WO2016017348A1 - ペレットの製造方法、鉄-ニッケル合金の製造方法 - Google Patents
ペレットの製造方法、鉄-ニッケル合金の製造方法 Download PDFInfo
- Publication number
- WO2016017348A1 WO2016017348A1 PCT/JP2015/068856 JP2015068856W WO2016017348A1 WO 2016017348 A1 WO2016017348 A1 WO 2016017348A1 JP 2015068856 W JP2015068856 W JP 2015068856W WO 2016017348 A1 WO2016017348 A1 WO 2016017348A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- pellets
- iron
- mixture
- nickel
- pellet
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B23/00—Obtaining nickel or cobalt
- C22B23/02—Obtaining nickel or cobalt by dry processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
- B22F9/16—Making metallic powder or suspensions thereof using chemical processes
- B22F9/18—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds
- B22F9/20—Making metallic powder or suspensions thereof using chemical processes with reduction of metal compounds starting from solid metal compounds
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21B—MANUFACTURE OF IRON OR STEEL
- C21B11/00—Making pig-iron other than in blast furnaces
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21B—MANUFACTURE OF IRON OR STEEL
- C21B13/00—Making spongy iron or liquid steel, by direct processes
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B1/00—Preliminary treatment of ores or scrap
- C22B1/14—Agglomerating; Briquetting; Binding; Granulating
- C22B1/24—Binding; Briquetting ; Granulating
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B1/00—Preliminary treatment of ores or scrap
- C22B1/14—Agglomerating; Briquetting; Binding; Granulating
- C22B1/24—Binding; Briquetting ; Granulating
- C22B1/2406—Binding; Briquetting ; Granulating pelletizing
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B5/00—General methods of reducing to metals
- C22B5/02—Dry methods smelting of sulfides or formation of mattes
- C22B5/10—Dry methods smelting of sulfides or formation of mattes by solid carbonaceous reducing agents
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C33/00—Making ferrous alloys
- C22C33/02—Making ferrous alloys by powder metallurgy
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C33/00—Making ferrous alloys
- C22C33/04—Making ferrous alloys by melting
Definitions
- the present invention relates to a method for producing pellets, and more particularly, to a method for producing pellets when processing in a nickel oxide ore refining process, and a method for producing an iron-nickel alloy using the method.
- limonite or saprolite As a smelting method of nickel oxide ore called limonite or saprolite, a dry smelting method that produces nickel mat using a smelting furnace, a dry smelting method that produces ferronickel using a rotary kiln or moving hearth furnace A hydrometallurgical process for producing mixed sulfide using an autoclave is known.
- nickel oxide ore When charging nickel oxide ore into the smelting process, pretreatment for pelletizing or slurrying the raw material ore is performed. Specifically, when nickel oxide ore is pelletized, that is, when pellets are produced, it is mixed with components other than the nickel oxide ore, for example, a binder and a reducing agent, and further adjusted for moisture, etc. Generally, it is generally made into a lump of about 10 to 30 mm (refers to pellets, briquettes, etc., hereinafter simply referred to as “pellets”).
- Ferronickel is an alloy of iron (Fe) and nickel (Ni), and is mainly used as a raw material of stainless steel. If the above-described pellet smelting reaction (reduction reaction) proceeds ideally, the pellet 1 Since one ferronickel grain is obtained for each, a relatively large ferronickel grain can be obtained.
- Patent Document 1 as a pretreatment method for producing ferronickel using a moving hearth furnace, a mixture containing a raw material containing nickel oxide and iron oxide and a carbonaceous reducing agent is mixed. A technique for adjusting the surplus carbon amount of the mixture in the mixing step is disclosed.
- the ferronickel When the size of the ferronickel particles obtained is small, the ferronickel is much smaller than the pellet size of about 10 mm to 30 mm in diameter and breaks down to about several mm or less. There is a problem that it becomes very difficult and the recovery rate decreases.
- an object of the present invention is to provide a method for producing a pellet capable of preventing the ferronickel obtained after the smelting reaction from becoming small particles.
- the present inventors have made extensive studies to solve the above-described problems.
- at least nickel oxide ore, a carbonaceous reducing agent, and iron oxide are mixed to form a mixture, and the total of nickel and iron in the total weight of the resulting pellets
- the smelting reaction can proceed effectively, and the pellets can suppress the breakage of ferronickel, an iron-nickel alloy obtained after the smelting reaction.
