, c, e and f is 1 or more.
- R 6 is OCF 2 or OC 2 F 4 ;
- R7 is a group selected from OC2F4, OC3F6, OC4F8, OC5F10 and OC6F12 , or two or three groups selected from these groups . It is a combination of two groups, g is an integer from 2 to 100.
- e is an integer from 1 to 200
- a, b, c, d and f are each independently an integer of 0 or more and 200 or less
- the repeating units enclosed in parentheses with a, b, c, d, e, or f can be present in any order in the formula.
- the R F is represented by the following formula (2-6): -(OCF 2 CF 2 CF 2 ) a -(OCF(CF 3 )CF 2 ) b -(OCF 2 CF(CF 3 )) c -(OCF 2 CF 2 ) d -(OCF(CF 3 )) e - (OCF 2 ) f - (2-6)
- a, b, c, d, e and f are each independently an integer of 0 to 200, The sum of a, b, c, d, e and f is 1 or more,
- the repeating units enclosed in parentheses with a, b, c, d, e, or f can be present in any order in the formula.
- the E 1 -Rf 1 and the E 2 -Rf 2 are each independently a group selected from the group consisting of -CF 3 , -CF 2 CF 3 , and -CF 2 CF 2 CF 3 , The lithium ion conductive material according to any one of aspects 1 to 6.
- a secondary battery comprising a positive electrode, an electrolyte layer and a negative electrode, At least one of the positive electrode, the electrolyte layer, and the negative electrode includes the lithium ion conductive material according to any one of aspects 1 to 7. Secondary battery.
- the lithium ion conductive material of the present disclosure has lithium ion conductivity, but has low reactivity to lithium.
- the lithium ion conductive material of the present disclosure includes a perfluoropolyether represented by the following formula (1) and LiI dissolved in the perfluoropolyether.
- PFPE Perfluoropolyether
- PFPE Perfluoropolyether
- the fluidity of the battery material improves due to the lubrication effect of PFPE.
- the filling rate of the battery material is likely to increase when the battery material is molded.
- PFPE has a large number of ether bonds, it is thought to have a high affinity for various battery materials, and is likely to exist appropriately with battery materials.
- PFPE has insulating properties, for example, by including PFPE in the separator layer (electrolyte layer) of a battery, the voltage resistance of the separator layer can be improved.
- the perfluoropolyether is represented by the following formula (1).
- Rf 1 and Rf 2 are each independently a C1-16 divalent alkylene group which may be substituted with one or more fluorine atoms
- E 1 and E 2 are each independently a fluorine group, a hydrogen group, a hydroxyl group, an aldehyde group, a carboxylic acid group, a C1-10 alkyl ester group, an amide group which may have one or more substituents
- R F is a divalent fluoropolyether group.
- Rf 1 and Rf 2 are each independently a C1-16 divalent alkylene group which may be substituted with one or more fluorine atoms.
- the "C1-16 divalent alkylene" in the C1-16 divalent alkylene group optionally substituted with one or more fluorine atoms is a straight chain, It may be a branched chain, and is preferably a straight or branched C1-6 fluoroalkylene group, particularly a C1-3 fluoroalkylene group, specifically -CF 2 CH 2 -, and It may be -CF 2 CF 2 CH 2 -, and more preferably a linear C1-6 perfluoroalkylene group, especially a C1-3 perfluoroalkylene group, specifically -CF 2 - , -CF 2 CF 2 -, and -CF 2 CF 2 CF 2 -.
- E 1 and E 2 each independently have a fluorine group, a hydrogen group, a hydroxyl group, an aldehyde group, a carboxylic acid group, a C1-10 alkyl ester group, or one or more substituents.
- This is a monovalent group selected from the group consisting of an amide group which may have one or more substituents, and an amino group which may have one or more substituents.
- E 1 and E 2 are each independently preferably a fluorine group.
- E 1 -Rf 1 and E 2 -Rf 2 are each independently a group selected from the group consisting of -CF 3 , -CF 2 CF 3 , and -CF 2 CF 2 CF 3 It may be.
- each occurrence of R F is independently a divalent fluoropolyether group.
