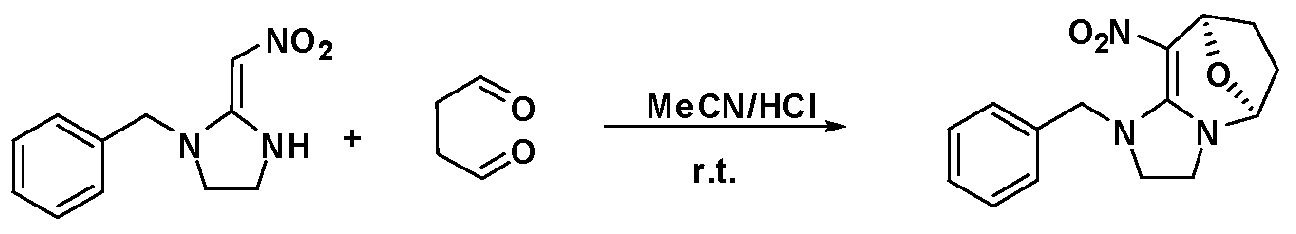WO2011069456A1 - 双联和氧桥杂环新烟碱化合物及其制备方法 - Google Patents
双联和氧桥杂环新烟碱化合物及其制备方法 Download PDFInfo
- Publication number
- WO2011069456A1 WO2011069456A1 PCT/CN2010/079591 CN2010079591W WO2011069456A1 WO 2011069456 A1 WO2011069456 A1 WO 2011069456A1 CN 2010079591 W CN2010079591 W CN 2010079591W WO 2011069456 A1 WO2011069456 A1 WO 2011069456A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- group
- formula
- carbonyl
- hours
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 C*C1C(*CC2)C2(C)NC(C*(C)=S)CCC1 Chemical compound C*C1C(*CC2)C2(C)NC(C*(C)=S)CCC1 0.000 description 4
- KATOGIJHEDYLRI-DHZHZOJOSA-N [O-][N+](/C=C1/N(Cc(cc2)ccc2Cl)CCN1)=O Chemical compound [O-][N+](/C=C1/N(Cc(cc2)ccc2Cl)CCN1)=O KATOGIJHEDYLRI-DHZHZOJOSA-N 0.000 description 1
- ALNDHUQPXHHNON-JXMROGBWSA-N [O-][N+](/C=C1/N(Cc(cn2)ccc2Cl)CCN1)=O Chemical compound [O-][N+](/C=C1/N(Cc(cn2)ccc2Cl)CCN1)=O ALNDHUQPXHHNON-JXMROGBWSA-N 0.000 description 1
- AMSZBACOVUMMSU-PKNBQFBNSA-N [O-][N+](/C=C1/N(Cc2ccccc2)CCN1)=O Chemical compound [O-][N+](/C=C1/N(Cc2ccccc2)CCN1)=O AMSZBACOVUMMSU-PKNBQFBNSA-N 0.000 description 1
- QCSJDGKVIIDILQ-OLZOCXBDSA-N [O-][N+](C1=C(N(Cc(cc2)ccc2Cl)CC2)N2[C@H]2O[C@@H]1CC2)=O Chemical compound [O-][N+](C1=C(N(Cc(cc2)ccc2Cl)CC2)N2[C@H]2O[C@@H]1CC2)=O QCSJDGKVIIDILQ-OLZOCXBDSA-N 0.000 description 1
- XFRZOMVOLXEWIA-WCQYABFASA-N [O-][N+](C1=C(N(Cc(cn2)ccc2Cl)CC2)N2[C@@H]2O[C@H]1CCC2)=O Chemical compound [O-][N+](C1=C(N(Cc(cn2)ccc2Cl)CC2)N2[C@@H]2O[C@H]1CCC2)=O XFRZOMVOLXEWIA-WCQYABFASA-N 0.000 description 1
- PNGGZPVAUFDZRO-OLZOCXBDSA-N [O-][N+](C1=C(N(Cc2ccccc2)CC2)N2[C@H]2O[C@@H]1CC2)=O Chemical compound [O-][N+](C1=C(N(Cc2ccccc2)CC2)N2[C@H]2O[C@@H]1CC2)=O PNGGZPVAUFDZRO-OLZOCXBDSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D498/08—Bridged systems
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/86—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms six-membered rings with one nitrogen atom and either one oxygen atom or one sulfur atom in positions 1,3
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/90—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having two or more relevant hetero rings, condensed among themselves or with a common carbocyclic ring system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/10—Anthelmintics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/14—Ectoparasiticides, e.g. scabicides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/12—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains three hetero rings
- C07D498/18—Bridged systems
Definitions
- Double and oxygen bridge heterocyclic neonicotinoid compound and preparation method thereof Double and oxygen bridge heterocyclic neonicotinoid compound and preparation method thereof
- the invention relates to a novel neonicotinoid insecticide, a preparation method and application thereof.
- the present invention relates to a bi- and oxygen-bridged heterocyclic neonicotinoid compound constructed from an imidacloprid nitromethylene analog and a dialdehyde, and a process for the preparation thereof.
- the neonicotinoid insecticide represented by imidacloprid has high insecticidal activity, wide insecticidal spectrum, low toxicity to mammals and aquatic animals, good systemic property and appropriate field stability and environmental friendliness. An important hot spot for the creation of new pesticides. Later, a series of nicotinic insecticides such as thiacloprid, clothianidin, thiamethoxam, acetamiprid, nitenpyram, and dinotefuran have been developed (see European patents 247477, 296453, 685477, 235725, 235725, 3 15826, 192060, 244777, 0386565, 580553, and 1031566, Japanese Patent Nos. 62292965, 8259568, 8291171, and 7242633).
- the present invention provides a new and more effective insecticide, solves the problem of resistance of neonicotinoid insecticides, expands the insecticidal spectrum, and solves the problems in the prior art.
- Another object of the invention is to provide protection for growing and harvested crops from insect attack and infestation.
- the invention provides an oxygen bridged heterocyclic neonicotinoid compound selected from the group consisting of a compound having the structure of formula (A) or (; B), or an optical isomer or agrochemically acceptable compound of said compound Accepted salt -
- an oxygen bridged heterocyclic neonicotinoid compound selected from the group consisting of a compound having the structure of formula (A) or (; B), or an optical isomer or agrochemically acceptable compound of said compound Accepted salt -
- A oxygen bridged heterocyclic neonicotinoid compound
- B an optical isomer or agrochemically acceptable compound of said compound Accepted salt -
- ⁇ is a five- or six-membered heterocyclic group containing nitrogen, oxygen and/or sulfur, a halogenated five- or six-membered heterocyclic group containing nitrogen, oxygen and/or sulfur, or a substituted or unsubstituted phenyl group, Wherein the substituent is one or more selected from the group consisting of halogen, CM halogenated fluorenyl or chloroalkoxy;
- R 3 are each independently H, d- 6 alkyl with, allyl, benzyl, d- 4 -d- 4 alkoxyalkyl group, alkoxy embankment - carbonyl, phenoxycarbonyl, C 2 - 6 alkynyl yl - carbonyl group, C 2 - 3 alkenyl - carbonyl group, C 3 - 6 cycloalkyl - carbonyl, benzoyl, or substituted with one or more substituents selected from a halogen atom, 4 alkyl, halo alkyl with D- 4, a benzoyl group substituted with a d- 4 methoxy group and a CM fluorenyl-carbonyl substituent, a furanylcarbonyl group or an N,N-dimethylcarbonyl group, or R 3 and a combination of -CH 2 -CH 2 -, -CH 2 -CH 2 -CH 2 - or -CH 2 -
- R is a substituent on a hetero atom selected from H, d- 6 alkyl, allyl, benzyl, phenyl, d- 4 methoxy-d- 4 alkyl, d- 4 alkoxy-carbonyl, phenoxycarbonyl , C 2 -6 alkynyl-carbonyl, C 2 _ 3 alkenyl-carbonyl, C 3 -6 cycloalkyl-carbonyl, benzoyl, or one or more selected from halogen atoms, C ⁇ halogenated fluorenyl a benzoyl group substituted with a d- 8 saturated or unsaturated fluorenyl group or a substituent of a decyloxy group and a fluorenyl-carbonyl group, a furanylcarbonyl group or a hydrazine, a fluorenyl-dimethylcarbonyl group;
- Y is nitro, cyano, trifluoromethyl, trifluoroacetyl or trifluoromethanesulfonyl.
