WO2018066427A1 - Trhアナログ及びアルンド酸を組み合わせてなる組成物、並びにアルンド酸の薬学的に許容される塩 - Google Patents
Trhアナログ及びアルンド酸を組み合わせてなる組成物、並びにアルンド酸の薬学的に許容される塩 Download PDFInfo
- Publication number
- WO2018066427A1 WO2018066427A1 PCT/JP2017/034926 JP2017034926W WO2018066427A1 WO 2018066427 A1 WO2018066427 A1 WO 2018066427A1 JP 2017034926 W JP2017034926 W JP 2017034926W WO 2018066427 A1 WO2018066427 A1 WO 2018066427A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- component
- composition
- acid
- pharmaceutically acceptable
- synthesis example
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 CCCC(CCCCC*)[N+]([N+](C(CCCCC*)CCN*)([O-])O)([O-])O Chemical compound CCCC(CCCCC*)[N+]([N+](C(CCCCC*)CCN*)([O-])O)([O-])O 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/513—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim having oxo groups directly attached to the heterocyclic ring, e.g. cytosine
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/194—Carboxylic acids, e.g. valproic acid having two or more carboxyl groups, e.g. succinic, maleic or phthalic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/20—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
- A61P25/10—Antiepileptics; Anticonvulsants for petit-mal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C51/00—Preparation of carboxylic acids or their salts, halides or anhydrides
- C07C51/41—Preparation of salts of carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C53/00—Saturated compounds having only one carboxyl group bound to an acyclic carbon atom or hydrogen
- C07C53/126—Acids containing more than four carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C53/00—Saturated compounds having only one carboxyl group bound to an acyclic carbon atom or hydrogen
- C07C53/126—Acids containing more than four carbon atoms
- C07C53/128—Acids containing more than four carbon atoms the carboxylic group being bound to a carbon atom bound to at least two other carbon atoms, e.g. neo-acids
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2200/00—Function of food ingredients
- A23V2200/30—Foods, ingredients or supplements having a functional effect on health
- A23V2200/322—Foods, ingredients or supplements having a functional effect on health having an effect on the health of the nervous system or on mental function
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2250/00—Food ingredients
- A23V2250/30—Other Organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/13—Crystalline forms, e.g. polymorphs
Definitions
- the present invention is a composition comprising a combination of a TRH analog and arundoic acid, particularly selected from dementia, Parkinson's disease, amyotrophic lateral sclerosis, progressive supranuclear palsy, multiple system atrophy, and triplet repeat disease.
- the present invention relates to a composition used for preventing and / or treating neurodegenerative diseases or cerebral infarction, and a composition used for improving learning disorders.
- the present invention also relates to pharmaceutically acceptable salts of arundoic acid.
- dementia According to the website of the Ministry of Health, Labor and Welfare, dementia is said to be in a state where it is unable to carry out daily life and social life due to chronic decline and disappearance of various mental functions once normally developed after birth. According to the website, the number of people with dementia has been increasing year by year since 1995, and the total population of people with dementia in 2015 was approximately 26.22 million, and those aged 65 years or older The prevalence rate for the general population is 8.4%. Furthermore, in 2020, the prevalence of the elderly population over 65 years old is predicted to be 8.9%.
- Symptoms of dementia include cognitive dysfunctions such as central memory (disabilities in learning new information and recalling previously learned information), as well as behavioral abnormalities and mental symptoms (abuse / violence, ⁇ , missing, delusions, etc.) appear.
- TRH thyroid stimulating hormone-releasing hormone
- TA-0910 which is an analog of TRH
- TRH thyroid stimulating hormone-releasing hormone
- TA-0910 has a higher improvement effect than TRH, and once passively administered TA-0910 to amnestic rats induced by hypoxia treatment.
- TRH thyroid stimulating hormone-releasing hormone
- TA-0910 also improves memory read-out in active avoidance learning trials by administering to rats that have destroyed the area (BF) containing the bilateral pallidal ventral side, anonymity, and preoptic area.
- BF area
- tartilelin is used for the prevention and treatment of shock symptoms
- nerve cells are assisted by glial cells such as astrocytes and Schwann cells for functions such as nutritional supplementation, waste excretion, and ion balance maintenance.
- Glial cells play an important role in the control of brain function by maintaining the ability of neurons to metabolize glutamate and ⁇ -aminobutyric acid and the ability to produce neuropeptides and cytokines, in addition to maintaining the cell functions of neurons.
- 2-propyloctanoic acid (Arundoic acid) is known (Patent Document 2)
- Mild Cognitive Impairment is one of the mild cognitive impairments that are precursors of dementia such as Alzheimer's disease, and it is necessary to start treatment at this stage or at an earlier stage, for example, Alzheimer's disease It is said that donepezil hydrochloride, an anticholinesterase inhibitor used as a therapeutic agent, has a low effect of suppressing the progression of Alzheimer's disease.
- an object of the present invention is to provide a composition for preventing (including suppressing progression) and / or treating neurodegenerative diseases and cerebral infarction including dementia.
- Another object of the present invention is to provide a composition for suppressing and / or improving the progression of learning disorders associated with neurodegenerative diseases including dementia and ischemic brain diseases, particularly spatial cognitive impairment.
- composition I-1 At least one selected from a compound represented by the following general formula (I), a pharmaceutically acceptable salt thereof, and a hydrate thereof (hereinafter also referred to as “TRH analog” in this specification) And (B) a composition comprising a combination of at least one selected from arundoic acid, a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable solvate thereof: (R is a hydrogen atom or an alkyl group having 1 to 6 carbon atoms.)
- R is a hydrogen atom or an alkyl group having 1 to 6 carbon atoms.
- composition according to I-1 which has a form in which component (A) and component (B) are mixed and adjusted at the time of use.
- the form includes a kit including a preparation containing at least the component (A) and a preparation containing the component (B).
- I-4. The composition according to any one of I-1 to I-3, wherein the component (A) is a hydrate of the compound represented by the general formula (I).
- I-5. The composition according to any one of I-1 to I-4, wherein R is a methyl group.
- I-6. (B) The composition according to any one of I-1 to I-5, wherein the component is arundoic acid.
- I-7 The composition according to any one of I-1 to I-5, wherein the component is arundoic acid.
- I-8. X 2+ is Ca 2+, Mg 2+, Fe 2+ , Ba 2+, + + H 3 N-NH 3, + + H 2 N NH 2, or + HN ⁇ NH a + A composition according to I-7 .
- I-9. The composition according to any one of I-1 to I-8, which is a pharmaceutical composition. I-10.
- a composition according to 1. I-11. The composition according to any one of I-1 to I-8, and I-10, which is a food composition.
- I-12. 12 The composition according to any one of I-1 to 11, which is used for improving a learning disorder.
- I-13. The composition according to I-12, wherein the learning disorder is a spatial cognitive disorder.
- (X 2+ is a divalent cation).
- Production method. II-7 The production method according to II-5, wherein the solvent is at least one selected from the group consisting of methyl tert-butyl ether, acetonitrile, and dichloromethane.
- a composition comprising the compound according to any one of II-1 to II-4 and one selected from pharmaceutically acceptable solvates thereof.
- the composition according to II-8, wherein the composition is used in combination with a TRH analog, a pharmaceutically acceptable salt thereof, and a hydrate thereof.
- the composition according to II-8 or II-9, wherein the composition is a pharmaceutical composition.
- II-11. The composition according to any one of II-8 to II-10, which is used to improve the function of astrocytes or to change reactive astrocytes into astrocytes. II-12.
- any one of II-8 to II-10 used for the treatment and / or prevention of neurodegenerative diseases, neurological dysfunction after cerebrospinal trauma, brain tumor, cerebrospinal disease associated with infection, or multiple sclerosis The composition according to item. II-13.
- II-14. The composition according to II-12, wherein the cerebrospinal disease associated with the infection is meningitis, brain abscess, Crotzfeld-Jakob disease, or AIDS dementia.
- II-15 The composition according to II-12, wherein the brain tumor is astrocytoma. II-16.
- TRH analog and arundoic acid can improve spatial memory impairment.
- learning impairment can be improved with a dose lower than that when TRH analog or arundoic acid is administered alone, and nerve cells that have fallen due to ischemia are regenerated. can do.
- FIG. 4 is a view showing NMR results of (R) -arundic acid. It is a figure which shows the result of NMR of the calcium arundo acid synthesized by the calcium chloride method. It is a figure which shows the result of the thermal analysis of the calcium arundo acid synthesized by the calcium chloride method.