- the present invention has been completed. That is, the present invention provides the following.
- the present invention is a method for producing pellets, which is used to produce an iron-nickel alloy and is produced by agglomerating a mixture obtained by mixing raw materials containing nickel oxide ore, At least a mixing treatment step of mixing the nickel oxide ore, a carbonaceous reducing agent, and iron oxide to form a mixture, and a pellet forming step of agglomerating the obtained mixture to form pellets; In the mixing treatment step, the mixture is generated so that the ratio of the total weight of nickel and iron in the total weight of the pellets to be formed is 30% by weight or more.
- the nickel oxide ore is limonite or saprolite, and in the mixing treatment step, the sum of nickel and iron in the total weight of the formed pellets.
- the present invention provides an iron-nickel alloy manufacturing method for manufacturing an iron-nickel alloy from nickel oxide ore, a pellet manufacturing process for manufacturing pellets from the nickel oxide ore, A reduction step of heating at a reduction temperature, and the pellet manufacturing step is obtained by mixing a nickel oxide ore, a carbonaceous reducing agent, and iron oxide to produce a mixture. And a pellet forming step of agglomerating the mixture to form pellets, and in the mixing treatment step, the ratio of the total weight of nickel and iron in the total weight of the formed pellets is 30% by weight or more.
- a method for producing an iron-nickel alloy characterized in that a mixture is produced.
- the ferronickel obtained after the smelting reaction is made to proceed by effectively proceeding the smelting reaction. Can be suppressed.
- Nickel Oxide Smelting Method a method for smelting nickel oxide ore as a raw material ore will be described.
- the nickel oxide ore which is the raw ore, is pelletized, and the pellet is reduced to produce metal (iron-nickel alloy (hereinafter also referred to as “ferronickel”) and slag
- ferrronickel iron-nickel alloy
- a smelting method for producing ferronickel by separating the metal and slag (a method for producing ferronickel) will be described as an example.
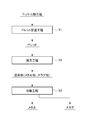
- the nickel oxide ore smelting method according to the present embodiment is a method of using nickel oxide ore pellets, charging the pellets into a smelting furnace (reduction furnace), and reducing and heating them.
- a pellet production step S1 for producing pellets from nickel oxide ore, and the obtained pellets are reduced by a predetermined reduction furnace. It has reduction process S2 heated at temperature, and separation process S3 which isolate
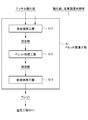
- FIG. 2 is a process flow diagram showing a process flow in the pellet manufacturing process S1.
- the pellet manufacturing process S1 includes a mixing process S11 in which raw materials containing nickel oxide ore are mixed, and pellet formation that forms (granulates) pellets that are agglomerated using the obtained mixture. It has process S12 and drying process S13 which dries the obtained pellet.
- the mixing treatment step S11 is a step of obtaining a mixture by mixing raw material powders containing nickel oxide ore. Specifically, in this mixing process step S11, at least nickel oxide ore as a raw material ore, a carbonaceous reducing agent, and iron oxide are mixed to obtain a mixture. In addition, a flux component, a binder, etc. can be added and mixed as needed.
- the particle diameter of these raw materials is not particularly limited, but for example, raw material powder having a particle diameter of about 0.2 mm to 0.8 mm is mixed to obtain a mixture.
- the nickel oxide ore is not particularly limited, but limonite or saprolite ore can be used.
- examples of the carbonaceous reducing agent include pulverized coal, pulverized coke, and the like. This carbonaceous reducing agent is preferably equivalent to the particle size of the nickel oxide ore described above.
- iron oxide for example, iron ore having an iron grade of about 50% or more, hematite obtained by wet refining of nickel oxide ore, and the like can be used.
- binder examples include bentonite, polysaccharides, resins, water glass, and dehydrated cake.
- flux component include calcium hydroxide, calcium carbonate, calcium oxide, silicon dioxide and the like.
- Table 1 below shows an example of the composition (% by weight) of some raw material powders. Note that the composition of the raw material powder is not limited to this.
- the next pellet forming step S12 is performed.
- the mixture is generated so that the total weight of nickel and iron contained in the pellets formed is equal to or greater than a predetermined ratio.
- the pellet is subjected to reduction heat treatment in the next step (reduction step S2) using the pellet. It is possible to effectively prevent the smelting reaction of, and the resulting ferronickel from becoming small particles.