- the oxygen bridge heterocyclic neonicotinoid compound is selected from the group consisting of
- oxygen bridge heterocyclic neonicotinoid compound is selected from the group consisting of
- the oxygen bridge heterocyclic neonicotinoid compound is an antagonist of an insect nicotinic acetylcholine receptor.
- the oxygen bridged heterocyclic neonicotinoid compounds (la) and (lb) are 2 to 30 times more active against imidacloprid resistant brown planthopper and whitefly.
- the present invention provides an agricultural composition comprising -
- the invention relates to the use of the agricultural composition for killing or preventing agricultural pests, Pests and pests that are harmful to animal health; or insecticide compositions used to kill or prevent agricultural pests, sanitary pests, and animal health hazards.
- the invention provides a pesticidal and/or pest control method comprising applying the above agricultural composition to a plant body that is or may be subject to pests, soil surrounding it, or the environment.
- the present invention relates to the use of the above compounds, their optical isomers or agrochemically acceptable salts, or a combination thereof, in the preparation of a pesticide composition.
- the present invention provides a process for the preparation of the above compound, its optical isomer or agrochemically acceptable salt, the method comprising the steps of:
- the compound of formula (a) is reacted with a compound of formula (a) or (c) in the presence of a catalytic acid at room temperature to produce a compound of formula (A) (B),
- R 3 , R 5 , R 6 , R 7 , R 8 , R 9 and Y are as defined above.
- the method comprises the steps of: - in acetonitrile, in the presence of a catalytic amount of an acid, at room temperature, at a temperature of from 2 to 24 hours to obtain a compound of formula (la):
- the inventors of the present invention have studied the imidacloprid nitromethylene structure of the existing imidacloprid nitromethylene-based neonicotinoid insecticide through the dialdehyde and the imidacloprid nitromethylene compound through long-term and in-depth research.
- a novel neonicotinoid compound was synthesized, which showed a marked increase in insecticidal activity and an expanded insecticidal spectrum.
- the inventors have completed the present invention.
- mercapto refers to a straight or branched alkyl group having from 1 to 6 carbon atoms, such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl. , tert-butyl, or the like.
- d- 4 methoxy refers to a straight or branched alkoxy group having from 1 to 4 carbon atoms, such as methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutylene.
- halogen means fluoro, chloro, bromo, or iodo.
- halogenated refers to a group substituted with one or more of the above halogen atoms, which may be the same or different, such as a trifluoromethyl group, a pentafluoroethyl group, or the like.
- five- or six-membered heterocyclic group refers to a five- or six-membered ring containing one or more heteroatoms selected from nitrogen, oxygen or sulfur, such as pyridyl, thiazolyl, pyrimidinyl, tetrahydrofuranyl, or Azolyl and the like.
- the compounds of the invention can be synthesized by the reaction steps described above.
- the compound of formula (Id) (ld)
- the synthesis of the compound of formula (2a) is as follows:
- the compounds of formula (2a)-(2e) can be prepared by the following reaction:
- active substance of the invention or “active compound of the invention” means a compound of the invention, an optical isomer or a pesticidally acceptable salt thereof, which has significantly enhanced insecticidal activity, and an expanded insecticidal activity. Spectrum.
- a pesticidally acceptable salt means that the anion of the salt is known and acceptable in forming the pharmaceutically acceptable salt of the pesticide.
- the salt is preferably water soluble.
- Suitable acid addition salts formed from the compounds of the formulae (A) and (B) include salts formed with inorganic acids, such as hydrochlorides, phosphates, sulfates, nitrates; and salts formed from organic acids, such as Acetate, benzoate.
- the active material of the present invention can be used for controlling and eliminating a wide range of agricultural and forestry plant pests, storing cereal pests, public health pests, and pests that are harmful to animal health.
- insecticide is a general term for substances having the action of controlling all the pests mentioned above. Examples of pests include, but are not limited to, Coleoptera: Sitophilus zeamais, ⁇ (2 0 //iTM castaneum), potato ticket melon
- Pests that are harmful to animal health include BoopMus micmphis, long-horned blood clams
- Hyalomama anatolicum cowhide! fe ⁇ ( Hypo derma spp. ), Fasciola hepatica, Moniezia blanchard, Oster nematode (Ostertagia spp.), Trypanosoma enansi, Babesia bigemina, etc.
- the compound of the invention has special effects especially for sucking and sucking mouthparts pests, such as aphids, leafhoppers, planthoppers, thrips, whiteflies and the like.
- Insecticide composition containing the active substance of the present invention is aphids, leafhoppers, planthoppers, thrips, whiteflies and the like.
- the active substance of the present invention can be prepared into a pesticide composition in a conventional manner.
- These active compounds can be formulated into conventional preparations such as solutions, emulsions, suspensions, powders, foams, pastes, granules; aerosols, natural and synthetic materials impregnated with active substances, Microcapsules in polymers, coatings for seeds, and preparations for use with combustion devices, such as smoking cartridges, smoked cans and smokers, as well as ULV cold mist and hot fog ( Warm mist) formulation.
- compositions can be produced by known methods, for example, by mixing the active compound with an extender which is a liquid or liquefied gas or solid diluent or carrier, and optionally a surfactant which is emulsified.
- an extender which is a liquid or liquefied gas or solid diluent or carrier
- a surfactant which is emulsified.
- Agents and/or dispersants and/or foam formers for example, when water is used as an extender, an organic solvent can also be used as an auxiliary.
- a liquid solvent as a diluent or a carrier, such as: aromatic hydrocarbons such as xylene, toluene or decylnaphthalene; chlorinated aromatic or chlorinated aliphatic hydrocarbons such as chlorobenzene, vinyl chloride or Dichloromethane; aliphatic hydrocarbons such as cyclohexane or paraffin, such as mineral oil fractions; alcohols such as ethanol or ethylene glycol and their ethers and lipids; ketones such as acetone, methyl ethyl ketone, methyl isobutyl Ketone or cyclohexanone; or less common polar solvents such as dimethylformamide and dimethyl sulfoxide, and water.
- aromatic hydrocarbons such as xylene, toluene or decylnaphthalene
- chlorinated aromatic or chlorinated aliphatic hydrocarbons such as chlorobenzene, vinyl chloride or Dichloromethane
- the diluent or carrier for the liquefied gas refers to a liquid which will become a gas at normal temperature and normal pressure, such as an aerosol propellant such as a halogenated hydrocarbon, and butyl hydrazine, propylene carbonate, nitrogen gas and carbon dioxide.