- the result of the XRPD analysis of the residual solid component obtained in Synthesis Examples 4 to 8 is shown.
- the result of the XRPD analysis of the solid component obtained from the supernatant liquid of the synthesis examples 4, 6, and 8 is shown.
- the result of the XRPD analysis of the solid component obtained in Synthesis Example 9 is shown.
- the result of 1 H NMR of the solid component obtained from the mother liquor in Synthesis Example 10 is shown.
- the result of the XRPD analysis of the solid component obtained from the mother liquor in Synthesis Example 10 is shown.
- the result of the DSC analysis and TGA analysis of the solid component obtained from the mother liquor in Synthesis Example 10 is shown.
- the polarization microscope image of the solid component obtained from the mother liquid in the synthesis example 10 is shown.
- the result of 1 H NMR of the solid component obtained from the mother liquor in Synthesis Example 11 is shown.
- the result of the XRPD analysis of the solid component obtained from the mother liquor and the solid component obtained from the suspension in Synthesis Example 11 is shown.
- the result of the DSC analysis and TGA analysis of the solid component obtained from the mother liquor in Synthesis Example 11 is shown.
- the polarization microscope image of the solid component obtained from the mother liquor in the synthesis example 11 is shown.
- the result of 1 H NMR of the compound of Synthesis Example 12 is shown.
- the result of the XRPD analysis of the compound of the synthesis example 12 is shown.
- the results of DSC analysis and TGA analysis of the compound of Synthesis Example 12 are shown.
- 12 shows a polarizing microscope image of the compound of Synthesis Example 12.
- Synthesis Example 12 shows the logical structural formula of the compound.
- the HPLC analysis result of the compound of Synthesis Example 12 is shown.
- the XRPD analysis result of the solid component obtained in batch No. 05 is shown.
- Batch No. The XRPD analysis results of the solid components obtained in 08, 10, 11, and 12 are shown.
- Batch No. The polarizing microscope image and XRPD analysis result of the solid component obtained in 08, 10, 11, and 12 are shown.
- the polarization microscope image and XRPD analysis result of the heat-processed compound of the synthesis example 12 are shown.
- the XRPD analysis result of the heat-processed synthesis example 12 compound is shown.
- the XRPD spectrum of the compound of Synthesis Example 12 is shown.
- the XRPD peak value of the compound of Synthesis Example 12 is shown.
- the XRPD spectrum of batch No. 05 is shown.
- the XRPD peak value of batch No. 05 is shown.
- the spectrum of XRPD of batch No. 08 is shown.
- the XRPD peak value of batch No. 08 is shown.
- the XRPD spectrum of batch No. 10 is shown.
- the XRPD peak value of batch No. 10 is shown.
- the spectrum of XRPD of batch No. 11 is shown.
- the XRPD peak value of batch No. 11 is shown.
- the spectrum of XRPD of batch No. 12 is shown.
- the XRPD peak value of batch No. 12 is shown.
- composition I-1 Composition Components and Combination Compositions
- the composition of the present invention comprises (A) at least one selected from TRH analogs, pharmaceutically acceptable salts thereof, and hydrates thereof, and (B) arundoic acid, A composition comprising a combination of at least one selected from pharmaceutically acceptable salts thereof and pharmaceutically acceptable solvates thereof, that is, a combined composition.
- the TRH analog refers to a compound represented by the following general formula (I): (R is a hydrogen atom or an alkyl group having 1 to 6 carbon atoms, preferably an alkyl group having 1 to 4 carbon atoms).
- R is preferably a hydrogen atom, a methyl group, an ethyl group, a propyl group, an isopropyl group, a butyl group, an isobutyl group, a sec-butyl group, or a tert-butyl group, more preferably a hydrogen atom, a methyl group, or An ethyl group, more preferably a methyl group.
- the compound represented by the above general formula (I) is most preferably a compound represented by the following formula (I-1), which is taltileline, or N-[(4S) -1-methyl-2,6 Also referred to as -dioxo-hexahydropyridine-4-carbonyl] -L-histidyl-L-prolinamide.
- the pharmaceutically acceptable salt of the compound represented by the general formula (I) or the formula (I-1) is not particularly limited.
- examples of such salts include inorganic acid addition salts such as hydrochloride, hydrobromide, sulfate, nitrate; and acetate, tartrate, maleate, succinate, citrate, methanesulfonate And organic acid addition salts such as malate, oxalate and benzenesulfonate. These salts can be produced, for example, by treating the compound represented by the general formula (I) with an acid.
- the solvent which forms a solvate with the compound represented by the above general formula (I) or the above formula (I-1) or a pharmaceutically acceptable salt thereof is not particularly limited.
- the solvent include water, ethanol, acetone, acetic acid, 1-propanol, 2-propanol, ethyl acetate, diethyl ether and the like.
- it is water or ethanol, More preferably, it is water.
- arundoic acid refers to a compound represented by the following formula:
- the arundo acid may be in R form, S form, and racemic form.
- Arundoic acid is sometimes called 2-propyloctanoic acid under the IUPAC name.
- the solvent that forms a solvate with arundo acid is not particularly limited.
- the solvent include methanol, ethanol, acetone, ethyl acetate, chloroform, diethyl ether, and acetonitrile.
- the pharmaceutically acceptable salt of arundo acid is not particularly limited.
- the salt include a sodium salt, a potassium salt, and a salt represented by the following general formula (II): (X 2+ is a divalent cation).
- Preferred as a pharmaceutically acceptable salt of arundoic acid is a compound represented by the above general formula (II), and in particular, X 2+ represents Ca 2+ , Mg 2+ , Fe 2+ , Ba 2+ , + H 3 N—NH.
- X 2+ represents Ca 2+ , Mg 2+ , Fe 2+ , Ba 2+ , + H 3 N—NH.
- a compound in which 3 + , + H 2 N ⁇ NH 2 + , or + HN ⁇ NH + is preferable, and a compound in which X 2+ is Ca 2+ is particularly preferable among the compounds described in the general formula (II).
- compositions of arundoic acid include enantiomers, amorphous and crystalline.
- X 2+ is Ca 2+
- the crystal has the typical peak of powder X-ray diffraction (XRPD) performed under the following conditions, the crystal 1 or 2 having the first to third peaks shown in Table 1, and At least one selected from the group consisting of crystals 3 to 5 which are the first peak to the fourth peak is preferable.
- the crystals 3 to 5 preferably have the first to seventh peaks shown in Table 2.
- the crystal 1 has the peak shown in FIG. 28, the crystal 2 has the peak shown in FIG. 29, the crystal 3 has the peak shown in FIG. 30, and the crystal 4 has the peak shown in FIG.
- the value of 2 ⁇ (°) described in this specification and the drawings may include an error of about ⁇ 0.2 °, preferably about ⁇ 0.1 °.
- the full width at half maximum (FWHM) described in the present specification and drawings may include an error of about ⁇ 0.1 °, preferably about ⁇ 0.05 °.
- the formula It is a compound represented by these.
- a pharmaceutically acceptable salt of arundoic acid can be produced according to the method described in Patent Document 2 or a synthesis method of arundoate described below.
- the solvent that forms a solvate with a pharmaceutically acceptable salt of arundoic acid is not particularly limited.
- the solvent include water, ethanol, acetone, acetic acid, 1-propanol, 2-propanol, ethyl acetate, diethyl ether and the like.
- it is water, ethanol, or a mixture thereof, more preferably water.
- composition targeted by the present invention is (I) When the component (A) and the component (B) are in a state of being mixed and adjusted in the same preparation (compound), (Ii) (A) component simple substance or preparation containing component (A) and (B) component simple substance or preparation containing component (B) are packaged as separate preparations, and both are combined (Kit) sold as a combination when used, (Iii) (A) component simple substance or preparation containing component (A) and (B) component simple substance or preparation containing component (B) are individual preparations, and these are combined to form one package.
- the “composition comprising the combination” means that the administration of the component (A) alone or the preparation containing the component (A) to the administration subject is the component (B) alone or the component (B). It suffices as long as it is performed simultaneously with or in parallel with administration of the contained preparation, and the form of the component (A) and the component (B) in the distribution stage including sales is not particularly limited.
- the administration of the component (A) alone or the preparation containing the component (A) to the administration subject precedes the administration of the component (B) alone or the preparation containing the component (B).
- the administration of the component (A) alone or the preparation containing the component (A) to the administration subject after the administration of the component (B) alone or the preparation containing the component (B). Is included.
