WO2021200531A1 - ガラスおよびガラスを含む物品 - Google Patents
ガラスおよびガラスを含む物品 Download PDFInfo
- Publication number
- WO2021200531A1 WO2021200531A1 PCT/JP2021/012476 JP2021012476W WO2021200531A1 WO 2021200531 A1 WO2021200531 A1 WO 2021200531A1 JP 2021012476 W JP2021012476 W JP 2021012476W WO 2021200531 A1 WO2021200531 A1 WO 2021200531A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- content
- glass
- sio
- zno
- zro
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C3/00—Glass compositions
- C03C3/04—Glass compositions containing silica
- C03C3/062—Glass compositions containing silica with less than 40% silica by weight
- C03C3/064—Glass compositions containing silica with less than 40% silica by weight containing boron
- C03C3/068—Glass compositions containing silica with less than 40% silica by weight containing boron containing rare earths
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C4/00—Compositions for glass with special properties
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2204/00—Glasses, glazes or enamels with special properties
Definitions
- the present invention relates to glass and articles containing glass.
- Glass is used in various articles to take advantage of its physical characteristics (for example, transparency, chemical, mechanical, thermal stability, etc.).
- articles having high water repellency are desired, but it is known that glass is generally a substance that is easily wetted and has low water repellency. Therefore, in articles that require water repellency, water repellency is imparted by forming a water-repellent coat on the glass surface or by applying microconcavo-convex processing on the glass surface (see, for example, Patent Document 1). rice field.
- the windshield of a car it is important to improve the water repellency of the windshield surface so that the adhering water droplets can be easily removed in order to secure the driver's field of vision.
- high water repellency is desirable from the viewpoint of suppressing the adhesion of water stains.
- meniscus generated by the high wettability of glass makes it difficult to accurately measure water.
- One aspect of the present invention is to provide a glass having high water repellency.
- One aspect of the present invention is in oxide-based glass composition
- SiO 2 content is 0 to 25%
- B 2 O 3 content is 0-35%
- P 2 O 5 content is 0-30%
- the total content of SiO 2 , B 2 O 3 and P 2 O 5 is 10 to 45%
- Al 2 O 3 content is 0 to 15%
- Li 2 O content is 0-2%
- Na 2 O content is 0-10%
- Rb 2 O content is 0-5%
- the total content of Li 2 O, Na 2 O, K 2 O, Rb 2 O and Cs 2 O is 0 to 15%.
- one aspect of the present invention relates to an article containing glass.
- the above items consist of a group consisting of window materials, windshields, cover glass, mirrors, tableware, physics and chemistry laboratory equipment, cooking utensils, washbasins, toilet bowls, tombstones, trinkets, crafts and arts, glass fibers and glass fiber moldings.
- the article of choice the glass having the above glass composition and having a wetness angle to water of 60 ° or more.
- an article containing a glass having high water repellency and a glass having high water repellency it is possible to provide an article containing a glass having high water repellency and a glass having high water repellency.
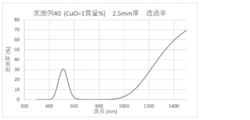
- the spectral transmittance curve obtained for the glass of Example 40 is shown.
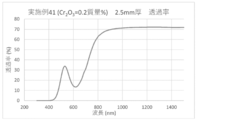
- the spectral transmittance curve obtained for the glass of Example 41 is shown.
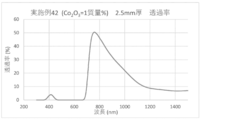
- the spectral transmittance curve obtained for the glass of Example 42 is shown.
- the spectral transmittance curve obtained for the glass of Example 43 is shown.
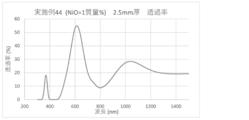
- the spectral transmittance curve obtained for the glass of Example 44 is shown.
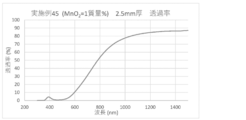
- the spectral transmittance curve obtained for the glass of Example 45 is shown.
- the spectral transmittance curve obtained for the glass of Example 46 is shown.
- the spectral transmittance curve obtained for the glass of Example 47 is shown.
- the wetting angle of soda lime glass which is widely used for window glass, bottle glass, etc., with respect to water is 40 ° or less.
- Soda lime glass is a glass containing about 70% by mass of SiO 2.
- the SiO 2 content is more than 70% by mass.
- Hydrophilicity and water repellency to water are determined by the surface free energy of the substance. Water repellency is generated by not inhibiting the surface tension of water, but since SiO 2 , which is a glass-forming oxide, has a relatively large surface energy, the wettability of water is enhanced.
- various components can be introduced into glass. For example, glass having various composition systems has been used for a long time as an optical glass that requires a unique refractive index characteristic as a lens or a prism.
- B 2 O 3 or P is used in addition to SiO 2. 2 O 5 glass is used.
- an antireflection coating is applied to reduce the reflection on the surface. Further, lenses having different characteristics are joined together.
- the present inventor has obtained a new finding that the wettability of glass is significantly changed by adjusting the glass composition through repeated studies. Specifically, it was found that the wettability of glass can be adjusted by adjusting the content of components that greatly affect the wettability. More specifically, it contains a large amount of a component that lowers surface energy, a component that is less reactive with water and has a lower solubility, and conversely has a large surface energy, a higher polarity, and is chemically reactive with water. It was clarified that the wettability of glass can be adjusted by suppressing the content of large components.
- the present inventor has obtained a glass having high water repellency and suitable for practical use, that is, the above glass.
- the glass will be described in more detail.
- the glass composition is expressed on an oxide basis with respect to the cationic component of the glass.
- the "oxide-based glass composition” refers to a glass composition obtained by converting all glass raw materials into those that are decomposed at the time of melting and exist as oxides in glass. Unless otherwise specified, the glass composition is displayed on a mass basis (mass%, mass ratio).
- the various components constituting the glass contain elements contained in the glass by known methods such as inductively coupled plasma emission spectroscopy (ICP-AES) and inductively coupled plasma mass spectrometry (ICP-MS). The amount (% by mass of the element) can be quantified.
- the content of this element By dividing the content of this element (mass% of the element) by the atomic weight, the content of each element in mol% can be obtained, and this value is used to determine the content (unit) in the oxide-equivalent glass composition. : Mass%) can be calculated.
- the anion component contained in the glass can be identified and quantified by a known analytical method such as an ion chromatography method or a non-dispersed infrared absorption method (ND-IR). For anion components other than oxygen, the content of% anion can be determined by a known method.