- an aerosol propellant such as a halogenated hydrocarbon, and butyl hydrazine, propylene carbonate, nitrogen gas and carbon dioxide.
- Solid supports can be used as natural minerals on the ground, such as kaolin, clay, talc, quartz, activated clay, montmorillonite, or diatomaceous earth, and ground synthetic minerals such as highly dispersed silicic acid, alumina and silicates.
- the solid carrier for the granules is a ground and graded natural stone, such as calcite, marble, pumice, sepiolite and dolomite, as well as inorganic and organic coarse powder synthetic particles, and organic materials such as sawdust, coconut shell, Corn cobs and granules of tobacco stems.
- Nonionic and anionic emulsified columns can be used as emulsifiers and I or foam formers.
- emulsifiers and I or foam formers polyoxyethylene-fatty acid esters, polyoxyethylene-fatty alcohol ethers, such as alkylaryl polyglycol ethers, mercaptosulfonates, alkyl sulfates, arylsulfonates, and white Protein hydrolysate.
- Dispersing agents include, for example, lignin sulfite waste liquid and methyl cellulose.
- Binders such as carboxymethylcellulose and natural and synthetic polymers in the form of powders, granules or emulsions such as gum arabic, polyvinyl alcohol and polyvinyl acetate may be used in the formulation.
- Colorants such as inorganic dyes such as iron oxide, oxidized diamonds and Prussian blue; organic dyes such as organic dyes such as azo dyes or metal phthalocyanine dyes; and trace nutrients such as iron, lanthanum, boron, copper may be used. , cobalt, aluminum and zinc salts, etc.
- the active compounds of the present invention may be present in a commercial preparation or in a dosage form prepared from these preparations in combination with other active compounds, including but not limited to: insecticides, baits, bactericides, Acaricides, killing lines, fungicides, growth control agents, etc.
- Insecticides include, for example, phosphates, carbamates, pyrethroids, chlorinated hydrocarbons, benzoylureas, nematodes, and microbial-derived substances such as avermectin.
- the active compounds of the invention may also be formulated as a mixture with synergists in their commercial preparations in the dosage forms prepared from these preparations.
- Synergists are compounds which increase the action of the active compound. Since the active compound itself is active, it is not necessary to add a synergist.
- These formulations usually contain from 0.001 to 99.99% by weight, preferably from 0.01 to 99.9% by weight, more preferably from 0.05 to 90% by weight, of the active compound of the invention, of the insecticidal composition.
- the concentration of the active compound in the dosage form prepared from the commercial preparation can be varied within a wide range.
- the concentration of active compound in the dosage form used may range from 0.000000 l to 100% (g/v), preferably between 0.0001 and 1%.
- Example 9 10-(4-Chlorobenzyl)-4-nitro-9-oxa-1 1,12-dihydroimidazo[2,3-albicyclo"3,3,11 ⁇ -3- Alkene (compound 2d)
- a 25% aqueous solution of glutaraldehyde and a catalytic amount of HC1 were placed in a 50 ml round bottom flask. Stir at room temperature and TLC is followed by reaction. After the completion of the reaction, the solvent was removed, and the residue was purified by column chromatography to yield pale yellow powdery product.
- Example 1 1 Insecticidal activity test of the compound of the present invention
- Aphids belong to the homopteran pests and have a sucker, which is a common crop pest.
- the soybean meal craccivora was used as the test object and tested by the dipping method.
- Test method Accurately weigh each sample, add DMSO 2 mL and 18 mL water, add three drops of emulsifier 2201 into a liquid, blank with DMSO 2 mL and 18 mL water, add three drops of emulsifier 2201. Soak a certain number of insects to be tested with broad bean leaves in the liquid for 3-5 seconds, remove and dry, transfer the test insects and foodstuffs into a clean container, place in a dry thermostat recovery room, and check the test insects after 24 hours. Poisoning deaths. The results are shown in Table 1 below.
- Rice brown planthopper is a homopteran pest with a sucker and is a common crop pest.
- the rice brown planthopper (; N//aparvato / wgew was used as the test object, and the microdropping method reported by Nagata was used.
- Procedure Select un-matted females with feathering for 2-3 days as test subjects, dilute the compounds to a series of concentrations with acetone, and test the insect paralysis with carbon dioxide, using a manual micro-dropper (Burkard Manufacturing Co. Ltd, Rickmansworth , UK) Drop the drug drop (0.08 L) before the test insect On the chest and back. Approximately 30 adults were treated at each concentration and each treatment was repeated 3 times. Take acetone as a control. The treated adults were housed on soilless cultivated rice seedlings in an incubator (20 x 20 x 10 cm) at a temperature of 25 ⁇ 1 V and 16 (L) / 8 (D) hours. The results were checked after 48 hours and LD 5 was calculated using standard probability analysis. value. The results are shown in Table 2 below.
- the second instar larvae were tested with the larvae of Pseudaktia separate Walker, and the leaf leaching method was used.
- the armyworm is an important heterophylla lepidopteran pest. It has a wide application range and is suitable for the determination of virulence, contact and residual effects such as stomach poisoning, contact and residual effects, and insect toxicology. For the screening test of new compounds, the test of the armyworm killing activity was carried out according to the method reported in the literature.
- Operation process of dip-leaf method Accurately weigh each sample, add DMSO (2 mL) and 18 mL of water separately, then add three drops of emulsifier 2201 (provided by Shanghai Pesticide Factory) to prepare liquid medicine, blank DMSO (2 mL) And 28 mL of clear water, add three drops of emulsifier 2201. Tear fresh corn leaves into small pieces, soak them in the liquid for about 5 seconds, take them out and dry them, put them into a 100 mL jar, and put about 20 second-instar larvae, white gauze for the jar. The rubber band was tightened, and the corn immersed in the liquid was continuously fed, and the mortality of the larva was checked after 5 days.
- the compound (la) was subjected to electrophysiological experiments and isotopic label substitution experiments, and the compound (la) inhibited the agonist reaction; the compound (la) expressed ⁇ ⁇ to the nicotinic acetylcholine receptor and oocyte of the United States.
- the 1/1 2 receptor has no agonistic effect; the compound (la) inhibits the agonist acetylcholine response, and these experiments indicate that the compound is an antagonist of nicotinic acetylcholine receptors (nAChRs).
- Example 13 Preparation of a Pesticide Composition Containing a Compound of the Invention
- the following components were prepared in proportion: 25% by weight of the compound la-le and 2a-2e; 5% polyoxyethylene sorbitol hexaoleate; 70% higher aliphatic hydrocarbon oil.
- the components were ground together in a sanding mill until the solid particles fell below about 5 microns.
- the resulting viscous suspension can be used as it is, but it can also be used after emulsification in water.
- the following components were prepared in proportion: 25% of the compounds la-le and 2a-2e; 3% hydrate attapulgit; 10% calcium lignosulfonate; 0.5% sodium dihydrogen phosphate; 61.5 %water.
- the components are ground together in a ball mill until the solid particles fall below about 10 microns. This aqueous suspension can be used directly.
