WO2015016189A1 - 非水二次電池用電解液および非水二次電池 - Google Patents
非水二次電池用電解液および非水二次電池 Download PDFInfo
- Publication number
- WO2015016189A1 WO2015016189A1 PCT/JP2014/069852 JP2014069852W WO2015016189A1 WO 2015016189 A1 WO2015016189 A1 WO 2015016189A1 JP 2014069852 W JP2014069852 W JP 2014069852W WO 2015016189 A1 WO2015016189 A1 WO 2015016189A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- group
- secondary battery
- aqueous secondary
- phosphorus
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0567—Liquid materials characterised by the additives
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/52—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron
- H01M4/525—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron of mixed oxides or hydroxides containing iron, cobalt or nickel for inserting or intercalating light metals, e.g. LiNiO2, LiCoO2 or LiCoOxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0017—Non-aqueous electrolytes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to an electrolytic solution for non-aqueous secondary batteries and a non-aqueous secondary battery using the same.
- a lithium ion secondary battery By using a lithium ion secondary battery, a large energy density can be obtained in charge and discharge as compared with a lead battery or a nickel cadmium battery.
- Applications to portable electronic devices such as mobile phones and laptop computers are widely spread using this characteristic.
- development of a secondary battery which is particularly lightweight and capable of obtaining a high energy density is in progress.
- their size, weight reduction, long life and reliability are strongly demanded.
- high reliability is essential, and coexistence of battery performance and reliability is further strongly demanded.
- a combination of a carbonate-based solvent such as propylene carbonate or diethyl carbonate and an electrolyte salt such as lithium hexafluorophosphate is widely used as an electrolytic solution of a lithium ion secondary battery. These are because the conductivity is high and the potential is stable.
- the present invention has been made in view of such problems, and an object of the present invention is to provide an electrolyte solution for a non-aqueous secondary battery and a secondary battery that realizes favorable storage characteristics while improving the flame retardancy.
- a non-aqueous secondary comprising an electrolyte, a phosphorus and a compound having a nitrogen atom, or a phosphorus-containing compound comprising a phosphorus and a compound having a halogen atom, a polymer compound having a weight average molecular weight of 500 or more, and a non-aqueous solvent
- adjacent substituents may form a ring, and at least one nitrogen atom or halogen atom is included in X and R 11 ).
- a plurality of R 21 each independently represent a monovalent substituent.
- the adjacent R 21 may be such that the substituents form a ring.
- N represents an integer of 2 or more, and the bonding ends are bonded to each other To form a ring)
- the nonaqueous secondary battery according to any one of [1] to [8], wherein the polymer compound has an oxygen atom, a nitrogen atom, or a halogen atom in at least one of a side chain and a main chain. Electrolyte solution.
- a non-aqueous secondary battery comprising the electrolytic solution for a non-aqueous secondary battery according to any one of [1] to [10], a positive electrode, and a negative electrode.
- each substitution The groups, etc. may be the same or different.
- a plurality of substituents and the like are adjacent to each other, they may be bonded to each other or condensed to form a ring.
- the electrolyte solution for non-aqueous secondary batteries of the present invention exhibits high storage characteristics even at high temperatures, and exhibits excellent flame retardancy.
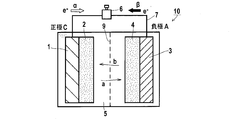
- FIG. 1 is a cross-sectional view schematically illustrating a mechanism of a lithium secondary battery according to a preferred embodiment of the present invention. It is sectional drawing which shows the specific structure of the lithium secondary battery which concerns on preferable embodiment of this invention. It is sectional drawing which shows the structure of CR2032 type coin battery typically.
- the electrolyte solution for non-aqueous secondary batteries of the present invention contains a specific phosphorus-containing compound and a specific amount of a polymer compound.
- the reason for exhibiting the above-mentioned excellent effect by this is presumed as follows.
- a polymer compound of a predetermined amount or more is added to the electrolyte solution for a secondary battery, it solidifies or has a high viscosity, which hinders the performance of the battery.
- a specific phosphorus-containing compound is added into the system together with the polymer compound, the viscosity can be lowered and it can be adapted to practical use.
- the performance relating to the capacity retention rate at high temperature storage and suppression of volume expansion of the battery is improved by selecting a particular phosphorus-containing compound.
- This is presumed to be the result of the action of the element contained in the phosphorus-containing compound, that the polarity of the molecule is not high, or the interaction with the polymer compound.
- the phosphorus-containing compound functions as a flame retardant and can impart flame retardancy to the electrolytic solution.
- the carbonized layer is formed on the surface of the electrolytic solution at the time of combustion due to the effect of applying the above-mentioned polymer compound, and the flame retardance is more effectively extracted.
- the phosphorus-containing compounds used in the present invention are compounds having phosphorus and nitrogen atoms in the molecule, or compounds having phosphorus and halogen atoms in the molecule.
- the phosphorus-containing compound may further have a carbon atom or an oxygen atom.
- the phosphorus-containing compound is preferably at least one of a phosphoric acid ester compound, a phosphoric acid amide compound, and a phosphazene compound.
- the said phosphorus containing compound is a phosphoric acid ester compound or a phosphoric acid amide compound
- the phosphoric acid ester compound and the phosphoric acid amide compound mean not only a compound having a phosphoric acid structure (derivative) but also a compound having a phosphonic acid structure (derivative) and a compound having a phosphinic acid structure (derivative) And a compound (derivative) having a phosphine oxide structure.
- X represents an oxygen atom, a sulfur atom, or N (R 12 ).
- R 12 represents a hydrogen atom or a monovalent substituent. Examples of the monovalent substituent include the following substituent T. Among them, R 12 is preferably an alkyl group having 1 to 12 carbon atoms, more preferably an alkyl group having 1 to 6 carbon atoms, and particularly preferably an alkyl group having 1 to 3 carbon atoms.
- R 11 represents a hydrogen atom or a monovalent substituent.
- the monovalent substituent include the following substituent T.
- R 11 adjacent substituents may form a ring.
- R 11 is a halogen atom (particularly preferably a fluorine atom), an alkyl group (preferably having 1 to 12 carbon atoms, more preferably 1 to 6 and particularly preferably 1 to 3), and an alkoxy group (1 to 6 carbon atoms) 12 is preferable, 1 to 6 is more preferable, 1 to 3 is particularly preferable, thioalkoxy group (having 1 to 12 carbon atoms is preferable, 1 to 6 is more preferable, 1 to 3 is particularly preferable), aryloxy group 6 to 22 carbon atoms are preferable, 6 to 14 carbon atoms are more preferable, arylthio group (preferably 6 to 22 carbon atoms, more preferably 6 to 14 carbon atoms), aralkyl group (preferably 7 to 23 carbon atoms, 7 to 15 carbon atoms More preferred),
- R 11 is an alkyl group, an alkoxy group, a thioalkoxy group, an aryl group, an aryloxy group, an arylthio group, an aralkyl group or an aralkyloxy group, it may particularly have a halogen atom (particularly a fluorine atom), It is preferable to have this.
- R 11 contains a halogen atom the number is preferably 1 to 20, more preferably 1 to 15, and still more preferably 1 to 12, in the molecule. It is particularly preferable that the number is 9 or more.
- R 11 contains a nitrogen atom, the number is preferably 1 to 6, more preferably 1 to 4 in the molecule.
- R 13 is a hydrogen atom or a monovalent substituent, and examples of the substituent T described later can be given.
- R 13 is preferably a hydrogen atom or an alkyl group having 1 to 12 carbon atoms, more preferably an alkyl group having 1 to 6 carbon atoms, and particularly preferably a hydrogen atom or an alkyl group having 1 to 3 carbon atoms.
- R 13 may be bonded to each other or condensed to form a ring. At this time, hetero atoms such as nitrogen atom, oxygen atom, and sulfur atom may be incorporated.
- the ring formed is preferably a 5- or 6-membered ring.
- the five-membered ring is preferably a compound containing a nitrogen-containing five-membered ring, such as pyrrole, imidazole, pyrazole, indazole, indole, benzimidazole, pyrrolidine, imidazolidine, pyrazolidine, indoline, carbazole, or derivatives thereof (all of them) N substitution) is mentioned.
- a nitrogen-containing five-membered ring such as pyrrole, imidazole, pyrazole, indazole, indole, benzimidazole, pyrrolidine, imidazolidine, pyrazolidine, indoline, carbazole, or derivatives thereof (all of them) N substitution
- 6-membered rings include piperidine, morpholine, piperazine, and derivatives thereof (all substituted with N).
- At least one nitrogen atom or halogen atom is contained in X and R 11 as a condition. In the present embodiment, this is an important factor, which brings about an excellent effect. For example, assuming that the X and R 11 do not contain a nitrogen atom nor a halogen atom, a trimethyl phosphoric acid, do not exhibit a sufficient effect in this (see below Comparative Example).
- Halogen content ( ⁇ F ) Number of halogen atoms / molecular weight
- the nitrogen content ( ⁇ N ) is preferably 0.05% or more and 6% or less, and more preferably 0.2% or more and 4% or less.
