WO2015029500A1 - 車載用カメラ - Google Patents
車載用カメラ Download PDFInfo
- Publication number
- WO2015029500A1 WO2015029500A1 PCT/JP2014/062075 JP2014062075W WO2015029500A1 WO 2015029500 A1 WO2015029500 A1 WO 2015029500A1 JP 2014062075 W JP2014062075 W JP 2014062075W WO 2015029500 A1 WO2015029500 A1 WO 2015029500A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- silicon dioxide
- vehicle
- hydrophilic film
- lens
- average particle
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/10—Optical coatings produced by application to, or surface treatment of, optical elements
- G02B1/18—Coatings for keeping optical surfaces clean, e.g. hydrophobic or photo-catalytic films
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C17/00—Surface treatment of glass, not in the form of fibres or filaments, by coating
- C03C17/006—Surface treatment of glass, not in the form of fibres or filaments, by coating with materials of composite character
- C03C17/007—Surface treatment of glass, not in the form of fibres or filaments, by coating with materials of composite character containing a dispersed phase, e.g. particles, fibres or flakes, in a continuous phase
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C17/00—Surface treatment of glass, not in the form of fibres or filaments, by coating
- C03C17/34—Surface treatment of glass, not in the form of fibres or filaments, by coating with at least two coatings having different compositions
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C17/00—Surface treatment of glass, not in the form of fibres or filaments, by coating
- C03C17/34—Surface treatment of glass, not in the form of fibres or filaments, by coating with at least two coatings having different compositions
- C03C17/3411—Surface treatment of glass, not in the form of fibres or filaments, by coating with at least two coatings having different compositions with at least two coatings of inorganic materials
- C03C17/3417—Surface treatment of glass, not in the form of fibres or filaments, by coating with at least two coatings having different compositions with at least two coatings of inorganic materials all coatings being oxide coatings
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03B—APPARATUS OR ARRANGEMENTS FOR TAKING PHOTOGRAPHS OR FOR PROJECTING OR VIEWING THEM; APPARATUS OR ARRANGEMENTS EMPLOYING ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ACCESSORIES THEREFOR
- G03B17/00—Details of cameras or camera bodies; Accessories therefor
- G03B17/02—Bodies
- G03B17/08—Waterproof bodies or housings
-
- H—ELECTRICITY
- H04—ELECTRIC COMMUNICATION TECHNIQUE
- H04N—PICTORIAL COMMUNICATION, e.g. TELEVISION
- H04N23/00—Cameras or camera modules comprising electronic image sensors; Control thereof
- H04N23/50—Constructional details
- H04N23/55—Optical parts specially adapted for electronic image sensors; Mounting thereof
-
- H—ELECTRICITY
- H04—ELECTRIC COMMUNICATION TECHNIQUE
- H04N—PICTORIAL COMMUNICATION, e.g. TELEVISION
- H04N25/00—Circuitry of solid-state image sensors [SSIS]; Control thereof
- H04N25/70—SSIS architectures; Circuits associated therewith
- H04N25/71—Charge-coupled device [CCD] sensors; Charge-transfer registers specially adapted for CCD sensors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60R—VEHICLES, VEHICLE FITTINGS, OR VEHICLE PARTS, NOT OTHERWISE PROVIDED FOR
- B60R2300/00—Details of viewing arrangements using cameras and displays, specially adapted for use in a vehicle
- B60R2300/80—Details of viewing arrangements using cameras and displays, specially adapted for use in a vehicle characterised by the intended use of the viewing arrangement
- B60R2300/802—Details of viewing arrangements using cameras and displays, specially adapted for use in a vehicle characterised by the intended use of the viewing arrangement for monitoring and displaying vehicle exterior blind spot views
-
- C—CHEMISTRY; METALLURGY
- C03—GLASS; MINERAL OR SLAG WOOL
- C03C—CHEMICAL COMPOSITION OF GLASSES, GLAZES OR VITREOUS ENAMELS; SURFACE TREATMENT OF GLASS; SURFACE TREATMENT OF FIBRES OR FILAMENTS MADE FROM GLASS, MINERALS OR SLAGS; JOINING GLASS TO GLASS OR OTHER MATERIALS
- C03C2217/00—Coatings on glass
- C03C2217/40—Coatings comprising at least one inhomogeneous layer
- C03C2217/43—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase
- C03C2217/46—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase characterized by the dispersed phase
- C03C2217/47—Coatings comprising at least one inhomogeneous layer consisting of a dispersed phase in a continuous phase characterized by the dispersed phase consisting of a specific material
- C03C2217/475—Inorganic materials
- C03C2217/478—Silica
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03B—APPARATUS OR ARRANGEMENTS FOR TAKING PHOTOGRAPHS OR FOR PROJECTING OR VIEWING THEM; APPARATUS OR ARRANGEMENTS EMPLOYING ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ACCESSORIES THEREFOR
- G03B2217/00—Details of cameras or camera bodies; Accessories therefor
Definitions
- the present invention relates to an in-vehicle camera that is attached to the outside of a vehicle and grasps a situation outside the vehicle, and more specifically, in-vehicle in which a hydrophilic film is formed on the lens surface in order to suppress disturbance of an image due to water droplet adhesion to the camera lens.
