WO2017163603A1 - 椎間板治療用組成物 - Google Patents
椎間板治療用組成物 Download PDFInfo
- Publication number
- WO2017163603A1 WO2017163603A1 PCT/JP2017/002925 JP2017002925W WO2017163603A1 WO 2017163603 A1 WO2017163603 A1 WO 2017163603A1 JP 2017002925 W JP2017002925 W JP 2017002925W WO 2017163603 A1 WO2017163603 A1 WO 2017163603A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composition
- nucleus pulposus
- intervertebral disc
- present
- group
- Prior art date
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 274
- 235000010443 alginic acid Nutrition 0.000 claims abstract description 102
- 229920000615 alginic acid Polymers 0.000 claims abstract description 102
- 239000002158 endotoxin Substances 0.000 claims abstract description 100
- 150000004781 alginic acids Chemical class 0.000 claims abstract description 90
- 229960001126 alginic acid Drugs 0.000 claims abstract description 89
- 239000000783 alginic acid Substances 0.000 claims abstract description 89
- 229910052751 metal Inorganic materials 0.000 claims abstract description 73
- 239000002184 metal Substances 0.000 claims abstract description 73
- 150000003839 salts Chemical class 0.000 claims abstract description 69
- 239000000243 solution Substances 0.000 claims description 104
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 claims description 87
- 235000010413 sodium alginate Nutrition 0.000 claims description 87
- 239000000661 sodium alginate Substances 0.000 claims description 87
- 229940005550 sodium alginate Drugs 0.000 claims description 87
- 238000000034 method Methods 0.000 claims description 85
- 238000011282 treatment Methods 0.000 claims description 65
- 238000011049 filling Methods 0.000 claims description 52
- 239000003431 cross linking reagent Substances 0.000 claims description 50
- 206010061246 Intervertebral disc degeneration Diseases 0.000 claims description 36
- 208000021600 intervertebral disc degenerative disease Diseases 0.000 claims description 32
- 230000007850 degeneration Effects 0.000 claims description 28
- 230000007547 defect Effects 0.000 claims description 27
- 238000012360 testing method Methods 0.000 claims description 26
- 208000018180 degenerative disc disease Diseases 0.000 claims description 25
- 239000007924 injection Substances 0.000 claims description 17
- 238000002347 injection Methods 0.000 claims description 17
- 230000002265 prevention Effects 0.000 claims description 17
- 230000006378 damage Effects 0.000 claims description 16
- 230000001629 suppression Effects 0.000 claims description 16
- 206010055040 Intervertebral disc injury Diseases 0.000 claims description 15
- 208000003618 Intervertebral Disc Displacement Diseases 0.000 claims description 14
- 238000000569 multi-angle light scattering Methods 0.000 claims description 12
- 206010050296 Intervertebral disc protrusion Diseases 0.000 claims description 11
- 229910021645 metal ion Inorganic materials 0.000 claims description 11
- 230000003412 degenerative effect Effects 0.000 claims description 9
- 208000034628 Celiac artery compression syndrome Diseases 0.000 claims description 8
- 206010041591 Spinal osteoarthritis Diseases 0.000 claims description 8
- 208000005801 spondylosis Diseases 0.000 claims description 8
- 208000018650 Intervertebral disc disease Diseases 0.000 claims description 7
- 150000001875 compounds Chemical class 0.000 claims description 7
- 238000000338 in vitro Methods 0.000 claims description 4
- 208000005198 spinal stenosis Diseases 0.000 claims description 4
- 201000008370 Discitis Diseases 0.000 claims 1
- 230000008929 regeneration Effects 0.000 abstract description 23
- 238000011069 regeneration method Methods 0.000 abstract description 23
- 230000001737 promoting effect Effects 0.000 abstract description 3
- 210000004027 cell Anatomy 0.000 description 104
- 210000001519 tissue Anatomy 0.000 description 50
- 238000005259 measurement Methods 0.000 description 38
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 30
- 238000011156 evaluation Methods 0.000 description 28
- 102000008186 Collagen Human genes 0.000 description 20
- 108010035532 Collagen Proteins 0.000 description 20
- 229920002683 Glycosaminoglycan Polymers 0.000 description 20
- 241001494479 Pecora Species 0.000 description 20
- 229920001436 collagen Polymers 0.000 description 20
- 239000000499 gel Substances 0.000 description 20
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 19
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 19
- 229960002591 hydroxyproline Drugs 0.000 description 19
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 19
- 239000000523 sample Substances 0.000 description 17
- 241000486679 Antitype Species 0.000 description 15
- 239000011324 bead Substances 0.000 description 14
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical group O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 13
- 102000016611 Proteoglycans Human genes 0.000 description 13
- 108010067787 Proteoglycans Proteins 0.000 description 13
- 229940072056 alginate Drugs 0.000 description 13
- 230000000694 effects Effects 0.000 description 13
- 239000000463 material Substances 0.000 description 13
- 239000002904 solvent Substances 0.000 description 13
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 12
- 239000011575 calcium Substances 0.000 description 12
- 229910052791 calcium Inorganic materials 0.000 description 11
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 10
- 210000000845 cartilage Anatomy 0.000 description 10
- 235000013305 food Nutrition 0.000 description 10
- 230000009467 reduction Effects 0.000 description 10
- 210000002966 serum Anatomy 0.000 description 10
- 102000000503 Collagen Type II Human genes 0.000 description 9
- 108010041390 Collagen Type II Proteins 0.000 description 9
- 230000001640 apoptogenic effect Effects 0.000 description 9
- 210000001188 articular cartilage Anatomy 0.000 description 9
- 238000005227 gel permeation chromatography Methods 0.000 description 9
- 235000003642 hunger Nutrition 0.000 description 9
- 239000002504 physiological saline solution Substances 0.000 description 9
- 230000037351 starvation Effects 0.000 description 9
- 241000199919 Phaeophyceae Species 0.000 description 8
- 239000007864 aqueous solution Substances 0.000 description 8
- 238000001723 curing Methods 0.000 description 8
- VWDWKYIASSYTQR-UHFFFAOYSA-N sodium nitrate Chemical compound [Na+].[O-][N+]([O-])=O VWDWKYIASSYTQR-UHFFFAOYSA-N 0.000 description 8
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 8
- 238000001356 surgical procedure Methods 0.000 description 8
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 7
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 7
- 230000030833 cell death Effects 0.000 description 7
- 230000005786 degenerative changes Effects 0.000 description 7
- 210000002744 extracellular matrix Anatomy 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 210000001032 spinal nerve Anatomy 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- 206010019909 Hernia Diseases 0.000 description 6
- 239000012901 Milli-Q water Substances 0.000 description 6
- 241000283973 Oryctolagus cuniculus Species 0.000 description 6
- 239000012530 fluid Substances 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 230000005764 inhibitory process Effects 0.000 description 6
- WEXRUCMBJFQVBZ-UHFFFAOYSA-N pentobarbital Chemical compound CCCC(C)C1(CC)C(=O)NC(=O)NC1=O WEXRUCMBJFQVBZ-UHFFFAOYSA-N 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 5
- 206010060738 Intervertebral discitis Diseases 0.000 description 5
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 239000008213 purified water Substances 0.000 description 5
- 239000012266 salt solution Substances 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- IAJILQKETJEXLJ-SQOUGZDYSA-N L-guluronic acid Chemical compound O=C[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O IAJILQKETJEXLJ-SQOUGZDYSA-N 0.000 description 4
- 241001512709 Lessonia <stramenopiles> Species 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 229930182555 Penicillin Natural products 0.000 description 4
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 4
- 108010059712 Pronase Proteins 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 230000032683 aging Effects 0.000 description 4
- AEMOLEFTQBMNLQ-UHFFFAOYSA-N beta-D-galactopyranuronic acid Natural products OC1OC(C(O)=O)C(O)C(O)C1O AEMOLEFTQBMNLQ-UHFFFAOYSA-N 0.000 description 4
- 230000010261 cell growth Effects 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- AEMOLEFTQBMNLQ-YBSDWZGDSA-N d-mannuronic acid Chemical compound O[C@@H]1O[C@@H](C(O)=O)[C@H](O)[C@@H](O)[C@H]1O AEMOLEFTQBMNLQ-YBSDWZGDSA-N 0.000 description 4
- 238000004925 denaturation Methods 0.000 description 4
- 230000036425 denaturation Effects 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 239000003480 eluent Substances 0.000 description 4
- 239000003102 growth factor Substances 0.000 description 4
- 239000000017 hydrogel Substances 0.000 description 4
- 230000001965 increasing effect Effects 0.000 description 4
- 208000014674 injury Diseases 0.000 description 4
- 238000000691 measurement method Methods 0.000 description 4
- 229940049954 penicillin Drugs 0.000 description 4
- 229920001282 polysaccharide Polymers 0.000 description 4
- 239000005017 polysaccharide Substances 0.000 description 4
- 150000004804 polysaccharides Chemical class 0.000 description 4
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 4
- 239000002994 raw material Substances 0.000 description 4
- 238000002271 resection Methods 0.000 description 4
- 239000004317 sodium nitrate Substances 0.000 description 4
- 235000010344 sodium nitrate Nutrition 0.000 description 4
- 229960005322 streptomycin Drugs 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 230000008733 trauma Effects 0.000 description 4
- 239000008215 water for injection Substances 0.000 description 4
- 241001474374 Blennius Species 0.000 description 3
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 3
- 241001466453 Laminaria Species 0.000 description 3
- 241001491708 Macrocystis Species 0.000 description 3
- 208000031481 Pathologic Constriction Diseases 0.000 description 3
- 239000006096 absorbing agent Substances 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- WYTGDNHDOZPMIW-RCBQFDQVSA-N alstonine Natural products C1=CC2=C3C=CC=CC3=NC2=C2N1C[C@H]1[C@H](C)OC=C(C(=O)OC)[C@H]1C2 WYTGDNHDOZPMIW-RCBQFDQVSA-N 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- 239000003708 ampul Substances 0.000 description 3
- 230000006907 apoptotic process Effects 0.000 description 3
- 239000001110 calcium chloride Substances 0.000 description 3
- 229910001628 calcium chloride Inorganic materials 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 230000006835 compression Effects 0.000 description 3
- 238000007906 compression Methods 0.000 description 3
- 230000003013 cytotoxicity Effects 0.000 description 3
- 231100000135 cytotoxicity Toxicity 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 239000012153 distilled water Substances 0.000 description 3
- 239000012091 fetal bovine serum Substances 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 3
- 210000004276 hyalin Anatomy 0.000 description 3
- 230000007062 hydrolysis Effects 0.000 description 3
- 238000006460 hydrolysis reaction Methods 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 230000006698 induction Effects 0.000 description 3
- 238000005342 ion exchange Methods 0.000 description 3
- 210000004705 lumbosacral region Anatomy 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 210000003205 muscle Anatomy 0.000 description 3
- 239000003960 organic solvent Substances 0.000 description 3
- 229960001412 pentobarbital Drugs 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 230000001172 regenerating effect Effects 0.000 description 3
- 230000035939 shock Effects 0.000 description 3
- 239000008279 sol Substances 0.000 description 3
- 238000010186 staining Methods 0.000 description 3
- 230000036262 stenosis Effects 0.000 description 3
- 208000037804 stenosis Diseases 0.000 description 3
- 239000011550 stock solution Substances 0.000 description 3
- 238000005303 weighing Methods 0.000 description 3
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 2
- 102000016284 Aggrecans Human genes 0.000 description 2
- 108010067219 Aggrecans Proteins 0.000 description 2
- 241000271566 Aves Species 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical class [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 241000700198 Cavia Species 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- 229920001287 Chondroitin sulfate Polymers 0.000 description 2
- 241000699800 Cricetinae Species 0.000 description 2
- 241000283086 Equidae Species 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 241000239218 Limulus Species 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 208000012902 Nervous system disease Diseases 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 229920001218 Pullulan Polymers 0.000 description 2
- 239000004373 Pullulan Substances 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 241000282887 Suidae Species 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 210000000577 adipose tissue Anatomy 0.000 description 2
- 229940069428 antacid Drugs 0.000 description 2
- 239000003159 antacid agent Substances 0.000 description 2
- 230000001458 anti-acid effect Effects 0.000 description 2
- 238000000149 argon plasma sintering Methods 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229920001222 biopolymer Polymers 0.000 description 2
- 239000012496 blank sample Substances 0.000 description 2
- 210000001185 bone marrow Anatomy 0.000 description 2
- BQRGNLJZBFXNCZ-UHFFFAOYSA-N calcein am Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(C)=O)=C(OC(C)=O)C=C1OC1=C2C=C(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(=O)C)C(OC(C)=O)=C1 BQRGNLJZBFXNCZ-UHFFFAOYSA-N 0.000 description 2
- 238000011088 calibration curve Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- -1 etc.) Substances 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- 210000004700 fetal blood Anatomy 0.000 description 2
- 229920001519 homopolymer Polymers 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 239000008176 lyophilized powder Substances 0.000 description 2
- 125000001288 lysyl group Chemical group 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 210000005036 nerve Anatomy 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 238000005192 partition Methods 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 235000019423 pullulan Nutrition 0.000 description 2
- 238000010188 recombinant method Methods 0.000 description 2
- OARRHUQTFTUEOS-UHFFFAOYSA-N safranin Chemical compound [Cl-].C=12C=C(N)C(C)=CC2=NC2=CC(C)=C(N)C=C2[N+]=1C1=CC=CC=C1 OARRHUQTFTUEOS-UHFFFAOYSA-N 0.000 description 2
- 239000012488 sample solution Substances 0.000 description 2
- 231100000241 scar Toxicity 0.000 description 2
- 230000037390 scarring Effects 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 230000009469 supplementation Effects 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- APKFDSVGJQXUKY-KKGHZKTASA-N Amphotericin-B Natural products O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1C=CC=CC=CC=CC=CC=CC=C[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-KKGHZKTASA-N 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 102000000412 Annexin Human genes 0.000 description 1
- 108050008874 Annexin Proteins 0.000 description 1
- 241000512260 Ascophyllum Species 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 229920002498 Beta-glucan Polymers 0.000 description 1
- 101100008049 Caenorhabditis elegans cut-5 gene Proteins 0.000 description 1
- 101710132601 Capsid protein Proteins 0.000 description 1
- 102000011727 Caspases Human genes 0.000 description 1
- 108010076667 Caspases Proteins 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 102000029816 Collagenase Human genes 0.000 description 1
- 108060005980 Collagenase Proteins 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 241001512723 Ecklonia Species 0.000 description 1
- 241000230129 Eisenia <Phaeophyceae> Species 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 101150021185 FGF gene Proteins 0.000 description 1
- 229920002148 Gellan gum Polymers 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 102100021866 Hepatocyte growth factor Human genes 0.000 description 1
- 101000898034 Homo sapiens Hepatocyte growth factor Proteins 0.000 description 1
- 101001076408 Homo sapiens Interleukin-6 Proteins 0.000 description 1
- 101000868152 Homo sapiens Son of sevenless homolog 1 Proteins 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102000004218 Insulin-Like Growth Factor I Human genes 0.000 description 1
- 108090000723 Insulin-Like Growth Factor I Proteins 0.000 description 1
- 101000844802 Lacticaseibacillus rhamnosus Teichoic acid D-alanyltransferase Proteins 0.000 description 1
- 241001260563 Lessonia nigrescens Species 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- PCZOHLXUXFIOCF-UHFFFAOYSA-N Monacolin X Natural products C12C(OC(=O)C(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 PCZOHLXUXFIOCF-UHFFFAOYSA-N 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 241000283977 Oryctolagus Species 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 206010033799 Paralysis Diseases 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- RYMZZMVNJRMUDD-UHFFFAOYSA-N SJ000286063 Natural products C12C(OC(=O)C(C)(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 RYMZZMVNJRMUDD-UHFFFAOYSA-N 0.000 description 1
- 108020004459 Small interfering RNA Proteins 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 229920002385 Sodium hyaluronate Polymers 0.000 description 1
- 201000002661 Spondylitis Diseases 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229960004821 amikacin Drugs 0.000 description 1
- LKCWBDHBTVXHDL-RMDFUYIESA-N amikacin Chemical compound O([C@@H]1[C@@H](N)C[C@H]([C@@H]([C@H]1O)O[C@@H]1[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O1)O)NC(=O)[C@@H](O)CCN)[C@H]1O[C@H](CN)[C@@H](O)[C@H](O)[C@H]1O LKCWBDHBTVXHDL-RMDFUYIESA-N 0.000 description 1
- 229960003942 amphotericin b Drugs 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940124599 anti-inflammatory drug Drugs 0.000 description 1
- 230000001754 anti-pyretic effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000002221 antipyretic Substances 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 229910052788 barium Inorganic materials 0.000 description 1
- DSAJWYNOEDNPEQ-UHFFFAOYSA-N barium atom Chemical compound [Ba] DSAJWYNOEDNPEQ-UHFFFAOYSA-N 0.000 description 1
- 238000012742 biochemical analysis Methods 0.000 description 1
- 239000000560 biocompatible material Substances 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000002798 bone marrow cell Anatomy 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 229910001424 calcium ion Inorganic materials 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 210000000038 chest Anatomy 0.000 description 1
- 210000001612 chondrocyte Anatomy 0.000 description 1
- 229960002424 collagenase Drugs 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000002124 endocrine Effects 0.000 description 1
- 238000011013 endotoxin removal Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 210000000968 fibrocartilage Anatomy 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- 238000001879 gelation Methods 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 239000000216 gellan gum Substances 0.000 description 1
- 235000010492 gellan gum Nutrition 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 238000007490 hematoxylin and eosin (H&E) staining Methods 0.000 description 1
- 210000003035 hyaline cartilage Anatomy 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000002055 immunohistochemical effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229940030980 inova Drugs 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 239000008274 jelly Substances 0.000 description 1
- 210000003041 ligament Anatomy 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000002690 local anesthesia Methods 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 229960004844 lovastatin Drugs 0.000 description 1
- PCZOHLXUXFIOCF-BXMDZJJMSA-N lovastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 PCZOHLXUXFIOCF-BXMDZJJMSA-N 0.000 description 1
- QLJODMDSTUBWDW-UHFFFAOYSA-N lovastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(C)C=C21 QLJODMDSTUBWDW-UHFFFAOYSA-N 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000005499 meniscus Effects 0.000 description 1
- 210000002901 mesenchymal stem cell Anatomy 0.000 description 1
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 229940035363 muscle relaxants Drugs 0.000 description 1
- 239000003158 myorelaxant agent Substances 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 208000004296 neuralgia Diseases 0.000 description 1
- 208000021722 neuropathic pain Diseases 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 230000000050 nutritive effect Effects 0.000 description 1
- 239000000014 opioid analgesic Substances 0.000 description 1
- 229940005483 opioid analgesics Drugs 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000000399 orthopedic effect Effects 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- 230000036407 pain Effects 0.000 description 1
- 229940124641 pain reliever Drugs 0.000 description 1
- 229960005489 paracetamol Drugs 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229940096701 plain lipid modifying drug hmg coa reductase inhibitors Drugs 0.000 description 1
- 229920000867 polyelectrolyte Polymers 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 235000010408 potassium alginate Nutrition 0.000 description 1
- 239000000737 potassium alginate Substances 0.000 description 1
- MZYRDLHIWXQJCQ-YZOKENDUSA-L potassium alginate Chemical compound [K+].[K+].O1[C@@H](C([O-])=O)[C@@H](OC)[C@H](O)[C@H](O)[C@@H]1O[C@@H]1[C@@H](C([O-])=O)O[C@@H](O)[C@@H](O)[C@H]1O MZYRDLHIWXQJCQ-YZOKENDUSA-L 0.000 description 1
- 238000007781 pre-processing Methods 0.000 description 1
- 229940071643 prefilled syringe Drugs 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- ZNZJJSYHZBXQSM-UHFFFAOYSA-N propane-2,2-diamine Chemical compound CC(C)(N)N ZNZJJSYHZBXQSM-UHFFFAOYSA-N 0.000 description 1
- 229940024999 proteolytic enzymes for treatment of wounds and ulcers Drugs 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229960002855 simvastatin Drugs 0.000 description 1
- RYMZZMVNJRMUDD-HGQWONQESA-N simvastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)C(C)(C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 RYMZZMVNJRMUDD-HGQWONQESA-N 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 239000000779 smoke Substances 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 229940010747 sodium hyaluronate Drugs 0.000 description 1
- YWIVKILSMZOHHF-QJZPQSOGSA-N sodium;(2s,3s,4s,5r,6r)-6-[(2s,3r,4r,5s,6r)-3-acetamido-2-[(2s,3s,4r,5r,6r)-6-[(2r,3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2- Chemical compound [Na+].CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 YWIVKILSMZOHHF-QJZPQSOGSA-N 0.000 description 1
- MSXHSNHNTORCAW-MPGIDXPLSA-M sodium;(3s,4s,5s,6r)-3,4,5,6-tetrahydroxyoxane-2-carboxylate Chemical compound [Na+].O[C@@H]1OC(C([O-])=O)[C@@H](O)[C@H](O)[C@@H]1O MSXHSNHNTORCAW-MPGIDXPLSA-M 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000012086 standard solution Substances 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 229960000707 tobramycin Drugs 0.000 description 1
- NLVFBUXFDBBNBW-PBSUHMDJSA-N tobramycin Chemical compound N[C@@H]1C[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N NLVFBUXFDBBNBW-PBSUHMDJSA-N 0.000 description 1
- 229950003937 tolonium Drugs 0.000 description 1
- HNONEKILPDHFOL-UHFFFAOYSA-M tolonium chloride Chemical compound [Cl-].C1=C(C)C(N)=CC2=[S+]C3=CC(N(C)C)=CC=C3N=C21 HNONEKILPDHFOL-UHFFFAOYSA-M 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 229940072358 xylocaine Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/54—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/442—Intervertebral or spinal discs, e.g. resilient
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/19—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles lyophilised, i.e. freeze-dried, solutions or dispersions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/20—Polysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/30—Joints
- A61F2/44—Joints for the spine, e.g. vertebrae, spinal discs
- A61F2/442—Intervertebral or spinal discs, e.g. resilient
- A61F2002/444—Intervertebral or spinal discs, e.g. resilient for replacing the nucleus pulposus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/715—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
- A61K31/734—Alginic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/20—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing organic materials
- A61L2300/23—Carbohydrates
- A61L2300/232—Monosaccharides, disaccharides, polysaccharides, lipopolysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/412—Tissue-regenerating or healing or proliferative agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
- A61L2400/06—Flowable or injectable implant compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/38—Materials or treatment for tissue regeneration for reconstruction of the spine, vertebrae or intervertebral discs
Definitions
- the present invention relates to a composition for treating an intervertebral disc, and particularly to a composition for supplementing the nucleus pulposus of an intervertebral disc.