- the halogen content ( ⁇ F ) is preferably 0.2% or more and 10% or less, and more preferably 1% or more and 6% or less.
- the phosphorus-containing compound is preferably a phosphazene compound, and more preferably a compound having a partial structure represented by the following formula (2).
- each R 21 independently represents a monovalent substituent.
- substituents may form a ring.
- n represents an integer of 2 or more, and the bonding ends may bond to form a ring.
- substituents of R 21 include the examples of substituent T described later, and among them, a halogen atom (particularly preferably a fluorine atom) and an alkyl group (preferably having a carbon number of 1 to 12, preferably 1 to 6), -3 is particularly preferable, an alkoxy group (preferably having 1 to 12 carbon atoms, more preferably 1 to 6 and particularly preferably 1 to 3), and a thioalkoxy group (preferably having 1 to 12 carbon atoms and 1 to 6)
- 1 to 3 is particularly preferable, aryloxy group (preferably having 6 to 22 carbon atoms, more preferably 6 to 14), arylthio group (preferably having 6 to 22 carbon atoms, and more preferably 6 to 14), aralkyl Group (preferably 7 to
- the phosphorus-containing compound is more preferably a cyclic phosphazene compound represented by the following formula (2-1) or (2-2).
- R a1 to R a6 are each independently a monovalent group, and examples of the group of R 21 can be mentioned, and a halogen atom (preferably a fluorine atom), an alkyl group (preferably having 1 to 12 carbon atoms, preferably 1 to 6) 1 to 3 are particularly preferable, an alkoxy group (preferably having 1 to 12 carbon atoms, more preferably 1 to 6 and particularly preferably 1 to 3), an -NCO group, an amino group (having 0 to 12 carbon atoms) The carbon number is preferably 0 to 6, more preferably 0 to 3).
- R a1 to R a6 may be the same or different.
- at least four of R a1 to R a6 are fluorine atoms, and more preferably at least five are fluorine atoms.
- Preferred amino groups are also the same as the N (R 13) 2.
- R b1 to R b8 are each independently a monovalent group, and preferable ones are the same as the above R a1 to R a6 .
- at least six of R b1 to R b8 are fluorine atoms, and more preferably at least seven are fluorine atoms.
- Preferred compounds of the above phosphorus-containing compounds are exemplified below, but the present invention is not construed as being limited thereby.
- the concentration of the phosphorus-containing compound in the electrolyte for a non-aqueous secondary battery is not particularly limited, but is preferably 0.5% by mass or more, and 1% by mass or more based on the total amount including the electrolyte. Is more preferable, and particularly preferably 1.5% by mass or more.
- the upper limit side is preferably 50% by mass or less, more preferably 30% by mass or less, and particularly preferably 20% by mass or less.
- polyacrylonitrile As the above-mentioned polymer compound used in the present invention, polyacrylonitrile, poly (meth) acrylic acid ester (the alkyl group constituting the ester preferably has 1 to 12 carbon atoms, more preferably 1 to 6 and more preferably 1 to 3) Particularly preferred) polyvinylidene fluoride, polysiloxane (preferably, polydimethylsiloxane which may be terminally and / or side-chain modified (preferably aryl modified, aralkyl modified, polyalkylene glycol modified), polytetramethoxysilane) And polyalkylene glycols (preferably polyethylene glycol, polypropylene glycol) are preferably selected from the group consisting of Among these, polyacrylonitrile, poly (meth) acrylic acid ester, polyvinylidene fluoride and polysiloxane are preferable.
- the above-mentioned polymer compound has an oxygen atom, a nitrogen atom or a halogen atom in at least one of the side chain and the main chain.
- the side chain contains an oxygen atom
- the side chain is preferably an acyl group (preferably having 1 to 12 carbon atoms, more preferably 1 to 6 carbon atoms, and particularly preferably 1 to 3 carbon atoms), an acyloxy group (1 carbon atoms) -12 is preferable, 1 to 6 is more preferable, and 1 to 3 is particularly preferable, or an alkoxy group (having 1 to 12 carbon atoms is preferable, 1 to 6 is more preferable, and 1 to 3 is particularly preferable), polyalkylene glycol And groups containing a group (preferably containing 3 to 100 alkylene glycol units).
- Examples of the group containing a nitrogen atom in the side chain include a group containing a cyano group or an amino group (more preferably 0 to 6 carbon atoms, particularly preferably 0 to 3 carbon atoms).
- Examples of those having a halogen atom in the side chain include a halogen atom (particularly preferably a fluorine atom) or a halogenated alkyl group (preferably having a carbon number of 1 to 12, more preferably 1 to 6, and particularly preferably 1 to 3).
- Be Examples of the structure containing an oxygen atom in the main chain include-( RA- O)-.
- R A is an alkylene group having 1 to 12 carbon atoms (preferably 1 to 6 carbon atoms, more preferably 1 to 3 carbon atoms).
- R A is an alkylene group having 1 to 12 carbon atoms (preferably 1 to 6 carbon atoms, more preferably 1 to 3 carbon atoms).
- RN is a hydrogen atom or an alkyl group (preferably having 1 to 12 carbon atoms, more preferably 1 to 6 carbon atoms, and particularly preferably 1 to 3 carbon atoms).
- the concentration of the above-mentioned polymer compound in the non-aqueous secondary battery electrolyte is not particularly limited, but is regulated to 20% by mass or less based on the total amount including the electrolyte. Furthermore, it is preferable that it is 10 mass% or less, and it is especially preferable that it is 8 mass% or less.
- the lower limit is 0.1% by mass or more, and more preferably 1% by mass or more.
- the weight average molecular weight of the polymer compound is preferably 500 or more, more preferably 1000 or more, and particularly preferably 3000 or more.
- the upper limit is preferably less than 1,000,000, more preferably 100,000 or less, and particularly preferably 50,000 or less.
- the molecular weight of the low molecular weight compound applies to the commercially available compound, the molecular weight calculated from the chemical structure described in the catalog.
- the method of determining molecular weight by mass spectrometry is applied after column separation by LC-MS.
- the weight average molecular weight in terms of standard polystyrene is measured by gel permeation chromatography (GPC).
- the above-mentioned polymer compound is dissolved in the solution. This is a meaning to distinguish it from being present in the liquid as a capsule or the like, and the term “dissolution” is dispersed as particles or suspended within a range that does not impair the effects of the present invention. Means that it may be settled. The dissolved state of the polymer compound can be confirmed by visual observation that the presence of a significant solid content is not recognized.
- the electrolyte used for the electrolyte solution of this invention is a salt of the metal ion which belongs to periodic table 1 group or 2 group.
- the material is suitably selected by the use purpose of electrolyte solution.
- lithium salt, potassium salt, sodium salt, calcium salt, magnesium salt and the like can be mentioned, and when used for a secondary battery etc., lithium salt is preferable from the viewpoint of output.
- a lithium salt may be selected as the metal ion salt.
- the lithium salt is preferably a lithium salt generally used for the electrolyte of a non-aqueous electrolytic solution for lithium secondary batteries, and is not particularly limited. For example, those described below are preferable.
- Inorganic lithium salts inorganic fluoride salts such as LiPF 6 , LiBF 4 , LiAsF 6 , LiSbF 6 etc; perhalogenates such as LiClO 4 , LiBrO 4 , LiIO 4 etc .; inorganic chloride salts such as LiAlCl 4 etc.
- (L-2) fluorine-containing organic lithium salt perfluoroalkanesulfonic acid salt such as LiCF 3 SO 3 ; LiN (CF 3 SO 2 ) 2 , LiN (CF 3 CF 2 SO 2 ) 2 , LiN (FSO 2 ) 2
- Perfluoroalkanesulfonylimide salts such as LiN (CF 3 SO 2 ) (C 4 F 9 SO 2 );
- Perfluoroalkanesulfonyl methide salts such as LiC (CF 3 SO 2 ) 3 ; Li [PF 5 (CF 2) CF 2 CF 3)], Li [PF 4 (CF 2 CF 2 CF 3) 2], Li [PF 3 (CF 2 CF 2 CF 3) 3], Li [PF 5 (CF 2 CF 2 CF 2 CF 3 )], Li [PF 4 ( CF 2 CF 2 CF 3 )], Li [PF 4 ( CF 2 CF 2 CF 3 )], Li [PF 3 (CF 2 CF 2 CF
- (L-3) oxalato borate salt lithium bis (oxalato) borate, lithium difluoro oxalato borate and the like.
- LiPF 6 , LiBF 4 , LiAsF 6 , LiSbF 6 , LiClO 4 , Li (Rf 1 SO 3 ), LiN (Rf 1 SO 2 ) 2 , LiN (FSO 2 ) 2 , and LiN (Rf 1 SO 2 ) 2 ) (Rf 2 SO 2 ) is preferable, and lithium such as LiPF 6 , LiBF 4 , LiN (Rf 1 SO 2 ) 2 , LiN (FSO 2 ) 2 , and LiN (Rf 1 SO 2 ) (Rf 2 SO 2 ) Imide salts are more preferred.
