WO2012176796A1 - Nk細胞の増幅方法 - Google Patents
Nk細胞の増幅方法 Download PDFInfo
- Publication number
- WO2012176796A1 WO2012176796A1 PCT/JP2012/065718 JP2012065718W WO2012176796A1 WO 2012176796 A1 WO2012176796 A1 WO 2012176796A1 JP 2012065718 W JP2012065718 W JP 2012065718W WO 2012176796 A1 WO2012176796 A1 WO 2012176796A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cells
- cell
- positive
- amplifying
- blood
- Prior art date
Links
- 210000000822 natural killer cell Anatomy 0.000 title claims abstract description 237
- 238000000034 method Methods 0.000 title claims abstract description 73
- 210000004027 cell Anatomy 0.000 claims abstract description 247
- 210000003958 hematopoietic stem cell Anatomy 0.000 claims abstract description 31
- 210000001744 T-lymphocyte Anatomy 0.000 claims abstract description 20
- 201000011510 cancer Diseases 0.000 claims abstract description 20
- 238000002659 cell therapy Methods 0.000 claims abstract description 16
- 108010002350 Interleukin-2 Proteins 0.000 claims abstract description 13
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 11
- 238000012258 culturing Methods 0.000 claims abstract description 11
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 11
- 210000000601 blood cell Anatomy 0.000 claims abstract description 9
- 208000015181 infectious disease Diseases 0.000 claims abstract description 4
- 230000003321 amplification Effects 0.000 claims description 75
- 238000003199 nucleic acid amplification method Methods 0.000 claims description 75
- 210000005087 mononuclear cell Anatomy 0.000 claims description 35
- 210000005259 peripheral blood Anatomy 0.000 claims description 35
- 239000011886 peripheral blood Substances 0.000 claims description 34
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 claims description 27
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 claims description 27
- 210000004369 blood Anatomy 0.000 claims description 13
- 239000008280 blood Substances 0.000 claims description 13
- 210000004700 fetal blood Anatomy 0.000 claims description 13
- 210000002966 serum Anatomy 0.000 claims description 13
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 claims description 10
- 238000002617 apheresis Methods 0.000 claims description 7
- 210000001185 bone marrow Anatomy 0.000 claims description 5
- 210000004504 adult stem cell Anatomy 0.000 claims description 3
- 210000001671 embryonic stem cell Anatomy 0.000 claims description 3
- 108010071390 Serum Albumin Proteins 0.000 claims description 2
- 102000007562 Serum Albumin Human genes 0.000 claims description 2
- 210000001165 lymph node Anatomy 0.000 claims description 2
- 238000000338 in vitro Methods 0.000 abstract 1
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 104
- 239000000203 mixture Substances 0.000 description 40
- 230000001472 cytotoxic effect Effects 0.000 description 38
- 238000000684 flow cytometry Methods 0.000 description 32
- 239000002609 medium Substances 0.000 description 30
- 210000004748 cultured cell Anatomy 0.000 description 28
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 26
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 26
- 102000000588 Interleukin-2 Human genes 0.000 description 11
- 239000011324 bead Substances 0.000 description 10
- 230000035755 proliferation Effects 0.000 description 10
- 206010004593 Bile duct cancer Diseases 0.000 description 9
- 208000022072 Gallbladder Neoplasms Diseases 0.000 description 9
- 208000003445 Mouth Neoplasms Diseases 0.000 description 9
- 208000026900 bile duct neoplasm Diseases 0.000 description 9
- 208000006990 cholangiocarcinoma Diseases 0.000 description 9
- 201000010175 gallbladder cancer Diseases 0.000 description 9
- 208000012987 lip and oral cavity carcinoma Diseases 0.000 description 9
- 239000000725 suspension Substances 0.000 description 8
- 101001023379 Homo sapiens Lysosome-associated membrane glycoprotein 1 Proteins 0.000 description 7
- 102100035133 Lysosome-associated membrane glycoprotein 1 Human genes 0.000 description 7
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 7
- 238000002474 experimental method Methods 0.000 description 7
- 238000005259 measurement Methods 0.000 description 7
- 239000002953 phosphate buffered saline Substances 0.000 description 7
- 238000005119 centrifugation Methods 0.000 description 6
- 210000000581 natural killer T-cell Anatomy 0.000 description 6
- 238000002054 transplantation Methods 0.000 description 6
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 5
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 5
- 239000006285 cell suspension Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 208000035473 Communicable disease Diseases 0.000 description 4
- 102000003812 Interleukin-15 Human genes 0.000 description 4
- 108090000172 Interleukin-15 Proteins 0.000 description 4
- 108010043610 KIR Receptors Proteins 0.000 description 4
- 102000002698 KIR Receptors Human genes 0.000 description 4
- 239000006143 cell culture medium Substances 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 239000012636 effector Substances 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- UZOVYGYOLBIAJR-UHFFFAOYSA-N 4-isocyanato-4'-methyldiphenylmethane Chemical compound C1=CC(C)=CC=C1CC1=CC=C(N=C=O)C=C1 UZOVYGYOLBIAJR-UHFFFAOYSA-N 0.000 description 3
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 3
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 3
- 108010065805 Interleukin-12 Proteins 0.000 description 3
- 102000013462 Interleukin-12 Human genes 0.000 description 3
- 102100030704 Interleukin-21 Human genes 0.000 description 3
- 108010002386 Interleukin-3 Proteins 0.000 description 3
- 102000000646 Interleukin-3 Human genes 0.000 description 3
- 108010002586 Interleukin-7 Proteins 0.000 description 3
- 102000000704 Interleukin-7 Human genes 0.000 description 3
- 102100029185 Low affinity immunoglobulin gamma Fc region receptor III-B Human genes 0.000 description 3
- 102000043129 MHC class I family Human genes 0.000 description 3
- 108091054437 MHC class I family Proteins 0.000 description 3
- 210000000173 T-lymphoid precursor cell Anatomy 0.000 description 3
- 230000024245 cell differentiation Effects 0.000 description 3
- 239000002458 cell surface marker Substances 0.000 description 3
- 239000000470 constituent Substances 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 238000012137 double-staining Methods 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 108700014844 flt3 ligand Proteins 0.000 description 3
- 230000005484 gravity Effects 0.000 description 3
- 239000001963 growth medium Substances 0.000 description 3
- 229940117681 interleukin-12 Drugs 0.000 description 3
- 108010074108 interleukin-21 Proteins 0.000 description 3
- 229940076264 interleukin-3 Drugs 0.000 description 3
- 229940100994 interleukin-7 Drugs 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 2
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 101001027081 Homo sapiens Killer cell immunoglobulin-like receptor 2DL1 Proteins 0.000 description 2
- 101000945340 Homo sapiens Killer cell immunoglobulin-like receptor 2DS1 Proteins 0.000 description 2
- 108091006905 Human Serum Albumin Proteins 0.000 description 2
- 102000008100 Human Serum Albumin Human genes 0.000 description 2
- 102100037363 Killer cell immunoglobulin-like receptor 2DL1 Human genes 0.000 description 2
- 102100033631 Killer cell immunoglobulin-like receptor 2DS1 Human genes 0.000 description 2
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 2
- 239000012980 RPMI-1640 medium Substances 0.000 description 2
- 108020004511 Recombinant DNA Proteins 0.000 description 2
- 125000003275 alpha amino acid group Chemical group 0.000 description 2
- 239000000427 antigen Substances 0.000 description 2
- 102000036639 antigens Human genes 0.000 description 2
- 108091007433 antigens Proteins 0.000 description 2
- 238000005138 cryopreservation Methods 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 238000007710 freezing Methods 0.000 description 2
- 230000008014 freezing Effects 0.000 description 2
- 238000011194 good manufacturing practice Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 210000000130 stem cell Anatomy 0.000 description 2
- 238000010257 thawing Methods 0.000 description 2
- 230000036962 time dependent Effects 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000011282 treatment Methods 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical class O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- YXHLJMWYDTXDHS-IRFLANFNSA-N 7-aminoactinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=C(N)C=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 YXHLJMWYDTXDHS-IRFLANFNSA-N 0.000 description 1
- 108700012813 7-aminoactinomycin D Proteins 0.000 description 1
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 1
- 238000012935 Averaging Methods 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 235000005956 Cosmos caudatus Nutrition 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 101000945333 Homo sapiens Killer cell immunoglobulin-like receptor 2DL3 Proteins 0.000 description 1
- 101000945337 Homo sapiens Killer cell immunoglobulin-like receptor 2DL5A Proteins 0.000 description 1
- 101000945335 Homo sapiens Killer cell immunoglobulin-like receptor 2DL5B Proteins 0.000 description 1
- 101000945490 Homo sapiens Killer cell immunoglobulin-like receptor 3DL2 Proteins 0.000 description 1
- 101001109501 Homo sapiens NKG2-D type II integral membrane protein Proteins 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 102100033634 Killer cell immunoglobulin-like receptor 2DL3 Human genes 0.000 description 1
- 102100033628 Killer cell immunoglobulin-like receptor 2DL5B Human genes 0.000 description 1
- 102100034840 Killer cell immunoglobulin-like receptor 3DL2 Human genes 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 1
- 102100022680 NKG2-D type II integral membrane protein Human genes 0.000 description 1
- 108010004222 Natural Cytotoxicity Triggering Receptor 3 Proteins 0.000 description 1
- 102100032852 Natural cytotoxicity triggering receptor 3 Human genes 0.000 description 1
- 101710160107 Outer membrane protein A Proteins 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 230000000735 allogeneic effect Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 229960004969 dalteparin Drugs 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000004043 dyeing Methods 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- 239000012997 ficoll-paque Substances 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 238000005755 formation reaction Methods 0.000 description 1
- 238000003125 immunofluorescent labeling Methods 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 238000000691 measurement method Methods 0.000 description 1
- 239000007758 minimum essential medium Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 210000001948 pro-b lymphocyte Anatomy 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 238000003908 quality control method Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000000941 radioactive substance Substances 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 229960001967 tacrolimus Drugs 0.000 description 1
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 210000003954 umbilical cord Anatomy 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0646—Natural killers cells [NK], NKT cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/17—Lymphocytes; B-cells; T-cells; Natural killer cells; Interferon-activated or cytokine-activated lymphocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/15—Natural-killer [NK] cells; Natural-killer T [NKT] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/0081—Purging biological preparations of unwanted cells
- C12N5/0087—Purging against subsets of blood cells, e.g. purging alloreactive T cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/23—Interleukins [IL]
- C12N2501/2302—Interleukin-2 (IL-2)
Definitions
- the present invention relates to a method for amplifying natural killer cells (NK cells) having high cytotoxic activity with high purity and high amplification factor, and a pharmaceutical composition containing NK cells obtained by the method.