- the preparation containing the component (A) means a preparation in which the component (A) is combined with other ingredients
- the “preparation containing the component (B)” means the ingredient (B). It means a preparation in which other components are combined, and is distinguished from a preparation composed of the component (A) alone and a preparation composed of the component (B) alone.
- the carrier or additive for formulation mentioned later is contained.
- the maximum daily dose of component (A) is 30 mg / kg, preferably 10 mg / kg, more preferably 3 mg / kg in terms of component (A).
- the minimum daily dose of component (A) is 0.1 mg / kg, preferably 0.5 mg / kg, more preferably 1 mg / kg.
- the range of the daily dose of the component (A) can be appropriately set from the values of the maximum dose and the minimum dose.
- the maximum daily dose of component (A) is 135 mg / human, preferably 100 mg / human, more preferably 50 mg / human, more preferably 40 mg / human, in terms of component (A), Even more preferred is 10 mg / human.
- the minimum daily dose of component (A) is 0.5 mg / human, preferably 1 mg / human, more preferably 2.5 mg / human, and even more preferably 5 mg / human.
- the range of the daily dose of the component (A) can be appropriately set from the values of the maximum dose and the minimum dose.
- Administration of the component (A) may be carried out once a day with the above dose, and if necessary, the dose is divided into 2, 3, 4 or 5 times a day, preferably It may be administered in two or three divided doses.
- the administration period of the component (A) can be administered for a period necessary for the prevention or treatment of the disease.
- the administration period is, for example, 1, 4, 10, 20, 30, or 50 weeks or more, and a more preferable administration period can be appropriately set from these.
- Administration can take place every other day, every other day, but preferably every day.
- the drug may be taken off for about 1 day every 5 to 7 days.
- the maximum daily dose of component (B) is 500 mg / kg, preferably 300 mg / kg, more preferably 100 mg / kg in terms of component (B).
- the minimum daily dose of component (B) is 3 mg / kg, preferably 10 mg / kg, more preferably 30 mg / kg.
- the range of the daily dose of the component (B) can be appropriately set from the values of the maximum dose and the minimum dose.
- the maximum daily dose of component (B) is 2500 mg / human, preferably 1000 mg / human, more preferably 500 mg / human, more preferably 100 mg / human in terms of component (B). is there.
- the minimum daily dose of component (B) is 3 mg / human, preferably 10 mg / human, more preferably 30 mg / human.
- the range of the daily dose of the component (B) can be appropriately set from the values of the maximum dose and the minimum dose.
- the ratio of the dose of component (B) to 1 part by weight of component (A) is 0.1 to 500 parts by weight, preferably 1 to 100 parts by weight, more preferably 3 to 50 parts by weight. Parts by weight, more preferably 10 to 30 parts by weight.
- Administration of the component (B) may be carried out once a day with the above dose, and if necessary, the dose is divided into 2, 3, 4 or 5 times a day, preferably It may be administered in two or three divided doses.
- the administration period of the component (B) is, for example, 1, 4, 10, 20, 30, or 50 weeks or more, and a more preferable administration period can be appropriately set. Administration can take place every other day, every other day, but preferably every day. The drug may be taken off for about 1 day every 5 to 7 days. Administration of component (B) can also be performed in the same manner as administration of component (A).
- (A) component and (B) component are each a single substance, or as a compounding agent which mix
- Carriers and additives used for the preparation of the preparation include various types commonly used for ordinary drugs according to the dosage form of the preparation, such as excipients, binders, disintegrants, lubricants, colorants, Examples include flavoring agents, flavoring agents, and surfactants.
- the dosage form in the case where the above combination preparation or preparation is orally administered is not particularly limited, but tablets, powders, granules, capsules (hard capsules and soft capsules) (Including capsules), liquids, pills, suspensions, jelly preparations, and emulsions.
- an injection, an instillation, a suppository, a nasal drop, a transpulmonary administration etc. can be illustrated.
- combination or preparation is an oral solid preparation such as a tablet, powder, granule, pill, capsule, etc.
- a carrier for example, lactose, sucrose, sodium chloride, glucose, urea, starch, carbonate
- Excipients such as calcium, kaolin, crystalline cellulose, silicic acid, methyl cellulose, glycerin, sodium alginate, gum arabic; simple syrup, pudou sugar solution, starch solution, gelatin solution, polyvinyl alcohol, polyvinyl ether, polyvinyl pyrrolidone, carboxymethyl cellulose, Binders such as shellac, methylcellulose, ethylcellulose, water, ethanol, potassium phosphate; dry starch, sodium alginate, agar powder, laminaran powder, sodium bicarbonate, calcium carbonate, polyoxyethylene sorbitan fatty acid ester Disintegrating agents such as tellurium, sodium lauryl sulfate, monostearate monoglyceride, starch, lact
- Moisturizers such as starch, lactose, kaolin, bentonite, colloidal silicic acid; lubricants such as purified talc, stearate, boric acid powder and polyethylene glycol can be used.
- the tablets include internal tablets (bare tablets, sugar-coated tablets, gelatin-encapsulated tablets, enteric-coated tablets, film-coated tablets, double tablets, multilayer tablets, etc.), chewable tablets (things to be ingested while chewing in the oral cavity) ), Oral tablets (including those taken if dissolved in the oral cavity such as troches), sublingual tablets, and buccal tablets.
- the compounding agent or preparation is an oral solid preparation of pills
- a carrier for example, excipients such as glucose, lactose, starch, cocoa butter, hydrogenated vegetable oil, kaolin, talc; Binders such as tragacanth powder and gelatin; disintegrating agents such as laminaran and agar can be used.
- the capsule is mixed with various carriers exemplified above and filled into a hard capsule or a soft capsule. Prepared.
- the compounding agent or preparation when the compounding agent or preparation is a liquid, it may be liquid, and may be an aqueous or oily suspension, solution, syrup, elixir, or drink.
- the liquid preparation is prepared according to a conventional method using ordinary additives.
- the container filled with the liquid agent is not limited as long as it can be sealed, and may be a glass container, an aluminum container, and a plastic container.
- carriers such as water, ethyl alcohol, macrogol, propylene glycol, ethoxylated isostearyl alcohol, polyoxylated isostearyl alcohol, polyoxyethylene sorbitan fatty acid esters, etc.
- Diluent such as sodium citrate, sodium acetate, sodium phosphate; buffer such as dipotassium phosphate, trisodium phosphate, sodium hydrogen phosphate, sodium citrate; sodium pyrosulfite, EDTA, thioglycol Stabilizers such as acid and thiolactic acid; saccharides such as mannitol, inositol, maltose, sucrose, and lactose can be used as a molding agent when freeze-dried.
- a sufficient amount of glucose or glycerin may be included in the pharmaceutical preparation to prepare an isotonic solution, and a normal solubilizing agent, soothing agent, local anesthetic, etc. may be added. Also good.
- a sufficient amount of glucose or glycerin may be included in the pharmaceutical preparation to prepare an isotonic solution, and a normal solubilizing agent, soothing agent, local anesthetic, etc. may be added. Also good.
- the above combination or preparation is an infusion, it can be prepared by dissolving the administered compound in an isotonic electrolyte infusion preparation based on physiological saline, Ringer's solution or the like.
- the component (A) alone or the preparation containing the component (A) and the component (B) alone or the preparation containing the component (B) are packaged as separate preparations and used in combination at the time of use.
- (A) component alone or a preparation containing component (A), (B) component alone, or (B) It can be administered prior to or in parallel with the administration of the formulation containing the ingredients.
- the component (B) alone or the preparation containing the component (B) is administered prior to or in parallel with the administration of the component (A) alone or the preparation containing the component (A). be able to. Of course, both can be administered simultaneously.
- Prior to administration of (B) component alone or a preparation containing (B) component when administering (A) component alone or (A) formulation containing component, (B) component alone, or Administration of the preparation containing the component (A) alone or the preparation containing the component (A) can be started immediately before to 3 days before the start of administration of the preparation containing the component (B).
- the treatment can be started immediately before to 2 days before, more preferably immediately before to 24 hours, more preferably immediately before to 12 hours, and most preferably immediately before to 3 hours.
- the administration period of the component (A) alone or the preparation containing the component (A) and the administration period of the component (B) alone or the preparation containing the component (B) are as described above.
- the compounding agent containing both the component (A) and the component (B) in the same preparation is only required to contain both the component (A) and the component (B). It can be prepared in combination with an agent.
- the mixing ratio of the component (A) and the component (B) is not particularly limited.
- the ratio of the dose of component (B) to 1 part by weight of component (A) is 1-1000 parts by weight, and the lower limit of the ratio is preferably 3, 10, or 30 parts by weight. It is.