- the spine is a rod-like skeleton with a series of vertebrae and supports the trunk and head.
- the vertebrae and vertebrae are connected by an intervertebral disc between the vertebrae.
- the intervertebral disc is a disc-like avascular tissue, and has a structure in which an annulus fibrosus surrounds the nucleus pulposus and endplates are arranged above and below.
- the nucleus pulposus of the intervertebral disc is composed of nucleus pulposus cells and the extracellular matrix thereof, and is a gel-like elastic structure containing a lot of water, and serves as a cushion for absorbing pressure applied between the vertebral bodies.
- the annulus fibrosus consists of a layered structure of fibrocartilage and a layer of collagen surrounding it, limiting the rotational movement between the vertebral bodies.
- the end plate is a hyaline cartilage tissue and firmly connects the intervertebral disc and the vertebral body.
- the nucleus pulposus located at the center of the intervertebral disc has a characteristic composition compared to the annulus fibrosus, end plate, and other cartilage tissues. That is, the main components of the extracellular matrix of the nucleus pulposus are water (70-90%; decreases with aging), type II collagen (20% of dry weight), proteoglycan (50% of dry weight), Compared with other cartilage tissues such as plates and articular cartilage, the ratio of proteoglycan to collagen is high (Non-patent Document 1). On the other hand, the extracellular matrix of other cartilage tissues such as articular cartilage has a higher proportion of collagen than proteoglycan.
- the function of the intervertebral disc as a shock absorber largely depends on its abundant water content. Such abundant water is maintained mainly by the negatively charged glycosaminoglycan bound to the proteoglycan core protein, which attracts water. Further, it is disclosed that the structure and size of proteoglycan present in the intervertebral disc is different from that of proteoglycan present in articular cartilage, and in particular, the difference in proteoglycan in nucleus pulposus was significant (Non-patent Document 2).
- the nucleus pulposus, annulus fibrosus, and endplate of the intervertebral disc have different structures and functions, and are maintained by groups of cells having different phenotypes.
- the nucleus pulposus cells present in the nucleus pulposus are round and create a proteoglycan-rich matrix. Cells present in the annulus are encased in a collagen fiber matrix.
- Non-patent Document 1 it has been reported that cells in such intervertebral discs have different phenotypes, and there are also phenotypic differences compared to articular chondrocytes.
- Intervertebral discs may be degenerated or damaged due to aging, trauma, disease, etc.
- Intervertebral disc degeneration is a state in which the number of cells, water content, extracellular matrix (type II collagen, aggrecan, etc.), etc. of the intervertebral disc have decreased, and the function of the intervertebral disc as a shock absorber cannot be performed as it progresses.
- Examples of intervertebral disc degeneration and intervertebral disc injury include intervertebral disc injuries due to intervertebral disc herniation, intervertebral disc disease, spinal degeneration, purulent intervertebral discitis, degenerative spondylosis, spinal stenosis, trauma, and the like.
- the annulus covering the nucleus pulposus deforms or cracks to form a hernia and protrudes out of the intervertebral disc, and the protruding nucleus pulposus compresses the spinal nerve, causing pain, paralysis, and the like.
- fibroblast-like cells may accumulate to form a tissue having different mechanical characteristics from the original nucleus pulposus. For this reason, the hernia recurrence rate is high after discectomy. Although the recurrence rate within 5 years after discectomy of the intervertebral disc is said to be about 4-15%, recent long-term data have shown that it will recur in the majority after 10 years.
- the hernia recurs reoperation is required, but the spinal nerve is buried in scar tissue formed after the first operation, and it is difficult to confirm the position of the spinal nerve. Even if the position of the spinal nerve can be confirmed, since the scar is thick and hard, it is extremely difficult to separate the spinal nerve from the surrounding tissue. Reoperation requires extremely difficult techniques. For this reason, there is a need to establish a surgical method in which hernia does not recur and scarring after discectomy.
- a treatment method that introduces polyelectrolyte materials into the disc space without removing the nucleus pulposus or annulus is proposed.
- One example is alginate (Patent Document 1).
- a method for enhancing the function of the intervertebral disc including injecting a cartilage protective material such as glycosaminoglycan into a site where it is required has been proposed.
- a cartilage protective material such as glycosaminoglycan
- the cartilage protective material is the parent of sodium alginate.
- Amphic derivatives are mentioned (Patent Document 2).
- a device for injecting an antacid into the intervertebral disc has been disclosed (Patent Document 3).
- an intervertebral disc filler may be optionally injected, and one of many examples of fillers is alginate crosslinked with calcium or barium (Patent Document 3).
- Patent Document 3 alginate crosslinked with calcium or barium
- hydrogels such as alginate are being studied as a nucleus pulposus filling material.
- hydrogels such as alginate As a nucleus pulposus filling material, its mechanical strength is a problem, and it is recommended that it retain its shape for a certain period of time when used in vivo.
- an object of the present invention is to provide a nucleus pulposus composition that can promote the regeneration of nucleus pulposus of the intervertebral disc. It is another object of the present invention to provide a nucleus pulposus composition that is relatively easy to fill and has little risk of complications such as compression of the spinal nerve.
- the present inventors examined the possibility of nucleus pulposus supplementation with a biocompatible material as a method for treating disc degeneration and disc damage.
- a biocompatible material such as a nucleus pulposus filling material
- the mechanical strength of hydrogels has been a problem, and when used in vivo It was also recommended that the shape be maintained for a certain period.
- the present inventors conversely injected a composition containing low endotoxin sodium alginate into the nucleus pulposus site in a sol state, and contacted with a cross-linking agent at the filling port of the composition on the surface of the intervertebral disc to prevent leakage.
- the present invention is as follows.
- the composition is applied to the nucleus pulposus site through the filling port of the composition on the surface of the intervertebral disc, and a part of the composition is cured, and the cross-linking agent is brought into contact with the filling port of the composition on the intervertebral disc surface.
- composition according to any one of [1] to [2] above wherein [4] The application of the composition to the nucleus pulposus site is performed by applying the composition to a nucleus pulposus defect formed by removing at least a part of the nucleus pulposus. [3] The composition according to any one of [3]. [5] The composition according to any one of [1] to [4] above, wherein the fluid composition has a viscosity of 100 mPa ⁇ s to 30000 mPa ⁇ s.
- [6A] The monovalent metal salt of low endotoxin alginic acid according to any one of [1] to [5A] above, wherein the weight average molecular weight (absolute molecular weight) measured by GPC-MALS method is 80,000 or more.
- [7] The composition according to any one of [1] to [6A] above, wherein the concentration of the monovalent metal salt of low endotoxin alginic acid is 0.5 w / v% to 5 w / v%. object.
- [7A] The composition according to any one of [1] to [6A] above, wherein the concentration of the monovalent metal salt of low endotoxin alginic acid is 0.5 w / w% to 5 w / w%. object.
- Composition Composition.
- the crosslinking agent is a divalent or higher-valent metal ion compound.
- composition according to [10] wherein the divalent or higher valent metal ion compound is at least one metal ion compound selected from the group consisting of Ca 2+ , Mg 2+ , Ba 2+ and Sr 2+ .
- intervertebral disc degeneration and / or intervertebral disc injury is selected from the group consisting of intervertebral disc herniation, intervertebral disc disease, spinal degeneration, suppurative intervertebral discitis, degenerative spondylosis, spinal canal stenosis, and intervertebral disc injury.
- a part of the composition is cured in vitro in a diameter of 6 mm according to Example 4 of the present specification using the same method and ratio of use of the cross-linking agent as filling the nucleus pulposus site.
- the above test tube is filled with 500 ⁇ L of low endotoxin sodium alginate and a cross-linking agent, and after standing for 1 hour, at least 50% of the volume of the composition in the test tube can be aspirated with a syringe equipped with a 21G needle.
- the composition having fluidity is any one of the above-mentioned [1] to [14A], wherein the composition has fluidity that can be injected with a 21G injection needle after the composition is allowed to stand at 20 ° C. for 1 hour.
- An intervertebral disk nucleus pulposus replacement kit comprising at least the composition according to any one of [1] to [14B] above and a crosslinking agent.
- a method for the treatment, prevention or suppression of recurrence of disc degeneration and / or disc damage A composition containing a monovalent metal salt of low endotoxin alginic acid and having fluidity is applied to the nucleus pulposus site of an intervertebral disc of a subject in need of the treatment, prevention or relapse suppression, Curing the portion of the applied composition.
- the intervertebral disc degeneration and / or intervertebral disc injury is at least selected from the group consisting of intervertebral disc herniation, intervertebral disc disease, spinal degeneration, purulent intervertebral discitis, degenerative spondylosis, spinal canal stenosis, and intervertebral disc injury.
- At least the intervertebral disc degeneration and / or intervertebral disc injury is selected from the group consisting of intervertebral disc herniation, intervertebral disc disease, spinal degeneration, suppurative intervertebral discitis, degenerative spondylosis, spinal canal stenosis, and intervertebral disc injury.
- a composition containing a monovalent metal salt of low endotoxin alginic acid and having fluidity is applied to a nucleus pulposus site of an intervertebral disc in a subject in need of treatment, prevention or relapse suppression of disc degeneration and / or disc damage.
- a monovalent metal salt of low endotoxin alginic acid for use in the treatment, prevention or relapse inhibition of disc degeneration and / or disc damage, which cures a portion of the applied composition.
- the present invention provides a nucleus pulposus composition that can promote the regeneration of the nucleus pulposus of an intervertebral disc. According to the composition of the present invention, it is also possible to suppress degenerative changes in the entire intervertebral disc tissue including not only the intervertebral disc nucleus pulposus but also the annulus fibrosus. In addition, the composition of the present invention has an effect of increasing the proportion of type II collagen-positive hyaline cartilage-like cells in the nucleus pulposus.
- the composition of the present invention is used as a nucleus pulposus replacement material in the prevention, treatment, or suppression of recurrence of a disease related to degenerative disc such as disc herniation or injured disc caused by trauma. Can do.
- composition of a preferred embodiment of the present invention can be injected into the nucleus pulposus site using a syringe or the like in a sol state, and is not only under direct view but also percutaneous nucleusectomy (about 5 mm).
- the spinal nerve may be compressed and damaged if it protrudes into the spinal canal, but the composition according to a more preferred embodiment of the present invention is limited to the surface. Because it gels, it is safe with less worry of such complications.
- composition of a particularly preferred embodiment of the present invention can prevent hernia recurrence and scarring after discectomy (resection). Also, in one of the preferred embodiments of the present invention, the burden on the intervertebral disc adjacent to the treated intervertebral disc is reduced by applying the composition of the present invention to the intervertebral disc nucleus having disc degeneration and / or intervertebral disc damage. This makes it possible to prevent and / or reduce the degeneration of adjacent discs.
- composition of the present invention satisfies any one or more of the above effects.
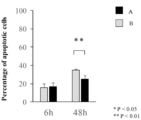
- the viable cell rate after 6 hours and 48 hours after the start of serum starvation is shown.
- Group A low endotoxin sodium alginate; and Group B: food grade sodium alginate.
- the apoptotic cell rate after 6 hours and 48 hours after the start of serum starvation is shown.
- Group A low endotoxin sodium alginate; and Group B: food grade sodium alginate.
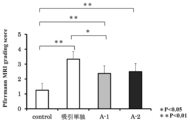
- Aspiration alone group and treatment group (Group A-1 and Group A-2). It is a graph which shows the histological evaluation result of the severity of intervertebral disc degeneration in 4 weeks after surgery. Normal control group, aspiration alone group, and treatment group (Group A-1 and Group A-2).
- A A stained photograph of an intervertebral disc tissue specimen 4 weeks after surgery. Normal control group, aspiration alone group, and treatment group (Group A-2).
- B shows a stained photograph of an intervertebral disc tissue specimen 12 weeks after the operation. Normal control group, aspiration alone group, and treatment group (Group A-2). It is a graph which shows the anti-Type IIcollagen antibody positive cell rate with respect to the number of cells in the disc tissue section at 4 weeks and 12 weeks after the operation.
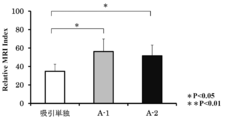
- Normal control group aspiration alone group, and treatment group (Group A-2). It is a graph which shows the evaluation result by the modified Boos classification
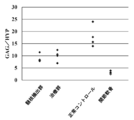
- the ratio of sulfated glycosaminoglycan (GAG) to hydroxyproline (HYP) in the intervertebral disc nucleus at 4 weeks after the operation is shown.
- GAG sulfated glycosaminoglycan
- HEP hydroxyproline
- composition of the present invention relates to a composition preferably used for nucleus pulposus replacement of an intervertebral disc.
- the composition of the present invention contains a monovalent metal salt of low endotoxin alginic acid that is applied to a nucleus pulposus site of a subject and used to cure a portion after application and has fluidity when applied to the nucleus pulposus site.
- a composition for intervertebral disc nucleus pulposus (sometimes referred to as “the composition of the present invention” in the present specification).
- Low endotoxin and “monovalent metal salt of alginic acid” are as described below.
- the intervertebral disc is a columnar tissue between the vertebrae connected to the spine.
- the intervertebral disc is a disc-like avascular tissue, and has a structure in which an annulus fibrosus surrounds the nucleus pulposus and endplates are arranged above and below.