- NK cells natural killer cells
- NK cells do not attack normal cells that express MHC class I molecules, but mainly attack cells in which the expression and decrease of MHC class I molecules are reduced.
- GVH Growth-versus-host
- Non-patent Document 3 From a single apheresis of normal adult peripheral blood, about 1 ⁇ 10 10 mononuclear cells can be collected, and assuming that the composition ratio of NK cells in the peripheral blood mononuclear cells is about 7%, 7 ⁇ 10 8 Individual NK cells are obtained (Non-patent Document 3). On the other hand, for transplantation of NK cells, 1 ⁇ 10 5 cells / kg to 2 ⁇ 10 7 cells / kg (Non-patent Document 1) or 5 ⁇ 10 5 cells / kg to 8.1 ⁇ 10 7 cells / kg ( Non-patent document 2) NK cells of the order are used. If a patient weighs 60 kg, 6 ⁇ 10 6 to 4.8 ⁇ 10 9 NK cells are required.
- NK cells obtained from a single apheresis of normal adult peripheral blood.
- the engraftment period of NK cells was not correlated with the number of NK cells administered, and was 2 to 189 days, and the median was only 10 days.
- transplantation of NK cells is frequent. This has to be repeated for the patient.
- NK cells obtained from a donor in a test tube to obtain NK cells sufficient to kill the target cells.
- Patent document 1 cultivated peripheral blood mononuclear cells of healthy individuals for 13 days in the presence of OKT3, which is an agonist antibody against human CD3, IL-2, and anti-CD16 antibody, to purify NK cells. Amplification was 81.2%, 130 times.
- Patent Document 2 describe peripheral blood mononuclear cells of healthy individuals by a method using a medium supplemented with IL-2, IL-15, anti-CD3 antibody, 5% human AB serum, tacrolimus and dalteparin.
- IL-2 peripheral blood mononuclear cells
- IL-15 peripheral blood mononuclear cells
- anti-CD3 antibody 5% human AB serum
- tacrolimus 5% human AB serum
- tacrolimus dalteparin
- the present invention provides a method for amplifying NK cells.
- the method for amplifying NK cells of the present invention comprises preparing a cell population containing NK cells, removing T cells from the cell population containing NK cells, and remaining cells from which the T cells have been removed. Culturing in a medium containing 2500 IU / mL to 2813 IU / mL of IL-2.
- the step of removing the T cells from the cell population containing the NK cells may be achieved by a step of removing CD3 positive cells.
- the NK cell amplification method of the present invention may include a step of removing hematopoietic progenitor cells from the cell population containing the NK cells.
- the step of removing the hematopoietic progenitor cells from the cell population containing the NK cells may be achieved by a step of removing CD34 positive cells.
- the medium may contain autologous serum, AB type serum, and / or serum albumin.
- the step of preparing the cell population containing the NK cells may be achieved by a step of separating mononuclear cells from blood cells collected from a subject.
- the blood cell may be collected from peripheral blood, umbilical cord blood, bone marrow and / or lymph node.
- the blood cell may be collected from peripheral blood by an apheresis method.
- the cell population containing the NK cell is a hematopoietic stem cell derived from any stem cell selected from the group consisting of embryonic stem cells, adult stem cells, and induced pluripotent stem (iPS) cells. And at least one selected from the group consisting of cord blood-derived hematopoietic stem cells, peripheral blood-derived hematopoietic stem cells, bone marrow blood-derived hematopoietic stem cells, cord blood mononuclear cells, and peripheral blood mononuclear cells May be prepared from cells.
- the donor of the cell population containing the NK cells may be derived from the recipient patient himself, a close relative of the patient, or an unrelated patient.
- the NK cells may be derived from a donor in which the recipient's major histocompatibility antigen (MHC) and killer immunoglobulin-like receptor (KIR) do not match.
- MHC major histocompatibility antigen
- KIR killer immunoglobulin-like receptor
- the present invention provides a pharmaceutical composition for cell therapy comprising NK cells prepared by the amplification method of the present invention.
- the pharmaceutical composition of the present invention may contain NK cell precursors, T cells, NKT cells, hematopoietic progenitor cells and the like in addition to the amplified NK cells.
- the pharmaceutical composition of the present invention may be used for treating infectious diseases and / or cancer.
- the pharmaceutical composition of the present invention may be administered to a patient having an HLA genotype different from that of NK cells prepared by the amplification method of the present invention.
- the present invention provides a step of preparing a cell population containing the NK cells, a step of removing T cells from the cell population containing the NK cells, and a remaining cell from which the T cells have been removed, from 2500 IU / mL to 2813 IU.
- a cell therapy comprising culturing in a medium containing / mL of IL-2 and transplanting NK cells amplified from the remaining cells to a patient.
- the cell therapy may include removing hematopoietic progenitor cells from the cell population containing the NK cells.
- the amplified NK cell may be transplanted together with an NK cell precursor, T cell, NKT cell, hematopoietic progenitor cell, and the like.
- the cell therapy of the present invention may be used to treat and / or prevent infection and / or cancer.
- the cell therapy of the present invention may comprise the step of transplanting into a patient having an HLA genotype different from the NK cells prepared by the amplification method of the present invention.
- the step of transplanting the NK cells into a patient may be achieved by administering the pharmaceutical composition of the present invention to the patient.
- the cell population containing the NK cells includes any hematopoietic stem cell derived from any of the group consisting of embryonic stem cells, adult stem cells and induced pluripotent stem (iPS) cells, and umbilical cord Prepared from at least one cell selected from the group consisting of blood-derived hematopoietic stem cells, peripheral blood-derived hematopoietic stem cells, bone marrow blood-derived hematopoietic stem cells, cord blood mononuclear cells, and peripheral blood mononuclear cells May be.
- the donor of the cell population containing the NK cells may be derived from the recipient patient himself, a close relative of the patient, or an unrelated patient.
- the NK cells may be derived from a donor in which the recipient's major histocompatibility antigen (MHC) and killer immunoglobulin-like receptor (KIR) do not match.
- MHC major histocompatibility antigen
- KIR killer immunoglobulin-like receptor
- NK cell refers to a CD3-negative CD56-positive mononuclear cell, and particularly has a cytotoxic activity against cells in which the expression of MHC class I molecules is low or the expression is lost.
- the cell population containing the NK cells can be prepared using various procedures known to those skilled in the art. For example, specific gravity centrifugation can be used when recovering mononuclear cells from blood such as umbilical cord blood and peripheral blood. NK cells can be collected using immunomagnetic beads. Furthermore, the NK cells can be isolated and identified using immunofluorescent staining with a specific antibody against a cell surface marker, using a FACS (Fluorescence activated cell sorter) or a flow cytometer.
- FACS Fluorescence activated cell sorter
- the NK cells may include cell surface antigen CD3 using immunomagnetic beads including, but not limited to, Dynabeads (trademark) manufactured by Dynal and CliniMACS (trademark) manufactured by Miltenyi Biotech.
- it may be prepared by separating and removing cells expressing CD34.
- a specific binding partner for T cells and / or hematopoietic progenitor cells may be used to selectively injure or kill T cells and / or hematopoietic progenitor cells.
- the step of removing the T cells from the mononuclear cells may be a step of removing other cell types such as hematopoietic progenitor cells, B cells and / or NKT cells together with the T cells.
- the step of removing hematopoietic progenitor cells from the mononuclear cells may be a step of removing other cell types such as T cells, B cells and / or NKT cells together with hematopoietic progenitor cells.
- mononuclear cells separated from umbilical cord blood and peripheral blood may be cryopreserved, thawed according to the time of transplantation into a patient, and used for amplification of NK cells.
- the mononuclear cells are frozen either during amplification by the NK cell amplification method of the present invention or after the amplification is completed, and are thawed according to the time of transplantation to the patient, and used for transplantation to the patient. May be. Any method known to those skilled in the art may be used for freezing and thawing blood cells. Any commercially available cell cryopreservation solution may be used for freezing the cells.
- the solution for suspending living NK cells is generally, for example, physiological saline, phosphate buffered saline (PBS), culture medium, serum or the like.
- the solution may contain pharmaceutically acceptable carriers as pharmaceuticals and quasi drugs.
- the NK cell therapy of the present invention can be applied to the treatment and / or prevention of various diseases sensitive to NK cells. Examples of the disease include, but are not limited to, oral cancer, gallbladder cancer, bile duct cancer, lung cancer, liver cancer, colon cancer, kidney cancer, bladder cancer, leukemia, and infections caused by viruses, bacteria, and the like.
- the cell therapy of the present invention may be performed alone or in combination with surgery, chemotherapy, radiation therapy or the like.
- NK cells may be transplanted, for example, by intravenous, arterial, subcutaneous, intraperitoneal administration or the like.
- Cell culture media for preparing NK cells of the present invention include KBM501 medium (Kohjin Bio Co., Ltd.), CellGro SCGM medium (Celgenics, Iwai Chemical Co., Ltd.), X-VIVO15 medium (Lonza, Takara Bio Inc.) Company), IMDM, MEM, DMEM, RPMI-1640 and the like.
- Interleukin-2 may be added to the medium at a concentration that can achieve the object of the present invention.
- the IL-2 concentration may be 2500 IU / mL to 2813 IU / mL.
- the IL-2 preferably has a human amino acid sequence, and is preferably produced by recombinant DNA technology for safety.
- the concentration of IL-2 may be indicated in domestic standard units (JRU) and international units (IU). Since 1 IU is about 0.622 JRU, 1750 JRU / mL is about 2813 IU / mL.
- the medium may be supplemented with the subject's autologous serum, human AB serum available from BioWhittaker and others, or donated human serum albumin available from the Japanese Red Cross.
- the autologous serum and the human type AB serum are preferably added at a concentration of 1 to 10%, and the donated human serum albumin is preferably added at a concentration of 1 to 10%.
- the subject may be a healthy person and a patient suffering from the disease.
- the medium may contain appropriate proteins, cytokines, antibodies, compounds and other components, provided that the amplification effect of NK cells is not impaired.
- the cytokines include interleukin 3 (IL-3), interleukin 7 (IL-7), interleukin 12 (IL-12), interleukin-15 (IL-15), and interleukin-21 (IL-21).
- SCF Stem cell factor
- Flt3L FMS-like tyrosine kinase 3 ligand
- the IL-3, IL-7, IL-12, IL-15, IL-21, SCF and Flt3L preferably have a human amino acid sequence, and are preferably produced by recombinant DNA technology for safety.