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Public Health (AREA)
- Pest Control & Pesticides (AREA)
- Zoology (AREA)
- Agronomy & Crop Science (AREA)
- Tropical Medicine & Parasitology (AREA)
- Plant Pathology (AREA)
- Dentistry (AREA)
- Wood Science & Technology (AREA)
- Environmental Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Plural Heterocyclic Compounds (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Pyridine Compounds (AREA)
Description
Claims
Priority Applications (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012542350A JP5600750B2 (ja) | 2009-12-09 | 2010-12-09 | エポキシ基含有複素環式ネオニコチノイド化合物及びその製造方法、農業用組成物及びその使用、害虫防除方法 |
| BR112012013846A BR112012013846A2 (pt) | 2009-12-09 | 2010-12-09 | composto relacionado á nicotina heterocíclico ligado por ponte de oxigênio ou um isômero óptico ou sal agroquimicamente aceitável do mesmo,composição agroquímica,(*)aplicação da composição agroquímica, método para preparar o composto relacionado á nicotina heterocíclico ligado por ponte de oxigênio ou um isômero óptico ou sal agroquimicamente aceitável do mesmo e método para controlar pragas |
| AU2010330474A AU2010330474B2 (en) | 2009-12-09 | 2010-12-09 | Divalent and oxabridged heterocyclic neonicotinoid compounds and preparation methods thereof |
| US13/514,460 US8809319B2 (en) | 2009-12-09 | 2010-12-09 | Substituted 8-oxa-10,11-dihydroimidazo[2,3-a]bicyclo[3.2.1]oct-3-enes and use thereof as an insecticide |
| CA2783504A CA2783504C (en) | 2009-12-09 | 2010-12-09 | Divalent and oxabridged heterocyclic nicotine related compounds and preparation methods thereof |
| MX2012006663A MX2012006663A (es) | 2009-12-09 | 2010-12-09 | Compuestos de neonicotinoides heterociclicos divalentes y compuestos oxa y metodos de preparacion de los mismos. |
| RU2012127868/04A RU2531920C2 (ru) | 2009-12-09 | 2010-12-09 | Двухвалентные и гетероциклические производные никотина с оксо-мостиком и способы их получения |
| PH1/2012/501159A PH12012501159A1 (en) | 2009-12-09 | 2010-12-09 | Substituted 8-oxa-10,11-dihydroimidazo[2,3-a]bicyclo[3.2.1]oct-3-enes and use thereof as an insecticide |
| EP10835491.1A EP2511279B1 (en) | 2009-12-09 | 2010-12-09 | Divalent and oxabridged heterocyclic neonicotinoid compounds and preparation methods thereof |
| UAA201208318A UA106256C2 (ru) | 2009-12-09 | 2010-12-09 | Двухвалентные гетероциклические родственные с никотином соединения, которые имеют кислородный мостик, и способы их приготовления |
| KR1020127017848A KR101504575B1 (ko) | 2009-12-09 | 2010-12-09 | 2가의 산소 브릿지된 헤테로사이클릭 네오니코티노이드 화합물 및 그의 제조방법 |
| IL220237A IL220237A (en) | 2009-12-09 | 2012-06-07 | Compounds closest to heterocyclic heterotinous nicotine compounds with oxygen bridges and methods for preparing them |
| ZA2012/05074A ZA201205074B (en) | 2009-12-09 | 2012-07-06 | Divalent and oxabridged heterocyclic neonicotinoid compounds and preparation methods thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN200910258534.3 | 2009-12-09 | ||
| CN200910258534.3A CN102093389B (zh) | 2009-12-09 | 2009-12-09 | 双联和氧桥杂环新烟碱化合物及其制备方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011069456A1 true WO2011069456A1 (zh) | 2011-06-16 |
Family
ID=44126675
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CN2010/079591 Ceased WO2011069456A1 (zh) | 2009-12-09 | 2010-12-09 | 双联和氧桥杂环新烟碱化合物及其制备方法 |
Country Status (18)
| Country | Link |
|---|---|
| US (1) | US8809319B2 (zh) |
| EP (1) | EP2511279B1 (zh) |
| JP (1) | JP5600750B2 (zh) |
| KR (1) | KR101504575B1 (zh) |
| CN (1) | CN102093389B (zh) |
| AU (1) | AU2010330474B2 (zh) |
| BR (1) | BR112012013846A2 (zh) |
| CA (1) | CA2783504C (zh) |
| CL (1) | CL2012001517A1 (zh) |
| CO (1) | CO6561790A2 (zh) |
| CR (1) | CR20120361A (zh) |
| IL (1) | IL220237A (zh) |
| MX (1) | MX2012006663A (zh) |
| PH (1) | PH12012501159A1 (zh) |
| RU (1) | RU2531920C2 (zh) |
| UA (1) | UA106256C2 (zh) |
| WO (1) | WO2011069456A1 (zh) |
| ZA (1) | ZA201205074B (zh) |
Cited By (72)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2377845A4 (en) * | 2008-12-19 | 2012-12-26 | Univ East China Science & Tech | HETEROCYCLIC NITROGEN OR OXYGEN COMPOUNDS WITH INSECTICIDAL EFFECT FROM DIALDEHYDE, AND THEIR PREPARATION AND USE |
| WO2013156331A1 (en) | 2012-04-16 | 2013-10-24 | Basf Se | Synergistic compositions comprising pyraclostrobin and an insecticidal compound |
| CN104082318A (zh) * | 2014-07-18 | 2014-10-08 | 江苏省绿盾植保农药实验有限公司 | 一种含有乙蒜素和环氧虫啶的复合杀虫剂及其应用 |
| WO2016071499A1 (en) | 2014-11-06 | 2016-05-12 | Basf Se | 3-pyridyl heterobicyclic compound for controlling invertebrate pests |
| JP2016514092A (ja) * | 2013-02-20 | 2016-05-19 | ビーエーエスエフ ソシエタス・ヨーロピアBasf Se | アントラニルアミド化合物、それらの混合物およびそれらの殺有害生物剤としての使用 |
| WO2016128261A2 (en) | 2015-02-11 | 2016-08-18 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| WO2016162371A1 (en) | 2015-04-07 | 2016-10-13 | Basf Agrochemical Products B.V. | Use of an insecticidal carboxamide compound against pests on cultivated plants |
| WO2016198611A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino heterocyclic compounds |
| WO2016198613A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino compounds |
| WO2017016883A1 (en) | 2015-07-24 | 2017-02-02 | Basf Se | Process for preparation of cyclopentene compounds |
| WO2017093163A1 (en) | 2015-11-30 | 2017-06-08 | Basf Se | Mixtures of cis-jasmone and bacillus amyloliquefaciens |
| WO2017140614A1 (en) | 2016-02-19 | 2017-08-24 | Basf Se | Method for controlling pests of soybean, corn, and cotton plants |
| WO2017153217A1 (en) | 2016-03-09 | 2017-09-14 | Basf Se | Spirocyclic derivatives |
| WO2017153218A1 (en) | 2016-03-11 | 2017-09-14 | Basf Se | Method for controlling pests of plants |
| WO2017167832A1 (en) | 2016-04-01 | 2017-10-05 | Basf Se | Bicyclic compounds |
| WO2017198588A1 (en) | 2016-05-18 | 2017-11-23 | Basf Se | Capsules comprising benzylpropargylethers for use as nitrification inhibitors |
| WO2018108671A1 (en) | 2016-12-16 | 2018-06-21 | Basf Se | Pesticidal compounds |