- the nucleus pulposus is a gel-like tissue present in the center of the intervertebral disc, and mainly contains nucleus pulposus cells, an extracellular matrix mainly composed of proteoglycan and type II collagen, and water. The nucleus pulposus is thought to have little self-repairing / regenerating ability.
- “Muscle nucleus replacement” means degeneration, reduction, or reduction of nucleus pulposus that has been degenerated, reduced, or removed by aging, trauma, infection, and surgical operations (eg, intervertebral discectomy (resection)). Compensation for the minute or removed part.
- the term “nuclear nucleus filling” is used in the same meaning as “nuclear nucleus filling”, and the “nuclear nucleus filling composition” of the present invention has the same meaning as “nuclear nucleus filling composition”. is there.
- “Muscle nucleus” refers to a site where the nucleus pulposus is present, a site where degeneration or reduction of the nucleus pulposus occurs, or a defect of the nucleus pulposus formed by removing at least a portion of the nucleus pulposus Including the peripheral part of the site where the nucleus pulposus is present.
- a “subject” is a human or non-human organism, such as avian and non-human mammals (eg, cows, monkeys, cats, mice, rats, guinea pigs, hamsters, pigs, dogs, rabbits, sheep, and horses). is there.
- avian and non-human mammals eg, cows, monkeys, cats, mice, rats, guinea pigs, hamsters, pigs, dogs, rabbits, sheep, and horses.
- “Applying” means filling the nucleus pulposus site with a sufficient amount of the composition of the present invention to fill the degeneration, reduction, removal, defect, etc. of the nucleus pulposus site of the intervertebral disc.
- Contains a monovalent metal salt of low endotoxin alginic acid means a monovalent metal salt of low endotoxin alginic acid in an amount sufficient to regenerate the nucleus pulposus at the site of the nucleus pulposus where the composition of the present invention is applied. Is contained.
- Intervertebral disc degeneration and / or intervertebral disc damage and “treatment, prevention or suppression of recurrence” are as described later.
- the composition of the present invention may be provided in a solution state using a solvent, or may be provided in a dry state such as a lyophilized body (in particular, a lyophilized powder).
- a dry state the composition of the present invention is used in a state having fluidity such as a solution using a solvent at the time of application.
- the solvent is not particularly limited as long as it is a solvent applicable to a living body.
- water for injection purified water, distilled water, ion-exchanged water (or deionized water), milli-Q water, physiological saline, phosphate buffered physiological Examples include saline (PBS).
- PBS saline
- PBS saline
- PBS water for injection, distilled water, physiological saline and the like that can be used for treatment of humans and animals.
- Monovalent metal salt of alginic acid is a water-soluble substance formed by ion exchange of the hydrogen atom of the 6-position carboxylic acid of alginic acid with monovalent metal ions such as Na + and K + . Salt. Specific examples of the monovalent metal salt of alginic acid include sodium alginate and potassium alginate. In particular, sodium alginate that is commercially available is preferable. A solution of a monovalent metal salt of alginic acid forms a gel when mixed with a crosslinking agent.
- Alginic acid used in the present invention is a biodegradable polymer polysaccharide, a polymer in which two types of uronic acids, D-mannuronic acid (M) and L-guluronic acid (G), are polymerized in a straight chain. It is. More specifically, D-mannuronic acid homopolymer fraction (MM fraction), L-guluronic acid homopolymer fraction (GG fraction), and D-mannuronic acid and L-guluronic acid are randomly arranged. This is a block copolymer in which the fractions (MG fraction) are bound arbitrarily.
- the composition ratio (M / G ratio) of D-mannuronic acid and L-guluronic acid of alginic acid varies depending on the type of organism mainly derived from seaweed, etc. It ranges from a high G type with an M / G ratio of about 0.4 to a high M type with an M / G ratio of about 5.
- Monovalent metal salt of alginic acid is a high molecular weight polysaccharide, and it is difficult to accurately determine the molecular weight. However, if the molecular weight is too low, the viscosity is low, and the adhesiveness of the applied site to the surrounding tissue may be weakened. In addition, when the molecular weight is too high, it is difficult to produce, the solubility is lowered, the viscosity is too high when handled in a solution state, the handling is bad, and the physical properties are difficult to maintain during long-term storage.
- the weight average molecular weight is in the range of 10,000 to 10,000,000, preferably 20,000 to 8,000,000, more preferably 50,000 to 5,000,000.
- a numerical range indicated using “to” indicates a range including the numerical values described before and after “to” as the minimum value and the maximum value, respectively.
- the value may vary depending on the measurement method.
- the weight average molecular weight measured by gel permeation chromatography (GPC) or gel filtration chromatography (also referred to as size exclusion chromatography) is preferably according to the effects shown in the examples of the present invention. It is 100,000 or more, more preferably 500,000 or more, and preferably 5 million or less, more preferably 3 million or less. The preferable range is 100,000 to 5,000,000, more preferably 500,000 to 3.5 million.
- the absolute weight average molecular weight can be measured.
- the weight average molecular weight (absolute molecular weight) measured by the GPC-MALS method is preferably 10,000 or more, more preferably 80,000 or more, and still more preferably 90,000 or more according to the effects shown in the examples of the present invention. It is preferably 1 million or less, more preferably 800,000 or less, further preferably 700,000 or less, and particularly preferably 500,000 or less.
- the preferable range is 10,000 to 1,000,000, more preferably 80,000 to 800,000, still more preferably 90,000 to 700,000, and particularly preferably 90,000 to 500,000.
- a measurement error of 10 to 20% or more may occur.
- the value may vary in the range of about 32 to 480,000 for 400,000, 400,000 to 600,000 for 500,000, and about 800 to 1,200,000 for 1,000,000.
- the molecular weight of the monovalent metal salt of alginic acid can be measured according to a conventional method. Typical conditions in the case of using gel permeation chromatography for molecular weight measurement are as described in the examples of the present specification. For example, GMPW-XL ⁇ 2 + G2500PW-XL (7.8 mm ID ⁇ 300 mm) can be used as the column.
- the eluent can be, for example, a 200 mM sodium nitrate aqueous solution, and pullulan can be used as a molecular weight standard. Can be used.
- GPC-MALS Typical conditions when GPC-MALS is used for molecular weight measurement are as described in the examples of this specification.
- the detector for example, an RI detector and a light scattering detector (MALS) can be used.
- the monovalent metal salt of alginic acid initially has a large molecular weight and a high viscosity when extracted from brown algae, but the molecular weight becomes small and the viscosity becomes low in the process of drying and purification by heat.
- Monovalent metal salts of alginic acid having different molecular weights can be produced by controlling conditions such as temperature in the production process, selecting brown algae as a raw material, and fractionating molecular weight in the production process. Furthermore, it is possible to obtain a monovalent metal salt of alginic acid having a target molecular weight by mixing with a monovalent metal salt of alginic acid having a different molecular weight or viscosity.
- the monovalent metal salt of alginic acid used in the present invention is preferably prepared by dissolving a monovalent metal salt of alginic acid in MilliQ water to give a solution having a concentration of 1 w / w%, and using a cone plate viscometer,
- the apparent viscosity when the viscosity is measured under the conditions is preferably 40 mPa ⁇ s to 800 mPa ⁇ s, more preferably 50 mPa ⁇ s to 600 mPa ⁇ s. It is desirable that the apparent viscosity measurement conditions follow the conditions described below. In the present specification, the “apparent viscosity” may be simply referred to as “viscosity”.
- the alginic acid used in the present invention may be naturally derived or synthetic, but is preferably naturally derived.
- naturally occurring alginic acid include those extracted from brown algae.
- brown alga containing alginic acid is nowadays in coastal areas around the world, seaweed that can actually be used as a raw material for alginic acid is limited, such as Lessonia in South America, Macrocystis in North America, Laminaria and Ascofilum in Europe, Australia Typical examples are Daviglia.
- brown algae used as a raw material for alginic acid include, for example, the genus Lesonia, the genus Macrocystis, the genus Laminaria (comb), the genus Ascophyllum, the genus Durvillea, Examples include the genus Eisenia and the genus Ecklonia.
- the monovalent metal salt of alginic acid used in the present invention is a monovalent metal salt of alginic acid with low endotoxin.
- Low endotoxin refers to a low endotoxin level that does not substantially cause inflammation or fever. More preferably, it is a monovalent metal salt of alginic acid treated with low endotoxin.
- the low endotoxin treatment can be performed by a known method or a method analogous thereto.
- the method of Takada et al. See, for example, JP-A-9-32001 for purifying sodium hyaluronate
- the method of Yoshida et al. Eg, JP-A-8-269102 for purifying ⁇ 1,3-glucan. Etc.
- a method of William et al. for example, see JP-T-2002-530440, etc.
- biopolymer salts such as alginate, gellan gum, etc.
- James et al. For example, international publication for purifying polysaccharides, etc.
- the low endotoxin treatment of the present invention is not limited thereto, but is washed, filtered with a filter (such as an endotoxin removal filter or a charged filter), ultrafiltration, a column (an endotoxin adsorption affinity column, a gel filtration column, a column with an ion exchange resin, etc.) ), Adsorption to hydrophobic substances, resin or activated carbon, organic solvent treatment (extraction with organic solvent, precipitation / precipitation by addition of organic solvent, etc.), surfactant treatment (for example, JP-A-2005-036036) It can be carried out by a known method such as a gazette) or a combination thereof. These processing steps may be appropriately combined with known methods such as centrifugation. It is desirable to select appropriately according to the type of alginic acid.
- a filter such as an endotoxin removal filter or a charged filter
- ultrafiltration such as an endotoxin removal filter or a charged filter
- a column an endotoxin
- the endotoxin level can be confirmed by a known method, and can be measured, for example, by a method using Limulus reagent (LAL), a method using Enspercy (registered trademark) ES-24S set (Seikagaku Corporation), or the like. .
- LAL Limulus reagent
- Enspercy registered trademark
- ES-24S set Seikagaku Corporation
- the method for treating the endotoxin of the monovalent metal salt of alginic acid contained in the composition of the present invention is not particularly limited.
- the endotoxin content of the monovalent metal salt of alginic acid is measured by the endorsin assay using the Limulus reagent (LAL).
- LAL Limulus reagent
- Low endotoxin-treated sodium alginate is commercially available, for example, Sea Matrix (registered trademark) (Mochida Pharmaceutical Co., Ltd.), PRONOVA TM UP LVG (FMC BioPolymer).
- composition of the present invention may be prepared using a solution of a monovalent metal salt of alginic acid.
- a solution of a monovalent metal salt of alginic acid can be prepared by a known method or a method analogous thereto. That is, the monovalent metal salt of alginic acid used in the present invention can be produced by a known method such as an acid method or a calcium method using the aforementioned brown algae.
- alginic acid can be obtained from these brown algae by extraction with an aqueous alkali solution such as an aqueous sodium carbonate solution and then adding an acid (for example, hydrochloric acid, sulfuric acid, etc.).
- a salt of alginic acid can be obtained by ion exchange.
- the solvent of the monovalent metal salt of alginic acid is not particularly limited as long as it is a solvent that can be applied to a living body.
- purified water distilled water, ion exchange water, milli-Q water, physiological saline, phosphate buffered physiological saline (PBS).
- PBS phosphate buffered physiological saline
- Milli-Q water can be used after sterilizing by filtration.
- composition of the present invention when the composition of the present invention is provided in a dry state such as a freeze-dried product, it can be prepared into a fluid solution using the above-mentioned solvent.
- all operations for obtaining the composition of the present invention be performed in an environment with low endotoxin levels and bacterial levels.
- the operation is preferably performed on a clean bench using a sterilizing instrument, and the instrument used may be treated with a commercially available endotoxin remover.
- composition of some embodiments of the present invention is a fluid liquid, ie, a solution.
- the composition of the present invention has fluidity when applied to the nucleus pulposus site.
- the composition of the present invention has fluidity that can be injected with a 21 G needle after the composition has been allowed to stand at 20 ° C. for 1 hour.
- the apparent viscosity of the composition of the present invention of this embodiment is not particularly limited as long as the effect of the present invention is obtained, but if the viscosity is too low, the adhesiveness to the surrounding tissue of the applied site may be weakened.
- the preferred range of the composition of the present invention is 10 mPa ⁇ s to 50000 mPa ⁇ s, more preferably 100 mPa ⁇ s to 30000 mPa ⁇ s, more preferably 200 mPa ⁇ s to 20000 mPa ⁇ s, and even more preferably 500 mPa ⁇ s to 20000 mPa ⁇ s. S, particularly preferably 700 mPa ⁇ s to 20000 mPa ⁇ s.
- composition of some embodiments of the present invention is a viscosity that can also be applied to a subject, such as with a syringe.
- the apparent viscosity of a composition containing a monovalent metal salt of alginic acid such as an aqueous solution of alginic acid can be measured according to a conventional method.
- a rotational viscometer method such as a coaxial double cylindrical rotational viscometer, a single cylindrical rotational viscometer (Brookfield viscometer), a cone-plate rotational viscometer (cone plate viscometer), etc.
- a rotational viscometer method such as a coaxial double cylindrical rotational viscometer, a single cylindrical rotational viscometer (Brookfield viscometer), a cone-plate rotational viscometer (cone plate viscometer), etc.
- the viscosity measurement is desirably performed at 20 ° C.
- the apparent viscosity of the composition is the apparent viscosity in a state that does not contain cells or the like in order to accurately measure the viscosity. It is preferable.
- the apparent viscosity of a composition containing a monovalent metal salt of alginic acid is particularly preferably measured using a cone plate viscometer.
- a cone plate viscometer For example, it is desirable to measure under the following measurement conditions.
- Sample solution is prepared using MilliQ water.
- the measurement temperature is 20 ° C.
- the rotational speed of the cone plate viscometer is 1 rpm when measuring a 1% solution of a monovalent metal alginate, and 0.5 rpm when measuring a 2% solution.
- the reading time is measured for 2 minutes in the case of measuring a 1% solution of a monovalent metal salt of alginic acid, and is taken as an average value from 1 minute to 2 minutes from the start.
- 2% solution measurement measure for 2.5 minutes and take the average value from the start 0.5 minutes to 2.5 minutes.
- the test value is the average of three measurements.
- the apparent viscosity of the composition of the present invention can be adjusted, for example, by controlling the concentration, molecular weight, M / G ratio, etc. of the monovalent metal salt of alginic acid.
- the apparent viscosity of the monovalent metal salt solution of alginic acid is high when the concentration of the monovalent metal salt of alginic acid in the solution is high, and low when the concentration is low.
- the viscosity is high when the molecular weight of the monovalent metal alginate is large, and the viscosity is low when the molecular weight is small.
- the apparent viscosity of the monovalent metal salt solution of alginic acid is affected by the M / G ratio, for example, an alginic acid having a preferable M / G ratio can be selected depending on the viscosity of the solution.
- the M / G ratio of alginic acid used in the present invention is about 0.1 to 5.0, preferably about 0.1 to 4.0, more preferably about 0.2 to 3.5.
- the type of brown algae used as a raw material affects the viscosity of a monovalent metal salt solution of alginic acid.
- the alginic acid used in the present invention is preferably derived from a brown algae of the genus Lessonia, Macrocystis, Laminaria, Ascofilum, Davilia, more preferably a brown alga of the genus Lessonia, particularly preferably Lessonia It is derived from Niscens (Lessonia nigrescens).
- composition of the present invention is characterized by containing a monovalent metal salt of low endotoxin alginic acid as an active ingredient.
- the present inventors have found for the first time that when a low endotoxin alginic acid monovalent metal salt is filled in a nucleus pulposus site of a living body, the monovalent metal salt of alginic acid itself exhibits a regeneration or therapeutic effect on nucleus pulposus tissue.
- the monovalent metal salt of low endotoxin alginic acid is contained in an amount capable of exerting a regeneration or therapeutic effect on nucleus pulposus tissue when applied to the affected area.
- the preferred monovalent metal salt concentration of alginic acid in the composition of the present invention is unclear because it is affected by the molecular weight, but preferably 0.5 w / v% to 5 w / v%, more preferably 1 w / v. It is v% to 5 w / v%, more preferably 1 w / v% to 3 w / v%, and particularly preferably 1.5 w / v% to 2.5 w / v%.
- the concentration of monovalent metal salt of alginic acid in the composition of the present invention is preferably 0.5 w / w% to 5 w / w%, more preferably 1 w / w% to 5 w / w%. More preferably, it may be 1 w / w% to 3 w / w%, and particularly preferably 1.5 w / w% to 2.5 w / w%.
- the endotoxin content of the composition is usually 500 EU / g or less, more preferably Is 300 EU / g or less, more preferably 150 EU / g or less, and particularly preferably 100 EU / g or less.
- composition of the present invention does not contain cells.
- the composition of some other embodiments of the present invention uses cells.
- the cells include nucleus pulposus cells, stem cells, stromal cells, mesenchymal stem cells, bone marrow stromal cells, etc.
- the origin is not particularly limited, but intervertebral disc nucleus pulposus, bone marrow, adipose tissue, umbilical cord blood, etc. Can be mentioned.
- examples of cells include ES cells and iPS cells.
- “Use cells” was prepared by collecting and concentrating target cells from the nucleus pulposus of the intervertebral disc, bone marrow, adipose tissue, umbilical cord blood, etc. It refers to adding cells to the composition of the present invention. Specifically, for example, 1 ⁇ 10 4 cells / ml or more, or 1 ⁇ 10 5 cells / ml or more, preferably 1 ⁇ 10 4 cells / ml to 1 ⁇ 10 7 cells / ml are used in the present invention. It is said to be contained in the composition. Cells may be obtained from the market and used.
- the composition of the present invention may contain a factor that promotes cell growth.
- factors include BMP, FGF, VEGF, HGF, TGF- ⁇ , IGF-1, PDGF, CDMP (cartilage-derived-morphogenetic protein), CSF, EPO, IL, PRP (Platelet® Rich® Plasma), Examples include SOX and IF.