- a hydrophilic film is formed on the lens surface in order to suppress disturbance of an image due to water droplet adhesion to the camera lens.
- the spread of in-vehicle cameras is accelerating in order to observe conditions outside the vehicle such as rearward confirmation and white line recognition.
- the in-vehicle camera may be installed inside the vehicle, but it is preferable to install it outside the vehicle because it is more suitable to monitor the surrounding conditions widely.
- water droplets adhere to the lens of the in-vehicle camera due to rain, snow, etc. and scatter the light incident on the camera, which makes it difficult to grasp the surrounding situation.
- Patent Document 1 discloses a technique in which a cleaning liquid is sprayed onto a lens of an in-vehicle camera to provide a mechanism for cleaning the lens surface.
- Patent Document 1 a cleaning device for injecting a cleaning liquid to a vehicle-mounted camera is required, and the cleaning liquid needs to be replenished. Therefore, in a vehicle provided with a cleaning device, a space for providing the cleaning device and a cleaning liquid tank is required, and wiring for driving the cleaning device and piping for the cleaning liquid are also required. In addition, the user who manages the vehicle needs to replenish the cleaning liquid.
- Such a cleaning apparatus that injects the cleaning liquid needs to secure a space in the vehicle, and the user needs to replenish the cleaning liquid. Therefore, a technique that does not require these has been required.
- the lens surface when the lens surface is made hydrophilic, the water droplet becomes a water film, so that the scattering of light by the water droplet is suppressed and the visibility is improved.
- the method for hydrophilizing the lens surface include a method of forming a titanium oxide-containing film on the surface.
- titanium oxide does not exhibit hydrophilicity unless irradiated with ultraviolet light, the effect is not exhibited during night driving.
- Patent Document 2 discloses a technique that exhibits hydrophilicity even when light is not irradiated. This uses a film composed of inorganic oxide particles such as silicon dioxide and voids on the lens surface. Inorganic oxides generally have higher hydrophilicity than organic substances. For example, a glass plate formed of silicon dioxide has a smaller contact angle with water and higher hydrophilicity than an acrylic resin plate or the like.
- the inventors have studied using a hydrophilic film composed of highly hydrophilic silicon dioxide particles and voids on the lens surface.
- the volume of water drops is a certain amount, so the entire surface of the film is water. Visibility is good because it becomes a film, but when small water droplets such as fog and light rain begin to adhere, there are both wet and non-wet parts even if it becomes a water film, in which case light is scattered It turned out that the field of view was hindered. If a certain number of small water droplets adhere as time passes, the entire surface becomes a water film, so the field of view is good, but there is a problem that the field of view is poor until the water film is formed.
- An object of the present invention is to provide an in-vehicle camera in which a hydrophilic film is formed which is easy to form a water film even if small water droplets adhere to the lens surface.
- a desired hydrophilic film can be formed by laminating a plurality of films having silicon dioxide particles having a plurality of sizes, leading to the present invention.
- the hydrophilicity of the silicon dioxide itself is improved by increasing the surface area due to the hydrophilicity of the silicon dioxide itself and the unevenness by forming a slight unevenness having optical influence on the surface of the hydrophilic film.
- the present invention relates to a vehicle-mounted camera provided on the outer periphery of a vehicle, from a silicon dioxide particle having an average particle diameter in a predetermined range on a surface of a lens provided on the outer surface of the camera, and a binder mainly composed of silicon dioxide.
- the average particle diameter of silicon dioxide present on the lens surface side is present on the side of the camera outer surface that is in contact with the air layer. It is characterized by being larger than the average particle diameter of silicon.
- silicon dioxide particles having an average particle diameter in a predetermined range and silicon dioxide as a main component are formed on the surface of a lens provided on the outer surface of the camera.
- a hydrophilic film made of a binder is provided, and among the silicon dioxide particles dispersed in the hydrophilic film, the average particle diameter of silicon dioxide present on the lens surface side is on the side in contact with the air layer on the outer surface of the camera.
- the hydrophilic film formed on the lens of the in-vehicle camera of the present invention has a binder mainly composed of silicon dioxide, silicon dioxide particles having an average particle diameter of 10 to 15 nm, and silicon dioxide having an average particle diameter of 40 to 100 nm. Consists of particles.
- silicon dioxide particles having an average particle size of 10 to 15 nm are referred to as small particles
- silicon dioxide particles having an average particle size of 40 to 100 nm are referred to as large particles.
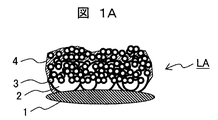
- the large particle 2 and the small particle 3 are held on the surface of the lens 1 by a binder 4 made of silicon dioxide. Since both the large irregularities due to the large-diameter particles 2 and the small irregularities due to the small-diameter particles 3 are provided, the surface area is large, and the hydrophilicity of the entire film becomes high.
- the film formed of only large-diameter particles has large surface irregularities, and is white and cloudy when visually observed due to scattering of light derived from the surface irregularities. In this case, the field of view may be hindered.
- the surface irregularity can be reduced by overlapping the layer of the small particle on the layer of the large particle, thereby reducing the turbidity of the film, and further providing fine irregularities formed by the small particle.
