WO2018143221A1 - 嵩密度の高い次亜塩素酸ナトリウム5水和物結晶粒子とその製造方法 - Google Patents
嵩密度の高い次亜塩素酸ナトリウム5水和物結晶粒子とその製造方法 Download PDFInfo
- Publication number
- WO2018143221A1 WO2018143221A1 PCT/JP2018/003044 JP2018003044W WO2018143221A1 WO 2018143221 A1 WO2018143221 A1 WO 2018143221A1 JP 2018003044 W JP2018003044 W JP 2018003044W WO 2018143221 A1 WO2018143221 A1 WO 2018143221A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sodium hypochlorite
- hypochlorite pentahydrate
- sodium
- crystal particles
- aqueous solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B11/00—Oxides or oxyacids of halogens; Salts thereof
- C01B11/04—Hypochlorous acid
- C01B11/06—Hypochlorites
- C01B11/062—Hypochlorites of alkali metals
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D9/00—Crystallisation
- B01D9/0004—Crystallisation cooling by heat exchange
- B01D9/0013—Crystallisation cooling by heat exchange by indirect heat exchange
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D9/00—Crystallisation
- B01D9/02—Crystallisation from solutions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D9/00—Crystallisation
- B01D9/0036—Crystallisation on to a bed of product crystals; Seeding
Definitions
- the present invention relates to sodium hypochlorite pentahydrate crystal particles and a method for producing the same.
- Sodium hypochlorite is generally marketed as an aqueous solution having a mass concentration of about 5 to 13% by mass (effective chlorine concentration of 5 to 12%), and has excellent sterilization and bleaching effects. It is used as a bactericidal agent for food, hot spring facilities, pools, food production, household use, etc., as a bleaching agent for food production, paper industry and textile industry.
- slime cleaning that prevents slime failures that occur in plant cooling circulating water systems, circulating water, wastewater treatment, etc. in various factories (slimation caused by algae, bacteria, etc., resulting in reduced thermal efficiency, obstruction of water piping, etc.) Used in drug applications.
- the bactericidal and bleaching effects of these sodium hypochlorites are attributed to their oxidizing power, and this oxidizing power is widely used not only in the field of organic synthesis but also in the production of food additives, electronic materials, pharmaceuticals and agricultural chemicals. ing.
- sodium hypochlorite is widely used in a wide variety of applications, but when performing an oxidation reaction in the field of organic synthesis on an industrial scale, a general sodium hypochlorite aqueous solution has low volumetric efficiency, There are drawbacks such as poor productivity and a lot of by-product wastewater. These problems seem to be solved by using a high-concentration sodium hypochlorite aqueous solution. However, since the sodium hypochlorite aqueous solution decomposes more rapidly as the concentration is higher at room temperature, it is 13 in Japan. It is not commercially available at a concentration of more than%.
- sodium hypochlorite crystals are also known, and it has been reported that monohydrate, 2.5 hydrate, pentahydrate, hexahydrate, etc. exist (“soda Handbook 1998, p361 "). Among them, sodium hypochlorite pentahydrate has a theoretical sodium hypochlorite mass concentration of 45%, which is about three times higher than that of sodium hypochlorite solution. Since there is a merit that the transportation volume can be greatly reduced and the transportation cost can be greatly reduced as compared with the aqueous sodium chlorite solution, a number of synthesis examples and application examples have been reported so far.
- Patent Document 1 discloses a method for industrially producing sodium hypochlorite pentahydrate
- Patent Document 2 discloses sodium chlorate contained in sodium hypochlorite pentahydrate.
- Patent Document 3 describes a method for producing a slurry solution of about 25 to 40% effective chlorine containing sodium hypochlorite pentahydrate.
- the crystal forms of the conventional sodium hypochlorite pentahydrate described in these documents are all described as needle-like (long needle-like) crystals having a large aspect ratio.
- sodium hypochlorite pentahydrate obtained in the example of Patent Document 1 also contains needle-like crystals having a high aspect ratio with an aspect ratio of 1.7 to 10.
- Patent Document 2 there is a description that a needle-like crystal having a major axis to minor axis ratio of 8 or more was obtained.
- sodium hypochlorite pentahydrate most frequently forms acicular crystals.
- a sodium hypochlorite composition made only from sodium hypochlorite pentahydrate crystals has a high density of needle crystals with high aspect ratio, which cannot be filled tightly into the container.
