WO2016148238A1 - 酸化抑制剤及びこれを用いた油脂含有飲食品 - Google Patents
酸化抑制剤及びこれを用いた油脂含有飲食品 Download PDFInfo
- Publication number
- WO2016148238A1 WO2016148238A1 PCT/JP2016/058502 JP2016058502W WO2016148238A1 WO 2016148238 A1 WO2016148238 A1 WO 2016148238A1 JP 2016058502 W JP2016058502 W JP 2016058502W WO 2016148238 A1 WO2016148238 A1 WO 2016148238A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- methyl
- compound
- oxidation inhibitor
- oxidation
- oils
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23D—EDIBLE OILS OR FATS, e.g. MARGARINES, SHORTENINGS OR COOKING OILS
- A23D9/00—Other edible oils or fats, e.g. shortenings or cooking oils
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23B—PRESERVATION OF FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES; CHEMICAL RIPENING OF FRUIT OR VEGETABLES
- A23B2/00—Preservation of foods or foodstuffs, in general
- A23B2/70—Preservation of foods or foodstuffs, in general by treatment with chemicals
- A23B2/725—Preservation of foods or foodstuffs, in general by treatment with chemicals in the form of liquids or solids
- A23B2/729—Organic compounds; Microorganisms; Enzymes
- A23B2/762—Organic compounds containing nitrogen
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23D—EDIBLE OILS OR FATS, e.g. MARGARINES, SHORTENINGS OR COOKING OILS
- A23D9/00—Other edible oils or fats, e.g. shortenings or cooking oils
- A23D9/007—Other edible oils or fats, e.g. shortenings or cooking oils characterised by ingredients other than fatty acid triglycerides
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L2/00—Non-alcoholic beverages; Dry compositions or concentrates therefor; Preparation or treatment thereof
- A23L2/52—Adding ingredients
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/115—Fatty acids or derivatives thereof; Fats or oils
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K15/00—Anti-oxidant compositions; Compositions inhibiting chemical change
- C09K15/04—Anti-oxidant compositions; Compositions inhibiting chemical change containing organic compounds
- C09K15/20—Anti-oxidant compositions; Compositions inhibiting chemical change containing organic compounds containing nitrogen and oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K15/00—Anti-oxidant compositions; Compositions inhibiting chemical change
- C09K15/04—Anti-oxidant compositions; Compositions inhibiting chemical change containing organic compounds
- C09K15/20—Anti-oxidant compositions; Compositions inhibiting chemical change containing organic compounds containing nitrogen and oxygen
- C09K15/22—Anti-oxidant compositions; Compositions inhibiting chemical change containing organic compounds containing nitrogen and oxygen containing an amide or imide moiety
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11B—PRODUCING, e.g. BY PRESSING RAW MATERIALS OR BY EXTRACTION FROM WASTE MATERIALS, REFINING OR PRESERVING FATS, FATTY SUBSTANCES, e.g. LANOLIN, FATTY OILS OR WAXES; ESSENTIAL OILS; PERFUMES
- C11B5/00—Preserving by using additives, e.g. anti-oxidants
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11B—PRODUCING, e.g. BY PRESSING RAW MATERIALS OR BY EXTRACTION FROM WASTE MATERIALS, REFINING OR PRESERVING FATS, FATTY SUBSTANCES, e.g. LANOLIN, FATTY OILS OR WAXES; ESSENTIAL OILS; PERFUMES
- C11B5/00—Preserving by using additives, e.g. anti-oxidants
- C11B5/0021—Preserving by using additives, e.g. anti-oxidants containing oxygen
- C11B5/0028—Carboxylic acids; Their derivates
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11B—PRODUCING, e.g. BY PRESSING RAW MATERIALS OR BY EXTRACTION FROM WASTE MATERIALS, REFINING OR PRESERVING FATS, FATTY SUBSTANCES, e.g. LANOLIN, FATTY OILS OR WAXES; ESSENTIAL OILS; PERFUMES
- C11B5/00—Preserving by using additives, e.g. anti-oxidants
- C11B5/0042—Preserving by using additives, e.g. anti-oxidants containing nitrogen
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11B—PRODUCING, e.g. BY PRESSING RAW MATERIALS OR BY EXTRACTION FROM WASTE MATERIALS, REFINING OR PRESERVING FATS, FATTY SUBSTANCES, e.g. LANOLIN, FATTY OILS OR WAXES; ESSENTIAL OILS; PERFUMES
- C11B5/00—Preserving by using additives, e.g. anti-oxidants
- C11B5/0042—Preserving by using additives, e.g. anti-oxidants containing nitrogen
- C11B5/005—Amines or imines
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
Definitions
- the present invention relates to an oxidation inhibitor. More specifically, the present invention relates to an oxidation inhibitor containing a substance in which a sphingoid and a carbonyl compound such as an aldehyde are bound as an active ingredient, an oil containing the oil, and a food containing the oil.