- BMP BMP
- FGF vascular endothelial growth factor
- VEGF vascular endothelial growth factor
- HGF vascular endothelial growth factor
- TGF- ⁇ TGF-1
- PDGF vascular endothelial growth factor
- CDMP cartilage-derived-morphogenetic protein
- CSF CSF
- EPO EPO
- IL IL
- PRP Platinum® Rich® Plasma
- SOX and IF SOX and IF.
- the composition of the present invention may contain a factor that suppresses cell death.
- factors that cause cell death include Caspase and TNF ⁇ .
- factors that suppress these include antibodies and siRNA. These factors that suppress cell death may be produced by recombinant methods or purified from protein compositions.
- the composition of some aspects of this invention does not contain the factor which suppresses these cell deaths. Even in the absence of a factor that suppresses cell death, the regeneration of the nucleus pulposus is sufficiently good, and it is safer than the case of positively suppressing cell death.
- the composition of the present invention does not contain any component that exerts a pharmacological action on the nucleus pulposus tissue of the intervertebral disc other than the monovalent metal salt of low endotoxin alginic acid. Even in a composition containing only a monovalent metal salt of low endotoxin alginic acid as an active ingredient, a sufficient nucleus pulposus regeneration or therapeutic effect can be exhibited.
- composition of the present invention other pharmaceutically active ingredients, conventional stabilizers, emulsifiers, osmotic pressure adjusting agents, buffers, isotonic agents, preservatives, soothing agents, coloring may be used as necessary.
- Ingredients usually used in medicine, such as an agent, can also be contained in the composition of the present invention.
- Curing the composition of the present invention The composition of the present invention is used to cure a portion after application to the nucleus pulposus site. “Curing part” refers to bringing a cross-linking agent into contact with a part of the composition of the present invention having fluidity to gel and harden a part rather than the whole composition in contact with the cross-linking agent. Preferably, a part of the composition of the present invention is cured by bringing a crosslinking agent into contact with at least a part of the surface of the composition of the present invention having fluidity.
- “partially harden the composition after application to the nucleus pulposus site” refers to the use of a cross-linking agent in the same manner as the filling to the nucleus pulposus site and the usage ratio.
- at least 50% of the volume of the composition in the test tube was in vitro after filling a 6 mm diameter test tube with 500 ⁇ L of low endotoxin sodium alginate and a cross-linking agent and allowing to stand for 1 hour.
- the non-gelled portion may be indicated by the fact that at least 50% of the volume of the composition in the test tube can be aspirated with a syringe with a 21G needle.
- the composition does not deviate even when a compressive force is applied from the cranial to caudal side of the intervertebral disc after filling because the composition shows such properties even after filling the nucleus pulposus site.
- “At least a portion of the surface of the composition” is, for example, an opening in the surface of the intervertebral disc leading to the nucleus pulposus, and preferably an opening in the surface of the disc used to apply the composition to the nucleus pulposus site, ie It is a filling port of the composition.
- the filling port of the composition on the surface of the intervertebral disc is, for example, an opening used for filling the surface of the intervertebral disc with a syringe needle or a scalpel, or a disc surface prepared by a scalpel or the like at the time of disc herniation.
- An opening is preferred.
- the intervertebral disc in this embodiment is preferably an annulus fibrosus.
- the composition of the present invention preferably does not contain an amount of a cross-linking agent that cures the composition prior to application to the target nucleus pulposus site.
- the composition of the present invention may contain an amount of a crosslinking agent that does not cure the composition even after a certain period of time.
- the fixed time is not particularly limited, but is preferably about 30 minutes to 12 hours.
- “Not containing a crosslinking agent in an amount that cures the composition” may be indicated, for example, by allowing the composition to stand at 20 ° C. for 1 hour and then injecting with a syringe with a 21G needle.
- the composition of some embodiments of the present invention does not include a crosslinker.
- the crosslinking agent is not particularly limited as long as it can fix the surface of the monovalent metal salt of alginic acid by crosslinking.
- the cross-linking agent include divalent or higher valent metal ion compounds such as Ca 2+ , Mg 2+ , Ba 2+ , and Sr 2+, and cross-linkable reagents having 2 to 4 amino groups in the molecule. More specifically, CaCl 2 , MgCl 2 , CaSO 4 , BaCl 2, etc. are used as divalent or higher valent metal ion compounds on the nitrogen atom as crosslinkable reagents having 2 to 4 amino groups in the molecule.
- a diaminoalkane that may have a lysyl group (—COCH (NH 2 ) — (CH 2 ) 4 —NH 2 ), that is, the diaminoalkane and its amino group are substituted with a lysyl group to form a lysylamino group.
- Specific examples include diaminoethane, diaminopropane, N- (lysyl) -diaminoethane, etc., but CaCl 2 solution is particularly preferred because of its availability and gel strength. Is preferable.
- the timing of bringing the crosslinking agent into contact with the surface of the composition of the present invention is preferably after the composition of the present invention is applied to the nucleus pulposus site.
- a method for bringing a crosslinking agent (for example, a metal ion having a valence of 2 or more) into contact with a part of the composition of the present invention is not particularly limited.
- a metal ion having a valence of 2 or more can be used with a syringe or a sprayer.
- a method of applying the solution to the surface of the composition can be used with a syringe or a sprayer.
- the cross-linking agent may be slowly applied to the filling port of the composition formed on the intervertebral disc for several seconds to several tens of seconds. Thereafter, if necessary, a treatment for removing the cross-linking agent remaining in the vicinity of the filling port may be added.
- the removal of the crosslinking agent may be, for example, washing of the application site with physiological saline or the like.
- the amount of the cross-linking agent used is preferably adjusted as appropriate in consideration of the application amount of the composition of the present invention, the size of the filling port of the composition on the surface of the intervertebral disc, the size of the application site of the nucleus pulposus of the intervertebral disc, and the like. In order not to exert a strong influence on the tissue around the filling port of the composition, the amount of the crosslinking agent used is adjusted so as not to be excessive.
- the amount of divalent or higher metal ion used is not particularly limited as long as it can solidify the surface of a composition containing a monovalent metal salt of alginic acid.
- the amount of CaCl 2 solution used is preferably about 0.3 to 5.0 ml. More preferably, it is about 0.5 ml to 3.0 ml.
- the amount of 100 mM CaCl 2 solution used is preferably about 0.3 to 10 ml, more Preferably, it is about 0.5 ml to 6.0 ml. It can be appropriately increased or decreased while observing the state of the composition of the present invention at the application site.
- the concentration is preferably 25 mM to 200 mM, more preferably 50 mM to 150 mM. desirable.
- the cross-linking agent remaining at the added site is removed by washing or the like.
- the fixed time for standing is not particularly limited, but it is preferable that the surface of the composition is gelled by standing for about 1 minute or longer, more preferably about 4 minutes or longer. Alternatively, it is preferable to stand for about 1 minute to about 10 minutes, more preferably about 4 minutes to about 10 minutes, about 4 minutes to about 7 minutes, more preferably about 5 minutes. It is desirable to keep the composition and the crosslinking agent in contact for a certain period of time, and a crosslinking agent may be appropriately added so that the liquid level of the composition does not dry.
- alginate beads are prepared by dropping a sodium alginate solution into a CaCl 2 solution and gelling it.
- alginic acid beads need to be applied while being pressed against the application site, but it is necessary to prepare a bead that matches the size of the application site, which is technically difficult to use in actual clinical practice.
- a CaCl 2 solution is used as a cross-linking agent, Ca ions on the surface of the beads come into contact with surrounding tissues, which causes a problem of calcium cytotoxicity.
- the composition of the present invention is in the form of a solution, it can be easily applied to application sites of any shape, and the entire application site can be covered with the composition. Good adhesion to tissue.
- the portion of the composition of the present invention that contacts the surrounding tissue can keep the calcium concentration low, and there are few problems of calcium cytotoxicity. Since the portion of the composition of the present invention that contacts the surrounding tissue is less affected by the cross-linking agent, the composition of the present invention can easily contact the cell or tissue at the application site.
- the composition of the present invention fuses with a living tissue so that it cannot be identified at the applied site after about 4 weeks after being applied to the nucleus pulposus site, and has high affinity to the living body.
- composition of the present invention When a composition of the present invention is applied to a nucleus pulposus site and partially gelated with a cross-linking agent, the composition of the present invention is partially cured at the affected area and localized in a state of being in close contact with the surrounding tissue. Leakage from the nuclear site can be prevented. In addition, when the composition of the present invention adheres to the surrounding tissue, the nucleus pulposus regeneration effect of the composition of the present invention is exerted more strongly.
- the hardened gel when the hardened gel is filled into the nucleus pulposus site, the hardened gel may protrude into the spinal canal and cause a serious neurological disorder.
- the solution-like composition of the present invention has such a low risk and a low risk of developing complications.
- composition of the present invention can be applied to human or non-human organisms such as birds and non-human mammals (eg, cows, monkeys, cats, mice, rats, guinea pigs, hamsters, pigs, dogs). , Rabbits, sheep, and horses) and used to promote nucleus pulposus regeneration.
- non-human mammals eg, cows, monkeys, cats, mice, rats, guinea pigs, hamsters, pigs, dogs.
- Rabbits, sheep, and horses used to promote nucleus pulposus regeneration.
- the form of the composition of the present invention is preferably a fluid liquid, that is, a solution.
- “having fluidity” means having a property of changing its form into an indeterminate form, and does not need to have a property of constantly flowing, such as a solution.
- the composition is allowed to stand at 20 ° C.
- composition of the present invention When the composition of the present invention is provided in a dry state such as a freeze-dried product, it can be made into a fluid composition as described above using a solvent or the like at the time of application.
- composition of the present invention in the form of a solution can be easily applied to the nucleus pulposus site of the intervertebral disc with a syringe, a gel pipette, a dedicated syringe, a dedicated injector, a filling device, or the like.
- a syringe of a pressure type or an electric type may be used.
- injecting with a syringe for example, it is preferable to use a 14G to 27G or 14G to 26G needle.
- the method of applying the composition of the present invention to the nucleus pulposus site is not particularly limited.
- the syringe is exposed after exposing the affected part directly under a known surgical technique, or under a microscope or an endoscope.
- the composition of the present invention can be applied to the nucleus pulposus site using a filling device or the like.
- a filling instrument needle or the like may be inserted from the annulus fibrosus surface toward the nucleus pulposus site, and the composition of the present invention may be applied.
- the composition of the present invention is in the form of a solution, it can be adapted to any shape of nucleus pulposus, such as reduction of nucleus pulposus, cavity or defect of nucleus pulposus, and reduction of nucleus pulposus, cavity or defect.
- the entire part can also be filled.
- Reduction of nucleus pulposus, cavity or defect in nucleus pulposus site may have been caused by degeneration or damage of intervertebral disc, or by surgical removal or aspiration of at least part of nucleus pulposus It can be a thing.
- the composition of the present invention is applied to a nucleus pulposus defect formed by removing at least part of the nucleus pulposus.
- the removal of at least a portion of the nucleus pulposus is not particularly limited, and may be, for example, an intervertebral discectomy performed directly, percutaneously, microscopically, or endoscopically.
- an incision of 2 cm to 10 cm is made on the back, the muscle is detached from the back surface of the posterior element of the spine called a vertebral arch, the ligament between the vertebral arches is removed, the nerve and disc herniation are confirmed, and the nerve is compressed
- It may be a method of extracting a hernia (love method).
- a method may be used in which the nucleus pulposus is irradiated with a laser to reduce the nucleus pulposus volume.
- composition of the present invention After application of the composition of the present invention to the nucleus pulposus site, a part of the composition can be cured with a crosslinking agent as in the previous operation.
- the application amount of the composition of the present invention may be determined according to the volume of the application site of the nucleus pulposus to be applied, and is not particularly limited. For example, 0.01 ml to 10 ml, more preferably 0.1 ml to 5 ml. More preferably, it is 0.2 ml to 3 ml.
- the composition of the present invention is applied to a nucleus pulposus defect, it is preferably injected so as to sufficiently fill the defect volume at the nucleus pulposus site.
- the frequency and frequency of application of the composition of the present invention can be increased or decreased according to symptoms and effects. For example, it may be applied only once, or may be continuously applied once a month to one year. Since alginic acid is a substance that does not originally exist in the animal body, the animal does not have an enzyme that specifically degrades alginic acid. Alginate is gradually degraded by normal hydrolysis in the animal body, but the degradation in the body is slower than that of polymers such as hyaluronic acid, and there are no blood vessels in the nucleus pulposus. When filled, long-lasting effects can be expected.
- composition of the present invention is not provided together with the cells and growth factors as described above, when the composition of the present invention is applied to the nucleus pulposus site, the cells, growth factors, cell death inhibitory factors described later, Other drugs may be used in combination.
- composition of the present invention exhibits an effect of promoting regeneration by suppressing degenerative changes in the entire intervertebral disc tissue and nucleus pulposus by applying it to the nucleus pulposus site. Therefore, the composition of the present invention is preferably used as a nucleus pulposus composition for intervertebral discs.
- One of the preferable embodiments of the composition of the present invention is a composition for suppressing degeneration of the intervertebral disc, more preferably a composition for suppressing degeneration of the nucleus pulposus of the intervertebral disc.
- “Degeneration of the intervertebral disc or nucleus pulposus” means that the number of intervertebral disc cells, water content, extracellular matrix (type II collagen, aggrecan, etc.), etc., decrease due to aging, etc., resulting in morphological changes and reduced function. This is a state in which it can be played, and when it progresses, it cannot function as a shock absorber of the intervertebral disc.
- “inhibition of denaturation” does not necessarily mean a state without denaturation, as long as the denaturation change is suppressed as compared with the case of no treatment.
- composition of the present invention is a composition for nucleus pulposus regeneration.
- the nucleus pulposus regeneration is intended to prevent the accumulation of fibroblast-like cells and regenerate the nucleus pulposus with a high ratio of nucleus pulposus cells, and the nucleus pulposus tissue rich in type II collagen and proteoglycan is regenerated. Is intended.
- the term nucleus pulposus regeneration also includes inhibiting degeneration of the nucleus pulposus.
- it is desirable that the composition of the nucleus pulposus regenerated by applying the composition of the present invention is close to the composition of natural normal nucleus pulposus.
- composition of a preferred embodiment of the present invention is used for the treatment, prevention or relapse suppression of disc degeneration and / or disc damage.
- treatment, prevention or suppression of recurrence refers to treatment, prevention, suppression of recurrence, reduction, suppression, improvement, elimination, decrease in incidence, delay in onset, suppression of progression, reduction in severity, relapse rate Includes reduction, delay in recurrence, and relief of clinical symptoms.
- Intervertebral disc degeneration and / or intervertebral disc injury is, for example, at least one selected from the group consisting of intervertebral disc herniation, spondylosis, spinal degeneration, purulent spondylitis, degenerative spondylosis, spinal stenosis, and intervertebral disc injury.
- a condition or disease is, for example, at least one selected from the group consisting of intervertebral disc herniation, spondylosis, spinal degeneration, purulent spondylitis, degenerative spondylosis, spinal stenosis, and intervertebral disc injury.
- the present invention provides a method for treating, preventing, or suppressing relapse of intervertebral disc degeneration and / or intervertebral disc injury using the composition of the present invention.
- the treatment method of the present invention is a method for treatment, prevention or relapse suppression of intervertebral disc degeneration and / or intervertebral disc damage, comprising a monovalent metal salt of low endotoxin alginic acid and having fluidity Applying to the nucleus pulposus site of the subject's intervertebral disc in need of treatment, prevention or relapse inhibition, and curing a portion of the applied composition.
- the treatment method of the present invention may include a step of removing at least a part of the nucleus pulposus before applying the composition of the present invention to the nucleus pulposus site.
- the intervertebral disc degeneration and / or intervertebral disc damage is at least one selected from the group consisting of intervertebral disc herniation, intervertebral disc disease, spinal degeneration, suppurative intervertebral discitis, degenerative spondylosis, spinal stenosis, intervertebral disc injury, for example. Is a condition or disease.
- the intervertebral disc degeneration and / or intervertebral disc injury is a herniated disc, in particular a lumbar disc herniation.
- one of several aspects of the present invention provides a method for suppressing degenerative changes in the intervertebral disc using the composition of the present invention. Moreover, in one of the preferable aspects of this invention, the method of reproducing
- a composition containing a monovalent metal salt of endotoxin alginic acid and having fluidity is applied to the nucleus pulposus site of a subject's intervertebral disc in need of inhibition of intervertebral disc degeneration or nucleus pulposus regeneration.
- Curing a portion of The method may include removing at least a portion of the nucleus pulposus prior to applying the composition of the present invention to the nucleus pulposus site.
- composition of the present invention The preferred embodiment of the composition of the present invention, the specific application method to the nucleus pulposus site of the intervertebral disc, the curing method of the composition, the meaning of the terms, etc. are as described above.
- the therapeutic method of the present invention may be performed by appropriately combining other intervertebral disc therapeutic methods and therapeutic agents.
- antibiotics such as streptomycin, penicillin, tobramycin, amikacin, gentamicin, neomycin, and amphotericin B, aspirin
- nonsteroidal antipyretic Concomitant drugs such as analgesics (NSAIDs), anti-inflammatory drugs such as acetaminophen, proteolytic enzymes, corticosteroids, HMG-CoA reductase inhibitors such as simvastatin and lovastatin may be filled.
- NSAIDs analgesics
- anti-inflammatory drugs such as acetaminophen, proteolytic enzymes, corticosteroids, HMG-CoA reductase inhibitors such as simvastatin and lovastatin
- simvastatin and lovastatin may be filled.
- muscle relaxants, opioid analgesics, neuropathic pain relievers and the like may be administered orally or parenterally in combination as necessary.
- the aforementioned cells may be applied to the nucleus pulposus site together with the composition of the present invention.
- the aforementioned factor that promotes cell growth may be applied to the nucleus pulposus site together with the composition of the present invention.
- an embodiment in which the composition of the present invention does not use a factor that promotes cell growth is also desirable.
- the composition of the present invention can promote regeneration of nucleus pulposus even when these cells and factors are not used.