- transportation efficiency and storage efficiency are lowered.
- a crystal with a low aspect ratio can be created by adding a process such as grinding the crystal in the conventional manufacturing process. If it is too fined such as 100 ⁇ m or less, it is difficult to increase the bulk density.
- a pulverization process it is necessary to introduce a pulverizer or the like, which not only increases labor, but also may cause decomposition of sodium hypochlorite pentahydrate due to frictional heat during pulverization. Therefore, it is hard to say that it is a realistic plan.
- Patent Document 3 in order to solve the problem of bulk density, a problem is solved by preparing a sodium hypochlorite aqueous solution slurry containing sodium hypochlorite pentahydrate. It contains unnecessary sodium chloride, sodium chlorate and sodium hydroxide, and there is a problem that these components affect the reaction.
- JP 2000-290003 A JP 2014-169215 A Special table 2015-533775 gazette JP-A-11-255503
- An object of the present invention is to control the shape of crystal particles of sodium hypochlorite pentahydrate so that the bulk density to the container is high and the transport efficiency is high, and the sodium hypochlorite 5 water with high bulk density is high. It is to provide a hydrate crystal particle and a production method thereof.
- the present inventors have not increased the number of production steps of the conventional method for producing sodium hypochlorite pentahydrate, and crystals of sodium hypochlorite pentahydrate have been increased. It is possible to produce crystal particles of sodium hypochlorite pentahydrate having a low aspect ratio, a high bulk density, and a nearly spherical shape by pulverizing the precipitated crystals during crystallization.
- the present invention was completed.
- the gist of the present invention is as follows. (1) Sodium hypochlorite pentahydrate crystal particles with an average aspect ratio of 2.5 or less and an average minor axis of 0.1 mm to 1.5 mm. (2) The sodium hypochlorite pentahydrate crystal particles according to (1), wherein the bulk density is 0.80 g / cm 3 or more. (3) Sodium hypochlorite pentahydrate crystal particles having a bulk density of 0.80 g / cm 3 or more.
- the aqueous solution containing sodium hypochlorite pentahydrate recovered in the second step is cooled to a cooling temperature of 0 to 26 ° C.
- Method for producing sodium acid pentahydrate crystal particles. The production method according to (4), wherein the stirring in the third step is performed at a stirring blade tip speed of 2.1 to 7.5 m / sec. (6) The pump circulation in the third step is performed by circulating a liquid amount 0.5 to 4.0 times the amount of the aqueous solution in the crystallization tank in one hour (4 ) Manufacturing method.
- the pump circulation in the third step circulates 0.5 to 4.0 times the amount of the aqueous solution in the crystallization tank in 1 hour, and stirring is performed by stirring.
- the round sodium hypochlorite pentahydrate crystal particles having a low aspect ratio according to the present invention have a high bulk density, and the filling amount into a bag or a container, that is, the volumetric efficiency is remarkably improved as compared with a conventional product. .
- the transportation volume is reduced and the transportation efficiency and containers are reduced. it can.
- the digital microscope photograph image of the sodium hypochlorite pentahydrate crystal particle of this invention of Example 1 is shown.
- the digital microscope photograph image of the sodium hypochlorite pentahydrate crystal particle of this invention of Example 2 is shown.
- crystallization of the comparative example 1 is shown.
- crystallization of the comparative example 2 is shown.
- crystallization of the comparative example 3 is shown.
- the distribution of the aspect ratio of the sodium hypochlorite pentahydrate crystal particles of Example 1 is shown.
- the distribution of the aspect ratio of the sodium hypochlorite pentahydrate crystal particles of Example 2 is shown.
- the distribution of the aspect ratio of the sodium hypochlorite pentahydrate crystal of Comparative Example 1 is shown.
- the distribution of the aspect ratio of the sodium hypochlorite pentahydrate crystal of Comparative Example 2 is shown.
- the distribution of the aspect ratio of the sodium hypochlorite pentahydrate crystal of Comparative Example 3 is shown.
- crystallization is shown.
- the sodium hypochlorite pentahydrate crystal particles have an average aspect ratio of 2.5 or less and a nearly spherical shape, and the bulk density is 0.80 g / cm 3 or more.
- the object is to provide sodium hypochlorite pentahydrate crystal particles capable of enhancing volumetric efficiency during transportation and a method for producing the same.