- animal fats such as pigs and cows
- oils and fats derived from natural materials such as fish oil and vegetable oil have been widely used.
- essential fatty acids such as linoleic acid, linolenic acid, arachidonic acid, eicosapentaenoic acid, and docosahexaenoic acid are subject to various metabolisms in vivo. It is converted into a compound such as prostaglandin and plays an important role in maintaining the homeostasis and function of the living body.
- unsaturated fatty acids are easily oxidized with oxygen in the air or dissolved in the solution, so that undesirable peroxides such as peroxides are easily generated.
- the oxidation reaction may be promoted by a small amount of metal such as iron or copper, ascorbic acid, or the like contained in the oil or fat-containing food or by a photochemical reaction. For this reason, even if nitrogen gas replacement or the like is performed, it is difficult to prevent quality deterioration due to flavor deterioration such as rot, coloring, and generation of a return odor of fat or oil-containing foods due to oxidation.
- oxidation inhibitor such as tocopherol, sorbic acid, soybean phospholipid, dibutylhydroxytoluene (BHT), butylhydroxyanisole (BHA).
- BHT dibutylhydroxytoluene
- BHA butylhydroxyanisole
- aminocarbonyl compound produced by an aminocarbonyl reaction between an amino acid and a saccharide is also known to be effective in suppressing an oxidation reaction such as auto-oxidation or thermal oxidation.
- aminocarbonyl compounds are generally highly polar and hydrophilic, they cannot be said to be suitable as oxidation inhibitors for fats and oils.
- Patent Document 1 discloses a fat and oil oxidation inhibitor containing dihydroxysphingosine as an active ingredient. However, coexistence of dihydroxysphingosine and ⁇ -tocopherol is required to fully demonstrate the effectiveness of such an oxidation inhibitor.
- An object of the present invention is to provide a novel oxidation inhibitor that has a strong oxidation inhibitory action and can prevent oxidation of unsaturated fatty acids, particularly polyunsaturated fatty acids.
- an aminocarbonyl compound formed by combining an amino group of a sphingoid and a carbonyl group of a carbonyl compound has a very strong oxidation-inhibiting action.
- the present invention has been completed.
- An oxidation inhibitor comprising an aminocarbonyl compound having a structure in which an amino group of a compound having a sphingoid base structure is bonded to a carbonyl group of a carbonyl compound as an active ingredient.
- the carbonyl compound is a compound selected from aldehydes, ketones, esters, and fatty acids.
- the carbonyl compound is propanal, propenal (acrolein), 2- / 3-hexenal, 2-pentenal, 2,4,7-decatrienal, 2-butenal, 2-butylfuran, acetaldehyde, 4,5 -Selected from the group consisting of epoxy-2-heptanal, butanal, methyl octanoate, methyl 9-oxononanoate, 3,6-nonadienal, 2,4-heptadienal, hexanal, 2-heptenal, heptanal, nonanal, pentanal, octanal
- the compound in which the carbonyl compound is selected from the group consisting of 2-propanone, 2-butanone, 2-pentanone, 2-hexanone, 2-heptanone, 2-octanone, 2-nonanone and 3-octen-2-one
- a compound having a sphingoid base structure is dihydrosphingosine, sphingosine, N, N-dimethylsphingosine, phytosphingosine, 4-sphingenin, 8-sphingenin, 4-hydroxy-8-sphingenine, 4,8-sphingadie
- a compound selected from the group consisting of nin, 9-methyl-4,8-sphingadienine, 4,8,10-sphingatrienin and 9-methyl-4,8,10-sphingatrienin The oxidation inhibitor according to any one of (1) to (5).
- An oil obtained by blending the oxidation inhibitor according to any one of (1) to (6).