- the medium may be replaced at any time after the start of culture, provided that the desired number of NK cells can be obtained, but is preferably every 3-5 days.
- the culture vessel includes, but is not limited to, a commercially available dish, flask, plate, and multiwell plate.
- the culture conditions are not particularly limited as long as they do not impair the amplification effect of NK cells, but culture conditions under 37 ° C., 5% CO 2 and saturated water vapor atmosphere are common. Since the object of the present invention is to prepare a large amount of NK cells, the longer the period of culturing in the medium, the more advantageous NK cells can be obtained.
- the culture period is not particularly limited, provided that NK cells are amplified to the desired number of cells.
- the cell population containing NK cells may contain NK cell precursors, T cells, NKT cells, hematopoietic progenitor cells and the like in addition to NK cells.
- Desired NK cells may be selected after amplification using, for example, specific gravity centrifugation, immunomagnetic beads, FACS, flow cytometry, and the like.
- the NK cells are anti-CD3 antibody, anti-CD16 antibody, anti-CD34 antibody, anti-CD56 antibody, anti-CD69 antibody, anti-CD94 antibody, anti-CD107a antibody, anti-KIR3DL1 antibody, anti-KIR3DL2 antibody, anti-KIR2DL3 antibody, anti-KIR2DL1 antibody.
- the anti-KIR2DS1 antibody, the anti-KIR2DL5 antibody, the anti-NKp46 antibody, the anti-NKp30 antibody, the anti-NKG2D antibody, and the like may be selectively separated from the cell population.
- the antibody may be a monoclonal antibody, a polyclonal antibody, or the like.
- the selection of NK cells may be performed by selectively removing T cells, NKT cells, hematopoietic progenitor cells and other cells.
- the production of the method and pharmaceutical composition of the present invention is preferably carried out under conditions (good manufacturing practice (GMP)) that conform to the manufacturing control and quality control rules of pharmaceuticals and quasi drugs.
- GMP good manufacturing practice
- the cytotoxic activity of the amplified NK cells can be evaluated by methods well known to those skilled in the art.
- the cytotoxic activity is generally quantified by measuring the radiation dose or fluorescence intensity after incubation of the NK cells (effector cells) and target cells labeled with a radioactive substance, a fluorescent dye or the like.
- the target cells may be, but are not limited to, K562 cells, acute myeloid leukemia cells, and chronic myeloid leukemia cells.
- the properties of the amplified NK cells can be examined using RT-PCR, solid-phase hybrid formation, ELISA, Western blot, immunoprecipitation, immunoturbidimetry, FACS, flow cytometry, etc. There is.
- NK cells whole blood of umbilical cord blood and peripheral blood
- preparation of autologous serum preparation of mononuclear cells from the whole blood
- measurement of the number of cells before and after the culture of the mononuclear cells and before and after the culture
- the measurement of the composition ratio of NK cells, T cells, hematopoietic progenitor cells, and other cell types in the mononuclear cells, calculation of amplification factor of NK cells, and statistical analysis of measurement error and significance are those skilled in the art. It may be carried out using any known method.
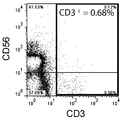
- FIG. 3 is a graph showing the results of double staining with antibodies against CD3 and CD56 and measurement by flow cytometry before removal of CD3-positive cells.
- 3 is a graph showing the results of measuring and averaging the change over time in the composition ratio of NK cells (CD3 negative / CD56 positive) isolated from 5 healthy individuals with respect to the whole cultured cells. Changes in the composition ratio of NK cells (CD3 negative / CD56 positive) isolated from 3 patients with advanced cancer (oral cancer, gallbladder cancer, and bile duct cancer) over time were measured by flow cytometry. Result diagram. Average growth curve of amplification factor of NK cells (CD3 negative / CD56 positive) isolated from 3 patients with advanced cancer (oral cancer, gallbladder cancer and bile duct cancer). The graph which compared the flow cytometry analysis result of CD69.
- NK cells Amplification of NK cells (1) 1. Materials and Methods (1) Blood collection from peripheral blood Peripheral blood was collected from healthy individuals and patients with advanced cancer (oral cancer, gallbladder cancer and bile duct cancer). This experiment was conducted with the approval of the Kyushu University Medical District Department Clinical Research Ethics Review Committee (approval number 22-176, approval date: March 31, 2011). Written consent has been obtained from the healthy person and the patient. Blood collection, cryopreservation, and thawing were performed by methods well known to those skilled in the art.
- the intermediate layer collected from one or two centrifuge tubes was collected in one new centrifuge tube, and the volume was adjusted to 50 mL with the diluent.
- the second centrifugation was performed under conditions of 500 ⁇ g, room temperature, 5 minutes, or 15 minutes.
- the supernatant was removed and the pellet was suspended in 30 mL of the diluent.
- the third centrifugation was performed under the conditions of 280 ⁇ g and room temperature for 10 minutes.
- the supernatant was removed, and the pellet was suspended in PBS supplemented with 2 mM EDTA and 0.1% BSA so that the cell concentration was 1 ⁇ 10 7 cells / mL (hereinafter referred to as “mononuclear”).
- Sphere suspension ”).
- CD3-negative cells The remaining cells in the suspension (hereinafter referred to as “CD3-negative cells”) are cell culture media (KBM501) supplemented with 5% autologous serum. 16025015, Kojin Bio Inc .; containing 1750 JRU / mL of IL-2 (hereinafter referred to as “KBM medium”), diluted to 5 ⁇ 10 5 cells / mL, and a 6-well culture plate (140675, nunc, Thermo Fisher Scientific Co., Ltd.). Cell culture was performed for 21 days at 37 ° C., 5% CO 2 and saturated water vapor atmosphere. Medium exchange was performed on the 5th, 9th, 13th and 17th days of culture. The cells were cultured without feeder cells.
- KBM501 cell culture media
- KBM medium 1750 JRU / mL of IL-2
- the cell number of the peripheral blood mononuclear cells was determined by measuring the number of living cells using a hemocytometer between the start of culture and the 21st day.
- Cell surface markers of the cells include anti-CD3 antibody (317308, BioLegend Japan), anti-CD16 antibody (556618, BD Pharmingen, Nippon Becton Dickinson), anti-CD56 antibody (304607, 318321, BioLegend Japan) , Anti-CD69 antibody (310905, BioLegend Japan), anti-KIR3DL1 / KIR3DL2 antibody (130-095-205, Miltenyi Biotech), anti-KIR2DL3 antibody (FAB2014P, R & D SYSTEMS, Cosmo Bio) KIR2DL1 / KIR2DS1 antibody (339505, BioLegend Japan, Inc.), anti-KIR2DL5 antibody (341003, BioLegend Japan Co., Ltd., anti-NKp46 antibody (331907, Bio
- FIG. 1A shows the experimental results of double staining with antibodies against CD3 and CD56 and measurement by flow cytometry before the removal of CD3-positive cells.
- FIG. 1B shows the experimental results of double staining with antibodies against CD3 and CD56 after removal of CD3 positive cells and measurement by flow cytometry.
- the composition ratio of CD3 positive cells the ratio of CD3 positive cells in the total cultured cells of each experimental group measured by the flow cytometry method is expressed as a percentage.
- the composition ratio (%) of CD3-positive cells was 69.37% before removal of CD3-positive cells, and 0.68% after removal of CD3-positive cells. As is apparent from these results, CD3-positive cells were significantly removed from the mononuclear cell suspension.
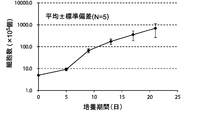
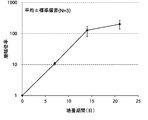
- FIG. 2A is a proliferation curve of the number of CD3-negative cells separated from mononuclear cells in the peripheral blood of five healthy subjects.
- FIG. 2B is an average growth curve of the number of CD3-negative cells isolated from mononuclear cells in the peripheral blood of five healthy subjects.
- the number of CD3-negative cells per 1 mL of peripheral blood collected from 5 healthy subjects is 5 days after culture, 9 days after culture, 13 days after culture, 17 days after culture and 21 days after culture. Measured. The standard deviation of each experimental condition was calculated from the measured values of experimental results repeated five times under the same conditions.
- CD3 negative cells continued to increase from the beginning of culture until 21st day. The rate of increase continued to increase until the 13th day and decreased after the 13th day.
- the number of CD3-negative cells increased from about 5 ⁇ 10 5 cells at the start of culture to about 700 ⁇ 10 5 cells after 21 days of culture.
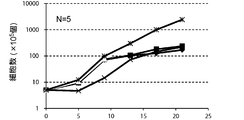
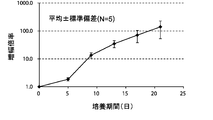
- FIG. 3A is a proliferation curve of each amplification factor of CD3-negative cells separated from mononuclear cells in the peripheral blood of five healthy subjects.
- FIG. 3B is an average growth curve of the magnification of CD3 negative cells isolated from mononuclear cells in the peripheral blood of 5 healthy subjects.
- the amplification factor is calculated by dividing the number of CD3 negative cells after 5 days, 9 days, 13 days, 17 days and 21 days by the number of CD3 negative cells at the start of culture. Calculated as the quotient. The standard deviation of each experimental condition was calculated from the measured values of experimental results repeated five times under the same conditions.
- the amplification factor of CD3 negative cells continued to increase from the beginning of the culture until the 21st day.
- the amplification factor continued to increase significantly until day 13, and increased to about 150 times after 21 days of culture.
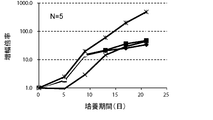
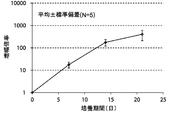
- FIG. 4A is a proliferation curve of each amplification factor of NK cells (CD3 negative / CD56 positive) isolated from mononuclear cells in the peripheral blood of five healthy subjects.
- FIG. 4B is an average growth curve of amplification factor of NK cells (CD3 negative / CD56 positive) isolated from mononuclear cells in peripheral blood of five healthy subjects.
- CD3-negative cells were double-stained with antibodies against CD3 and CD56 and analyzed by flow cytometry.
- the amplification factor was calculated as the quotient obtained by dividing the number of NK cells after 7 days, 14 days and 21 days by the number of NK cells at the start of culture.
- the standard deviation of each experimental condition was calculated from the measured values of experimental results repeated five times under the same conditions.
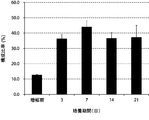
- the amplification factor of NK cells continued to increase from the beginning of culture until the 21st day.
- the amplification factor continued to increase significantly up to day 14, and increased to about 400 times after 21 days of culture.
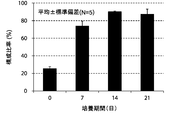
- FIG. 5A shows the results of an experiment in which the change over time in the composition ratio of NK cells (CD3 negative / CD56 positive) isolated from 5 healthy subjects to the whole cultured cells was measured by a flow cytometry method.
- FIG. 5B shows an experiment in which the change over time in the average value of the composition ratio of NK cells (CD3 negative / CD56 positive) isolated from 5 healthy subjects to the whole cultured cells was measured by flow cytometry and averaged. It is a result. 5A and 5B, CD3 negative cells were double-stained with antibodies against CD3 and CD56 and analyzed by flow cytometry.
- the ratio of NK cells in the total cultured cells of each experimental group, measured by flow cytometry, is expressed as a percentage.
- the vertical axis of the graph is the composition ratio (%) of NK cells (CD3 negative / CD56 positive) to the whole cultured cells, and the horizontal axis is the number of culture days.
- the standard deviation of each experimental condition was calculated from the measured values of experimental results repeated five times under the same conditions.
- the composition ratio of NK cells continued to increase from the beginning of culture until the 21st day.
- the composition ratio of the NK cells continued to increase significantly until the 14th day and increased to about 90% after 14 days of culture.
- the present invention has been shown to selectively amplify NK cells over time.
- FIG. 6A shows the composition ratio of NK cells (CD3 negative / CD56 positive) isolated from three patients with advanced cancer (oral cancer, gallbladder cancer and bile duct cancer) to the whole cultured cells. It is the experimental result which measured the change with time by the flow cytometry method.
- FIG. 6B is an average growth curve of amplification factor of NK cells (CD3 negative / CD56 positive) isolated from 3 patients with advanced cancer (oral cancer, gallbladder cancer and bile duct cancer).
- the “constituent ratio of NK cells” the ratio of NK cells in the total cultured cells of each experimental group, measured by flow cytometry, is expressed as a percentage.
- FIG. 6A shows the composition ratio of NK cells (CD3 negative / CD56 positive) isolated from three patients with advanced cancer (oral cancer, gallbladder cancer and bile duct cancer) to the whole cultured cells. It is the experimental result which measured the change with time by the flow cytometry method.
- FIG. 6B is an
- the vertical axis represents the composition ratio (%) of NK cells (CD3 negative / CD56 positive) with respect to the whole cultured cells, and the horizontal axis represents the number of culture days.
- “Amplification magnification of NK cells” represents the result of dividing the number of NK cells after amplification by the number of NK cells present in peripheral blood mononuclear cells before amplification.
- the vertical axis represents the amplification factor of NK cells
- the horizontal axis represents the number of culture days. The standard deviation of each experimental condition was calculated from the measured values of the experimental results repeated three times under the same conditions. As shown in FIG.
- the composition ratio of NK cells continued to increase remarkably from the beginning of the culture to the 14th day, and increased to about 85% after the 14-day culture.
- the amplification factor of NK cells continued to increase remarkably from the beginning of the culture to the 14th day, and increased to about 140 times after the 14-day culture.
- the proportion of NK cells decreased due to the proliferation of CD3-positive cells.
- the proliferation of the CD3 positive cells hardly affected the amplification of NK cells. From the above results, it was shown that NK cells isolated from patients with advanced cancer (oral cancer, gallbladder cancer and bile duct cancer) are amplified over time. In addition, it was suggested that the present invention can amplify NK cells isolated from patients suffering from cancer, infectious diseases, etc. over time.
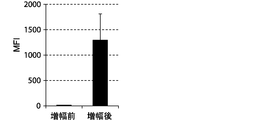
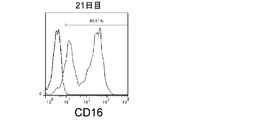
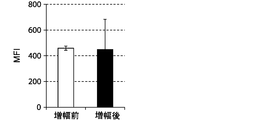
- FIGS. 7, 9 and 11 show graphs comparing the results of flow cytometry analysis of each cell surface marker. Moreover, the graph of the measured value of the mean fluorescence intensity (MFI) which compared the flow cytometry analysis result of CD69 and CD16 to FIG. 8 and 10 is shown. The standard deviation of each experimental condition was calculated from the measured values of the experimental results repeated three times under the same conditions.
- the cells amplified by the method of the present invention strongly expressed CD69, KIR2DL3, KIR2DL1 / KIR2DS1, KIR2DL5, NKp30, and NKG2D as compared to the cells before amplification. .
- the expression of CD69 was about 100%.
- the cells prepared by the method of the present invention were shown to express differentiation markers as NK cells.
- the NK cells have high cytotoxic activity.
- NK cells can be selectively and efficiently amplified by removing CD3-positive cells, ie, T cells, and then culturing them in the KBM medium. It was suggested that a large amount of NK cells can be prepared not only from healthy subjects but also from patients suffering from cancer, infectious diseases and the like. Moreover, it was suggested that the method of the present invention can remarkably amplify not only peripheral blood-derived NK cells but also cells derived from other tissues / organs, particularly umbilical cord blood-derived NK cells.
- NK cells were prepared from healthy individuals according to the method described in Example 1.
- CellGro SCGM 2001, Cellogenics, Iwai Chemicals
- 2500 IU / mL IL-2 AF-200-02-2, PeproTech, Toyobo Co., Ltd.
- 5% autologous serum "CellGro medium”
- the NK cells were amplified in the KBM medium and the CellGro medium according to the method described in Example 1.
- FIG. 12 is a growth curve of amplification factor of NK cells cultured in KBM medium and CellGro medium.
- the amplification factor was calculated as the quotient obtained by dividing the number of NK cells after 7 days, 14 days and 21 days by the number of NK cells at the start of culture. The standard deviation of each experimental condition was calculated from the measured values of the experimental results repeated twice under the same conditions.
- the amplification factor of NK cells continued to increase in the KBM medium and CellGro medium from the start of culture until the 21st day. After 21 days of culture, the amplification factor was about 670 times in the KBM medium and about 140 times in the CellGro medium.
- NK cells are sufficiently amplified in the KBM medium and the CellGro medium.
- NK cells can be amplified in media containing 2500 IU / mL to 2813 IU / mL IL-2 regardless of the type of cell culture media.
- Cytotoxic activity of amplified NK cells Materials and Methods (1) Quantification of cytotoxic activity NK cells were prepared according to the method described in Example 1 and used as effector cells. K562 cells (chronic myeloid leukemia cells) were prepared by methods well known to those skilled in the art and used as target cells. The cytotoxic activity of amplified NK cells and non-amplified NK cells (hereinafter referred to as “non-amplified NK cells”) was quantified by methods well known to those skilled in the art.
- the target cells are cultured in RPMI-1640 medium supplemented with 3-3'-dioctadesiloxacarbocyanine (D4292, Sigma-Aldrich Japan Co., Ltd.) (final concentration: 0.01 mM) for 10 minutes.
- the target cells were washed three times with PBS ( ⁇ ) and serum-free IMDM medium after labeling.
- the effector cells and the target cells were seeded in a round bottom 96-well culture plate and co-cultured in serum-free IMDM medium for 2 hours.
- the ratio of effector cells to target cells (E: T ratio) was adjusted to 3: 1, 2: 1, 1: 1, 1: 5, and 1:10. Cytotoxic activity (%) was quantified by flow cytometry using anti-MHC class I antibody (311409, BioLegend Japan) and 7-amino-actinomycin D (A9400, Sigma-Aldrich Japan). .
- NK cell differentiation marker NK cells were amplified according to the method described in Example 1. At the start of culture, after culturing for 3 days, after culturing for 7 days, after culturing for 14 days and after culturing for 21 days, the NK cells and the K562 cells were co-cultured at a 2: 1 E: T ratio for 2 hours. . Thereafter, the composition ratio of CD107a positive cells in the NK cells was analyzed by flow cytometry using an anti-CD107a antibody (328606, BioLegend Japan).
- FIG. 13 is a graph showing the experimental results of examining the cytotoxic activity of peripheral blood-derived NK cells amplified by the method of the present invention against K562.
- the vertical axis represents cytotoxic activity (unit:%).
- the white bar indicates the cytotoxic activity of non-amplified NK cells, and the black bar indicates the cytotoxic activity of amplified NK cells.
- the horizontal axis represents the E: T ratio between amplified NK cells or non-amplified NK cells and K562 cells. When the E: T ratio was 3: 1, the cytotoxic activity was about 30% for unamplified NK cells and about 110% for amplified NK cells.
- the cytotoxic activity was about 20% for unamplified NK cells and about 107% for amplified NK cells.
- the E: T ratio was 1: 1, the cytotoxic activity was about 10% for unamplified NK cells and about 100% for amplified NK cells.
- the E: T ratio was 1: 5 and 1:10, the cytotoxic activity of the amplified NK cells was about 25% and about 15%, respectively.
- FIG. 14 shows the results of experiments in which the change over time in the composition ratio of CD107a-positive cells isolated from healthy subjects to the whole cultured cells was measured by flow cytometry. The standard deviation of each experimental condition was calculated from the measured values of experimental results repeated five times under the same conditions.
- “constituent ratio of CD107a positive cells” the ratio of CD107a positive cells in the total cultured cells of each experimental group, measured by flow cytometry, is expressed as a percentage.
- the vertical axis represents the composition ratio (%) of CD107a positive cells to the whole cultured cells, and the horizontal axis represents the number of culture days. The composition ratio of CD107a positive cells was increased to about 35% by the third day from the start of the culture, and the composition ratio was maintained even on the 21st day.
- NK cells amplified according to the present invention have high cytotoxic activity. Therefore, it was shown that the present invention can selectively and efficiently amplify NK cells having high cytotoxic activity without using feeder cells, NK cells transfected with foreign molecules, or the like. It was also suggested that NK cells have high cytotoxic activity when amplified from cells derived from other tissues and organs, particularly cells derived from umbilical cord blood, as well as cells derived from peripheral blood.
- NK cell amplification (3) (repeated removal of CD3 positive cells) After the experiments of Examples 1 to 3, CD3 positive cells increased non-selectively as NK cell amplification experiments were repeated, and the composition ratio of CD3 positive cells to the whole cultured cells was as shown in the results of this example. The knowledge that it may exceed 30% was obtained. The frequency of non-selective increase of CD3-positive cells was about 30% of the experiments in which NK cells were amplified using peripheral blood mononuclear cells collected by apheresis from patients with advanced cancer ( Data is not shown.) Therefore, in order to selectively amplify NK cells, it was attempted to repeat the step of removing CD3-positive cells.
- NK cells were amplified and cell number and cell surface markers were analyzed.
- Mononuclear cell suspensions were prepared from patients with advanced cancer (oral cancer, gallbladder cancer and bile duct cancer). Removal of CD3 positive cells was performed once or twice.
- CD3-negative cells were cultured in the KBM medium for 14 days.
- FIG. 15 is a bar graph showing the composition ratio of NK cells (CD3 negative / CD56 positive) with respect to the whole cultured cells after removal of CD3 positive cells once and twice.
- the error bar for each experimental condition indicates the standard error of the measured value of the experimental result repeated three times under the same condition.
- the composition ratio of NK cells, CD3 positive cells and other cells the percentages of NK cells, CD3 positive cells and other cells in the total cultured cells of each experimental group, measured by flow cytometry, are percentages. expressed.
- the vertical axis of the graph is the composition ratio (%) of NK cells, CD3 positive cells and other cells to the whole cultured cells, and the horizontal axis is the number of removals of CD3 positive cells.
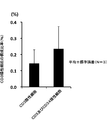
- the composition ratio (%) of the NK cells to the whole cultured cells was about 50% when the CD3 positive cells were removed once and about 65% when the CD3 positive cells were removed twice.
- NK cells were amplified and cell number and cell surface markers were analyzed.
- Mononuclear cell suspensions were prepared from patients with advanced cancer (oral cancer, gallbladder cancer and bile duct cancer). After removal of CD3 positive cells, hematopoietic progenitor cells were removed.
- the removal of the hematopoietic progenitor cells is performed by removing cells expressing CD34 (CD34 positive cells), biotinylated anti-CD34 antibody (343523, BioLegend Japan Co., Ltd.) and magnetic beads (Dynabeads biotin binder, 110-47, Life Technology Japan Co., Ltd.). Briefly, the CD34 positive cells were reacted with the biotinylated anti-CD34 antibody. Thereafter, centrifugation was performed, the supernatant was removed, and a suspension of cells bound with the antibody was prepared. The magnetic beads are washed once with 0.1% BSA was added PBS, the cells 10 7 per 50 ⁇ L is added to the suspension.
- the suspension containing the magnetic beads was stirred with a rotator at 4 ° C. for 30 minutes.
- the magnetic beads were separated from the suspension by a magnet, and CD34 positive cells were removed.
- the remaining cells hereinafter referred to as “CD3 and CD34 negative cells” in the suspension were cultured in the KBM medium for 14 days.
- an anti-CD34 antibody (343505, BioLegend Japan Co., Ltd.) was additionally used.
- FIG. 16A is a bar graph showing the composition ratio of CD34 positive cells in CD3 negative cells before amplification and CD3 and CD34 negative cells.
- FIG. 16B is a bar graph showing the composition ratio of CD3 positive cells in CD3 negative cells before amplification and CD3 and CD34 negative cells.
- the error bar for each experimental condition indicates the standard error of the measured value of the experimental result repeated three times under the same condition.
- the composition ratio of CD34 positive cells and CD3 positive cells the ratio of CD34 positive cells and CD3 positive cells in all cells of each experimental group, measured by flow cytometry, is expressed as a percentage.
- the vertical axis of the graph represents the composition ratio (%) of CD34 positive cells and CD3 positive cells before amplification to the whole cells.
- the horizontal axis of the graph indicates the cell type of each experimental group for amplification.
- the composition ratio (%) of CD34 positive cells before amplification was about 0.15% for CD3 negative cells and about 0.02% for CD3 and CD34 negative cells.
- the composition ratio (%) of CD3 positive cells before amplification was about 0.15% for CD3 negative cells and about 0.25% for CD3 and CD34 negative cells.
- FIG. 17 is a bar graph showing the composition ratio of NK cells (CD3 negative / CD56 positive) to the whole cultured cells in the amplified CD3 negative cells and CD3 and CD34 negative cells.
- the error bar for each experimental condition indicates the standard error of the measured value of the experimental result repeated three times under the same condition.
- the composition ratio of NK cells, CD3 positive cells and other cells the percentages of NK cells, CD3 positive cells and other cells in the total cultured cells of each experimental group, measured by flow cytometry, are percentages. expressed.
- the vertical axis of the graph represents the composition ratio (%) of NK cells, CD3 positive cells and other cells to the whole cultured cells.
- the horizontal axis of the graph indicates the cell type of each experimental group used for amplification.
- the composition ratio (%) of NK cells after amplification to the whole cultured cells was about 60% for CD3 negative cells and about 90% for CD3 and CD34 negative cells.
- NK cells CD3 negative / CD56 positive
- the composition ratio of NK cells was remarkably increased by removing CD3 positive cells and CD34 positive cells.
- NK cells are amplified using peripheral blood mononuclear cells collected by apheresis
- NK cells are amplified with high purity by removing CD3-positive cells and CD34-positive cells. It was shown that it can be done.
- NK cells could be prepared in large quantities by removing CD3-positive cells (T cells) from peripheral blood-derived mononuclear cells. Further, the cells amplified by the method of the present invention had a very high cytotoxic activity, as revealed by the experimental results of this example. Furthermore, NK cells could be prepared with high purity by removing CD3-positive cells (T cells) and CD34-positive cells (hematopoietic progenitor cells) from mononuclear cells derived from peripheral blood.
- cytotoxic activity of NK cells is low in the currently reported NK cell amplification methods.
- NK cells derived from peripheral blood of healthy subjects
- Patent Literature 1). Tanaka, J .;
- the purity is 96.8%
- the amplification factor is 277 times
- the present invention is remarkably superior to the prior art because the cytotoxic activity of NK cells is high and there is no risk of the feeder cells being mixed into the final product. Therefore, the present invention is useful for preparing NK cells having high cytotoxic activity in large quantities with high purity from collected blood cells.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Biomedical Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biotechnology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Genetics & Genomics (AREA)
- Wood Science & Technology (AREA)
- Epidemiology (AREA)
- Cell Biology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Hematology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Virology (AREA)
- Developmental Biology & Embryology (AREA)
- Molecular Biology (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Abstract
Description
1.材料及び方法
(1)末梢血からの採血
末梢血が、健常者と、進行癌(口腔癌、胆嚢癌及び胆管癌)の患者とから採取された。本実験は、九州大学医系地区部局臨床研究倫理審査委員会の承認(承認番号22?176、承認日:平成23年3月31日)を得て実施された。書面による同意が、前記健常者及び前記患者から得られている。採血、凍結保存、及び、解凍は当業者に周知の方法で行われた。
得られた血液は、常温に保たれた希釈液(1mM EDTA及び2%ウシ胎仔血清が添加されたPBS)で2倍希釈され、各遠心管に、希釈血20ないし35mLが、10ないし15mLのFicoll Paque(比重1.077)に重層された。遠心は、500×g、室温で20分間行われ、ブレーキをかけずに停止された。遠心上清(血漿部分)は数mLを残して除去され、中間層が回収された。遠心管1ないし2本から回収された前記中間層が1本の新たな遠心管に集められ、前記希釈液により体積が50mLに調整された。2回目の遠心は、500×g、室温、5分間又は15分間の条件で行われた。上清は除去され、ペレットが、前記希釈液30mLに懸濁された。3回目の遠心は、280×g、室温10分間の条件で行われた。上清は除去され、ペレットは、細胞濃度が1×107個/mLになるように、2mM EDTAと、0.1%BSAとが添加されたPBSに懸濁された(以下、「単核球懸濁液」という。)。
抗CD3抗体が固定化された磁気ビーズ(Dynabeads CD3)は、0.1%BSAが添加されたPBSで1回洗浄された後、前記単核球懸濁液に細胞107個あたり50μLが添加された。前記ビーズを含む単核球懸濁液は、4°Cで30分間ローテーターにて攪拌された。その後、前記磁気ビーズは磁石によって前記懸濁液から分離され、CD3を細胞表面に発現する細胞(CD3陽性細胞)が除去された。
前記懸濁液中の残りの細胞(以下、「CD3陰性細胞」という。)は、5%自家血清が添加された細胞培養用培地(KBM501、16025015、コージンバイオ株式会社;1750JRU/mLのIL-2含有)(以下、「KBM培地」という。)で5×105個/mLになるように希釈され、6ウェルの培養プレート(140675、nunc、サーモフィッシャーサイエンティフィック株式会社)に播種された。細胞培養は、37°C、5%CO2及び飽和水蒸気雰囲気下で21日間行われた。培地交換が、培養5日目、9日目、13日目及び17日目に行われた。前記細胞は、フィーダー細胞なしで培養された。
前記末梢血単核球の細胞数は、培養開始時から21日目までの間に血球計算盤を用いて生細胞数を計測することにより決定された。前記細胞の細胞表面マーカーは、抗CD3抗体(317308、BioLegend Japan 株式会社)、抗CD16抗体(556618、BD Pharmingen、日本ベクトン・ディッキンソン株式会社)、抗CD56抗体(304607、318321、BioLegend Japan 株式会社)、抗CD69抗体(310905、BioLegend Japan 株式会社)、抗KIR3DL1/KIR3DL2抗体(130-095-205、ミルテニーバイオテク株式会社)、抗KIR2DL3抗体(FAB2014P、R&D SYSTEMS社、コスモ・バイオ株式会社)、抗KIR2DL1/KIR2DS1抗体(339505、BioLegend Japan 株式会社)、抗KIR2DL5抗体(341303、BioLegend Japan 株式会社)、抗NKp46抗体(331907、BioLegend Japan 株式会社)、抗NKp30抗体(325207、BioLegend Japan 株式会社)、及び、抗NKG2D抗体(320805、BioLegend Japan 株式会社)を用いて、フロー・サイトメトリー法で解析された。
(1)健常者のNK細胞の増幅
図1Aは、CD3陽性細胞の除去前に、CD3及びCD56に対する抗体で2重染色し、フロー・サイトメトリー法で測定した実験結果である。図1Bは、CD3陽性細胞の除去後に、CD3及びCD56に対する抗体で2重染色しフロー・サイトメトリー法で測定した実験結果である。「CD3陽性細胞の構成比率」では、フロー・サイトメトリー法により計測された、各実験群の全培養細胞中のCD3陽性細胞の割合が百分率で表される。CD3陽性細胞の構成比率(%)は、CD3陽性細胞の除去前には69.37%であり、CD3陽性細胞の除去後には0.68%であった。これらの結果から明らかなとおり、CD3陽性細胞は単核球懸濁液から顕著に除去された。
図6Aは、進行癌(口腔癌、胆嚢癌及び胆管癌)の患者3人から分離されたNK細胞(CD3陰性/CD56陽性)の培養細胞全体に対する構成比率の経時的な変化をフロー・サイトメトリー法で測定した実験結果である。図6Bは、進行癌(口腔癌、胆嚢癌及び胆管癌)の患者3人から分離されたNK細胞(CD3陰性/CD56陽性)の増幅倍率の平均増殖曲線である。「NK細胞の構成比率」では、フロー・サイトメトリー法により計測された、各実験群の全培養細胞中のNK細胞の割合が百分率で表される。図6Aのグラフでは、縦軸は培養細胞全体に対するNK細胞(CD3陰性/CD56陽性)の構成比率(%)で、横軸は培養日数である。「NK細胞の増幅倍率」では、増幅後のNK細胞の細胞数を増幅前に末梢血単核球中に存在したNK細胞の細胞数で除算した結果が表される。図6Bのグラフでは、縦軸はNK細胞の増幅倍率で、横軸は培養日数である。各実験条件の標準偏差は、同一条件で3回繰り返した実験結果の測定値から算出された。図6Aに示されるとおり、NK細胞の構成比率は、培養開始時から14日目まで顕著に増大し続け、14日間培養後には約85%に増大した。また、図6Bに示されるとおり、NK細胞の増幅倍率は、培養開始時から14日目まで顕著に増大し続け、14日間培養後には約140倍に増大した。21日間培養後には、CD3陽性細胞が増殖したために、NK細胞の構成比率が低下した。しかし、前記CD3陽性細胞の増殖は、NK細胞の増幅にはほとんど影響しなかった。以上の結果から、進行癌(口腔癌、胆嚢癌及び胆管癌)の患者から分離されたNK細胞は、経時的に増幅することが示された。また、本発明は、癌、感染症等に罹患した患者から分離されたNK細胞を経時的に増幅できることが示唆された。
図7、9及び11に、各細胞表面マーカーのフロー・サイトメトリー解析結果を比較したグラフを示す。また、図8及び10に、CD69及びCD16のフロー・サイトメトリー解析結果を比較した平均蛍光強度(MFI)の測定値のグラフを示す。各実験条件の標準偏差は、同一条件で3回繰り返した実験結果の測定値から算出された。図7ないし11から明らかなとおり、本発明の方法で増幅された細胞は、増幅前の細胞と比較して、CD69、KIR2DL3、KIR2DL1/KIR2DS1、KIR2DL5、NKp30、及び、NKG2Dを強く発現していた。特に、前記増幅された細胞では、CD69の発現は、約100%であった。これらの図から明らかなとおり、本発明の方法で調製された細胞は、NK細胞としての分化マーカーを発現することが示された。また、前記NK細胞は、高い細胞傷害活性を備えていることが示唆された。
1.材料及び方法
NK細胞は実施例1で説明された方法に従って健常者から調製された。2500IU/mLのIL-2(AF-200-02-2、PeproTech、東洋紡績株式会社)と、5%自家血清とを添加したCellGro SCGM(2001、セルジェニックス、岩井化学薬品株式会社)(以下、「CellGro培地」という。)が細胞培養用培地として調製された。前記NK細胞は、実施例1で説明された方法に従って前記KBM培地及び前記CellGro培地で増幅された。
図12は、KBM培地と、CellGro培地とで培養されたNK細胞の増幅倍率の増殖曲線である。前記増幅倍率は、7日間培養後、14日間培養後及び21日間培養後のNK細胞の細胞数を、培養開始時のNK細胞の細胞数で除算した商として算出された。各実験条件の標準偏差は、同一条件で2回繰り返した実験結果の測定値から算出された。NK細胞の増幅倍率は、KBM培地及びCellGro培地で、培養開始時から21日目まで増大し続けた。21日間培養後、前記増幅倍率は、KBM培地では約670倍であり、CellGro培地では約140倍であった。
1.材料及び方法
(1)細胞傷害活性の定量
NK細胞が、実施例1で説明された方法に従って調製され、エフェクター細胞として用いられた。K562細胞(慢性骨髄性白血病細胞)が当業者に周知の方法で調製され、標的細胞として用いられた。増幅されたNK細胞と、増幅されていないNK細胞(以下、「非増幅NK細胞」という。)との細胞傷害活性が、当業者に周知の方法で定量された。簡潔には、前記標的細胞は、3-3’-ジオクタデシロキサカルボシアニン(D4292、シグマ アルドリッチ ジャパン株式会社)(終濃度:0.01mM)を添加したRPMI-1640培地で10分間培養することによって標識された。前記標的細胞は、標識後、PBS(-)及び無血清IMDM培地を用いて3回洗浄された。前記エフェクター細胞と、前記標的細胞とは、丸底の96ウェルの培養プレートに播種され、無血清IMDM培地で2時間共培養された。エフェクター細胞と標的細胞との比(E:T比)は、3対1、2対1、1対1、1対5、及び、1対10に調製された。細胞傷害活性(%)は、抗MHCクラスI抗体(311409、BioLegend Japan 株式会社)及び7-アミノ-アクチノマイシンD(A9400、シグマ アルドリッチ ジャパン株式会社)を用いてフロー・サイトメトリー法によって定量された。
NK細胞は、実施例1で説明された方法に従って増幅された。培養開始時、3日間培養後、7日間培養後、14日間培養後及び21日間培養後に、前記NK細胞と、前記K562細胞とは、2対1のE:T比で2時間共培養された。その後、前記NK細胞におけるCD107a陽性細胞の構成比率が、抗CD107a抗体(328606、BioLegend Japan 株式会社)を用いてフロー・サイトメトリー法で解析された。
(1)細胞傷害活性の定量
図13は、本発明の方法で増幅された末梢血由来NK細胞のK562に対する細胞傷害活性を調べた実験結果を示すグラフである。縦軸は細胞傷害活性(単位:%)である。白色棒は非増幅NK細胞の細胞傷害活性を示し、黒色棒は増幅されたNK細胞の細胞傷害活性を示す。横軸は、増幅されたNK細胞又は非増幅NK細胞と、K562細胞とのE:T比である。E:T比が3対1であるとき、前記細胞傷害活性は、非増幅NK細胞では約30%であり、増幅されたNK細胞では約110%であった。E:T比が2対1であるとき、前記細胞傷害活性は、非増幅NK細胞では約20%であり、増幅されたNK細胞では約107%であった。E:T比が1対1であるとき、前記細胞傷害活性は、非増幅NK細胞では約10%であり、増幅されたNK細胞では約100%であった。E:T比が、1対5及び1対10であるとき、増幅されたNK細胞の前記細胞傷害活性は、それぞれ、約25%及び約15%であった。
図14は、健常者から分離されたCD107a陽性細胞の培養細胞全体に対する構成比率の経時的な変化をフロー・サイトメトリー法で測定した実験結果である。各実験条件の標準偏差は、同一条件で5回繰り返した実験結果の測定値から算出された。「CD107a陽性細胞の構成比率」では、フロー・サイトメトリー法により計測された、各実験群の全培養細胞中のCD107a陽性細胞の割合が百分率で表される。図14のグラフでは、縦軸は培養細胞全体に対するCD107a陽性細胞の構成比率(%)で、横軸は培養日数である。CD107a陽性細胞の構成比率は、培養開始時から3日目までに約35%まで増大され、前記構成比率は、21日目でも維持された。
実施例1ないし3の実験後、さらにNK細胞の増幅実験を重ねるなかで、CD3陽性細胞が非選択的に増大し、CD3陽性細胞の培養細胞全体に対する構成比率が本実施例の結果のように30%を超える場合があるとの知見が得られた。このCD3陽性細胞の非選択的な増大の頻度は、進行癌の患者よりアフェレーシス法で採取された末梢血単核球細胞を利用してNK細胞を増幅した実験のうち約30%であった(データは示されない。)。そこで、NK細胞を選択的に増幅するために、CD3陽性細胞を除去するステップを繰り返すことが試みられた。
実施例1で説明された方法に従って、NK細胞は増幅され、細胞数及び細胞表面マーカーが解析された。単核球懸濁液は進行癌(口腔癌、胆嚢癌及び胆管癌)の患者から調製された。CD3陽性細胞の除去が、1回又は2回実施された。CD3陰性細胞は前記KBM培地で14日間培養された。
図15は、CD3陽性細胞の1回除去及び2回除去後のNK細胞(CD3陰性/CD56陽性)の培養細胞全体に対する構成比率を示す棒グラフである。各実験条件の誤差棒は同一条件で3回繰り返した実験結果の測定値の標準誤差を示す。NK細胞、CD3陽性細胞及び他の細胞の構成比率では、フロー・サイトメトリー法により計測された、各実験群の全培養細胞中のNK細胞、CD3陽性細胞及び他の細胞の割合それぞれが百分率で表される。グラフの縦軸は培養細胞全体に対するNK細胞、CD3陽性細胞及び他の細胞の構成比率(%)であり、横軸はCD3陽性細胞の除去回数である。NK細胞の培養細胞全体に対する構成比率(%)は、CD3陽性細胞の1回除去では約50%であり、CD3陽性細胞の2回除去では約65%であった。
1.材料及び方法
実施例1で説明された方法に従って、NK細胞は増幅され、細胞数及び細胞表面マーカーが解析された。単核球懸濁液は進行癌(口腔癌、胆嚢癌及び胆管癌)の患者から調製された。CD3陽性細胞の除去後、造血前駆細胞が除去された。前記造血前駆細胞の除去は、CD34を細胞表面に発現する細胞(CD34陽性細胞)を、ビオチン化抗CD34抗体(343523、BioLegend Japan 株式会社)と、磁気ビーズ(Dynabeads biotin binder、110-47、ライフテクノロジーズジャパン株式会社)とを用いて行われた。簡潔には、前記CD34陽性細胞と、前記ビオチン化抗CD34抗体とが反応された。その後、遠心分離が行われ、上清が除去され、前記抗体が結合した細胞の懸濁液が調製された。前記磁気ビーズは、0.1%BSAが添加されたPBSで1回洗浄後、細胞107個あたり50μLが前記懸濁液に添加された。前記磁気ビーズを含む懸濁液は、4°Cで30分間ローテーターにて攪拌された。前記磁気ビーズは磁石によって前記懸濁液から分離され、CD34陽性細胞が除去された。前記懸濁液中の残りの細胞(以下、「CD3及びCD34陰性細胞」という。)は前記KBM培地で14日間培養された。フロー・サイトメトリー法での計測では、抗CD34抗体(343505、BioLegend Japan 株式会社)が追加的に用いられた。
図16Aは、増幅前のCD3陰性細胞と、CD3及びCD34陰性細胞とにおけるCD34陽性細胞の構成比率を示す棒グラフである。図16Bは、増幅前のCD3陰性細胞と、CD3及びCD34陰性細胞とにおけるCD3陽性細胞の構成比率を示す棒グラフである。各実験条件の誤差棒は同一条件で3回繰り返した実験結果の測定値の標準誤差を示す。CD34陽性細胞及びCD3陽性細胞の構成比率では、フロー・サイトメトリー法により計測された、各実験群の全細胞中のCD34陽性細胞及びCD3陽性細胞の割合が百分率で表される。グラフの縦軸は細胞全体に対する増幅前のCD34陽性細胞及びCD3陽性細胞の構成比率(%)である。グラフの横軸は、増幅用の各実験群の細胞タイプを示す。増幅前のCD34陽性細胞の構成比率(%)は、CD3陰性細胞では約0.15%であり、CD3及びCD34陰性細胞では約0.02%であった。増幅前のCD3陽性細胞の構成比率(%)は、CD3陰性細胞では約0.15%であり、CD3及びCD34陰性細胞では約0.25%であった。
以上の実験結果から明らかなとおり、末梢血由来の単核球からCD3陽性細胞(T細胞)を除去することによって、NK細胞を大量に調製することができた。また本発明の方法で増幅された細胞は、本実施例の実験結果で明らかにされたとおり、非常に高い細胞傷害活性を有した。さらに、末梢血由来の単核球からCD3陽性細胞(T細胞)及びCD34陽性細胞(造血前駆細胞)を除去することによって、NK細胞を高純度で調製することができた。
Claims (11)
- NK細胞を含む細胞集団を調製するステップと、
前記NK細胞を含む細胞集団からT細胞を除去するステップと、
前記T細胞が除去された残りの細胞を、2500IU/mLないし2813IU/mLのIL-2を含む培地で培養するステップとを含むことを特徴とする、NK細胞の増幅方法。 - 前記NK細胞を含む細胞集団から前記T細胞を除去するステップは、CD3陽性細胞を除去するステップによって達成されることを特徴とする、請求項1に記載のNK細胞の増幅方法。
- 前記NK細胞を含む細胞集団から造血前駆細胞を除去するステップを含むことを特徴とする、請求項1又は2に記載のNK細胞の増幅方法。
- 前記NK細胞を含む細胞集団から前記造血前駆細胞を除去するステップは、CD34陽性細胞を除去するステップによって達成されることを特徴とする、請求項3に記載のNK細胞の増幅方法。
- 前記培地は、自家血清、AB型血清、及び/又は、血清アルブミンを含むことを特徴とする、請求項1ないし4のいずれか1つに記載のNK細胞の増幅方法。
- 前記NK細胞を含む細胞集団を調製するステップは、被験者から採取された血球細胞から単核球を分離するステップによって達成されることを特徴とする、請求項1ないし5のいずれか1つに記載のNK細胞の増幅方法。
- 前記血球細胞は、末梢血、臍帯血、骨髄及び/又はリンパ節から採取されることを特徴とする、請求項6に記載のNK細胞の増幅方法。
- 前記血球細胞は末梢血からアフェレーシス法により採取されることを特徴とする、請求項7に記載のNK細胞の増幅方法。
- 前記NK細胞を含む細胞集団は、胚性幹細胞、成体幹細胞及び人工多能性幹(iPS)細胞からなるグループから選択されるいずれかの幹細胞由来の造血幹細胞と、臍帯血由来の造血幹細胞と、末梢血由来の造血幹細胞と、骨髄血由来の造血幹細胞と、臍帯血単核球と、末梢血単核球とからなる群から選択される少なくとも1種類の細胞から調製されることを特徴とする、請求項1ないし5のいずれか1つに記載の方法。
- 請求項1ないし9のいずれか1つに記載の増幅方法によって調製されるNK細胞を含むことを特徴とする、細胞療法のための医薬品組成物。
- 感染症及び/又は癌を治療するために用いられることを特徴とする、請求項10に記載の医薬品組成物。
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| HK14103574.2A HK1190432B (en) | 2011-06-24 | 2012-06-20 | Method for amplifying nk cells |
| EP12801859.5A EP2725100B1 (en) | 2011-06-24 | 2012-06-20 | Method for amplifying nk cells |
| CA2840161A CA2840161C (en) | 2011-06-24 | 2012-06-20 | Method for amplifying nk cells |
| CN201280031188.5A CN103620022B (zh) | 2011-06-24 | 2012-06-20 | Nk细胞的扩增方法 |
| KR1020147001579A KR101963920B1 (ko) | 2011-06-24 | 2012-06-20 | Nk 세포의 증폭 방법 |
| AU2012274478A AU2012274478B2 (en) | 2011-06-24 | 2012-06-20 | Method for amplifying NK cells |
| US14/129,143 US9404083B2 (en) | 2011-06-24 | 2012-06-20 | Method for amplifying NK cells |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011-140725 | 2011-06-24 | ||
| JP2011140725 | 2011-06-24 | ||
| JP2012-021972 | 2012-02-03 | ||
| JP2012021972A JP5572863B2 (ja) | 2011-06-24 | 2012-02-03 | Nk細胞の増幅方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012176796A1 true WO2012176796A1 (ja) | 2012-12-27 |
Family
ID=47422628
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/065718 WO2012176796A1 (ja) | 2011-06-24 | 2012-06-20 | Nk細胞の増幅方法 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US9404083B2 (ja) |
| EP (1) | EP2725100B1 (ja) |
| JP (1) | JP5572863B2 (ja) |
| KR (1) | KR101963920B1 (ja) |
| CN (1) | CN103620022B (ja) |
| AU (1) | AU2012274478B2 (ja) |
| CA (1) | CA2840161C (ja) |
| MY (1) | MY161389A (ja) |
| WO (1) | WO2012176796A1 (ja) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018207900A1 (ja) * | 2017-05-12 | 2018-11-15 | 米満 吉和 | 高活性nk細胞、およびその利用 |
| JP2019137696A (ja) * | 2019-05-20 | 2019-08-22 | 米満 吉和 | 高活性nk細胞、およびその利用 |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5511039B1 (ja) * | 2013-05-22 | 2014-06-04 | 国立大学法人九州大学 | Nk細胞の調製方法 |
| JP6164650B2 (ja) * | 2014-01-20 | 2017-07-19 | 国立大学法人九州大学 | Nk細胞の調製方法 |
| WO2015132415A1 (en) * | 2014-03-07 | 2015-09-11 | Emercell Sas | Pooled nk cells from ombilical cord blood and their uses for the treatment of cancer and chronic infectious disease |
| KR101697473B1 (ko) | 2014-11-26 | 2017-01-18 | 주식회사 녹십자랩셀 | T 세포를 이용한 자연살해세포의 배양방법 |
| WO2016122014A1 (ko) * | 2015-01-27 | 2016-08-04 | 한국생명공학연구원 | 자연살해세포의 대량생산 방법 및 상기 방법으로 수득된 자연살해세포의 항암제로서의 용도 |
| CN106222141B (zh) * | 2016-10-17 | 2018-10-19 | 湖南丰晖生物科技有限公司 | Nk细胞培养液和细胞培养方法 |
| EP3633029A4 (en) | 2017-05-26 | 2021-06-09 | Green Cross Lab Cell Corporation | METHOD OF DEVELOPING A NATURAL KILLER CELL USING A T-CELL |
| MY199514A (en) | 2018-02-01 | 2023-11-02 | Nkmax Co Ltd | Method of producing natural killer cells and composition for treating cancer |
| WO2019152663A1 (en) * | 2018-02-01 | 2019-08-08 | Nkmax Co., Ltd. | Method of producing natural killer cells and composition for treating cancer |
| KR102765799B1 (ko) * | 2018-02-01 | 2025-02-13 | 주식회사 엔케이맥스 | Cd56+ 자연살해세포를 포함하는 항암용 조성물 |
| JP6543375B1 (ja) * | 2018-03-27 | 2019-07-10 | 株式会社ガイアバイオメディシン | ケモカインレセプターと細胞接着分子を発現するcd3陰性細胞の集団、およびその利用 |
| CN110628714B (zh) * | 2018-06-21 | 2023-03-28 | 精准生技股份有限公司 | 用于体外扩增自然杀手细胞及自然杀手t细胞的无血清细胞培养液 |
| CN109161527A (zh) * | 2018-09-25 | 2019-01-08 | 深圳市五零生命科技有限公司 | 一种高效的nk细胞扩增方法 |
| AU2019381526B2 (en) * | 2018-11-14 | 2023-02-23 | Green Cross Lab Cell Corporation | Method for culturing cord blood-derived natural killer cells using transformed T-cells |
| WO2020152661A1 (ja) | 2019-01-21 | 2020-07-30 | 株式会社ガイアバイオメディシン | Nk細胞を含む細胞集団の製造方法 |
| JP6838750B2 (ja) * | 2019-01-21 | 2021-03-03 | 株式会社ガイアバイオメディシン | Nk細胞を含む細胞集団の製造方法 |
| KR102234394B1 (ko) * | 2019-03-08 | 2021-03-31 | 신지섭 | 타가면역세포배양방법, 그 방법으로 얻어진 면역세포배양액 및 이를 포함하는 면역세포치료제 |
| KR102216710B1 (ko) * | 2019-03-27 | 2021-02-17 | 신지섭 | Nk세포배양배지용 첨가조성물, 상기 첨가조성물을 이용한 nk세포배양방법 및 상기 배양방법으로 얻어진 피부트러블개선용 화장료조성물 |
| CN111154721B (zh) * | 2020-01-14 | 2023-10-17 | 深圳格泰赛尔生物科技有限公司 | Nk细胞扩增方法 |
| US20230040477A1 (en) * | 2020-01-19 | 2023-02-09 | Board Of Regents, The University Of Texas System | T-cell death associated gene 8 (tdag8) modulation to enhance cellular cancer therapies |
| AU2021401052A1 (en) | 2020-12-18 | 2023-06-22 | Century Therapeutics, Inc. | Chimeric antigen receptor systems with adaptable receptor specificity |
| WO2023081813A1 (en) | 2021-11-05 | 2023-05-11 | St. Jude Children's Research Hospital, Inc. | Zip cytokine receptors |
| WO2023240182A1 (en) | 2022-06-08 | 2023-12-14 | St. Jude Children's Research Hospital, Inc. | Disruption of kdm4a in t cells to enhance immunotherapy |
| WO2024059787A1 (en) | 2022-09-16 | 2024-03-21 | St. Jude Children's Research Hospital, Inc. | Disruption of asxl1 in t cells to enhance immunotherapy |
| WO2025022372A1 (ja) * | 2023-07-21 | 2025-01-30 | 株式会社ガイアバイオメディシン | 細胞集団の製造方法 |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007103901A2 (en) * | 2006-03-06 | 2007-09-13 | Government Of The United States Of America, Represented By The Secretary, Department Of Health And Human Services | Autologous natural killer cells and lymphodepleting chemotherapy for the treatment of cancer |
| JP2007297292A (ja) | 2006-04-28 | 2007-11-15 | Kyorin Pharmaceut Co Ltd | 1−アルコキシイソキノリン誘導体又はその水和物の製造方法 |
| WO2008153150A1 (ja) * | 2007-06-15 | 2008-12-18 | Medinet Co., Ltd. | Nk細胞を含む細胞集団の培養方法及び当該細胞集団の利用 |
| WO2010013947A2 (ko) * | 2008-07-29 | 2010-02-04 | 주식회사 녹십자 | 자연살해세포의 증식방법 |
| WO2011030851A1 (ja) * | 2009-09-11 | 2011-03-17 | タカラバイオ株式会社 | ナチュラルキラー細胞の製造方法 |
| JP2011517944A (ja) * | 2008-04-09 | 2011-06-23 | マックスサイト インコーポレーティッド | 新規に単離された細胞の治療組成物の操作および送達 |
| JP2011140504A (ja) | 2006-08-28 | 2011-07-21 | Hisamitsu Pharmaceut Co Inc | 爪用貼付剤 |
| JP2011239701A (ja) * | 2010-05-14 | 2011-12-01 | Tella Inc | 樹状細胞の培養方法 |
| JP2013006793A (ja) | 2011-06-24 | 2013-01-10 | Tella Inc | Nk細胞を増幅するための組成物及び方法 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030068306A1 (en) * | 2001-09-14 | 2003-04-10 | Dilber Mehmet Sirac | Medium |
| US7544355B2 (en) * | 2002-03-13 | 2009-06-09 | Universita Degli Studi Di Perugia | Methods and compositions for allogeneic transplantation |
| CA2510787A1 (en) * | 2002-12-23 | 2004-07-08 | Innate Pharma | Pharmaceutical compositions having an effect on the proliferation of nk cells and a method using the same |
| US7435596B2 (en) | 2004-11-04 | 2008-10-14 | St. Jude Children's Research Hospital, Inc. | Modified cell line and method for expansion of NK cell |
| WO2006050270A2 (en) * | 2004-11-02 | 2006-05-11 | The Government Of The United States Of America As Represented By The Secretary Department Of Health & Human Services | Compositions and methods for treating hyperproliferative disorders |
| JP4275680B2 (ja) | 2006-04-28 | 2009-06-10 | 裕 照沼 | リンパ球の活性・増殖に係る培養方法 |
| EP2052075A4 (en) * | 2006-08-23 | 2010-05-26 | Binex Co Ltd | METHOD OF PREPARING ACTIVATED LYMPHOCYTES FOR IMMUNOTHERAPY |
| NZ599825A (en) * | 2007-09-28 | 2014-10-31 | Anthrogenesis Corp | Tumor suppression using human placental perfusate and human placenta-derived intermediate natural killer cells |
| PT2411507T (pt) | 2009-03-26 | 2020-01-09 | Cellprotect Nordic Pharmaceuticals Ab | Expansão de células nk |
| DK3184109T3 (da) | 2009-12-29 | 2021-02-15 | Gamida Cell Ltd | Fremgangsmåder til forbedring af natural killer-cellers proliferation og aktivitet |
| KR101039843B1 (ko) | 2010-08-30 | 2011-06-09 | 주식회사 엔케이바이오 | 자기활성화 림프구 배양용 배지 조성물 및 이를 이용한 자기활성화 림프구 배양방법 |
-
2012
- 2012-02-03 JP JP2012021972A patent/JP5572863B2/ja active Active
- 2012-06-20 EP EP12801859.5A patent/EP2725100B1/en active Active
- 2012-06-20 AU AU2012274478A patent/AU2012274478B2/en active Active
- 2012-06-20 CA CA2840161A patent/CA2840161C/en active Active
- 2012-06-20 US US14/129,143 patent/US9404083B2/en active Active
- 2012-06-20 KR KR1020147001579A patent/KR101963920B1/ko active Active
- 2012-06-20 CN CN201280031188.5A patent/CN103620022B/zh active Active
- 2012-06-20 MY MYPI2013004630A patent/MY161389A/en unknown
- 2012-06-20 WO PCT/JP2012/065718 patent/WO2012176796A1/ja active Application Filing
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2007103901A2 (en) * | 2006-03-06 | 2007-09-13 | Government Of The United States Of America, Represented By The Secretary, Department Of Health And Human Services | Autologous natural killer cells and lymphodepleting chemotherapy for the treatment of cancer |
| JP2007297292A (ja) | 2006-04-28 | 2007-11-15 | Kyorin Pharmaceut Co Ltd | 1−アルコキシイソキノリン誘導体又はその水和物の製造方法 |
| JP2011140504A (ja) | 2006-08-28 | 2011-07-21 | Hisamitsu Pharmaceut Co Inc | 爪用貼付剤 |
| WO2008153150A1 (ja) * | 2007-06-15 | 2008-12-18 | Medinet Co., Ltd. | Nk細胞を含む細胞集団の培養方法及び当該細胞集団の利用 |
| JP2011517944A (ja) * | 2008-04-09 | 2011-06-23 | マックスサイト インコーポレーティッド | 新規に単離された細胞の治療組成物の操作および送達 |
| WO2010013947A2 (ko) * | 2008-07-29 | 2010-02-04 | 주식회사 녹십자 | 자연살해세포의 증식방법 |
| WO2011030851A1 (ja) * | 2009-09-11 | 2011-03-17 | タカラバイオ株式会社 | ナチュラルキラー細胞の製造方法 |
| JP2011239701A (ja) * | 2010-05-14 | 2011-12-01 | Tella Inc | 樹状細胞の培養方法 |
| JP2013006793A (ja) | 2011-06-24 | 2013-01-10 | Tella Inc | Nk細胞を増幅するための組成物及び方法 |
Non-Patent Citations (10)
| Title |
|---|
| "Research Ethics Committee of Departments", MEDICAL FACILITIES OF KYUSHU UNIVERSITY, 31 March 2011 (2011-03-31) |
| ALICI, E. ET AL., BLOOD, vol. 111, 2008, pages 3155 |
| CARLENS, S. ET AL., HUM. IMMUNOL., vol. 62, 2001, pages 1092 |
| CHO, D.; CAMPANA, D., KOREAN J. LAB. MED., 2009, pages 29 - 89 |
| FUJISAKI, H. ET AL., CANCER RES., vol. 69, 2009, pages 4010 |
| KOHJIN BIO CO., LTD.: "KBM501 Soshiki Baiyo Kanren Baichi no Kaihatsu", HANBAI, 28 July 2010 (2010-07-28), XP055139607, Retrieved from the Internet <URL:http://web.archive.org/web/20100728100847/http://www.kohjin-bio.co.jp/products/?id=1269767360-072037> [retrieved on 20120911] * |
| MALE,V. ET AL.: "Immature NK Cells, Capable of Producing IL-22, Are Present in Human Uterine Mucosa", JOURNAL OF IMMUNOLOGY, vol. 185, no. 7, 2010, pages 3913 - 3918, XP055139608 * |
| MILLERS ET AL., BLOOD, vol. 105, 2005, pages 3051 |
| RUBNITZ ET AL., J. CLIN. ONCOL., vol. 28, 2010, pages 955 |
| See also references of EP2725100A4 |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2018207900A1 (ja) * | 2017-05-12 | 2018-11-15 | 米満 吉和 | 高活性nk細胞、およびその利用 |
| JP2018193303A (ja) * | 2017-05-12 | 2018-12-06 | 米満 吉和 | 高活性nk細胞、およびその利用 |
| US11723924B2 (en) | 2017-05-12 | 2023-08-15 | Yoshikazu Yonemitsu | Highly active NK cell and use thereof |
| TWI846668B (zh) * | 2017-05-12 | 2024-07-01 | 米満吉和 | 高活性nk細胞及其利用 |
| US12329817B2 (en) | 2017-05-12 | 2025-06-17 | Yoshikazu Yonemitsu | Highly active NK cell and use thereof |
| JP2019137696A (ja) * | 2019-05-20 | 2019-08-22 | 米満 吉和 | 高活性nk細胞、およびその利用 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20140120072A1 (en) | 2014-05-01 |
| CA2840161C (en) | 2019-02-12 |
| MY161389A (en) | 2017-04-14 |
| JP2013027385A (ja) | 2013-02-07 |
| CA2840161A1 (en) | 2012-12-27 |
| EP2725100B1 (en) | 2019-09-04 |
| EP2725100A1 (en) | 2014-04-30 |
| JP5572863B2 (ja) | 2014-08-20 |
| AU2012274478B2 (en) | 2017-03-30 |
| KR101963920B1 (ko) | 2019-03-29 |
| AU2012274478A8 (en) | 2016-07-28 |
| CN103620022B (zh) | 2015-09-30 |
| EP2725100A4 (en) | 2015-01-07 |
| AU2012274478A1 (en) | 2014-02-20 |
| HK1190432A1 (en) | 2014-07-04 |
| KR20140051263A (ko) | 2014-04-30 |
| CN103620022A (zh) | 2014-03-05 |
| US9404083B2 (en) | 2016-08-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5572863B2 (ja) | Nk細胞の増幅方法 | |
| JP5511039B1 (ja) | Nk細胞の調製方法 | |
| US12329817B2 (en) | Highly active NK cell and use thereof | |
| KR102534472B1 (ko) | 케모카인 리셉터와 세포 접착 분자를 발현하는 cd3 음성 세포의 집단, 및 그 이용 그리고 그 제조 방법 | |
| JP5989016B2 (ja) | Nk細胞の増幅方法 | |
| JP2013006793A (ja) | Nk細胞を増幅するための組成物及び方法 | |
| TWI757709B (zh) | 含有nk細胞之細胞集團之製造方法 | |
| JP6164650B2 (ja) | Nk細胞の調製方法 | |
| JP6697611B2 (ja) | 高活性nk細胞、およびその利用 | |
| JP2020108405A (ja) | 高活性nk細胞、およびその利用 | |
| HK1190432B (en) | Method for amplifying nk cells | |
| JP2023153286A (ja) | Nk細胞を含む細胞集団の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12801859 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2840161 Country of ref document: CA |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14129143 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 20147001579 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2012274478 Country of ref document: AU Date of ref document: 20120620 Kind code of ref document: A |