- the hydrophilicity can be improved.
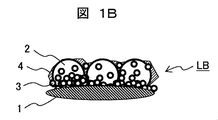
- Hydrophilic film in which small-sized particles and large-sized particles are mixed As shown in the film LB in FIG. 1B, this is a hydrophilic film formed so that the large-sized particles 2 and the small-sized particles 3 coexist in the entire film. It is a structure in which the small particle 3 is inserted between the large particles 2.
- the film formed only by the large-diameter particles 2 has large surface irregularities, and is white and cloudy when visually observed due to scattering of light derived from the surface irregularities, which may hinder the visibility. Therefore, by filling the gaps between the large-diameter particles 2 with the small-diameter particles 3, the surface irregularities can be reduced, and hence turbidity can be reduced. Further, by providing fine irregularities formed between the small-diameter particles 3, the hydrophilicity of the film can be reduced. Improvement can be achieved.
- the gap formed between the large-diameter particles and the small-diameter particles inside the membrane is highly hydrophilic because it spreads water droplets and forms a water membrane by taking water into the membrane by capillary action.
- the surface area is large and the hydrophilicity is high.
- the gap between the large-diameter particles and the small-diameter particles inside the membrane widens the water droplets by trying to take water into the membrane by capillarity to form a water membrane, thereby increasing the hydrophilicity. Therefore, since the membrane (C) has a membrane shape that further improves the hydrophilicity of the membranes (A) and (B), the hydrophilicity is further improved.
- the hydrophilic film formed on the lens of the vehicle-mounted camera of the present invention is composed of silicon dioxide particles and binders having different sizes. Since the lens on the outermost surface of the vehicle-mounted camera is made of glass because importance is attached to the abrasion resistance, the present invention will be described mainly below with respect to the glass lens. These resins may be used.
- grains of this invention use the particle
- the material is silicon dioxide is that the material is excellent in transparency and excellent in hydrophilicity. It is also the reason why it is selected because of its high hardness and high transparency. Further, since the refractive index is about 1.5, which is almost the same as that of glass, the reflectance is low and the transmittance is high.
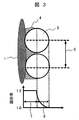
- FIG. 3 is an explanatory diagram showing the relationship between the cross section of the hydrophilic film and the refractive index.
- FIG. 1A to 1C and FIG. 2 show a state in which the lens is laid sideways, but FIG. 3 shows a state in which the lens 1 is erected so that the optical axis is horizontal.
- the region 7 filled with the silicon dioxide particles and the binder from the lens surface is considered in the thickness direction of the hydrophilic film.
- the refractive index is approximately 1.5.
- an air layer having a refractive index of 1.0 is mixed, resulting in a region 8 in which silicon dioxide and air are mixed.
- the refractive index decreases from 1.5, and is approximately 1.0 at the right end of the mixed region 8. That is, the refractive index near the surface of the hydrophilic film is lower than that of glass.
- the hydrophilic film of the present invention is composed of a binder mainly composed of silicon dioxide particles and silicon dioxide.
- the binder is mainly composed of silicon dioxide formed by curing a silicon compound having a hydrolyzable group by hydrolysis.
- silica sol As a silicon compound having a hydrolyzable group, first, silica sol can be mentioned. This is a substance that is hydrolyzed by heating and changes to silicon dioxide. Silica sol is generally prepared using tetraalkoxysilane as a raw material. When tetraalkoxysilane is heated for several hours under weakly acidic conditions, part of the alkoxysilane group is hydrolyzed to form a hydroxyl group, which reacts with the nearby alkoxysilane group to form a silicon-oxygen-silicon bond. A silica sol having a molecular weight of several thousand is obtained.
- tetraalkoxysilane examples include tetramethoxysilane, tetraethoxysilane, tetrapropoxysilane, and tetrabutoxysilane.
- silicon compounds containing chlorine groups such as silicon tetrachloride are also included.
- silicon compounds having a hydrolyzable group as a raw material for the binder include 3-aminopropyltrimethoxysilane, 3-aminopropyltriethoxysilane, N- (2-aminoethyl) -3-aminopropyltrimethoxy.
- Silane N- (2-aminoethyl) -3-aminopropyltriethoxysilane, N-phenyl-3-aminopropyltrimethoxysilane, N-phenyl-3-aminopropyltriethoxysilane, 3-chloropropyltrimethoxysilane 3-chloropropyltriethoxysilane, 3-mercaptopropyltrimethoxysilane, 3-mercaptopropyltriethoxysilane, vinyltrimethoxysilane, vinyltriethoxysilane, 3-glucidoxypropyltrimethoxysilane, 3-glucidoxy Propi Triethoxysilane, 3-methacryloxypropyl trimethoxy silane, 3-methacryloxypropyl triethoxysilane, and the like.
- 3-aminopropyltrimethoxysilane having an amino group 3-aminopropyltriethoxysilane, N- (2-aminoethyl) -3-aminopropyltrimethoxysilane, N- (2-aminoethyl)- 3-Aminopropyltriethoxysilane, N-phenyl-3-aminopropyltrimethoxysilane, and N-phenyl-3-aminopropyltriethoxysilane have amino groups in the binder by hydrolysis. When the vehicle-mounted camera on which this film is formed is left under acidic conditions, the amino group becomes an ammonium salt structure and the hydrophilicity increases.