- the fat according to (7), wherein the blending amount of the oxidation inhibitor is 1 ppt or more with respect to the fat.
- the oxidation inhibitor according to the present invention has a strong oxidation-inhibiting ability and can suppress the oxidation of fats and oils, particularly fats and oils containing a large amount of polyunsaturated fatty acids, thereby preventing alteration due to the oxidation of fats and oils. Furthermore, the oxidation inhibitor according to the present invention is applicable to food ingredients other than fats and oils.
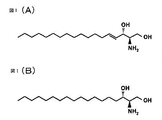
- FIG. 1 (A) shows sphingosine
- FIG. 1 (B) shows dihydrosphingosine
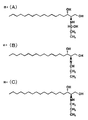
- 2A is a compound in which the carbonyl carbon of the carbonyl compound is covalently bonded to the amino group in the sphingoid base, and the carbonyl oxygen is reduced to the hydroxyl group
- FIG. 2B is derived from the carbonyl oxygen in FIG. Compound in which hydroxyl group is eliminated as water together with hydrogen of amino group
- FIG. 2C shows a compound in which the double bond in FIG. 2B is reduced. It is a graph which shows the result of having measured the time-dependent change of the oxygen absorption amount by the oxidation inhibitor of this invention.
- FIG. 1 (B) shows dihydrosphingosine
- 2A is a compound in which the carbonyl carbon of the carbonyl compound is covalently bonded to the amino group in the sphingoid base, and the carbonyl oxygen is reduced to the hydroxyl group
- FIG. 4A shows a compound in which the carbonyl carbon of propanal is covalently bonded to the amino group of dihydrosphingosine and the carbonyl oxygen is reduced to a hydroxyl group
- FIG. 4B shows the hydroxyl group derived from the carbonyl oxygen in FIG. Compound released as water together with amino group hydrogen
- FIG. 4C shows a compound in which the double bond of FIG. 4B is reduced.
- the present invention relates to an oxidation inhibitor comprising as an active ingredient an aminocarbonyl compound having a structure in which an amino group of a compound having a sphingoid base structure and a carbonyl group of a carbonyl compound are bonded.
- the compound having a sphingoid base structure in the present invention is typically a sphingoid which is a kind of long-chain amino alcohol.
- the sphingoid that can be used in the present invention include dihydrosphingosine, sphingosine, N, N-dimethylsphingosine, phytosphingosine, 4-sphingenin, 8-sphingenin, 4-hydroxy-8-sphingenine, and 4,8-sphingadie. Nin, 9-methyl-4,8-sphingadienine, 4,8,10-sphingatrienin, 9-methyl-4,8,10-sphingatrienine and the like.
- FIG. 1 (A) and 1 (B) are diagrams showing the chemical structures of typical examples of compounds having a sphingoid base structure, FIG. 1 (A) is the structure of sphingosine, and FIG. 1 (B) is dihydrosphingosine. The structure of is shown.
- Sphingoid can be prepared by hydrolyzing a sphingolipid containing a sphingoid base.
- Sphingolipids are widely present in animals and plants, and have a structure in which ceramide in which a sphingoid base, which is a long-chain base component, and a fatty acid are bonded with an acid amide bond has a common structure, and a sugar or phosphoric acid and a base are further bonded thereto.
- a sugar-linked glycoside is called a sphingoglycolipid and a sphingophospholipid combined with a phosphate and a base.
- the compound having a sphingoid base structure in the present invention can be prepared from sphingolipids obtained from plants such as cereals and beans, animals such as milk and cow brain, or microorganisms.
- dihydrosphingosine can be prepared by hydrolysis from sphingomyelin, which is a sphingophospholipid present in milk, lactosylceramide or glucosylceramide, which is a glycosphingolipid.
- the carbonyl compound in the present invention may be a compound having a carbonyl group capable of reacting with an amino group in the base of a compound having a sphingoid base structure, and examples thereof include aldehydes, ketones, esters, and fatty acids. .
- aldehydes examples include propanal, propenal (acrolein), 2- / 3-hexenal, 2-pentenal, 2,4,7-decatrienal, 2-butenal, acetaldehyde, 4,5-epoxy-2-heptanal , Butanal, 3,6-nonadienal, 2,4-heptadienal, hexanal, 2-heptenal, heptanal, nonanal, pentanal, and octanal can be used.