- the round sodium hypochlorite pentahydrate crystal particles of the present invention have an average aspect ratio represented by the ratio of the major axis L to the minor axis S (L / S) of the digital microscope (stock) Manufactured by Tech Co., Ltd., Hide Micron 3)
- the range is 1 ⁇ L / S ⁇ 2.5. Since the bulk density tends to be higher as the aspect ratio is smaller, the aspect ratio is more preferably in the range of 1.0 ⁇ L / S ⁇ 2.3, and 1.0 ⁇ L / S ⁇ 2.0. More preferably, it is in the range.
- Such sodium hypochlorite pentahydrate crystal particles, which are the target substance of the present invention show that the bulk density is remarkably improved as compared with the conventional product when filled into a container as a product.
- the aspect ratio is determined as the ratio of the minor axis (S) to the major axis (L) of the crystal grains (hereinafter referred to as L / S ratio).
- the minor axis is a short side of a rectangle that minimizes the length of one side of the circumscribed rectangle of the target region of the observation image of the particle to be measured.
- the major axis is a long side of a rectangle having a maximum length of one side among the circumscribed rectangles of the target region. These can be measured using generally available image analysis software (eg, “Image-J”).
- the average aspect ratio value is calculated as the number average of the aspect ratio (L / S) of 300 or more particles measured by the above method.
- the average minor axis is calculated as the average number of S.
- crystal grains having a minor diameter of 0.01 mm or less that cannot be observed with a digital microscope are not taken into consideration in the content of the present invention.
- the round sodium hypochlorite pentahydrate crystal particles of the present invention are rounded by circulating and / or stirring in the crystallization tank during the crystallization of sodium hypochlorite pentahydrate.
- the shape is close to roundness. Thereby, the angle of repose of the powder is 60 degrees or less, which is in a range that does not cause a problem when the raw material is charged.
- the circulation by a powerful pump is too strong, the grown crystal particles will collapse, forming fine crystals of 0.1 mm or less and reducing the bulk density. It is preferable to form crystals having an average minor axis of 0.1 mm or more and 1.5 mm or less. More preferably, the size of the crystal grains is 0.3 mm to 0.7 mm in terms of average minor axis.
- the bulk density of the present invention was measured as heavy bulk density. Specifically, according to JIS R 9301-2-3: 1999 (alumina powder-Part 2: Physical property measurement method-3: Light bulk density and heavy bulk density), a sample is filled in a specified container by a specified method. After that, the cylinder containing the sample was dropped 100 times from a height of about 30 mm to compress the sample, and calculation was performed from the mass and volume of the compressed sample.
- the method for producing sodium hypochlorite pentahydrate crystal particles of the present invention includes a chlorination step (first step), a sodium chloride separation step (second step), a crystallization step (third step), And a solid-liquid separation step (fourth step).
- first step a chlorination step
- second step a sodium chloride separation step
- third step a crystallization step
- fourth step a solid-liquid separation step
- the present invention is not limited to the first to fourth steps, and other steps may be included.
- a step of partially recycling the separation liquid in the second step or the separation liquid obtained in the fourth step to the chlorination step may be included. It is also possible to add sodium hydroxide to increase the precipitation effect.
- each step will be described in detail.
- Chlorination process (1st process) In the chlorination step, a sodium hypochlorite aqueous solution is obtained by reacting an aqueous sodium hydroxide solution with chlorine gas.
- the reaction process of the present invention may be batch treatment or continuous treatment, but it is desirable to continuously supply sodium hydroxide and chlorine in order to coarsen the sodium chloride particles and increase the productivity.
- the reaction tank may be a single tank, but in order to prevent the formation of sodium chlorate due to local heat generation in contact with chlorine, a continuous tank reactor in which multiple tanks such as two tanks and three tanks are connected. Thus, it is desirable to divide and supply chlorine to the respective reaction tanks for reaction.
- chlorine gas may be supplied to the reaction as it is, local heat generation can be suppressed by supplying it diluted with nitrogen or air.
- the starting sodium hydroxide aqueous solution preferably has a concentration of 40 to 48% by mass, and the reaction temperature is not particularly limited, but is preferably 15 to 32 ° C. If it is this range, the production
- chlorination proceeds until the sodium hypochlorite concentration is 23 to 27 mass%, the sodium chloride concentration is 22 to 27 mass%, and the sodium hydroxide concentration is 1.0 to 2.0 mass%.