- R F preferably represents formula (2): -(OC 6 F 12 ) a -(OC 5 F 10 ) b -(OC 4 F 8 ) c -(OC 3 R Fa 6 ) d -(OC 2 F 4 ) e -(OCF 2 ) f - (2 )
- R Fa is independently a hydrogen atom, a fluorine atom, or a chlorine atom at each occurrence
- a, b, c, d, e and f are each independently an integer from 0 to 200
- the sum of a, b, c, d, e and f is 1 or more
- the order of existence of each repeating unit enclosed in parentheses with a, b, c, d, e or f is arbitrary in the formula, However, when all R Fa are hydrogen atoms or chlorine atoms, at least one of a, b, c, e and f is 1 or more.
- R Fa is preferably a hydrogen atom or a fluorine atom, more preferably a fluorine atom.
- the sum of a, b, c, d, e and f is preferably 5 or more, more preferably 10 or more, and may be, for example, 15 or more or 20 or more.
- the sum of a, b, c, d, e and f is preferably 200 or less, more preferably 100 or less, even more preferably 60 or less, and may be, for example, 50 or less or 30 or less.
- repeating units may be linear or branched.
- -(OC 6 F 12 )- is -(OCF 2 CF 2 CF 2 CF 2 CF 2 CF 2 )-, -(OCF(CF 3 )CF 2 CF 2 CF 2 )-, -(OCF 2 CF (CF 3 )CF 2 CF 2 CF 2 )-, -(OCF 2 CF 2 CF (CF 3 )CF 2 CF 2 )-, -(OCF 2 CF 2 CF 2 CF (CF 3 )CF 2 )-, and , -(OCF 2 CF 2 CF 2 CF 2 CF (CF 3 )) -.
- -(OC 5 F 10 )- is -(OCF 2 CF 2 CF 2 CF 2 CF 2 )-, -(OCF(CF 3 )CF 2 CF 2 CF 2 )-, -(OCF 2 CF(CF 3 ) It may be any one of CF 2 CF 2 )-, -(OCF 2 CF 2 CF(CF 3 )CF 2 )-, and -(OCF 2 CF 2 CF 2 CF(CF 3 ))-.
- -(OC 3 F 6 )- (that is, in the above formula (2), when R Fa is a fluorine atom), -(OCF 2 CF 2 CF 2 )-, -(OCF(CF 3 )CF 2 ) -, and -(OCF 2 CF (CF 3 )) -.
- -(OC 2 F 4 )- may be either -(OCF 2 CF 2 )- or -(OCF(CF 3 ))-.
- R F may be a group represented by any of the following formulas (2-1) to (2-5), each occurrence independently.
- R 6 is OCF 2 or OC 2 F 4
- R7 is a group selected from OC2F4, OC3F6, OC4F8, OC5F10 and OC6F12 , or two or three groups selected from these groups . It is a combination of two groups, g is an integer from 2 to 100. ];
- f is an integer from 1 to 200
- a, b, c, d and e are each independently an integer of 0 to 200
- the repeating units enclosed in parentheses with a, b, c, d, e, or f can be present in any order in the formula.
- e and f are each independently an integer preferably from 5 to 200, more preferably from 10 to 200. Further, the sum of c, d, e, and f is preferably 5 or more, more preferably 10 or more, and may be, for example, 15 or more or 20 or more.
- the above formula (2-2) preferably represents -(OCF 2 CF 2 CF 2 CF 2 ) c -(OCF 2 CF 2 CF 2 ) d -(OCF 2 CF 2 ) e -(OCF 2 CF 2 CF 2 ) 2 ) It is a group represented by f -.
- formula (2-2) may be a group represented by -(OC 2 F 4 ) e -(OCF 2 ) f -.
- R 6 is preferably OC 2 F 4 .
- R 7 is preferably a group selected from OC 2 F 4 , OC 3 F 6 and OC 4 F 8 , or independently selected from these groups. It is a combination of two or three groups, more preferably a group selected from OC 3 F 6 and OC 4 F 8 .