- Pellet formation process S12 is a process of forming (granulating) the mixture of the raw material powder obtained in mixing process process S11 into the pellet which is a lump. Specifically, water necessary for agglomeration is added to the mixture obtained in the mixing process step S11, for example, an agglomerate production apparatus (rolling granulator, compression molding machine, extrusion molding machine, etc.), etc. Or pellets are formed by hand.
- the shape of the pellet is not particularly limited, but may be spherical, for example.
- the size of the lump to be pelletized is not particularly limited.
- the size of the pellet spherical pellet charged in a reduction furnace or the like in the reduction process through a drying process and a pre-heat treatment described later
- the diameter is about 10 mm to 30 mm.
- the mixing process step S11 a mixture is prepared in order to form pellets in which the total weight of nickel and iron is a predetermined ratio or more.
- the pellets obtained in the pellet forming step S12 contain metal components of nickel and iron at a predetermined ratio, and the reduction heating in the subsequent reduction step S2 using the pellets.
- the smelting reaction of the pellets can proceed effectively, and the resulting ferronickel can be prevented from becoming small particles. Details will be described later.
- Drying process process S13 is a process of drying the pellet which is the lump obtained in pellet formation process S12.
- the formed pellets (lumps) contain excessive amounts of water, for example, about 50% by weight, and are in a sticky state. Therefore, in order to facilitate the handling of the pellets, in the drying process step S13, for example, the drying process is performed so that the solid content of the pellets is about 70% by weight and the moisture is about 30% by weight.
- the drying process for the pellets in the drying process step S13 is not particularly limited.
- hot air of 300 ° C. to 400 ° C. is blown onto the pellets to dry the pellets.
- the temperature of the pellet at the time of this drying process is less than 100 degreeC.
- Table 2 below shows an example of the composition (parts by weight) in the solid content of the pellets after the drying treatment. Note that the composition of the pellets after the drying treatment is not limited to this.
- pellet manufacturing step S1 a mixture of raw material powders containing nickel oxide ore, which is a raw material ore, is granulated (agglomerated) into pellets, and dried to produce pellets.
- the size of the pellets obtained is about 10 mm to 30 mm, and the pellets have such strength that the shape can be maintained, for example, the ratio of the pellets that collapse even when dropped from a height of 1 m is about 1% or less. Is done.
- Such pellets can withstand impacts such as dropping when charged into the reduction furnace in the subsequent reduction step S2, and can maintain the shape of the pellets. Since an appropriate gap is formed between them, the smelting reaction in the reduction step S2 proceeds appropriately.
- this pellet manufacturing process S1 you may make it provide the pre-heating process which pre-heats the pellet which is the lump which performed the drying process in the drying process S13 mentioned above to predetermined
- pre-heat treatment on the lump after the drying treatment to produce pellets, even when the pellets are reduced and heated at a high temperature of about 1400 ° C. in the reduction step S2, for example.
- the proportion of the collapsing pellets out of all the pellets charged in the reduction furnace can be made as small as less than 10%, and the shape can be maintained with 90% or more of the pellets.
- the pellets after the drying treatment are preheated to a temperature of 350 ° C. to 600 ° C.
- pre-heat treatment is preferably performed at a temperature of 400 ° C. to 550 ° C.
- the water of crystallization contained in the nickel oxide ore constituting the pellet can be reduced, and a reduction of about 1400 ° C. Even when the temperature is rapidly increased by charging the furnace, it is possible to suppress the collapse of the pellet due to the detachment of the crystal water.
- the thermal expansion of particles such as nickel oxide ore, carbonaceous reducing agent, iron oxide, binder, and flux component constituting the pellet proceeds slowly in two stages.
- the treatment time for the pre-heat treatment is not particularly limited, and may be appropriately adjusted according to the size of the mass containing nickel oxide ore.
- the size of the obtained pellet is about 10 mm to 30 mm. If it is a lump, the processing time can be about 10 to 60 minutes.
- the pellets obtained in the pellet manufacturing step S1 are heated to a predetermined reduction temperature.
- the smelting reaction proceeds to generate metal and slag.
- the reduction heat treatment in the reduction step S2 is performed using a smelting furnace (reduction furnace) or the like, and a pellet containing nickel oxide ore is charged into a reduction furnace heated to a temperature of about 1400 ° C., for example.
- nickel oxide and iron oxide in the pellet are first reduced and metalized in the vicinity of the surface of the pellet where the reduction reaction proceeds easily in a short time of about 1 minute, for example. It becomes an alloy (ferronickel) and forms a shell.
