WO2012074054A1 - 活性炭粉末とその製造方法、及び電気二重層キャパシタ - Google Patents
活性炭粉末とその製造方法、及び電気二重層キャパシタ Download PDFInfo
- Publication number
- WO2012074054A1 WO2012074054A1 PCT/JP2011/077803 JP2011077803W WO2012074054A1 WO 2012074054 A1 WO2012074054 A1 WO 2012074054A1 JP 2011077803 W JP2011077803 W JP 2011077803W WO 2012074054 A1 WO2012074054 A1 WO 2012074054A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- activated carbon
- carbon powder
- cellulose acetate
- range
- powder according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/30—Electrodes characterised by their material
- H01G11/32—Carbon-based
- H01G11/42—Powders or particles, e.g. composition thereof
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/30—Active carbon
- C01B32/312—Preparation
- C01B32/336—Preparation characterised by gaseous activating agents
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/24—Electrodes characterised by structural features of the materials making up or comprised in the electrodes, e.g. form, surface area or porosity; characterised by the structural features of powders or particles used therefor
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/30—Electrodes characterised by their material
- H01G11/32—Carbon-based
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01G—CAPACITORS; CAPACITORS, RECTIFIERS, DETECTORS, SWITCHING DEVICES, LIGHT-SENSITIVE OR TEMPERATURE-SENSITIVE DEVICES OF THE ELECTROLYTIC TYPE
- H01G11/00—Hybrid capacitors, i.e. capacitors having different positive and negative electrodes; Electric double-layer [EDL] capacitors; Processes for the manufacture thereof or of parts thereof
- H01G11/22—Electrodes
- H01G11/30—Electrodes characterised by their material
- H01G11/32—Carbon-based
- H01G11/34—Carbon-based characterised by carbonisation or activation of carbon
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/12—Surface area
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/14—Pore volume
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2006/00—Physical properties of inorganic compounds
- C01P2006/16—Pore diameter
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/13—Energy storage using capacitors
Definitions
- the present invention relates to activated carbon powder and a method for producing the same.
- the present invention also relates to an electric double layer capacitor using the activated carbon powder as an electrode active material.
- Activated carbon is widely used in various industrial fields for purposes such as air purification, radioactive material adsorption, iodine trap, methane occlusion, hydrogen occlusion, purified water production, solvent recovery, decolorization, water treatment, and gas mask applications. It's being used. In recent years, it is also used as an electrode active material for capacitors (electric double layer capacitors, lithium ion capacitors), and there is a demand for higher functionality.
- An electric double layer capacitor is an energy storage device using an electric double layer formed at an interface between an electrode and an electrolyte.
- the activated carbon as the electrode active material used in this electric double layer capacitor is preferably larger in specific surface area because the interface with the electrolytic solution becomes wider and the capacitance increases. Therefore, activated carbon having a large specific surface area is used as the electrode active material of the electric double layer capacitor.
- the activated carbon is generally roughly classified into activated carbon fibers made of fibrous activated carbon particles and activated carbon powder made of non-fibrous (granular) activated carbon particles.
- Non-Patent Document 1 describes that activated carbon for an electric double layer capacitor requires pores of 2 nm or more to form the same electric double layer capacitance at low temperature and room temperature (p.80). . Also, p. Table 7 in 79 shows phenol-based activated carbon fibers having a specific surface area of ⁇ 2500 m 2 / g, an average pore diameter of 20 to 40 mm (2 to 4 nm), and a cumulative pore volume of 0.5 to 1.5 cc / g. Are listed. However, the phenol-based activated carbon fiber described in Non-Patent Document 1 is an activated carbon fiber obtained by carbonization activation of a special phenol resin fiber (novoloid fiber) (p.73-75).
- This non-patent document 1 also describes rayon-based activated carbon fibers using rayon (cellulose) fibers as raw materials and acrylic-based activated carbon fibers using polyacrylonitrile fibers as raw materials other than phenol-based activated carbon fibers.
- the fiber has a specific surface area as small as 1000 to 1500 m 2 / g and an average pore diameter as small as 14 mm (1.4 nm).
- An acrylic activated carbon fiber also has a specific surface area as small as 700 to 1200 m 2 / g, an average pore diameter as small as 10 mm (1.0 nm), and a cumulative pore volume of up to 1.1 cc / g. .
- Patent Document 1 discloses an activated carbon powder for an electric double layer capacitor having a phosphorus atom content of 1000 to 20000 ppm, a BET specific surface area of 1600 to 2200 m 2 / g, and an average pore diameter of 1.7 to 2.1 nm. Therefore, a phosphorus compound composite activated carbon powder having a pore diameter of 1.4 to 2.0 nm and a pore volume of 0.25 cm 3 / g or more is described. However, the upper limit of the pore volume is preferably described as 0.5 cm 3 / g or less ([0032]).
- Patent Document 1 as a method for producing the above-mentioned activated carbon powder, a first heating step in which an activated carbon raw material and phosphoric acid are kneaded at 130 to 170 ° C., molded, and heated at 100 to 230 ° C. is then included. And a second heating step of heating at 400 to 600 ° C., followed by firing at 800 ° C. or higher in an inert gas atmosphere to combine activated carbon and a phosphorus compound.
- activated carbon materials include hardwoods, softwoods and their waste, corn ears, coffee beans, rice fir, fruit seeds, fruit shells and other debris such as molasses and lignin, coal, tar, pitch , Asphalt and petroleum residues.
- Patent Document 2 describes a method for producing activated carbon powder by carbonizing cellulose acetate (cellulose acylate) to produce a carbide, and activating the obtained carbide. There is no description of the specific surface area, average pore diameter and pore volume of the powder. However, the activated carbon powder obtained in the example of Patent Document 2 has an iodine adsorption amount of about 1144 mg / g, and it is presumed that the specific surface area is not so high.
- the phenol-based activated carbon fiber described in Non-Patent Document 1 has a large specific surface area, average pore diameter, and pore volume, and is considered to be one of useful materials as an electrode active material for electric double layer capacitors. It is done.