- a hydrophilic film is produced by preparing a coating solution necessary for forming a hydrophilic film, applying it to a lens, and drying by heating.
- the configuration of the hydrophilic film of the present invention is roughly divided into three types, and each will be described.
- A Hydrophilic film in which small-diameter particles are in the surface layer and large-diameter particles are in the interface layer with the lens When this film is formed, a coating solution containing large-diameter particles is first applied and then dried. A coating liquid containing small-diameter particles is applied thereon and dried.
- B Hydrophilic film in which small-diameter particles and large-diameter particles are mixed. This film is produced by preparing a coating liquid in which large-diameter particles and small-diameter particles are mixed, and applying and drying the coating liquid.
- (C) A hydrophilic film in which small-diameter particles are present throughout the film and large-diameter particles are in the interface layer with the lens. This film first forms the film of (B) above, and a coating liquid containing small-diameter particles thereon. After coating, it is dried to produce.
- Coating liquid preparation A coating liquid is manufactured by mixing a binder liquid and the dispersion of a silicon dioxide particle. A method for preparing these two types of solutions will be described below.
- Binder liquid preparation First, a method for preparing a silica sol solution will be described. In this method, a compound such as tetraethoxysilane described above is partially hydrolyzed with an acid such as acetic acid, and then converted into a silica sol having an average molecular weight of several thousands by a subsequent polymerization reaction.
- a 3-aminopropyltrimethoxysilane solution is produced by dissolving 3-aminopropyltrimethoxysilane in an alcohol such as ethanol.
- an alcohol such as ethanol.
- a dispersion of silicon dioxide particles is prepared. Silicon dioxide has a specific gravity of about 2.5, which is relatively small among inorganic oxides. Therefore, a dispersion of silicon dioxide particles can be prepared by adding a dispersant such as ethylene glycol monoalkyl ester, diethylene glycol monoalkyl ester, ethylene glycol monoalkyl ether, or diethylene glycol monoalkyl ether to the solvent. This is mixed with the binder liquid to prepare a coating liquid for forming a hydrophilic film.
- a dispersant such as ethylene glycol monoalkyl ester, diethylene glycol monoalkyl ester, ethylene glycol monoalkyl ether, or diethylene glycol monoalkyl ether
- the solvent of the binder liquid and the silicon dioxide particle dispersion is preferably an alcohol having a hydroxyl group or ethylene glycol monoalkyl ether.
- the solvent of the coating solution is alcohol or ethylene glycol monoalkyl ether which dissolves in water or partially dissolves.
- the boiling point is preferably 140 ° C. or lower in order to improve throughput.
- Suitable solvents include methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol, t-butanol, 1-pentanol, 2-pentanol, 3-pentanol, and t-pen. Examples include ethanol and 2-ethoxyethanol.
- the arithmetic average roughness (Ra) of the hydrophilic film needs a certain size in order to form surface irregularities by the particles, increase the surface area, and improve the hydrophilicity.
- the lower limit of Ra of the hydrophilic film is 2.5 nm, and this value ensures sufficient hydrophilicity. Since the lower limit of the average particle diameter of the particles used is 10 nm, it is considered that the particles are 2.5 nm because they are buried in the gap between the binder and other particles.
- the in-vehicle camera needs to transmit light in the visible region (approximately 400 to 700 nm).
- the scattering was 5% or less of the irradiation light when Ra was 50 nm.
- the scattering was easily abrupt and the scattering was irradiated when Ra was 55 nm.
- the light transmittance increased to about 10%, and the light transmittance decreased by 10% or more. Scattering was particularly strong at short wavelengths, and the transmittance decreased with shorter wavelengths.
- the upper limit of Ra is preferably 50 nm. From the above, it was found that the range of Ra is preferably 2.5 to 50 nm.
- Rv the maximum depth of the surface roughness of the hydrophilic film. If the depth is too small, the hydrophilicity decreases. This means that the depth needs to be increased to some extent for water to enter the capillarity from the gaps between the particles, which is 6 nm in the experiment. On the other hand, when the depth is too large, specifically, when the depth is 16 nm or more, it is considered that dirt other than water enters and the hydrophilicity is lowered.
- Rp the maximum peak of the surface roughness of the hydrophilic film
- the film thickness is preferably a structure in which about 1 to 2 large-diameter particles overlap in the film thickness direction, and 1 to 5 small-diameter particles overlap. Since the large particles have an average particle size of 40 to 100 nm and the small particles have an average particle size of 10 to 15 nm, the thickness of the hydrophilic film is preferably about 50 to 250 nm. As a result, a water film is formed in such a way that water enters the gaps between the particles by capillary action, so that hydrophilicity is improved.
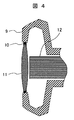
- FIG. 1 A schematic cross-sectional view of the vehicle-mounted camera of the present invention is shown in FIG.
- a lens 11 coated with a hydrophilic film is attached to the camera housing 9 via a packing 10.
- a CCD element 12 is provided inside the lens 11 to read image information that has entered through the lens 11 and send it to an image processing device (not shown in this figure).