- the shell as the shell is formed, the slag component in the pellet is gradually melted to form a liquid phase slag.
- metal ferronickel metal
- slag ferronickel slag
- the slag in the pellet is melted to form a liquid phase, but the metal and slag that have already been separated and produced do not mix with each other. It becomes a mixture mixed as a separate phase.
- the volume of this mixture is shrunk to a volume of about 50% to 60% compared to the pellets to be charged.
- the “daruma shape” is a shape in which a metal solid phase and a slag solid phase are joined.
- the mixture has the largest particle size, and therefore, when recovering from the reduction furnace, there is less time for recovery and the reduction in the metal recovery rate is suppressed. can do.
- the surplus carbonaceous reducing agent described above is not limited to the one mixed in the pellets in the pellet manufacturing step S1, but for example, coke or the like is spread on the hearth of the reduction furnace used in the reduction step S2. You may prepare by.
- the pellet manufacturing step S1 it is formed when mixing at least the nickel oxide ore, the carbonaceous reducing agent, and the iron oxide.
- the mixture is generated so that the total weight of nickel and iron contained in the pellets is a predetermined amount or more.
- the separation step S3 the metal and slag generated in the reduction step S2 are separated and the metal is recovered. Specifically, the metal phase is separated and recovered from the mixture containing the metal phase (metal solid phase) and the slag phase (slag solid phase) obtained by the reduction heat treatment on the pellets.
- the obtained metal phase and slag phase can be easily separated because of poor wettability, and the above-mentioned “dharma” mixture is dropped, for example, with a predetermined drop, or The metal phase and the slag phase can be easily separated from the “daruma-like” mixture by applying an impact such as applying a predetermined vibration during sieving.
- the metal phase (ferronickel) is recovered by separating the metal phase and the slag phase.
- the pellet manufacturing process S1 is obtained by mixing the raw material containing nickel oxide ore, the pellet forming process S12 that agglomerates the obtained mixture to form pellets that are agglomerated, and And a drying treatment step S13 for drying the pellets.
- mixing process step S11 in mixing at least nickel oxide ore, a carbonaceous reducing agent, and iron oxide, it is made into the pellet formed in the next pellet formation process S12.
- a mixture is produced
- the mixture is prepared such that the total weight of the metal components of nickel and iron contained in the pellet is 30% by weight or more.
- the pellet obtained by preparing the mixture in this way and agglomerating the mixture has a high concentration of iron oxide and nickel oxide in the pellet, and is charged into the reduction furnace in the subsequent reduction step S2. Then, iron oxide and nickel oxide in the pellet are rapidly reduced to an iron-nickel alloy, that is, ferronickel (metal) to form a shell.
- the formation of the shell in the reduction heat treatment in the reduction step S2 is important for ideally proceeding the smelting reaction, and thus, one mixture for one charged pellet. It is obtained as (a mixture in which one metal solid phase and one slag solid phase are mixed), and ferronickel particles that are the largest in particle size can be effectively obtained. Thereby, when recovering ferronickel from the reduction furnace, there is little effort of recovery and it is possible to suppress a decrease in recovery rate. Moreover, it is more preferable to prepare a mixture so that the total weight of the metal components of nickel and iron contained in the pellet is 35% by weight or more, thereby obtaining ferronickel particles that stably maximize the particle size. be able to.
- the ratio of the metal component of nickel and iron contained in the pellet is not particularly limited as long as the total weight is 30% by weight or more.
- the carbonaceous material is used.
- the upper limit is preferably 55% by weight or less.
- the Ni quality contained in these ores is as low as about 1%. Therefore, the total weight of the above-described metal components (nickel and iron) when iron oxide such as iron ore is added is particularly preferably 30% by weight or more and 45% by weight or less, whereby the ferronickel in the resulting ferronickel It can suppress that Ni quality falls.
- the present embodiment when producing pellets used for the smelting reaction in the reduction step S2, at least nickel oxide ore, carbonaceous reducing agent, and iron oxide are formed into pellets to be formed.
- a mixture is prepared by mixing so that the total weight of nickel and iron contained is 30% by weight or more, and the mixture is agglomerated into pellets.
- the resulting mixture was kneaded by hand while adding water to form a spherical mass so that the finished pellet size was about 10 mm to 30 mm.
- the lump was dried to a solid content of 70% by weight and moisture of about 30% by weight to form pellets.