- the upper limit of the ratio is preferably 100, 300, or 1000 parts by weight.
- the prepared combination preparation may be administered once a day according to the dosage form so as to be within the range of the daily dose of the component (A) and the component (B).
- the dose may be divided into 2, 3, 4, or 5 times a day, preferably 2 or 3 times a day so as to be within the above dose range.
- composition I-2 Pharmaceutical composition I-1.
- the combination composition described in 1 can be used as a pharmaceutical composition.
- the pharmaceutical composition of this aspect is used for the prevention of neurodegenerative diseases or cerebral infarction such as dementia, Parkinson's disease, amyotrophic lateral sclerosis, progressive supranuclear palsy, multiple system atrophy, triplet repeat disease and the like. Or it can be used for treatment.
- prevention includes suppressing and / or delaying the onset of symptoms.
- Treatment also includes reducing and / or eliminating the onset of symptoms.
- “dementia” includes dementia due to frontotemporal type such as Alzheimer's disease, cerebrovascular dementia, Pick's disease, dementia with Lewy bodies, and infectious diseases (spirochetes, HIV viruses, prions, etc.). included. Also included are mild cognitive impairments such as MCI. Dementia is preferably mild cognitive impairment, Alzheimer's disease, or cerebrovascular dementia.
- multiple system atrophy includes striatal nigra degeneration, olive bridge cerebellar atrophy, Shy-Drager syndrome, and the like. Preferably, it is olive bridge cerebellar atrophy.
- triplet repeat disease includes Huntington's chorea, Friedrich's ataxia, spinocerebellar degeneration type 1 and the like.
- Cerebral infarction refers to a state in which nerve cells and / or glial cells have been killed or can be killed by ischemia of brain tissue.
- the “cerebral infarction” of this embodiment includes subacute to chronic cerebral infarction and prolonged cerebral infarction.
- the “cerebral infarction” of the present embodiment is preferably a cerebral infarction from the subacute phase to the chronic phase, and a prolonged cerebral infarction.
- the pharmaceutical composition of this embodiment can be used to improve learning disorders.
- Learning disorder means that the input of new memory and / or reading (recall) of input memory is not normally performed.
- the “learning disorder” is preferably a spatial cognitive disorder or a memory disorder non-linguistic learning disorder, and more preferably a “spatial cognitive disorder”.
- the dosage, administration method, and formulation of the pharmaceutical composition of this embodiment are the same as those in I-1. According to the explanation.
- I-3 Food composition I-1.
- the combination composition described in can be used as a food composition.
- the dosage and administration method of the food composition of this embodiment are as described in I-1. According to the explanation.
- the dosage form of the food composition of this embodiment is the above-mentioned I-1. According to the description of the orally administered combination drug or preparation described in 1. above.
- the expressions “dose” and “administration method” can be read as “intake amount” and “intake method”, respectively.
- the food composition of this embodiment includes general foods, health functional foods (functional display foods, nutritional functional foods, foods for specified health use).
- the definition and classification of functional health foods are as stipulated in the Japanese Health Promotion Law and the Food Sanitation Law.
- the food composition of this aspect is the above I-3. Can be used in the same applications as the pharmaceutical composition described in 1. above.
- the relationship with the disease can be changed to a display format that does not conflict with the domestic law. For example, to maintain the youthfulness of the brain, keep the brain healthy, prevent forgetfulness, restore memory, prevent memory loss, prevent the loss of adults (especially the elderly), etc. Can be used as a display.
- the present invention includes arundoic acid, pharmaceutically acceptable salts thereof, and pharmaceutically acceptable salts thereof.
- a composition comprising at least one selected from solvates, wherein the composition is combined with at least one selected from TRH analogs, pharmaceutically acceptable salts thereof, and hydrates thereof. The composition used is included. The description of the above-described combination compositions 1-1 to 1-3 and the use of the combination composition are all incorporated in the composition of this section.
- arundoate Arundoate, production method thereof, and use of arundoate II-1.
- Arundoate Examples of the arundoate in this embodiment include salts represented by the following general formula (II): (X 2+ is a divalent cation).
- Preferred as a pharmaceutically acceptable salt of arundoic acid is a compound represented by the above general formula (II), and in particular, X 2+ represents Ca 2+ , Mg 2+ , Fe 2+ , Ba 2+ , + H 3 N—NH.
- X 2+ represents Ca 2+ , Mg 2+ , Fe 2+ , Ba 2+ , + H 3 N—NH.
- a compound in which 3 + , + H 2 N ⁇ NH 2 + , or + HN ⁇ NH + is preferable, and a compound in which X 2+ is Ca 2+ is particularly preferable among the compounds described in the general formula (II).
- Pharmaceutically acceptable salts of arundoic acid include enantiomers, amorphous and crystalline.
- X 2+ is Ca 2+
- it is preferably a crystal.
- the crystal has the above I-1.
- the crystals described in (1) are preferred.
- the solvent that forms a solvate with a pharmaceutically acceptable salt of arundoic acid is not particularly limited.
- the solvent include water, ethanol, acetone, acetic acid, 1-propanol, 2-propanol, ethyl acetate, diethyl ether and the like.
- it is water, ethanol, or a mixture thereof, more preferably water.
- Method for synthesizing the salt represented by the general formula (II) is not limited as long as the salt can be synthesized.
- I-1 Is dissolved in an aqueous sodium hydroxide solution of about 0.1 to 1.5 N and mixed with about 0.5 to 2 M of calcium chloride to form a solid.
- An arunate can be obtained by removing the solvent component from the solid and washing and drying as necessary.
- arundoic acid is dissolved in a lower alcohol (such as methanol or ethanol), and an equivalent amount of calcium carbonate is added to this solution.
- the precipitated calcium salt is collected by filtration and washed with alcohol.
- the precipitated calcium salt can be redissolved in purified water, and the purified water can be evaporated and then washed with alcohol to obtain recrystallization.
- the equivalent e.q. indicates the amount of base (mol) that can just neutralize 1 mol of an arundoic acid carboxyl group.
- the above I-1 Is dissolved in an aqueous sodium hydroxide solution of about 0.1 to 1.5 N, and then mixed with aqueous ammonia.
- the solvent component can be removed to obtain a solid component, and the solid component can be washed and dried as necessary to obtain an arundoate.
- the above I-1 in the solvent such as methyl tert-butyl ether, acetonitrile, dichloromethane, etc., preferably in methyl tert-butyl ether, the above I-1. Is mixed with about 0.4 to 0.6 equivalents of calcium hydroxide. The temperature of the mixture is increased to, for example, about 40 ° C. to 60 ° C., maintained at the increased temperature for about 12 hours to 24 hours, and then cooled to room temperature (about 18 ° C. to about 32 ° C.). Maintain for 0.5-2 hours. After the maintenance, since the mixed solution becomes a suspension, a calcium salt as a solid component can be obtained by centrifugation. Moreover, about the supernatant liquid after centrifugation, calcium salt can be obtained as a solid component by making it dry.
- the calcium salt synthesized by this method is the above-mentioned I-1. It becomes the crystal 1 of description.
- the arundoate synthesized by the above method can be further recrystallized.
- the recrystallization can be performed by an evaporation method or a slurry method, but is preferably a slurry method.
- the solvent is acetonitrile
- the solvent is evaporated at room temperature (about 18 to 32 ° C.), preferably about 23 to 28 ° C. for about 16 to 24 hours.
- the solvent is water
- the water is evaporated at about 20 to 55 ° C. for about 16 to 72 hours.
- acetonitrile is used as a solvent, the above I-1.
- compositions comprising a salt represented by the general formula (II), or a pharmaceutically acceptable solvate thereof, is prepared according to the above II-1. Or a pharmaceutically acceptable solvate thereof.
- the above II-1 wherein the composition comprises a salt represented by the general formula (II) or a pharmaceutically acceptable solvate thereof, wherein the composition comprises a TRH analog, a pharmaceutically acceptable salt thereof, And compositions used in combination with at least one selected from hydrates thereof.
- the dosage, administration method, formulation form, and the like of the composition of this embodiment are described in I-1. According to the description about the component (B) described in 1.
- the above II-1 According to the description about the component (B) described in 1.
- a composition comprising a salt represented by the general formula (II) or a pharmaceutically acceptable solvate thereof, a TRH analog, a pharmaceutically acceptable salt thereof, and a hydrate thereof.
- a salt represented by the general formula (II) or a pharmaceutically acceptable solvate thereof for at least one selected combination, the above I-1.