- the “anion%” is a value calculated by “(the number of anions of interest / the total number of anions of the glass component) ⁇ 100” and means the molar percentage of the amount of anions of interest to the total amount of anions.
- all of the anion components that is, 100 anion%) are oxygen ions.
- the oxygen content is the valence of the cation component contained in the glass and the cation%.
- the content (unit: anion%) calculated by the following method is adopted from the content, the valence of the anion component other than oxygen, and the content in% of the anion. That is, from the results of identification and quantitative analysis by a known method, the total of "valence x content" (hereinafter, “total cation valence”) of the cation component contained in the glass is calculated. For the anion component excluding oxygen, the total of "valence x content" (hereinafter, “total anion valence excluding oxygen”) is calculated from the results of identification and quantitative analysis by a known method.
- the value calculated as "[(total cation valence)-(total anion valence excluding oxygen)] / 2" is the oxygen content (specifically, the oxygen ion in the anion component). It can also be used as a ratio (unit: anion%)).
- the “cation%” is a value calculated by “(the number of cations of interest / the total number of cations of the glass component) ⁇ 100” and means the molar percentage of the amount of cations of interest to the total amount of cations.
- the formal valence of each cation is used.
- the formal valence is the valence required for the oxide to maintain its electrical neutrality when the valence of the oxygen ions (anions) that make up the oxide is -2 for the oxide of the cation of interest. Yes, it can be uniquely obtained from the chemical formula of the oxide.
- the valence of anions for example, the valence of oxygen ions is -2, the valence of fluorine ions is -1, and the valence of chlorine ions is -1
- the idea is that each ion accepts an electron and has a closed shell structure.
- Formal valence based on for example, oxygen ion accepts two electrons to form a closed shell structure, and fluorine ion and chlorine ion accept one electron to form a closed shell structure).
- the content of the anionic component is the content (unit: mass%) of each element in the external division with respect to the total content of 100% by mass of the oxide-based glass composition of the cation component obtained as described above. It can also be specified. Further, in the present invention and the present specification, the content of the constituent component is 0% or not contained or introduced, which means that the constituent component is substantially not contained, and the constituent component is at an unavoidable impurity level. It is permissible to be included.
- SiO 2- Na 2 O-CaO-based glass is used as the window glass and the container glass.
- These glasses have a wetting angle of about 30 ° with respect to water and are usually recognized as hydrophilic substances. It is presumed that increasing the water repellency of the glass itself has not been studied because there is no significant difference in the wetting angle of the glass containing 60% or more of SiO 2 even if the other components are adjusted.
- glass using a network former other than SiO 2 has been conventionally used, and by containing various components, the refractive index and the dispersion of the refractive index (refraction by the wavelength of light) are used. It has been used for lenses, prisms, optical fibers, etc.
- the main component of the network former may be SiO 2 , B 2 O 3 , or P 2 O 5 .
- B 2 O 3 is particularly effective because it can contain a large amount of La 2 O 3, which will be described later.
- the B 2 O 3 content is 35% or less, preferably 25% or less.
- the B 2 O 3 content is 0% or more, preferably 8% or more.
- the B 2 O 3 content may be 32% or less, 27% or less, 22% or less, 18% or less, or 15% or less.
- SiO 2 is effective as a glass network former. From the viewpoint of suppressing the decrease in water repellency, the SiO 2 content is 25% or less, preferably 8% or less. The SiO 2 content is 0% or more, preferably 1% or more. From the viewpoint of improving the alkali resistance and / or the chemical durability of the glass, the SiO 2 content may be 2% or more, 4% or more, 10% or more, 15% or more or 20% or more.
- P 2 O 5 is a useful component from the viewpoint of containing a large amount of components such as BaO, CaO, and Nb 2 O 5, which will be described later.
- the P 2 O 5 content is 0% or more, and 30% or less from the viewpoint of improving chemical durability.
- Glass from the point of view of the alkali resistance improving, the content of P 2 O 5 is preferably 5% or less, and more preferably 3% or less.
- the total content of SiO 2 , B 2 O 3 and P 2 O 5 is in the range of 10 to 45% from the viewpoint of suppressing the decrease in water repellency, and is in the range of 10 to 45%. It is preferably in the range of 40%, more preferably in the range of 15-30%.
- the GeO 2 content is 0% or more.
- GeO 2 has a function of stabilizing glass as a network former, but since it is a rare component, its content is 5% or less, preferably 2% or less, and more preferably less than 1%. ..
- the Al 2 O 3 content is 0% or more. Since Al 2 O 3 is a component that can strengthen the structure of glass, it is effective in improving chemical durability and mechanical strength. From the viewpoint of suppressing the crystallization tendency for stable production, the Al 2 O 3 content is 15% or less. From the viewpoint of improving the alkali resistance of the glass, the content of Al 2 O 3 may be more than 0%, 1% or more, or 3% or more.
- Li 2 O, Na 2 O, K 2 O, Rb 2 O, and Cs 2 O are 0% or more, respectively. These are components that lower the melting temperature of glass and promote vitrification. From the viewpoints of suppressing the decrease in water repellency, suppressing the decrease in chemical durability, and suppressing the decrease in mechanical strength, the Li 2 O content is 2% or less, the Na 2 O content is 10% or less, and the K 2 O content is The Rb 2 O content is 10% or less, the Rb 2 O content is 5% or less, and the Cs 2 O content is 5% or less.
- the total content of Li 2 O, Na 2 O, K 2 O, Rb 2 O and Cs 2 O suppresses the decrease in water repellency and the decrease in chemical durability. It is in the range of 0 to 20% from the viewpoint of suppression and suppression of decrease in mechanical strength. It is preferably 16% or less, more preferably 9% or less, still more preferably 6% or less. From the viewpoint of improving the chemical durability of glass, the total content of Li 2 O, Na 2 O, K 2 O, Rb 2 O and Cs 2 O (Li 2 O + Na 2 O + K 2 O + Rb 2 O + Cs 2 O) is determined. It may be 3% or less, 2% or less, 1% or less, or 0.3% or less.
- the ZnO content is 0% or more, preferably 3% or more, and more preferably 5% or more.
- ZnO is a component that enhances water repellency, and is a component that can realize stable vitrification even if its content is large.
- the ZnO content is 60% or less, preferably 55% or less, and more preferably 35% or less. be.