- the size (diameter) of the obtained pellet was about 17 mm.
- the total weight of nickel and iron contained in the pellets was 35% by weight.
- the formed pellets were charged into a reduction furnace heated to a reduction temperature of 1400 ° C. and subjected to reduction heat treatment. Then, after 10 minutes had passed since charging in the reduction furnace (the reduction reaction was terminated), the state was observed, and the number of ferronickel particles obtained was counted.
- the number of ferronickel grains becomes more than 10 when splitting during the smelting reaction (reduction reaction)
- the occurrence of splitting was evaluated by measuring the number of ferronickel.
- the number of ferronickel particles was 100 or more, many ferronickel particles were as small as 1 mm or less, so measurement was interrupted when the number was 100 or more.
- the number of ferronickel particles obtained was 10, and the Ni content in the ferronickel was 1.7% by weight.
- Example 1 the smelting reaction could be effectively advanced, and the ferronickel obtained after the smelting reaction could be prevented from breaking into small particles.
- the size (diameter) of the obtained pellet was about 17 mm, and the total weight of nickel and iron contained in the pellet was 40% by weight.
- the number of ferronickel particles obtained was 10, and the Ni content in the ferronickel was 1.5% by weight.
- Example 2 the smelting reaction could be effectively advanced, and the ferronickel obtained after the smelting reaction could be prevented from breaking into small particles.
- the size (diameter) of the obtained pellet was about 17 mm, and the total weight of nickel and iron contained in the pellet was 30% by weight.
- the number of ferronickel particles obtained was 10, and the Ni content in the ferronickel was 1.7% by weight.
- Example 3 the smelting reaction could be effectively advanced, and the ferronickel obtained after the smelting reaction could be prevented from breaking into small particles.
- the size (diameter) of the obtained pellet was about 17 mm, and the total weight of nickel and iron contained in the pellet was 45% by weight.
- the number of ferronickel particles obtained was 10, and the Ni content in the ferronickel was 1.3% by weight.
- Example 4 the smelting reaction could be effectively advanced, and the ferronickel obtained after the smelting reaction could be prevented from breaking into small particles.
- the size (diameter) of the obtained pellet was about 17 mm, and the total weight of nickel and iron contained in the pellet was 25% by weight.
- the number of ferronickel grains obtained was 83, which was divided into small grains.
- the Ni content in the ferronickel was 2.0% by weight.
- the size (diameter) of the obtained pellet was about 17 mm, and the total weight of nickel and iron contained in the pellet was 20% by weight.
- the number of ferronickel particles obtained was 100 or more and was divided into small particles.
- the Ni content in the ferronickel was 4.0% by weight.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Mechanical Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Environmental & Geological Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- Geology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Manufacture And Refinement Of Metals (AREA)
- Manufacture Of Iron (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2015297793A AU2015297793B2 (en) | 2014-08-01 | 2015-06-30 | Method for producing pellets and method for producing iron-nickel alloy |