- the finished liquid is a slurry in which supersaturated sodium chloride is deposited.
- Sodium chloride separation step (second step)
- a solution obtained by solid-liquid separation of sodium chloride from the chlorination end solution is used as a mother liquor in the next crystallization step.
- the sodium hypochlorite aqueous solution produced by chlorination contains a large amount of by-produced sodium chloride crystals.
- solid-liquid separation is performed by a centrifuge or a filter.
- the purpose is to reduce the amount of heat removal in the crystallization tank of the next step by pre-cooling, and the filtrate temperature should be within the crystallization start temperature + 2 ° C., more preferably within the crystallization start temperature +0 to + 1 ° C. Is preferred.
- the precooling temperature is set to be equal to or lower than the crystallization start temperature, crystals are precipitated in the heat exchanger, and line freezing is likely to occur.
- the concentration of the sodium hypochlorite solution after separation of sodium chloride is preferably 24% by mass or more, more preferably 30% by mass or more and 34% by mass or less. In the case of 34% by mass or more, the sodium hypochlorite solution is supersaturated, so that it scales on the surface of the cooler and may deteriorate the heat transfer efficiency.
- Crystallization process (third process) In the crystallization step, crystallization is performed by introducing the sodium hypochlorite aqueous solution (mother solution) obtained in the second step into a crystallization apparatus.
- the crystallization tank is not particularly limited, but a tank-type crystallization apparatus is desirable, and it is further preferable to include a pump and a cooler for circulating the fluid in the crystallization tank. If necessary, soft water is added to the sodium hypochlorite separation liquid obtained in the previous step to adjust the concentration of sodium hypochlorite.
- the concentration of the sodium hypochlorite solution is preferably 28% or more, and more preferably 28% or more and 34% or less from the viewpoint of crystallization efficiency.
- Sodium dichlorite pentahydrate is obtained by cooling the diluted and adjusted separation liquid in the subsequent crystallization step.
- this operation is performed in a batch process, there is no problem even if a seed crystal is not added, but it is preferable to add a seed crystal in order to control the crystal shape.
- the cooling start temperature (the temperature of the mother liquor) is in the range of 10 to 26 ° C.
- the mother liquor is continuously added while cooling in the presence of seed crystals.
- the cooling start temperature for adding the seed crystal is more preferably in the range of 12 to 24 ° C., and further preferably 16 to 24 ° C.
- the crystallization temperature is not particularly limited, but it may be cooled at a constant temperature or at a constant cooling rate.
- cooling at a constant cooling rate it is preferably 1 to 4 ° C./hour. With this set speed, it is possible to prevent crystal scales adhering to the wall of the crystallization tank, and not only increase the stirring power due to the formation of fine crystals, but also prevent deterioration of liquid drainage during solid-liquid separation, as well as affect productivity.
- the mother liquor can be cooled without exerting any effect.
- the circulation flow rate at the pump is preferably such that a liquid amount of about 0.5 to 4.0 times the amount of the sodium hypochlorite aqueous solution in the crystallization tank is circulated in one hour. It is preferable to circulate a liquid amount of about 1.0 to 3.0 times.
- the tip speed of the stirring blade is preferably set to 2.1 to 7.5 m / sec.
- the stirring blade is gently stirred at a tip speed of 2.0 m / second or less, a crystal having a high aspect ratio is generated. In order to achieve the optimum balance, it is particularly preferable that the tip speed of the stirring blade is 3.0 to 7.0 m / sec.
- the above two agitation, pump circulation and agitation are performed simultaneously, and the circulation flow rate at the pump is about 0.5 to 4.0 times the sodium hypochlorite aqueous solution amount in the crystallization tank in 1 hour.
- Crystallization may be performed while stirring with the tip speed of the stirring blade set at 2.1 to 7.5 m / sec while circulating the liquid amount.
- the temperature finally reached in the crystallization step is preferably a temperature at which sodium hypochlorite pentahydrate precipitates but sodium chloride does not precipitate.
- the temperature finally reached in this step is preferably 0 to 16 ° C. If it is this temperature, a moderate slurry density
- the cooling end temperature is more preferably 2 to 14 ° C., and further preferably 4 to 12 ° C.
- the crystallization time is not particularly defined, but considering productivity, it is preferably within 12 hours, particularly preferably within 10 hours.
