WO2014104188A1 - 電気化学解析装置および電気化学システム - Google Patents
電気化学解析装置および電気化学システム Download PDFInfo
- Publication number
- WO2014104188A1 WO2014104188A1 PCT/JP2013/084877 JP2013084877W WO2014104188A1 WO 2014104188 A1 WO2014104188 A1 WO 2014104188A1 JP 2013084877 W JP2013084877 W JP 2013084877W WO 2014104188 A1 WO2014104188 A1 WO 2014104188A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- frequency
- electrochemical
- rectangular wave
- electrochemical cell
- analysis apparatus
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01R—MEASURING ELECTRIC VARIABLES; MEASURING MAGNETIC VARIABLES
- G01R23/00—Arrangements for measuring frequencies; Arrangements for analysing frequency spectra
- G01R23/16—Spectrum analysis; Fourier analysis
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01R—MEASURING ELECTRIC VARIABLES; MEASURING MAGNETIC VARIABLES
- G01R31/00—Arrangements for testing electric properties; Arrangements for locating electric faults; Arrangements for electrical testing characterised by what is being tested not provided for elsewhere
- G01R31/36—Arrangements for testing, measuring or monitoring the electrical condition of accumulators or electric batteries, e.g. capacity or state of charge [SoC]
- G01R31/389—Measuring internal impedance, internal conductance or related variables
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/28—Electrolytic cell components
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/416—Systems
- G01N27/4161—Systems measuring the voltage and using a constant current supply, e.g. chronopotentiometry
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/42—Methods or arrangements for servicing or maintenance of secondary cells or secondary half-cells
- H01M10/48—Accumulators combined with arrangements for measuring, testing or indicating the condition of cells, e.g. the level or density of the electrolyte
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- Embodiments of the present invention relate to an electrochemical analysis device for measuring characteristics of an electrochemical cell including a plurality of electrodes and an electrolyte, and an electrochemical system including the electrochemical analysis device.
- the impedance measurement of an electrochemical cell including a plurality of electrodes and an electrolyte is widely used for elucidating the mechanism of the electrochemical reaction.
- an impedance measurement method an AC impedance method is known in which the frequency of a sinusoidal signal to be applied to an electrochemical cell to be measured is scanned.
- a frequency characteristic analyzer (FRA: Frequency Response Analyzer) and a potentiostat are used.
- the FRA outputs a frequency response signal for applying a sine wave signal having a predetermined frequency to the electrochemical cell.
- the potentiostat controls the voltage (current) applied to the electrochemical cell based on the frequency signal from the FRA.
- impedances at a plurality of frequencies that is, frequency characteristics of the impedance are acquired.
- the impedance trajectory represented by a complex plane with the frequency characteristics of impedance as the resistance component on the Z ′ (real impedance) axis and the reactance component (usually capacitive) on the Z ′′ (imaginary impedance) axis is the Nyquist plot (call Cole plot).
- the Nyquist plot shown in FIG. 1 shows an interface resistance R int composed of an electrolyte resistance R s , a charge transfer resistance and a film resistance, an associated capacitance C such as an electric double layer, and a charge carrier diffusion Z w .
- a simple electrochemical reaction in an electrochemical cell using a reference electrode is composed of ion movement in the electrolyte, charge transfer reaction at the electrode interface, and accompanying ion diffusion.
- the semicircular locus is a locus in which at least two semicircles overlap.
- the electrochemical cell is a lithium ion secondary battery
- the lithium ion electrolyte component and the organic solvent in the electrolyte are decomposed due to deterioration of the active material itself, such as a change in crystal structure, and the decomposition of the negative electrode and the positive electrode as electrolyte decomposition products. It is presumed that the resistance increases because it deposits on the surface in the form of organic or inorganic substances and the insertion / extraction of lithium ions is inhibited.
- renewable energy such as solar power generation or wind power generation
- a large-scale power storage system is indispensable for stable power supply using renewable energy.
- the large-scale power storage system has a large capacity secondary battery as its main component.
- Large capacity secondary batteries have very low internal resistance.
- a very expensive large-capacity potentiostat is required.
- the internal resistance of the secondary battery is 10 m ⁇
- a potentiostat signal current of 300 A is required to control the voltage to 3 V
- the internal resistance is 1 m ⁇
- the signal current of 3000 A is required. Also, voltage control is not easy.
- Japanese Patent Application Laid-Open No. 2003-090869 discloses a measuring apparatus that obtains impedances at a plurality of frequencies by applying a signal in which a sine wave having a plurality of frequencies is superimposed to a battery and Fourier-transforming a response signal. It is disclosed.
- Japanese Patent Application Laid-Open No. 2012-185167 discloses that in a power storage device having a plurality of batteries, impedance is measured by applying a pseudo sine wave signal from one battery to another battery.
- an embodiment of the present invention aims to provide an electrochemical analysis device having a simple configuration capable of acquiring impedance characteristics of an electrochemical cell, and an electrochemical system including the electrochemical analysis device. .
- An electrochemical analysis apparatus generates a rectangular wave signal having a first frequency and applies it to an electrochemical cell including a plurality of electrodes and an electrolyte, and the electrochemical for the rectangular wave signal.
- a Fourier transform unit that performs a Fourier transform on the response signal of the cell and calculates a frequency characteristic including a second frequency component that is an integer multiple of the first frequency, and a frequency characteristic calculated by the Fourier transform unit.
- An electrochemical system includes an electrochemical cell including a plurality of electrodes and an electrolyte, a power controller that generates a rectangular wave signal having a first frequency and applies the square wave signal to the electrochemical cell, A Fourier transform unit that Fourier-transforms a response signal of the electrochemical cell with respect to a rectangular wave signal to calculate a frequency characteristic including a second frequency component that is an integer multiple of the first frequency, and the Fourier transform unit calculates A calculation unit that calculates an impedance characteristic of the electrochemical cell based on the frequency characteristic.
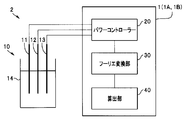
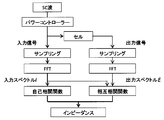
- the electrochemical system 2 of the first embodiment includes an electrochemical cell 10 and an electrochemical analysis device 1.
- the electrochemical analysis device 1 includes a power controller 20 that generates a signal to be applied to the electrochemical cell 10, a Fourier transform unit 30, and a calculation unit 40.
- the electrochemical system 2 is a system for analyzing a redox reaction of hexacyanoiron.
- the electrochemical cell 10 includes a working electrode (WE) 11 made of glassy carbon, a counter electrode (CE) 12 made of platinum wire, a reference electrode (RE) 13 made of silver / silver chloride and 3M-NaCl, and an electrolyte 14. And including.
- the electrolyte 14 is an aqueous solution composed of 5 mM K 4 [Fe (CN) 6 ], 5 mM K 3 [Fe (CN) 6 ], and 0.5 M KNO 3 .
- the power controller 20 generates a rectangular wave signal having a voltage of the first frequency f1 based on the reference electrode (RE) 13 and applies it to the working electrode (WE) 11 and the counter electrode (CE) 12 of the electrochemical cell 10. .
- a signal based on current may be used.
- a rectangular wave signal is generated based on the counter electrode (CE) without using the reference electrode (RE) 13 and applied to the working electrode (WE) 11 and the counter electrode (CE) 12 of the electrochemical cell 10.
- the power controller 20 may apply a rectangular wave of current.
- the power controller 20 that outputs a simple rectangular wave signal may have a configuration in which a DC power supply is combined with an ON / OFF switch that operates at a predetermined cycle.
- the Fourier transform unit 30 performs a Fourier transform on the response signal of the electrochemical cell 10 to the rectangular wave signal applied by the power controller 20, and a second frequency (3f1, 5f1, 7f1,...) That is an odd multiple of the first frequency (f1). ) Component for calculating frequency characteristics (input signal and output signal spectrum).
- the calculation unit 40 is an arithmetic circuit that calculates impedance characteristics including impedances and phase differences at a plurality of frequencies of the electrochemical cell 10 based on the input / output spectrum calculated by the Fourier transform unit 30.
- the Fourier transform unit 30 and the calculation unit 40 may be an integrated circuit, for example, a central processing circuit (CPU: Central Processing Unit) that also controls the entire electrochemical system 2.
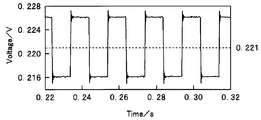
- the midpoint potential is 0.221 V vs. the potential (immersion potential) between the working electrode (WE) 11 and the reference electrode (RE) 13 when no signal is applied.
- the voltage amplitude is 10 mV.
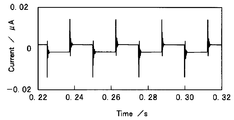
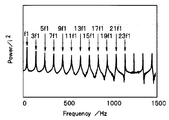
- 4 and 5 show the response signal of the electrochemical cell 10 to the rectangular wave signal.
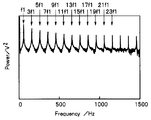
- 6 and 7 show the input / output spectrum (power spectrum notation) calculated by the Fourier transform unit 30 from the response signal.
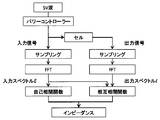
- FIG. 8A is a flowchart for calculating impedance (Z ′, Z ′′) in frequency of the calculation unit 40 when a current rectangular wave is used.
- the generated rectangular wave signal is applied to an electrochemical cell using a power controller.
- the input signal current and the output signal voltage are sampled, and the obtained data is subjected to Fourier transform to obtain the input spectrum I and the output spectrum E.
- the cross-correlation function / The impedance (Z ′, Z ′′) at each frequency is calculated by the autocorrelation function.
- FIG. 8B is a flowchart for calculating the impedance (Z ′, Z ′′) at the frequency of the calculation unit 40 when the voltage rectangular wave is used.
- the generated rectangular wave signal is applied to the electrochemical cell using the power controller.
- the input signal voltage and the output signal current are sampled, and the obtained data is subjected to Fourier transform to obtain an input spectrum E and an output spectrum I. Further, from the cross-correlation function and autocorrelation function of the spectrum, the autocorrelation function /
- the impedance (Z ′, Z ′′) at each frequency is calculated by the cross correlation function.
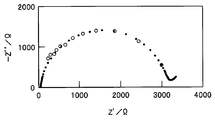
- FIG. 9 is a Nyquist plot of the electrochemical cell 10.
- the black circles indicate the impedance obtained by the normal AC impedance method.
- the frequency of a sine wave (voltage amplitude: 10 mV) was scanned from 100 kHz to 1 Hz using a frequency characteristic analyzer and a potentiostat.
- the electrochemical system 2 can acquire the impedance characteristics of the electrochemical cell 10 similar to the AC impedance method, while having a simple configuration without a frequency characteristic analyzer and a potentiostat. By analyzing the acquired impedance characteristics using an equivalent circuit model, it is possible to grasp the characteristics and the like of each component such as an electrode and an electrolyte constituting the electrochemical cell 10.
- the intensity of the second frequency multiplied by n in the input / output spectrum calculated by the Fourier transform unit 30 is 1 / n of the intensity of the first frequency. For this reason, it is difficult to acquire a high frequency component in a system with many noise components. For example, in the power spectrum shown in FIG. 7, the frequency component higher than the frequency 23f1 cannot be used for the analysis using the equivalent circuit model because the variation is large and the reliability is low.
- the input / output spectrum calculated by the Fourier transform unit 30 preferably includes a plurality of second frequency (f2) components, and includes three or more second frequency (f2) components. It is particularly preferred.
- the second frequency f2 can be acquired up to 23 times the frequency (23f3) of the first frequency f1. That is, impedances at a total of 12 types of frequencies were obtained.
- the second frequency f2 can be acquired up to 7f1
- the second frequency f2 can be acquired only as 3f1.
- the sampling rate is at least 100 times the first frequency f1.
- noise is likely to occur at the portion where the positive / negative of the current in the power controller 20 is reversed.
- the power controller 20 of the electrochemical analysis device 2 can use a DC power source that is cheaper than the AC power source. Furthermore, the power controller 20 that generates a DC rectangular wave may have a simple configuration in which ON / OFF control is performed only on a signal having a predetermined potential or current value.
- the input / output spectrum calculated by the Fourier transform unit 30 included only a component having a frequency (3f1, etc.) that is an odd multiple of the first frequency (f1).
- frequencies (2f1, 4f1,...) That are integer multiples of the first frequency (f1) are included due to the effect of the response speed of the cell 10. In some cases.
- the input / output spectrum calculated by the Fourier transform unit 30 may include a component having a frequency that is an integral multiple of the first frequency (f1).
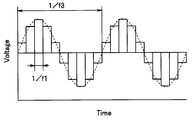
- the rectangular wave signal output from the power controller 20 is not limited to a waveform with a very steep rise.
- the rectangular wave signal output from the power controller 20 is also regarded as a so-called sawtooth wave that changes with a certain slope as the frequency is increased.
- the rectangular wave signal output from the power controller 20 is not limited to a waveform having a very steep rise and fall.
- the rectangular wave signal output from the power controller 20 is also regarded as a so-called triangular wave that changes with a certain inclination as the frequency is increased. That is, the rectangular wave in this embodiment is a concept including a sawtooth wave and a triangular wave.
- the rectangular wave signal output from the power controller 20 may be positively converted into a sawtooth signal by using a delay circuit such as an LC circuit.
- the input / output spectrum calculated by the Fourier transform unit 30 includes a frequency component that is an integral multiple of the first frequency (f1).
- the power controller 20 is configured by a rectangular wave signal having the first frequency (f1), which is lower than the first frequency (f1).
- the signal of the frequency (f3) is applied, and the Fourier transform unit calculates an input / output spectrum including the component of the third frequency (f3).
- the Fourier transform unit 30 includes a component of the first frequency (f1) of the rectangular wave signal, a component of an odd multiple of the frequency (3f1, 5f1, 7f1,...) Higher than the first frequency (f1), and the first An input / output spectrum including a component of the third frequency (f3) lower than the frequency (f1) is calculated. Furthermore, an input / output spectrum includes a component having an odd multiple of the third frequency (f3).
- the power controller 20 includes a pseudo sine having a third frequency (f3) lower than the first frequency (f1), which is configured by a rectangular wave signal having the first frequency (f1). Generate a wave signal.
- the electrochemical analysis apparatus 1A (electrochemical system 2A) according to the present modification can detect a frequency component based on the third frequency (f3) in addition to the effect of the electrochemical analysis apparatus 1 (electrochemical system 2). Improves the accuracy of impedance analysis.
- the pseudo sine wave can be regarded as a sine wave. That is, when acquiring low-frequency impedance, the power controller 20 can be used as a sine wave generator.
- noise may occur when switching from a positive voltage to a negative voltage and when switching from a negative voltage to a positive voltage.
- noise may occur when switching from a positive current to a negative current and when switching from a negative current to a positive current.
- the power controller 20 applies rectangular wave signals having a plurality of first frequencies f1A and f1B, and the Fourier transform unit 30 has a plurality of first frequencies.
- An input / output spectrum including a third frequency component that is an odd multiple of each of the frequencies is calculated.
- the Fourier transform unit 30 calculates It is the Nyquist plot of the electrochemical cell 10 which plotted the data acquired from the input-output spectrum by the white circle. Black circles are the same data as shown in FIG. 9 and acquired by the normal AC impedance method.
- f1A 5 Hz
- 3f1A 15 Hz
- 5f1A 25 Hz
- 7f1A 35 Hz
- 9f1A 45 Hz
- f1B 50 Hz
- 3f1B 150 Hz
- 5f1B 250 Hz
- 7f1B 350 Hz
- 9f1B 450 Hz
- f1C 250 Hz
- Impedances Z ′ and Z ′′ at a total of 16 frequencies of 3f1C 750 Hz
- 5f1C 1250 Hz
- 7f1C 1750 Hz
- f1D 500 Hz
- 3f1D 1500 Hz were obtained.
- the electrochemical analysis apparatus 1B (electrochemical system 2B) according to the present modification can acquire highly accurate impedance at more frequencies in addition to the effects of the electrochemical analysis apparatus 1 (electrochemical system 2).
- the frequency of the plurality of rectangular wave signals output from the power controller 20 it is preferable to set the frequency of the plurality of rectangular wave signals output from the power controller 20 so that the odd multiples of the frequency do not become the same. That is, when the frequency of an odd multiple of the frequency of a plurality of rectangular wave signals becomes the same, the impedance analysis may become unstable due to the error of the component acquired at that frequency. Furthermore, by setting the frequencies so as not to be the same, impedances at more frequencies can be acquired.
- the electrochemical analysis device 1C (electrochemical system 2C) of the second embodiment is similar in basic configuration to the electrochemical analysis device 1 (electrochemical system 2) of the first embodiment. .
- description of the component of the same function is abbreviate
- the electrochemical cell 10A is a large-capacity secondary battery (hereinafter referred to as “battery”) 10C, and the power controller 20C is called an inverter by those skilled in the art.
- the battery 10C is, for example, a lithium ion battery including a positive electrode 11C containing lithium cobalt oxide or the like, a negative electrode 12C containing a carbon material or the like, and an electrolyte 14C in which LiPF 6 is dissolved in cyclic and chain carbonates.
- Electrochemical cell 10A may be a power storage unit that can temporarily store electricity.
- the electrochemical system 2C is a large-scale power storage system of the power system 100.
- the power generation unit 50 such as a wind power generation unit or a solar power generation unit
- the load unit 60 such as a factory or home
- the battery 10C of the electrochemical system 2C is used. Is supplied to the load unit 60.
- the generated power amount exceeds the power consumption amount, the battery 10C is charged.
- the motor is the power generation unit 50 and the load unit 60. That is, when electric power is supplied, the motor is driven, and electric power is generated using the rotation of the motor.
- FIG. 14 and 15 are Nyquist plots in which the impedance characteristics of the battery 10C obtained by the electrochemical system 2C are indicated by white circles, and the impedance characteristics obtained by the normal AC impedance method are indicated by black circles. Five types of Nyquist plots are shown when the charging rate (SOC: State Of Charge) is 90% to 10%.
- SOC State Of Charge
- FIG. 14 is a Nyquist plot of the new battery 10
- FIG. 15 is a Nyquist plot after 200 cycles of the charge / discharge test (deteriorated battery).
- offset current 0.5 A, amplitude ⁇ 0.5 A
- offset current 0.5 A, amplitude ⁇ 0.5 A
- the internal resistance of the battery 10C was as extremely low as 10 m ⁇ , it was not easy to measure using a normal AC impedance method.
- the impedance characteristic of the battery 10C obtained by the electrochemical system 2C is in good agreement with the impedance characteristic obtained by the normal AC impedance method. That is, the same effect as that of the electrochemical system 2 of the first embodiment can be obtained in the electrochemical system 2 ⁇ / b> C in which the electrochemical cell is the battery 10. Furthermore, the battery 10C has an extremely low internal resistance of 10 m ⁇ , but it was easy to obtain impedance characteristics in the electrochemical system 2C.
- the diameter of the semicircle (R int ) is larger than that in FIG. 14, indicating that the internal resistance has increased. That is, the calculation unit 40 can further quantitatively detect the deterioration of the battery 10C from the impedance characteristics. Moreover, the calculation part 40 can also detect separately the deterioration degree of the positive electrode 11C, the negative electrode 12C, and an electrolyte by analyzing using an equivalent circuit. Furthermore, the calculation unit 40 can also detect the charging rate of the battery 10C in a state where the deterioration of the battery 10C does not change.
- Electrochemical system 2C can easily obtain impedance characteristics even when the internal resistance of the battery is 10 m ⁇ or less. Furthermore, it is easy to obtain impedance characteristics even for a battery having an internal resistance of 1 m ⁇ or less, which is extremely difficult to measure by a normal AC impedance method.
- the electrochemical system 2C has one battery 10C as an electrochemical cell.
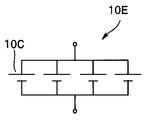
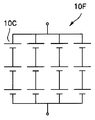
- the electrochemical cell is a battery unit 10D in which a plurality of batteries 10C shown in FIG. 16A are connected in series, a battery unit 10E in which a plurality of batteries 10C shown in FIG. 16B are connected in parallel, or a plurality of batteries 10C shown in FIG.
- the internal resistances of the battery units 10E and 10F in which the batteries are connected in parallel are lower than the internal resistances of the respective batteries 10C.
- the electrochemical system 2C it is easy to obtain impedance characteristics.
- the cell is not limited to a lithium secondary battery as long as it is an electricity storage device that can store electricity, and various secondary batteries and capacitors can also be used.
Landscapes
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Electrochemistry (AREA)
- Molecular Biology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Mathematical Physics (AREA)
- Secondary Cells (AREA)
- Measurement Of Resistance Or Impedance (AREA)
- Charge And Discharge Circuits For Batteries Or The Like (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/758,052 US9977059B2 (en) | 2012-12-27 | 2013-12-26 | Electrochemical analysis apparatus and electrochemical system |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-285550 | 2012-12-27 | ||
| JP2012285550A JP6226261B2 (ja) | 2012-12-27 | 2012-12-27 | 電気化学システム |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014104188A1 true WO2014104188A1 (ja) | 2014-07-03 |
Family
ID=51021262
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/084877 Ceased WO2014104188A1 (ja) | 2012-12-27 | 2013-12-26 | 電気化学解析装置および電気化学システム |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US9977059B2 (enExample) |
| JP (1) | JP6226261B2 (enExample) |
| WO (1) | WO2014104188A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022249708A1 (ja) * | 2021-05-24 | 2022-12-01 | 日置電機株式会社 | 信号注入装置およびインピーダンス測定装置 |
| WO2022249709A1 (ja) * | 2021-05-24 | 2022-12-01 | 日置電機株式会社 | インピーダンス測定装置 |
| JP2022180277A (ja) * | 2021-05-24 | 2022-12-06 | 日置電機株式会社 | インピーダンス測定装置 |
| JP2022180295A (ja) * | 2021-05-24 | 2022-12-06 | 日置電機株式会社 | 信号注入装置およびインピーダンス測定装置 |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12075957B2 (en) * | 2014-03-24 | 2024-09-03 | Intelligent Cleaning Equipment Holdings Co. Ltd. | Floor cleaning machines having intelligent systems, associated sub-assemblies incorporating intelligent systems, and associated methods of use |
| US10076220B2 (en) * | 2014-03-24 | 2018-09-18 | International Cleaning Equipment Holdings Co., Ltd. | Floor cleaning machines having intelligent systems, associated sub-assemblies incorporating intelligent systems, and associated methods of use |
| JP6508729B2 (ja) | 2016-12-02 | 2019-05-08 | トヨタ自動車株式会社 | 電池状態推定装置 |
| JP6614176B2 (ja) | 2017-02-09 | 2019-12-04 | トヨタ自動車株式会社 | 電池状態推定装置 |
| JP6575548B2 (ja) | 2017-03-22 | 2019-09-18 | トヨタ自動車株式会社 | 電池状態推定装置 |
| US11051670B2 (en) | 2017-04-13 | 2021-07-06 | Intelligent Cleaning Equipment Holdings Co. Ltd. | Floor cleaning machines having intelligent systems, associated sub-assemblies incorporating intelligent systems, and associated methods of use |
| JP6806002B2 (ja) | 2017-08-24 | 2020-12-23 | トヨタ自動車株式会社 | 温度推定装置 |
| JP6881156B2 (ja) | 2017-08-24 | 2021-06-02 | トヨタ自動車株式会社 | インピーダンス推定装置 |
| DE102017128566A1 (de) * | 2017-12-01 | 2019-06-06 | Endress+Hauser Flowtec Ag | Sensoranordnung zur Anordnung an einer Prozessanlage, sowie Verfahren zum Betrieb der Sensoranordnung und Prozessanlage |
| JP7157908B2 (ja) * | 2018-12-20 | 2022-10-21 | トヨタ自動車株式会社 | 電池容量の推定方法および電池容量の推定装置 |
| JP7157909B2 (ja) | 2018-12-20 | 2022-10-21 | トヨタ自動車株式会社 | 電池容量の推定方法、および電池容量の推定装置 |
| JP7172838B2 (ja) * | 2019-04-26 | 2022-11-16 | 株式会社デンソー | 電池監視装置 |
| JP7226147B2 (ja) | 2019-07-04 | 2023-02-21 | 株式会社デンソー | 電池監視装置 |
| JP7522542B2 (ja) | 2019-07-17 | 2024-07-25 | 株式会社デンソー | 電池監視装置 |
| JP7259614B2 (ja) | 2019-07-19 | 2023-04-18 | 株式会社デンソー | 電池監視装置 |
| JP7205410B2 (ja) | 2019-07-26 | 2023-01-17 | 株式会社デンソー | 電池監視装置 |
| JP7167898B2 (ja) * | 2019-10-30 | 2022-11-09 | 株式会社デンソー | 電池監視装置 |
| JP2021078295A (ja) * | 2019-11-12 | 2021-05-20 | 株式会社東芝 | 充電装置、電池診断システム、及び、充電方法 |
| DE112021000699T5 (de) | 2020-01-24 | 2022-11-24 | Denso Corporation | Batteriemessvorrichtung |
| JP2022007515A (ja) * | 2020-06-26 | 2022-01-13 | 株式会社デンソー | 電池診断システム |
| KR102496569B1 (ko) * | 2021-04-13 | 2023-02-06 | 포항공과대학교 산학협력단 | 임피던스 특성을 결정하는 방법 및 디바이스 |
| JP7517256B2 (ja) | 2021-06-10 | 2024-07-17 | 株式会社デンソー | 電池測定装置及び電池状態測定方法 |
| CN119096148A (zh) | 2022-02-27 | 2024-12-06 | 株式会社电知 | 阻抗测定装置、测定方法以及二次电池诊断系统 |
| EP4624958A1 (en) | 2022-11-24 | 2025-10-01 | Nuvoton Technology Corporation Japan | Impedance detection device and impedance detection method |
| JPWO2024166301A1 (enExample) | 2023-02-09 | 2024-08-15 | ||
| JPWO2024185073A1 (enExample) | 2023-03-08 | 2024-09-12 | ||
| JPWO2024189905A1 (enExample) | 2023-03-16 | 2024-09-19 | ||
| DE102024201193A1 (de) * | 2024-02-09 | 2025-08-14 | Schaeffler Technologies AG & Co. KG | Vereinfachtes Verfahren zur Impedanzanalyse elektrischer Bauteile |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01167679A (ja) * | 1987-12-23 | 1989-07-03 | Advantest Corp | インピーダンス測定装置 |
| JP2002330752A (ja) * | 2001-05-08 | 2002-11-19 | Sanden Corp | 微生物数測定装置 |
| JP2003090869A (ja) * | 2001-07-09 | 2003-03-28 | Yokogawa Electric Corp | インピーダンスの測定装置 |
| JP2005180927A (ja) * | 2003-12-16 | 2005-07-07 | Horiba Ltd | インピーダンス測定装置 |
| JP2012015932A (ja) * | 2010-07-05 | 2012-01-19 | Tamagawa Seiki Co Ltd | 正弦波発生回路 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4947130A (en) | 1987-12-23 | 1990-08-07 | Advantest Corporation | Impedance measuring apparatus |
| JPH08125441A (ja) * | 1994-10-20 | 1996-05-17 | Fujitsu Ltd | 波形発生装置 |
| JP3367320B2 (ja) * | 1996-02-26 | 2003-01-14 | 新神戸電機株式会社 | 密閉型鉛蓄電池の劣化判定方法及び装置 |
| JP5367604B2 (ja) * | 2003-08-22 | 2013-12-11 | 古河電気工業株式会社 | 二次電池の内部インピーダンスを測定する方法及び装置 |
| JP4999353B2 (ja) | 2006-04-26 | 2012-08-15 | パナソニック株式会社 | 蓄電装置、携帯機器及び電動車両 |
-
2012
- 2012-12-27 JP JP2012285550A patent/JP6226261B2/ja active Active
-
2013
- 2013-12-26 WO PCT/JP2013/084877 patent/WO2014104188A1/ja not_active Ceased
- 2013-12-26 US US14/758,052 patent/US9977059B2/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH01167679A (ja) * | 1987-12-23 | 1989-07-03 | Advantest Corp | インピーダンス測定装置 |
| JP2002330752A (ja) * | 2001-05-08 | 2002-11-19 | Sanden Corp | 微生物数測定装置 |
| JP2003090869A (ja) * | 2001-07-09 | 2003-03-28 | Yokogawa Electric Corp | インピーダンスの測定装置 |
| JP2005180927A (ja) * | 2003-12-16 | 2005-07-07 | Horiba Ltd | インピーダンス測定装置 |
| JP2012015932A (ja) * | 2010-07-05 | 2012-01-19 | Tamagawa Seiki Co Ltd | 正弦波発生回路 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2022249708A1 (ja) * | 2021-05-24 | 2022-12-01 | 日置電機株式会社 | 信号注入装置およびインピーダンス測定装置 |
| WO2022249709A1 (ja) * | 2021-05-24 | 2022-12-01 | 日置電機株式会社 | インピーダンス測定装置 |
| JP2022180277A (ja) * | 2021-05-24 | 2022-12-06 | 日置電機株式会社 | インピーダンス測定装置 |
| JP2022180295A (ja) * | 2021-05-24 | 2022-12-06 | 日置電機株式会社 | 信号注入装置およびインピーダンス測定装置 |
| JP7698556B2 (ja) | 2021-05-24 | 2025-06-25 | 日置電機株式会社 | インピーダンス測定装置 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20160195577A1 (en) | 2016-07-07 |
| JP2014126532A (ja) | 2014-07-07 |
| JP6226261B2 (ja) | 2017-11-08 |
| US9977059B2 (en) | 2018-05-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6226261B2 (ja) | 電気化学システム | |
| JP6370581B2 (ja) | 電気化学解析装置および電気化学システム | |
| Luo et al. | Rapid prediction of the state of health of retired power batteries based on electrochemical impedance spectroscopy | |
| Monem et al. | Lithium-ion batteries: Evaluation study of different charging methodologies based on aging process | |
| Wang et al. | Fast calculation of broadband battery impedance spectra based on S transform of step disturbance and response | |
| CN100495060C (zh) | 用于测量电池容量的方法 | |
| Zhao et al. | A measurement method for determination of dc internal resistance of batteries and supercapacitors | |
| CN106970266A (zh) | 一种锂离子电池的eis快速测量方法 | |
| Yokoshima et al. | Application of electrochemical impedance spectroscopy to ferri/ferrocyanide redox couple and lithium ion battery systems using a square wave as signal input | |
| CN108663631A (zh) | 一种锂离子电池组电化学阻抗谱在线测量装置 | |
| JP2000019234A (ja) | パルス電流の電圧応答信号を用いた電池容量測定方法及び電池容量測定装置 | |
| Yokoshima et al. | Impedance measurements of kilowatt-class lithium ion battery modules/cubicles in energy storage systems by square-current electrochemical impedance spectroscopy | |
| CN102288830A (zh) | 一种单片机产生spwm信号激励的蓄电池阻抗检测装置 | |
| Bohlen | Impedance-based battery monitoring | |
| Huang et al. | Online impedance measurement of operational batteries utilizing Sinc function signal injection via DC-DC power converter | |
| Luo et al. | AC impedance technique for dynamic and static state of charge analysis for Li-ion battery | |
| Watanabe et al. | Determination of electrochemical impedance of lithium-ion battery from charge curve by wavelet transformation | |
| Yan et al. | Battery impedance measurement using pseudo random binary sequences | |
| Thanapalan et al. | Advanced EIS techniques for performance evaluation of Li-ion cells | |
| Peng et al. | A High-Precision and Fast Measurement Method for Li-Ion Battery EIS | |
| CN119395119A (zh) | 一种筛选高倍率性能电极的方法及其应用 | |
| Wang et al. | Comparative study on fast calculation methods of broadband electrochemical impedance spectroscopy of power batteries | |
| Bechara et al. | Embedded spectroscopy: Potentialities and constraints for onboard battery diagnostics | |
| Hossain et al. | Battery Impedance Measurement Using Electrochemical Impedance Spectroscopy Board | |
| JP2014106038A (ja) | 電池インピーダンス測定装置 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13868212 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14758052 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13868212 Country of ref document: EP Kind code of ref document: A1 |