- the present invention also relates to the use of a monovalent metal salt of low endotoxin alginic acid to produce the composition of the present invention.
- the use of the present invention is the use of a monovalent metal salt of low endotoxin alginic acid for the manufacture of a composition for the treatment, prevention or relapse inhibition of disc degeneration and / or disc damage, wherein said composition comprises It is used to harden a part of the nucleus pulposus after application and has fluidity when applied to the nucleus pulposus site.
- the present invention further provides a composition comprising a monovalent metal salt of low endotoxin alginic acid and having fluidity to the nucleus pulposus of an intervertebral disc of a subject in need of treatment, prevention or relapse suppression of disc degeneration and / or disc damage.
- a monovalent metal salt of low endotoxin alginic acid for use in the treatment, prevention or relapse inhibition of disc degeneration and / or disc damage, applying to the site and curing a portion of the applied composition.
- kits The present invention provides a nucleus pulposus replacement kit for an intervertebral disc.
- the kit of the present invention can contain the composition of the present invention.
- the composition of the present invention included in the kit of the present invention is in a solution state or a dry state, but is preferably in a dry state, more preferably a lyophilized body, and particularly preferably a lyophilized powder. .
- a solvent for dissolution for example, water for injection
- the kit of the present invention may further contain a crosslinking agent.
- the kit of the present invention can further contain a cross-linking agent, a syringe, an injection needle, a gel pipette, a dedicated filler, an instruction manual, and the like.
- kits include (1) a vial in which a lyophilized form of low endotoxin sodium alginate is enclosed (2) an ampoule in which a solvent such as water for injection is encapsulated as a solution, (3) a calcium chloride solution as a cross-linking agent, etc. 2
- An ampoule or the like enclosing a metal ion compound having a valence higher than that in a single pack can be provided.
- a monovalent metal salt of alginic acid is sealed in one chamber of a syringe composed of two chambers that are integrally molded and separated by a partition, and a solvent or a cross-linking agent as a solution is filled in the other chamber.
- the kit is configured to enclose a solution containing it so that the partitions in both chambers can be easily opened at the time of use, and to mix and dissolve both at the time of use.
- a monovalent metal salt solution of alginic acid is sealed in a prefilled syringe and used as a kit that can be filled as it is without any preparation operation at the time of use.
- the alginic acid solution and the cross-linking agent are enclosed in separate syringes to form a kit bundled in one pack. Or it is good also as a kit containing the ampule etc. which filled the vial filled with the monovalent metal salt solution of alginic acid, and the crosslinking agent.
- the “composition of the present invention”, “crosslinking agent”, “syringe” and the like are as described above.
- This kit can be used, for example, for the treatment method of the present invention.
- Example 1 Effect of low endotoxin sodium alginate on human intervertebral disc cells 1- (1) Isolation and culture of non-degenerative human intervertebral disc cells The nucleus pulposus tissue was removed from human non-degenerative intervertebral disc tissue and cultured in a DMEM (Dulbecco's modified Eagle's medium) medium containing 0.25% collagenase (Wako) at 37 ° C. After time treatment, nucleus pulposus cells were isolated.
- DMEM Dynamic Eagle's medium
- Wako collagenase
- the nucleus pulposus cells obtained were treated with DMEM containing 1% penicillin / streptomycin, 1.25 ⁇ g / ml fungizone (fungizone, Invitrogen) and 10% FBS (fetal bovine serum) at 37 ° C., 5% CO 2 , 20%. Cells were cultured under O 2 conditions and passage 2 cells were used in the experiments.
- DMEM fetal bovine serum
- alginate beads (A) Sodium alginate treated with low endotoxin shown in Table 1 below, and (B) Food grade sodium alginate (Wako Pure Chemical Industries, Ltd.), 199 The human nucleus pulposus cells were cultured using two types of sodium alginate (Mochida Pharmaceutical Co., Ltd.).
- a 2 w / v% sodium alginate solution was prepared using Milli-Q water.
- the human nucleus pulposus cells 4.0 ⁇ 10 6 cells obtained in (1) above were suspended in 1.0 ml of these sodium alginate solutions.
- This cell suspension was dropped into a 102 mM calcium chloride aqueous solution using a 22 gauge needle and allowed to stand for 10 minutes to prepare beads encapsulating nucleus pulposus cell-containing sodium alginate solution.
- the obtained beads were washed twice with 0.9% physiological saline, and then placed in a DMEM culture solution containing 1% penicillin / streptomycin, 1.25 ⁇ g / ml fungizone, 10% FBS at 37 ° C., 5% CO2. 2. Three-dimensional (3D) culture under 20% O 2 conditions. Cells were collected 48 hours, 7 days, 14 days, and 28 days after the start of culture and used for the following evaluation.
- Group A low endotoxin sodium alginate
- Group B food grade sodium alginate
- FITC + / PI ⁇ was an early apoptotic cell
- FITC + / PI + was a late apoptotic cell
- these were combined to make an apoptotic cell.
- Group A low endotoxin sodium alginate
- Group B food grade sodium alginate
- Beads were collected on the 7th day of 1- (2) 3D culture, and the beads were washed twice with PBS. Serum starvation was induced by adding beads to DMEM without serum, 1% penicillin / streptomycin, 1.25 ⁇ g / ml fungizone medium and incubating at 37 ° C., 5% CO 2 , 20% O 2 . Cells were collected 6 hours and 48 hours after the start of serum starvation. As described above, evaluation using a confocal laser microscope and evaluation using a flow cytometer were performed.
- group A low endotoxin sodium alginate
- group B food grade sodium alginate
- the induction of serum starvation in this study is a test that assumes the environment of the human nucleus pulposus nucleus in an avascular field and in a low-nutrition environment. It was suggested that cultures of nucleus pulposus cells with low endotoxin sodium alginate are more resistant to apoptosis under serum starvation induction compared to food grade sodium alginate. In other words, it was suggested that low endotoxin sodium alginate does not induce apoptosis of nucleus pulposus cells and retains cell viability when filled in the nucleus pulposus site of the intervertebral disc as compared with food grade sodium alginate.
- Example 2 Application of low endotoxin sodium alginate solution to rabbit disc nucleus pulposus defect model Two types of low endotoxin sodium alginate solutions were filled in the rabbit intervertebral disc nucleus nucleus defect model, and the effects were evaluated.
- a cone plate type rotational viscometer visco-viscoelasticity measuring device Rheostress RS600 (Thermo Haake GmbH) sensor: 35/1
- the number of revolutions was 1 rpm when measuring a 1 w / w% sodium alginate solution and 0.5 rpm when measuring a 2 w / w% sodium alginate solution.
- the reading time is 2 minutes when measuring 1 w / w% solution, and the average value from 1 minute to 2 minutes from the start, and 2.5 minutes when measuring 2 w / w% solution, and 0.5 minutes from the start.
- the average value was up to 2.5 minutes.
- the average value of three measurements was taken as the measurement value.
- the measurement temperature was 20 ° C.
- the weight average molecular weight of each sodium alginate was measured by two types of measurement methods, gel permeation chromatography (GPC) and GPC-MALS.
- the measurement conditions are as follows.
- Each low endotoxin sodium alginate was dissolved in milli-Q water to prepare a 2 w / v% solution.
- An injection needle was inserted from the side of the intervertebral disc toward the nucleus pulposus, and 20 ⁇ l of a low endotoxin sodium alginate solution was injected into the nucleus pulposus defect.
- a 102 mM calcium chloride aqueous solution was applied to the side of the intervertebral disc from which the injection needle had been removed (referred to as the A-1 group and A-2 group, respectively, and these are collectively referred to as the treatment group).
- the group that performed only nucleus pulposus aspiration was designated as the aspiration alone group (aspiration alone group).
- Group A-2 was also evaluated for 12 weeks after surgery. Eight cases were performed in each group.
- the aspiration alone group and the treatment group were significantly compared with the normal control group at 4 weeks after the operation.
- the score was high, indicating the degeneration of the disc.
- the A-1 group had a significantly lower score than the aspiration alone group, and degeneration was suppressed.
- the MRI index of the treatment group (Group A-1 and Group A-2) was significantly higher than that of the aspiration alone group, and the degenerative change was suppressed (FIG. 4).
- the MRI index of the A-2 group was significantly higher than that of the aspiration alone group.
- Grade 1 Mildly serpentine with rupture Grade 2: moderately serpentine with rupture Grade 3: severely serpentine with mildly reversed Grade 4: severely reversed contour Grade 5: Indistinct
- the anti-Type I collagen antibody-positive cell ratio relative to the number of cells in the sections was 4 weeks after the operation in the normal control group, the suction alone group, and the treatment group (Group A-1 and Group A-2). There was no difference between them.
- the anti-type II collagen antibody-positive cell rate was significantly lower in the aspiration alone group and the treatment group (Group A-1 and Group A-2) than the normal control group at 4 weeks after the operation.
- the extracellular organ production seen in normal intervertebral disc tissue was reduced.
- the treatment group (Groups A-1 and A-2) had a significantly higher anti-type II II collagen antibody-positive cell rate than the aspiration alone group.
- FIG. 7 shows a graph of the anti-type II collagen antibody positive cell rate against the number of cells in the intervertebral disc nucleus pulposus tissue sections of the normal control group, the suction alone group, and the A-2 group at 4 weeks and 12 weeks after the operation.
- Type II collagen antibody-positive cells indicate the presence of hyaline cartilage-like cells.
- low endotoxin sodium alginate solution suppresses the degenerative change of the entire intervertebral disc tissue and nucleus pulposus and promotes regeneration by filling the nucleus pulposus defect part.
- low endotoxin sodium alginate A-1 and A-2 showed the same degree of suppression and regeneration effect of intervertebral disc degeneration.
- the 2% solution of A-2 has a higher viscosity than the 2% solution of A-1, so it is difficult to reversely flow from the filling part when filling the nucleus pulposus site. It had the advantage of being easy to stick. It was found that the 2% solution of A-2 had an appropriate viscosity for filling and excellent handleability.
- Example 3 Examination of injection method of low endotoxin sodium alginate solution 3- (1) Examination of injection method of low endotoxin sodium alginate solution Low endotoxin sodium alginate solution was injected into sheep cadaver lumbar spine and evaluated by the following two injection methods (i) and (ii) .
- Example 2 According to the description in Example 2, a 2% low endotoxin sodium alginate solution of A-2 described in Example 2 was injected into the part of the nucleus pulposus, and then 100 mM chloride was placed near the hole of the injection needle on the surface of the intervertebral disc. A method in which a calcium solution is brought into contact (sodium alginate is cured at the portion in contact with the calcium chloride solution). (Ii) A-2 low endotoxin sodium alginate solution 2% and 100 mM calcium chloride solution 1: 1 simultaneously filled into the part of the nucleus pulposus nucleus (the entire sodium alginate injected into the nucleus pulposus is cured) . (Ii) was carried out by placing a low endotoxin sodium alginate solution and a calcium chloride solution in separate syringes and simultaneously injecting them into the defect of the intervertebral disc nucleus with a 22G needle.
- the technique (i) had no difficulty in the injection procedure when it was performed on a sheep cadaver disc.
- the method (ii) was considered to have low reproducibility with respect to making the mixing ratio of the low endotoxin sodium alginate solution and the calcium chloride solution uniform.
- the method (ii) after filling the disc nucleus pulposus site with the method (ii) and applying compressive force to the intervertebral disc from the caudal side, there is a phenomenon that the hardened gel deviates from the hole on the side of the disc where the gel was injected. It was. In the method (i), no deviation from the intervertebral disc was observed.
- the method (i) has an advantage that the calcium concentration in the intervertebral disc can be reduced and the cytotoxicity can be reduced as compared with the method (ii). Further, the method (ii) has a risk of causing a serious neurological disorder when the cured gel protrudes into the spinal canal. However, the method (i) has a low risk.
- the method (i) that is, the method in which the calcium chloride solution is applied to the intervertebral disc surface after injecting the low endotoxin sodium alginate solution into the nucleus pulposus nucleus site, is a method suitable for nucleus pulposus supplementation using low endotoxin sodium alginate. It was thought that.
- the technique of hardening the vicinity of the injection port on the surface of the intervertebral disc with a cross-linking agent is the compressive force applied to the intervertebral disc by changing the posture or walking after filling with the sodium alginate solution. It was suggested that this is a filling method that can withstand the stretching force.
- Example 4 Examination of properties after injection of low endotoxin sodium alginate solution After injecting a low endotoxin sodium alginate solution into the intervertebral disc nucleus, when the calcium chloride solution is brought into contact with the vicinity of the sodium alginate solution inlet on the surface of the intervertebral disc and the contact area is hardened, the injected low endotoxin sodium alginate solution becomes the intervertebral disc nucleus In order to predict what kind of property it is, the following examination was performed in vitro.
- Example 4 Test method Put the sodium alginate solution into each of the micro test tubes (diameter 6mm, height 25mm) by the following three methods X, Y, and Z, and leave the test tubes beside. After 1 hour, 24 hours, 48 hours, and 1 week, the properties of the test substance in the test tube were evaluated. In the sheep internuclear disc nucleusectomy model of Example 5, the nucleus was removed by excising the annulus fibrosus at 5 mm x 3 mm. Therefore, the size is relatively close to this in vivo test, and manipulation in a test tube is possible. A 6 mm diameter micro test tube was selected as the possible size.
- Example 2 A-2 low endotoxin sodium alginate was dissolved in physiological saline and used as a 2 w / v% solution. The test was performed at room temperature of 20 ° C.
- the properties of Group X were almost the same from 1 hour to 1 week later. That is, the surface portion of the sodium alginate solution in the test tube having a surface portion of about 2 to 3 mm was in a gel state, but no lumps were observed except for the surface, and the solution was in a sol state. Most of the sol-like portion could be sucked with a syringe equipped with a 21G needle, but the viscosity was higher than that of the 2 w / v% sodium alginate solution, and it took time for suction.
- the properties of the Y group were almost the same from 1 hour to 1 week later. That is, most of the inside of the test tube was gelled in a jelly shape, and a liquid such as water was slightly observed. It was speculated that this was a “separation” phenomenon where the gel contracted and the liquid separated and exuded. The gelled part could not be sucked with a syringe with a 21G needle.
- the properties of the Z group were almost the same from 1 hour to 1 week later. That is, a white and turbid gelled mass having a diameter of about 5 mm was formed, and the other part became a liquid like water. This was also presumed to be a detachment phenomenon. The gelled part could not be sucked with a syringe with a 21G needle.
- the method of group X is a method simulating the injection method into the intervertebral nucleus pulposus performed in the embodiment of the present invention, and the sodium alginate solution is sol-like even when the sodium alginate solution is injected into the intervertebral nucleus pulposus. It was predicted to exist at.
- both the Y group and the Z group exist in a gel state, and as confirmed in Example 3 of the present invention, when compressive force is applied from the cranial and caudal side after the gel is filled in the intervertebral disc nucleus pulposus There was a concern that the cured gel could deviate from the side of the disc into which the gel was injected.
- Example 5 Application of low endotoxin sodium alginate solution to sheep intervertebral disc nucleus pulposus defect model
- a sheep endocrine nucleus pulposus defect model was filled with a low endotoxin sodium alginate solution and the effect was evaluated. The following evaluation was performed using L1 / 2, L2 / 3, L3 / 4, and L4 / 5 of 7 discs of sheep (male, Suffolk) weighing 40 kg to 60 kg.
- the size of the excision of the annulus was examined at 5 mm ⁇ 3 mm and 10 mm ⁇ 3 mm, and 5 mm ⁇ 3 mm in which progression of intervertebral disc degeneration was observed was selected according to the amount of nucleus pulposus removal.
- the amount of nucleus pulposus was examined in four types: 0.02 g, 0.05 g, 0.1 g, and 0.2 g.
- the amount of ovine nucleus pulposus 0.1 g was 1.2 g when converted to humans, and since it was the closest to the amount of nucleus pulposus in human clinical practice, 0.1 g was selected as the amount of nucleus pulposus.
- pentobarbital was overdose and euthanized, the lumbar spine was excised, and the disc tissue was collected.
- the intervertebral disc height index is calculated by dividing the intervertebral disc height (the average value of the anterior, central and posterior disc heights) by the front and rear diameters of the disc (Eur Spine J. 2014, 23 (1): 19 -26.)
- the intervertebral disc height index in the nucleus pulposus group was significantly lower than that in the normal control group.
- the disc height index in the treatment group was not significantly different from that in the normal control group. From this, it was found that the decrease in the intervertebral disc height due to the nucleotomy was suppressed by filling the nucleus pulposus with the low endotoxin sodium alginate solution (FIG. 9).
- the ratio of anti-Type ⁇ I collagen antibody-positive cells to the number of cells in the section at 4 weeks after the operation was 10% or less in the normal control group, whereas the nucleus pulposus group and the treatment group Showed significantly higher values.
- the percentage of anti-Type I collagen antibody positive cells was significantly lower than in the nucleus pulposus group.
- the ratio of anti-Type II collagen antibody positive cells to the number of cells in the sections was about 60% in the normal control group and the treatment group, and no difference was observed.
- the nucleus pulposus group showed about 40%, and a significant difference was observed in comparison with the normal control group and in comparison with the treatment group (FIG. 10).
- the proportion of anti-Type II collagen antibody-positive cells is lower than normal, but by filling with low endotoxin sodium alginate solution, it is comparable to the normal control group at 4 weeks after surgery. It was shown to recover.
- the composition of the present invention can be preferably used particularly for filling of the nucleus pulposus after discectomy.
- the decrease in the intervertebral disc height index due to the removal of the nucleus pulposus was suppressed, suggesting the possibility of preventing and / or reducing the degeneration of the intervertebral disc adjacent to the treated intervertebral disc.
- Example 6 Examination of composition of sheep nucleus pulposus nucleus The main components of the extracellular matrix of the nucleus pulposus of the intervertebral disc are water, type II collagen, and proteoglycan, and it is said that the ratio of proteoglycan to collagen is higher than other cartilage tissues such as intervertebral disc endplate and articular cartilage .