- the lens is slightly turbid due to large silicon dioxide particles, and has a higher haze than a transparent lens.
- the CCD element by disposing the CCD element as close as possible to the surface of the lens, it is possible to suppress a decrease in transmittance due to turbidity as much as possible.
- the haze of the lens 11 of the in-vehicle camera of the present invention is 1 or less. However, when the CCD element 12 is separated from the lens by about 4 mm, the input light quantity is reduced by about 2% from the light quantity entering the CCD element 12 by 3 mm. . Since the distance between the CCD element 12 and the lens 11 is 3 mm or less, or the light transmittance difference is 0.5% or less when the lens element 11 is in contact with the lens 11, the distance between the CCD element and the lens is preferably 3 mm or less. [Use of the vehicle-mounted camera of the present invention] The vehicle-mounted camera of the present invention can be used as a back monitor for rearward confirmation.
- Another possible application is to recognize the white line on the road and automatically control the accelerator, brakes, and steering wheel using it as a mark, and function as the eyes of a system that runs automatically.
- it can be used as a monitor that can check the rear and diagonally rearward conditions that have been relied on with conventional door mirrors and rearview mirrors.
- it can also be used as a remote operation monitor for crane vehicles, excavation vehicles, dump trucks, etc. that automatically operate in a poor environment.
- it can be used as a monitor for remote control of unmanned railway vehicles.
- Example 1 describes the contents relating to the hydrophilic film formed on the lens of the vehicle-mounted camera of the present invention and the lens.
- (1) Preparation of coating liquid for hydrophilic film formation After mixing silicon dioxide particles (30 parts by weight) having an average particle size of 10 to 15 nm and ethylene glycol monobutyrate (5 parts by weight), ethanol (965 parts by weight) with stirring. ) Is added little by little. This liquid is a particle mixed liquid.
- Tetraethoxysilane 70 parts by weight is dissolved in ethanol (980 parts by weight), a very small amount of nitric acid is added, and the mixture is heated at 50 ° C. for about 1 hour.
- a silica sol solution 1000 parts by weight having a silicon concentration of about 1% by weight and volatilization of the solvent and a silicon dioxide concentration after heat curing of about 2% by weight is obtained.
- the whole amount of the particle mixture prepared above is added, and the coating solution containing silicon dioxide particles having an average particle diameter of 10 to 15 nm prepared in this manner is designated as coating solution A.
- a coating liquid containing silicon dioxide particles having an average particle diameter of 70 to 100 nm is prepared.
- the coating solution A was prepared by using silicon dioxide particles having an average particle size of 70 to 100 nm (30 parts by weight) instead of silicon dioxide particles having an average particle size of 10 to 15 nm (30 parts by weight). It was prepared in the same manner as in the above.
- a coating liquid containing silicon dioxide particles having an average particle diameter of 70 to 100 nm is referred to as a coating liquid C.
- (2) Formation of hydrophilic film on lens Using the coating liquid A and coating liquid C, a hydrophilic film is formed on the lens.
- application by the dip method is described. First, the lens is fixed to the lifting jig of the dip device so that the light transmission surface of the lens is horizontal.
- the coating liquid C is used as the coating liquid.
- the lens is inserted into a container containing the coating liquid C so that the lens is immersed in the coating liquid C, and then the lens is pulled up from the coating liquid C at a lifting speed of 0.75 mm / second. After pulling up, it is put in a constant temperature bath at 80 ° C. for 1 hour, taken out, and cooled to room temperature.
- hydrophilic film hydrophilic film-1 having a structure corresponding to LA in FIG. 1A is formed on the lens.
- Fig. 5 shows a cross-sectional photograph of hydrophilic membrane-1. A structure in which small-sized particles are present on large-sized particles can be confirmed.
- the binder made of silicon dioxide is not shown by an arrow because it is too thin to be confirmed in this photograph. Also, the subsequent photographs do not show a silicon dioxide binder for the same reason.
- coating solution D Equal amounts of the coating liquid A and the coating liquid C prepared in Example 2 are mixed. This is designated coating solution D.
- Coating liquid D is applied to the lens and heated in the same manner as coating liquid C.
- a hydrophilic film hydrophilic film-2 having a structure corresponding to LB in FIG. 1B is formed on the lens.
- FIG. 1B A cross-sectional photograph of hydrophilic membrane-2 is shown in FIG. A structure in which large particles and small particles are present in the same manner as in FIG. 1B can be confirmed.
- a coating liquid containing silicon dioxide particles having an average particle diameter of 40 to 50 nm is prepared by using large particles having an average particle diameter of 40 to 50 nm instead of large particles having an average particle diameter of 70 to 100 nm in Example 2.
- the coating solution A was prepared by using silicon dioxide particles having an average particle size of 40-50 nm (30 parts by weight) instead of silicon dioxide particles having an average particle size of 10-15 nm (30 parts by weight). It was prepared in the same manner as in the above.
- a coating liquid containing silicon dioxide particles having an average particle diameter of 40 to 50 nm is referred to as a coating liquid B. Equal amounts of the coating liquid A and the coating liquid B produced above are mixed. This is designated as coating liquid E.