| WO2018162312A1 (en) | 2017-03-10 | 2018-09-13 | Basf Se | Spirocyclic derivatives |
| WO2018166855A1 (en) | 2017-03-16 | 2018-09-20 | Basf Se | Heterobicyclic substituted dihydroisoxazoles |
| WO2018177970A1 (en) | 2017-03-31 | 2018-10-04 | Basf Se | Process for preparing chiral 2,3-dihydrothiazolo[3,2-a]pyrimidin-4-ium compounds |
| WO2018177781A1 (en) | 2017-03-28 | 2018-10-04 | Basf Se | Pesticidal compounds |
| WO2018192793A1 (en) | 2017-04-20 | 2018-10-25 | Basf Se | Substituted rhodanine derivatives |
| WO2018197466A1 (en) | 2017-04-26 | 2018-11-01 | Basf Se | Substituted succinimide derivatives as pesticides |
| WO2018206479A1 (en) | 2017-05-10 | 2018-11-15 | Basf Se | Bicyclic pesticidal compounds |
| US10149477B2 (en) | 2014-10-06 | 2018-12-11 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2018224455A1 (en) | 2017-06-07 | 2018-12-13 | Basf Se | Substituted cyclopropyl derivatives |
| WO2018229202A1 (en) | 2017-06-16 | 2018-12-20 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2018234202A1 (en) | 2017-06-19 | 2018-12-27 | Basf Se | Substituted pyrimidinium compounds and derivatives for combating animal pests |
| WO2018234488A1 (en) | 2017-06-23 | 2018-12-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019042932A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING RICE PARASITES IN RICE |
| EP3453706A1 (en) | 2017-09-08 | 2019-03-13 | Basf Se | Pesticidal imidazole compounds |
| WO2019072906A1 (en) | 2017-10-13 | 2019-04-18 | Basf Se | IMIDAZOLIDINE PYRIMIDINIUM COMPOUNDS FOR CONTROL OF HARMFUL ANIMALS |
| WO2019121159A1 (en) | 2017-12-21 | 2019-06-27 | Basf Se | Pesticidal compounds |
| WO2019121143A1 (en) | 2017-12-20 | 2019-06-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019134840A1 (en) | 2018-01-05 | 2019-07-11 | Basf Se | Control of pests of soybean plants with mesoionic compounds |
| WO2019137995A1 (en) | 2018-01-11 | 2019-07-18 | Basf Se | Novel pyridazine compounds for controlling invertebrate pests |
| WO2019145140A1 (en) | 2018-01-09 | 2019-08-01 | Basf Se | Silylethynyl hetaryl compounds as nitrification inhibitors |
| WO2019166558A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of pyrazole propargyl ethers as nitrification inhibitors |
| WO2019166561A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of alkoxypyrazoles as nitrification inhibitors |
| WO2019166560A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of n-functionalized alkoxy pyrazole compounds as nitrification inhibitors |
| WO2019175712A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New uses for catechol molecules as inhibitors to glutathione s-transferase metabolic pathways |
| WO2019175713A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New catechol molecules and their use as inhibitors to p450 related metabolic pathways |
| WO2019185413A1 (en) | 2018-03-27 | 2019-10-03 | Basf Se | Pesticidal substituted cyclopropyl derivatives |
| WO2019211106A1 (en) | 2018-04-30 | 2019-11-07 | Basf Se | Control of pests of soybean plants with mesoionic compounds |
| WO2019219529A1 (en) | 2018-05-15 | 2019-11-21 | Basf Se | Mixtures comprising benzpyrimoxan and oxazosulfyl and uses and methods of applying them |
| WO2019224092A1 (en) | 2018-05-22 | 2019-11-28 | Basf Se | Pesticidally active c15-derivatives of ginkgolides |
| WO2020002472A1 (en) | 2018-06-28 | 2020-01-02 | Basf Se | Use of alkynylthiophenes as nitrification inhibitors |
| WO2020020777A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of substituted 2-thiazolines as nitrification inhibitors |
| WO2020020765A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of a substituted thiazolidine compound as nitrification inhibitor |
| US10556844B2 (en) | 2015-02-06 | 2020-02-11 | Basf Se | Pyrazole compounds as nitrification inhibitors |
| EP3613736A1 (en) | 2018-08-22 | 2020-02-26 | Basf Se | Substituted glutarimide derivatives |
| EP3628158A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| EP3628156A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method for controlling pests of sugarcane, citrus, rapeseed, and potato plants |
| EP3628157A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| WO2020064492A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling pests by seed treatment application of a mesoionic compound or mixture thereof |
| EP3643705A1 (en) | 2018-10-24 | 2020-04-29 | Basf Se | Pesticidal compounds |
| WO2020109039A1 (en) | 2018-11-28 | 2020-06-04 | Basf Se | Pesticidal compounds |
| WO2020126591A1 (en) | 2018-12-18 | 2020-06-25 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| EP3696177A1 (en) | 2019-02-12 | 2020-08-19 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2020239517A1 (en) | 2019-05-29 | 2020-12-03 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| EP3766879A1 (en) | 2019-07-19 | 2021-01-20 | Basf Se | Pesticidal pyrazole derivatives |
| EP3769623A1 (en) | 2019-07-22 | 2021-01-27 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| US11053175B2 (en) | 2015-05-12 | 2021-07-06 | Basf Se | Thioether compounds as nitrification inhibitors |
| US11142514B2 (en) | 2015-10-02 | 2021-10-12 | Basf Se | Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents |
| WO2022167488A1 (en) | 2021-02-02 | 2022-08-11 | Basf Se | Synergistic action of dcd and alkoxypyrazoles as nitrification inhibitors |
| WO2022243523A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of an n-functionalized alkoxy pyrazole compound as nitrification inhibitor |
| WO2022243521A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of ethynylpyridine compounds as nitrification inhibitors |
| WO2022268810A1 (en) | 2021-06-21 | 2022-12-29 | Basf Se | Metal-organic frameworks with pyrazole-based building blocks |
| WO2022268813A1 (en) | 2021-06-24 | 2022-12-29 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023046853A1 (en) | 2021-09-23 | 2023-03-30 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023203066A1 (en) | 2022-04-21 | 2023-10-26 | Basf Se | Synergistic action as nitrification inhibitors of dcd oligomers with alkoxypyrazole and its oligomers |