- Sodium hypochlorite pentahydrate crystal separation process (4th process)
- sodium hypochlorite pentahydrate crystals obtained in the crystallization step are separated using a solid-liquid separator such as a centrifuge, and sodium hypochlorite pentahydrate is obtained. Crystal grains are obtained. Crystallization of sodium hypochlorite pentahydrate crystallized from an aqueous solution containing sodium hypochlorite pentahydrate cooled in the crystallization process, using a continuous or batch centrifuge type, centrifugal effect Solid-liquid separation at 1000-3500G. If necessary, the crystal surface is washed with a solution containing water or an inorganic substance and subjected to surface treatment to obtain sodium hypochlorite pentahydrate crystal particles.
- Example 1 In the chlorination step (first step), a two-stage CSTR (continuous stirred tank reactor) reaction tank (capacity 3.5 m 3 ⁇ 2 tank) equipped with a stirrer, a scrubber and an external circulation type cooler was used. To this, a 48 mass% sodium hydroxide aqueous solution was added at 860 kg / hr as a raw material, and chlorine gas diluted to 1/2 concentration with air was added to the scrubber so that the residual sodium hydroxide concentration was 2 mass%. The solution was introduced while adjusting the supply amount, and chlorination was carried out while cooling to a reaction temperature of 24 to 30 ° C. At this time, the residence time in the reaction vessel was about 720 minutes.
- CSTR continuous stirred tank reactor
- the reactant slurry extracted from the reaction tank of the chlorination step at 1188 kg / hr was subjected to solid-liquid separation using a centrifuge.
- 934 kg of sodium hypochlorite aqueous solution (filtrate 1) consisting of 254 kg / hr of precipitated sodium chloride, sodium hypochlorite having a concentration of 35% by mass, and sodium chloride having a concentration of 5.4% by mass. / Hr was obtained.
- Soft water was added to the filtrate 1 to adjust the sodium hypochlorite concentration to 31.9 mass%, the sodium chloride concentration 4.9 mass%, and the sodium hydroxide concentration 1.5 mass%.
- the filtrate 1 is added to a titanium crystallization tank (capacity 7 m 3 ) equipped with a stirrer, jacket, coil cooler and external circulation pump while adjusting the temperature to 22 ° C. 8942 kg was charged, and at this time, the temperature difference ⁇ T between the temperature of the filtrate 1 and the refrigerant temperature was 3 while stirring at a tip speed of 4.5 m / sec with a stirrer at 1.5 times / hr using a spiral pump. Cooling was started so as to reach ⁇ 4 ° C., and cooling was performed over 4 hours until reaching 12 ° C. Crystal formation was observed from 19 ° C. in the crystallization tank.
- the separation step (fourth step) the slurry extracted from the crystallization tank in the crystallization step (third step) was solid-liquid separated at 1500 G with a centrifugal separator while maintaining the temperature of the crystallization tank at 14 ° C. As a result, 2850 kg of high purity sodium hypochlorite pentahydrate sodium hypochlorite pentahydrate crystals were obtained. The composition and properties of the obtained sodium hypochlorite pentahydrate crystal particles are shown in Table 1. The average aspect ratio was 1.65.
- Example 2 (Invention Example) The same operations as in the chlorination step (first step) and sodium chloride separation step (second step) in Example 1 were performed, and the sodium hypochlorite concentration was 32.2 mass% and the sodium chloride concentration was 5.1 mass. %, And a filtrate 2 having a sodium hydroxide concentration of 1.3% by mass was obtained.
- the temperature of the filtrate obtained is adjusted to 22 ° C. in a titanium crystallization tank (capacity 7 m 3 ) equipped with a stirrer, jacket, coil cooler and external circulation pump. Then, 8781 kg of the filtrate 2 was added, and at this time, the mixture was cooled so that the temperature difference ⁇ T between the temperature of the filtrate 2 and the refrigerant temperature became 3 to 4 ° C. while stirring at a tip speed of 7.5 m / sec using a stirrer. And cooled over 4 hours until reaching 12 ° C. Crystal formation was observed from 17 ° C. in the crystallization tank.
- the separation step (fourth step) the slurry extracted from the crystallization tank in the crystallization step (third step) was solid-liquid separated at 1500 G with a centrifugal separator while maintaining the temperature of the crystallization tank at 14 ° C. As a result, 2790 kg of high-purity sodium hypochlorite pentahydrate sodium hypochlorite pentahydrate crystals were obtained. The composition and properties of the obtained sodium hypochlorite pentahydrate crystal particles are shown in Table 1. The average aspect ratio was 2.16.