- Coating liquid E is applied to the lens and heated in the same manner as coating liquid C.
- a hydrophilic film hydrophilic film-3 having a structure corresponding to LB in FIG. 1B is formed on the lens.
- the coating liquid D is applied and heated in the same manner as the coating liquid C.
- a hydrophilic film (hydrophilic film-2) having a structure corresponding to LB in FIG. 1B is formed on the lens.
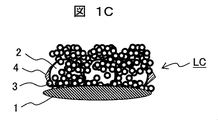
- the coating liquid A is similarly applied onto the hydrophilic film-2 and heated to form a hydrophilic film (hydrophilic film-4) having a structure corresponding to FIG. 1C on the lens.
- FIG. 1 A cross-sectional photograph of hydrophilic membrane-4 is shown in FIG. A structure in which large particles and small particles are present in the same manner as the LC in FIG. 1C can be confirmed.
- (3) Evaluation of hydrophilicity Each of the contact angles of hydrophilic membrane-1 to hydrophilic membrane-4 produced as described above with water was 5 ° or less. Since the contact angle of the lens with water before forming the hydrophilic film was about 30 °, the hydrophilicity of the formed film was confirmed. (Comparative Example 1) (1) Formation of hydrophilic film on the lens Only the coating liquid A prepared in Example 1 was applied to the lens in the same manner as in Example 1 and heated, whereby a hydrophilic film having a structure corresponding to FIG. Membrane-5) is formed. A cross-sectional photograph of this hydrophilic film is shown in FIG.
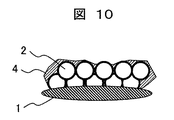
- Example 6 only the coating liquid B prepared in Example 1 is applied to the lens in the same manner as in Example 1 and heated to form a hydrophilic film (hydrophilic film-6) having a structure corresponding to FIG. 10 on the lens. .
- a cross-sectional photograph of this hydrophilic film is shown in FIG.
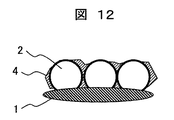
- Example 2 only the coating liquid C prepared in Example 1 is applied to the lens in the same manner as in Example 1 and heated to form a hydrophilic film (hydrophilic film-7) having a structure corresponding to FIG. 12 on the lens. .
- a cross-sectional photograph of this hydrophilic film is shown in FIG. (2) Evaluation of hydrophilicity
- the contact angles of the hydrophilic films-5 to 7 prepared as described above with water were all 5 ° or less. Since the contact angle of the lens with water before forming the hydrophilic film was about 30 °, the hydrophilicity of the formed film was confirmed.
- the lens on which the hydrophilic film is formed is held at a position 50 mm away from the steam outlet of the steam humidifier (HLF-350 manufactured by Hitachi Living Supply Co.) for a certain period of time.
- the exposed surface was placed on a printed material in the Mincho style, and the visibility of letters was visually evaluated to evaluate the degree of cloudiness.
- the exposure time is short, when the vapor adheres as water droplets, the lens becomes cloudy and the characters become difficult to see.
- the exposure time becomes long when the vapor becomes a water film, the lens does not become cloudy and the characters can be easily seen.
- the higher the hydrophilicity the more easily the steam becomes a water film, so that characters can be easily seen in a short time. In this way, it was decided to evaluate hydrophilicity based on the visibility of characters.
- hydrophilic film-1 to hydrophilic film-4 were legible in an exposure time of 2 seconds.
- the hydrophilic film 5 cannot be read unless exposed for 10 seconds, and the hydrophilic film-6 and hydrophilic film-7 are difficult to read unless exposed for 15 seconds.
- a hydrophilic film having large-sized particles on the lens side and small-sized particles on the air layer side, or a hydrophilic film in which large-sized particles and small-sized particles are mixed is compared with a hydrophilic film composed of particles of almost the same size. It was found to be highly hydrophilic. In particular, it has been found that when a small amount of water droplets adheres, the water droplets are easily formed into a water film, and fogging due to the slight water droplet adhesion can be suppressed.
- Comparative Example 2 The lenses coated with hydrophilic membrane-1 to hydrophilic membrane-4 produced in Examples 1 to 4 and hydrophilic membrane-5 to hydrophilic membrane-7 produced in Comparative Example 1 were attached to the in-vehicle camera shown in FIG.
- the lens portion of these in-vehicle cameras is held at a position 50 mm away from the steam outlet of the humidifier used in Comparative Example 1 for a certain period of time.
- the white line on the road 10 m away from the lens is the in-vehicle camera. It was investigated whether it could be confirmed visually with a monitor that displayed image information.
- the hydrophilicity was evaluated based on whether or not the characters could be read.
- the white line on the road was evaluated instead of the characters.
- hydrophilic film-1 to hydrophilic film-4 were able to recognize white lines after an exposure time of 2 seconds.
- the hydrophilic film-5 to hydrophilic film-7 could not be confirmed as white lines unless exposed for 10 seconds.
- the hydrophilic film having large-sized particles on the lens side and small-sized particles on the air layer side, or the hydrophilic film in which large-sized particles and small-sized particles coexist are composed of particles of almost the same size. It was found that the hydrophilicity is higher than that of the hydrophilic membrane. In particular, it has been found that when a small amount of water droplets adheres, the water droplets are easily formed into a water film, and fogging due to the slight water droplet adhesion can be suppressed.