- the phenol-based activated carbon fiber described in Non-Patent Document 1 uses a novoloid fiber, which is a special phenol resin fiber, as a raw material, and therefore has low versatility and a problem in production cost.
- the fibrous activated carbon particles are more likely to have gaps between the particles when formed into the electrodes than the non-fibrous activated carbon particles, the activated carbon fibers are likely to have a lower packing density than the activated carbon powder.
- the activated carbon powder described in Patent Document 1 is inferior to the phenol-based activated carbon fiber described in Non-Patent Document 1 in terms of specific surface area, average pore diameter, and pore volume.
- the object of the present invention is that the specific surface area, average pore diameter, and pore volume characteristics are equal to or better than those of conventional phenol-based activated carbon fibers, and can be advantageously used as an electrode active material for electric double layer capacitors.
- An object of the present invention is to provide an activated carbon powder and a method for producing the same.
- Another object of the present invention is to provide an electric double layer capacitor having a large electric capacity.
- the present inventor heated the cellulose acetate to carbonize, and the carbonized product obtained in the carbonization step is heated at a temperature higher by 50 ° C. than the temperature at which the cellulose acetate is carbonized in the carbonization step to remain in the carbide.
- the BET specific surface area is in the range of 1600 to 3000 m 2 / g by using an acetic acid removing step for volatilizing and removing the acetic acid component to be removed and an activation step for activating the carbide from which acetic acid has been removed in the acetic acid removing step.
- an activated carbon powder having an average pore diameter in the range of 2.0 to 4.0 nm and a total pore volume in the range of 1.0 to 3.0 cm 3 / g can be obtained. I found it.
- the activated carbon powder was confirmed to show a high capacitance when used as an electrode active material of an electric double layer capacitor, and the present invention was completed.
- the present invention has a BET specific surface area in the range of 1600 to 3000 m 2 / g, an average pore diameter in the range of 2.0 to 4.0 nm, and a total pore volume of 1.0 to
- the activated carbon powder is in the range of 3.0 cm 3 / g.
- Preferred embodiments of the activated carbon powder of the present invention are as follows.
- the BET specific surface area is in the range of 2100 to 3000 m 2 / g, particularly in the range of 2600 to 3000 m 2 / g.
- the average pore diameter is in the range of 2.2 to 2.8 nm.
- the total volume of the pores is in the range of 1.1 to 2.5 cm 3 / g.
- the average aspect ratio is 5 or less, more preferably 3 or less, and particularly preferably 2 or less.
- the average particle size is in the range of 1 to 30 ⁇ m.
- the present invention is also an electric double layer capacitor including a positive electrode, a negative electrode, and an electrolytic solution, wherein at least one of the positive electrode and the negative electrode includes the activated carbon powder of the present invention.
- the present invention further includes a carbonization step in which cellulose acetate is heated to carbonize, and the carbide obtained in the carbonization step is heated to a temperature higher by 50 ° C. than the temperature at which cellulose acetate is carbonized in the carbonization step.
- the method for producing the activated carbon powder of the present invention also includes an acetic acid removing step for volatilizing and removing the remaining acetic acid component, and an activation step for activating the carbide from which acetic acid has been removed in the acetic acid removing step.
- the preferable aspect of the manufacturing method of the activated carbon powder of the said invention is as follows. (1) In the carbonization step, cellulose acetate is heated and carbonized in the presence of a phosphorus compound. (2) The cellulose acetate is cellulose acetate containing a phosphorus compound. (3) The cellulose acetate contains a phosphorus compound in the range of 0.1 to 5.0% by mass as the amount of phosphorus. (4) The cellulose acetate is a mixture of cellulose acetate substantially free of a phosphorus compound and cellulose acetate containing a phosphorus compound. (5) A mixture of cellulose acetate and a phosphorus compound is heated to carbonize the cellulose acetate.
- cellulose acetate is heated and carbonized at a temperature of 250 to 350 ° C. in an inert gas atmosphere.
- the carbide is heated at a temperature of 380 to 700 ° C. in an inert gas atmosphere.
- the carbide is heated at a temperature of 800 to 1100 ° C. in a gas atmosphere selected from the group consisting of carbon dioxide gas, water vapor, oxygen gas, hydrogen chloride gas, ammonia gas and air.
- the activated carbon powder of the present invention exhibits a high capacitance under contact with the electrolytic solution of the electric double layer capacitor. Moreover, the activated carbon powder of the present invention is non-fibrous and has a uniform particle shape and particle size compared to the activated carbon fiber, so that the packing density can be increased. Therefore, the electric double layer capacitor using the activated carbon powder of the present invention as an electrode active material exhibits a high electric capacity. In addition, the electrode active material for electric double layer capacitors is required to be reduced in cost, and from this point, the activated carbon powder of the present invention that can be produced using cellulose acetate as a raw material is more advantageous than activated carbon fibers. .
- the activated carbon powder of the present invention has a large BET specific surface area, it can be advantageously used as an activated carbon powder for gas phase adsorption, gas storage, water purification, or decolorization. Further, by using the production method of the present invention, there is an advantage that high-performance activated carbon powder can be advantageously produced industrially using cellulose acetate which is usually discarded as a raw material.
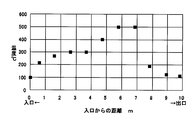
- FIG. 6 is a graph showing a temperature distribution in a roller hearth kiln used for producing activated carbon powders of Examples 3 to 5.
- the activated carbon powder of the present invention consists of fine activated carbon particles.
- the activated carbon particles are non-fibrous, and the average aspect ratio (ratio between the major axis and the minor axis of the activated carbon particles: major axis / minor axis) is generally 5 or less, preferably 3 or less, particularly preferably 2 or less.