- the ZnO content may be 10% or more, 15% or more, 20% or more, 25% or more, 30%, 35% or more, 40% or more or 45% or more. Further, in one form, the ZnO content may be 40% or less, 32% or less, 24% or less, 16% or less, 8% or less, or 4% or less.
- the contents of MgO, CaO, SrO, and BaO are 0% or more, respectively. These are effective components for stable vitrification for any network former. From the viewpoint of suppressing the decrease in water repellency, the MgO content is 20% or less, the CaO content is 25% or less, the SrO content is 25% or less, and the BaO content is 30% or less.
- the La 2 O 3 content is 0% or more, preferably 15% or more.
- La 2 O 3 is a component having a great effect of enhancing water repellency.
- the La 2 O 3 content is 50% or less, preferably 45% or less.
- the La 2 O 3 content may be 10% or more, 15% or more, 20% or more, 25% or more, 33% or more, or 40% or more.
- Y 2 O 3 and Gd 2 O 3 are 0% or more, respectively.
- Y 2 O 3 and Gd 2 O 3 are components that can bring about the same effect as La 2 O 3.
- the Y 2 O 3 content is 15% or less, and the Gd 2 O 3 content is 25% or less.
- Y 2 O 3 content is 1% or more, may be 3% or more, or more than 4%.
- the Gd 2 O 3 content may be 1% or more, 3% or more, or 4% or more.
- the Yb 2 O 3 content is 0% or more.
- the Yb 2 O 3 content can bring about the same effect as La 2 O 3 , Y 2 O 3 , and Gd 2 O 3 , but since it is a rare component, its content is 10% or less. It is preferably 5% or less.
- the CeO 2 content is 0% or more.
- the effect of shielding ultraviolet rays can be obtained by adding a small amount of CeO 2.
- the color of the glass can also be adjusted by adding a small amount of CeO 2. From the viewpoint of suppressing the decrease in devitrification resistance, the CeO 2 content is 5% or less.
- Ga 2 O 3 and In 2 O 3 are 0% or more, respectively.
- Ga 2 O 3 and In 2 O 3 can bring about the same effect as La 2 O 3 , Y 2 O 3 , and Gd 2 O 3 , but since they are rare components, their contents are 5% or less, respectively. Is.
- the ZrO 2 content is 0% or more, preferably 1% or more.
- ZrO 2 is a component that can greatly improve chemical durability and mechanical strength (for example, hardness) without significantly reducing water repellency. From the viewpoint of suppressing an increase in the crystallization tendency due to an increase in the melting temperature, the ZrO 2 content is 15% or less, preferably 8% or less.
- the ZrO 2 content may be 2% or more, 4% or more, 5% or more, 6% or more, or 7% or more. This is because ZrO 2 is a particularly useful component for improving the chemical durability and / or alkali resistance of glass.
- the TiO 2 content is 0% or more.
- TiO 2 is a component that can be contained in a large amount, and is a component that can improve chemical durability and mechanical strength. From the viewpoint of suppressing the decrease in transmittance, the TiO 2 content is 20% or less. Further, the TiO 2 content may be 2% or more, 4% or more, 6% or more, 8% or more, 10% or more, 12% or more, 16% or more or 20% or more. This is because TiO 2 is a useful component for improving the chemical durability and / or the alkali resistance of glass.
- the SnO 2 content is 0% or more.
- SnO 2 is a component that can bring about a clarification effect by adding a small amount. It can also have the effect of increasing water repellency. From the viewpoint of suppressing the decrease in meltability, the SnO 2 content is 10% or less.
- the Nb 2 O 5 content is 0% or more, and may be 1% or more, 3% or more, 6% or more, 10% or more, or 14% or more.
- Nb 2 O 5 can preferably be contained in a large amount in the glass having P 2 O 5 as a network, thereby providing a water-repellent effect.
- the Nb 2 O 5 content is 30% or less, and may be 20% or less, 15% or less, or 10% or less.
- the Ta 2 O 5 content is 0% or more. Ta 2 O 5 can bring about the effect of increasing the stability of the glass with a small amount of addition.
- the Ta 2 O 5 content may be greater than 0%, greater than 1%, greater than or equal to 3%, greater than or equal to 6%, or greater than or equal to 12%. From the viewpoint of suppressing the crystallization tendency, the Ta 2 O 5 content is 15% or less, and may be 10% or less, 5% or less, or 0%.
- the WO 3 content is 0% or more. WO 3 can bring about an improvement in chemical durability with a small amount of addition.
- the WO 3 content may be greater than 0%, greater than 1%, greater than or equal to 2%, or greater than or equal to 4%. From the viewpoint of suppressing an increase in melting temperature and suppressing a tendency to crystallize, the WO 3 content is 15% or less, and may be 10% or less, 5% or less, or 0%.
- the Bi 2 O 3 content is 0% or more.
- Bi 2 O 3 is a component capable of increasing the meltability without lowering the water repellency. From the viewpoint of suppressing the decrease in mechanical strength and suppressing the decrease in crystallization tendency, the Bi 2 O 3 content is 20% or less, and may be 14% or less, 9% or less, or 4% or less.
- the Fe 2 O 3 content is in the range of 0 to 10%, and is the sum of V 2 O 5 , Cr 2 O 3 , MnO 2 , Co 2 O 3 , NiO, CuO, MoO, Au 2 O 3 and Ag 2 O.
- the content (V 2 O 5 + Cr 2 O 3 + MnO 2 + Co 2 O 3 + NiO + CuO + MoO + Au 2 O 3 + Ag 2 O) is in the range of 0 to 3%.
- the color of glass can be adjusted by appropriately adding one or more of Fe, V, Cr, Mn, Co, Ni, Cu, Mo, Au and Ag, which are coloring components.
- glasses of various colors can be obtained by adjusting the melting temperature, atmosphere, heat treatment color development, etc. to adjust the color. ..
- a coloring component such as Nd 2 O 3 can be mentioned, and further, Eu 2 O 3 , Er 2 O 3 , Sm 2 O 3, and the like can be mentioned. You can also do it.
- the Sb 2 O 3 content is 0% or more. Up to 1% of Sb 2 O 3 can be added, which can promote the clarification action during melting.
- the glass can be obtained by blending, melting, and molding various glass raw materials.
- oxides, hydrates, phosphates, carbonates, nitrates, sulfates, fluorides and the like can be used.
- Specific examples of glass raw materials include oxides such as SiO 2 , ZnO, La 2 O 3 , ZrO 2 , hydrates such as H 3 BO 3 , nitrates such as Sr (NO 3 ) 2 , and carbonates such as BaCO 3. Salt and the like can be mentioned.