- the ovine intervertebral nucleus pulposus defect model was filled with a low endotoxin sodium alginate solution, and biochemical analysis of nucleus pulposus tissue was performed and evaluated at 4 weeks after the operation in accordance with the above literature.
- the nucleus pulposus of the intervertebral disc and the left and right cartilage tissues (articular cartilage) of the femur were collected, and after pretreatment of the specimen, sulfated glycosaminoglycan (GAG) and hydroxyproline (HYP ) was measured.
- GAG glycosaminoglycan
- HEP hydroxyproline
- Pretreatment of the sample is freeze-dried, and after dry weight measurement, 1 mL of pronase solution (20 mM HEPES buffer (pH 7.5) containing 1 mg / mL of pronase (Calbiochem)) is added to the dried sample. And digested at 60 ° C. for 3 hours with stirring every other hour. Centrifugation was performed at 8,000 ⁇ g for 10 minutes, and the obtained supernatant was used as a sample stock solution for measurement. Refrigerated until measurement.
- GAG sulfated glycosaminoglycan
- the measurement sample stock solution was diluted 100 times with purified water, and the resulting diluted solution was used as a measurement sample.
- the blank sample was diluted 100 times with the pronase solution.
- the measurement wavelength was 620 nm, and the amount of GAG in the sample was calculated using a calibration curve prepared using chondroitin-6-sulfate (CS-6) in the measurement kit as a standard.
- Hydroxyproline was measured as follows. The measurement sample stock solution was diluted 100 times with purified water, and the resulting diluted solution was used as a measurement sample. The blank sample was diluted 100 times with the pronase solution. A sample for measurement of 50 ⁇ L was collected in a vial for hydrolysis, added with the same amount of concentrated hydrochloric acid, sealed, and then hydrolyzed at 120 ° C. for 16 hours. Three hydrolyzed samples were prepared per specimen. 20 ⁇ L of the hydrolyzed sample and 100 ⁇ L of the HYP standard solution were collected in a well plate, depressurized at 40 ° C. for 15 hours, and dried.
- the average value of GAG / HYP in the normal control group was 17.8, which was higher than that of untreated articular cartilage 3.1.
- the average value of GAG / HYP in the nucleus pulposus group and the treatment group was lower than that in the normal control group.
- the average value of GAG / HYP tended to be slightly higher in the treatment group than in the nucleus pulposus group.
- the nucleus pulposus nucleus has a higher sulfated glycosaminoglycan (GAG) / hydroxyproline (HYP) value than the articular cartilage, that is, the ratio of components constituting the tissue is different. It was suggested that the tissue characteristics were different from those of articular cartilage. It was considered that the composition of the present invention may restore such a characteristic composition of the nucleus pulposus.
- GAG glycosaminoglycan
- HEP hydroxyproline
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Transplantation (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Pharmacology & Pharmacy (AREA)
- Neurology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Molecular Biology (AREA)
- Dermatology (AREA)
- Cardiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Materials For Medical Uses (AREA)
- Medicinal Preparation (AREA)
- Prostheses (AREA)
- Polysaccharides And Polysaccharide Derivatives (AREA)
Abstract
Description
本発明者らは、このような知見に基づきさらに検討を重ね、本発明を完成するに至った。
[1] 対象の髄核部位に適用し、適用後に一部分を硬化するように用いられ、髄核部位への適用時に流動性を有する、低エンドトキシンアルギン酸の1価金属塩を含有する、椎間板の髄核補填用組成物。
[1A] 低エンドトキシンアルギン酸の1価金属塩を含有し、髄核部位への適用時に流動性を有する、対象の髄核部位への適用後に一部分を硬化するように用いられる、椎間板の髄核補填用組成物。
[2] 前記組成物の硬化を、前記組成物の表面の少なくとも一部分に架橋剤を接触させることで行う、上記[1]または[1A]に記載の組成物。
[3] 前記組成物の髄核部位への適用を、椎間板表面の組成物の充填口を介して行い、前記組成物の一部分の硬化を、椎間板表面の組成物の充填口に架橋剤を接触させることで行う、上記[1]~[2]のいずれか1項に記載の組成物。
[4] 前記組成物の髄核部位への適用を、髄核の少なくとも一部を除去することで形成した髄核欠損部に、前記組成物を適用することで行う、上記[1]~[3]のいずれか1項に記載の組成物。
[5] 前記流動性を有する組成物の粘度が、100mPa・s~30000mPa・sである、上記[1]~[4]のいずれか1項に記載の組成物。
[5A] 前記流動性を有する組成物の見掛け粘度が、コーンプレート型粘度計を用いた20℃の条件での測定により、100mPa・s~30000mPa・sである、上記[1]~[4]のいずれか1項に記載の組成物。
[6] 前記低エンドトキシンアルギン酸の1価金属塩は、GPC-MALS法により測定された重量平均分子量が8万以上である、上記[1]~[5A]のいずれか1項に記載の組成物。
[6A] 前記低エンドトキシンアルギン酸の1価金属塩は、GPC-MALS法により測定された重量平均分子量(絶対分子量)が8万以上である、上記[1]~[5A]のいずれか1項に記載の組成物。
[7] 前記組成物は、低エンドトキシンアルギン酸の1価金属塩の濃度が0.5w/v%~5w/v%である、上記[1]~[6A]のいずれか1項に記載の組成物。
[7A] 前記組成物は、低エンドトキシンアルギン酸の1価金属塩の濃度が0.5w/w%~5w/w%である、上記[1]~[6A]のいずれか1項に記載の組成物。
[8] 前記組成物は、前記対象の髄核部位に適用する前に、前記組成物を硬化させる量の架橋剤を含有しない、上記[1]~[7A]のいずれか1項に記載の組成物。
[9] 前記組成物は、細胞を含有しない、上記[1]~[8]のいずれか1項に記載の組成物。
[10] 架橋剤が2価以上の金属イオン化合物である、上記[2]~[9]のいずれか1項に記載の組成物。
[10A] 2価以上の金属イオン化合物がCa2+、Mg2+、Ba2+、Sr2+からなる群より選ばれる少なくとも1つの金属イオン化合物である、上記[10]に記載の組成物。
[11] 前記組成物が、椎間板変性および/または椎間板損傷の治療、予防または再発抑制のために用いられる、上記[1]~[10A]のいずれか1項に記載の組成物。
[12] 前記椎間板変性および/または椎間板損傷が、椎間板ヘルニア、椎間板症、脊椎変性辷り症、化膿性椎間板炎、変形性脊椎症、脊柱管狭窄症、及び椎間板損傷からなる群から選択される少なくとも1種である、上記[11]に記載の組成物。
[13] 前記組成物が、髄核部位への適用前に乾燥状態である、上記[1]~[12]のいずれか1項に記載の組成物。
[13A] 前記組成物が、髄核部位への適用前に乾燥状態または溶液状態である、上記[1]~[12]のいずれか1項に記載の組成物。
[14] 前記乾燥状態の低エンドトキシンアルギン酸の1価金属塩が、凍結乾燥体である、上記[13]または[13A]に記載の組成物。
[14A] 前記組成物の一部分の硬化が、髄核部位への充填と同様の架橋剤の使用方法および使用比率を用いて、本明細書の実施例4に準じて、in vitroで、直径6mmの試験管に低エンドトキシンアルギン酸ナトリウム500μLおよび架橋剤を充填して1時間静置後に、試験管内の組成物の容量の少なくとも5割が21Gの注射針をつけたシリンジで吸引できることで示される、上記[1]~[14]のいずれか1項に記載の組成物。
[14B] 前記流動性を有する組成物は、組成物を20℃で1時間静置した後に、21Gの注射針で注入できる流動性を有する、上記[1]~[14A]のいずれか1項に記載の組成物。
[15] 上記[1]ないし[14B]のいずれか1項に記載の組成物、および架橋剤を少なくとも含む、椎間板の髄核補填用キット。
[16] 椎間板変性および/または椎間板損傷の治療、予防または再発抑制のための方法であって、
低エンドトキシンアルギン酸の1価金属塩を含有し、流動性を有する組成物を、前記治療、予防または再発抑制を必要とする対象の椎間板の髄核部位に適用し、
適用した前記組成物の一部分を硬化することを含む、前記方法。
[17] 前記椎間板変性および/または椎間板損傷が、椎間板ヘルニア、椎間板症、脊椎変性辷り症、化膿性椎間板炎、変形性脊椎症、脊柱管狭窄症、及び椎間板損傷からなる群から選択される少なくとも1種である、上記[16]に記載の方法。
[18] 椎間板変性および/または椎間板損傷の治療、予防または再発抑制のための組成物を製造するための低エンドトキシンアルギン酸の1価金属塩の使用であって、
前記組成物が、対象の髄核部位に適用され、適用後に一部分を硬化するように用いられ、髄核部位への適用時に流動性を有する、前記使用。
[19] 前記椎間板変性および/または椎間板損傷が、椎間板ヘルニア、椎間板症、脊椎変性辷り症、化膿性椎間板炎、変形性脊椎症、脊柱管狭窄症、及び椎間板損傷からなる群から選択される少なくとも1種である、上記[18]に記載の使用。
[20] 低エンドトキシンアルギン酸の1価金属塩を含有し、流動性を有する組成物を、椎間板変性および/または椎間板損傷の治療、予防または再発抑制を必要とする対象の椎間板の髄核部位に適用し、適用した組成物の一部を硬化する、椎間板変性および/または椎間板損傷の治療、予防または再発抑制において使用されるための低エンドトキシンアルギン酸の1価の金属塩。
1.本発明の組成物
本発明は、椎間板の髄核補填に好ましく用いられる組成物に関する。
本発明の組成物は、対象の髄核部位に適用し、適用後に一部分を硬化するように用いられ、髄核部位への適用時に流動性を有する、低エンドトキシンアルギン酸の1価金属塩を含有する、椎間板の髄核補填用組成物である(本明細書において、「本発明の組成物」という場合がある)。
「アルギン酸の1価金属塩」は、アルギン酸の6位のカルボン酸の水素原子を、Na+やK+などの1価金属イオンとイオン交換することでつくられる水溶性の塩である。アルギン酸の1価金属塩としては、具体的には、アルギン酸ナトリウム、アルギン酸カリウムなどを挙げることができるが、特には、市販品により入手可能なアルギン酸ナトリウムが好ましい。アルギン酸の1価金属塩の溶液は、架橋剤と混合したときにゲルを形成する。
分子量測定にゲル浸透クロマトグラフィーを用いる場合の代表的な条件は、本明細書の実施例に記載のとおりである。カラムは、例えば、GMPW-XL×2+G2500PW-XL(7.8mm I.D.×300mm)を用いることができ、溶離液は、例えば、200mM硝酸ナトリウム水溶液とすることができ、分子量標準としてプルランを用いることができる。
本発明で用いるアルギン酸の1価金属塩は、低エンドトキシンのアルギン酸の1価金属塩である。低エンドトキシンとは、実質的に炎症、または発熱を惹起しない程度にまでエンドトキシンレベルが低いことをいう。より好ましくは、低エンドトキシン処理されたアルギン酸の1価金属塩であることが望ましい。
本発明の組成物は、アルギン酸の1価金属塩の溶液を用いて調製してもよい。アルギン酸の1価金属塩の溶液は、公知の方法またはそれに準じる方法により調製することができる。すなわち、本発明で用いられるアルギン酸の1価金属塩は、前述の褐藻類を用いて、酸法、カルシウム法など公知の方法により製造することができる。具体的には、例えば、これらの褐藻類から、炭酸ナトリウム水溶液などのアルカリ水溶液を用いて抽出した後、酸(例えば、塩酸、硫酸など)を添加することによってアルギン酸を得ることができ、アルギン酸のイオン交換によりアルギン酸の塩を得ることができる。前述のとおり、低エンドトキシン処理を行う。アルギン酸の1価金属塩の溶媒は、生体へ適用可能な溶媒であれば特に限定されないが、例えば、精製水、蒸留水、イオン交換水、ミリQ水、生理食塩水、リン酸緩衝生理食塩水(PBS)などが挙げられる。これらは、滅菌されていることが好ましく、低エンドトキシン処理されたものが好ましい。例えば、ミリQ水をろ過滅菌して用いることができる。
また、本発明の組成物を得るための操作は全てエンドトキシンレベル、および、細菌レベルの低い環境下で行うことが望ましい。例えば、操作はクリーンベンチで、滅菌器具を使用して行うことが好ましく、使用する器具を市販のエンドトキシン除去剤で処理してもよい。
本発明のいくつかの態様の組成物は、流動性のある液体状、すなわち、溶液状である。本発明の組成物は、髄核部位への適用時に流動性を有する。本発明の態様の1つでは、好ましくは、本発明の組成物は、組成物を20℃で1時間静置した後に、21Gの注射針で注入できる流動性を有する。この態様の本発明の組成物の見掛け粘度は、本発明の効果が得られれば、特に限定されないが、粘度が低すぎると適用した部位の周辺組織への密着性が弱くなる恐れがあるため、好ましくは10mPa・s以上、より好ましくは100mPa・s以上、さらに好ましくは200mPa・s以上、とりわけ好ましくは500mPa・s以上である。見掛け粘度が高すぎると取扱性が悪くなる恐れがあるため、好ましくは50000mPa・s以下、より好ましくは20000mPa・s以下であり、さらに好ましくは10000mPa・s以下である。見掛け粘度が20000mPa・s以下のときシリンジ等での適用がより容易に行える。しかし、見掛け粘度が20000mPa・s以上であっても加圧型や電動型の充填器具やその他の手段を用いて適用可能である。本発明の組成物の好ましい範囲は、10mPa・s~50000mPa・s、より好ましくは、100mPa・s~30000mPa・s、さらに好ましくは200mPa・s~20000mPa・s、またさらに好ましくは500mPa・s~20000mPa・s、とりわけ好ましくは700mPa・s~20000mPa・sである。別の好ましい態様では、500mPa・s~10000mPa・s、あるいは2000mPa・s~10000mPa・sであってもよい。本発明のいくつかの態様の組成物は、シリンジ等で対象に適用することもできる粘度である。
本発明の組成物は、低エンドトキシンアルギン酸の1価金属塩を有効成分として含有することを特徴とする。本発明者らは、低エンドトキシンアルギン酸1価金属塩を生体の髄核部位に充填した場合に、アルギン酸の1価金属塩自体が髄核組織の再生または治療効果を発揮することを初めて見出した。有効成分として含有するとは、低エンドトキシンアルギン酸の1価金属塩が患部に適用された際に、髄核組織の再生または治療効果を発揮できる量で含有されていればよく、少なくとも、組成物全体の0.1w/v%以上であることが好ましく、より好ましくは0.5w/v%以上、さらに好ましくは、1w/v%である。本発明の組成物中の好ましいアルギン酸の1価金属塩濃度は、分子量の影響を受けるので、一概にはいえないが、好ましくは0.5w/v%~5w/v%、より好ましくは1w/v%~5w/v%であり、さらに好ましくは、1w/v%~3w/v%で、とりわけ好ましくは1.5w/v%~2.5w/v%である。また、別の態様では、本発明の組成物中のアルギン酸の1価金属塩濃度は、好ましくは、0.5w/w%~5w/w%、より好ましくは1w/w%~5w/w%であり、さらに好ましくは、1w/w%~3w/w%で、とりわけ好ましくは1.5w/w%~2.5w/w%であってもよい。
本発明の別のいくつかの態様の組成物は、細胞を用いる。
細胞としては、例えば、髄核細胞、幹細胞、間質細胞、間葉系幹細胞、骨髄間質細胞などが挙げられ、由来は特に限定されないが、椎間板髄核、骨髄、脂肪組織、臍帯血などを挙げることができる。また、細胞として、ES細胞およびiPS細胞を挙げることもできる。
本発明の組成物は、髄核部位への適用後に一部分を硬化するように用いられる。
「一部分を硬化する」とは、流動性を有する本発明の組成物の一部分に架橋剤を接触させて、架橋剤と接触した組成物の全体ではなく一部分をゲル化し、固めることをいう。好ましくは、流動性を有する本発明の組成物の表面の少なくとも一部分に架橋剤を接触させることで本発明の組成物の一部分を硬化する。本発明のいくつかの態様では、「組成物を髄核部位への適用後に一部分を硬化させる」とは、髄核部位への充填と同様の架橋剤の使用方法および使用比率を用いて、本明細書の実施例4に準じて、in vitroで、直径6mmの試験管に低エンドトキシンアルギン酸ナトリウム500μLおよび架橋剤を充填して1時間静置後に、試験管内の組成物の容量の少なくとも5割はゲル化しておらず、ゲル化していない部分は、試験管内の組成物の容量の少なくとも5割が21Gの注射針をつけたシリンジで吸引できることで示されてもよい。組成物が髄核部位への充填後にもこのような性状を示すことにより、充填後に椎間板の頭尾側から圧縮力をかけた場合でも組成物が逸脱することがないと考えられる。「組成物の表面の少なくとも一部分」は、例えば、髄核へつながる椎間板の表面の開口部であり、好ましくは、髄核部位に組成物を適用するのに使用した椎間板の表面の開口部、すなわち組成物の充填口である。組成物の表面の少なくとも一部分をゲル化して固めることで、椎間板から組成物が漏れ出すのを効果的に防ぐことができる。椎間板表面の組成物の充填口は、例えば、椎間板の表面にシリンジの針やメスで作製した組成物の充填に用いられた開口部、あるいは、椎間板ヘルニア摘出時にメス等により作製された椎間板表面の開口部であることが好ましい。この態様における椎間板とは好ましくは線維輪である。
本発明の組成物は、ヒト、またはヒト以外の生物、例えば、トリおよび非ヒト哺乳動物(例えば、ウシ、サル、ネコ、マウス、ラット、モルモット、ハムスター、ブタ、イヌ、ウサギ、ヒツジ、およびウマ)の椎間板の髄核部位に適用し、髄核の再生を促進するために用いられる。
本発明の組成物の粘度が高い場合には、シリンジで適用するのが困難になるため、加圧型や電動型などのシリンジを用いてもよい。シリンジなどを使用しなくても、例えば、へら、棒などで髄核部位の欠損部へ適用してもよい。シリンジで注入する場合、例えば、14G~27Gまたは14G~26Gの針を使用するのが好ましい。