- the activated carbon powder of the present invention has a BET specific surface area of 1600 to 3000 m 2 / g, preferably 2100 to 3000 m 2 / g, more preferably 2300 to 3000 m 2 / g, particularly preferably 2600. It is in the range of ⁇ 3000 m 2 / g.
- an electric double layer is formed at the interface between the activated carbon powder and the electrolyte. Therefore, the larger the BET specific surface area of the activated carbon powder, the greater the capacitance. Get higher. Therefore, it is advantageous that the activated carbon powder is in contact with the electrolyte solution with a large specific surface area. For this reason, as described below, the average pore diameter and pore volume of the activated carbon powder are important.
- the activated carbon powder of the present invention has an average pore diameter in the range of 2.0 to 4.0 nm, preferably in the range of 2.0 to 3.5 nm, more preferably in the range of 2.0 to 2.8 nm, particularly preferably. It is in the range of 2.2 to 2.8 nm.
- the average pore diameter is an indicator of the ease of penetration of the electrolyte into the pores of the activated carbon powder that occurs when the activated carbon powder is placed in contact with the electrolyte of the electric double layer capacitor. That is, the larger the average pore diameter, the easier it is for the electrolyte to enter the pores of the activated carbon powder.
- the average pore diameter is excessively large, the number of pores that can be formed in the activated carbon powder decreases, and therefore the BET specific surface area of the activated carbon powder tends to decrease.
- the activated carbon powder of the present invention has a total pore volume in the range of 1.0 to 3.0 cm 3 / g, preferably in the range of 1.1 to 2.5 cm 3 / g.
- the total pore volume is also an indicator of the ease of entry of the electrolyte into the pores of the activated carbon powder that occurs when the activated carbon powder is placed in contact with the electrolyte of the electric double layer capacitor. That is, the larger the total pore volume, the greater the amount of penetration of the electrolytic solution into the pores of the activated carbon powder. However, when the total pore volume becomes excessively large, the strength of the activated carbon powder becomes weak.
- the activated carbon powder of the present invention preferably has an iodine adsorption amount in the range of 1600 to 2300 mg / g, and more preferably in the range of 2000 to 2300 mg / g.
- the ratio (BET specific surface area / iodine adsorption amount) of the BET specific surface area expressed in units of m 2 / g to the iodine adsorption amount value expressed in units of mg / g is 1.1 to 2.0. It is preferably in the range, more preferably in the range of 1.1 to 1.5.
- the activated carbon powder of the present invention preferably has an average particle size in the range of 1 to 30 ⁇ m, and more preferably in the range of 3 to 20 ⁇ m.
- the activated carbon powder of the present invention may contain a phosphorus compound.
- the amount of the phosphorus compound contained in the activated carbon powder is preferably 5.0% by mass or less as the amount of phosphorus, more preferably in the range of 0.3 to 1% by mass, and 0.3 to 0.7%. It is particularly preferable to be in the range of mass%.
- the activated carbon powder of the present invention is heated, for example, by carbonizing the cellulose acetate by heating cellulose acetate, and by heating the carbide obtained in the carbonizing step at a temperature 50 ° C. or higher than the temperature when carbonizing the cellulose acetate in the carbonizing step.
- it can be produced by a method including an acetic acid removing step of volatilizing and removing the acetic acid component remaining in the carbide and an activation step of activating the carbide from which acetic acid has been removed in the acetic acid removing step.
- the cellulose acetate used as a starting material preferably has a degree of substitution of acetic acid in the range of 2 to 3.
- the cellulose acetate is preferably cellulose mainly composed of triacetyl cellulose.
- limiting in the form of a cellulose acetate Any form of flake form, a powder form, and a lump form may be sufficient.
- Cellulose acetate may contain a plasticizer.
- plasticizers include phosphate esters, esters of carboxylic acids and alcohols, and polyesters.
- phosphate esters include triphenyl phosphate, tricresyl phosphate, cresyl diphenyl phosphate, octyl diphenyl phosphate, diphenyl biphenyl phosphate, trioctyl phosphate, and tributyl phosphate.
- Examples of carboxylic acids include phthalic acid, citric acid, oleic acid, ricinoleic acid and sebacic acid.
- alcohols examples include aliphatic alcohols (preferably aliphatic alcohols having 1 to 6 carbon atoms), glycolic acid, glycols (preferably glycols having 2 to 3 carbon atoms), glycerol, diglycerol, pentaerythritol. And dipentaerythritol.
- esters include esters of phthalic acid with aliphatic alcohols (eg, dimethyl phthalate, diethyl phthalate, dibutyl phthalate, dioctyl phthalate, diethyl hexyl phthalate) and esters of citric acid with aliphatic alcohols (eg, acetyl citrate) Triethyl, acetyltributyl citrate).
- esters include polyesters of aromatic dicarboxylic acids and glycols.
- Cellulose acetate is used as a support for photographic films and as a protective film material for polarizing plates for liquid crystal display devices.
- cellulose acetate recovered from defective products produced in the production process of photographic films and the production process of polarizing plates for liquid crystal display devices can be used as a raw material.
- the carbonization step it is preferable to heat and carbonize cellulose acetate in the presence of a phosphorus compound.
- the phosphorus compound is preferably added to the starting cellulose acetate before heating. That is, the starting material is preferably cellulose acetate containing a phosphorus compound, a mixture of cellulose acetate substantially free of a phosphorus compound and cellulose acetate containing a phosphorus compound, or a mixture of cellulose acetate and a phosphorus compound.
- the phosphorus compound-containing cellulose acetate preferably contains the phosphorus compound in the range of 0.1 to 5.0% by mass, more preferably 0.1 to 3.0% by mass as the phosphorus amount, It is particularly preferable that the content be in the range of 0.1 to 1.0% by mass.
- the phosphorus compound is preferably a phosphate ester. Examples of the phosphate ester are as described above.
- the phosphorus compound is preferably molecularly dispersed in cellulose acetate.
- the cellulose acetate substantially free of a phosphorus compound means one having a phosphorus content of less than 0.1% by mass, particularly less than 0.01% by mass.