| WO2024028243A1 (en) | 2022-08-02 | 2024-02-08 | Basf Se | Pyrazolo pesticidal compounds |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102197808B (zh) * | 2011-03-31 | 2013-10-30 | 上海生农生化制品有限公司 | 环氧虫啶水分散粒剂及其制备方法 |
| CN102197804A (zh) * | 2011-03-31 | 2011-09-28 | 上海生农生化制品有限公司 | 环氧虫啶悬浮剂及其制备方法 |
| CN102197807A (zh) * | 2011-03-31 | 2011-09-28 | 上海生农生化制品有限公司 | 环氧虫啶可湿性粉剂及其制备方法 |
| CN102326583B (zh) * | 2011-07-19 | 2013-09-18 | 陕西上格之路生物科学有限公司 | 一种含环氧虫啶和有机磷类杀虫剂的杀虫组合物 |
| CN102293208B (zh) * | 2011-08-29 | 2013-05-15 | 陕西上格之路生物科学有限公司 | 一种含环氧虫啶和噻嗪酮的杀虫组合物 |
| CN102405919B (zh) * | 2011-12-25 | 2013-10-02 | 上海生农生化制品有限公司 | 环氧虫啶与甲氨基阿维菌素苯甲酸盐的杀虫剂组合物剂及用途 |
| CN103242323B (zh) * | 2012-02-06 | 2017-05-10 | 华东理工大学 | 具有杀虫活性的含氮(硫)桥环化合物、制备及用途 |
| CN102440252B (zh) * | 2012-02-13 | 2013-09-18 | 陕西上格之路生物科学有限公司 | 一种含环氧虫啶和吡唑类杀虫剂的杀虫组合物 |
| CN102657189B (zh) * | 2012-05-08 | 2014-04-30 | 陕西上格之路生物科学有限公司 | 一种含环氧虫啶的杀虫组合物 |
| CN102986701B (zh) * | 2012-12-18 | 2014-04-16 | 上海市农业科学院 | 一种含环氧虫啶和氟啶脲的杀虫组合物及其用途 |
| CN104054721B (zh) * | 2013-03-20 | 2017-09-29 | 上海生农生化制品有限公司 | 乙螨唑和环氧虫啶杀螨组合物 |
| CN105557709B (zh) * | 2014-10-11 | 2018-08-14 | 南京农业大学 | 八元氧桥杂环化合物作为杀虫剂增效剂的用途 |
| CN109535177B (zh) * | 2018-11-07 | 2020-05-12 | 北京市农林科学院 | 一种三酮化合物及其应用 |
| CN111165505A (zh) * | 2020-02-24 | 2020-05-19 | 华东理工大学 | 八元氧桥杂环化合物作为蜜蜂选择性杀虫剂增效剂的用途 |
| CN112010873A (zh) * | 2020-08-20 | 2020-12-01 | 上海生农生化制品股份有限公司 | 一种环氧虫啶的合成方法 |
| CN116076499B (zh) * | 2023-03-02 | 2024-03-22 | 石河子大学 | 3-甲基-1-丁醇在制备双斑长跗萤叶甲雌虫引诱剂中的应用 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101747320A (zh) * | 2008-12-19 | 2010-06-23 | 华东理工大学 | 二醛构建的具有杀虫活性的含氮或氧杂环化合物及其制备方法 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0192060B1 (de) | 1985-02-04 | 1991-09-18 | Nihon Bayer Agrochem K.K. | Heterocyclische Verbindungen |
| JPH0717621B2 (ja) | 1986-03-07 | 1995-03-01 | 日本バイエルアグロケム株式会社 | 新規ヘテロ環式化合物 |
| DE3638121A1 (de) | 1986-05-30 | 1987-12-03 | Bayer Ag | 1,2,3,6-tetrahydro-5-nitro-pyrimidin-derivate |
| JPH0710865B2 (ja) | 1987-06-26 | 1995-02-08 | 日本バイエルアグロケム株式会社 | ニトロ置換ヘテロ環式化合物及び殺虫剤 |
| JP2583429B2 (ja) | 1987-11-06 | 1997-02-19 | 日本バイエルアグロケム株式会社 | イミダゾリン類及び殺虫剤 |
| JP2610988B2 (ja) | 1989-03-09 | 1997-05-14 | 日本バイエルアグロケム 株式会社 | 新規ヘテロ環式化合物及び殺虫剤 |
| TW240163B (en) | 1992-07-22 | 1995-02-11 | Syngenta Participations Ag | Oxadiazine derivatives |
| JPH07242633A (ja) | 1994-03-03 | 1995-09-19 | Ishihara Sangyo Kaisha Ltd | 5−アミノエチルアミノメチル−2−クロロピリジンの製造方法 |
| JP4136000B2 (ja) | 1994-06-03 | 2008-08-20 | 三井化学株式会社 | 殺虫性テトラヒドロフラン系化合物 |
| JPH08259568A (ja) | 1995-03-27 | 1996-10-08 | Mitsui Toatsu Chem Inc | 殺虫性テトラヒドロピリミジン誘導体 |
| JP3722512B2 (ja) | 1995-04-20 | 2005-11-30 | 三井化学株式会社 | 殺虫性5−{(テトラヒドロ−3−フラニル)メチル}−4−ニトロイミノパーヒドロ−1,3,5−オキサジアジン誘導体 |
| CA2305734A1 (en) | 1997-10-20 | 1999-04-29 | Taisho Pharmaceutical Co., Ltd. | 2-phenoxyaniline derivatives |
| CZ301369B6 (cs) * | 1998-03-13 | 2010-02-03 | Syngenta Participations Ag | Herbicidne aktivní deriváty 3-hydroxy-4-aryl-5-oxopyrazolinu |
| US20060258648A1 (en) * | 2003-04-30 | 2006-11-16 | Olivier Bezencon | 9-Azabicyclo'3.3.1 inon-6-ee derivatives with a heteroatom at the 3-position as renin inhibitors |
| CN1295228C (zh) * | 2004-11-23 | 2007-01-17 | 华东理工大学 | 硝基亚甲基衍生物及其用途 |
| WO2007101369A1 (fr) * | 2006-03-09 | 2007-09-13 | East China University Of Science And Technology | Méthode de préparation et utilisation de composés présentant une action biocide |
| CN101045728B (zh) * | 2006-03-28 | 2012-03-21 | 华东理工大学 | 一类具有高杀虫活性化合物的制备方法及用途 |
-
2009
- 2009-12-09 CN CN200910258534.3A patent/CN102093389B/zh active Active
-
2010
- 2010-12-09 UA UAA201208318A patent/UA106256C2/ru unknown
- 2010-12-09 RU RU2012127868/04A patent/RU2531920C2/ru not_active IP Right Cessation
- 2010-12-09 EP EP10835491.1A patent/EP2511279B1/en active Active
- 2010-12-09 JP JP2012542350A patent/JP5600750B2/ja active Active
- 2010-12-09 AU AU2010330474A patent/AU2010330474B2/en not_active Ceased
- 2010-12-09 KR KR1020127017848A patent/KR101504575B1/ko active Active
- 2010-12-09 PH PH1/2012/501159A patent/PH12012501159A1/en unknown
- 2010-12-09 WO PCT/CN2010/079591 patent/WO2011069456A1/zh not_active Ceased
- 2010-12-09 CA CA2783504A patent/CA2783504C/en active Active
- 2010-12-09 BR BR112012013846A patent/BR112012013846A2/pt active IP Right Grant
- 2010-12-09 MX MX2012006663A patent/MX2012006663A/es active IP Right Grant
- 2010-12-09 US US13/514,460 patent/US8809319B2/en active Active
-
2012
- 2012-06-07 IL IL220237A patent/IL220237A/en active IP Right Grant
- 2012-06-08 CL CL2012001517A patent/CL2012001517A1/es unknown
- 2012-06-29 CR CR20120361A patent/CR20120361A/es unknown
- 2012-07-06 ZA ZA2012/05074A patent/ZA201205074B/en unknown
- 2012-07-06 CO CO12113709A patent/CO6561790A2/es active IP Right Grant
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101747320A (zh) * | 2008-12-19 | 2010-06-23 | 华东理工大学 | 二醛构建的具有杀虫活性的含氮或氧杂环化合物及其制备方法 |
Non-Patent Citations (2)
| Title |
|---|
| See also references of EP2511279A4 * |
| SHAO, XU-SHENG ET AL.: "Divalent and Oxabridged Neonicotinoids Constructed by Dialdehydes and Nitromethylene Analogues of Imidacloprid: Design, Synthesis, Crystal Structure, and Insecticidal Activities", JOURNAL OF AGRICULTURAL AND FOOD CHEMISTRY, vol. 58, no. 5, 2010, pages 2696 - 2702, XP008140274, Retrieved from the Internet <URL:http://Pubs.acs.org> [retrieved on 20091112], DOI: doi:10.1021/jf902531y * |
Cited By (79)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2377845A4 (en) * | 2008-12-19 | 2012-12-26 | Univ East China Science & Tech | HETEROCYCLIC NITROGEN OR OXYGEN COMPOUNDS WITH INSECTICIDAL EFFECT FROM DIALDEHYDE, AND THEIR PREPARATION AND USE |
| WO2013156331A1 (en) | 2012-04-16 | 2013-10-24 | Basf Se | Synergistic compositions comprising pyraclostrobin and an insecticidal compound |
| JP2016514092A (ja) * | 2013-02-20 | 2016-05-19 | ビーエーエスエフ ソシエタス・ヨーロピアBasf Se | アントラニルアミド化合物、それらの混合物およびそれらの殺有害生物剤としての使用 |
| CN104082318A (zh) * | 2014-07-18 | 2014-10-08 | 江苏省绿盾植保农药实验有限公司 | 一种含有乙蒜素和环氧虫啶的复合杀虫剂及其应用 |