- ketones examples include 2-propanone, 2-butanone, 2-pentanone, 2-hexanone, 2-heptanone, 2-octanone, 2-nonanone, 3-octen-2-one and 2,4-octadiene-2- On can be used.
- esters examples include methyl 10-oxo-8-decenoate, methyl heptanoate, methyl 10-oxodecanoate, methyl nonanoate, methyl 8-oxooctanoate, methyl octoate, methyl 9-oxononanoate, and furan octane.
- Methyl acid and methyl 13-oxo-9,11-tridecandienoate can be used.
- fatty acids examples include hexanoic acid, heptanoic acid, octanoic acid, and nonanoic acid.
- a particularly preferred carbonyl compound in the present invention is propanal or propenal (acrolein).
- the oxidation inhibitor of the present invention can be obtained by aminocarbonyl reaction between the compound having a sphingoid base structure and a compound having a carbonyl group capable of reacting with an amino group in the base.
- FIGS. 2A to 2C show sphingosine and a carbonyl compound (R 1 —CO—R 2 ), which are compounds having a sphingoid base structure.
- R 1 —CO—R 2 are compounds having a sphingoid base structure.
- the aminocarbonyl reaction according to the present invention can be carried out under the same conditions as general aminocarbonyl reactions.
- a compound having a sphingoid base structure in an approximately equimolar ratio and a compound having a carbonyl group are mixed in a suitable solvent such as a phosphate buffer, and then at atmospheric pressure and 60 ° C. to 120 ° C. for 10 minutes to 2 minutes. You just have to react for a while.
- the reaction product can be separated and purified by column chromatography or other general methods.
- the oxidation inhibitor of the present invention is a kind of aminocarbonyl compound, it has the effect of suppressing oxidation reactions such as auto-oxidation and thermal oxidation possessed by the aminocarbonyl compound. Furthermore, since the oxidation inhibitor of the present invention has a long-chain base structure, it is oil-soluble unlike conventionally known aminocarbonyl compounds, and is useful for antioxidants of fats and oils.
- the antioxidant of the present invention can prevent oxidation of fats and oils by being added to the fats and oils.
- the oxidation inhibitor of the present invention is preferably added to a normal temperature oil or fat, or a fat or oil heated to a temperature higher than the melting point, and mixed or dispersed.
- the antioxidant of this invention is 1ppt or more with respect to fats and oils It is preferable to contain.
- the addition amount is less than 1 ppt, the oxidation suppressing effect is lowered.
- a remarkable effect can be obtained as the amount added is increased.
- the oxidation inhibitor of the present invention can be added to food and drink other than fats and oils. Although there is no restriction
- the stirring and mixing conditions are not particularly limited as long as the oxidation inhibitor of the present invention can be mixed uniformly. It is also possible.
- the solution containing the oxidation inhibitor of the present invention may be used after concentration or lyophilization with a reverse osmosis membrane (RO membrane) or the like, as necessary, so that it can be easily used as a raw material for food and drink.
- RO membrane reverse osmosis membrane
- the oxidation inhibitor of the present invention can be sterilized normally used in the manufacture of pharmaceuticals, foods and drinks, or feeds, and in the case of a powdered oxidation inhibitor, dry heat sterilization is also possible. is there. Therefore, the oxidation inhibitor of the present invention can be used in the manufacture of various forms of pharmaceuticals, foods and drinks, and feeds such as liquid, gel, powder and granules.
- the oxidation inhibitor of the present invention can be added to fats and oils, in particular fats and oils containing polyunsaturated fatty acids, or foods containing the same, thereby suppressing the return odor and maintaining the flavor of the food. Moreover, in order to suppress a flavor deterioration for a long period, the expiration date of the fats and foods which mix
- a known oil-soluble oxidation inhibitor for example, ⁇ -carotene
- the fats and oils to which the oxidation inhibitor of the present invention is added can be used alone as ordinary edible fats and oils, margarine, spreads and other oily foods, salad dressings, cookies, butter cakes, infant formulas and other powdered milk or coffee It can also be used as a raw material for foods such as creams and creams such as whipped cream. Foods made from fats and oils to which the oxidation inhibitor of the present invention is added are prevented from being oxidized during storage, and the rise in peroxide value (hereinafter also referred to as “POV”) accompanying storage and the generation of oxidized odors are suppressed. Is done.