アルギン酸は動物の体内に元来存在しない物質であるため、動物はアルギン酸を特異的に分解する酵素を保有していない。アルギン酸は動物体内においては、通常の加水分解により徐々に分解されるが、ヒアルロン酸等のポリマーに比べ体内の分解が緩やかであり、また髄核内には血管が存在しないため、髄核内に充填した場合、長期間の効果持続が期待できる。
本発明は、前記本発明の組成物を用いる、椎間板変性および/または椎間板損傷の治療、予防または再発抑制のための方法を提供する。好ましくは、本発明の治療方法は、椎間板変性および/または椎間板損傷の治療、予防または再発抑制のための方法であって、低エンドトキシンアルギン酸の1価金属塩を含有し、流動性を有する組成物を、前記治療、予防または再発抑制を必要とする対象の椎間板の髄核部位に適用し、適用した前記組成物の一部分を硬化することを含む。
本発明は、椎間板の髄核補填用キットを提供する。
本発明のキットには、本発明の組成物を含めることができる。本発明のキットに含める本発明の組成物は、溶液状態または乾燥状態であるが、好ましくは、乾燥状態であり、より好ましくは、凍結乾燥体であり、特に好ましくは、凍結乾燥粉体である。また、本発明の組成物が乾燥状態のときは溶解用の溶媒(例えば、注射用水)を含むことが望ましい。
本発明のキットは、さらに、架橋剤を含んでいてよい。
本発明のキットは、さらに、架橋剤、シリンジ、注射針、ゲル用ピペット、専用充填器、取り扱い説明書等を含めることができる。
1-(1)非変性ヒト椎間板細胞の単離・培養
ヒト非変性椎間板組織から髄核組織を取り出し、0.25%コラゲナーゼ(Wako)を含むDMEM(Dulbecco’s modified Eagle’s medium)培地で37℃、4時間処理し、髄核細胞を単離した。得られた髄核細胞を1%ペニシリン/ストレプトマイシン、1.25μg/ml ファンギゾン(fungizone,Invitrogen)、10%FBS(fetal bovine serum)を含むDMEMを培養液として37℃、5%CO2、20%O2の条件下で培養し、2継代の細胞を実験で使用した。
下記表1に示す(A)低エンドトキシン処理されたアルギン酸ナトリウムと、(B)食品グレード(commercial grade)のアルギン酸ナトリウム(和光純薬工業(株)、199-09961)の2種類のアルギン酸ナトリウム(持田製薬(株))とをそれぞれ用いて、ヒト髄核細胞を培養し、比較を行った。
培養開始から48時間、7日、14日、および28日後にビーズを回収し、4℃の55mMクエン酸ナトリウム水溶液中にビーズを20分間浸すことでビーズを溶解し、遠心分離により細胞を回収した。5μMのCalcein AMと1.5μMのpropidium iodide(PI)により、得られた細胞を染色し、共焦点レーザー顕微鏡(オリンパス、FV300)で観察した。Calcein AM陽性細胞を生細胞、PI陽性細胞を死細胞とし、ImageJ(National Institutes of Health, Bethesda, MD, USA)を用いて、生細胞率を算定した。細胞を回収した各時点においてn=5とした。
1-(3)と同様に、培養開始から48時間、7日、14日、および28日後にそれぞれビーズを回収し、ビーズをPBSで2回洗浄後細胞を回収し、3.6×105個の細胞をAnnexin V-fluorescein isothiocyanate(FITC) Apoptosis Detection Kit II(BD Biosciences, San Jose, CA, USA)によりラベリングし、フローサイトメーター(FACS Cant; BD biosciences, CA, USA)を用いてアポトーシス細胞を計測した。FITC+/PI-を早期アポトーシス細胞、FITC+/PI+を後期アポトーシス細胞とし、これらを合わせてアポトーシス細胞とした。また、FITC-/PI-を生細胞として、全細胞に対する生細胞の比率、および、全細胞に対するアポトーシス細胞の比率を評価した。細胞を回収した各時点においてn=5とした。
ヒト椎間板内は無血管領域であり低栄養の環境下にあるため、椎間板内の環境を想定した血清飢餓下で細胞培養試験を実施し、評価した。
ウサギ椎間板髄核欠損モデルに対して、2種類の低エンドトキシンアルギン酸ナトリウム溶液をそれぞれ充填し、効果を評価した。
体重3.2~3.5kgの日本白色家兎に対し、ペントバルビタールによる静脈麻酔と1%キシロカインの局所麻酔を行い、18G針を用いて椎間板の髄核組織を吸引し、椎間板髄核欠損モデルを作製した。L2/3、および、L4/5椎間板髄核に対し吸引を行い、椎間板欠損モデルとし、L3/4は吸引を行わず、正常椎間板とした(正常コントロール群)。
低エンドトキシンアルギン酸ナトリウムは、次の2種類を用いた。いずれもエンドトキシン含量は、50g/EU未満であった。各低エンドトキシンアルギン酸ナトリウムの見掛け粘度及び重量平均分子量は表2のとおりである。アルギン酸ナトリウムの見掛け粘度測定は、日本薬局方(第16版)の粘度測定法に従い、回転粘度計法(コーンプレート型回転粘度計)を用いて測定した。具体的な測定条件は以下のとおりである。試料溶液の調製は、MilliQ水を用いて行った。測定機器は、コーンプレート型回転粘度計(粘度粘弾性測定装置レオストレスRS600(Thermo Haake GmbH)センサー:35/1)を用いた。回転数は、1w/w%アルギン酸ナトリウム溶液測定時は1rpm、2w/w%アルギン酸ナトリウム溶液測定時は0.5rpmとした。読み取り時間は、1w/w%溶液測定時は、2分間測定し、開始1分から2分までの平均値とし、2w/w%溶液測定時は、2.5分間測定し、開始0.5分から2.5分までの平均値とした。3回の測定の平均値を測定値とした。測定温度は20℃とした。
試料に溶離液を加え溶解後、0.45μmメンブランフィルターろ過したものを測定溶液とした。
(1)ゲル浸透クロマトグラフィー(GPC)測定
[測定条件(相対分子量分布測定)]
カラム:TSKgel GMPW-XL×2+G2500PW-XL(7.8mm I.D.×300mm×3本)
溶離液:200mM硝酸ナトリウム水溶液
流量:1.0mL/min
濃度:0.05%
検出器:RI検出器
カラム温度:40℃
注入量:200μL
分子量標準:標準プルラン、グルコース
[屈折率増分(dn/dc)測定(測定条件)]
示唆屈折率計:Optilab T-rEX
測定波長:658nm
測定温度:40℃
溶媒:200mM硝酸ナトリウム水溶液
試料濃度:0.5~2.5mg/mL(5濃度)
カラム:TSKgel GMPW-XL×2+G2500PW-XL(7.8mm I.D.×300mm×3本)
溶離液:200mM硝酸ナトリウム水溶液
流量:1.0mL/min
濃度:0.05%
検出器:RI検出器、光散乱検出器(MALS)
カラム温度:40℃
注入量:200μL
椎間板の側面から髄核へ向かって注射針を挿入し、低エンドトキシンアルギン酸ナトリウム溶液20μlを髄核欠損部へ注入した。注射針を抜いた椎間板の側面に、102mM塩化カルシウム水溶液を数秒かけた(それぞれA-1群、A-2群といい、これらを合わせて治療群という)。
7.0-Tesla MR scanner (Unity Inova, Varian)により、椎間板のT2強調矢状断像を撮影した。椎間板のT2強調MRI画像により、椎間板の変性による変化が確認できる。椎間板変性の重症度を評価するため、Pfirrmann分類を用いてスコア化した。本分類は、MRIにおける椎間板変性を5段階で評価した指標である(グレード1:正常~グレード5:高度に変性)。椎間板変性の評価基準を表3に示す(Spine (Phila Pa 1976). 2001;26(17) 1873-8)。
Analyze 10.0 software (AnalyzeDirect, Overland Park, KS, USA)を用いて、矢状断におけるMRI index(髄核の平均信号強度と髄核面積の積)を測定し定量的に評価した。正常コントロール群の椎間板のMRI indexを100としたときの、各群におけるMRI indexの割合で評価した(Spine (Phila Pa 1976). 2005 Jan 1;30(1):15-24.参照)。
A-2群についての術後12週の評価においても、A-2群のMRI indexは、吸引単独群と比較して有意に高値であった。
MRI撮像後に椎間板の組織標本を作製した。10%ホルムアルデヒドでサンプルを固定、10%EDTA(pH7.5)で脱灰処理し、パラフィンに包埋した。矢状断5μm厚のパラフィン切片をキシレンにより脱パラフィンし、アルコール処理、水洗いした後、HE染色、サフラニン-O染色を施した。線維輪における変性変化の分類であるNishimuraらの分類(Spine (Phila Pa 1976). 1998;23(14):1531-8)を用いて、椎間板組織全体における変性度をスコア化した。Nishimuraらの分類は以下のとおりである。
グレード2:破裂を伴い中程度に湾曲(moderately serpentine with rupture)
グレード3:低度に逆転を伴い高度に湾曲(severely serpentine with mildly reversed)
グレード4:高度に逆転した形状(severely reversed contour)
グレード5:不明瞭(indistinct)
2-(5)で作製した組織標本に対して、抗Type I collagen抗体および抗type II collagen抗体を用いて免疫組織学的染色を行い、椎間板髄核において無作為に選択した5視野において陽性細胞数を計測した。
3-(1)低エンドトキシンアルギン酸ナトリウム溶液の注入方法の検討
次の2種類の注入方法(i)(ii)により、低エンドトキシンアルギン酸ナトリウム溶液をヒツジ屍体(Cadaver)腰椎へ注入し、評価を行った。
(ii)A-2の低エンドトキシンアルギン酸ナトリウム2%溶液と100mM塩化カルシウム溶液とを同時に1:1で椎間板髄核部分摘出部へ充填する手法(髄核部位へ注入するアルギン酸ナトリウム全体が硬化する)。
(ii)は、低エンドトキシンアルギン酸ナトリウム溶液と塩化カルシウム溶液をそれぞれ別のシリンジに入れ、22G針で椎間板髄核の欠損部に同時に注入することにより実施した。
3-(1)において(i)の手法で作製したヒツジカダバーの椎間板(A-2の低エンドトキシンアルギン酸ナトリウム2%溶液を椎間板髄核部分摘出部へ注入後、椎間板表面の注射針の穴付近に100mM塩化カルシウム溶液を接触させたもの)について、充填から約1時間後に、Instron5943(インストロン社)を用いて、椎間板に頭尾側から、-300N~300Nの軸圧縮・伸張力で、1000回繰り返し圧縮力および伸張力をかけて、アルギン酸ナトリウム溶液を注入した穴からの逸脱がないかを観察した。このとき、アルギン酸ナトリウム溶液は、視認性向上のために0.05%トルイジンブルーで呈色したものを用いた。
椎間板髄核に低エンドトキシンアルギン酸ナトリウム溶液を注入後、椎間板表面のアルギン酸ナトリウム溶液注入口付近に塩化カルシウム溶液を接触させ、その接触部を硬化させたとき、注入した低エンドトキシンアルギン酸ナトリウム溶液が椎間板髄核内でどのような性状であるかを予測するため、in vitroで以下の検討を行った。
下記のX、Y、Zの3種の方法で、ミクロ試験管(直径6mm、高さ25mm)にアルギン酸ナトリウム溶液をそれぞれ入れて、試験管を横にして静置し、1時間後、24時間後、48時間後、1週間後に、試験管内の被験物質の性状を評価した。実施例5のヒツジ椎間板髄核摘出モデルにおいて、線維輪を5mm×3mmで切除して髄核摘出を行っているため、このin vivo試験に比較的サイズが近く、かつ、試験管内での操作が可能なサイズとして直径6mmのミクロ試験管を選択した。実施例2のA-2の低エンドトキシンアルギン酸ナトリウムを生理食塩水で溶解し、2w/v%溶液として用いた。試験は室温20℃で行った。
試験に用いた2w/v%アルギン酸ナトリウム溶液は、生理食塩水と比較して粘度が高く、バイアルを傾けると液面がゆっくり移動し、21Gの注射針をつけたシリンジで時間をかけて吸うことができた。
ヒツジ椎間板髄核欠損モデルに対して、低エンドトキシンアルギン酸ナトリウム溶液を充填し、効果を評価した。体重40kg~60kgのヒツジ(雄、サフォーク種)の7頭の椎間板のL1/2、L2/3、L3/4、及びL4/5を用いて以下の評価を行った。
ヒツジに対して麻酔を行い、電気メスを用いて椎間板を露出した。椎間板の線維輪を5mm×3mmで切除、除去し、その穴から鉗子を挿入し髄核を0.10g除去し、椎間板髄核欠損モデルを作製した。ここで、線維輪の切除の大きさ、及び、髄核摘出量は、事前に、ヒツジ椎間板髄核摘出による椎間板変性試験を行い決定した。線維輪の切除の大きさは、5mm×3mm、及び10mm×3mmで検討し、髄核摘出量に応じて椎間板変性の進行を認めた5mm×3mmを選択した。髄核摘出量は、0.02g、0.05g、0.1g、及び0.2gの4種類で検討した。ヒツジ髄核摘出量0.1gは、ヒトに換算すると1.2gとなり、最もヒト臨床での髄核摘出量に近いため、髄核摘出量は、0.1gを選択した。
実施例2のA-2の低エンドトキシンアルギン酸ナトリウムをミリQ水で2w/v%溶液に調製し、その0.10mlをシリンジで、5-(1)で作製したヒツジ椎間板髄核欠損部へ注入した。注入したアルギン酸ナトリウム溶液の表面に、102mM塩化カルシウム水溶液を数秒かけた。約5分間放置した後、塩化カルシウム溶液をかけた部位を生理食塩水で洗浄し、縫合した。これを治療群とした(n=11)。また、5-(1)で椎間板髄核摘出のみ行い縫合を行った群を髄核摘出群(n=10)とした。無処置のヒツジ椎間板を正常コントロール群(n=7)とした。
術後4週時にペントバルビタールを過量投与し安楽死させ、腰椎を切除し、椎間板組織を回収した。
前記2-(5)の記載に準じて、HE染色、サフラニンO染色を施し、椎間板の組織標本を作製した。Boos分類をもとに改変された分類(Eur Spine J. 2014 Jan; 23(1):19-26. Spine 2002 Vol.27, No.23 p.2631-2644)を用いて、椎間板の変性度を評価した。分類を表5に示す。椎間板の項目は最大20ポイント、椎体終板の項目は最大16ポイントであり、合計を最大36ポイントとして評価した。
前記5-(3)で作製した組織標本に対して、抗Type I collagen抗体および抗Type II collagen抗体を用いて免疫組織学的染色を行い、椎間板髄核において無作為に選択した5視野において陽性細胞数を計測した。
椎間板の髄核の細胞外マトリックスの主成分は、水分、タイプ II コラーゲン、プロテオグリカンであり、椎間板終板や関節軟骨など他の軟骨組織と比較して、コラーゲンに対するプロテオグリカンの割合が高いといわれている。このコラーゲンに対するプロテオグリカンの比を、ヒドロキシプロリン(Hydroxyproline: HYP)に対する硫酸化グリコサミノグリカン(sulfated glycosaminoglycans: GAG)の比でみた文献が存在する(European Cells and Materials Vol.8. 2004 p.58-64)。
ヒツジ椎間板髄核欠損モデルに対して、低エンドトキシンアルギン酸ナトリウム溶液を充填し、術後4週時に、上記文献に準じて髄核組織の生化学的分析を行い、評価を行った。
体重35kg~60kgのヒツジ(雄、サフォーク種)の2頭の椎間板のL1/2、L2/3、L3/4、および、L4/5を試験に用いた。実施例5に準じて、ヒツジ椎間板髄核欠損モデルを作製し、2w/v%低エンドトキシンアルギン酸ナトリウム溶液を充填し、治療群とした(n=4)。実施例5に準じて、椎間板髄核摘出のみ行い縫合を行った群を髄核摘出群とした(n=4)。無処置のヒツジ椎間板(T12/L1、L5/6)を正常コントロール群とした(n=4)。術後4週時に椎間板髄核、および、無処置の大腿骨左右の軟骨組織(関節軟骨)を回収し、検体の前処理をした後、硫酸化グリコサミノグリカン(GAG)およびヒドロキシプロリン(HYP)の測定を行った。
検体乾燥重量あたりのGAG量およびHYP量(μg/mg dry weight)を得て、HYPに対するGAGの比(GAG/HYP)を求めた。各群n=4について、平均値および標準偏差を求めた(表6)。また、各群の散布図を図11に示す。
Claims (17)
- 対象の髄核部位に適用し、適用後に一部分を硬化するように用いられ、髄核部位への適用時に流動性を有する、低エンドトキシンアルギン酸の1価金属塩を含有する、椎間板の髄核補填用組成物。
- 前記組成物の硬化を、前記組成物の表面の少なくとも一部分に架橋剤を接触させることで行う、請求項1に記載の組成物。
- 前記組成物の髄核部位への適用を、椎間板表面の組成物の充填口を介して行い、前記組成物の一部分の硬化を、椎間板表面の組成物の充填口に架橋剤を接触させることで行う、請求項1または2に記載の組成物。
- 前記組成物の一部分の硬化が、髄核部位への充填と同様の架橋剤の使用方法および使用比率を用いて、本明細書の実施例4に準じて、in vitroで、直径6mmの試験管に低エンドトキシンアルギン酸ナトリウム500μLおよび架橋剤を充填して1時間静置後に、試験管内の組成物の容量の少なくとも5割が21Gの注射針をつけたシリンジで吸引できることで示される、請求項1~3のいずれか1項に記載の組成物。
- 前記組成物の髄核部位への適用を、髄核の少なくとも一部を除去することで形成した髄核欠損部に、前記組成物を適用することで行う、請求項1~4のいずれか1項に記載の組成物。
- 前記流動性を有する組成物の見掛け粘度が、コーンプレート型粘度計を用いた20℃の条件での測定により、100mPa・s~30000mPa・sである、請求項1~5のいずれか1項に記載の組成物。
- 前記低エンドトキシンアルギン酸の1価金属塩は、GPC-MALS法により測定された重量平均分子量(絶対分子量)が8万以上である、請求項1~6のいずれか1項に記載の組成物。
- 前記組成物は、低エンドトキシンアルギン酸の1価金属塩の濃度が0.5w/w%~5w/w%である、請求項1~7のいずれか1項に記載の組成物。
- 前記組成物は、前記対象の髄核部位に適用する前に、前記組成物を硬化させる量の架橋剤を含有しない、請求項1~8のいずれか1項に記載の組成物。
- 前記流動性を有する組成物は、組成物を20℃で1時間静置した後に、21Gの注射針で注入できる流動性を有する、請求項1~9のいずれか1項に記載の組成物。
- 前記組成物は、細胞を含有しない、請求項1~10のいずれか1項に記載の組成物。
- 架橋剤が2価以上の金属イオン化合物である、請求項2~11のいずれか1項に記載の組成物。
- 前記組成物が、椎間板変性および/または椎間板損傷の治療、予防または再発抑制のために用いられる、請求項1~12のいずれか1項に記載の組成物。
- 前記椎間板変性および/または椎間板損傷が、椎間板ヘルニア、椎間板症、脊椎変性辷り症、化膿性椎間板炎、変形性脊椎症、脊柱管狭窄症、及び椎間板損傷からなる群から選択される少なくとも1種である、請求項13に記載の組成物。
- 前記組成物が、髄核部位への適用前に乾燥状態または溶液状態である、請求項1~14のいずれか1項に記載の組成物。
- 前記乾燥状態の低エンドトキシンアルギン酸の1価金属塩が、凍結乾燥体である、請求項15に記載の組成物。
- 請求項1ないし16のいずれか1項に記載の組成物、および架橋剤を少なくとも含む、椎間板の髄核補填用キット。
Priority Applications (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP23212370.3A EP4374886A1 (en) | 2016-03-23 | 2017-01-27 | Composition for treating intervertebral disc |
| EP17769650.7A EP3434291B1 (en) | 2016-03-23 | 2017-01-27 | Composition for treating intervertebral disc |
| ES17769650T ES2972523T3 (es) | 2016-03-23 | 2017-01-27 | Composición para el tratamiento del disco intervertebral |
| JP2018507084A JP6487110B2 (ja) | 2016-03-23 | 2017-01-27 | 椎間板治療用組成物 |
| US16/086,081 US20200289547A1 (en) | 2016-03-23 | 2017-01-27 | Composition for treating intervertebral disc |
| BR122021024709-9A BR122021024709B1 (pt) | 2016-03-23 | 2017-01-27 | Composição e kit |
| BR112018069060A BR112018069060A2 (pt) | 2016-03-23 | 2017-01-27 | composição para tratar o disco intervertebral |
| CA3018152A CA3018152A1 (en) | 2016-03-23 | 2017-01-27 | Composition comprising a salt of alginic acid for treating intervertebral disc |
| CN201780018143.7A CN109069692A (zh) | 2016-03-23 | 2017-01-27 | 椎间盘治疗用组合物 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016058396 | 2016-03-23 | ||
| JP2016-058396 | 2016-03-23 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2017163603A1 true WO2017163603A1 (ja) | 2017-09-28 |
Family
ID=59901106
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/002925 WO2017163603A1 (ja) | 2016-03-23 | 2017-01-27 | 椎間板治療用組成物 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20200289547A1 (ja) |