- the phosphorus compound-containing cellulose acetate mixed with the cellulose acetate substantially free of the phosphorus compound is as described above.
- the mixing ratio of cellulose acetate substantially free of phosphorus compound and phosphorus compound-containing cellulose acetate is preferably in the range of 30:70 to 70:30 by mass ratio.
- Examples of phosphorus compounds mixed with cellulose acetate include phosphoric acid, phosphates and phosphate esters.
- Phosphoric acid includes orthophosphoric acid and condensed phosphoric acid.
- Examples of phosphates include ammonium salts, alkali metal salts, and alkaline earth metal salts.
- Examples of the phosphate ester are as described above.
- the phosphorus compound is preferably a phosphate ester.
- the mixture of cellulose acetate and phosphorus compound preferably contains the phosphorus compound in the range of 0.1 to 5.0% by mass, more preferably in the range of 0.1 to 3.0% by mass. It is particularly preferable that it be contained in the range of 0.1 to 1.0 mass%.
- the cellulose acetate may be a phosphorus compound-containing cellulose acetate or a cellulose acetate substantially free of a phosphorus compound.
- the carbonization step it is preferable to carbonize cellulose acetate by heating it at a temperature of 250 to 350 ° C. in an inert gas atmosphere.
- the inert gas include noble gases such as nitrogen gas and argon gas, helium gas, xenon gas, and neon gas.
- the heating time is at least until the cellulose acetate becomes a carbide, but is usually in the range of 5 to 180 minutes, for example, in the range of 5 to 30 minutes or 30 to 120 minutes.
- cellulose acetate is usually carbonized after it is once melted and then solidified.
- the raw material cellulose acetate contains a phosphorus compound
- the phosphorus compound can be uniformly dispersed in the carbide.
- pores are formed by volatilizing and removing acetic acid remaining in the carbide in the subsequent acetic acid removing step. In this carbonization step, part of acetic acid is usually removed by volatilization.
- the acetic acid removal step it is preferable to remove the carbide by heating the carbide in an inert gas atmosphere to volatilize the acetic acid component.
- the heating temperature is generally in the range of 380 to 700 ° C, preferably in the range of 500 to 650 ° C.
- the inert gas include noble gases such as nitrogen gas and argon gas, helium gas, xenon gas, and neon gas.
- the heating time is generally in the range of 10 minutes to 10 hours, preferably in the range of 30 minutes to 5 hours.
- many passages formed when the acetic acid component volatilizes from the carbide are formed as pores in the carbide.
- the formation of a large number of pores in the carbide in the acetic acid removing step can be confirmed by measuring the iodine adsorption amount of the carbide.
- the carbide before the acetic acid removal step usually has an iodine adsorption amount of 100 mg / g or less, but the carbide after the acetic acid removal step usually has an iodine adsorption amount in the range of 300 to 800 mg / g, particularly 400 to 800 mg / g. The range will be significantly larger.
- an oxidizing gas such as carbon dioxide gas, water vapor, oxygen, or air is mixed with the above inert gas, pore formation may be promoted.
- the amount of the oxidizing gas used is preferably 20% by mass or less, particularly preferably 5 to 15% by mass with respect to the total amount of the inert gas and the oxidizing gas. .
- the oxidizing gas may be mixed with an inert gas in the carbonization step, and the acetic acid removal step may be continuously performed in the same atmosphere.
- the amount of acetic acid remaining in the carbide after the acetic acid removal step is preferably 20% by mass or less, particularly preferably 10% by mass or less as the acetic acid content with respect to the total amount of carbides. preferable.
- the volatile gas containing acetic acid generated in the carbonization step and the acetic acid removal step may be burned or liquefied and recovered.
- the recovered liquid containing acetic acid can be used as a raw material for agricultural products, industrial acetic acid and fuel. Further, by adding the recovered liquid to the raw material cellulose acetate, the formation of carbide pores in the acetic acid removing step is promoted, and the characteristics of the activated carbon are improved.
- the activation treatment is preferably performed by heating the carbide in the presence of an activation gas.
- the activation gas include carbon dioxide gas, water vapor, oxygen gas, hydrogen chloride gas, ammonia gas, and air.
- the activation gas carbon dioxide gas and water vapor are preferable, and water vapor is particularly preferable.
- the heating temperature is generally in the range of 800 to 1100 ° C., preferably in the range of 900 to 1100 ° C.
- the heating time is generally in the range of 10 minutes to 10 hours, preferably in the range of 30 minutes to 5 hours.
- a mixed gas containing carbon monoxide gas and carbon dioxide gas When carbon dioxide gas is used as the activation gas, what is discharged in the activation process is a mixed gas containing carbon monoxide gas and carbon dioxide gas.
- the discharged mixed gas may be recovered and used as an activation gas.
- the mixed gas it is preferable to convert the carbon monoxide gas contained in the mixed gas into carbon dioxide gas in advance to increase the amount of carbon dioxide gas in the mixed gas.
- a method for converting carbon monoxide gas in the mixed gas into carbon dioxide gas a method in which the mixed gas is brought into contact with the oxidation catalyst in the presence of oxygen, a method in which the mixed gas is brought into contact with the shift catalyst in the presence of water vapor, and a mixed gas Can be mentioned in the presence of oxygen.
- a known heating furnace can be used for carrying out the carbonization step, the acetic acid removal step and the activation step.
- the heating furnace may be a batch type or a continuous type.
- Examples of the batch heating furnace include a charcoal kiln type carbonization furnace, a stirring type carbonization furnace, a trolley type carbonization furnace, and a fluidized bed type carbonization furnace.
- In the continuous heating furnace there is no particular limitation on the method of conveying the object to be heated in the furnace. Examples of the conveyance method of the object to be heated include a roller type, a belt conveyor type, a fluidized bed type, a rotary kiln type, and a screw conveyor type. In terms of production efficiency, it is preferable to use a continuous heating furnace.