- POV peroxide value
- the fats and oils to which the oxidation inhibitor of the present invention is added are used as oils for fried foods such as tempura, potato chips, donuts, fried chicken, fried foods, etc.
- the deterioration of the oil is less likely to occur even if used for a long time. Therefore, the fats and oils to which the oxidation inhibitor of the present invention is added are suitable as fried oil when producing a large amount of fried food for business use.
- frying oil palm oil or the like having a relatively low content of polyunsaturated fatty acids is used in order to avoid oxidation of the oil, but by using the oxidation inhibitor of the present invention, polyvalent unsaturated is used.
- soybean oil and rapeseed oil containing a large amount of saturated fatty acids as frying oil. Furthermore, when fried food is prepared using fats and oils to which the oxidation inhibitor of the present invention is added, oxidation of the prepared fried food is also suppressed.
- Example 1 Sphingosine and propanal were dissolved and mixed in a phosphate buffer and heat treated at 100 ° C. for 1 hour. Formation of a compound in which the amino group of sphingosine and the carbonyl group of propanal were bonded was confirmed by liquid chromatography / mass spectrum (hereinafter also referred to as “LC / MS”). The product was purified by silica gel column chromatography to obtain an oxidation inhibitor (Example Product 1) of this example having a purity of 95%.
- Example 2 Ethanol was added to buttersarum powder and immersed overnight, and the filtrate was collected by suction filtration. The residue was immersed in ethanol overnight and the extraction was repeated. The filtrate was concentrated and then dissolved in chloroform / methanol / water (10: 5: 3, v / v / v), followed by liquid-liquid partitioning overnight. The lower chloroform layer was collected and concentrated. Further, the solvent was completely removed to obtain buttersalam lipid.
- SPM sphingomyelin
- Dihydrosphingosine and 2-pentenal were dissolved and mixed in a phosphate buffer and heat-treated at 100 ° C. for 1 hour.
- LC / MS confirmed the formation of a compound in which the amino group of dihydrosphingosine and the carbonyl group of 2-pentenal were bonded.
- the product was purified by silica gel column chromatography to obtain an oxidation inhibitor (Example product 2) of this example having a purity of 90%.
- Example 1 The oxidation inhibition effect of each oxidation inhibitor obtained in Example 1 and Example 2 was evaluated by an oxidation experiment.
- Each of Example Product 1, Example Product 2 and ⁇ -tocopherol (1 mg) as an oxidation inhibitor control was mixed with fish oil triglyceride (hereinafter also referred to as “fish oil TG”) (99 mg) to prepare an analytical sample.
- Fish oil TG 100 mg was used as a control containing no oxidation inhibitor.
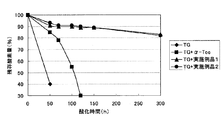
- the analytical sample was precisely weighed into an analytical vial (5 mL), and then capped with a butyl septum rubber and an aluminum sealed vial. After incubating in the dark at 40 ° C., 40 ⁇ L of air at the top of the vial was collected at regular intervals and injected into a gas chromatograph (GC) of a thermal conductivity detector (TCD) device. Since the oxygen peak in the air decreases with oxidation, the amount of oxygen absorbed by lipid oxidation was calculated from the change in the peak ratio of oxygen and nitrogen. The transition of the average value of each measured value is shown in FIG. The vertical axis of the graph represents the amount of residual oxygen (%), and the horizontal axis represents the oxidation time (hours). The fatty acid composition and analysis conditions of fish oil TG used in the experiment are shown below.
- Fish oil TG contains a lot of highly unsaturated fatty acids such as 20: 5n-3 (EPA) and 22: 6n-3 (DHA) (Table 1), so it is very susceptible to oxidation. Of oxygen was consumed by oxidation. On the other hand, the oxygen absorption rate when Example Product 1 or Example Product 2 is added to fish oil TG is clearly slower than that when no product is added (only fish oil TG), and 300 hours after the start of measurement. But most of the oxygen remained. On the other hand, the oxygen absorption rate when ⁇ -tocopherol was added to fish oil TG was slower than that when no addition was made, but it was clear compared with the case where Example product 1 or Example product 2 was added. It was fast. From the above, it was revealed that when Example Product 1 or Example Product 2 was added to fish oil TG, an excellent oxidation inhibition effect was obtained.