- the combination of two or three groups independently selected from OC 2 F 4 , OC 3 F 6 and OC 4 F 8 is not particularly limited, but includes, for example, -OC 2 F 4 OC 3 F 6 -, -OC 2 F 4 OC 4 F 8 -, -OC 3 F 6 OC 2 F 4 -, -OC 3 F 6 OC 3 F 6 -, -OC 3 F 6 OC 4 F 8 -, -OC 4 F 8 OC 4 F 8 -, -OC 4 F 8 OC 3 F 6 -, -OC 4 F 8 OC 2 F 4 -, -OC 2 F 4 OC 2 F 4 OC 3 F 6 -, -OC 2 F 4 OC 2 F 4 OC 3 F 6 -, -OC 2 F 4 OC 2 F 4 OC 4 F 8 -, -OC 2 F 4 OC 3 F 6 -, -OC 2 F 4 OC 2 F 4 OC 4 F 8 -, -OC 2 F 4 OC 3 F 6 -
- g is preferably an integer of 3 or more, more preferably 5 or more.
- the above g is preferably an integer of 50 or less.
- OC 2 F 4 , OC 3 F 6 , OC 4 F 8 , OC 5 F 10 and OC 6 F 12 may be either straight chain or branched chain. , preferably linear.
- the above formula (2-3) is preferably -(OC 2 F 4 -OC 3 F 6 ) g - or -(OC 2 F 4 -OC 4 F 8 ) g -.
- f is preferably an integer of 1 or more and 100 or less, more preferably 5 or more and 100 or less.
- the sum of a, b, c, d, e and f is preferably 5 or more, more preferably 10 or more, for example 10 or more and 100 or less.
- the above R F is a group represented by the above formula (2-1).
- the above R F is a group represented by the above formula (2-2).
- the above R F is a group represented by the above formula (2-3).
- the above R F is a group represented by the above formula (2-4).
- the above R F is a group represented by the above formula (2-5).
- the ratio of e to f may be 0.5 to 4, preferably 0.6 to 3, and more preferably 0.7 to 4. 2, and even more preferably 0.8 to 1.4.
- e/f ratio a ratio of e to f
- the e/f ratio may be 0.5 to 4, preferably 0.6 to 3, and more preferably 0.7 to 4. 2, and even more preferably 0.8 to 1.4.
- the R F is represented by the following formula (2-6): -(OCF 2 CF 2 CF 2 ) a -(OCF(CF 3 )CF 2 ) b -(OCF 2 CF(CF 3 )) c -(OCF 2 CF 2 ) d -(OCF(CF 3 )) e - (OCF 2 ) f - (2-6)
- a, b, c, d, e and f are each independently an integer of 0 to 200, The sum of a, b, c, d, e and f is 1 or more,
- the repeating units enclosed in parentheses with a, b, c, d, e, or f can be present in any order in the formula.
- It may be a group represented by
- R F is represented by the following formula (2-7): -(OCF 2 CF 2 ) d -(OCF(CF 3 )) e -(OCF 2 ) f - (2-7)
- d, e and f are each independently an integer of 0 to 200, The sum of d, e and f is 1 or more, The repeating units suffixed with d, e or f and enclosed in parentheses can be present in any order in the formula.
- the ratio of d to f may be 0.5 to 4, preferably 0.6 to 3, and more preferably 0.7 to 4. 2, and even more preferably 0.8 to 1.4.
- d/f ratio 0.5 to 4 or less, lubricity and chemical stability are further improved.
- the smaller the d/f ratio the better the lubricity.
- the stability of the compound can be further improved.
- the larger the d/f ratio the more stable the fluoropolyether structure is. In this case, the value of f is preferably 0.8 or more.
- the number average molecular weight of the R F moiety is not particularly limited, but is, for example, 500 to 30,000, preferably 1,500 to 30,000, more preferably 2,000. ⁇ 10,000.
- the number average molecular weight of R F is a value measured by 19 F-NMR.
- lithium salts that can be used as battery materials include halides (LiF, LiCl, LiBr, LiI), imide salts (LiTFSI), and the like.
- LiI is particularly easy to dissolve in the above-mentioned PFPE, and easily causes PFPE to exhibit lithium ion conductivity.
- Iodine is less electronegative compared to fluorine, chlorine and bromine. That is, since LiI has a property of dissociating lithium ions more easily than LiF, LiCl, and LiBr, it is considered that LiI has a high solubility in the above-mentioned PFPE.
- the concentration of LiI in PFPE is not particularly limited, and may be adjusted as appropriate depending on the desired lithium ion conductivity.
- the molar concentration of LiI in PFPE may be at least 0.01M, at least 0.02M, at least 0.03M, at least 0.04M, or at least 0.05M, and at most the saturation concentration.