- the description of the combination composition described in is incorporated herein by reference.
- composition II-4 Pharmaceutical composition II-3.
- the composition described in 1 can be used as a pharmaceutical composition.
- the pharmaceutical composition can be used, for example, for the use described in Patent Document 2 and for the treatment and / or prevention of other cranial nervous system diseases.
- the pharmaceutical composition of this embodiment can be used to improve the function of astrocytes or to change reactive astrocytes into astrocytes.
- the pharmaceutical composition of this embodiment is used for the treatment and / or prevention of neurodegenerative diseases, neurological dysfunction after cerebrospinal trauma, brain tumors, cerebrospinal diseases associated with infection, or multiple sclerosis.
- the “neurodegenerative disease” in this embodiment refers to the above I-2. According to the explanation. Dementia, Parkinson's disease, amyotrophic lateral sclerosis, progressive supranuclear palsy, or multisystem atrophy is preferred.
- the “brain tumor” is a glioma derived from glial cells, preferably astrocytoma.
- the “cerebrospinal disease associated with infection” is not limited as long as it is a disease that develops along with microbial infection.
- meningitis meningitis, brain abscess, Clotsfeld-Jakob disease, or cerebrospinal disease associated with AIDS.
- prevention and treatment in this embodiment are the above-mentioned I-2. According to the explanation.
- the dosage, administration method, formulation form and the like of the pharmaceutical composition of this embodiment are the same as those in I-1. According to the description about the component (B) described in 1.
- Food composition above II-2 The composition described in can be used as a food composition.
- the dosage and administration method of the food composition of this embodiment are as described in I-1. According to the description about the component (B) described in 1. Moreover, the dosage form of the food composition of this embodiment is the above-mentioned I-1. According to the description of the preparation of the (B) component to be administered orally described in 1. Furthermore, the expressions “dose” and “administration method” can be read as “intake amount” and “intake method”, respectively.
- the food composition of this embodiment includes general foods, health functional foods (functional display foods, nutritional functional foods, foods for specified health use).
- the definition and classification of functional health foods are as stipulated in the Japanese Health Promotion Law and the Food Sanitation Law.
- the food composition of this aspect is the above II-4. Can be used in the same applications as the pharmaceutical composition described in 1. above.
- the relationship with the disease can be changed to a display format that does not conflict with the domestic law. For example, expressions such as smoothing daily activities, reducing forgetfulness, easily speaking words, reducing the number of lost children (especially elderly people), etc. may be used as an indication of the use of the food composition. it can.
- Example 1 1. experimental method 1) For animal experiments, male Crl: Wistar rats of 7 weeks of age (body weight 200-240 g) were used in the experiments with an acclimatization period of 1 week or more. Rats are kept free of food (oriental yeast, CRF-1) and water in the breeding room at room temperature 23 ⁇ 1 ° C, humidity 55 ⁇ 5%, 12-hour lighting (6: 30-18: 30) Raised.
- male Crl Wistar rats of 7 weeks of age (body weight 200-240 g) were used in the experiments with an acclimatization period of 1 week or more. Rats are kept free of food (oriental yeast, CRF-1) and water in the breeding room at room temperature 23 ⁇ 1 ° C, humidity 55 ⁇ 5%, 12-hour lighting (6: 30-18: 30) Raised.
- arundoic acid was dissolved in 300 mg (306 ⁇ L) of arundoic acid by adding 1.61 mL of 1N NaOH, and 48.084 mL of water or 0.5% CMC (carboxymethylcellulose) was added to make a total volume of 50 mL. .
- CMC carboxymethylcellulose
- a 10-fold concentrated arundo acid solution was prepared and prepared at the time of use.
- Taltilelin was dissolved in distilled water at a dose of 3 ml / kg at a dose of 3 mg / kg or 10 mg / kg.
- the test drug was orally administered for 28 days (including weekends and holidays) from one week after the preparation of the four-vessel occlusion model.
- Group 1 is a group that has not been treated for four-vessel occlusion
- groups 2-5 are groups that have been treated for four-vessel occlusion.
- 1 group Sham control (distilled water 2 ml / kg / day)
- group 2 group control (distilled water 5 ml / kg / day)
- group 3 group arundoic acid (30 mg / kg / day) administration
- 4 group tartilelin (10 mg / kg / day) administration
- 5 groups Arundoic acid (10 mg / kg / day) and taltileline (3 mg / kg / day) combined use group
- the tip of a bipolar coagulator (Mizuho Medical Industry, MICROID) tweezers is inserted into the alar foramina site at both ends of the first cervical vertebra, and the vertebral artery ascending to the base of the brain is bilaterally electrocauterized and cut. The skin was sutured. Subsequently, the rat was fixed back to the experimental operating table in the supine position, the skin of the ventral neck was incised, the bilateral common carotid artery was separated from the surrounding tissue, the silk thread was looped, and the skin was sutured . After the operation, the rats were returned to their cages and observed to confirm that no abnormal behavior was observed.
- MICROID Bipolar coagulator
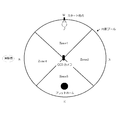
- Zone 1 to Zone 4 indicate four divided areas of the circular pool.
- E, W, S, and N indicate the directions of east, west, south, and north.
- a CCD camera was installed in the center of the water maze, and the white color of the rat's hair was automatically detected by a personal computer connected to the CCD camera, and the trajectory of swimming was analyzed.
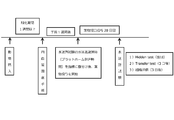
- the experiment schedule is shown in FIG. Specifically, the experiment proceeded according to the following procedure. 1) 40 male Crl: Wistar rats of 8 weeks old (body weight 200-240 g) were purchased. Body weight was measured at the time of purchase. The acclimatization period was one week or longer. 2) After vascular occlusion surgery (24 hours after vertebral artery cauterization surgery, 10 minutes of common carotid artery occlusion), general symptoms were observed after resumption of blood flow. 3) One week after the resumption of blood flow, the Morris water maze test performed 30-minute interval and cut-off 180 second Hidden test 5 trials, and the grouping was performed using the swimming escape latency (platform arrival time) as an index.
- a catheter was inserted from the heart into the carotid artery and 50 mL or more of heparin saline was flowed. Subsequently, at least 50 mL of 4% paraformaldehyde-phosphate buffer was flowed. The reflux operation was performed by ligating the aorta. After perfusion fixation, the brain was removed and fixed by osmosis in 4% paraformaldehyde-phosphate buffer. (3) HE staining A continuous frozen section having a thickness of 20 ⁇ m is prepared from this brain with a cryostat. Every other serial section was stained with hematoxylin and eosin. The degree of shed neurons in tissues stained under a light microscope was evaluated semi-quantitatively. 7) The data obtained in the Morris water maze test was compared between two groups by Dunnett's multiple comparison method to determine the p-value.
- Table 4 shows the Morris water maze test results of each rat at the time of grouping and after 28 days of administration of the test drug.
- Table 5 shows the results of Hidden test and Transfer test of each group after administration of the test drug.
- Table 4 shows the 2-5 groups of rats treated with the 4-vessel occlusion compared to the 1 group of rats not treated with the 4-vessel occlusion. It has been shown that the place learning ability has decreased. Table 5 shows the effect of each test drug. In the 3 groups administered with arundoic acid alone and in the 4 groups administered alone with tartyrelin, no improvement in the arrival time was observed in the Hidden test. Moreover, it could not be said that the total moving distance was remarkably improved as compared with the control group 2. On the other hand, in group 5 administered with a combination of arundoic acid and tartyrelin, the time to reach the platform in the Hidden test was remarkably shortened, which was comparable to that of group 1 as the Sham control group.
- the total travel distance was also significantly shorter than the 2-4 groups. From this, it was considered that the place learning ability and place memory ability (spatial learning ability and spatial memory ability) lost by the four-vessel occlusion can be recovered by co-administering arundoic acid and taltileline.
- the hippocampal CA1 neurons were detached 28 days after the treatment of the four-vessel occlusion.
- the dropout was also observed in tartyrelin administered (treated) after delayed neuronal death, but delayed neuronal death was minimal with arundoic acid and a combination of arundoic acid and tartyrelin. there were.
- alundic acid did not affect spatial learning ability and spatial memory ability in the Morris water maze test, despite promoting regeneration of hippocampal CA1 neurons.
- co-administration of arundoic acid and taltilelin improved spatial learning / memory ability.
- FT-IR was measured by the ATR method with an ATR attachment (iD5ATR) attached to an FT-IR apparatus (NicoletiS5 FT-IR manufactured by Thermo Fisher Scientific). Measurement was requested from Osaka Shinyaku Co., Ltd.