- EPA EPA
- DHA 6n-3
- Example 3 A reaction solution obtained by allowing protease to act on a 10% aqueous solution of whey protein concentrate (WPC) was extracted with a chloroform-methanol (2: 1) solution, concentrated, and further extracted with acetone to obtain a phospholipid fraction. Obtained. The obtained phospholipid fraction was subjected to silica gel chromatography, and step-extracted with a chloroform-methanol solution was lyophilized to obtain purified sphingomyelin. The purified sample was fractionated by thin layer chromatography, developed with Dittmer reagent, and then quantified using a densitometer. As a result, the sphingomyelin content was 95.2%.
- WPC whey protein concentrate
- the sphingomyelin was subjected to acid hydrolysis using hydrochloric acid.
- the degradation product was purified using silica gel column chromatography to obtain 99% highly pure dihydrosphingosine.
- Dihydrosphingosine and propenal were dissolved and mixed in a phosphate buffer and heat-treated at 100 ° C. for 1 hour.
- LC / MS confirmed the formation of a compound in which the amino group of dihydrosphingosine and the carbonyl group of propenal were bonded.
- the product was purified by silica gel column chromatography to obtain an oxidation inhibitor (Example Product 3) of this example having a purity of 92%.
- Example 4 A mixture of 5 to 6 mg of sphingomyelin with 1.5 ml of 0.1 M Tris buffer (pH 7.4) containing 0.03 M CaCl 2 was sonicated for 10 seconds, 3 mg of phospholipase C derived from C. perfringens and diethyl ether 1.5 ml was added. The mixture was shaken vigorously and then incubated at room temperature for 3 hours with frequent shaking. 3 ml of ether was added, the mixture was shaken and centrifuged, and the ether layer was removed. The mixture was extracted again with 3 ml of ether. The entire ether extract was washed with distilled water, centrifuged, and the ether solution was concentrated to dryness under a nitrogen stream to remove a trace amount of water to obtain a ceramide mixture.
- Dihydrosphingosine and propanal were dissolved and mixed in a phosphate buffer and heat-treated at 100 ° C. for 1 hour.
- LC / MS confirmed the formation of a compound (FIGS. 4A to 4C) in which the amino group of sphingosine and the carbonyl group of propanal were bonded.
- the product was purified by silica gel column chromatography to obtain an oxidation inhibitor (Example Product 4) of this example having a purity of 92%.
- Example 5 Phytosphingosine and 2-pentanone were dissolved and mixed in a phosphate buffer and heat treated at 100 ° C. for 1 hour. LC / MS confirmed the formation of a compound in which the amino group of phytosphingosine and the carbonyl group of 2-pentanone were bonded. The product was purified by silica gel column chromatography to obtain an oxidation inhibitor (Example Product 5) of this example having a purity of 89%.
- Example 6 Dihydrosphingosine and methyl octanoate were dissolved and mixed in a phosphate buffer, and heat-treated at 100 ° C. for 1 hour. LC / MS confirmed the formation of a compound in which the amino group of dihydrosphingosine and the carbonyl group of methyl octoate were bonded. The product was purified by silica gel column chromatography to obtain an oxidation inhibitor (Example Product 6) of this example having a purity of 86%.
- Example 7 Sphingosine and hexanoic acid were dissolved and mixed in a phosphate buffer and heat-treated at 100 ° C. for 1 hour. LC / MS confirmed the formation of a compound in which the amino group of sphingosine and the carbonyl group of hexanoic acid were bonded. The product was purified by silica gel column chromatography to obtain an oxidation inhibitor (Example Product 7) of this example having a purity of 92%.
- Example 2 The oxidation inhibitory effect of Examples 3 to 7 on fish oil was evaluated by measuring the peroxide value (POV) and sensory evaluation. Each Example product (1 mg) was mixed with fish oil TG (99 mg) to prepare an analysis sample. Fish oil TG (100 mg) was used as a control containing no oxidation inhibitor. The analytical sample was precisely weighed into an analytical vial (5 mL) and then capped with a butyl septum rubber and an aluminum sealed vial. Incubated for 2 months in the dark at 40 ° C. Samples after incubation were evaluated by measuring POV and a panel of 8 flavors. In sensory evaluation, the return odor of the additive-free fish oil used as a control was set to 5 points, and the score was evaluated. That is, a lower score indicates that there is no return odor and the flavor is better. The results are shown in Table 2.