- the light transmittance before the vapor exposure was about 96%. When exposed for 2 seconds, it decreased to about 92%, but when exposed for 5 seconds, it recovered to about 94%, and when exposed for 10 seconds, it recovered to about 95%.
- the light transmittance before the vapor exposure was about 96%. However, when exposed for 2 seconds, it decreased to about 80%, and when exposed for 5 seconds, it decreased to about 70%. After that, it recovered, but even after exposure for 10 seconds, it recovered only to 85%.
- a hydrophilic film having large-diameter particles on the lens side and small-diameter particles on the air layer side, or a hydrophilic film in which large-diameter particles and small-diameter particles coexist can suppress a decrease in light transmittance at the initial stage of water droplet adhesion. Became clear.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- General Physics & Mathematics (AREA)
- Multimedia (AREA)
- Optics & Photonics (AREA)
- Dispersion Chemistry (AREA)
- Composite Materials (AREA)
- Signal Processing (AREA)
- Laminated Bodies (AREA)
- Surface Treatment Of Optical Elements (AREA)
- Mechanical Engineering (AREA)
- Camera Bodies And Camera Details Or Accessories (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020167004837A KR20160035042A (ko) | 2013-08-30 | 2014-05-01 | 차량 탑재용 카메라 |
| EP14840521.0A EP3040772A4 (en) | 2013-08-30 | 2014-05-01 | Vehicle-mounted camera |
| CN201480046419.9A CN105474088A (zh) | 2013-08-30 | 2014-05-01 | 车载用摄像机 |
| US14/914,897 US9713984B2 (en) | 2013-08-30 | 2014-05-01 | Vehicle-mounted camera |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-179284 | 2013-08-30 | ||
| JP2013179284A JP6214281B2 (ja) | 2013-08-30 | 2013-08-30 | 車載用カメラ |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015029500A1 true WO2015029500A1 (ja) | 2015-03-05 |
Family
ID=52586082
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/062075 Ceased WO2015029500A1 (ja) | 2013-08-30 | 2014-05-01 | 車載用カメラ |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US9713984B2 (enExample) |
| EP (1) | EP3040772A4 (enExample) |
| JP (1) | JP6214281B2 (enExample) |
| KR (1) | KR20160035042A (enExample) |
| CN (1) | CN105474088A (enExample) |
| TW (1) | TWI580595B (enExample) |
| WO (1) | WO2015029500A1 (enExample) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6749093B2 (ja) * | 2015-12-02 | 2020-09-02 | 日揮触媒化成株式会社 | 超親水性被膜付基材と、その塗布液および製造方法 |
| CN106161965A (zh) * | 2016-09-06 | 2016-11-23 | 广东创我科技发展有限公司 | 一种摄像机及摄像方法 |
| JP6593360B2 (ja) * | 2017-01-30 | 2019-10-23 | トヨタ自動車株式会社 | 車両用光学システム |
| WO2019136433A1 (en) * | 2018-01-08 | 2019-07-11 | 3M Innovative Properties Company | Protective film and method of use thereof |
| JP2020016862A (ja) * | 2018-07-27 | 2020-01-30 | 京セラ株式会社 | カメラ装置、カメラシステム、移動体およびカメラ装置の製造方法 |
| JP7301508B2 (ja) | 2018-08-08 | 2023-07-03 | キヤノン株式会社 | 接合レンズ、およびそれを有する光学系、および光学機器、および接合レンズの製造方法 |
| JP2020148845A (ja) * | 2019-03-12 | 2020-09-17 | マクセル株式会社 | レンズ、レンズの製造方法、および、レンズユニット |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001051108A (ja) * | 1999-08-09 | 2001-02-23 | Toto Ltd | 浴室用防曇鏡及び浴室設備並びに浴室 |
| JP2002002766A (ja) * | 2000-06-21 | 2002-01-09 | Toto Ltd | 防曇性を有する加熱容器用蓋 |
| JP2002080829A (ja) * | 2000-09-07 | 2002-03-22 | Toto Ltd | 親水性部材、その製造方法、およびその製造のためのコーティング剤 |
| JP2004123996A (ja) | 2002-10-07 | 2004-04-22 | Hitachi Ltd | 有機樹脂上に形成される親水膜,撥水膜 |
| JP2009265473A (ja) * | 2008-04-28 | 2009-11-12 | Konica Minolta Opto Inc | レンズ、撮像レンズ及び撮像装置 |
| JP2011240910A (ja) | 2009-09-29 | 2011-12-01 | Denso Corp | 車載光学センサカバー |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH101913A (ja) | 1996-06-17 | 1998-01-06 | Mitsui Eng & Shipbuild Co Ltd | 吊り橋 |
| WO2003039855A1 (en) * | 2001-11-08 | 2003-05-15 | Nippon Sheet Glass Company, Limited | Article coated with coating film, and functional article coated with coating film using the same |