- Comparative Example 1 In order to confirm the effect of the circulation and stirring, a comparative example in which the circulation was not performed in the third step and the stirring speed was slow was examined.
- the sodium hypochlorite aqueous solution of Table 1 obtained by chlorination of sodium hydroxide in the first step and obtained through the second step was converted from 22 ° C. (cooling start temperature) to 240 ° C. Without circulating until reaching 12 ° C.
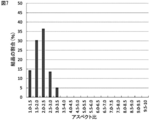
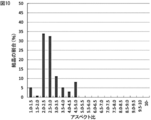
- the distribution of the aspect ratio of the obtained sodium hypochlorite pentahydrate crystal particles is shown in FIG. Crystals with an aspect ratio of 9.0 or more accounted for 60% of the total, and thin needles were obtained.
- Comparative Example 2 Aspect ratio and bulk density were measured using sodium hypochlorite pentahydrate solid type (product code 15591-65) manufactured by Nacalai Tesque. The composition and properties are shown in Table 1. The average aspect ratio was 4.29.
- FIG. 9 shows the aspect ratio distribution of the sodium hypochlorite pentahydrate crystal of Comparative Example 2. Thin needles distributed in an aspect ratio range of 2.5 to 6.0 were obtained.
- the sodium hypochlorite pentahydrate of the present invention is a round sodium hypochlorite pentahydrate crystal particle having a low aspect ratio compared to the conventional production method, Compared with the conventional product whose bulk density is remarkably improved, the transportation efficiency can be improved and the number of containers can be reduced by reducing the volume during transportation.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- Inorganic Chemistry (AREA)
- Crystallography & Structural Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Treatment Of Water By Oxidation Or Reduction (AREA)
- Detergent Compositions (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US16/481,725 US20190367360A1 (en) | 2017-02-02 | 2018-01-30 | Sodium hypochlorite pentahydrate crystal grains having high bulk density and method for producing same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017017784A JP6477739B2 (ja) | 2017-02-02 | 2017-02-02 | 嵩密度の高い次亜塩素酸ナトリウム5水和物結晶粒子とその製造方法 |
| JP2017-017784 | 2017-02-02 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018143221A1 true WO2018143221A1 (ja) | 2018-08-09 |
Family
ID=63040678
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/003044 Ceased WO2018143221A1 (ja) | 2017-02-02 | 2018-01-30 | 嵩密度の高い次亜塩素酸ナトリウム5水和物結晶粒子とその製造方法 |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US20190367360A1 (enExample) |
| JP (1) | JP6477739B2 (enExample) |
| WO (1) | WO2018143221A1 (enExample) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116199593A (zh) * | 2023-01-05 | 2023-06-02 | 浙江天瑞化学有限公司 | 一种dl-丙氨酸的结晶方法 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63294953A (ja) * | 1987-01-21 | 1988-12-01 | ノベル・チエミ・アーベー | 結晶質物質の製造方法 |
| JP2000290003A (ja) * | 1999-04-01 | 2000-10-17 | Nippon Light Metal Co Ltd | 次亜塩素酸ソーダ5水和物の製造法 |
| JP2014088276A (ja) * | 2012-10-30 | 2014-05-15 | Toagosei Co Ltd | 次亜塩素酸ソーダ水溶液の製造方法及び製造装置 |
| JP2014169215A (ja) * | 2013-02-06 | 2014-09-18 | Kaneka Corp | 次亜塩素酸ナトリウム5水和物結晶およびその製造方法 |