- a TGIDTA device RivetiS5 FT-IR manufactured by Thermo Fisher Scientific
- a DSC device RivetiS5 FT-IR manufactured by Thermo Fisher Scientific
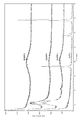
- FIG. 4A shows the FT-IR result of arundoic acid
- FIG. 4B shows the FT-IR result of the obtained compound.
- the results of thermal analysis (thermogravimetry TG, differential thermal analysis DTA, differential scanning calorimetry DSC) of the obtained compound are shown in FIG.
- the results of elemental analysis were C: 48.5%, H: 7.8%, N: ⁇ 0.3, Na: 19.5, and Ca: 4.77.
- Synthesis example 2 (calcium carbonate method) Arundic acid is dissolved in a lower alcohol (such as methanol or ethanol), and an equivalent amount of calcium carbonate Ca (CO 3 ) 2 is added to this solution. The precipitated calcium salt is collected by filtration and washed with alcohol. The precipitated calcium salt is redissolved in purified water, and the purified water is evaporated and then washed with alcohol to obtain recrystallization.
- a lower alcohol such as methanol or ethanol
- Example 3 The calcium arundoate was synthesized by the following method.
- the unit 1 eq (equivalent) indicates the amount of base (mol) that can just neutralize 1 mol of the aldundic carboxyl group. 1.
- Reagents The reagents shown in Table 9 below were used for the synthesis of calcium arundoate in this section.
- DSC Differential scanning calorimetry
- TGA Thermogravimetric analysis
- Q5000IR Thermal gravimetric analysis
- Samples (3 ⁇ 5 mg), placed in an open airtight platinum pan, 25 mL / min of N 2 presence at 10 °C / min 300 °C from 30 ° C. or in 10 ° C. in N 2 presence of 25 mL / min The mixture was heated until the weight% at 80 minutes reached 80% of the weight at the time of charging.
- Polarized light microscope The polarizing microscope used was LV100 PL (Nikon) equipped with a 5-megapixel CCD.
- ICP-OES Inductively coupled plasma atomic emission spectroscopy
- Karl Fischer (KF) titration A volume KF titrator (Volumetric Karl Fischer (V20, Mettler-Toledo)) was charged with methanol as a solvent, and 100 mg of a sample was added. Titrate to end point with HYDRANAL- Composite 5.
- Reference Synthesis Examples 1-7 102 ⁇ L (100 mg) of arundoic acid was dissolved in 500 ⁇ l of ethanol, acetone, or methanol. Then 22.1 mg Ca (OH) 2 , 0.35 g / ml CaCl 2 (100 ⁇ l) or 0.54 g / mL NaOH aqueous solution 50 ⁇ L was added (Reference Synthesis Example 1: Combination of ethanol and CaCl 2 , Reference Synthesis Example 1 : Combination of ethanol and Ca (OH) 2 Reference synthesis example 3: Combination of acetone and CaCl 2 Reference synthesis example 4: Combination of acetone and Ca (OH) 2 Reference synthesis example 5: Combination of methanol and CaCl 2 Reference synthesis example 6: combination of methanol and Ca (OH) 2 , reference synthesis example 7: combination of methanol and NaOH). The vial after addition was warmed to 50 ° C. and incubated at 50 ° C. for 2 hours. In Reference Synthesis Examples 1
- Synthesis Examples 4 to 8 and Reference Synthesis Examples 8 to 12 22.12 mg Ca (OH) 2 was weighed into each of multiple 2 mL vials. Thereto was added 102 ⁇ L (100 mg) of arundoic acid. 500 ⁇ L of different solvent was added to each vial containing Ca (OH) 2 and arundoic acid. As solvents, MtBE (Synthesis Example 4), ACN (Synthesis Example 5) , THF (Synthesis Example 6), DCM (Synthesis Example 7), and heptane (Synthesis Example 8) were used. The vial after solvent addition was warmed to 50 ° C. and incubated at 50 ° C. for 18 hours.
- the vial was then cooled to 25 ° C. and incubated at 25 ° C. for 1 hour.
- the liquid in the vial after the incubation became a suspension.
- the suspension was centrifuged to separate the residual solid component and the supernatant.
- the residual solid component and the supernatant were dried at 30 ° C. for 3 hours using a vacuum oven.
- Each solid component obtained after drying was analyzed by XRPD. Table 11 shows the properties of Synthesis Examples 4 to 8.
- Reference synthesis example 13 102 ⁇ L (100 mg) of arundoic acid was dissolved in 500 ⁇ L of acetone, and 50 ⁇ L of 0.54 g / mL NaOH aqueous solution was added to this solution. This solution was warmed to 50 ° C. and incubated at 50 ° C. for 2 hours. 0.35 mg / mL CaCl 2 aqueous solution (100 ⁇ L) was added to the solution after the incubation. An oily component appeared by this reaction. Furthermore, 400 ⁇ L of water was added thereto and incubated at 50 ° C. for 2 hours, then cooled to 25 ° C. and incubated at 25 ° C. for 20 hours. The oily component remained unchanged and did not solidify.
- the suspension was centrifuged to separate the residual solid component and the supernatant (mother liquor).
- the mother liquor was dried in a suction oven at 30 ° C. for 17 hours. As a result, a gel-like solid was obtained.
- the residual solid component of the suspension and the solid component obtained from the mother liquor were analyzed by XRPD.
- Synthesis Example 10 As the calcium arundoate synthesis system, the scale-up was attempted using 200 mg of arundoic acid using the system of Synthesis Example 4 above.
- Table 13 shows the properties of the compound obtained in Synthesis Example 10. The results of 1 H NMR, XRPD, DSC, TGA and PLM are shown in FIGS. 9 to 12, respectively.
- Synthesis example 11 Next, an attempt was made to scale up the system of Synthesis Example 9 using 500 mg of arundoic acid. 510 ⁇ l (500 mg) of arundoic acid was dissolved in 500 ⁇ L of THF, and 250 ⁇ L of 0.54 g / mL NaOH aqueous solution was added to this solution. This solution was warmed to 50 ° C. and incubated at 50 ° C. for 3 hours. The solution became clear. Thereafter, 500 ⁇ L of 0.33 g / mL CaCl 2 aqueous solution was added to the solution after the incubation. A suspension was obtained by this reaction. This suspension was maintained at 50 ° C. for 3 hours, then cooled to 25 ° C.
- Table 14 shows the properties of the compound obtained in Synthesis Example 11. The results of 1 H NMR, XRPD, DSC, TGA, and PLM are shown in FIGS. 13 to 16, respectively.
- Synthesis example 12 As a method for synthesizing calcium arundate, the system of Synthesis Example 4 was simple and a highly pure compound could be obtained. Therefore, an attempt was made to synthesize from 6 g of arundoic acid using this system.
- Synthesis Example 12 compound Each solid component obtained after drying was analyzed for 1 H NMR, XRPD, DSC, TGA, PLM, logical structure, purity, and solubility.
- the calcium content in the solid component was measured by inductively coupled plasma atomic emission spectroscopy (ICP-OES).
- the water content was measured by Karl Fischer (KF) titration.
- Table 15 shows properties of the compound obtained in Synthesis Example 12, Table 16 shows purity, and Table 17 shows solubility.
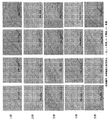
- the results of 1 H NMR, XRPD, DSC, TGA, PLM, logical structure, and HPLC are shown in FIGS. 17 to 22, respectively.
- FIG. 28 shows a table in which each peak of XRPD of the compound of Synthesis Example 12 is quantified. According to the DSC scan of the compound of Synthesis Example 12 (FIG. 19), one endothermic peak appeared at 151.1 ° C. TGA scan showed a 4.6% weight loss from 35 ° C to 150 ° C. By observation with a polarizing microscope, the compound of Synthesis Example 12 was amorphous (FIG. 20).
- the calcium content of the compound of Synthesis Example 12 was 10.34% (Table 15).
- the arundoic acid content of the solid component was 83.85%, and the ratio of arundoic acid to calcium was 1: 0.57.
- the water content was about 5.03% (Table 15). This result suggested that a slight amount of water remained in the synthesized calcium arundo acid salt.
- the theoretical calcium content is the theoretical structure of calcium salt shown in FIG. 21 (calciumcalc (R) -2-propyloctanoate (S) -2-propyloctanoate, Chemical Formula: C22H42CaO4, molecular weight: 410.64, Elemental Analysis: C, 64.35; H , 10.31; Ca, 9.76; O, 15.58).