| CA2956509A CA2956509C (en) | 2014-08-01 | 2015-06-30 | Method for producing pellets and method for producing iron-nickel alloy |
| US15/328,692 US9938604B2 (en) | 2014-08-01 | 2015-06-30 | Method for producing pellets and method for producing iron-nickel alloy |
| CN201580039607.3A CN106536765B (zh) | 2014-08-01 | 2015-06-30 | 颗粒的制造方法、铁镍合金的制造方法 |
| EP15827988.5A EP3173496B1 (en) | 2014-08-01 | 2015-06-30 | Method for producing iron-nickel alloy |
| PH12017500172A PH12017500172B1 (en) | 2014-08-01 | 2017-01-30 | Method for producing pellets and method for producing iron-nickel alloy |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014157577A JP6179478B2 (ja) | 2014-08-01 | 2014-08-01 | ペレットの製造方法、鉄−ニッケル合金の製造方法 |
| JP2014-157577 | 2014-08-01 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016017348A1 true WO2016017348A1 (ja) | 2016-02-04 |
Family
ID=55217252
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/068856 Ceased WO2016017348A1 (ja) | 2014-08-01 | 2015-06-30 | ペレットの製造方法、鉄-ニッケル合金の製造方法 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US9938604B2 (enExample) |
| EP (1) | EP3173496B1 (enExample) |
| JP (1) | JP6179478B2 (enExample) |
| CN (1) | CN106536765B (enExample) |
| AU (1) | AU2015297793B2 (enExample) |
| CA (1) | CA2956509C (enExample) |
| PH (1) | PH12017500172B1 (enExample) |
| WO (1) | WO2016017348A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2017188344A1 (ja) * | 2016-04-27 | 2017-11-02 | 住友金属鉱山株式会社 | 酸化鉱石の製錬方法 |
| JP2018150571A (ja) * | 2017-03-09 | 2018-09-27 | 住友金属鉱山株式会社 | 酸化鉱石の製錬方法、ペレット及び容器の製造方法 |
| US11479832B2 (en) | 2016-04-22 | 2022-10-25 | Sumitomo Metal Mining Co., Ltd. | Method for smelting oxide ore |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6943075B2 (ja) * | 2017-08-18 | 2021-09-29 | 住友金属鉱山株式会社 | 酸化鉱石の製錬方法、還元炉 |
| CN108971509B (zh) * | 2018-07-31 | 2021-10-08 | 上海工程技术大学 | 一种可控粒径的铁镍合金纳米材料的制备方法 |
| CN110732679B (zh) * | 2019-11-06 | 2022-07-01 | 合肥学院 | 一种基于红土镍矿制备的纳米零价铁镍复合材料及其方法 |
| CN115057644A (zh) * | 2022-08-02 | 2022-09-16 | 盐城工学院 | 一种提高风冷镍铁渣反应活性的方法 |
| WO2024254658A1 (pt) * | 2023-06-13 | 2024-12-19 | Tecnored Desenvolvimento Tecnologico S.A. | Aglomerado sólido prensado a frio para produção de ligas ferro-níquel, processo de fabricação do mesmo, e, processo de produção de uma liga ferro-níquel |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS4910110A (enExample) * | 1972-05-29 | 1974-01-29 | ||
| JPH0625770A (ja) * | 1991-12-03 | 1994-02-01 | Inco Ltd | ラテライト鉱石の低温熱的品質改良方法 |
| JP2007169774A (ja) * | 2005-11-25 | 2007-07-05 | Jfe Steel Kk | 焼結鉱の製造方法 |
| JP2012062505A (ja) * | 2010-09-14 | 2012-03-29 | Kobe Steel Ltd | 塊成物の製造方法 |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2960396A (en) * | 1957-12-23 | 1960-11-15 | P M Associates | Pelletization of iron ore concentrates |
| US3525604A (en) * | 1966-10-21 | 1970-08-25 | Edward M Van Dornick | Process for refining pelletized metalliferous materials |
| JPS53131215A (en) * | 1977-04-18 | 1978-11-15 | Nippon Steel Corp | Granulating method |
| US4659374A (en) * | 1985-06-14 | 1987-04-21 | Dow Corning Corporation | Mixed binder systems for agglomerates |
| JP4348152B2 (ja) | 2002-10-18 | 2009-10-21 | 株式会社神戸製鋼所 | フェロニッケルおよびフェロニッケル精錬原料の製造方法 |
| WO2004035847A1 (ja) * | 2002-10-18 | 2004-04-29 | Kabushiki Kaisha Kobe Seiko Sho | フェロニッケルおよびフェロニッケル精錬原料の製造方法 |
| CN100478461C (zh) * | 2006-12-22 | 2009-04-15 | 昆明贵金属研究所 | 一种转底炉-电炉联合法处理红土镍矿生产镍铁方法 |
| JP5420935B2 (ja) | 2008-04-09 | 2014-02-19 | 株式会社神戸製鋼所 | 粒状金属鉄の製造方法 |
| CN100584971C (zh) * | 2008-11-13 | 2010-01-27 | 马和平 | 氧化镍矿精选工艺 |