- Each step of the carbonization step, the acetic acid removal step and the activation step may be performed sequentially using separate heating furnaces, or may be performed continuously using one heating furnace. Further, the carbonization step and the acetic acid removal step may be continuously performed using one heating furnace, and the activation step may be performed using another heating furnace, or the acetic acid removal step and the activation step may be performed using one heating furnace. The carbonization step may be performed using another heating furnace.
- the carbonization step and the acetic acid removal step be performed using a continuous heating furnace in which the object to be heated in the furnace is conveyed by a roller or a belt conveyor.
- a continuous heating furnace it is preferable to use a roller-type roller hearth kiln as a method for conveying an object to be heated because it is easy to control the temperature in the furnace.
- the first heating area adjusted to a temperature of 250 to 350 ° C. in the furnace, and the second heating area adjusted to a temperature of 380 to 700 ° C. higher than the first heating area by 50 ° C. or more
- It is preferable to perform the carbonization step and the acetic acid removal step by conveying the heat-resistant container containing the raw material cellulose acetate from the first region to the second region. Two or more heat-resistant containers can be stacked to obtain a homogeneous carbide.
- the activation step is preferably performed using a rotary kiln in order to increase production efficiency.
- a pulverizer such as a ball mill, a disk mill, a bead mill, and a jet mill can be used. It is preferable to use a ball mill (particularly a planetary ball mill) or a disk mill (particularly a stone mill type disc mill) as the grinding device.
- the grinding media for these mills are preferably made of alumina, ceramic or zirconia in order to prevent the metal from being mixed into the activated carbon.
- a stainless steel sieve or a cyclone classifier can be used.
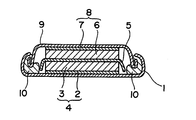
- FIG. 1 is a cross-sectional view of an example of an electric double layer capacitor according to the present invention.
- the electric double layer capacitor shown in FIG. 1 is an electric double layer capacitor generally called a coin type.
- an electric double layer capacitor includes a positive electrode container 1, a positive electrode sheet 4 loaded on the bottom surface of the positive electrode container 1, and a positive electrode sheet 4 formed by press-bonding a positive electrode current collector 2 and a positive electrode active material sheet 3.
- Both the positive electrode active material sheet 3 and the negative electrode active material sheet 6 are generally composed of a mixture of an electrode active material, a binder, and a conductive material.
- the activated carbon powder of the present invention is used for at least one (preferably both) of the positive electrode active material sheet 3 and the negative electrode active material sheet 6.
- the binder include polytetrafluoroethylene and polyvinylidene fluoride.
- the conductive material include acetylene black and carbon black.
- an organic solvent solution containing an electrolyte is generally used.
- the electrolyte include tetraalkylammonium hexafluorophosphate, tetraalkylphosphonium hexafluorophosphate, tetraalkylphosphonium tetrafluoroborate and tetraalkylammonium tetrafluoroborate. These electrolytes may be used individually by 1 type, and may use 2 or more types together.
- organic solvent examples include alkylene carbonates such as propylene carbonate and ethylene carbonate, ⁇ -butyrolactone, dimethylformamide, dimethyl sulfoxide, acetonitrile, tetrahydrofuran, dimethoxyethane, and methyl formate. These organic solvents may be used individually by 1 type, and may use 2 or more types together.
- concentration of the electrolyte in the electrolytic solution is generally in the range of 0.5 to 2.0 mol / L.
- a metal is generally used as a material for the positive electrode container 1, the positive electrode current collector 2, the negative electrode current collector 7, and the negative electrode container 9, a metal is generally used. Examples of metals include aluminum and stainless steel.
- a porous sheet is generally used as the separator 5, a porous sheet is generally used. Examples of the porous sheet include a glass wool sheet and a non-woven sheet. Resin is used for the material of the gasket 10. Examples of the resin include polypropylene, polyethylene, polybutylene, and polyamide.
- the electric double layer capacitor of the present invention is not limited to a coin-type electric double layer capacitor.
- the electric double layer capacitor of the present invention may be a wound type electric double layer capacitor.
- the wound type electric double layer capacitor is an electrode roll produced by winding a separator between a long positive electrode active material sheet and a long negative electrode active material sheet, and an electrolytic solution. Is an electric double layer capacitor that is sealed in a container.
- Example 1 ⁇ Manufacture of activated carbon powder> Cellulose acetate (phosphorus content: 0.1 to 5% by mass) containing triphenyl phosphate was pulverized into flakes and placed in a heat-resistant container. The container was covered with a thermometer, a nitrogen gas inlet and a gas outlet. Subsequently, it heated so that the internal temperature of a heat-resistant container might be 300 degreeC, supplying nitrogen gas to the nitrogen gas inlet of a heat-resistant container. The cellulose acetate flakes in the heat-resistant container dissolved and became liquid, and then carbonized to produce carbides (carbonization step).
- the internal temperature of the heat-resistant container was raised to 600 ° C. and held at that temperature for 1 hour to remove acetic acid remaining on the carbide (acetic acid removal step).
- acetic acid removal step After heating, the mixture was allowed to cool to room temperature, the lid was removed, and the carbide was taken out. It was 580 mg / g when the iodine adsorption amount of the carbide
- the acetic acid content in the carbide after the acetic acid removing step was 6.0% by mass.
- the carbide obtained in the acetic acid removing step was put into a rotary kiln furnace equipped with a nitrogen gas inlet, a carbon dioxide gas inlet, and a gas outlet.
- the rotary kiln furnace is rotated at a rotation speed of 1 rpm, and while the carbon dioxide gas is supplied to the carbon dioxide gas inlet at a flow rate of 16 L / min, the furnace temperature is raised to 1050 ° C. and held at that temperature for 3 hours.
- the carbide was activated (activation process). Then, it was allowed to cool, and when the furnace temperature reached 800 ° C., the supply of carbon dioxide gas was stopped, nitrogen gas was supplied to the nitrogen gas inlet, and when the furnace temperature reached 100 ° C.