- fluorides such as ZnF 2 , MgF 2 , and AlF 3
- a glass raw material by adding fluorides such as ZnF 2 , MgF 2 , and AlF 3 as a glass raw material at the time of glass production, a glass containing fluorine can be obtained.
- the type and content of the anionic component introduced into the glass can be adjusted according to the type and amount of the glass raw material added.
- the anion component include oxygen (O 2- ), fluorine (F ⁇ ), chlorine (Cl ⁇ ) and the like.
- oxygen (O 2- ) may be 100 anion% of the anion component, and one or more other anion components may be contained in addition to oxygen.
- the introduction of fluorine (F ⁇ ) can contribute to the improvement of water repellency.
- the content of fluorine (F ⁇ ) is preferably 30 anions or less.
- the fluorine content is preferably 10% by mass or less.
- chlorine (Cl ⁇ ) can be introduced into glass by using chlorides such as AuCl 3 and AgCl as a glass raw material, for example.
- the glass can be amorphous glass in one form and crystal-containing glass (crystallized glass) in another form. Further, the above glass can be used as a molded body molded into various shapes such as a plate by an arbitrary molding method, can be used as a coating film (glass layer), or can be used as a glass powder. .. It can be used as a sintered body of glass powder, can be used as a glass fiber, or can be used as a molded body of glass fiber. However, the glass does not include the glass used for the optical element selected from the group consisting of the lens and the prism and the glass used for the optical fiber.
- the wetting angle of the glass with respect to water is 60 ° or more, preferably 65 ° or more, more preferably 70 ° or more, further preferably 75 ° or more, and more preferably 80 ° or more. Is more preferable.
- the wetting angle of the glass with respect to water can be, for example, 120 ° or less, 110 ° or less, 100 ° or less, 98 ° or less, or 95 ° or less.
- the wetting angle with water exceeds the above-exemplified value because the larger the value of the wetting angle with respect to water is, the better the water repellency is.
- the wetting angle of the glass with respect to water is such that 0.1 ml ⁇ 0.02 ml of water is dropped on the glass surface in a measurement environment where the ambient temperature is 20 ° C. ⁇ 5 ° C. and the relative humidity is 50% ⁇ 20%. , The contact angle measured within 30 seconds after dropping. The arithmetic mean of the values obtained by the three measurements is taken as the wetting angle of the glass with respect to water.
- the three measurements can be carried out at three different locations on the glass surface, or the measurement can be carried out repeatedly after removing the water dropped for the previous measurement by drying or the like.
- the wetting angle is measured on the surface.
- the arithmetic average roughness Ra of the surface of the glass to be measured has a wetting angle of more than 0.2 ⁇ m, the wetting angle with water is measured on the surface polished after grinding and polishing so that Ra is 0.2 ⁇ m or less. It shall be.
- the arithmetic mean roughness Ra is a surface texture parameter defined in JIS B 0601: 2013. By determining the wetting angle with respect to water on a surface having Ra of 0.2 ⁇ m or less, the water repellency of the glass itself can be evaluated more accurately.
- the glass can also have excellent water resistance in one form.
- the water resistance can be evaluated by the water resistance Dw obtained based on the Japan Optical Glass Industry Association standard JOBIS-06: 2019 "Measuring method of chemical durability of optical glass (powder method)".
- the water resistance Dw thus obtained can be grade 1.
- the glass may also have excellent mechanical strength in one form.
- the mechanical strength can be evaluated by, for example, hardness, and as an example, Knoop hardness can be evaluated.
- the hardness of the glass can be, for example, 450 or more, and is 500 or more, as the Knoop hardness required based on the Japan Optical Glass Industry Association standard JOBIS-09: 2019 "Method for measuring Knoop hardness of optical glass". It is preferably 550 or more, and more preferably 550 or more.
- the Knoop hardness of the glass can be, for example, 850 or less, 800 or less, or 750 or less, but may exceed the values exemplified here. Glass with a large Knoop hardness value is preferable because it is less likely to be scratched.
- the glass transition temperature Tg As an index of heat resistance, the glass transition temperature Tg can be mentioned.
- the glass transition temperature Tg is obtained as follows. In the differential scanning calorimetry, when the temperature of the glass sample is raised, the endothermic behavior accompanying the change in specific heat, that is, the endothermic peak appears, and when the temperature is further raised, the exothermic peak appears. In the differential scanning calorimetry, a differential scanning calorimetry curve (DSC curve) is obtained in which the horizontal axis is the temperature and the vertical axis is the amount corresponding to the exothermic endothermic of the sample. The intersection of the tangent line and the baseline at the point where the slope becomes maximum when the endothermic peak appears from the baseline on this curve is defined as the glass transition temperature Tg.
- DSC curve differential scanning calorimetry curve
- the glass transition temperature Tg can be measured by using a sample obtained by sufficiently pulverizing glass in a mortar or the like and using a differential scanning calorimeter at a heating rate of 10 ° C./min.
- the glass transition temperature Tg of soda lime glass is about 540 ° C.
- the glass can be a glass having a glass transition temperature exceeding 550 ° C.
- the coefficient of linear expansion ⁇ is determined by using a thermomechanical analyzer (TMA; Thermomechanical Analysis) in accordance with JOBIS-16: 2019 “Method for measuring the coefficient of linear expansion of optical glass near room temperature”. It is a value measured by. Glass having an average linear expansion coefficient ⁇ of 70 ⁇ 10 -7 / ° C.
- the glass can be a glass having an average coefficient of linear expansion ⁇ of 70 ⁇ 10 -7 / ° C. or less at ⁇ 30 to 70 ° C.
- the water repellency, chemical properties, mechanical properties, thermal properties, etc. can be appropriately adjusted to obtain desirable physical properties according to the application.
- the glass can also have excellent alkali resistance in one form.
- alkali resistance the mass corresponding to 1 cubic centimeter of optical glass is used by using the sample preparation method described in the Japan Optical Glass Industry Association Standard JOGIS-06: 2019 “Measuring Method of Chemical Durability of Optical Glass (Powder Method)”. Mass reduction rate when powdered glass (particle size 425 to 600 ⁇ m) was placed in a platinum basket and immersed in a NaOH aqueous solution having a concentration of 0.01 mol / l at a liquid temperature of 50 ° C. for 15 hours (hereinafter, “in the powder method alkali resistance test”. It can be described as "mass reduction rate").