- Recrystallization screening 9-1 Recrystallization by slurry method or evaporation method Synthesis Example 12 An attempt was made to recrystallize the compound by the slurry method or evaporation method. Table 18 shows each solvent for recrystallization. 30 mg of the compound of Synthesis Example 12 was suspended at 25 ° C. in 500 ⁇ l of each solvent. After stirring for 1 hour, in the system using THF, MtBE, ethanol, and 2-propanol as solvents, the solution became clear. For these systems, recrystallization was attempted by evaporation. In the evaporation method, the solution was contacted with air at 25 ° C. for 21 hours to evaporate the solvent.
- the system in which crystals were obtained by recrystallization was a batch No. with ACN as the solvent. 05, and batch No. with H 2 O as solvent. It was 08, 10, 11, 12.
- FIG. 23 and FIG. Batch Nos. 05 and 10 showed the peak pattern CI (FIG. 23).
- Batch Nos. 08, 11 and 12 showed C-II different from the peak pattern C-I of Batch No. 10 and Synthesis Example 12 (FIG. 24).
- Figure 25 shows the batch number. The polarization microscope images of 08, 10, 11, and 12 are shown.
- Fig. 29 shows the XRPD peak value of batch No. ⁇ 05
- Fig. 30 shows the XRPD peak value of batch No. 08
- Fig. 31 shows the XRPD peak value of batch No. 10
- Fig. 32 shows The XRPD peak value of batch No. 11 is shown
- the XRPD peak value of batch No. 12 is shown in FIG.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Neurology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Pain & Pain Management (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Physical Education & Sports Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Psychology (AREA)
- Heart & Thoracic Surgery (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Cardiology (AREA)
- Nutrition Science (AREA)
- Mycology (AREA)
- Polymers & Plastics (AREA)
- Food Science & Technology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Coloring Foods And Improving Nutritive Qualities (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Priority Applications (17)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2017340594A AU2017340594B2 (en) | 2016-10-03 | 2017-09-27 | Composition comprising combination of TRH analog with arundic acid, and pharmaceutically acceptable salt of arundic acid |
| CN202110788643.7A CN113559102B (zh) | 2016-10-03 | 2017-09-27 | 包括trh类似物和丙基辛酸的组合的组合物和丙基辛酸的药学上可接受的盐 |
| BR112019006429A BR112019006429A2 (pt) | 2016-10-03 | 2017-09-27 | composição compreendendo combinação de análogo de trh com ácido arúndico, e sal farmaceuticamente aceitável de ácido arúndico |
| JP2018543858A JP6596697B2 (ja) | 2016-10-03 | 2017-09-27 | Trhアナログ及びアルンド酸を組み合わせてなる組成物、並びにアルンド酸の薬学的に許容される塩 |
| EP17858265.6A EP3520795B1 (en) | 2016-10-03 | 2017-09-27 | Composition comprising combination of trh analog with arundic acid, and pharmaceutically acceptable salt of arundic acid |
| SG11201902599SA SG11201902599SA (en) | 2016-10-03 | 2017-09-27 | Composition comprising combination of trh analog with arundic acid, and pharmaceutically acceptable salt of arundic acid |
| EA201990826A EA201990826A1 (ru) | 2017-09-26 | 2017-09-27 | Композиция, содержащая комбинацию trh-аналога с арундовой кислотой, и фармацевтически приемлемая соль арундовой кислоты |
| KR1020197011464A KR20190065315A (ko) | 2016-10-03 | 2017-09-27 | Trh 아날로그 및 아룬드산을 조합하여 이루어지는 조성물, 그리고 아룬드산의 약학적으로 허용되는 염 |
| MX2019003837A MX2019003837A (es) | 2016-10-03 | 2017-09-27 | Composición que comprende una combinación del análogo de tirotropina (trh) con ácido arúndico, y la sal farmacéuticamente aceptable del acido arúndico. |
| US16/338,594 US10828303B2 (en) | 2016-10-03 | 2017-09-27 | Composition comprising combination of TRH analog with arundic acid, and pharmaceutically acceptable salt of arundic acid |
| NZ752311A NZ752311B2 (en) | 2016-10-03 | 2017-09-27 | Composition comprising combination of trh analog with arundic acid, and pharmaceutically acceptable salt of arundic acid |
| CN201780072845.3A CN110267661B (zh) | 2016-10-03 | 2017-09-27 | 包括trh类似物和丙基辛酸的组合的组合物和丙基辛酸的药学上可接受的盐 |
| CA3039027A CA3039027A1 (en) | 2016-10-03 | 2017-09-27 | Composition comprising combination of trh analog with arundic acid, and pharmaceutically acceptable salt of arundic acid |
| PH12019500636A PH12019500636A1 (en) | 2016-10-03 | 2019-03-22 | Composition comprising combination of trh analog with arundic acid, and pharmaceutically acceptable salt of arundic acid |
| IL265688A IL265688A (en) | 2016-10-03 | 2019-03-28 | A preparation containing a combination of a trh analog with arundic acid, and a pharmaceutically acceptable salt of arundic acid |
| CONC2019/0003255A CO2019003255A2 (es) | 2016-10-03 | 2019-04-01 | Composición que comprende una combinación de un anáologo de trh con ácido arúndico y una sal farmacéuticamente aceptable de ácido arúndico |
| ZA2019/02341A ZA201902341B (en) | 2016-10-03 | 2019-04-12 | Composition comprising combination of trh analog with arundic acid, and pharmaceutically acceptable salt of arundic acid |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016-195505 | 2016-10-03 | ||
| JP2016195505 | 2016-10-03 | ||
| JP2017-184731 | 2017-09-26 | ||
| JP2017184731 | 2017-09-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018066427A1 true WO2018066427A1 (ja) | 2018-04-12 |
Family
ID=61832036
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/034926 Ceased WO2018066427A1 (ja) | 2016-10-03 | 2017-09-27 | Trhアナログ及びアルンド酸を組み合わせてなる組成物、並びにアルンド酸の薬学的に許容される塩 |
Country Status (16)
| Country | Link |
|---|---|
| US (1) | US10828303B2 (enExample) |
| EP (1) | EP3520795B1 (enExample) |
| JP (2) | JP6596697B2 (enExample) |
| KR (1) | KR20190065315A (enExample) |
| CN (2) | CN110267661B (enExample) |
| AU (1) | AU2017340594B2 (enExample) |
| BR (1) | BR112019006429A2 (enExample) |
| CA (1) | CA3039027A1 (enExample) |
| CO (1) | CO2019003255A2 (enExample) |
| IL (1) | IL265688A (enExample) |
| MX (1) | MX2019003837A (enExample) |
| PH (1) | PH12019500636A1 (enExample) |
| SG (1) | SG11201902599SA (enExample) |
| TW (1) | TW201818939A (enExample) |
| WO (1) | WO2018066427A1 (enExample) |
| ZA (1) | ZA201902341B (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB201907591D0 (en) * | 2019-05-29 | 2019-07-10 | Eolas Res Limited | Composition and use |
| IT202000015895A1 (it) | 2020-07-27 | 2022-01-27 | Univ Cattolica Del Sacro Cuore | L’impiego di acido arundico nella terapia della sclerosi multipla |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62234029A (ja) * | 1985-12-27 | 1987-10-14 | Tanabe Seiyaku Co Ltd | 中枢神経賦活剤 |
| WO2005105722A1 (ja) * | 2004-04-28 | 2005-11-10 | Ono Pharmaceutical Co., Ltd. | (2r)−2−プロピルオクタン酸とアミンとからなる結晶 |