| JP2004309628A (ja) * | 2003-04-03 | 2004-11-04 | Tokai Rika Co Ltd | 反射鏡 |
| WO2005103172A2 (en) * | 2004-04-15 | 2005-11-03 | Avery Dennison Corporation | Dew resistant coatings |
| US8425058B2 (en) * | 2006-11-14 | 2013-04-23 | Alpine Electronics, Inc. | Optical unit, image pickup device using the optical unit, and on-vehicle image display device using the image pickup device |
| EP2426781A4 (en) * | 2009-04-30 | 2013-05-22 | Bridgestone Corp | SEMICONDUCTOR ELECTRODE, SOLAR CELL WITH A SEMICONDUCTOR ELECTRODE AND METHOD FOR PRODUCING SEMICONDUCTOR ELECTRODE |
| JP5056919B2 (ja) | 2009-09-29 | 2012-10-24 | 株式会社デンソー | 車載光学センサカバー及び車載光学センサ装置 |
-
2013
- 2013-08-30 JP JP2013179284A patent/JP6214281B2/ja not_active Expired - Fee Related
-
2014
- 2014-05-01 US US14/914,897 patent/US9713984B2/en not_active Expired - Fee Related
- 2014-05-01 CN CN201480046419.9A patent/CN105474088A/zh active Pending
- 2014-05-01 KR KR1020167004837A patent/KR20160035042A/ko not_active Ceased
- 2014-05-01 EP EP14840521.0A patent/EP3040772A4/en not_active Withdrawn
- 2014-05-01 WO PCT/JP2014/062075 patent/WO2015029500A1/ja not_active Ceased
- 2014-05-21 TW TW103117777A patent/TWI580595B/zh active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001051108A (ja) * | 1999-08-09 | 2001-02-23 | Toto Ltd | 浴室用防曇鏡及び浴室設備並びに浴室 |
| JP2002002766A (ja) * | 2000-06-21 | 2002-01-09 | Toto Ltd | 防曇性を有する加熱容器用蓋 |
| JP2002080829A (ja) * | 2000-09-07 | 2002-03-22 | Toto Ltd | 親水性部材、その製造方法、およびその製造のためのコーティング剤 |
| JP2004123996A (ja) | 2002-10-07 | 2004-04-22 | Hitachi Ltd | 有機樹脂上に形成される親水膜,撥水膜 |
| JP2009265473A (ja) * | 2008-04-28 | 2009-11-12 | Konica Minolta Opto Inc | レンズ、撮像レンズ及び撮像装置 |
| JP2011240910A (ja) | 2009-09-29 | 2011-12-01 | Denso Corp | 車載光学センサカバー |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3040772A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| US9713984B2 (en) | 2017-07-25 |
| KR20160035042A (ko) | 2016-03-30 |
| JP6214281B2 (ja) | 2017-10-18 |
| CN105474088A (zh) | 2016-04-06 |
| EP3040772A1 (en) | 2016-07-06 |
| TW201509712A (zh) | 2015-03-16 |
| EP3040772A4 (en) | 2017-01-25 |
| US20160207460A1 (en) | 2016-07-21 |
| JP2015049281A (ja) | 2015-03-16 |
| TWI580595B (zh) | 2017-05-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6214281B2 (ja) | 車載用カメラ | |
| JP6794322B2 (ja) | ウインドシールドおよびその製造方法 | |
| JP6670397B2 (ja) | ウインドシールド、ウインドシールド用ガラス製品及び防曇部材 | |
| EP0882686B1 (en) | Nonfogging and stainproof glass articles | |
| TWI579341B (zh) | 低折射率膜形成用組成物及使用此之低折射率膜之形成方法 | |
| JP6598750B2 (ja) | 光学部材及び光学部材の製造方法 | |
| US20210199855A1 (en) | Optical member and method for manufacturing optical member | |
| JP5157143B2 (ja) | 反射防止膜付き基体 | |
| JP6909660B2 (ja) | ウインドシールド | |
| US20230057817A1 (en) | Transparent laminate | |
| JP7376239B2 (ja) | 親水性部材並びにこれを用いたレンズ、車載用カメラ、樹脂フィルム及び窓 | |
| CN108572404B (zh) | 光学构件、摄像设备和光学构件的制造方法 | |
| JP2018077260A (ja) | 親水レンズ | |
| TW201700615A (zh) | 低折射率膜形成用液體組成物 | |
| JPH0812375A (ja) | 撥水性物品及びその製造方法 | |
| JP2020032719A (ja) | 部材および部材の製造方法 | |
| JP7118614B2 (ja) | 光学部材、撮像装置、及び光学部材の製造方法 | |
| WO2017107185A1 (en) | Composition, method of making composition, and article | |
| JP2013105846A (ja) | 防曇性膜を有する光学部品、半導体装置 | |
| WO2017183700A1 (ja) | ウインドシールド | |
| WO2015029214A1 (ja) | 親水膜を有する光学部品 | |
| JP2001318423A (ja) | カメラ用保護カバー | |
| EP0883035A1 (en) | Carrier for electrophotography and developer using the carrier |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201480046419.9 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14840521 Country of ref document: EP Kind code of ref document: A1 |
|
| REEP | Request for entry into the european phase |
Ref document number: 2014840521 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2014840521 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 20167004837 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14914897 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |