WO2011021560A1 - 非焼成含炭塊成鉱およびその製造方法 - Google Patents
非焼成含炭塊成鉱およびその製造方法 Download PDFInfo
- Publication number
- WO2011021560A1 WO2011021560A1 PCT/JP2010/063685 JP2010063685W WO2011021560A1 WO 2011021560 A1 WO2011021560 A1 WO 2011021560A1 JP 2010063685 W JP2010063685 W JP 2010063685W WO 2011021560 A1 WO2011021560 A1 WO 2011021560A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- carbon
- porosity
- raw material
- unfired
- containing agglomerated
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B1/00—Preliminary treatment of ores or scrap
- C22B1/14—Agglomerating; Briquetting; Binding; Granulating
- C22B1/24—Binding; Briquetting ; Granulating
- C22B1/242—Binding; Briquetting ; Granulating with binders
- C22B1/244—Binding; Briquetting ; Granulating with binders organic
- C22B1/245—Binding; Briquetting ; Granulating with binders organic with carbonaceous material for the production of coked agglomerates
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21B—MANUFACTURE OF IRON OR STEEL
- C21B5/00—Making pig-iron in the blast furnace
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21B—MANUFACTURE OF IRON OR STEEL
- C21B5/00—Making pig-iron in the blast furnace
- C21B5/008—Composition or distribution of the charge
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B1/00—Preliminary treatment of ores or scrap
- C22B1/14—Agglomerating; Briquetting; Binding; Granulating
- C22B1/24—Binding; Briquetting ; Granulating
- C22B1/242—Binding; Briquetting ; Granulating with binders
- C22B1/243—Binding; Briquetting ; Granulating with binders inorganic
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P10/00—Technologies related to metal processing
- Y02P10/20—Recycling
Definitions
- the present invention relates to a non-fired carbon-containing agglomerated mineral for a blast furnace that is manufactured by mixing, forming, and curing an iron-containing raw material and a carbon-containing raw material.
- the present invention relates to a non-fired carbon-containing agglomerated ore for blast furnace having a carbon content (TC) of 18 to 25% by mass and a porosity of 20 to 30%, and a method for producing the same.
- TC carbon content
- a method for producing an unfired carbon-containing agglomerated mineral a method is known in which iron-making dust is granulated into pellets, and then the pellets are cured and hardened.
- the particle size distribution of the dust is adjusted to an appropriate range, a binder such as quick lime and cement and 5 to 15% of water are added, and the mixture is granulated with a disk pelletizer or the like. I have pellets.
- a carbon interior non-fired agglomerated mineral is manufactured by blending an iron-containing iron raw material and a carbon-based carbon material, adding a binder, kneading, molding, and curing.
- This carbon-incorporated non-calcined agglomerated mineral has 80 to 120% of the theoretical carbon amount required to reduce iron oxide contained in the iron oxide-containing raw material into metallic iron.
- the binder is selected so that the crushing strength at room temperature is 7850 kN / m 2 or more, and mixing, molding, and curing are performed. Since the reduction reaction occurs due to the carbon contained in the iron oxide in the unfired carbon-containing agglomerated mineral, the reduction rate can be improved.
- Patent Document 2 focusing on the fact that the particle size and carbon content of the carbonaceous material greatly affect the hot strength in the reduction temperature region as well as the cold strength of the carbon-containing unfired carbon-containing agglomerated mineral, A method for producing an unfired carbon-containing agglomerated mineral for blast furnaces with a strength of 50 kg / cm 2 or more is proposed.
- a finely divided iron-containing raw material containing 40% by mass or more of iron and a finely divided carbonaceous material containing 10% by mass or more of carbon are added with a hydraulic binder, mixed while adjusting moisture,
- the median diameter of the finely divided carbonaceous material is 100 to 150 ⁇ m.
- the unfired agglomerated blast furnace ore requires proper moisture in the granulation and molding processes.
- cement-based binders exhibit strength due to hydration reaction, they have a disadvantage that the amount of water of crystallization is higher than that of other raw materials and the explosion characteristics in a blast furnace are inferior.
- the converter dust generated at the ironworks is collected by a non-combustion gas processing device and mixed with carbon powder as an iron raw material to produce pellets.
- This pellet is partially reduced to reduced iron in a rotary hearth type reducing furnace and reused.
- the recovered converter dust contains a lot of moisture, and has poor handleability and miscibility with other powders. For this reason, converter dust is used after being dried, but if it is too dry, there is a problem that metal iron having a large specific surface area reacts with air in a fine particle state in converter dust and generates heat of oxidation.
- Patent Document 3 discloses a method for recycling converter dust.
- the powder containing iron oxide and the powder containing carbon are mixed with the converter dust collected by the non-combustion dust collector of the converter gas, and the moisture content of the mixture is adjusted to 17 to 27% by mass.
- the mixture is molded to produce a compact having a porosity of 40 to 54%, and the compact is reduced in a rotary hearth type reduction furnace.
- this method has an effect of preventing oxidation heat generation when the compact is reduced in the rotary hearth type reduction furnace. For this reason, this method is not directly useful for improving the explosion characteristics in blast furnaces having different operating conditions such as temperature.
- Patent Document 4 discloses a method for reducing iron oxide using a rotary hearth type reducing furnace. This method includes a step of placing a molded body containing metal oxide and carbon on a moving hearth, heating the molded body with heat from the upper combustion gas, and firing and reducing, and ferric oxide.
- the porosity of the molded body containing is adjusted to a specific value. By adjusting the porosity of the molded body to a specific value, volume expansion that occurs when iron oxide is reduced from hematite to magnetite is absorbed by the pores. For this reason, there is little powdering and stable reduction is possible.
- this method is also effective in preventing pulverization when the compact is reduced in a rotary hearth type reduction furnace, but it is directly useful for preventing pulverization in blast furnaces with different operating conditions such as temperature and reduction pattern. It is not a thing.
- the porosity and carbon content of a carbon-containing agglomerated ore that is optimal for efficient blast furnace operation are specified, and an unfired carbon-containing agglomerated ore for blast furnaces that enables efficient blast furnace operation and It aims at providing the manufacturing method.
- the inventors of the present invention have intensively studied the porosity and carbon content of unfired carbon-containing agglomerated ore for blast furnaces. As a result, the following characteristics can be realized by controlling the blending conditions and manufacturing conditions so that the porosity of the unfired carbon-containing agglomerated mineral is 20-30% and the carbon content is 18-25% by mass. It has been found that an unfired carbon-containing agglomerated mineral can be provided.
- A Excellent pulverization resistance against explosion or reduced pulverization by steam
- b High reducibility of iron oxide in unfired carbon-containing agglomerated minerals
- c Surrounding iron ore (iron-based charge)
- Promotion of reduction The blending conditions to be controlled are raw material particle size, fine carbon content, high crystal water ore blending amount, cement amount, and the like.
- the non-fired carbon-containing agglomerated ore for a blast furnace is obtained by mixing and kneading an iron-containing raw material, a carbon-containing raw material, and a binder, forming a kneaded product, and then obtaining the molded product.
- the carbon content (TC) is 18 to 25% by mass and the porosity is 20 to 30%.
- a method for producing a non-fired carbon-containing agglomerated ore for a blast furnace according to an aspect of the present invention is a method of mixing a kneaded material, a carbon-containing material, and a binder, kneading, forming a kneaded product, and obtaining a molded product.
- a step of curing the shaped body to obtain a non-fired carbonaceous agglomerated mineral, and the carbon content (TC) of the non-fired carbonaceous agglomerated mineral is 18 to 25% by mass. 1 and selected from the group consisting of raw material moisture, raw material particle size, fine coke content, high crystal water ore content, and binder content so that the porosity is 20-30%. Or two or more compounding conditions are adjusted.
- the method for producing a non-fired carbon-containing agglomerated mineral for a blast furnace since a non-fired process is applied, it is possible to save energy and reduce CO 2 compared to the fired process. Further, the dust generated in the iron making process can be recycled as an iron-containing raw material and a carbon material by a relatively inexpensive and simple method.
- the non-fired carbon-containing agglomerated ore for blast furnace of this embodiment is a method of mixing and kneading iron-containing raw material, carbon-containing raw material, and binder, forming a kneaded product to obtain a molded product, and then curing the molded product Manufactured by.
- the carbon content (TC) is 18 to 25% by mass, and the porosity is 20 to 30%.
- the carbon content (TC) of the unfired carbon-containing agglomerated mineral is 18 to 25% by mass.
- the carbon content (TC) of the unfired carbon-containing agglomerated mineral is preferably 20 to 23% by mass, more preferably 22 to 23% by mass.
- the porosity of the unfired carbon-containing agglomerated mineral is set to 20 to 30%.
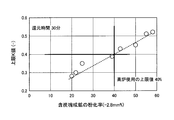
- the porosity is less than 20%, the effect of improving the reduction rate is limited (FIGS. 7 and 8).
- the pulverization rate in a blast furnace increases, and the upper limit of the pulverization rate required for the raw material used in a blast furnace may be exceeded (FIG. 1).
- the porosity exceeds 30%, the effect of improving the reduction rate is saturated (FIGS. 7 and 8).
- the cold crushing strength is reduced, and the minimum cold crushing strength necessary for use in a blast furnace cannot be obtained (FIG. 6).
- the porosity of the unfired carbon-containing agglomerated mineral is preferably 23 to 27%, more preferably 24 to 26%.
- the method for producing a non-fired carbon-containing agglomerated ore for a blast furnace includes a step of forming a molded body to obtain a molded body by mixing and kneading the iron-containing raw material, the carbon-containing raw material, and the binder, and molding the kneaded product. Then, there is a step of curing the molded body.
- the raw material moisture, the raw material particle size, the carbon content (TC) of the unfired carbon-containing agglomerated mineral is 18 to 25% by mass, and the porosity is 20 to 30%.
- One or two or more compounding conditions selected from the group consisting of fine powder coke amount, high crystal water ore compounding amount, and binder compounding amount are adjusted.
- iron-containing raw materials used in the present embodiment sintered dust generated in the iron making process, iron-containing dust such as fired dust obtained by firing oil-containing sludge, pellet feed having a smaller particle size than powdered iron ore for sintering, etc.
- iron-containing dust such as fired dust obtained by firing oil-containing sludge, pellet feed having a smaller particle size than powdered iron ore for sintering, etc.
- examples thereof include fine powder iron ore and high crystal water ore containing a large amount of crystal water.
- Examples of the carbon-containing raw material used in this embodiment include blast furnace primary ash, coke dust, fine coke, and anthracite.
- raw material moisture is also referred to as free water, and means the amount of water contained in raw material of molded product after molding (before curing).
- the porosity can be increased.
- the powdering rate explosiveness
- the raw material particle size means a weighted average value of the weight-based median diameter d50 of the iron-containing raw material and the carbon-containing raw material to be used.
- the porosity can be reduced.
- the pulverization rate explosiveness
- fine powder coke means fine powdery coke having a median diameter d50 value of 100 ⁇ m or less based on weight.
- the amount of fine coke as a carbon-containing raw material is increased, the porosity can be increased.
- the amount of fine coke is too small, there is a problem that the powdering rate (explosiveness) is increased. For this reason, it is preferable to adjust the amount of fine coke in the range of 10 to 30%.
- the high crystal water ore means ores containing 5% or more of crystal water such as lobe river, yandi couzina, maramanba and the like.
- crystal water such as lobe river, yandi couzina, maramanba and the like.
- binders used in the present embodiment include fine powders mainly composed of blast furnace granulated slag and aging binders composed of alkali stimulants, quicklime, Portland cement, bentonite, and the like.
- the binder blending amount (addition amount) can be appropriately determined in consideration of other blending conditions and the like. If the amount of the binder is too small, it is difficult to sufficiently maintain the cold rolling strength of the unfired carbon-containing agglomerated mineral. Moreover, when there is too much binder compounding quantity, the amount of slag of a non-baking carbon-containing agglomerated mineral will increase, and the air permeability of a furnace lower part will become unstable. For this reason, the effect of reducing the reducing material ratio stably cannot be obtained. From the above viewpoint, a particularly preferable binder content is 5 to 19% by mass.
- the iron-containing raw material and the carbon-containing raw material cut out from the raw material hopper are put together with a binder such as cement into a wet ball mill or a Reidege mixer and mixed. And after kneading, it is kneaded.
- the kneaded material obtained by sufficiently kneading is formed by a pan pelletizer or a briquette machine.
- the molded body is cured in the sun for several days until the strength required for handling is developed in the primary curing yard. Thereafter, in the secondary curing yard, the molded body is cured in the sun to sufficiently develop the strength by using a binder such as cement.
- the non-baking carbon-containing agglomerate for blast furnaces is manufactured by the above. And it is supplied to a blast furnace and used.
- the production process in order to set the carbon content (TC) of the unfired carbon-containing agglomerated mineral to 18 to 25% by mass and the porosity to 20 to 30%, the production process (pellet, briquette), blending conditions It can be carried out by adjusting (raw material moisture, raw material particle size, fine coke amount, high crystal water ore compounding amount, binder compounding amount). In particular, it can be carried out by adjusting one or two or more blending conditions selected from the group consisting of raw material moisture, raw material particle size, fine coke amount, high crystal water ore blending amount, and binder blending amount. In general, pellet molding is more porous than briquette molding, but either may be selected according to the raw material conditions.
- the porosity increases as the raw material moisture, the amount of fine carbon (the amount of fine coke), and the amount of high crystal water ore increase.
- the carbon in the non-fired carbon-containing agglomerated mineral of the present embodiment reduces iron oxide in the non-fired carbon-containing agglomerated mineral, but the surplus carbon further reduces the surrounding iron ore in the blast furnace. For this reason, a reduction rate can be improved (FIGS. 7 and 8).
- CO gas reducing gas
- the reducing gas reducing power is weakened in the upper layer of the ore layer, and the reduction of the ore may not proceed sufficiently.
- Reduction efficiency can be greatly improved. Since the reduction efficiency in the upper layer of the ore layer that is difficult to be reduced can be greatly improved, the reduction efficiency in the entire blast furnace is greatly improved. For this reason, it is possible to reduce the amount of reducing material larger than the amount of coke equivalent to the excess amount of carbon in the unfired carbon-containing agglomerated mineral of this embodiment (FIG. 7).
- the ratio of reducing materials related to the operation of the blast furnace including the pulverized coal blown from the tuyere can be reduced. Since the reducing material ratio can be reduced, the amount of CO 2 generated in the iron making process can be reduced, and the environmental load can be reduced.
- Example 1 Manufacture of unfired carbon-containing agglomerated minerals
- iron-containing raw materials, carbon-containing raw materials, and binders mixing, hydration, kneading, molding (granulation), curing are performed while adjusting the blending amount, particle size, and moisture content of the raw materials as shown in Table 1.
- An unfired carbon-containing agglomerated mineral was produced.
- the porosity was measured by the water method (JIS K2151 conformity) which substitutes with water and measures an apparent specific gravity.
- Table 1 shows the types of iron-containing raw materials used, types of carbon-containing raw materials, raw material moisture content, raw material particle size (average value), types and blending amounts of crystal water ore, binder types and blending amounts, and The carbon content and porosity of unfired carbon-containing agglomerated minerals are also shown.
- the unfired carbon-containing agglomerated mineral of this embodiment It was found that can be manufactured.
- the granulation equipment is not particularly limited, and may be any material having functions of kneading, hydrating, granulating, and product sieving of raw materials, and kneading machines, granulating machines, etc. are particularly limited. is not.
- Example 2 (Influence of porosity) Non-fired carbon-containing agglomerated minerals with different porosity were prepared, and the effect of porosity on the pulverization phenomenon of unfired carbon-containing agglomerated minerals in the furnace was investigated.
- the same iron-containing raw material and carbon-containing raw material as in Example 1 were pulverized, mixed and kneaded together with cement (binder) to form a kneaded product.
- the obtained molded body was cured for a predetermined period to produce a non-fired carbon-containing agglomerated mineral having a carbon content (TC) of 15,25% by mass.
- TC carbon content
- the blending amount of the iron-containing raw material and the carbon-containing raw material is set to a constant value, and the porosity is adjusted to 5%, 10%, 15%, 20%, 25%, 30 by adjusting the molding pressure and the cement amount at the time of compression molding. %, 35%, and 40% unfired carbon-containing agglomerated minerals were produced. Further, the brand of the carbon-containing raw material was finely adjusted according to the change in the cement blending amount so that the carbon content (TC) was constant at 15% by mass or 25% by mass.
- the powdering property was evaluated by the following method using a reduced powdering test (JIS M8720).
- JIS M8720 a reduced powdering test
- a sample of 500 g was heated in N 2 and held at 550 ° C. in a reducing gas containing 30% CO for a predetermined time.
- the measurement sample was prepared by setting the reduction time at 550 ° C. to 1, 10, 30, 60 minutes.
- a rotational impact of 900 rotations was applied to the measurement sample using a rotation tester.
- the ratio of particles of 2.8 mm or less (powdering rate (-2.8 mm%)) of the measurement sample after applying the rotational impact is measured, and this powdered rate (-2.8 mm%) is used to reduce powder. Sex was evaluated.
- the explosibility was measured by the following method with reference to a method for testing thermal cracking of iron ore (ISO 8371: Iron ores-Determination of description index).
- ISO 8371 Iron ores-Determination of description index.
- a 500 g sample was rapidly heated in N 2 to a maximum temperature of 700 ° C.
- samples for measurement were prepared at heating rates of 5 ° C./min, 50 ° C./min, 500 ° C./min, and 1000 ° C./min.
- the pulverization phenomenon caused by gas generation due to evaporation and gasification of contained water and crystal water (from iron ore and cement) is called explosion.
- the pulverization phenomenon caused by the generation of voids or volume expansion or internal stress accompanying reduction is referred to as reduction pulverization.
- the powdering rate tends to decrease as the porosity increases, but the effect of the reduction time is large. Reduced powdering is caused by volume expansion and internal stress during the reduction of hematite to magnetite and is most intensely powdered around 550 ° C. For this reason, it is known that the reduction powdering depends on the residence time around 550 ° C. In other words, when the reduction is 550 ° C. or higher, the powdering becomes rather small. From this, the residence time at 550 ° C. affects the reduction powdering together with the porosity.
- the rotary hearth type reduction furnace has a high heating rate of 1000 ° C./min and a residence time of 550 ° C.
- the residence time at 550 ° C. in the blast furnace is 10 minutes (center part) to 60 minutes (peripheral part), and there is a problem that the reduction powdering rate becomes high.
- Example 3 (Allowable powder reduction rate)
- the upper K value needs to be 0.4 or less. From the upper limit of the upper K value, the allowable range of the reduced powdering rate of the unfired carbon-containing agglomerated ore was examined. The blending amount of the raw materials was adjusted so that the carbon content (TC) was 25% by mass, and non-fired carbon-containing agglomerated minerals having different porosities were produced in the same manner as in Example 2. The pulverization rate ( ⁇ 2.8 mm%) was measured in the same manner as in the evaluation method of reduced powder property of Example 2.
- the upper K value was measured by the following method.
- a blast furnace with an internal volume of 4500 m 3 short-term tests were conducted using various unfired carbon-containing agglomerated minerals having different porosity (ie, pulverization rate).
- An unfired carbon-containing agglomerated amount of 10% by weight of the total iron-based charge was mixed and charged into the ore layer.
- the operating conditions under the base condition of the blast furnace were a reducing material ratio of 480 kg / tp and an ore to coke weight ratio of 5.0.
- the ventilation resistance value (upper K value) at the upper part of the shaft was calculated from the measured value of the pressure probe installed on the furnace wall. The obtained results are shown in Table 4 and FIG.
- FIG. 3 shows the relationship between the pulverization rate of the unfired carbon-containing agglomerated mineral and the upper K value.
- the upper K value needs to be 0.4 or less. From the relationship between the pulverization rate (reduction pulverization rate) of the unfired carbon-containing agglomerated mineral shown in FIG. 3 and the upper K value, the non-fired carbon-containing agglomerated mineral with a carbon content of 25% by weight is pulverized. It can be seen that when the rate (reduction powdering rate) exceeds 40%, the upper K value increases to more than 0.4, and stable operation becomes difficult. Therefore, it is important to reduce the pulverization rate (reduction pulverization rate) to 40% or less in the unfired carbon-containing agglomerated mineral having a carbon content of 25% by weight.
- the powdering rate when the porosity is 20% or more, the powdering rate is a relatively low value.
- the porosity becomes less than 20% with 20% as a boundary the reduction powderization rate increases rapidly. In particular, this tendency is remarkable when the reduction time is 30 minutes or more.
- the powdering rate reduced powdering rate
- the powdering rate can be suppressed to 40% or less even when the reduction time is 60 minutes. It is considered that when the porosity is increased, the internal stress resulting from the volume expansion of the unfired carbon-containing agglomerated minerals is dispersed by the pores, and reduced powdering is suppressed.
- the reduction time should be as short as possible and the porosity should be 20% or more.
- the rotary hearth type reducing furnace has a high heating rate of 1000 ° C./min, and the unfired carbon-containing agglomerated minerals are easy to explode.
- the heating rate of the blast furnace is 5 ° C./min (peripheral part) to 50 ° C./min (central part). For this reason, referring to FIG. 2, it may be considered that the problem of pulverization (explosibility) does not occur when an unfired carbon-containing agglomerated mineral having a carbon content of 15 mass% is used in a blast furnace.
- Example 4 (Influence of carbon content) Next, the influence of the carbon content (TC) of the unfired carbon-containing agglomerated ore was examined.
- Non-calcined carbon-containing agglomerated minerals having different carbon content (TC) and porosity were prepared, and the influence of carbon content (TC) on pulverization (explosive properties) was investigated.
- the carbon content (TC) is 15% by mass, 18 except that the blending amount of the iron-containing raw material and the carbon-containing raw material, the molding pressure at the time of compression molding, and the amount of cement are adjusted.
- Non-fired carbon-containing agglomerated minerals having a porosity of 10%, 20%, 30%, and 40% were prepared.
- the explosibility was evaluated by measuring the powdering rate ( ⁇ 6.3 mm%) in the same manner as the explosive evaluation method of Example 2 except that the heating rate (temperature increase rate) was 50 ° C./min. .
- the heating rate of 50 ° C./min is the most severe temperature rise condition in the blast furnace. The obtained results are shown in Table 5 and FIG.
- Example 5 (Explosive tolerance) As described above, in order to perform stable operation in an actual blast furnace, the upper K value needs to be 0.4 or less. From the upper limit of the upper K value, the allowable range of explosibility of the unfired carbon-containing agglomerated ore was examined.