| US10149477B2 (en) | 2014-10-06 | 2018-12-11 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| WO2016071499A1 (en) | 2014-11-06 | 2016-05-12 | Basf Se | 3-pyridyl heterobicyclic compound for controlling invertebrate pests |
| US10556844B2 (en) | 2015-02-06 | 2020-02-11 | Basf Se | Pyrazole compounds as nitrification inhibitors |
| WO2016128261A2 (en) | 2015-02-11 | 2016-08-18 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| US10701937B2 (en) | 2015-02-11 | 2020-07-07 | Basf Se | Pesticidal mixture comprising a pyrazole compound, an insecticide and a fungicide |
| WO2016162371A1 (en) | 2015-04-07 | 2016-10-13 | Basf Agrochemical Products B.V. | Use of an insecticidal carboxamide compound against pests on cultivated plants |
| US11053175B2 (en) | 2015-05-12 | 2021-07-06 | Basf Se | Thioether compounds as nitrification inhibitors |
| WO2016198611A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino heterocyclic compounds |
| WO2016198613A1 (en) | 2015-06-11 | 2016-12-15 | Basf Se | N-(thio)acylimino compounds |
| WO2017016883A1 (en) | 2015-07-24 | 2017-02-02 | Basf Se | Process for preparation of cyclopentene compounds |
| US11142514B2 (en) | 2015-10-02 | 2021-10-12 | Basf Se | Imino compounds with a 2-chloropyrimidin-5-yl substituent as pest-control agents |
| WO2017093163A1 (en) | 2015-11-30 | 2017-06-08 | Basf Se | Mixtures of cis-jasmone and bacillus amyloliquefaciens |
| WO2017140614A1 (en) | 2016-02-19 | 2017-08-24 | Basf Se | Method for controlling pests of soybean, corn, and cotton plants |
| WO2017153217A1 (en) | 2016-03-09 | 2017-09-14 | Basf Se | Spirocyclic derivatives |
| WO2017153218A1 (en) | 2016-03-11 | 2017-09-14 | Basf Se | Method for controlling pests of plants |
| WO2017167832A1 (en) | 2016-04-01 | 2017-10-05 | Basf Se | Bicyclic compounds |
| WO2017198588A1 (en) | 2016-05-18 | 2017-11-23 | Basf Se | Capsules comprising benzylpropargylethers for use as nitrification inhibitors |
| WO2018108671A1 (en) | 2016-12-16 | 2018-06-21 | Basf Se | Pesticidal compounds |
| WO2018162312A1 (en) | 2017-03-10 | 2018-09-13 | Basf Se | Spirocyclic derivatives |
| WO2018166855A1 (en) | 2017-03-16 | 2018-09-20 | Basf Se | Heterobicyclic substituted dihydroisoxazoles |
| WO2018177781A1 (en) | 2017-03-28 | 2018-10-04 | Basf Se | Pesticidal compounds |
| EP3978504A1 (en) | 2017-03-31 | 2022-04-06 | Basf Se | Chiral 2,3-dihydrothiazolo[3,2-a]pyrimidine derivatives for combating animal pests |
| WO2018177970A1 (en) | 2017-03-31 | 2018-10-04 | Basf Se | Process for preparing chiral 2,3-dihydrothiazolo[3,2-a]pyrimidin-4-ium compounds |
| WO2018192793A1 (en) | 2017-04-20 | 2018-10-25 | Basf Se | Substituted rhodanine derivatives |
| WO2018197466A1 (en) | 2017-04-26 | 2018-11-01 | Basf Se | Substituted succinimide derivatives as pesticides |
| WO2018206479A1 (en) | 2017-05-10 | 2018-11-15 | Basf Se | Bicyclic pesticidal compounds |
| WO2018224455A1 (en) | 2017-06-07 | 2018-12-13 | Basf Se | Substituted cyclopropyl derivatives |
| WO2018229202A1 (en) | 2017-06-16 | 2018-12-20 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2018234202A1 (en) | 2017-06-19 | 2018-12-27 | Basf Se | Substituted pyrimidinium compounds and derivatives for combating animal pests |
| WO2018234488A1 (en) | 2017-06-23 | 2018-12-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019042932A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING RICE PARASITES IN RICE |
| WO2019043183A1 (en) | 2017-08-31 | 2019-03-07 | Basf Se | METHOD FOR CONTROLLING PESTS OF RICE IN RICE |
| EP3453706A1 (en) | 2017-09-08 | 2019-03-13 | Basf Se | Pesticidal imidazole compounds |
| WO2019072906A1 (en) | 2017-10-13 | 2019-04-18 | Basf Se | IMIDAZOLIDINE PYRIMIDINIUM COMPOUNDS FOR CONTROL OF HARMFUL ANIMALS |
| WO2019121143A1 (en) | 2017-12-20 | 2019-06-27 | Basf Se | Substituted cyclopropyl derivatives |
| WO2019121159A1 (en) | 2017-12-21 | 2019-06-27 | Basf Se | Pesticidal compounds |
| WO2019134840A1 (en) | 2018-01-05 | 2019-07-11 | Basf Se | Control of pests of soybean plants with mesoionic compounds |
| WO2019145140A1 (en) | 2018-01-09 | 2019-08-01 | Basf Se | Silylethynyl hetaryl compounds as nitrification inhibitors |
| WO2019137995A1 (en) | 2018-01-11 | 2019-07-18 | Basf Se | Novel pyridazine compounds for controlling invertebrate pests |
| WO2019166558A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of pyrazole propargyl ethers as nitrification inhibitors |
| WO2019166561A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of alkoxypyrazoles as nitrification inhibitors |
| WO2019166560A1 (en) | 2018-02-28 | 2019-09-06 | Basf Se | Use of n-functionalized alkoxy pyrazole compounds as nitrification inhibitors |
| WO2019175713A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New catechol molecules and their use as inhibitors to p450 related metabolic pathways |
| WO2019175712A1 (en) | 2018-03-14 | 2019-09-19 | Basf Corporation | New uses for catechol molecules as inhibitors to glutathione s-transferase metabolic pathways |
| WO2019185413A1 (en) | 2018-03-27 | 2019-10-03 | Basf Se | Pesticidal substituted cyclopropyl derivatives |
| WO2019211106A1 (en) | 2018-04-30 | 2019-11-07 | Basf Se | Control of pests of soybean plants with mesoionic compounds |
| WO2019219529A1 (en) | 2018-05-15 | 2019-11-21 | Basf Se | Mixtures comprising benzpyrimoxan and oxazosulfyl and uses and methods of applying them |
| WO2019224092A1 (en) | 2018-05-22 | 2019-11-28 | Basf Se | Pesticidally active c15-derivatives of ginkgolides |
| WO2020002472A1 (en) | 2018-06-28 | 2020-01-02 | Basf Se | Use of alkynylthiophenes as nitrification inhibitors |
| WO2020020777A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of substituted 2-thiazolines as nitrification inhibitors |
| WO2020020765A1 (en) | 2018-07-23 | 2020-01-30 | Basf Se | Use of a substituted thiazolidine compound as nitrification inhibitor |
| EP3613736A1 (en) | 2018-08-22 | 2020-02-26 | Basf Se | Substituted glutarimide derivatives |