- LiI dissolved in PFPE is not limited to a state in which LiI, which is a lithium salt, is added and dissolved in PFPE, but also a state in which a Li source and an I source are separately added to PFPE. This also includes cases where LiI is dissolved in PFPE as a result of dissolution.
- the lithium ion conductive material of the present disclosure may contain other additive components in addition to the above-mentioned PFPE and LiI. Various other additive components may be employed depending on the desired performance.
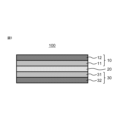
- a secondary battery 100 includes a positive electrode 10, an electrolyte layer 20, and a negative electrode 30, and at least one of the positive electrode 10, the electrolyte layer 20, and the negative electrode 30
- One includes the lithium ion conductive material of the present disclosure described above.
- the shape of the positive electrode active material layer 11 is not particularly limited, and may be, for example, a sheet-like positive electrode active material layer having a substantially flat surface.
- the thickness of the positive electrode active material layer 11 is not particularly limited, and may be, for example, 0.1 ⁇ m or more, 1 ⁇ m or more, or 10 ⁇ m or more, or 2 mm or less, 1 mm or less, or 500 ⁇ m or less.
- positive electrode active material those known as positive electrode active materials for secondary batteries may be used.
- a material whose potential for intercalating and releasing lithium ions (charge/discharge potential) is relatively noble is used as a positive electrode active material, and a relatively base material is used as a negative electrode active material as described below.
- the positive electrode active material may be, for example, at least one selected from various lithium-containing compounds, elemental sulfur, sulfur compounds, and the like.
- Lithium-containing compounds as positive electrode active materials include lithium cobalt oxide, lithium nickel oxide, Li 1 ⁇ Ni 1/3 Co 1/3 Mn 1/3 O 2 ⁇ , lithium manganate, and spinel-based lithium compounds (Li 1+x Mn 2-x-y M y O 4 (M is one or more selected from Al, Mg, Co, Fe, Ni and Zn), lithium titanate, phosphorous
- Various lithium-containing oxides such as acid metal lithium (such as LiMPO 4 , where M is one or more selected from Fe, Mn, Co, and Ni) may be used.
- the positive electrode active material contains a lithium-containing oxide containing at least Li, at least one of Ni, Co, and Mn, and O as constituent elements.
- One type of positive electrode active material may be used alone, or two or more types may be used in combination.
- the shape of the positive electrode active material may be any shape commonly used as a positive electrode active material of batteries.
- the positive electrode active material may be in the form of particles.
- the positive electrode active material may be hollow, have voids, or be porous.
- the positive electrode active material may be a primary particle or a secondary particle formed by agglomerating a plurality of primary particles.
- the average particle diameter D50 of the positive electrode active material may be, for example, 1 nm or more, 5 nm or more, or 10 nm or more, or may be 500 ⁇ m or less, 100 ⁇ m or less, 50 ⁇ m or less, or 30 ⁇ m or less.
- the average particle diameter D50 referred to in the present application is a particle diameter (median diameter) at an integrated value of 50% in a volume-based particle size distribution determined by a laser diffraction/scattering method.
- a protective layer containing an ion-conductive oxide may be formed on the surface of the positive electrode active material. This makes it easier to suppress reactions between the positive electrode active material and sulfide (for example, sulfide solid electrolyte described below).
- ion conductive oxides include Li 3 BO 3 , LiBO 2 , Li 2 CO 3 , LiAlO 2 , Li 4 SiO 4 , Li 2 SiO 3 , Li 3 PO 4 , Li 2 SO 4 , Li 2 TiO 3 , Li 4 Ti 5 O 12 , Li 2 Ti 2 O 5 , Li 2 ZrO 3 , LiNbO 3 , Li 2 MoO 4 , Li 2 WO 4 and the like.
- the ion conductive oxide may have some elements replaced with a doping element such as P or B.
- the coverage (area ratio) of the protective layer on the surface of the positive electrode active material may be, for example, 70% or more, 80% or more, or 90% or more.
- the thickness of the protective layer may be, for example, 0.1 nm or more or 1 nm or more, or 100 nm or less or 20 nm or less.
- the electrolyte that may be included in the positive electrode active material layer 11 may be a solid electrolyte, a liquid electrolyte (electrolyte solution), or a combination thereof.
- a solid electrolyte a liquid electrolyte (electrolyte solution), or a combination thereof.
- high effects are likely to be obtained when the positive electrode active material layer 11 contains at least a solid electrolyte as an electrolyte.
- even higher effects are likely to be obtained when the positive electrode active material layer 11 includes at least a solid electrolyte and the above-mentioned lithium ion conductive material of the present disclosure as an electrolyte.
- the solid electrolyte one known as a solid electrolyte for secondary batteries may be used.
- the solid electrolyte may be an inorganic solid electrolyte or an organic polymer electrolyte.
- inorganic solid electrolytes have excellent ionic conductivity and heat resistance.
- oxide solid electrolytes such as lithium lanthanum zirconate, LiPON, Li 1+X Al X Ge 2-X (PO 4 ) 3 , Li-SiO glass, and Li-Al-S-O glass.
- Li 2 S-P 2 S 5 Li 2 S-SiS 2 , LiI-Li 2 S-SiS 2 , LiI-Si 2 S-P 2 S 5 , Li 2 S-P 2 S 5 -LiI-LiBr, Sulfide solids such as LiI-Li 2 S-P 2 S 5 , LiI-Li 2 SP 2 O 5 , LiI-Li 3 PO 4 -P 2 S 5 , Li 2 S-P 2 S 5 -GeS 2
- An example is an electrolyte.
- sulfide solid electrolytes especially sulfide solid electrolytes containing at least Li, S, and P as constituent elements, have high performance.
- the solid electrolyte may be amorphous or crystalline.
- the solid electrolyte may be in the form of particles, for example.
- One type of solid electrolyte may be used alone, or two or more types may be used in combination.
- the electrolyte may contain predetermined carrier ions (for example, lithium ions).
- the electrolyte may be, for example, a non-aqueous electrolyte.
- the composition of the electrolytic solution may be the same as that known as the composition of electrolytic solutions for secondary batteries.
- an electrolyte in which a lithium salt is dissolved in a carbonate solvent at a predetermined concentration can be used.
- carbonate solvents include fluoroethylene carbonate (FEC), ethylene carbonate (EC), and dimethyl carbonate (DMC).
- Examples of the lithium salt include LiPF 6 and the like.
- Examples of the conductive additive that can be included in the positive electrode active material layer 11 include vapor grown carbon fiber (VGCF), acetylene black (AB), Ketjen black (KB), carbon nanotube (CNT), and carbon nanofiber (CNF). ); and metal materials such as nickel, aluminum, and stainless steel.
- the conductive aid may be, for example, in the form of particles or fibers, and its size is not particularly limited. One type of conductive aid may be used alone, or two or more types may be used in combination.
- binders that can be included in the positive electrode active material layer 11 include butadiene rubber (BR) binders, butylene rubber (IIR) binders, acrylate butadiene rubber (ABR) binders, styrene butadiene rubber (SBR) binders, and polyfluoride binders.
- BR butadiene rubber
- IIR acrylate butadiene rubber
- SBR styrene butadiene rubber
- polyfluoride binders examples include vinylidene (PVdF) binders, polytetrafluoroethylene (PTFE) binders, and polyimide (PI) binders.
- PVdF vinylidene
- PTFE polytetrafluoroethylene
- PI polyimide
- the positive electrode 10 may include a positive electrode current collector 12 in contact with the positive electrode active material layer 11 described above.
- the positive electrode current collector 12 can be any one commonly used as a positive electrode current collector for batteries. Further, the positive electrode current collector 12 may be in the form of a foil, a plate, a mesh, a punched metal, a foam, or the like.
- the positive electrode current collector 12 may be made of metal foil or metal mesh. In particular, metal foil has excellent handling properties.
- the positive electrode current collector 12 may be made of a plurality of foils. Examples of metals constituting the positive electrode current collector 12 include Cu, Ni, Cr, Au, Pt, Ag, Al, Fe, Ti, Zn, Co, and stainless steel.
- the positive electrode current collector 12 may contain Al.
- the positive electrode current collector 12 may have some kind of coating layer on its surface for the purpose of adjusting resistance or the like.
- the positive electrode current collector 12 may be a metal foil or a base material on which the above-mentioned metal is plated or vapor-deposited.
- the positive electrode current collector 12 is made of a plurality of metal foils, there may be some kind of layer between the plurality of metal foils.
- the thickness of the positive electrode current collector 12 is not particularly limited. For example, it may be 0.1 ⁇ m or more or 1 ⁇ m or more, or 1 mm or less or 100 ⁇ m or less.
- the electrolyte layer 20 is disposed between the positive electrode 10 and the negative electrode 30 and can function as a separator.
- the electrolyte layer 20 contains at least an electrolyte, and may optionally further contain a binder or the like.
- the electrolyte layer 20 may also contain various other additives.
- the content of each component in the electrolyte layer 20 is not particularly limited, and may be determined as appropriate depending on the desired battery performance.
- the shape of the electrolyte layer 20 is not particularly limited, and may be in the form of a substantially flat sheet, for example.
- the thickness of the electrolyte layer 20 is not particularly limited, and may be, for example, 0.1 ⁇ m or more or 1 ⁇ m or more, or 2 mm or less or 1 mm or less.
- the electrolyte included in the electrolyte layer 20 may be appropriately selected from those exemplified as electrolytes that can be included in the positive electrode active material layer.
- the performance of the electrolyte layer 20 containing a solid electrolyte, especially a sulfide solid electrolyte, and more particularly, a sulfide solid electrolyte containing at least Li, S, and P as constituent elements is high. Further, even higher effects are likely to be obtained when the electrolyte layer 20 includes at least a solid electrolyte and the above-mentioned lithium ion conductive material of the present disclosure as an electrolyte.
- the solid electrolyte When the electrolyte is a solid electrolyte, the solid electrolyte may be amorphous or crystalline. When the electrolyte is a solid electrolyte, the solid electrolyte may be in the form of particles, for example. One type of electrolyte may be used alone, or two or more types may be used in combination.
- binder that may be included in the electrolyte layer 20 may be appropriately selected, for example, from among those exemplified as binders that may be included in the positive electrode active material layer.
- the negative electrode 30 is not particularly limited in its configuration as long as it can function appropriately as a negative electrode of a secondary battery. As shown in FIG. 1, the negative electrode 30 may include a negative electrode active material layer 31 and a negative electrode current collector 32.
- the negative electrode active material layer 31 contains at least a negative electrode active material, and may optionally further contain an electrolyte, a conductive aid, a binder, and the like.
- the negative electrode active material layer 31 may also contain various other additives.
- the content of each component in the negative electrode active material layer 31 may be determined as appropriate depending on the desired battery performance. For example, the content of the negative electrode active material may be 40% by mass or more, 50% by mass or more, 60% by mass or more, or 70% by mass or more, assuming that the entire negative electrode active material layer 31 (total solid content) is 100% by mass. , 100% by mass or less, or 90% by mass or less.
- the shape of the negative electrode active material layer 31 is not particularly limited, and may be, for example, a sheet-like negative electrode active material layer having a substantially flat surface.
- the thickness of the negative electrode active material layer 31 is not particularly limited, and may be, for example, 0.1 ⁇ m or more, 1 ⁇ m or more, or 10 ⁇ m or more, or 2 mm or less, 1 mm or less, or 500 ⁇ m or less.
- negative electrode active material various materials can be employed whose potential for intercalating and releasing lithium ions (charging and discharging potential) is lower than that of the above-mentioned positive electrode active material.
- negative electrode active materials include silicon-based active materials such as Si, Si alloys, and silicon oxide; carbon-based active materials such as graphite and hard carbon; various oxide-based active materials such as lithium titanate; metallic lithium, lithium alloys, etc. It may be at least one selected from the following.
- One type of negative electrode active material may be used alone, or two or more types may be used in combination.
- the electrolyte that may be included in the negative electrode active material layer 31 may be a solid electrolyte, a liquid electrolyte (electrolytic solution), or a combination thereof.
- high effects are likely to be obtained when the negative electrode active material layer 31 contains at least a solid electrolyte as an electrolyte.
- even higher effects are likely to be obtained when the negative electrode active material layer 31 includes at least a solid electrolyte and the above-described lithium ion conductive material of the present disclosure as an electrolyte.
- the negative electrode active material layer 31 may include a solid electrolyte, particularly a sulfide solid electrolyte, and further a sulfide solid electrolyte containing Li 2 SP 2 S 5 .
- the negative electrode 30 may include a negative electrode current collector 32 in contact with the negative electrode active material layer 31 described above.
- the negative electrode current collector 32 any general negative electrode current collector for batteries can be employed.
- the negative electrode current collector 32 may be in the form of a foil, a plate, a mesh, a punched metal, a foam, or the like.
- the negative electrode current collector 32 may be a metal foil or a metal mesh, or a carbon sheet. In particular, metal foil has excellent handling properties.
- the negative electrode current collector 32 may be made of a plurality of foils or sheets.
- the secondary battery 100 may have each of the above-mentioned configurations housed inside an exterior body.
- the exterior body any known exterior body for batteries can be used.
- a plurality of secondary batteries 100 may be arbitrarily electrically connected and arbitrarily stacked on top of each other to form an assembled battery.
- the assembled battery may be housed inside a known battery case.
- the secondary battery 100 may also include other obvious configurations such as necessary terminals. Examples of the shape of the secondary battery 100 include a coin shape, a laminate shape, a cylindrical shape, and a square shape.
- the secondary battery 100 can be manufactured by applying a known method. For example, it can be manufactured as follows. However, the method for manufacturing the secondary battery 100 is not limited to the following method, and each layer may be formed by dry molding or the like, for example.
- a negative electrode slurry is obtained by dispersing the negative electrode active material and the like constituting the negative electrode active material layer in a solvent.
- the solvent used in this case is not particularly limited, and water and various organic solvents can be used. Then, using a doctor blade or the like, apply the negative electrode slurry to the surface of the negative electrode current collector or the electrolyte layer described below, and then dry it to form a negative electrode active material layer on the surface of the negative electrode current collector or electrolyte layer.
- Each layer is laminated so that the electrolyte layer is sandwiched between the negative electrode and the positive electrode to obtain a laminate having a negative electrode current collector, a negative electrode active material layer, an electrolyte layer, a positive electrode active material layer, and a positive electrode current collector in this order.
- the electrolyte layer may be obtained by, for example, molding an electrolyte mixture containing an electrolyte and a binder, or may be obtained by press molding.
- a separator serving as an electrolyte layer may be sandwiched between a negative electrode active material layer and a positive electrode active material layer.
- the laminate may be further press-molded. Other members such as terminals are attached to the laminate as necessary.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Electrochemistry (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Inorganic Chemistry (AREA)
- Materials Engineering (AREA)
- Secondary Cells (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2024528980A JPWO2023243718A1 (enExample) | 2022-06-17 | 2023-06-16 | |
| CN202380047089.4A CN119547224A (zh) | 2022-06-17 | 2023-06-16 | 锂离子传导材料和二次电池 |
| KR1020247041626A KR20250009532A (ko) | 2022-06-17 | 2023-06-16 | 리튬 이온 전도 재료 및 이차 전지 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022098325 | 2022-06-17 | ||
| JP2022-098325 | 2022-06-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2023243718A1 true WO2023243718A1 (ja) | 2023-12-21 |
Family
ID=89191447
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2023/022430 Ceased WO2023243718A1 (ja) | 2022-06-17 | 2023-06-16 | リチウムイオン伝導材料及び二次電池 |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JPWO2023243718A1 (enExample) |
| KR (1) | KR20250009532A (enExample) |
| CN (1) | CN119547224A (enExample) |
| TW (1) | TWI880244B (enExample) |
| WO (1) | WO2023243718A1 (enExample) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018084265A1 (ja) * | 2016-11-02 | 2018-05-11 | ダイキン工業株式会社 | 電極および電気化学デバイス |
| JP2018515893A (ja) * | 2015-05-21 | 2018-06-14 | ザ ユニバーシティ オブ ノース カロライナ アット チャペル ヒルThe University Of North Carolina At Chapel Hill | アルカリバッテリーの為のハイブリッド型固体シングルイオン伝導性電解質 |
| JP2019524977A (ja) * | 2016-07-29 | 2019-09-05 | ブルー カレント、インコーポレイテッド | 柔軟な固体状イオン伝導性複合材料および製造方法 |
| WO2021060541A1 (ja) * | 2019-09-27 | 2021-04-01 | 富士フイルム株式会社 | 無機固体電解質含有組成物、全固体二次電池用シート、全固体二次電池用電極シート及び全固体二次電池、並びに、全固体二次電池用シート及び全固体二次電池の製造方法 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10308587B2 (en) * | 2015-02-03 | 2019-06-04 | Blue Current, Inc. | Functionalized fluoropolymers and electrolyte compositions |
| FR3035544B1 (fr) | 2015-04-21 | 2017-04-14 | Rhodia Operations | Electrolyte polymere solide et dispositifs electrochimiques le comprenant |
| US11196087B2 (en) | 2017-05-19 | 2021-12-07 | Panasonic Intellectual Property Management Co., Ltd. | Nonaqueous electrolyte containing perfluoropolyether and nitrile compound, and secondary battery including the same |
-
2023
- 2023-06-16 JP JP2024528980A patent/JPWO2023243718A1/ja active Pending
- 2023-06-16 WO PCT/JP2023/022430 patent/WO2023243718A1/ja not_active Ceased
- 2023-06-16 TW TW112122602A patent/TWI880244B/zh active
- 2023-06-16 CN CN202380047089.4A patent/CN119547224A/zh active Pending
- 2023-06-16 KR KR1020247041626A patent/KR20250009532A/ko active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018515893A (ja) * | 2015-05-21 | 2018-06-14 | ザ ユニバーシティ オブ ノース カロライナ アット チャペル ヒルThe University Of North Carolina At Chapel Hill | アルカリバッテリーの為のハイブリッド型固体シングルイオン伝導性電解質 |
| JP2019524977A (ja) * | 2016-07-29 | 2019-09-05 | ブルー カレント、インコーポレイテッド | 柔軟な固体状イオン伝導性複合材料および製造方法 |
| WO2018084265A1 (ja) * | 2016-11-02 | 2018-05-11 | ダイキン工業株式会社 | 電極および電気化学デバイス |
| WO2021060541A1 (ja) * | 2019-09-27 | 2021-04-01 | 富士フイルム株式会社 | 無機固体電解質含有組成物、全固体二次電池用シート、全固体二次電池用電極シート及び全固体二次電池、並びに、全固体二次電池用シート及び全固体二次電池の製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN119547224A (zh) | 2025-02-28 |
| KR20250009532A (ko) | 2025-01-17 |
| TWI880244B (zh) | 2025-04-11 |
| TW202408065A (zh) | 2024-02-16 |
| JPWO2023243718A1 (enExample) | 2023-12-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN104247135B (zh) | 锂离子二次电池 | |
| JP5253785B2 (ja) | ドライポリマー電解質および全固体型ポリマー電池 | |
| CN112041955A (zh) | 蓄电装置用正极及蓄电装置 | |
| TWI880244B (zh) | 鋰離子傳導材料及二次電池 | |
| JP6870422B2 (ja) | イオン導電性固体電解質及び全固体アルカリ金属イオン二次電池 | |
| TWI871671B (zh) | 二次電池 | |
| TWI871672B (zh) | 二次電池 | |
| TWI898226B (zh) | 二次電池及其製造方法 | |
| JP7556371B2 (ja) | リチウムイオン伝導材料及びリチウムイオン二次電池 | |
| US20250149636A1 (en) | Lithium ion battery | |
| JP6805929B2 (ja) | イオン導電性固体電解質と全固体アルカリ金属イオン二次電池 | |
| JP7459982B1 (ja) | リチウムイオン伝導材料、リチウムイオン二次電池、及びリチウムイオン伝導材料の製造方法 | |
| JP7708140B2 (ja) | 二次電池システム | |
| WO2019208733A1 (ja) | 蓄電デバイス用正極及び蓄電デバイス | |
| US20250149634A1 (en) | Lithium-ion battery | |
| KR20240095017A (ko) | 활물질 2차 입자, 부극 합재, 이들의 제조 방법, 및, 이차 전지 | |
| JP2025076981A (ja) | リチウムイオン電池 | |
| JP2023167546A (ja) | 電解液及びリチウムイオン二次電池 | |
| WO2019208734A1 (ja) | 蓄電デバイス用正極及び蓄電デバイス |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 23824008 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2024528980 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202380047089.4 Country of ref document: CN |
|
| ENP | Entry into the national phase |
Ref document number: 20247041626 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020247041626 Country of ref document: KR |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWP | Wipo information: published in national office |
Ref document number: 202380047089.4 Country of ref document: CN |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 23824008 Country of ref document: EP Kind code of ref document: A1 |