| JP2015124108A (ja) * | 2013-12-26 | 2015-07-06 | 昭和電工株式会社 | 高純度次亜塩素酸ナトリウム5水和物および次亜塩素酸ナトリウム水溶液の製造方法 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016201397A1 (en) * | 2015-06-10 | 2016-12-15 | Olin Corporation | Sodium hypochlorite compositions |
-
2017
- 2017-02-02 JP JP2017017784A patent/JP6477739B2/ja active Active
-
2018
- 2018-01-30 US US16/481,725 patent/US20190367360A1/en not_active Abandoned
- 2018-01-30 WO PCT/JP2018/003044 patent/WO2018143221A1/ja not_active Ceased
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63294953A (ja) * | 1987-01-21 | 1988-12-01 | ノベル・チエミ・アーベー | 結晶質物質の製造方法 |
| JP2000290003A (ja) * | 1999-04-01 | 2000-10-17 | Nippon Light Metal Co Ltd | 次亜塩素酸ソーダ5水和物の製造法 |
| JP2014088276A (ja) * | 2012-10-30 | 2014-05-15 | Toagosei Co Ltd | 次亜塩素酸ソーダ水溶液の製造方法及び製造装置 |
| JP2014169215A (ja) * | 2013-02-06 | 2014-09-18 | Kaneka Corp | 次亜塩素酸ナトリウム5水和物結晶およびその製造方法 |
| JP2015124108A (ja) * | 2013-12-26 | 2015-07-06 | 昭和電工株式会社 | 高純度次亜塩素酸ナトリウム5水和物および次亜塩素酸ナトリウム水溶液の製造方法 |
Non-Patent Citations (4)
| Title |
|---|
| ADAM, L. C.: "Hypochlorite Ion Decomposition: Effects of Temperature, Ionic Strength, and Chloride Ion", INORGANIC CHEMISTRY, vol. 38, 20 February 1999 (1999-02-20), pages 1299 - 1304, XP055530434 * |
| ASAWA, TOMOTAKE ET AL.: "The Placing of Crystalline Sodium Hypochlorite Pentahydrate on the Market", JOURNAL OF ION EXCHANGE, vol. 27, 21 September 2016 (2016-09-21), pages 42 - 46, XP055530440 * |
| OOSHIMA, HIROSHI: "Industrial Crystallization : Crystal Size Distribution and Crystal Polymorphs 'MechanismofNucleation", THE SOCIELY OF POWDER TECHNOLOGY, vol. 38, 2001, pages 251 - 259 * |
| TER HORST, J. H. ET AL.: "Fundamentals of Industrial Crystallization", HANDBOOK OF CRYSTAL GROWTH, 2015, pages 1317 - 1349 * |
Also Published As
| Publication number | Publication date |
|---|---|
| US20190367360A1 (en) | 2019-12-05 |
| JP2018123035A (ja) | 2018-08-09 |
| JP6477739B2 (ja) | 2019-03-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102602965B (zh) | 从含锂卤水中直接制备高纯度锂化合物的方法 | |
| JP2014530160A (ja) | 硫酸マグネシウム | |
| CN102203012B (zh) | 氯化钠生产方法 | |
| CN104837763A (zh) | 次氯酸钠组合物 | |
| US4747917A (en) | Scale-free process for purifying concentrated alkali metal halide brines containing sulfate ions as an impurity | |
| TWI635043B (zh) | 次氯酸鈉水溶液之製造方法 | |
| JP6477739B2 (ja) | 嵩密度の高い次亜塩素酸ナトリウム5水和物結晶粒子とその製造方法 | |
| US8951305B2 (en) | Method of producing naturally purified salt products | |
| WO2019012859A1 (ja) | 次亜塩素酸ナトリウム水溶液、及びこれを得るための次亜塩素酸ナトリウム5水和物結晶、並びに次亜塩素酸ナトリウム水溶液の製造方法 | |
| JP6443865B2 (ja) | 次亜塩素酸ナトリウム5水和物の結晶体およびその製造方法 | |
| US4390512A (en) | Process for producing calcium hypochlorite | |
| CA2573628C (en) | Manufacture of high-strength, low-salt hypochlorite bleach | |
| US20210024355A1 (en) | Solid bleach and processes for making solid bleach | |
| GB2104053A (en) | Production of nickel and cobalt sulphates and chlorides | |
| JP2985362B2 (ja) | 臭化マンガン水溶液の製造方法 | |
| JPS58135105A (ja) | 次亜塩素酸カルシウムの製造法 | |
| US20210024354A1 (en) | Solid bleach and processes for making solid bleach | |
| JP2952726B2 (ja) | 臭化マンガン水溶液の製造方法 | |
| JPS5820703A (ja) | 次亜塩素酸ソーダ水溶液の製造方法、及びその装置 | |
| JPS605005A (ja) | 次亜塩素酸カルシウムの製法 | |
| JPS5926901A (ja) | 次亜塩素酸カルシウムの製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18747610 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18747610 Country of ref document: EP Kind code of ref document: A1 |