| JP2006143708A (ja) * | 2004-10-19 | 2006-06-08 | Ono Pharmaceut Co Ltd | 神経変性疾患治療用医薬 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6223429A (ja) * | 1985-07-24 | 1987-01-31 | Ishikawajima Harima Heavy Ind Co Ltd | 水素同位体の回収・貯蔵・供給装置 |
| EP0632008B1 (en) | 1993-06-01 | 1998-02-04 | Ono Pharmaceutical Co., Ltd. | Pentanoic acid derivatives |
| JPH0782166A (ja) | 1993-09-14 | 1995-03-28 | Tanabe Seiyaku Co Ltd | 抗ショック剤 |
| US9016221B2 (en) * | 2004-02-17 | 2015-04-28 | University Of Florida Research Foundation, Inc. | Surface topographies for non-toxic bioadhesion control |
| TW202216656A (zh) * | 2008-05-19 | 2022-05-01 | 美商Prtk Spv2公司 | 四環素化合物之甲苯磺酸鹽及同素異形體 |
| EP2443120B1 (en) * | 2009-06-16 | 2016-11-02 | RSPR Pharma AB | Crystalline form of pemirolast |
| CN102786414B (zh) * | 2012-08-15 | 2014-07-16 | 四川大学 | 一类用于治疗和/或预防神经退行性相关疾病的化合物 |
-
2017
- 2017-09-27 MX MX2019003837A patent/MX2019003837A/es unknown
- 2017-09-27 KR KR1020197011464A patent/KR20190065315A/ko not_active Ceased
- 2017-09-27 BR BR112019006429A patent/BR112019006429A2/pt not_active IP Right Cessation
- 2017-09-27 WO PCT/JP2017/034926 patent/WO2018066427A1/ja not_active Ceased
- 2017-09-27 SG SG11201902599SA patent/SG11201902599SA/en unknown
- 2017-09-27 US US16/338,594 patent/US10828303B2/en active Active
- 2017-09-27 AU AU2017340594A patent/AU2017340594B2/en not_active Ceased
- 2017-09-27 JP JP2018543858A patent/JP6596697B2/ja active Active
- 2017-09-27 CA CA3039027A patent/CA3039027A1/en not_active Abandoned
- 2017-09-27 EP EP17858265.6A patent/EP3520795B1/en active Active
- 2017-09-27 CN CN201780072845.3A patent/CN110267661B/zh active Active
- 2017-09-27 CN CN202110788643.7A patent/CN113559102B/zh active Active
- 2017-10-02 TW TW106133988A patent/TW201818939A/zh unknown
-
2019
- 2019-03-22 PH PH12019500636A patent/PH12019500636A1/en unknown
- 2019-03-28 IL IL265688A patent/IL265688A/en unknown
- 2019-04-01 CO CONC2019/0003255A patent/CO2019003255A2/es unknown
- 2019-04-12 ZA ZA2019/02341A patent/ZA201902341B/en unknown
- 2019-07-16 JP JP2019131162A patent/JP2019203007A/ja active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS62234029A (ja) * | 1985-12-27 | 1987-10-14 | Tanabe Seiyaku Co Ltd | 中枢神経賦活剤 |
| WO2005105722A1 (ja) * | 2004-04-28 | 2005-11-10 | Ono Pharmaceutical Co., Ltd. | (2r)−2−プロピルオクタン酸とアミンとからなる結晶 |
| JP2006143708A (ja) * | 2004-10-19 | 2006-06-08 | Ono Pharmaceut Co Ltd | 神経変性疾患治療用医薬 |
Non-Patent Citations (5)
| Title |
|---|
| JOHANSEN F. F. ET AL., ACTA NEUROPATHOLOGICA, vol. 61, 1983, pages 135 - 140 |
| KWON, MYOUNG-OK ET AL.: "List of drugs in development for neurodegenerative diseases", NEURODEGENERATIVE DIS, vol. 1, 2004, pages 113 - 152, XP009092961, ISSN: 1660-2854, DOI: 10.1159/000081242 * |
| PULSINELLI, W.A.; BRIERLEY, J.B., STROKE, vol. 10, 1979, pages 267 |
| See also references of EP3520795A4 |
| YAMAMURA M ET AL., JAPAN. J. PHARMACOL., vol. 55, 1991, pages 241 - 253 |
Also Published As
| Publication number | Publication date |
|---|---|
| PH12019500636A1 (en) | 2019-10-28 |
| CN110267661A (zh) | 2019-09-20 |
| TW201818939A (zh) | 2018-06-01 |
| CA3039027A1 (en) | 2018-04-12 |
| KR20190065315A (ko) | 2019-06-11 |
| US10828303B2 (en) | 2020-11-10 |
| AU2017340594B2 (en) | 2020-09-10 |
| CN113559102B (zh) | 2023-01-03 |
| ZA201902341B (en) | 2020-08-26 |
| CO2019003255A2 (es) | 2019-04-12 |
| EP3520795B1 (en) | 2023-06-14 |
| US20190240221A1 (en) | 2019-08-08 |
| IL265688A (en) | 2019-05-30 |
| JP2019203007A (ja) | 2019-11-28 |
| BR112019006429A2 (pt) | 2019-06-25 |
| AU2017340594A1 (en) | 2019-04-18 |
| EP3520795A4 (en) | 2020-05-13 |
| CN110267661B (zh) | 2021-08-03 |
| EP3520795A1 (en) | 2019-08-07 |
| JPWO2018066427A1 (ja) | 2019-07-25 |
| JP6596697B2 (ja) | 2019-10-30 |
| CN113559102A (zh) | 2021-10-29 |
| SG11201902599SA (en) | 2019-05-30 |
| NZ752311A (en) | 2020-12-18 |
| MX2019003837A (es) | 2019-06-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN109152933B (zh) | 诱导抗细胞凋亡bcl-2家族蛋白的降解的化合物及其用途 | |
| JP5841672B2 (ja) | N−ベンジルアニリン誘導体及びその使用 | |
| CN105001165A (zh) | 取代的二氨基嘧啶其组合物,和用其治疗的方法 | |
| CN103561770B (zh) | 硫辛酰基化合物及其用于治疗缺血性伤害的用途 | |
| JP2930245B2 (ja) | 1,2,3,4―テトラヒドロ―9―アクリジナミンの誘導体 | |
| JP2016511273A (ja) | D−グルシトール,1−デオキシ−1−(メチルアミノ)−,1−(6−アミノ−3,5−ジフルオロピリジン−2−イル)−8−クロロ−6−フルオロ−1,4−ジヒドロ−7−(3−ヒドロキシアゼチジン−1−イル)−4−オキソ−3−キノリンカルボン酸塩の結晶型 | |
| CN102234287B (zh) | 硝基咪唑类化合物、其制备方法和用途 | |
| KR20210097100A (ko) | 2-(1-아실옥시-n-펜틸)벤조산 및 염기성 아미노산 또는 아미노구아니딘이 형성하는 염, 이의 제조 방법 및 용도 | |
| JP6596697B2 (ja) | Trhアナログ及びアルンド酸を組み合わせてなる組成物、並びにアルンド酸の薬学的に許容される塩 | |
| CN108239095A (zh) | 一类吡喃并咔唑生物碱及其制备方法和其药物组合物与用途 | |
| WO2019119832A1 (zh) | 用于代谢性疾病治疗的化合物及其制备方法和应用 | |
| TWI462915B (zh) | 一種組織胺h3受體拮抗劑的新穎富馬酸鹽 | |
| JP2002527350A (ja) | 発作を治療するのに有用なアミノ酸誘導体 | |
| CN113166071A (zh) | 尼拉帕利盐 | |
| WO1994029303A1 (en) | Heterocyclic chemistry | |
| JP2004517818A (ja) | 細胞障害抑制剤 | |
| CN118139863A (zh) | 单酰基甘油脂肪酶抑制剂的结晶形式 | |
| NZ752311B2 (en) | Composition comprising combination of trh analog with arundic acid, and pharmaceutically acceptable salt of arundic acid | |
| HK40005518A (en) | Composition comprising combination of trh analog with arundic acid, and pharmaceutically acceptable salt of arundic acid | |
| JPWO2003087091A1 (ja) | キノキサリンジオン誘導体無水物の新規結晶 | |
| TW202448418A (zh) | 發炎性腸道疾病之治療 | |
| WO2020156360A1 (zh) | 胆碱酯酶抑制剂多晶型及其应用 | |
| CN118724879A (zh) | 一种氘代稠环化合物及其制备方法与用途 | |
| EA050218B1 (ru) | Производные 6-метилурацила, обладающие антихолинэстеразной активностью, и их применение | |
| JP2002516611A (ja) | 結晶性(−)−3r,4r−トランス−7−メトキシ−2,2−ジメチル−3−フェニル−4−{4−〔2−(ピロリジン−1−イル)エトキシ〕フェニル}クロマン水素マレエート |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17858265 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2018543858 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 265688 Country of ref document: IL |
|
| ENP | Entry into the national phase |
Ref document number: 3039027 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: NC2019/0003255 Country of ref document: CO |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112019006429 Country of ref document: BR |
|
| WWP | Wipo information: published in national office |
Ref document number: NC2019/0003255 Country of ref document: CO |
|
| ENP | Entry into the national phase |
Ref document number: 2017340594 Country of ref document: AU Date of ref document: 20170927 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 20197011464 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2017858265 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 112019006429 Country of ref document: BR Kind code of ref document: A2 Effective date: 20190329 |