- the carbon content (TC) is 20% by mass in the same manner as in Example 2 except that the blending amount of the iron-containing material and the carbon-containing material, the molding pressure at the time of compression molding, and the amount of cement are adjusted.
- the non-fired carbon-containing agglomerated minerals having various values of porosity were prepared.
- the explosibility was evaluated by measuring the powdering rate ( ⁇ 6.3 mm%) in the same manner as the explosive evaluation method of Example 2 except that the heating rate (temperature increase rate) was 50 ° C./min. .
- the upper K value was measured in the same manner as in Example 3 using the unfired carbon-containing agglomerated mineral in an amount of 10% by mass based on the total iron-based charge. The obtained results are shown in Table 6 and FIG.
- FIG. 5 shows the relationship between the explosibility and the upper K value of an unfired carbon-containing agglomerated mineral having a carbon content (TC) of 20% by mass.
- FIG. 5 shows that when the non-fired carbon-containing agglomerated ore is used in an amount of 10% by mass with respect to the total iron-based charge in the blast furnace, the explosibility of the non-fired carbon-containing agglomerated ore is the ventilation of the blast furnace.
- the effect on sex was investigated. From the relationship between the explosibility of the unfired carbon-containing agglomerated ore and the upper K value shown in Fig. 5, when the explosibility exceeds 30%, the upper K value rises above 0.4 and is stable. It can be seen that efficient operation becomes difficult. Therefore, it is important to reduce the explosiveness to 30% or less.
- FIG. 2 it was found from FIG. 2 that the greater the porosity, the smaller the explosiveness. Further, FIG. 1 also shows that the porosity needs to be 20% or more. Referring to FIG. 4, it can be seen that in an unfired carbon-containing agglomerated mineral having a porosity of 20% or more, when the carbon content (TC) is 25% by mass or less, the explosiveness is 30% or less. For this reason, it turns out that it is necessary to make carbon content (TC) into 25 mass% or less.
- Example 6 Cold crushing strength, 1000 ° C reduction rate and reducing material ratio in BIS furnace
- the carbon content (TC) is 15% by mass, 18 except that the blending amount of the iron-containing raw material and the carbon-containing raw material, the molding pressure at the time of compression molding, and the amount of cement are adjusted.
- Non-calcined carbon-containing agglomerated minerals with a mass%, 25 mass%, 26 mass% and porosity of 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40% were produced. .
- ⁇ Batteries for blast furnace are required to have sufficient strength to withstand handling such as transfer and sizing until charging into the blast furnace.
- the cold crushing strength of the unfired carbon-containing agglomerated mineral was measured as an index of such strength.
- the cold crushing strength was measured as follows according to JIS M8718 “Iron Ore Pellet Crushing Strength Test Method”. A compressive load was applied to one sample at a specified pressure plate speed, the load value when the sample broke was measured, and the average value of 100 samples was evaluated as the cold crushing strength. The obtained results are shown in Table 7 and FIG.
- FIG. 6 shows the relationship between the porosity and cold crushing strength of unfired carbon-containing agglomerated minerals.
- the cold crushing strength does not depend on the carbon content, and is almost determined by the difference in porosity. I understand that. In any carbon-containing agglomerated ore with any carbon content, it is difficult to maintain 100 kg / cm 2, which is the lower limit of the cold crushing strength necessary for use in a blast furnace, at a porosity of 30% or more. It was. Therefore, from the viewpoint of cold crushing strength, the porosity should be 30% or less.
- the cold crushing strength is low at a porosity of 20% or more. Below 100 kg / cm 2 (lower limit for blast furnace use). For this reason, the carbon content should be 25% by mass or less.
- the amount of coke blended is excessively large, the amount of binder that penetrates into open pores in the coke increases. Therefore, when the carbon content exceeds 25% by mass, it is considered difficult to efficiently develop the strength with the binder.