- the activated charcoal (activated carbon) was taken out of the product.
- the obtained activated carbon was pulverized by a planetary ball mill using a zirconia container and balls, and then classified to obtain activated carbon powder.
- the phosphorus content of the obtained activated carbon powder was 0.56% by mass.
- the average particle diameter of the activated carbon powder was 3.8 ⁇ m.
- a coin-type electric double layer capacitor was manufactured and the capacitance was measured as follows.
- (1) Production of Coin Type Electric Double Layer Capacitor 10 mg of activated carbon powder, 4 mg of acetylene black and 2 mg of polytetrafluoroethylene (PTFE) were weighed and kneaded in a mortar.
- the obtained kneaded material was molded into a circular sheet having a diameter of 16 mm and used as an active material sheet.
- this active material sheet was pressure-bonded to a mesh-like aluminum current collector to produce an electrode sheet.
- Two electrode sheets were prepared, one being a positive electrode sheet and the other being a negative electrode sheet. Subsequently, the positive electrode sheet and the negative electrode sheet were heat-dried under reduced pressure.
- the heated and dried positive electrode sheet and negative electrode sheet were put in a glove box in an argon gas atmosphere, and a coin-type electric double layer capacitor as shown in FIG. 1 was manufactured in the glove box. That is, the positive electrode sheet was laminated in the positive electrode container so that the bottom surface of the positive electrode container and the aluminum current collector of the electrode sheet were in contact, and then a glass wool separator was laminated on the positive electrode sheet. Next, after the electrolytic solution (propylene carbonate solution containing 1.5 mol / L triethylmethylammonium hexafluorophosphate) is dropped into the glass wool separator as it is, the electrolytic solution is sufficiently infiltrated into the separator. The negative electrode sheet was laminated on the separator so that the separator surface and the active material sheet of the negative electrode sheet were in contact with each other. Finally, a negative electrode container was placed on the negative electrode sheet and sealed with a gasket.
- the electrolytic solution propylene carbonate solution containing 1.5 mol / L triethylmethyl
- the coin-type electric double layer capacitor was charged with a constant current of 1 mA (current density per electrode area: 0.5 mA / cm 2 ) until the voltage reached 3.0V.
- the charged coin-type electric double layer capacitor is discharged at a constant current of 1 mA until the voltage reaches 0 V, and a discharge curve is created in which the relationship between the discharge voltage and the discharge time of the coin-type electric double layer capacitor is plotted. did. From the slope of the discharge curve, the capacitance of the activated carbon powder was calculated according to a conventional method.
- Example 2 In the production of the activated carbon powder of Example 1, activated carbon powder was produced in the same manner as in Example 1 except that the heating temperature in the acetic acid removing step was 400 ° C.
- the carbide after the acetic acid removing step had an iodine adsorption amount of 386 mg / g and an acetic acid content of 6.0% by mass.
- the obtained activated carbon powder had a phosphorus content of 0.58% by mass and an average particle size of 3.7 ⁇ m.
- the obtained activated carbon powder was measured in the same manner as in Example 1 for the BET specific surface area, average pore diameter, pore total volume, iodine adsorption amount and capacitance. Table 1 shows the results.
- Example 1 The electrostatic capacity of the commercially available activated carbon powder for electric double layer capacitors was measured in the same manner as in Example 1. Table 1 shows the results, the specific surface area of the activated carbon powder, the total pore volume, and the iodine adsorption amount.
- the activated carbon powder according to the present invention has a higher BET specific surface area and a total pore volume than the commercially available activated carbon powder, and is used as an electrode active material for an electric double layer capacitor. It was confirmed that a high capacitance was exhibited.
- Example 3 An activated carbon powder was obtained in the same manner as in Example 1 except that cellulose acetate containing a polyester of phenylene dicarboxylic acid and ethylene diol in the range of 10 to 15% by mass was used instead of triphenyl phosphate.
- the carbide after the acetic acid removing step had an iodine adsorption amount of 500 mg / g and an acetic acid content of 5.0% by mass.
- the obtained activated carbon powder had a BET specific surface area of 2200 m 2 / g, an average pore diameter of 2.3 nm, a total pore volume of 1.30 cm 3 / g, and an iodine adsorption amount of 1800 mg / g.
- Example 4 Roller hearth kiln (length: 10 m) in which a flake pulverized product of cellulose acetate (phosphorus content: 1.5% by mass) containing triphenyl phosphate was placed in a heat-resistant container and the furnace temperature was adjusted to the temperature distribution shown in FIG. The carbonization step and the acetic acid removal step were continuously carried out at a speed of 1 m / hour.
- the horizontal axis indicates the distance from the entrance of the roller hearth kiln, and the vertical axis indicates the temperature at the distance.
- the obtained carbide had an iodine adsorption of 476 mg / g and an acetic acid content of 5.4% by mass.
- the obtained carbide was put into an electric furnace, and the activation process was performed by heating at 850 ° C. for 3 hours while introducing water vapor into the electric furnace at a rate of 0.5 mL / min.
- the activated carbide was pulverized in the same manner as in Example 1 and then classified to obtain activated carbon powder.
- the obtained activated carbon powder had a BET specific surface area of 2100 m 2 / g, an average pore diameter of 2.32 nm, a total pore volume of 1.19 cm 3 / g, and an iodine adsorption amount of 1803 mg / g.
- Example 5 In the activation step, activated carbon powder was obtained in the same manner as in Example 4 except that the heating time was 4 hours.
- the obtained activated carbon powder had a BET specific surface area of 2435 m 2 / g, an average pore diameter of 2.44 nm, a total pore volume of 1.48 cm 3 / g, and an iodine adsorption amount of 1837 mg / g.
- activated carbon powder was obtained in the same manner as in Example 4 except that carbon dioxide gas was introduced into the electric furnace at a rate of 200 mL / min and heated at a temperature of 950 ° C. for 3 hours.