| EP (2) | EP4374886A1 (ja) |
| JP (4) | JP6487110B2 (ja) |
| CN (1) | CN109069692A (ja) |
| BR (2) | BR122021024709B1 (ja) |
| CA (1) | CA3018152A1 (ja) |
| ES (1) | ES2972523T3 (ja) |
| WO (1) | WO2017163603A1 (ja) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018164128A1 (ja) * | 2017-03-07 | 2018-09-13 | 持田製薬株式会社 | アルギン酸液剤 |
| WO2019182015A1 (ja) | 2018-03-22 | 2019-09-26 | 持田製薬株式会社 | 非ステロイド性抗炎症性化合物結合アルギン酸誘導体 |
| CN111867645A (zh) * | 2018-01-31 | 2020-10-30 | 国立大学法人神户大学 | 椎间盘退变的治疗剂及椎间盘细胞培养材料 |
| WO2021145325A1 (ja) * | 2020-01-14 | 2021-07-22 | 持田製薬株式会社 | アルギン酸塩を使用した対象の処置のための組合せ及び方法 |
| WO2022092298A1 (ja) * | 2020-11-01 | 2022-05-05 | 雄平 島田 | 脊髄損傷の治療剤 |
| WO2022102093A1 (ja) * | 2020-11-13 | 2022-05-19 | 国立大学法人北海道大学 | 椎間板性疼痛抑制用組成物 |
| WO2022163872A1 (ja) * | 2021-01-29 | 2022-08-04 | 国立大学法人北海道大学 | 椎間板再生用組成物 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7435438B2 (ja) * | 2018-12-26 | 2024-02-21 | 東レ株式会社 | 多孔質膜、複合膜及び多孔質膜の製造方法 |
| EP4072608A1 (en) | 2019-12-12 | 2022-10-19 | Instituto de Engenharia Biomédica (INEB) | Fetal decellularized nucleus pulposus material and methods for obtaining pharmaceutic compositions to be used in the treatment of intervertebral disc degeneration and back pain |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993013136A1 (en) | 1991-12-20 | 1993-07-08 | Howmedica Inc. | Ultra-pure polysaccharide materials for medical use |
| JPH08269102A (ja) | 1995-03-30 | 1996-10-15 | Shiseido Co Ltd | エンドトキシンフリーのβ1,3−グルカン及びその製造法並びに医療用ゲル素材 |
| US5589591A (en) | 1986-07-03 | 1996-12-31 | Advanced Magnetics, Inc. | Endotoxin-free polysaccharides |
| JPH09324001A (ja) | 1996-04-02 | 1997-12-16 | Kyowa Hakko Kogyo Co Ltd | ヒアルロン酸ナトリウムの精製法 |
| JPH10503667A (ja) * | 1994-05-24 | 1998-04-07 | スミス アンド ネフュー ピーエルシー | 椎間板インプラント |
| JP2002530440A (ja) | 1998-11-13 | 2002-09-17 | シーピー ケルコ ユー.エス.インク. | エンドトキシンレベルが低い生体高分子塩、その生体高分子組成物およびこれを製造する方法 |
| US20030069639A1 (en) | 2001-04-14 | 2003-04-10 | Tom Sander | Methods and compositions for repair or replacement of joints and soft tissues |
| JP2005036036A (ja) | 2003-07-16 | 2005-02-10 | Tanabe Seiyaku Co Ltd | エンドトキシン除去方法 |
| WO2006136905A2 (en) * | 2005-06-20 | 2006-12-28 | Giuseppe Calvosa | Biocompatible composition for replacing/regenerating tissues |
| US20070150060A1 (en) | 2005-12-27 | 2007-06-28 | Sdgi Holdings, Inc | Rehydration and restoration of intervertebral discs with polyelectrolytes |
| WO2008102855A1 (ja) | 2007-02-21 | 2008-08-28 | Mochida Pharmaceutical Co., Ltd. | 軟骨疾患治療用組成物 |
| JP2008543449A (ja) * | 2005-06-17 | 2008-12-04 | アボット・ラボラトリーズ | 変性脊椎疾患の改良型治療方法 |
| US20090082719A1 (en) | 2005-05-03 | 2009-03-26 | Yeung Jeffrey E | Injection device for the intervertebral disc |
| WO2013027854A1 (ja) | 2011-08-23 | 2013-02-28 | 持田製薬株式会社 | 軟骨再生用組成物 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH11253547A (ja) * | 1998-03-11 | 1999-09-21 | Kunio Ishikawa | 細胞遮断膜 |
| EP2082755A1 (de) * | 2008-01-16 | 2009-07-29 | CellMed AG | Monolithische in-situ vernetzte Alginatimplantate |
| JP6196283B2 (ja) | 2015-12-10 | 2017-09-13 | トヨタ自動車株式会社 | 燃料電池用電極、燃料電池用電極の製造方法、固体高分子形燃料電池、および触媒インクの製造方法 |
-
2017
- 2017-01-27 BR BR122021024709-9A patent/BR122021024709B1/pt active IP Right Grant
- 2017-01-27 JP JP2018507084A patent/JP6487110B2/ja active Active
- 2017-01-27 US US16/086,081 patent/US20200289547A1/en active Pending
- 2017-01-27 CN CN201780018143.7A patent/CN109069692A/zh active Pending
- 2017-01-27 ES ES17769650T patent/ES2972523T3/es active Active
- 2017-01-27 EP EP23212370.3A patent/EP4374886A1/en active Pending
- 2017-01-27 BR BR112018069060A patent/BR112018069060A2/pt not_active Application Discontinuation
- 2017-01-27 CA CA3018152A patent/CA3018152A1/en active Pending
- 2017-01-27 EP EP17769650.7A patent/EP3434291B1/en active Active
- 2017-01-27 WO PCT/JP2017/002925 patent/WO2017163603A1/ja active Application Filing
-
2019
- 2019-02-20 JP JP2019027933A patent/JP6907254B2/ja active Active
-
2021
- 2021-06-29 JP JP2021107910A patent/JP7314207B2/ja active Active
-
2023
- 2023-07-11 JP JP2023113813A patent/JP2023134610A/ja active Pending
Patent Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5589591A (en) | 1986-07-03 | 1996-12-31 | Advanced Magnetics, Inc. | Endotoxin-free polysaccharides |
| WO1993013136A1 (en) | 1991-12-20 | 1993-07-08 | Howmedica Inc. | Ultra-pure polysaccharide materials for medical use |
| JPH10503667A (ja) * | 1994-05-24 | 1998-04-07 | スミス アンド ネフュー ピーエルシー | 椎間板インプラント |
| JPH08269102A (ja) | 1995-03-30 | 1996-10-15 | Shiseido Co Ltd | エンドトキシンフリーのβ1,3−グルカン及びその製造法並びに医療用ゲル素材 |
| JPH09324001A (ja) | 1996-04-02 | 1997-12-16 | Kyowa Hakko Kogyo Co Ltd | ヒアルロン酸ナトリウムの精製法 |
| JP2002530440A (ja) | 1998-11-13 | 2002-09-17 | シーピー ケルコ ユー.エス.インク. | エンドトキシンレベルが低い生体高分子塩、その生体高分子組成物およびこれを製造する方法 |
| US20030069639A1 (en) | 2001-04-14 | 2003-04-10 | Tom Sander | Methods and compositions for repair or replacement of joints and soft tissues |
| JP2005036036A (ja) | 2003-07-16 | 2005-02-10 | Tanabe Seiyaku Co Ltd | エンドトキシン除去方法 |
| US20090082719A1 (en) | 2005-05-03 | 2009-03-26 | Yeung Jeffrey E | Injection device for the intervertebral disc |
| JP2008543449A (ja) * | 2005-06-17 | 2008-12-04 | アボット・ラボラトリーズ | 変性脊椎疾患の改良型治療方法 |
| WO2006136905A2 (en) * | 2005-06-20 | 2006-12-28 | Giuseppe Calvosa | Biocompatible composition for replacing/regenerating tissues |
| US20070150060A1 (en) | 2005-12-27 | 2007-06-28 | Sdgi Holdings, Inc | Rehydration and restoration of intervertebral discs with polyelectrolytes |
| WO2008102855A1 (ja) | 2007-02-21 | 2008-08-28 | Mochida Pharmaceutical Co., Ltd. | 軟骨疾患治療用組成物 |
| WO2013027854A1 (ja) | 2011-08-23 | 2013-02-28 | 持田製薬株式会社 | 軟骨再生用組成物 |
Non-Patent Citations (19)
| Title |
|---|
| .: "Japanese Pharmacopoeia, 16th ed.", . |
| ACTA BIOMATERIA, vol. 10, 2014, pages 1646 - 1662 |
| APPL. MICROBIOL. BIOTECHNOL., vol. 40, 1994, pages 638 - 643 |
| EUR SPINE J, vol. 16, 2007, pages 1892 - 1898 |
| EUR SPINE J., vol. 23, no. 1, 2014, pages 19 - 26 |
| EUR SPINE J., vol. 23, no. 1, January 2014 (2014-01-01), pages 19 - 26 |
| EUROPEAN CELLS AND MATERIALS, vol. 8, 2004, pages 58 - 64 |
| JOURNAL OF ANATOMY, vol. 221, 2012, pages 480 - 496 |
| JOURNAL OF ORTHOPAEDIC RESEARCH, vol. 7, 1989, pages 146 - 151 |
| JOURNAL OF THE MECHANICAL BEHAVIOR OF BIOMEDICAL MATERIALS, vol. 29, 2014, pages 56 - 67 |
| JOURNAL OF THE MECHANICAL BEHAVIOR OF BIOMEDICAL MATERIALS, vol. 4, 2011, pages 1196 - 1205 |
| MATERIALS SCIENCE AND ENGINEERING, vol. 63, 2016, pages 198 - 210 |
| NISHIMURA ET AL., SPINE (PHILA PA 1976, vol. 23, no. 14, 1998, pages 1531 - 8 |
| OSTEOARTHRITIS AND CARTILAGE, vol. 17, 2009, pages 1377 - 1384 |
| SPINE (PHILA PA 1976, vol. 26, no. 17, 2001, pages 1873 - 8 |
| SPINE (PHILA PA 1976, vol. 30, no. 1, 1 January 2005 (2005-01-01), pages 15 - 24 |
| SPINE J, vol. 13, no. 3, 2013, pages 243 - 262 |
| SPINE, vol. 27, no. 23, 2002, pages 2631 - 2644 |
| WOESSNER JF JR, ARCH BIOCHEM BIOPHYS, vol. 93, 1961, pages 440 - 447 |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018164128A1 (ja) * | 2017-03-07 | 2018-09-13 | 持田製薬株式会社 | アルギン酸液剤 |
| US11969437B2 (en) | 2017-03-07 | 2024-04-30 | Mochida Pharmaceutical Co., Ltd. | Alginate liquid preparation |
| CN111867645A (zh) * | 2018-01-31 | 2020-10-30 | 国立大学法人神户大学 | 椎间盘退变的治疗剂及椎间盘细胞培养材料 |
| CN111867645B (zh) * | 2018-01-31 | 2022-05-27 | 国立大学法人神户大学 | 椎间盘退变的治疗剂及椎间盘细胞培养材料 |
| US11951231B2 (en) | 2018-01-31 | 2024-04-09 | Kinki University | Therapeutic agent for intervertebral disc degeneration and material for culturing inter vertebral disc cells |
| WO2019182015A1 (ja) | 2018-03-22 | 2019-09-26 | 持田製薬株式会社 | 非ステロイド性抗炎症性化合物結合アルギン酸誘導体 |
| WO2021145325A1 (ja) * | 2020-01-14 | 2021-07-22 | 持田製薬株式会社 | アルギン酸塩を使用した対象の処置のための組合せ及び方法 |
| WO2022092298A1 (ja) * | 2020-11-01 | 2022-05-05 | 雄平 島田 | 脊髄損傷の治療剤 |
| WO2022102093A1 (ja) * | 2020-11-13 | 2022-05-19 | 国立大学法人北海道大学 | 椎間板性疼痛抑制用組成物 |
| WO2022163872A1 (ja) * | 2021-01-29 | 2022-08-04 | 国立大学法人北海道大学 | 椎間板再生用組成物 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP6487110B2 (ja) | 2019-03-20 |
| JP2023134610A (ja) | 2023-09-27 |
| US20200289547A1 (en) | 2020-09-17 |
| CN109069692A (zh) | 2018-12-21 |
| BR112018069060A2 (pt) | 2019-01-29 |
| JPWO2017163603A1 (ja) | 2019-01-31 |
| ES2972523T3 (es) | 2024-06-13 |
| BR122021024709B1 (pt) | 2023-01-03 |
| JP7314207B2 (ja) | 2023-07-25 |
| CA3018152A1 (en) | 2017-09-28 |
| EP4374886A1 (en) | 2024-05-29 |
| JP2021154163A (ja) | 2021-10-07 |
| JP6907254B2 (ja) | 2021-07-21 |
| EP3434291A1 (en) | 2019-01-30 |
| EP3434291B1 (en) | 2023-11-29 |
| EP3434291A4 (en) | 2019-10-23 |
| JP2019088900A (ja) | 2019-06-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6487110B2 (ja) | 椎間板治療用組成物 | |
| JP5425134B2 (ja) | 軟骨疾患治療用組成物 | |
| RU2698210C2 (ru) | Состав fgf-18 в альгинатных/коллагеновых гидрогелях | |
| JP5491866B2 (ja) | 関節疾患治療用組成物 | |
| US20160067309A1 (en) | Composition for regeneration of cartilage | |
| JP7191007B2 (ja) | 線維軟骨組織損傷治療用組成物 | |
| WO2022102093A1 (ja) | 椎間板性疼痛抑制用組成物 | |
| US20230080690A1 (en) | Cartilage damage treatment material utilizing bone marrow fluid | |
| TWI835690B (zh) | 用於治療節段性骨缺損的可注射型水膠 | |
| TW202246491A (zh) | 椎間盤再生用組成物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2018507084 Country of ref document: JP |
|
| ENP | Entry into the national phase |
Ref document number: 3018152 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 122021015253 Country of ref document: BR |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112018069060 Country of ref document: BR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2017769650 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2017769650 Country of ref document: EP Effective date: 20181023 |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17769650 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 112018069060 Country of ref document: BR Kind code of ref document: A2 Effective date: 20180919 |