| EP3628158A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| WO2020064480A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Pesticidal mixture comprising a mesoionic compound and a biopesticide |
| WO2020064492A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling pests by seed treatment application of a mesoionic compound or mixture thereof |
| EP3628156A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method for controlling pests of sugarcane, citrus, rapeseed, and potato plants |
| EP3628157A1 (en) | 2018-09-28 | 2020-04-01 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| WO2020064408A1 (en) | 2018-09-28 | 2020-04-02 | Basf Se | Method of controlling insecticide resistant insects and virus transmission to plants |
| EP3643705A1 (en) | 2018-10-24 | 2020-04-29 | Basf Se | Pesticidal compounds |
| WO2020083733A1 (en) | 2018-10-24 | 2020-04-30 | Basf Se | Pesticidal compounds |
| WO2020109039A1 (en) | 2018-11-28 | 2020-06-04 | Basf Se | Pesticidal compounds |
| WO2020126591A1 (en) | 2018-12-18 | 2020-06-25 | Basf Se | Substituted pyrimidinium compounds for combating animal pests |
| EP3696177A1 (en) | 2019-02-12 | 2020-08-19 | Basf Se | Heterocyclic compounds for the control of invertebrate pests |
| WO2020239517A1 (en) | 2019-05-29 | 2020-12-03 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2021013561A1 (en) | 2019-07-19 | 2021-01-28 | Basf Se | Pesticidal pyrazole and triazole derivatives |
| EP3766879A1 (en) | 2019-07-19 | 2021-01-20 | Basf Se | Pesticidal pyrazole derivatives |
| EP3769623A1 (en) | 2019-07-22 | 2021-01-27 | Basf Se | Mesoionic imidazolium compounds and derivatives for combating animal pests |
| WO2022167488A1 (en) | 2021-02-02 | 2022-08-11 | Basf Se | Synergistic action of dcd and alkoxypyrazoles as nitrification inhibitors |
| WO2022243523A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of an n-functionalized alkoxy pyrazole compound as nitrification inhibitor |
| WO2022243521A1 (en) | 2021-05-21 | 2022-11-24 | Basf Se | Use of ethynylpyridine compounds as nitrification inhibitors |
| WO2022268810A1 (en) | 2021-06-21 | 2022-12-29 | Basf Se | Metal-organic frameworks with pyrazole-based building blocks |
| WO2022268813A1 (en) | 2021-06-24 | 2022-12-29 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023046853A1 (en) | 2021-09-23 | 2023-03-30 | Syngenta Crop Protection Ag | Insect, acarina and nematode pest control |
| WO2023203066A1 (en) | 2022-04-21 | 2023-10-26 | Basf Se | Synergistic action as nitrification inhibitors of dcd oligomers with alkoxypyrazole and its oligomers |
| WO2024028243A1 (en) | 2022-08-02 | 2024-02-08 | Basf Se | Pyrazolo pesticidal compounds |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2013513553A (ja) | 2013-04-22 |
| CR20120361A (es) | 2012-12-18 |
| US20120245126A1 (en) | 2012-09-27 |
| CN102093389A (zh) | 2011-06-15 |
| PH12012501159A1 (en) | 2012-11-05 |
| JP5600750B2 (ja) | 2014-10-01 |
| EP2511279B1 (en) | 2015-11-11 |
| UA106256C2 (ru) | 2014-08-11 |
| US8809319B2 (en) | 2014-08-19 |
| KR20120094111A (ko) | 2012-08-23 |
| CO6561790A2 (es) | 2012-11-15 |
| ZA201205074B (en) | 2013-03-27 |
| AU2010330474B2 (en) | 2015-01-29 |
| IL220237A (en) | 2017-08-31 |
| MX2012006663A (es) | 2012-10-15 |
| RU2531920C2 (ru) | 2014-10-27 |
| IL220237A0 (en) | 2012-07-31 |
| EP2511279A4 (en) | 2013-05-01 |
| CA2783504A1 (en) | 2011-06-16 |
| CA2783504C (en) | 2013-07-09 |
| BR112012013846A2 (pt) | 2015-09-15 |
| KR101504575B1 (ko) | 2015-03-30 |
| CL2012001517A1 (es) | 2013-01-11 |
| AU2010330474A1 (en) | 2012-07-26 |
| CN102093389B (zh) | 2014-11-19 |
| RU2012127868A (ru) | 2014-01-20 |
| EP2511279A1 (en) | 2012-10-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2011069456A1 (zh) | 双联和氧桥杂环新烟碱化合物及其制备方法 | |
| JP5771150B2 (ja) | ジアルデヒドを用いて形成された殺虫活性を有する窒素含有複素環式化合物または酸素含有複素環式化合物およびその製造方法 | |
| CN101045728B (zh) | 一类具有高杀虫活性化合物的制备方法及用途 | |
| WO2006056108A1 (en) | Nitromethylene derivatives and their use | |
| CN101492444B (zh) | 具有杀虫活性的含氮杂环化合物、其制备及用途 | |
| CN102070607A (zh) | 具有杀虫活性的吡啶氮氧化物类新烟碱化合物及其用途 | |
| CN101906096B (zh) | 3,4-二氢吡啶-2-酮杂环类化合物及其用途 | |
| CN101875653B (zh) | 1,2,3-3h吡啶杂环化合物的制备及用途 | |
| CN104557963B (zh) | 二醛构建的具有杀虫活性的含氮或氧杂环化合物及其制备方法 | |
| CN103518745B (zh) | 二醛构建的具有杀虫活性的含氮或氧杂环化合物及其制备方法 | |
| CN103570729B (zh) | 环烯酮构建的二环新烟碱化合物及其制备方法和应用 | |
| CN110256414A (zh) | 具有杀虫活性的二硫环戊烯酮类化合物及其合成 | |
| CN106632300B (zh) | 具有杀虫活性的多取代异噁唑类化合物及其制备方法 | |
| CN104119268A (zh) | 具有杀虫活性的含三氟乙酰基新烟碱化合物的制备与用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10835491 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2783504 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13514460 Country of ref document: US Ref document number: 220237 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012001517 Country of ref document: CL Ref document number: 2012542350 Country of ref document: JP Ref document number: MX/A/2012/006663 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1201002721 Country of ref document: TH |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12012501159 Country of ref document: PH |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010835491 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010330474 Country of ref document: AU Ref document number: CR2012-000361 Country of ref document: CR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1669/MUMNP/2012 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12113709 Country of ref document: CO |
|
| ENP | Entry into the national phase |
Ref document number: 20127017848 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012127868 Country of ref document: RU Ref document number: A201208318 Country of ref document: UA |
|
| ENP | Entry into the national phase |
Ref document number: 2010330474 Country of ref document: AU Date of ref document: 20101209 Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112012013846 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112012013846 Country of ref document: BR Kind code of ref document: A2 Effective date: 20120608 |