- Rf 1 and Rf 2 each represent a perfluoroalkyl group.
- the electrolyte used for electrolyte solution may be used individually by 1 type, and may combine 2 or more types arbitrarily.
- the electrolyte (preferably, an ion of a metal belonging to Group 1 or Group 2 of the periodic table or a metal salt thereof) in the electrolytic solution is added in such an amount that the preferred salt concentration described in the preparation of the electrolytic solution below Is preferred.
- the salt concentration is appropriately selected depending on the purpose of use of the electrolytic solution, but generally it is 10% by mass to 50% by mass, and more preferably 15% by mass to 30% by mass in the total mass of the electrolytic solution.
- the molar concentration is preferably 0.5M to 1.5M.
- what is necessary is just to be calculated by salt conversion with the metal applied suitably, when evaluating as a density
- the non-aqueous solvent used in the present invention is preferably an aprotic organic solvent, and more preferably an aprotic organic solvent having 2 to 10 carbon atoms.
- the non-aqueous solvent is preferably a compound having an ether group, a carbonyl group, an ester group or a carbonate group.
- the above compound may have a substituent, and examples thereof include a substituent T described later.
- non-aqueous solvents examples include ethylene carbonate, propylene carbonate, butylene carbonate, dimethyl carbonate, diethyl carbonate, ethyl methyl carbonate, methyl propyl carbonate, ⁇ -butyrolactone, ⁇ -valerolactone, 1,2-dimethoxyethane, tetrahydrofuran, 2 -Methyltetrahydrofuran, tetrahydropyran, 1,3-dioxolane, 4-methyl-1,3-dioxolane, 1,3-dioxane, 1,4-dioxane, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate, butyric acid Methyl, methyl isobutyrate, methyl trimethylacetate, ethyl trimethylacetate, acetonitrile, glutaronitrile, adiponitrile, methoxyacetonitrile, 3-
- At least one member selected from the group consisting of ethylene carbonate, propylene carbonate, dimethyl carbonate, diethyl carbonate and ethyl methyl carbonate is preferred, and in particular, high viscosity (high dielectric constant) solvents such as ethylene carbonate or propylene carbonate (eg, ratio More preferred is a combination of a dielectric constant ⁇ ⁇ 30) and a low viscosity solvent (eg, viscosity ⁇ 1 mPa ⁇ s) such as dimethyl carbonate, ethyl methyl carbonate or diethyl carbonate.
- high viscosity solvents such as ethylene carbonate or propylene carbonate
- a low viscosity solvent eg, viscosity ⁇ 1 mPa ⁇ s
- the electrolytic solution of the present invention preferably contains various functional additives.
- Examples of the function developed by this additive include improvement in flame retardancy, improvement in cycle characteristics, and improvement in capacity characteristics. Below, the example of the functional additive which it is preferable to apply to this invention is shown.
- aromatic compounds include biphenyl compounds and alkyl-substituted benzene compounds.
- the biphenyl compound has a partial structure in which two benzene rings are linked by a single bond, and the benzene ring may have a substituent, and preferred substituents are alkyl groups having 1 to 4 carbon atoms (for example, Methyl, ethyl, propyl, t-butyl and the like), and an aryl group having 6 to 10 carbon atoms (eg, phenyl, naphthyl and the like).
- biphenyl compound examples include biphenyl, o-terphenyl, m-terphenyl, p-terphenyl, 4-methylbiphenyl, 4-ethylbiphenyl and 4-tert-butylbiphenyl.
- the alkyl-substituted benzene compound is preferably a benzene compound substituted with an alkyl group having 1 to 10 carbon atoms, and specifically, ethylbenzene, isopropylbenzene, cyclohexylbenzene, t-amylbenzene, t-butylbenzene, tethyrahydronaphthalene It can be mentioned.
- Halogen-containing compound (B) As a halogen atom which a halogen containing compound has, a fluorine atom, a chlorine atom, or a bromine atom is preferable, and a fluorine atom is more preferable.
- the number of halogen atoms is preferably 1 to 6, and more preferably 1 to 3.
- the halogen-containing compound is preferably a carbonate compound substituted with a fluorine atom, a polyether compound having a fluorine atom, or a fluorine-substituted aromatic compound.
- the halogen-substituted carbonate compound may be either linear or cyclic, but from the viewpoint of ion conductivity, cyclic carbonate compounds having high coordination ability with electrolyte salt (eg, lithium ion) are preferable, and 5-membered cyclic carbonate compounds are particularly preferable. preferable. Preferred specific examples of the halogen-substituted carbonate compound are shown below. Among these, compounds of Bex1 to Bex4 are particularly preferable, and Bex1 is particularly preferable.
- ⁇ Polymerizable compound (C)> Carbon as a polymerizable compound - preferably a compound having a carbon double bond, selected vinylene carbonate, carbonate compounds having a double bond such as vinyl ethylene carbonate, acrylate group, methacrylate group, cyanoacrylate group, from ArufaCF 3 acrylate groups Compounds having a group and compounds having a styryl group are preferable, and carbonate compounds having a double bond or compounds having two or more polymerizable groups in the molecule are more preferable.
- phosphorus containing compound (D) As a phosphorus containing compound, it is a thing other than the said specific phosphorus containing compound, and a phosphoric acid ester compound and a phosphazene compound are preferable. Preferred examples of the phosphoric acid ester compound include triphenyl phosphate and tribenzyl phosphate. As a phosphorus containing compound, the compound represented by following formula (D2) or (D3) is also preferable.
- R D4 to R D11 represent monovalent substituents.
- monovalent substituents preferred are halogen atoms such as alkyl group, aryl group, alkoxy group, aryloxy group, amino group, fluorine, chlorine, bromine and the like.
- At least one of the substituents of R D4 to R D11 is preferably a fluorine atom, and more preferably a substituent consisting of an alkoxy group, an amino group or a fluorine atom.
- the compound represented by following formula (E1) and (E2) is preferable.
- X 1 and X 2 each independently represent -O- or -C (Ra) (Rb)-.
- Ra and Rb each independently represent a hydrogen atom or a substituent.
- the substituent is preferably an alkyl group having 1 to 8 carbon atoms, a fluorine atom, or an aryl group having 6 to 14 carbon atoms.
- ⁇ represents an atomic group necessary to form a 5- to 6-membered ring.
- the skeleton of ⁇ may contain, in addition to carbon atoms, sulfur atoms, oxygen atoms, and the like.
- ⁇ may be substituted, and the substituent includes a substituent T, preferably an alkyl group, a fluorine atom or an aryl group.
- the silicon-containing compound is preferably a compound represented by the following formula (F1) or (F2).
- R F1 represents an alkyl group, an alkenyl group, an acyl group, an acyloxy group or an alkoxycarbonyl group.
- R F2 represents an alkyl group, an alkenyl group, an alkynyl group or an alkoxy group. A plurality of R F1 and R F2 in one formula may be different or the same.
- nitrile compound (G) As a nitrile compound, the compound represented by following formula (G) is preferable.
- R G1 to R G3 each independently represent a hydrogen atom, an alkyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, a cyano group, a carbamoyl group, a sulfonyl group, a halogen atom, or a phosphonyl group.
- Preferred examples of each substituent can be referred to the examples of substituent T.
- compounds in which any one or more of R G1 to R G3 have a plurality of nitrile groups containing a cyano group are preferable.
- Ng represents an integer of 1 to 8;
- Specific examples of the compound represented by the formula (G) include acetonitrile, propionitrile, isobutyronitrile, succinonitrile, malononitrile, glutaronitrile, adiponitrile, 2-methylglutanonitrile, hexanetricarbonitrile, propane Tetracarbonitrile and the like are preferable. Particularly preferred are succinonitrile, malononitrile, glutaronitrile, adiponitrile, 2-methylglutanonitrile, hexanetricarbonitrile, propanetetracarbonitrile.
- the metal complex compound is preferably a transition metal complex or a rare earth complex.
- complexes represented by any of the following formulas (H-1) to (H-3) are preferable.
- X H and Y H each represent a methyl group, n-butyl group, bis (trimethylsilyl) amino group, or a thioisocyanate group, and X H and Y H are condensed to form a cyclic alkenyl group (butadiene Metal-lacycle) may be formed.
- M H represents a transition element or a rare earth element. Specifically, M H is preferably Fe, Ru, Cr, V, Ta, Mo, Ti, Zr, Hf, Y, La, Ce, Sw, Nd, Lu, Er, Yb, Gd.
- m H and n H are integers that satisfy 0 ⁇ m H + n H ⁇ 3.
- n H + m H is preferably 1 or more. When n H and m H are 2 or more, two or more groups defined therein may be different from each other.
- the metal complex compound is also preferably a compound having a partial structure represented by the following formula (H-4).
- M H represents a transition element or a rare earth element and has the same meaning as in formulas (H-1) to (H-3).