| CN101886171A (zh) * | 2009-05-14 | 2010-11-17 | 宝山钢铁股份有限公司 | 红土镍矿含碳球团预还原富集镍的方法 |
| CN102816922B (zh) * | 2011-06-10 | 2014-05-14 | 上海梅山钢铁股份有限公司 | 一种烧结原料组合物及烧结造块方法 |
| CN102409127A (zh) * | 2011-11-22 | 2012-04-11 | 唐山奥特斯科技有限公司 | 金属氧化物高温高料层直接还原工艺 |
| US9920397B2 (en) | 2012-06-22 | 2018-03-20 | Cerro Matoso Sa | Removal of ferric iron as hematite at atmospheric pressure |
| CN103436766B (zh) * | 2013-07-13 | 2016-04-20 | 瞿立双 | 一种含镍铬合金钢的制备方法 |
| CN105658820B (zh) * | 2013-08-19 | 2018-04-06 | 鲁道夫安东尼奥M·戈麦斯 | 用于生产和还原氧化铁团块的方法 |
-
2014
- 2014-08-01 JP JP2014157577A patent/JP6179478B2/ja active Active
-
2015
- 2015-06-30 CN CN201580039607.3A patent/CN106536765B/zh active Active
- 2015-06-30 EP EP15827988.5A patent/EP3173496B1/en active Active
- 2015-06-30 AU AU2015297793A patent/AU2015297793B2/en active Active
- 2015-06-30 US US15/328,692 patent/US9938604B2/en not_active Expired - Fee Related
- 2015-06-30 WO PCT/JP2015/068856 patent/WO2016017348A1/ja not_active Ceased
- 2015-06-30 CA CA2956509A patent/CA2956509C/en not_active Expired - Fee Related
-
2017
- 2017-01-30 PH PH12017500172A patent/PH12017500172B1/en unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS4910110A (enExample) * | 1972-05-29 | 1974-01-29 | ||
| JPH0625770A (ja) * | 1991-12-03 | 1994-02-01 | Inco Ltd | ラテライト鉱石の低温熱的品質改良方法 |
| JP2007169774A (ja) * | 2005-11-25 | 2007-07-05 | Jfe Steel Kk | 焼結鉱の製造方法 |
| JP2012062505A (ja) * | 2010-09-14 | 2012-03-29 | Kobe Steel Ltd | 塊成物の製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3173496A4 * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11479832B2 (en) | 2016-04-22 | 2022-10-25 | Sumitomo Metal Mining Co., Ltd. | Method for smelting oxide ore |
| WO2017188344A1 (ja) * | 2016-04-27 | 2017-11-02 | 住友金属鉱山株式会社 | 酸化鉱石の製錬方法 |
| US11608543B2 (en) | 2016-04-27 | 2023-03-21 | Sumitomo Metal Mining Co., Ltd. | Oxide ore smelting method |
| JP2018150571A (ja) * | 2017-03-09 | 2018-09-27 | 住友金属鉱山株式会社 | 酸化鉱石の製錬方法、ペレット及び容器の製造方法 |
| JP7035322B2 (ja) | 2017-03-09 | 2022-03-15 | 住友金属鉱山株式会社 | 酸化鉱石の製錬方法、ペレット及び容器の製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| PH12017500172A1 (en) | 2017-07-10 |
| CA2956509A1 (en) | 2016-02-04 |
| US9938604B2 (en) | 2018-04-10 |
| EP3173496A1 (en) | 2017-05-31 |
| US20170211166A1 (en) | 2017-07-27 |
| CA2956509C (en) | 2017-07-04 |
| CN106536765B (zh) | 2021-03-02 |
| CN106536765A (zh) | 2017-03-22 |
| JP6179478B2 (ja) | 2017-08-16 |
| EP3173496B1 (en) | 2019-12-18 |
| AU2015297793A1 (en) | 2017-02-23 |
| EP3173496A4 (en) | 2017-08-23 |
| AU2015297793B2 (en) | 2017-07-13 |
| JP2016035084A (ja) | 2016-03-17 |
| PH12017500172B1 (en) | 2018-10-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6179478B2 (ja) | ペレットの製造方法、鉄−ニッケル合金の製造方法 | |
| JP5842967B1 (ja) | ペレットの製造方法、鉄−ニッケル合金の製造方法 | |
| JP5858105B1 (ja) | ニッケル酸化鉱の製錬方法 | |
| JP5958576B1 (ja) | サプロライト鉱の製錬方法 | |
| JP6314781B2 (ja) | ニッケル酸化鉱の製錬方法 | |
| JP6780284B2 (ja) | ペレットの製造方法、及びニッケル酸化鉱石の製錬方法 | |
| JP5839090B1 (ja) | ニッケル酸化鉱の製錬方法、ペレットの装入方法 | |
| JP6303901B2 (ja) | ニッケル酸化鉱の製錬方法 | |
| JP5858101B2 (ja) | ペレットの製造方法、ニッケル酸化鉱の製錬方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15827988 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 15328692 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 2956509 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12017500172 Country of ref document: PH |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2015297793 Country of ref document: AU Date of ref document: 20150630 Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015827988 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015827988 Country of ref document: EP |