- the obtained activated carbon powder had a BET specific surface area of 2775 m 2 / g, an average pore diameter of 3.77 nm, a total pore volume of 2.61 cm 3 / g, and an iodine adsorption amount of 2111 mg / g.
- Example 7 (Influence of phosphorus compound contained in cellulose acetate as starting material)
- the triphenyl phosphate used in Example 1 is contained in 100 parts by mass of the pulverized cellulose acetate containing polyester of phenylenedicarboxylic acid and ethylenediol used in Example 3 in the range of 10 to 15% by mass.
- 100 parts by mass of a flake pulverized product of cellulose acetate (phosphorus amount: 0.1 to 5% by mass) was added and mixed.
- An activated carbon powder was obtained by treating in the same manner as in Example 1 except that the obtained mixture (phosphorus content: 0.05 to 2.5% by mass) was used as a starting material.
- Table 2 shows the BET specific surface area, average pore diameter, total pore volume, and iodine adsorption amount of the obtained activated carbon powder together with the results of the activated carbon powder obtained in Example 3.
- Example 8 Influence of phosphorus compound contained in cellulose acetate as starting material 10 parts by mass of triphenyl phosphate was added to 100 parts by mass of a pulverized product obtained by pulverizing cellulose acetate containing a polyester of phenylene dicarboxylic acid and ethylene diol used in Example 3 in a range of 10 to 15% by mass. Part was added and mixed. An activated carbon powder was obtained by treating in the same manner as in Example 1 except that the obtained mixture (phosphorus content: 1% by mass) was used as a starting material. Table 2 shows the BET specific surface area, average pore diameter, total pore volume, and iodine adsorption amount of the obtained activated carbon powder.
- the activated carbon powder obtained shows high values for all of the BET specific surface area, average pore diameter, total pore volume, and iodine adsorption amount. It turns out that there is a tendency.
Landscapes
- Engineering & Computer Science (AREA)
- Power Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Materials Engineering (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Carbon And Carbon Compounds (AREA)
- Electric Double-Layer Capacitors Or The Like (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012521889A JP5099277B1 (ja) | 2010-12-03 | 2011-12-01 | 活性炭粉末とその製造方法、及び電気二重層キャパシタ |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010270411 | 2010-12-03 | ||
| JP2010-270411 | 2010-12-03 | ||
| JP2011-158980 | 2011-07-20 | ||
| JP2011158980 | 2011-07-20 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012074054A1 true WO2012074054A1 (ja) | 2012-06-07 |
Family
ID=46171979
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/077803 Ceased WO2012074054A1 (ja) | 2010-12-03 | 2011-12-01 | 活性炭粉末とその製造方法、及び電気二重層キャパシタ |
Country Status (3)
| Country | Link |
|---|---|
| JP (2) | JP5099277B1 (enExample) |
| TW (1) | TW201236039A (enExample) |
| WO (1) | WO2012074054A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014091653A (ja) * | 2012-11-05 | 2014-05-19 | Nankai Kogyo Kk | 活性炭の製造方法 |
| JP2015160775A (ja) * | 2014-02-27 | 2015-09-07 | 南開工業株式会社 | 活性炭の製造方法 |
| WO2015146459A1 (ja) * | 2014-03-27 | 2015-10-01 | Jx日鉱日石エネルギー株式会社 | 活性炭、活性炭の製造方法および活性炭の処理方法 |
| WO2016031423A1 (ja) * | 2014-08-28 | 2016-03-03 | Jx日鉱日石エネルギー株式会社 | 活性炭、活性炭の炭素原料およびこれらの製造方法 |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012074054A1 (ja) * | 2010-12-03 | 2012-06-07 | 南開工業株式会社 | 活性炭粉末とその製造方法、及び電気二重層キャパシタ |
| CN104934231A (zh) * | 2015-06-19 | 2015-09-23 | 中国第一汽车股份有限公司 | 一种超级电容器电极材料 |
| KR102020126B1 (ko) * | 2017-12-19 | 2019-09-10 | 주식회사 티씨케이 | 전극소재용 활성탄의 제조방법 |
| US20220266138A1 (en) * | 2021-02-22 | 2022-08-25 | Neople Inc. | Method and apparatus for providing touch screen interface |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH111314A (ja) * | 1997-06-10 | 1999-01-06 | Dainippon Ink & Chem Inc | 球状活性炭素材及びその製造方法 |
| JP2002033249A (ja) * | 2000-05-09 | 2002-01-31 | Mitsubishi Chemicals Corp | 電気二重層キャパシタ用活性炭 |
| JP2006075708A (ja) * | 2004-09-09 | 2006-03-23 | Optonix Seimitsu:Kk | 球状超微粒子及びその製造方法 |
| JP2007266248A (ja) * | 2006-03-28 | 2007-10-11 | Osaka Gas Co Ltd | 電気二重層キャパシタ用炭素材料、電気二重層キャパシタ用電極、及び電気二重層キャパシタ |