- the POV of the fish oil TG with no addition after 2 months of storage was 1.5 kg / meq
- the POV of the fish oil TG with each example product added was 0.4 or 0. It was 0.5 kg / meq.
- the return odor was suppressed in the fish oil to which the products of the examples were added, compared to the fish oil TG without addition. From the above results, the oxidation stability is improved by adding the oxidation inhibitor of each example product to the fish oil TG, the effect of suppressing the generation of a return odor due to the oxidation of unsaturated fatty acids in the fish oil, and preventing flavor deterioration It became clear that there was.
- Example 4 The effect of inhibiting the oxidation of Examples 1 to 4 on the photodegradation of soybean oil was evaluated by sensory evaluation.
- Example products 1 to 4 (1 mg) were mixed with soybean oil TG (99 mg) to obtain analytical samples.
- soybean oil TG 100 mg was used as a control containing no oxidation inhibitor.
- the analytical sample was precisely weighed in an analytical vial (5 mL), stoppered with a butyl septum rubber and an aluminum seal vial, and incubated in a 5 ° C. showcase (3500 lux) for 7 days. The sample after the incubation was evaluated by a taste panel of 8 people.
- the return odor of the additive-free soybean oil used as a control was set to 5 points, and the score was evaluated. That is, a lower score indicates that there is no return odor and the flavor is better.
- Table 4 The results are shown in Table 4.
- the present invention has a strong oxidation-inhibiting ability, and can be used as an oxidation inhibitor that can inhibit the oxidation of fats and oils, particularly fats and oils that contain a large amount of polyunsaturated fatty acids, and prevent alteration due to oxidation of fats and oils. is there.
- the present invention can also be used for food ingredients other than oxidized fats and oils.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Food Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Wood Science & Technology (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nutrition Science (AREA)
- Materials Engineering (AREA)
- Zoology (AREA)
- Mycology (AREA)
- Emergency Medicine (AREA)
- Fats And Perfumes (AREA)
- Food Preservation Except Freezing, Refrigeration, And Drying (AREA)
- Edible Oils And Fats (AREA)
- Anti-Oxidant Or Stabilizer Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/558,826 US11252974B2 (en) | 2015-03-19 | 2016-03-17 | Oxidation inhibitor, and food and drink containing fats and oils using same |
| EP16765067.0A EP3272835A4 (en) | 2015-03-19 | 2016-03-17 | Oxidation inhibitor, and food and drink containing fats and oils using same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015056511A JP6232008B2 (ja) | 2015-03-19 | 2015-03-19 | 酸化抑制剤及びこれを用いた油脂含有飲食品 |
| JP2015-056511 | 2015-03-19 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016148238A1 true WO2016148238A1 (ja) | 2016-09-22 |
Family
ID=56919066
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2016/058502 Ceased WO2016148238A1 (ja) | 2015-03-19 | 2016-03-17 | 酸化抑制剤及びこれを用いた油脂含有飲食品 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US11252974B2 (enExample) |
| EP (1) | EP3272835A4 (enExample) |
| JP (1) | JP6232008B2 (enExample) |
| TW (1) | TWI711600B (enExample) |
| WO (1) | WO2016148238A1 (enExample) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH05320048A (ja) * | 1992-05-19 | 1993-12-03 | Shiseido Co Ltd | 抗酸化剤 |
| JPH08259988A (ja) * | 1995-03-23 | 1996-10-08 | Snow Brand Milk Prod Co Ltd | 酸化防止剤及びこれを用いた油脂の酸化防止方法 |