- R 1H and R 2H are hydrogen, an alkyl group (preferably 1 to 6 carbon atoms), an alkenyl group (preferably 2 to 6 carbon atoms), an alkynyl group (preferably 2 to 6 carbon atoms), an aryl group (preferably carbon atoms) Represents 6 to 14), a heteroaryl group (preferably 3 to 6 carbon atoms), an alkylsilyl group (preferably 1 to 6 carbon atoms), or a halogen.
- R 1H and R 2H may be linked to each other.
- R 1H and R 2H may be linked together to form a ring.
- Preferred examples of R 1H and R 2H include examples of the substituent T described later.
- q H represents an integer of 1 to 4 and is preferably an integer of 2 to 4. More preferably, it is 2 or 4. When q H is 2 or more, a plurality of groups defined there may be the same or different.
- the metal complex compound is also preferably a compound represented by any of the following formulas.
- the central metal M h is particularly preferably Ti, Zr, ZrO, Hf, V, Cr, Fe, Ce, and most preferably Ti, Zr, Hf, V, Cr.
- R 3h , R 5h , R 7h to R 10h represent substituents.
- an alkyl group, an alkoxy group, an aryl group, an alkenyl group and a halogen atom are preferable, and an alkyl group having 1 to 6 carbon atoms, an alkoxy group having 1 to 6 carbon atoms, an aryl group having 6 to 12 carbon atoms, and 2 carbon atoms
- Alkenyl groups of -6 are more preferred, with methyl, ethyl, propyl, isopropyl, isobutyl, t-butyl, perfluoromethyl, methoxy, phenyl and ethenyl being preferred.
- R 33h , R 55h R 33h and R 55h each represent a hydrogen atom or a substituent of R 3h .
- Y h Y h is preferably an alkyl group having 1 to 6 carbon atoms or a bis (trialkylsilyl) amino group, and more preferably a methyl group or a bis (trimethylsilyl) amino group.
- L h , m h , o h l h, m h, o h represents an integer of 0-3, an integer of 0 to 2 is preferred.
- l h , m h and o h are 2 or more, the plurality of structural parts defined there may be the same as or different from each other.
- L h is preferably an alkylene group or an arylene group, more preferably a cycloalkylene group having 3 to 6 carbon atoms or an arylene group having 6 to 14 carbon atoms, and still more preferably cyclohexylene or phenylene.
- the imide compound is preferably a sulfoneimide compound having a perfluoro group from the viewpoint of oxidation resistance, and specific examples include a perfluorosulfoimide lithium compound. Specific examples of the imide compound include the following structures, and more preferred are Cex1 and Cex2.
- the electrolytic solution of the present invention may contain at least one selected from the negative electrode film-forming agent, a flame retardant, an overcharge inhibitor and the like, in addition to the above.
- the content ratio of these functional additives in the non-aqueous electrolyte is not particularly limited, but preferably 0.001% by mass to 10% by mass with respect to the whole non-aqueous electrolyte (including the electrolyte).
- the above exemplary compounds may have an optional substituent T.
- substituent T include the following.
- An alkyl group preferably an alkyl group having 1 to 20 carbon atoms, such as methyl, ethyl, isopropyl, t-butyl, pentyl, heptyl, 1-ethylpentyl, benzyl, 2-ethoxyethyl, 1-carboxymethyl and the like
- alkenyl A group preferably an alkenyl group having 2 to 20 carbon atoms, such as vinyl, allyl, oleyl etc.
- an alkynyl group preferably an alkynyl group having 2 to 20 carbon atoms such as ethynyl, butadiynyl, phenylethynyl etc
- a cycloalkyl group preferably a cycloalkyl group having 3 to 20 carbon atoms, such as cyclopropyl, cyclopentyl, cycl
- An acyl group preferably an acyl group having 1 to 20 carbon atoms, such as acetyl, propionyl, butyryl, benzoyl etc.
- an acyloxy group preferably an acyloxy group having 1 to 20 carbon atoms, such as acetyloxy, benzoyl Oxy and the like
- carbamoyl groups preferably having 1 to 20 carbon atoms
- Ruvamoyl group for example, N, N-dimethylcarbamoyl, N-phenylcarbamoyl and the like
- acylamino group preferably an acylamino group having 1 to 20 carbon atoms, for example, acetylamino, benzoylamino and the like
- sulfonamide group preferably A sulfamoyl group having 0 to 20 carbon atoms, such as methane sulfonamide, benzene sulfonamide, N-methyl methane sul
- the compound or the substituent / connecting group or the like contains an alkyl group, an alkylene group, an alkenyl group, an alkenylene group or the like, these may be cyclic or linear, or may be linear or branched and substituted as described above It may be substituted or unsubstituted.
- the aryl group, the heterocyclic group and the like may be monocyclic or fused ring, and may be substituted or unsubstituted as well.
- Each substituent defined in the present specification may be substituted via the following linking group L within the scope of achieving the effects of the present invention.
- the alkyl group / alkylene group, the alkenyl group / alkenylene group may further have the following hetero linking group in the structure.
- the linking group L a hydrocarbon linking group (an alkylene group having 1 to 10 carbon atoms (more preferably 1 to 6 carbon atoms, more preferably 1 to 3 carbon atoms) and an alkenylene group having 2 to 10 carbon atoms (more preferably carbon atoms) Number 2 to 6, more preferably 2 to 4), an arylene group having 6 to 22 carbon atoms (more preferably 6 to 10 carbon atoms)], hetero linking group [carbonyl group (-CO-), ether group (-O) -), A thioether group (-S-), an imino group (-NR N- )), or a linking group combining these is preferred.
- R N is defined as above, the number of atoms constituting the linking group is preferably from 1 to 36, 1 to more preferably from 24, more that 1 to 12 Preferably, it is 1 to 6, particularly preferably.
- the number of linking atoms in the linking group is preferably 10 or less, and more preferably 8 or less.
- the lower limit is 1 or more, preferably 2 or more.
- the non-aqueous electrolytic solution of the present invention can be prepared by dissolving each of the above components in the non-aqueous electrolytic solution solvent, including an example using a lithium salt as a metal ion salt, by a conventional method.
- non-water refers to substantially free of water, and may contain a trace amount of water as long as the effects of the invention are not impaired.
- concentration of water is preferably 200 ppm (mass basis) or less, more preferably 100 ppm or less, and still more preferably 20 ppm or less. There is no particular lower limit value, but it is practical that it is 1 ppm or more in consideration of unavoidable contamination.
- the viscosity of the electrolytic solution of the present invention is not particularly limited, but is preferably 1000 mPa ⁇ s or less at 25 ° C., more preferably 100 to 0.1 mPa ⁇ s, and 10 to 0.5 mPa ⁇ s. Is particularly preferred.
- Viscosity refers to the value measured by the following method.
- One mL of the sample is placed in a rheometer (CLS 500), and measured using Steel Cone 4 cm / 2 in diameter (both made by TA Instrumentsnts).
- CLS 500 rheometer
- Steel Cone 4 cm / 2 in diameter both made by TA Instrumentsnts.
- the sample is previously kept warm until the temperature becomes constant at the measurement start temperature, and the measurement is started thereafter.
- the measurement temperature is 25 ° C.
- the lithium ion secondary battery 10 of the present embodiment includes the electrolyte 5 for non-aqueous secondary batteries of the present invention and the positive electrode C capable of inserting and releasing lithium ions (positive electrode current collector 1, positive electrode active material layer 2) And a negative electrode A (negative electrode current collector 3, negative electrode active material layer 4) capable of insertion and release or dissolution and deposition of lithium ions.
- the separator 9 disposed between the positive electrode and the negative electrode, the collector terminal (not shown), the exterior case, etc. (Not shown) may be included.
- a protective element may be attached to the inside of the battery and / or the outside of the battery.
- Battery shape There is no restriction
- FIG. 2 is an example of a bottomed cylindrical lithium secondary battery 100.
- This battery is a bottomed cylindrical lithium secondary battery 100 in which the positive electrode sheet 14 and the negative electrode sheet 16 wound together through the separator 12 are wound and housed in the outer can 18.
- the ratio of the area T of the largest surface (the product of the width and height of the external dimension excluding the terminal portion, unit cm 2 ) and the thickness T (unit cm) of the battery outline The value of 2S / T is preferably 100 or more, and more preferably 200 or more.
- the lithium secondary battery of the present embodiment is configured to include the electrolyte solution 5, the electrode mixtures C and A of the positive electrode and the negative electrode, and the basic member 9 of the separator, as described with reference to FIG. Each of these members will be described below.
- Electrode mixture The electrode mixture is obtained by applying a dispersion of an active material, a conductive agent, a binder, a filler, and the like on a current collector (electrode base material), and in a lithium battery, the active material is a positive electrode active material It is preferable to use a negative electrode mixture in which the positive electrode mixture and the active material are negative electrode active materials. Next, each component etc. in the dispersion (composition for electrodes) which comprises an electrode mixture material are demonstrated.
- a transition metal oxide for the positive electrode active material it is preferable to use a transition metal oxide for the positive electrode active material, and among them, it has a transition element M a (one or more elements selected from Co, Ni, Fe, Mn, Cu, V). Is preferred.
- mixed elements M b (elements of Group 1 (Ia) other than lithium, elements of Group 2 (IIa), Al, Ga, In, Ge, Sn, Pb, Sb, Bi, Si) , P, B, etc.) may be mixed.
- Specific transition metal oxides including, for example, those represented by any of the following formulas (MA) to (MC) as the transition metal oxides, or V 2 O 5 , MnO 2 as the other transition metal oxides, etc. Can be mentioned.
- a particulate positive electrode active material may be used as the positive electrode active material. Specifically, a transition metal oxide capable of reversibly inserting and releasing lithium ions can be used, but it is preferable to use the above-mentioned specific transition metal
- the transition metal oxides, oxides containing the above transition element M a is preferably exemplified.
- a mixed element M b (preferably Al) may be mixed.
- the amount of mixing is preferably 0 to 30 mol% with respect to the amount of transition metal. It is more preferable to be synthesized by mixing so that the molar ratio of Li / M a is 0.3 to 2.2.
- Transition metal oxide represented by the formula (MA) (layered rock salt type structure)
- those represented by the following formula are preferable.
- M 1 has the same meaning as Ma above.
- a represents 0 to 1.2, preferably 0.1 to 1.15, and more preferably 0.6 to 1.1.
- b represents 1 to 3 and is preferably 2.
- a part of M 1 may be substituted by the above mixed element M b .
- the transition metal oxide represented by the above formula (MA) typically has a layered rock salt type structure.
- the transition metal oxide is more preferably one represented by the following formulae.
- g has the same meaning as a.
- j represents 0.1 to 0.9.
- i represents 0 to 1; However, 1-ji is 0 or more.
- k is the same as b above.
- Specific examples of the above transition metal compounds include LiCoO 2 (lithium cobaltate [LCO]), LiNi 2 O 2 (lithium nickelate), LiNi 0.85 Co 0.01 Al 0.05 O 2 (nickel cobalt aluminum acid) Lithium [NCA], LiNi 0.33 Co 0.33 Mn 0.33 O 2 (lithium nickel manganese cobaltate [NMC]), and LiNi 0.5 Mn 0.5 O 2 (lithium manganese nickelate).
- transition metal oxides represented by the formula (MA) partially overlap, but when the notation is changed and shown, those represented below are also mentioned as preferable examples.
- M 2 has the same meaning as Ma above.
- c represents 0 to 2, preferably 0.1 to 1.15, and more preferably 0.6 to 1.5.
- d represents 3 to 5 and is preferably 4.
- the transition metal oxide represented by formula (MB) is more preferably one represented by each of the following formulas.
- (MB-1) Li m Mn 2 O n
- (MB-2) Li m Mn p Al 2-p O n
- (MB-3) Li m Mn p Ni 2-p O n
- m is the same as c. n is synonymous with d. p represents 0 to 2; Specific examples of the transition metal compound are LiMn 2 O 4 and LiMn 1.5 Ni 0.5 O 4 .
- transition metal oxide represented by the formula (MB) those represented by the following may also be mentioned as preferable examples.
- the electrode containing Ni is more preferable from the viewpoint of high capacity and high output.
- e represents 0 to 2, preferably 0.1 to 1.15, and more preferably 0.5 to 1.5.
- f represents 1 to 5 and is preferably 0.5 to 2.
- M 3 represents one or more elements selected from V, Ti, Cr, Mn, Fe, Co, Ni, and Cu. M 3 may be substituted by other metals such as Ti, Cr, Zn, Zr, Nb, etc., in addition to the mixed element M b described above. Specific examples thereof include olivine-type iron phosphates such as LiFePO 4 and Li 3 Fe 2 (PO 4 ) 3 , iron pyrophosphates such as LiFeP 2 O 7 , cobalt phosphates such as LiCoPO 4 , Li 3 Examples include monoclinic Nasacon type vanadium phosphate salts such as V 2 (PO 4 ) 3 (lithium vanadium phosphate).
- the a, c, g, m and e values representing the composition of Li are values that change due to charge and discharge, and are typically evaluated by the values of stable states when containing Li.
- the composition of Li is shown as a specific value, but this also changes depending on the operation of the battery.
- an active material containing nickel and / or manganese can be used at a high potential, so that the capacity can be increased, and even when used at a high potential, it is particularly preferable because the capacity retention rate is high.
- the positive electrode active material a material that can maintain normal use at a positive electrode potential (Li / Li + reference) of 3.5 V or more, more preferably 3.8 V or more, and 4 V or more It is more preferably present, 4.25 V or more, and still more preferably 4.3 V or more. There is no particular upper limit, but 5 V or less is practical. By setting the above range, cycle characteristics and high rate discharge characteristics can be improved.
- being able to maintain normal use means that the electrode material does not deteriorate and become unusable even when charging is performed at that voltage, and this potential is also referred to as a normally usable potential.
- positive electrode potential (negative electrode potential) + (battery voltage).
- the negative electrode potential is 1.55 V.
- graphite is used as the negative electrode, the negative electrode potential is 0.1 V.
- the battery voltage is observed to calculate the positive electrode potential.
- the average particle size of the positive electrode active material used in the non-aqueous secondary battery of the present invention is not particularly limited, but is preferably 0.1 ⁇ m to 50 ⁇ m. No particular limitation is imposed on the specific surface area, preferably from 0.01m 2 / g ⁇ 50m 2 / g by BET method.
- the pH of the supernatant liquid when 5 g of the positive electrode active material is dissolved in 100 ml of distilled water is preferably 7 or more and 12 or less.
- a well-known pulverizer or classifier is used.
- a mortar, a ball mill, a vibration ball mill, a vibration mill, a satellite ball mill, a planetary ball mill, a swirl flow jet mill, a sieve or the like is used.
- the positive electrode active material obtained by the above-mentioned firing method may be used after washing with water, an acidic aqueous solution, an alkaline aqueous solution and an organic solvent.
- the compounding quantity of a positive electrode active material is not specifically limited, It is preferable that it is 60-98 mass% in 100 mass% of solid components in the dispersoid (mixture) for comprising an active material layer, and 70-95 mass. More preferably, it is%.
- the electrolyte solution for non-aqueous secondary batteries of the present invention in combination with a high potential positive electrode.
- a high potential positive electrode usually the battery characteristics tend to be greatly degraded.
- the non-aqueous electrolyte of the present invention is compounded by combining the above-mentioned specific phosphorus-containing compound and the polymer, so the reduction is suppressed. Good performance can be maintained.
- anode Active Material materials capable of reversibly inserting and releasing lithium ions are preferable, and there is no particular limitation, and carbonaceous materials, metal oxides such as tin oxide and silicon oxide, metal complex oxides, lithium Examples thereof include a simple substance, a lithium alloy such as a lithium aluminum alloy, and a metal capable of forming an alloy with lithium such as Sn or Si. One of these may be used alone, or two or more of these may be used in any combination and ratio. Among them, carbonaceous materials or lithium composite oxides are preferably used from the viewpoint of reliability.
- the metal composite oxide is preferably capable of absorbing and desorbing lithium, and preferably contains titanium and / or lithium as a component from the viewpoint of high current density charge / discharge characteristics.
- the carbonaceous material used as the negative electrode active material is a material substantially consisting of carbon.
- Examples thereof include petroleum pitch, natural graphite, artificial graphite such as vapor grown graphite, and carbonaceous materials obtained by firing various synthetic resins such as PAN-based resin and furfuryl alcohol resin.
- various carbon fibers such as PAN-based carbon fiber, cellulose-based carbon fiber, pitch-based carbon fiber, vapor-grown carbon fiber, dehydrated PVA-based carbon fiber, lignin carbon fiber, glassy carbon fiber, activated carbon fiber, mesophase fine Spheres, graphite whiskers, flat graphite and the like can also be mentioned.
- These carbonaceous materials can also be divided into non-graphitizable carbon materials and graphitic carbon materials according to the degree of graphitization. Further, it is preferable that the carbonaceous material have the spacing, density, and size of crystallite described in JP-A-62-22066, JP-A-2-6856 and JP-A-3-45473.
- the carbonaceous material does not have to be a single material, and it is preferable to use a mixture of natural graphite and artificial graphite described in JP-A-5-90844, or graphite having a coating layer described in JP-A-6-4516. You can also.
- the metal oxide and metal complex oxide which are the negative electrode active material used in the non-aqueous secondary battery of this invention contain these at least 1 sort (s).
- a metal oxide and metal complex oxide an amorphous oxide is preferable, and chalcogenide which is a reaction product of a metal element and an element of periodic table group 16 is also preferably used.
- amorphous is an X-ray diffraction method using CuK ⁇ radiation, and means one having a broad scattering band having an apex in a region of 20 ° to 40 ° in 2 ⁇ value, and a crystalline diffraction line May be included.
- the strongest intensity of the crystalline diffraction lines observed at 40 degrees or more and 70 degrees or less in 2 ⁇ value is 100 times or less of the diffraction line intensity at the top of the broad scattering band observed at 20 degrees or more and 40 degrees or less in 2 ⁇ values Is preferably, it is more preferably 5 times or less, and particularly preferably not having a crystalline diffraction line.
- amorphous oxides of semimetal elements and chalcogenides are more preferable, and elements of periodic table group 13 (IIIB) to group 15 (VB), Particularly preferred are oxides of one of Al, Ga, Si, Sn, Ge, Pb, Sb and Bi alone or a combination of two or more thereof, and chalcogenides.
- oxides of one of Al, Ga, Si, Sn, Ge, Pb, Sb and Bi alone or a combination of two or more thereof and chalcogenides.
- preferable amorphous oxides and chalcogenides include, for example, Ga 2 O 3 , SiO, GeO, SnO, SnO 2 , PbO, PbO 2 , Pb 2 O 3 , Pb 2 O 4 , Pb 3 O 4 , and the like.
- Sb 2 O 3 , Sb 2 O 4 , Sb 2 O 5 , Bi 2 O 3 , Bi 2 O 4 , SnSiO 3 , GeS, SnS, SnS 2 , PbS, PbS 2 , Sb 2 S 3 , Sb 2 S 5 , SnSiS 3 etc. are mentioned preferably. They may also be complex oxides with lithium oxide, such as Li 2 SnO 2 .
- the average particle size of the negative electrode active material used in the non-aqueous secondary battery of the present invention is preferably 0.1 ⁇ m to 60 ⁇ m.
- grinders and classifiers are used to achieve the desired particle size.
- a mortar, a ball mill, a sand mill, a vibrating ball mill, a satellite ball mill, a planetary ball mill, a swirl flow jet mill, a sieve or the like is suitably used.
- wet pulverization in the presence of water or an organic solvent such as methanol can also be carried out as necessary.
- the classification method is not particularly limited, and a sieve, an air classifier or the like can be used as required. Classification can be used both dry and wet.
- the chemical formula of the compound obtained by the above-mentioned firing method can be calculated from the mass difference of the powder before and after firing as a measurement method using inductively coupled plasma (ICP) emission spectroscopy and as a simple method.
- ICP inductively coupled plasma
- a negative electrode active material which can be used in combination with an amorphous oxide negative electrode active material mainly composed of Sn, Si and Ge, a carbon material capable of inserting and extracting lithium ion or lithium metal, lithium, Preferred examples include lithium alloys and metals that can be alloyed with lithium.
- the electrolyte of the present invention is preferably combined with a high potential negative electrode (preferably lithium titanium oxide, potential 1.55 V vs. Li metal), and a low potential negative electrode (preferably carbon material, silicon-containing material, potential about 0) It exhibits excellent properties in any of the combinations with (1 V vs. Li metal).
- Metal or metal oxide negative electrode preferably Si, Si oxide, Si / Si oxide, Sn, Sn oxide, Sn oxide, SnB x P y O z , Cu
- Metal or metal oxide negative electrode preferably Si, Si oxide, Si / Si oxide, Sn, Sn oxide, Sn oxide, SnB x P y O z , Cu
- It can also be preferably used in a battery using, as a negative electrode, / Sn and a plurality of composites thereof, and a composite of these metals or metal oxides and a carbon material.
- the electrically conductive material it is preferable to use an electron conductive material that does not cause a chemical change in the configured secondary battery, and any known electrically conductive material can be used.
- natural graphite scaling graphite, flake graphite, earthy graphite etc.
- artificial graphite carbon black, acetylene black, ketjen black, carbon fiber and metal powder (copper, nickel, aluminum, silver And the like), metal fibers or polyphenylene derivatives (described in JP-A-59-20,971), etc.
- metal fibers or polyphenylene derivatives described in JP-A-59-20,971), etc.
- the amount of the conductive agent added is preferably 11 to 50% by mass, and more preferably 2 to 30% by mass. In the case of carbon and graphite, 2 to 15% by mass is particularly preferable.
- Binder polysaccharide, thermoplastic resin, polymer having rubber elasticity, etc. may be mentioned. Among them, for example, starch, carboxymethylcellulose, cellulose, diacetylcellulose, methylcellulose, hydroxyethylcellulose, hydroxypropylcellulose Water solubility of sodium alginate, polyacrylic acid, sodium polyacrylate, polyvinylphenol, polyvinyl methyl ether, polyvinyl alcohol, polyvinyl pyrrolidone, polyacrylonitrile, polyacrylamide, polyhydroxy (meth) acrylate, styrene-maleic acid copolymer, etc.
- Polymer polyvinyl chloride, polytetrafluoroethylene, polyvinylidene fluoride, tetrafluoroethylene-hexafluoropropylene copolymer, vinyl (Polyamide, polypropylene, ethylene-propylene-diene-polymer (EPDM), sulfonated EPDM, polyvinyl acetal resin, methyl methacrylate, 2-ethylhexyl acrylate, etc.
- the binder may be used singly or in combination of two or more.
- the amount of addition of the binder is small, the holding power and the cohesion of the electrode mixture become weak.
- the amount is too large, the electrode volume increases and the capacity per electrode unit volume or unit mass decreases.
- the addition amount of the binder is preferably 1 to 30% by mass, and more preferably 2 to 10% by mass.
- the electrode mixture may contain a filler.
- a filler As the material for forming the filler, it is preferable to use a fibrous material which does not cause a chemical change in the secondary battery of the present invention. Usually, fibrous fillers made of materials such as olefin polymers such as polypropylene and polyethylene, glass and carbon are used. The amount of the filler added is not particularly limited, but it is preferably 0 to 30% by mass in the dispersion.
- the current collector of the positive and negative electrodes an electron conductor which does not cause a chemical change in the non-aqueous electrolyte secondary battery of the present invention is used.
- the current collector of the positive electrode in addition to aluminum, stainless steel, nickel, titanium etc., it is preferable to treat the surface of aluminum or stainless steel with carbon, nickel, titanium or silver, and among them, aluminum, aluminum alloy More preferable.
- a collector of a negative electrode aluminum, copper, stainless steel, nickel, and titanium are preferable, and aluminum, copper, and a copper alloy are more preferable.
- the shape of the current collector is usually a film sheet, but a net, a punch, a lath body, a porous body, a foam, a molded body of a fiber group, and the like can also be used.
- the thickness of the current collector is not particularly limited, but is preferably 1 ⁇ m to 500 ⁇ m. Further, it is also preferable to make the current collector surface uneven by surface treatment.
- An electrode mixture of a lithium secondary battery is formed by members appropriately selected from these materials.
- the separator used in the non-aqueous secondary battery of the present invention is made of a material having mechanical strength, ion permeability, and oxidation / reduction resistance at the contact surface of the positive electrode and the negative electrode, which electrically insulates the positive electrode and the negative electrode.
- a material having mechanical strength, ion permeability, and oxidation / reduction resistance at the contact surface of the positive electrode and the negative electrode, which electrically insulates the positive electrode and the negative electrode.
- porous polymer materials, inorganic materials, organic-inorganic hybrid materials, glass fibers, etc. are used.
- These separators preferably have a shutdown function for securing reliability, that is, have a function of closing a gap at 80 ° C. or more to increase resistance and interrupting current, and the closing temperature is 90 ° C. or more and 180 ° C. or less Is preferred.
- the shape of the pores of the separator is usually circular or elliptical, and the size is 0.05 ⁇ m to 30 ⁇ m, preferably 0.1 ⁇ m to 20 ⁇ m. Furthermore, it may be a rod-like or irregular-shaped hole as in the case of the drawing method or the phase separation method.
- the ratio of these gaps, ie, the porosity is 20% to 90%, preferably 35% to 80%.
- polymer material what used single materials, such as a cellulose nonwoven fabric, polyethylene, a polypropylene, and what used 2 or more types of composite materials may be used. What laminated two or more types of microporous films which changed a pore size, a porosity, the blockade temperature of a pore, etc. is preferable.
- oxides such as alumina and silicon dioxide, nitrides such as aluminum nitride and silicon nitride, and sulfates such as barium sulfate and calcium sulfate are used, and those having a particle shape or fiber shape are used.
- a thin film such as a non-woven fabric, a woven fabric and a microporous film is used.
- the thin film shape one having a pore diameter of 0.01 ⁇ m to 1 ⁇ m and a thickness of 5 ⁇ m to 50 ⁇ m is suitably used.
- a separator in which a composite porous layer containing particles of the above-mentioned inorganic substance is formed on the surface layer of a positive electrode and / or a negative electrode using a resin binder.
- alumina particles having a 90% particle diameter of less than 1 ⁇ m may be formed as porous layers on both sides of the positive electrode using a fluorine resin binder.
- any shape such as a sheet shape, a square shape, and a cylinder shape can be applied.
- a mixture of a positive electrode active material and a negative electrode active material is mainly used by being coated (coated), dried and compressed on a current collector.
- FIG. 2 shows an example of a bottomed cylindrical lithium secondary battery 100.
- This battery is a bottomed cylindrical lithium secondary battery 100 in which the positive electrode sheet 14 and the negative electrode sheet 16 wound together through the separator 12 are wound and housed in the outer can 18.
- 20 in the figure is an insulating plate
- 22 is a sealing plate
- 24 is a positive electrode current collector
- 26 is a gasket
- 28 is a pressure sensitive valve body
- 30 is a current interrupting element.
- hatching is changed in consideration of visibility, but each member corresponds to the whole view by reference numeral.
- a slurry or paste negative electrode mixture is prepared by mixing a negative electrode active material and a binder, filler, etc., which is optionally used, dissolved in an organic solvent.
- the obtained negative electrode mixture is uniformly applied over the entire surfaces of both surfaces of the metal core as a current collector, and then the organic solvent is removed to form a negative electrode mixture layer.
- the laminate of the current collector and the negative electrode mixture layer is rolled by a roll press machine or the like to a predetermined thickness, and a negative electrode sheet (electrode sheet) is obtained.
- the coating method of each agent, the drying of the coated material, and the method of forming the positive and negative electrodes may be carried out according to a standard method.
- a cylindrical battery is described as an example, but the present invention is not limited thereto.
- a separator After processing into a sheet battery as it is, or inserting it into a rectangular can after folding it into a rectangular can, electrically connecting the can and the sheet, an electrolyte is injected and the opening is sealed using a sealing plate. You may form a prismatic battery.
- the safety valve can be used as a sealing plate for sealing the opening.
- the sealing member may be equipped with various conventionally known safety elements.
- a fuse, a bimetal, a PTC element or the like is suitably used as the overcurrent protection element.
- a method of cutting the battery can, a method of cracking the gasket, a method of cracking the sealing plate, or a method of cutting with the lead plate can be used as a measure for raising the internal pressure of the battery can.
- the charger may be provided with a protective circuit incorporating an overcharge or overdischarge countermeasure, or may be connected independently.
- the can and the lead plate can be made of an electrically conductive metal or alloy.
- metals such as iron, nickel, titanium, chromium, molybdenum, copper, aluminum or alloys thereof are suitably used.
- the method of welding the cap, the can, the sheet, and the lead plate can use a known method (eg, direct current or alternating current electric welding, laser welding, ultrasonic welding).
- the sealing compound for sealing can use conventionally known compounds and mixtures, such as asphalt.
- a secondary battery called a lithium battery is a secondary battery (lithium ion secondary battery) that utilizes absorption and desorption of lithium for charge and discharge reactions, and a secondary battery (lithium metal secondary battery) that utilizes precipitation and dissolution of lithium are roughly divided into In the present invention, application as a lithium ion secondary battery is preferable.
- the non-aqueous secondary battery of the present invention is applicable to various applications because it can produce a secondary battery with good cycle performance.
- the application mode is not particularly limited, for example, when installed in an electronic device, a laptop computer, a pen input computer, a mobile computer, an e-book player, a mobile phone, a cordless handset, a pager, a handy terminal, a mobile fax, a mobile phone Copy, portable printer, headphone stereo, video movie, LCD TV, handy cleaner, portable CD, mini disc, electric shaver, transceiver, electronic organizer, calculator, memory card, portable tape recorder, radio, backup power supply, memory card etc
- Other consumer products include automobiles, electric vehicles, motors, lighting devices, toys, game machines, road conditioners, watches, strobes, cameras, medical devices (pace makers, hearing aids, shoulder machines, etc.). Furthermore, it can be used for various military and space applications. It can also be combined with a solar cell.
- Example 1 (Preparation of electrolyte) LiPF 6 was added to a solution in which ethylene carbonate (EC) and ethyl methyl carbonate (EMC) were mixed at a volume ratio of 1: 2 so as to be 1 mol / L, to prepare a reference electrolyte preparation liquid.
- EC ethylene carbonate
- EMC ethyl methyl carbonate
- Each of the polyacrylonitrile and the phosphorus-containing compound was added to this electrolytic solution preparation solution at the addition amount described in the table, and the electrolytic solution of the example was prepared by stirring.
- the comparative example without an additive used the said electrolyte solution preparation liquid as it was as electrolyte solution.
- an electrolyte combustion test and a cell characteristic test described later were conducted.
- the viscosity at 25 ° C. of the prepared electrolytic solution of the present invention was all 100 mPa ⁇ s or less, and the water content measured by Karl Fischer method (JIS K 0113) was 20 pp
- Active material active material: lithium nickel manganese cobaltate (LiNi 1/3 Mn 1/3 Co 1/3 O 2 ) [NMC] 85 mass%, conductive additive: carbon black 7.5 mass%, binder: PVDF 7.
- the negative electrode was prepared with 5% by mass of active material: 85% by mass of graphite, 7.5% by mass of conductive additive: carbon black, and 7.5% by mass of binder: PVDF.
- the separator is a polypropylene porous membrane 24 ⁇ m thick.
- the electrolyte flame resistance evaluation and 2032 type coin battery (diameter: 20 mm, height: 3.2 mm, top cover, bottom cover: made of SUS) for each test electrolyte A thickness of 250 ⁇ m, see FIG. 3) was prepared, and the following battery characteristic items were evaluated.
- the volume was measured.
- the ratio of the discharge capacity after this one month to the initial discharge capacity was taken as the capacity retention rate (%).
- the ratio of the increase in battery volume after the test to the volume at the time of battery preparation was defined as the volume increase during the charge life test. This volume increase is 0% or more and less than 1% A 1% or more to less than 3% B 3% or more C Evaluated as. It is preferable that the volume does not increase during normal charge storage.
- this volume increase means the decomposition of the components of the electrolytic solution and the accompanying gasification, and the smaller the increase, the more preferable the deterioration is suppressed.
- confirmation of the change of the performance by high temperature storage can be evaluated as performance as it is in a high temperature environment as it is, and can also be evaluated from the viewpoint of durability at normal temperature as a deterioration acceleration test.
- Test No. Tests with numbers starting with: c are comparative examples.
- TMP trimethyl phosphate
- TEP triethyl phosphate
- PAN polyacrylonitrile
- 1 It represents the result of the flame retardancy evaluation of the electrolytic solution.
- * 2 Weight average molecular weight
- Example 2 Preparation of electrolyte
- An electrolyte was prepared in the same manner as in Example 1 except that the polymer and the phosphorus-containing compound described in Table 2 were used, and each performance evaluation test of the secondary battery was performed.
- the same 2032 type coin battery as that of Example 1 was manufactured and used as a secondary battery in its material and structure. The results are as follows.
- Example 3 A secondary battery for test was produced in the same manner as in Example 1 except that the positive electrode active material was changed from NMC to LiCoO 2 (LCO).
- the flame retardancy evaluation and the high temperature storage characteristics were tested in the same manner as in Example 1 using this, the following results were obtained. From this result, it is understood that the phosphorus-containing compound of the present invention produces a significant difference particularly when combined with a high potential positive electrode material (NMC or the like), and a remarkable effect is exhibited.
- evaluation results are shown by an index (relative value) when the performance of c31 or c41 is 100 except for flame retardancy.
- Example 4 A sample of the electrolytic solution was prepared in the same manner as in the above test 101 except that the phosphorus-containing compound was changed from C1-1 to C1-14, C1-19, C2-11, and C2-17. The flame retardancy and high temperature storage characteristics (capacity retention rate, volume increase) were evaluated for each electrolyte solution. As a result, all showed the favorable result of test 101 and a level substantially the same.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Secondary Cells (AREA)
- Battery Electrode And Active Subsutance (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013157190A JP2015026589A (ja) | 2013-07-29 | 2013-07-29 | 非水二次電池用電解液および非水二次電池 |
| JP2013-157190 | 2013-07-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015016189A1 true WO2015016189A1 (ja) | 2015-02-05 |
Family
ID=52431722
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/069852 Ceased WO2015016189A1 (ja) | 2013-07-29 | 2014-07-28 | 非水二次電池用電解液および非水二次電池 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP2015026589A (enExample) |
| WO (1) | WO2015016189A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111864196A (zh) * | 2019-04-30 | 2020-10-30 | Sk新技术株式会社 | 锂二次电池 |
| JP2022116941A (ja) * | 2021-01-29 | 2022-08-10 | トヨタ自動車株式会社 | リチウムイオン二次電池用非水系電解液およびリチウムイオン二次電池 |
| WO2023193230A1 (zh) * | 2022-04-08 | 2023-10-12 | 宁德时代新能源科技股份有限公司 | 电解液、二次电池、电池模块、电池包和用电装置 |
| WO2024095972A1 (ja) * | 2022-10-31 | 2024-05-10 | 富士フイルム株式会社 | 非水電解液二次電池用電解液、非水電解液二次電池、及び非水電解液二次電池の製造方法 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102015106811B4 (de) * | 2015-04-30 | 2022-02-03 | VON ARDENNE Asset GmbH & Co. KG | Verwendung einer Folienstruktur in einem Energiespeicher und Energiespeicher |
| DE102015218653A1 (de) * | 2015-09-28 | 2017-03-30 | Wacker Chemie Ag | Cyclische Phosphonamide als Elektrolytbestandteil für Lithium-Ionen-Batterien |
| KR20180129492A (ko) | 2017-05-26 | 2018-12-05 | 삼성전자주식회사 | 포스파이트계 첨가제를 포함하는 리튬이차전지 |
| JP7375511B2 (ja) * | 2019-12-02 | 2023-11-08 | 株式会社Gsユアサ | 非水電解質、非水電解質蓄電素子及びその製造方法 |
| US20230109546A1 (en) * | 2020-02-28 | 2023-04-06 | Zeon Corporation | Electrolyte solution for electrochemical devices, plastic composition, use and production method |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS61133582A (ja) * | 1984-12-04 | 1986-06-20 | Hitachi Ltd | 二次電池 |
| JPH09293534A (ja) * | 1996-04-26 | 1997-11-11 | Nippon Telegr & Teleph Corp <Ntt> | リチウム電池用電解液組成物 |
| JPH1021958A (ja) * | 1996-07-04 | 1998-01-23 | Shin Kobe Electric Mach Co Ltd | リチウムイオン二次電池 |
| JPH11185803A (ja) * | 1997-12-22 | 1999-07-09 | Sony Corp | 非水電解液二次電池及びその製造方法 |
| JP2000164247A (ja) * | 1998-11-24 | 2000-06-16 | Mitsui Chemicals Inc | 非水電解液およびそれを用いた二次電池 |
| JP2002252030A (ja) * | 2001-02-22 | 2002-09-06 | Fuji Photo Film Co Ltd | 電解質組成物及びその製造方法、非水電解質二次電池 |
| JP2002359000A (ja) * | 2001-03-28 | 2002-12-13 | Toshiba Corp | 非水電解液および非水電解液二次電池 |
| JP2007080651A (ja) * | 2005-09-14 | 2007-03-29 | Bridgestone Corp | ポリマーゲル電解質及びそれを用いたポリマー電池 |
| JP2012089468A (ja) * | 2010-09-22 | 2012-05-10 | Fujifilm Corp | 非水二次電池用電解液及びリチウム二次電池 |
| JP2013033663A (ja) * | 2011-08-02 | 2013-02-14 | Mitsubishi Chemicals Corp | 非水系電解液及びそれを用いた非水系電解液電池 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009158460A (ja) * | 2007-12-05 | 2009-07-16 | Sony Corp | 非水電解液二次電池 |
| US9123973B2 (en) * | 2010-12-22 | 2015-09-01 | Samsung Sdi Co., Ltd. | Electrolyte for lithium secondary battery and lithium secondary battery comprising the same |
| JP5780481B2 (ja) * | 2011-01-24 | 2015-09-16 | 積水化学工業株式会社 | 電解質、電解質膜、リチウムイオン二次電池及びホスファゼン化合物 |
| JP5777982B2 (ja) * | 2011-09-02 | 2015-09-16 | 株式会社Nttファシリティーズ | 非水電解液電池 |
| JP5801765B2 (ja) * | 2012-06-20 | 2015-10-28 | 富士フイルム株式会社 | 非水二次電池用電解液および非水電解液二次電池 |
| JP2014078434A (ja) * | 2012-10-11 | 2014-05-01 | Fujifilm Corp | 非水電解液二次電池 |
-
2013
- 2013-07-29 JP JP2013157190A patent/JP2015026589A/ja not_active Abandoned
-
2014
- 2014-07-28 WO PCT/JP2014/069852 patent/WO2015016189A1/ja not_active Ceased
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS61133582A (ja) * | 1984-12-04 | 1986-06-20 | Hitachi Ltd | 二次電池 |
| JPH09293534A (ja) * | 1996-04-26 | 1997-11-11 | Nippon Telegr & Teleph Corp <Ntt> | リチウム電池用電解液組成物 |
| JPH1021958A (ja) * | 1996-07-04 | 1998-01-23 | Shin Kobe Electric Mach Co Ltd | リチウムイオン二次電池 |
| JPH11185803A (ja) * | 1997-12-22 | 1999-07-09 | Sony Corp | 非水電解液二次電池及びその製造方法 |
| JP2000164247A (ja) * | 1998-11-24 | 2000-06-16 | Mitsui Chemicals Inc | 非水電解液およびそれを用いた二次電池 |
| JP2002252030A (ja) * | 2001-02-22 | 2002-09-06 | Fuji Photo Film Co Ltd | 電解質組成物及びその製造方法、非水電解質二次電池 |
| JP2002359000A (ja) * | 2001-03-28 | 2002-12-13 | Toshiba Corp | 非水電解液および非水電解液二次電池 |
| JP2007080651A (ja) * | 2005-09-14 | 2007-03-29 | Bridgestone Corp | ポリマーゲル電解質及びそれを用いたポリマー電池 |
| JP2012089468A (ja) * | 2010-09-22 | 2012-05-10 | Fujifilm Corp | 非水二次電池用電解液及びリチウム二次電池 |
| JP2013033663A (ja) * | 2011-08-02 | 2013-02-14 | Mitsubishi Chemicals Corp | 非水系電解液及びそれを用いた非水系電解液電池 |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111864196A (zh) * | 2019-04-30 | 2020-10-30 | Sk新技术株式会社 | 锂二次电池 |
| JP2022116941A (ja) * | 2021-01-29 | 2022-08-10 | トヨタ自動車株式会社 | リチウムイオン二次電池用非水系電解液およびリチウムイオン二次電池 |
| JP7678675B2 (ja) | 2021-01-29 | 2025-05-16 | トヨタ自動車株式会社 | リチウムイオン二次電池用非水系電解液およびリチウムイオン二次電池 |
| WO2023193230A1 (zh) * | 2022-04-08 | 2023-10-12 | 宁德时代新能源科技股份有限公司 | 电解液、二次电池、电池模块、电池包和用电装置 |
| CN117223141A (zh) * | 2022-04-08 | 2023-12-12 | 宁德时代新能源科技股份有限公司 | 电解液、二次电池、电池模块、电池包和用电装置 |
| EP4283745A4 (en) * | 2022-04-08 | 2024-05-01 | Contemporary Amperex Technology Co., Limited | ELECTROLYTE SOLUTION, SECONDARY BATTERY, BATTERY MODULE, BATTERY PACK AND ELECTRICAL DEVICE |
| WO2024095972A1 (ja) * | 2022-10-31 | 2024-05-10 | 富士フイルム株式会社 | 非水電解液二次電池用電解液、非水電解液二次電池、及び非水電解液二次電池の製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2015026589A (ja) | 2015-02-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6376709B2 (ja) | 非水二次電池用電解液および非水二次電池、これらに用いられる添加剤 | |
| JP6071600B2 (ja) | 非水二次電池用電解液および非水二次電池、電解液用添加剤 | |
| JP2014127354A (ja) | 非水二次電池用電解液および非水二次電池、電解液用添加剤 | |
| JP6154145B2 (ja) | 非水二次電池用電解液および非水二次電池 | |
| JP6047458B2 (ja) | 非水二次電池 | |
| JP6485956B2 (ja) | 非水電解液および非水二次電池 | |
| WO2015016189A1 (ja) | 非水二次電池用電解液および非水二次電池 | |
| JP6238411B2 (ja) | 非水二次電池用電解液および非水二次電池 | |
| WO2015016187A1 (ja) | 非水二次電池用電解液および非水二次電池 | |
| CN105393397B (zh) | 非水电解液及非水二次电池 | |
| JP6588965B2 (ja) | 電解液および非水二次電池 | |
| JP2014235986A (ja) | 非水二次電池用電解液および非水二次電池 | |
| JP5886160B2 (ja) | 非水二次電池用電解液、非水二次電池電解液用キット及び非水電解液二次電池 | |
| JP2016162523A (ja) | 非水二次電池用電解液および非水二次電池 | |
| JPWO2018135395A1 (ja) | 非水二次電池用電解液、非水二次電池及び金属錯体 | |
| WO2014157533A1 (ja) | 非水二次電池および非水二次電池用電解液 | |
| JPWO2018016519A1 (ja) | 非水二次電池用電解液および非水二次電池 | |
| JP2014063668A (ja) | 非水二次電池用電解液及び二次電池 | |
| JP6391028B2 (ja) | 電解液、リチウムイオン電池およびリチウムイオンキャパシタ | |
| JP6583915B2 (ja) | 非水電解液および非水二次電池 | |
| JPWO2018016444A1 (ja) | 非水二次電池用電解液および非水二次電池 | |
| JP2014220053A (ja) | 非水二次電池および非水二次電池用電解液 | |
| JP2015026587A (ja) | 非水二次電池用電解液、非水二次電池電解液用添加剤及び非水二次電池 | |
| WO2014156428A1 (ja) | 非水二次電池用電解液及び非水二次電池 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14831166 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14831166 Country of ref document: EP Kind code of ref document: A1 |