| JP2008201664A (ja) * | 2007-01-24 | 2008-09-04 | Fujifilm Corp | 活性炭製造方法及び廃棄フイルムのリサイクルシステム |
| WO2009063966A1 (ja) * | 2007-11-16 | 2009-05-22 | Asahi Kasei Kabushiki Kaisha | 非水系リチウム型蓄電素子 |
| JP2009269764A (ja) * | 2008-04-10 | 2009-11-19 | Kansai Coke & Chem Co Ltd | アルカリ賦活炭およびその製造方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5102855A (en) * | 1990-07-20 | 1992-04-07 | Ucar Carbon Technology Corporation | Process for producing high surface area activated carbon |
| JP2000100437A (ja) * | 1998-09-24 | 2000-04-07 | Daicel Chem Ind Ltd | 結着剤、積層体およびそれを用いたリチウム二次電池 |
| US20030157014A1 (en) * | 2000-04-27 | 2003-08-21 | Qing Wang | Pyrolyzed hard carbon material, preparation and its applications |
| JP4037673B2 (ja) * | 2002-02-20 | 2008-01-23 | ジ ェ ニ ス 株式会社 | 電磁波吸収体、およびその製造方法 |
| US20100324201A1 (en) * | 2007-06-29 | 2010-12-23 | Michigan Technological University | Process of forming radicalized polymer intermediates and radicalized polymer intermediate compositions |
| WO2012074054A1 (ja) * | 2010-12-03 | 2012-06-07 | 南開工業株式会社 | 活性炭粉末とその製造方法、及び電気二重層キャパシタ |
-
2011
- 2011-12-01 WO PCT/JP2011/077803 patent/WO2012074054A1/ja not_active Ceased
- 2011-12-01 JP JP2012521889A patent/JP5099277B1/ja not_active Expired - Fee Related
- 2011-12-02 TW TW100144407A patent/TW201236039A/zh unknown
-
2012
- 2012-09-10 JP JP2012198621A patent/JP5598683B2/ja not_active Expired - Fee Related
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH111314A (ja) * | 1997-06-10 | 1999-01-06 | Dainippon Ink & Chem Inc | 球状活性炭素材及びその製造方法 |
| JP2002033249A (ja) * | 2000-05-09 | 2002-01-31 | Mitsubishi Chemicals Corp | 電気二重層キャパシタ用活性炭 |
| JP2006075708A (ja) * | 2004-09-09 | 2006-03-23 | Optonix Seimitsu:Kk | 球状超微粒子及びその製造方法 |
| JP2007266248A (ja) * | 2006-03-28 | 2007-10-11 | Osaka Gas Co Ltd | 電気二重層キャパシタ用炭素材料、電気二重層キャパシタ用電極、及び電気二重層キャパシタ |
| JP2008201664A (ja) * | 2007-01-24 | 2008-09-04 | Fujifilm Corp | 活性炭製造方法及び廃棄フイルムのリサイクルシステム |
| WO2009063966A1 (ja) * | 2007-11-16 | 2009-05-22 | Asahi Kasei Kabushiki Kaisha | 非水系リチウム型蓄電素子 |
| JP2009269764A (ja) * | 2008-04-10 | 2009-11-19 | Kansai Coke & Chem Co Ltd | アルカリ賦活炭およびその製造方法 |
Non-Patent Citations (1)
| Title |
|---|
| HIROSHI YANAI ET AL., THE NIKKAN KOGYO SHINBUN, 27 July 1996 (1996-07-27), pages 90 * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014091653A (ja) * | 2012-11-05 | 2014-05-19 | Nankai Kogyo Kk | 活性炭の製造方法 |
| JP2015160775A (ja) * | 2014-02-27 | 2015-09-07 | 南開工業株式会社 | 活性炭の製造方法 |
| WO2015146459A1 (ja) * | 2014-03-27 | 2015-10-01 | Jx日鉱日石エネルギー株式会社 | 活性炭、活性炭の製造方法および活性炭の処理方法 |
| WO2016031423A1 (ja) * | 2014-08-28 | 2016-03-03 | Jx日鉱日石エネルギー株式会社 | 活性炭、活性炭の炭素原料およびこれらの製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201236039A (en) | 2012-09-01 |
| JP2013042146A (ja) | 2013-02-28 |
| JP5099277B1 (ja) | 2012-12-19 |
| JP5598683B2 (ja) | 2014-10-01 |
| JPWO2012074054A1 (ja) | 2014-05-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5598683B2 (ja) | 活性炭粉末製造原料用の炭化物の製造方法 | |
| JP4432493B2 (ja) | 活性炭の製造方法、分極性電極及び電気二重層キャパシタ | |
| US20170053748A1 (en) | Carbon activation method and energy storage device thereof | |
| JP6037498B2 (ja) | 金属酸化物担持炭素紙の製造方法及び金属酸化物担持炭素紙 | |
| JP2019108269A (ja) | 高活性表面積を有する活性炭 | |
| CN105122407A (zh) | 双电层电容器电极用活性炭及其制造方法 | |
| KR101459729B1 (ko) | 흑연 복합재 및 그 제조 방법 | |
| CN103782421A (zh) | 含表面氟化b型氧化钛粉末的锂离子电池用负极材料及其制造方法、和使用其的锂离子电池 | |
| JP6571043B2 (ja) | 炭素繊維の製造方法、炭素繊維及び電気二重層キャパシタ用電極 | |
| JP2017014079A (ja) | 活性炭の製造方法、活性炭及び電気二重層キャパシタ用電極材料 | |
| JP2013249234A (ja) | 活性炭とその製造方法 | |
| WO2016031423A1 (ja) | 活性炭、活性炭の炭素原料およびこれらの製造方法 | |
| JP6895825B2 (ja) | 多孔質焼成体の作製方法 | |
| CN102496475A (zh) | 一种基于石墨烯的超级电容电极片及其制备方法 | |
| CN119660701A (zh) | 磷酸铁锂正极材料及其制备方法、电化学装置 | |
| KR102139098B1 (ko) | 전극활물질 및 그의 제조방법 | |
| JP3664331B2 (ja) | 黒鉛微結晶 | |
| JP2000228193A (ja) | 非水系二次電池用炭素質負極活物質及び非水系二次電池 | |
| JP5573404B2 (ja) | 電気二重層キャパシタ電極用活性炭の製造方法 | |
| KR20200065994A (ko) | 캐패시터용 전극재의 제조방법 | |
| JP2016150870A (ja) | 活性炭の製造方法及び活性炭を含む電極 | |
| KR102157482B1 (ko) | 전극활물질 및 그의 제조방법 | |
| CN111600022A (zh) | 一种二氧化锡包覆镍钴锰酸锂材料及其制备方法和应用 | |
| JP4394209B2 (ja) | 活性炭の製造方法 | |
| JP4069822B2 (ja) | 活性炭の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012521889 Country of ref document: JP |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11845463 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11845463 Country of ref document: EP Kind code of ref document: A1 |