- the mass reduction rate of the glass in the powder method alkali resistance test is preferably 0.10% by mass or less, and more preferably less than 0.02% by mass.
- the content of the glass component such as P 2 O 5 is set to the range described above, it becomes easy to obtain a glass having excellent alkali resistance.
- One aspect of the present invention relates to an article containing glass.
- the above items consist of a group consisting of window materials, windshields, cover glass, mirrors, tableware, physics and chemistry laboratory equipment, cooking utensils, washbasins, toilet bowls, tombstones, trinkets, crafts and arts, glass fibers and glass fiber moldings.
- the article of choice the glass having the above glass composition and having a wetness angle to water of 60 ° or more.
- the glass composition and physical properties of the glass contained in the above article are as described above for the glass according to one aspect of the present invention.
- the above items consist of a group consisting of window materials, windshields, cover glass, mirrors, tableware, physics and chemistry laboratory equipment, cooking utensils, washstands, toilet bowls, tombstones, trinkets, crafts and arts, glass fibers and glass fiber moldings.
- the above items include window glass for buildings such as residences, showcase glass, window materials such as show window glass, car front glass, bus front glass, truck front glass, and various worker front glasses.
- the article can be an article having a surface layer containing the glass.
- glass having excellent water repellency is most effective in the windshield of an automobile. Due to its high water repellency and sliding property, raindrops can be easily removed even at low speeds. In addition, the slip of the wiper is improved, and the durability of the wiper itself can be improved as well as the quiet operation.
- glass having excellent water repellency for the windshield of automobiles and the like it is possible to obtain a comfortable field of view stably for a long period of time. These effects are also effective for side windows, rear windows, side mirrors, window materials for monitor cameras, and the like.
- Water deposit is considered to be a phenomenon that occurs due to the bond between the Si component contained in tap water and the like and the SiO 2 glass.
- the glass can be a glass with low reactivity with Si, which can suppress the generation of water deposits.
- the total weight tends to be larger, but it is possible to obtain the merit of improving sound insulation.
- glass with excellent water repellency can also be used as a window material for trains and airplanes.
- Glass having excellent water repellency is also preferable as a window material for general buildings because it is effective in improving visibility in rainy weather and can reduce residual dirt, so that it can be used for a long period of time. Further, by reducing the content of the coloring component exemplified above, it is possible to obtain a glass having high transmittance and less coloring.
- glass having excellent water repellency and water resistance is suitable for a mirror for a bathroom. It is possible to maintain excellent performance even for long-term use, as well as features that water droplets do not easily remain and are easy to see. It is also effective for a visual mirror installed outdoors.
- it is preferable content of B 2 O 3 is 25% or less.
- the above glass is melted, dip-coated, and subjected to predetermined slow cooling, it is possible to impart water repellency to the surface of various shapes of glass. can.
- the glass can be a glass having a small surface free energy, the strength of adhesion to various substances can be reduced.
- the surface with a small amount of free energy is soft to the touch, and fingerprint marks are less likely to adhere to it, making it easier to remove the adhered surface. Taking advantage of this characteristic, it can be used as a window material of a fingerprint authentication device, a cover glass of a smartphone, and the like.
- the glass can also be used for tableware and containers, and since it is easy to drain water after washing, it is also effective in reducing the cleaning load and improving hygiene.
- the glass can be a glass having a relatively high refractive index and a large light dispersion. When such a glass is used as a glass, a glittering glow of light can be obtained. In addition, glass with high hardness is not easily scratched, so that long-term transparency can be obtained. Glass having a relatively large specific density is advantageous for providing an article having a high profound feeling. With glass having a small thermal expansion, it is possible to reduce the occurrence of cracks due to a sudden temperature change.
- a glass having a total content of ZnO and La 2 O 3 (ZnO + La 2 O 3 ) of 60% or more tends to have a smaller thermal conductivity and specific heat than soda lime glass.
- Glass with low thermal conductivity and specific heat has a high heat-retaining effect and has the effect of reducing the temperature difference at the moment of touch.
- the high water repellency of the surface is effective in reducing water dripping when pouring hot water.
- the high water repellency of the surface of cups and coffee cups can reduce dripping.
- the surface of the glass can be surface-processed according to the application.
- water wetting can be further suppressed by imparting fine irregularities to the surface by pickpocketing the surface with # 60, # 320, # 800, # 1200 abrasive grains, or softening press processing. It can also provide an excellent water-repellent effect as a window glass material.
- the glass can be crystal-containing glass.
- Crystal-containing glass is also generally called crystallized glass, and is less likely to break because the progress of scratches tends to be suppressed as compared with amorphous glass materials.
- By adjusting the glass composition and the crystallization conditions it is possible to obtain crystal-containing glass having desired water repellency, mechanical strength, and chemical durability, which is useful as a building material such as an outer wall, a floor material, and a roofing material. Further, by using the crystal-containing glass for a toilet bowl, a wash basin, or the like, the effect of reducing the adhesion of dirt and water stain can be obtained.
- the above glass can have a higher refractive index than soda lime glass.
- Glass having a high refractive index has a feature of large reflection of light.
- Such glass is preferred as a decorative glass for outdoor decoration.
- the above glass can be appropriately colored and its durability can be improved by adjusting the composition.
- a tombstone By using such glass as a tombstone, it is difficult for dirt to adhere, it is easy to clean, and it can be kept clean for a long period of time. You can get possible tombstones.
- a water-repellent coat can be formed by using the above glass as a powder material and applying it to various materials. Further, such a coat can contribute to the improvement of heat resistance as well as the improvement of water repellency.
- Enamel using crystallized glass powder as a glaze can suppress the adhesion of scorching.
- the glass having excellent water repellency it is possible to suppress the ingress of water in a narrow gap due to the capillary phenomenon, and by adjusting the glass composition according to the target liquid, the surface tension, the wetting of the liquid, and the ingress are adjusted. It is also possible to do.
- the memory may become difficult to see due to the wetting of the meniscus, which may make accurate measurement difficult. Accurate weighing becomes possible.
- the wetting angle with respect to water is about 90 ° (for example, about 85 ° to 95 °). This point also applies to measuring utensils for cooking, which is a kind of cooking utensils.
- the above-mentioned glass is fiber-molded and the resulting fiber can be used for various molded bodies for ships, glass wool heat insulating material with high heat insulating property, etc. due to its property of being hard to get wet with water.
- Glass fiber made of glass with excellent alkali resistance is useful as glass fiber for cement strengthening. Further, since glass having excellent alkali resistance has high resistance to alkaline soapy water, it is also useful as a material for a glass bathroom mirror, a material for a surface layer of a bathroom mirror, and the like.
- Example 1 the raw materials for introducing each component were weighed so as to have the glass composition shown in Table 1 (Table 1-1 to Table 1-4) below, respectively. It was mixed well and used as a compounding raw material. As a raw material, oxides of each component were used except for Example 8. In Example 8, ZnO and ZnF 2 were used as Zn raw materials, and oxides of each component were used as other raw materials.
- a glass plate was prepared by melting 100 to 200 g of the compounding raw material at 1200 ° C. to 1400 ° C. using a platinum crucible, pouring it into an iron mold, and annealing after molding.
- the glass constituting the glass plates of Examples 12, 22, 26, and 28 is crystal-containing glass, and the glass constituting the glass plates of other examples is amorphous glass.
- the wetting angle with respect to water, water resistance Dw, and Knoop hardness Hk were determined by the methods described above.
- the mass reduction rate in the powder method alkali resistance test was determined by the method described above.
- the glass plate of Example 10 when the sliding property of water was measured in a measurement environment with an ambient temperature of 20 ° C. ⁇ 5 ° C. and a relative humidity of 50% ⁇ 20%, the inclination angle (sliding) of the surface of the glass plate with respect to the horizontal plane was measured. Water angle) It was confirmed that 0.1 ml of water droplets slipped down at 40 °.
- the unit of content is mass%. Further, “A” is the total content of SiO 2 , B 2 O 3 and P 2 O 5 (SiO 2 + B 2 O 3 + P 2 O 5 ), and “B” is ZnO, MgO, CaO, SrO, BaO.
- C is Li 2 O, Na 2 O, K 2
- the total content of O, Rb 2 O and Cs 2 O Li 2 O, Na 2 O, K 2
- the total content of O, Rb 2 O and Cs 2 O Li 2 O + Na 2 O + K 2 O + Rb 2 O + Cs 2 O
- the wetting angle of stainless steel SUS304 with respect to water was similarly determined and found to be 68 °.
- Reference Example 2 for PTFE (polytetrafluoroethylene), which is generally recognized as a water-repellent material, the wetting angle with respect to water was similarly determined and found to be 90 °.
- the glass of the example has excellent water repellency as well as a material known as a material having high water repellency.
- Comparative Example 1 is an optical glass (BSC7 equivalent material manufactured by HOYA Corporation) in which SiO 2 exceeds 60%.
- the SiO 2 content is high, and the total content of Li 2 O, Na 2 O, K 2 O, Rb 2 O and Cs 2 O (Li 2 O + Na 2 O + K 2 O + Rb 2 O + Cs 2 O) is also high.
- the P 2 O 5 content is high, and the total content of SiO 2 , B 2 O 3 and P 2 O 5 (SiO 2 + B 2 O 3 + P 2 O 5 ) is also high.
- Comparative Example 6 is a commercially available soda lime glass having a SiO 2 content of more than 70% and a total content of Li 2 O, Na 2 O, K 2 O, Rb 2 O and Cs 2 O (Li 2 O + Na 2). O + K 2 O + Rb 2 O + Cs 2 O) is also common.
- the wetting angle with respect to water was less than 60 °, and the water repellency was inferior to that of the glass of Example.
- a glass plate having a thickness of 2.5 mm was prepared by the above method, and the spectral transmittance of the prepared glass was measured to obtain a spectral transmittance curve (FIGS. 1 to 8). From the spectral transmittance curves shown in FIGS. 1 to 8, it was possible to change the absorption characteristics of the glass in various ways by adding components useful for color adjustment, that is, the color of the glass can be easily adjusted. It can be confirmed that it was there.
- the SiO 2 content is 0 to 25%
- the B 2 O 3 content is 0 to 35%
- the P 2 O 5 content is 0 to 30 on a mass basis.
- SiO 2 , B 2 O 3 and P 2 O 5 total content (SiO 2 + B 2 O 3 + P 2 O 5 ) is 10 to 45%
- Al 2 O 3 content is 0 to 15%
- Li 2 O content is 0 to 2%
- Na 2 O content is 0 to 10%
- K 2 O content is 0 to 10%
- Rb 2 O content is 0 to 5%
- Cs 2 O content is 0 to 0.
- Li 2 O, Na 2 O, K 2 O, Rb 2 O and Cs 2 O total content Li 2 O + Na 2 O + K 2 O + Rb 2 O + Cs 2 O
- MgO content 0 ⁇ 20% CaO content of 0 to 25%, SrO content 0 to 25%, BaO content 0 to 30%, ZnO content of 0 ⁇ 60%
- La 2 O 3 content of 0 to 50 % La 2 O 3 content of 0 to 50 %
- Y 2 O 3 content 0 to 15% Gd 2 O 3 content 0 to 25%, Yb 2 O 3 content 0 to 10%, CeO 2 content 0 to 10%, ZrO 2
- the content is 0 to 15%, the TiO 2 content is 0 to 20%, the SnO 2 content is 0 to 10%, the Nb 2 O 5 content is 0 to 30%, and the Ta 2 O 5 content is 0 to 15.
- WO 3 content 0 to 15%, Bi 2 O 3 content 0 to 20%, Ga 2 O 3 content 0 to 5%, In 2 O 3 content 0 to 5%, GeO 2 Content is 0 to 5%, Sb 2 O 3 content is 0 to 1%, ZnO, MgO, CaO, SrO, BaO, Y 2 O 3 , La 2 O 3 , Gd 2 O 3 , ZrO 2 , TiO 2 , Al 2 O 3, Nb 2 O 5, Ta 2 O 5, WO 3 and the total content of Bi 2 O 3 (ZnO + MgO + CaO + SrO + BaO + Y 2 O 3 + La 2 O 3 + Gd 2 O 3 + ZrO 2 + TiO 2 + Al 2 O 3 + Nb 2 O 5 + Ta 2 O 5 + WO 3 + Bi 2 O 3 ) is 55 to 90%, Fe 2 O 3 content is 0 to 10%, V 2 O 5 , Cr 2 O 3 , MnO 2 , Co 2 O 3 , NiO
- the above glass can show excellent water repellency.
- an article comprising glass consist of a group consisting of window materials, windshields, cover glasses, mirrors, tableware, physics and chemistry experimental equipment, cooking utensils, washstands, toilet bowls, tombstones, trinkets, crafts and arts, glass fibers, and glass fiber moldings.
- the article to be selected, the above glass has a SiO 2 content of 0 to 25%, a B 2 O 3 content of 0 to 35%, and a P 2 O 5 content on a mass basis in an oxide-based glass composition.
- the amount is 0 to 30%, the total content of SiO 2 , B 2 O 3 and P 2 O 5 (SiO 2 + B 2 O 3 + P 2 O 5 ) is 10 to 45%, and the Al 2 O 3 content is 0 to 0. 15%, Li 2 O content 0 to 2%, Na 2 O content 0 to 10%, K 2 O content 0 to 10%, Rb 2 O content 0 to 5%, Cs 2 O The content is 0 to 5%, and the total content of Li 2 O, Na 2 O, K 2 O, Rb 2 O and Cs 2 O (Li 2 O + Na 2 O + K 2 O + Rb 2 O + Cs 2 O) is 0 to 15%.
- MgO content 0 to 20%, CaO content 0 to 25%, SrO content 0 to 25%, BaO content 0 to 30%, ZnO content 0 to 60%, La 2 O 3 content
- the amount is 0 to 50%, the Y 2 O 3 content is 0 to 15%, the Gd 2 O 3 content is 0 to 25%, the Yb 2 O 3 content is 0 to 10%, and the CeO 2 content is 0 to 0.
- the amount is 0 to 15%, the WO 3 content is 0 to 15%, the Bi 2 O 3 content is 0 to 20%, the Ga 2 O 3 content is 0 to 5%, and the In 2 O 3 content is 0 to 0.
- the glass and the glass contained in the article may have the following forms.
- the total content of SiO 2 , B 2 O 3 and P 2 O 5 (SiO 2 + B 2 O 3 + P 2 O 5 ) is 10-35%, ZnO, MgO, CaO, SrO, BaO, Y 2 Total content of O 3 , La 2 O 3 , Gd 2 O 3 , ZrO 2 , TiO 2 , Al 2 O 3 , Nb 2 O 5 , Ta 2 O 5 , WO 3 and Bi 2 O 3 (ZnO + MgO + CaO + SrO + BaO + Y 2 O 3) + La 2 O 3 + Gd 2 O 3 + ZrO 2 + TiO 2 + Al 2 O 3 + Nb 2 O 5 + Ta 2 O 5 + WO 3 + Bi 2 O 3 ) can be 55 to 85%.
- the SiO 2 content is 1 to 8%
- the B 2 O 3 content is 8 to 25%
- the ZnO content is 3 to 35%
- the La 2 O 3 content is 15 to 45%
- the ZrO 2 content is contained.
- the amount is 1 to 8%
- the total content of SiO 2 , B 2 O 3 and P 2 O 5 (SiO 2 + B 2 O 3 + P 2 O 5 ) is 15 to 30%, ZnO, MgO, CaO, SrO, BaO.
- the wetting angle of the glass with water can be 80 ° or more.
- the water resistance Dw of the glass can be grade 1.
- the Knoop hardness Hk of the glass can be 450 or more or 550 or more.
- the mass reduction rate of the glass in the powder method alkali resistance test can be 0.10 mass% or less.
- the embodiments disclosed this time are exemplary in all respects and not restrictive.
- the scope of the present invention is shown by the claims rather than the above description, and it is intended to include all modifications within the meaning and scope equivalent to the claims.
- the glass according to one aspect of the present invention can be obtained by adjusting the composition described in the specification with respect to the above-exemplified glass composition.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Glass Compositions (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US17/915,688 US20230139239A1 (en) | 2020-03-31 | 2021-03-25 | Glass and article including glass |
| JP2022512060A JPWO2021200531A1 (enExample) | 2020-03-31 | 2021-03-25 | |
| CN202180023257.7A CN115335338A (zh) | 2020-03-31 | 2021-03-25 | 玻璃及包含玻璃的物品 |
| CN202510131101.0A CN119822632A (zh) | 2020-03-31 | 2021-03-25 | 玻璃及包含玻璃的物品 |
| EP21782335.0A EP4129941A4 (en) | 2020-03-31 | 2021-03-25 | GLASS AND ARTICLE CONTAINING GLASS |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020064876 | 2020-03-31 | ||
| JP2020-064876 | 2020-03-31 | ||
| JP2020-178263 | 2020-10-23 | ||
| JP2020178263 | 2020-10-23 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021200531A1 true WO2021200531A1 (ja) | 2021-10-07 |
Family
ID=77927456
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/012476 Ceased WO2021200531A1 (ja) | 2020-03-31 | 2021-03-25 | ガラスおよびガラスを含む物品 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20230139239A1 (enExample) |
| EP (1) | EP4129941A4 (enExample) |
| JP (1) | JPWO2021200531A1 (enExample) |
| CN (2) | CN115335338A (enExample) |
| TW (1) | TW202142508A (enExample) |
| WO (1) | WO2021200531A1 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4398516A4 (en) * | 2021-12-27 | 2025-01-15 | Samsung Electronics Co., Ltd. | Split tunneling method and device |
| TW202335991A (zh) * | 2021-12-28 | 2023-09-16 | 日商Hoya股份有限公司 | 玻璃、玻璃物品、光學玻璃、玻璃元件 |
| EP4450467B1 (en) * | 2023-02-28 | 2025-12-10 | Nitto Boseki Co., Ltd. | Glass composition for glass fibers, glass fibers, woven glass fiber fabric, and glass-fiber-reinforced resin composition |
| CN116395959B (zh) * | 2023-03-17 | 2024-10-22 | 鲁米星特种玻璃科技股份有限公司 | 一种铝酸盐玻璃及其制备方法和应用 |
| CN117658456B (zh) * | 2023-11-02 | 2025-07-18 | 中国建筑材料科学研究总院有限公司 | 稀土离子掺杂硼硅酸盐闪烁体玻璃及其制备方法和应用 |
| CN117941679B (zh) * | 2024-03-26 | 2024-05-31 | 安徽正合雅聚新材料科技有限公司 | 一种载银玻璃抗菌剂及其透明抗菌树脂产品和制品 |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0230640A (ja) * | 1988-07-19 | 1990-02-01 | Nippon Electric Glass Co Ltd | ファイバープレート用芯ガラス |
| JPH10226533A (ja) * | 1997-02-10 | 1998-08-25 | Nikon Corp | 放射線遮蔽ガラス |
| WO2007043280A1 (ja) * | 2005-10-13 | 2007-04-19 | Ohara Inc. | 放射線遮蔽ガラス |
| JP2008500935A (ja) * | 2004-05-29 | 2008-01-17 | ショット アクチエンゲゼルシャフト | ナノガラス粉末、特に平均の粒子径が1μm以下の多成分ガラス粉末、並びにその使用 |
| JP2011178634A (ja) * | 2010-03-03 | 2011-09-15 | Okuno Chemical Industries Co Ltd | 耐酸性を有する無鉛低融点ガラス |
| JP2012221591A (ja) * | 2011-04-04 | 2012-11-12 | Ohara Inc | 発光素子及び発光素子用基板材料 |
| JP2013203627A (ja) * | 2012-03-29 | 2013-10-07 | Asahi Glass Co Ltd | ガラス組成物 |
| JP2017001327A (ja) | 2015-06-12 | 2017-01-05 | Jxエネルギー株式会社 | 撥水部材 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2545642B2 (ja) * | 1990-09-26 | 1996-10-23 | 松下電器産業株式会社 | ガラス |
| US5122484A (en) * | 1991-05-23 | 1992-06-16 | Corning Incorporated | Zinc phosphate low temperature glasses |
| JP3750984B2 (ja) * | 2000-05-31 | 2006-03-01 | Hoya株式会社 | 光学ガラスおよび光学製品の製造方法 |
| JP4017832B2 (ja) * | 2001-03-27 | 2007-12-05 | Hoya株式会社 | 光学ガラスおよび光学部品 |
| JP3943348B2 (ja) * | 2001-06-06 | 2007-07-11 | 株式会社オハラ | 光学ガラス |
| US7049251B2 (en) * | 2003-01-21 | 2006-05-23 | Saint-Gobain Technical Fabrics Canada Ltd | Facing material with controlled porosity for construction boards |
| CN110835227A (zh) * | 2018-08-17 | 2020-02-25 | 成都光明光电股份有限公司 | 光学玻璃、由其制备而成的玻璃预制件或光学元件及光学仪器 |
-
2021
- 2021-03-25 CN CN202180023257.7A patent/CN115335338A/zh active Pending
- 2021-03-25 JP JP2022512060A patent/JPWO2021200531A1/ja active Pending
- 2021-03-25 EP EP21782335.0A patent/EP4129941A4/en active Pending
- 2021-03-25 CN CN202510131101.0A patent/CN119822632A/zh active Pending
- 2021-03-25 US US17/915,688 patent/US20230139239A1/en active Pending
- 2021-03-25 WO PCT/JP2021/012476 patent/WO2021200531A1/ja not_active Ceased
- 2021-03-26 TW TW110111053A patent/TW202142508A/zh unknown
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0230640A (ja) * | 1988-07-19 | 1990-02-01 | Nippon Electric Glass Co Ltd | ファイバープレート用芯ガラス |
| JPH10226533A (ja) * | 1997-02-10 | 1998-08-25 | Nikon Corp | 放射線遮蔽ガラス |
| JP2008500935A (ja) * | 2004-05-29 | 2008-01-17 | ショット アクチエンゲゼルシャフト | ナノガラス粉末、特に平均の粒子径が1μm以下の多成分ガラス粉末、並びにその使用 |
| WO2007043280A1 (ja) * | 2005-10-13 | 2007-04-19 | Ohara Inc. | 放射線遮蔽ガラス |
| JP2011178634A (ja) * | 2010-03-03 | 2011-09-15 | Okuno Chemical Industries Co Ltd | 耐酸性を有する無鉛低融点ガラス |
| JP2012221591A (ja) * | 2011-04-04 | 2012-11-12 | Ohara Inc | 発光素子及び発光素子用基板材料 |
| JP2013203627A (ja) * | 2012-03-29 | 2013-10-07 | Asahi Glass Co Ltd | ガラス組成物 |
| JP2017001327A (ja) | 2015-06-12 | 2017-01-05 | Jxエネルギー株式会社 | 撥水部材 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP4129941A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN115335338A (zh) | 2022-11-11 |
| CN119822632A (zh) | 2025-04-15 |
| EP4129941A1 (en) | 2023-02-08 |
| US20230139239A1 (en) | 2023-05-04 |
| TW202142508A (zh) | 2021-11-16 |
| EP4129941A4 (en) | 2024-04-24 |
| JPWO2021200531A1 (enExample) | 2021-10-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2021200531A1 (ja) | ガラスおよびガラスを含む物品 | |
| JP7433149B2 (ja) | 金属酸化物濃度勾配を有するガラスおよびガラスセラミック | |
| US12024461B2 (en) | Ion exchangeable, opaque gahnite-spinel glass ceramics with high hardness and modulus | |
| JP7336440B2 (ja) | 高い硬度および弾性率を有するイオン交換可能な透明ガーナイト-スピネル・ガラスセラミックス | |
| US10934209B2 (en) | Glass-based articles having improved fracture performance | |
| JP7084880B2 (ja) | 透明な近赤外線遮蔽ガラスセラミック | |
| US20200407268A1 (en) | Glass-ceramic and methods of making the same | |
| EA037679B1 (ru) | Лист стекла, приближающийся по своим характеристикам к нейтральному независимо от толщины | |
| EP1154961B1 (en) | COLOURLESS INORGANIC GLASSES WITH A SHARP OPTICAL ABSORPTION CUTOFF BETWEEN 370 AND 425 nm; PRODUCTS FROM SAID GLASSES | |
| JP2019522623A (ja) | 多色感光性ガラス系部品および製造方法 | |
| TWI705048B (zh) | 在一定深度處具有高應力數值的玻璃基製品 | |
| JP2025183320A (ja) | ガラスを含む物品の製造方法 | |
| JP2024052506A (ja) | 化学強化ガラス及びその製造方法 | |
| JP7729345B2 (ja) | 化学強化ガラス | |
| US11358897B2 (en) | Black b-spodumene glass ceramics with an optimized color package | |
| CZ2009446A3 (cs) | Rubínové sklo barvené zlatem | |
| RU2820480C1 (ru) | Микрокристаллическое стекло, изделие из микрокристаллического стекла и способ их изготовления | |
| Aparisi et al. | Obtaining smooth, white floor tile glazes from zirconium-free frits | |
| JPH06263475A (ja) | 鉛含有ガラス及びこのガラスを用いたガラス容器 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21782335 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2022512060 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2021782335 Country of ref document: EP Effective date: 20221031 |