| WO2001042390A1 (fr) * | 1999-12-06 | 2001-06-14 | Akihiko Niina | Stabilisant pour composes insatures ou pate contenant ces composes et procede de stabilisation correspondant |
| JP2013147636A (ja) * | 2011-12-22 | 2013-08-01 | Snow Brand Milk Products Co Ltd | 酸化抑制剤およびこれを用いた油脂含有飲食品 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2343273A (en) * | 1938-07-20 | 1944-03-07 | Marchant Calculating Machine | Calculating machine |
| JP2006516280A (ja) * | 2003-01-20 | 2006-06-29 | ネーデルランドセ オルガニサティエ フォール トエゲパストナトールヴェテンシャッペリク オンデルゾエク ティエヌオー | 血漿コレステロール及びトリアシルグリセロールレベルを減少するためにスフィンゴ脂質を使用する方法 |
| JP2008504330A (ja) | 2004-06-29 | 2008-02-14 | ヤド テクノロジーズ ゲゼルシャフト ミット ベシュレンクテル ハフツング | スフィンゴ脂質由来医薬組成物 |
| US8436196B2 (en) * | 2008-09-30 | 2013-05-07 | Kaneka Corporation | Type I natural ceramide derivative and method for producing same |
| US8853452B2 (en) | 2009-02-10 | 2014-10-07 | The Administrators Of The Tulane Educational Fund | Compounds, their syntheses, compositions, and methods to treat cancer |
| JP5825672B2 (ja) | 2011-12-22 | 2015-12-02 | 高砂香料工業株式会社 | 高純度セラミド類の製造方法 |
-
2015
- 2015-03-19 JP JP2015056511A patent/JP6232008B2/ja active Active
-
2016
- 2016-03-17 WO PCT/JP2016/058502 patent/WO2016148238A1/ja not_active Ceased
- 2016-03-17 US US15/558,826 patent/US11252974B2/en active Active
- 2016-03-17 EP EP16765067.0A patent/EP3272835A4/en not_active Withdrawn
- 2016-03-18 TW TW105108389A patent/TWI711600B/zh not_active IP Right Cessation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH05320048A (ja) * | 1992-05-19 | 1993-12-03 | Shiseido Co Ltd | 抗酸化剤 |
| JPH08259988A (ja) * | 1995-03-23 | 1996-10-08 | Snow Brand Milk Prod Co Ltd | 酸化防止剤及びこれを用いた油脂の酸化防止方法 |
| WO2001042390A1 (fr) * | 1999-12-06 | 2001-06-14 | Akihiko Niina | Stabilisant pour composes insatures ou pate contenant ces composes et procede de stabilisation correspondant |
| JP2013147636A (ja) * | 2011-12-22 | 2013-08-01 | Snow Brand Milk Products Co Ltd | 酸化抑制剤およびこれを用いた油脂含有飲食品 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3272835A4 * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3272835A4 (en) | 2018-08-29 |
| US20180077946A1 (en) | 2018-03-22 |
| TW201643131A (zh) | 2016-12-16 |
| JP6232008B2 (ja) | 2017-11-15 |
| US11252974B2 (en) | 2022-02-22 |
| EP3272835A1 (en) | 2018-01-24 |
| JP2016175983A (ja) | 2016-10-06 |
| TWI711600B (zh) | 2020-12-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2025237A1 (en) | Lecithin and LC-PUFA | |
| JP7129723B2 (ja) | 高度不飽和脂肪酸のトリグリセリドを含む組成物 | |
| JP4955813B2 (ja) | 飲食品の呈味向上剤 | |
| JP6891032B2 (ja) | 分離型液体調味料 | |
| JP2013147636A (ja) | 酸化抑制剤およびこれを用いた油脂含有飲食品 | |
| KR101275406B1 (ko) | 시효변화된 식품 오일을 재생시키기 위한 방법 | |
| JP2008069184A (ja) | 油脂組成物 | |
| JP2009284859A (ja) | 飲食品の甘味増強剤および甘味増強方法 | |
| JP2016026487A (ja) | 油相及び水相を含有する液体調味料の製造方法 | |
| JP6232008B2 (ja) | 酸化抑制剤及びこれを用いた油脂含有飲食品 | |
| JP3813197B2 (ja) | 酸化防止剤及びこれを用いた油脂の酸化防止方法 | |
| US9332783B2 (en) | Metal-complexing aroma compounds for use in aroma stabilization | |
| JP4463586B2 (ja) | 大豆油の精製方法 | |
| JP2009225724A (ja) | アラキドン酸類含有組成物 | |
| JP7652535B2 (ja) | 酸化抑制剤 | |
| Hadolin et al. | Stabilisation of butter with rosemary antioxidants | |
| JP3762026B2 (ja) | 抗酸化剤及び該抗酸化剤を配合した油脂含有食品 | |
| JPH08239685A (ja) | ドコサヘキサエン酸類の安定化剤 | |
| JP2022088348A (ja) | 水中油型乳化組成物 | |
| JP2005264077A (ja) | 共役型トリエン酸含有油脂組成物及びその油脂の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16765067 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15558826 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2016765067 Country of ref document: EP |