- the BIS furnace reducing material ratio and 1000 are as follows.
- the reduction rate in ° C was measured.
- An uncalcined carbon-containing agglomerated mineral in an amount of 10% by weight of the total iron-based charge was uniformly mixed into the ore layer and charged into the BIS furnace so as to be layered with the coke layer.
- the BIS furnace is a test apparatus for simulating the countercurrent reaction of the blast furnace shaft portion, and is composed of a reaction tube in which sintered ore and coke are charged in layers, and a vertically moving electric furnace.
- the charging amount was adjusted so that the weight ratio of iron oxide to carbon was 5.0. Then, a bosch gas amount and composition gas corresponding to an operation with a reducing material ratio of 480 kg / tp and a pulverized coal injection ratio of 150 kg / tp was supplied to the BIS furnace to reduce the ore. The shaft efficiency and heat storage zone temperature of the BIS furnace were measured, and the thermal mass balance was calculated from these measured values. The reducing material ratio of the BIS furnace was obtained from the thermal mass balance. Moreover, after finishing the reduction
- FIGS. 7 and 8 show the reducing material ratio and the 1000 ° C. reduction rate in the BIS furnace when the unfired carbon-containing agglomerated mineral in an amount of 10% by weight of the total iron-based charge is uniformly mixed into the ore layer.
- FIGS. 7 and 8 it can be seen that the higher the carbon content, the higher the 1000 ° C. reduction rate and the lower the reducing material ratio.
- the carbon content is 15%, the reduction rate of 1000 ° C. is remarkably lowered, and the efficiency of blast furnace operation is lowered. For this reason, the lower limit value of the carbon content (TC) is set to 18%.
- the porosity is increased and the 1000 ° C. reduction rate is improved. Even if the carbon content (TC) is 18% by mass, the reduction ratio reaches 75% when the porosity is 20%, and the reducing material ratio is 470 kg / tp or less. Reached. However, when the porosity was less than 20%, the effect of improving the 1000 ° C. reduction rate and reducing the reducing material ratio was limited, and was almost the same as the condition without the non-fired carbon-containing agglomerated ore. Moreover, when the porosity exceeded 30%, it turned out that the effect of improving a 1000 degreeC reduction rate and reducing a reducing material ratio is saturated. Therefore, it is understood that the porosity of the carbon-containing agglomerated minerals having a carbon content (TC) of 18% by mass and 25% by mass may be 20% or more and 30% or less.
- the carbon content (TC) of 18 to 25 masses is the most effective in achieving the most effective effects of pulverization rate, explosiveness, cold crushing strength, reduction rate and reducing material ratio in blast furnace operation.
- an unfired carbon-containing agglomerated mineral having a porosity of 20 to 30% may be used.
- the non-fired carbon-containing agglomerated ore according to one aspect of the present invention is not only a non-fired carbon-containing agglomerated mineral when used in a blast furnace, but also the reduction rate of iron-containing raw materials for main blast furnaces such as sintered ores. Has a sufficient carbon content to improve. Furthermore, while maintaining a cold crushing strength of 100 kg / cm 2 or more required as a raw material for a blast furnace, it is excellent in hot strength in a reduction temperature range as compared with the conventional one. For this reason, the reducing material ratio (coke ratio) at the time of blast furnace operation can be reduced significantly.
- the non-calcined carbonaceous mass Naruko manufacturing method according to an embodiment of the present invention, as compared to the firing process, energy saving, it is possible to lower CO 2 reduction.
- the dust generated in the iron making process can be recycled as an iron-containing raw material and a carbonaceous material by a relatively inexpensive and simple method. Therefore, one aspect of the present invention can be suitably applied to the technical field related to the carbon-containing agglomerated mineral used in the blast furnace.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Metallurgy (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Environmental & Geological Engineering (AREA)
- Geology (AREA)
- Geochemistry & Mineralogy (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Mechanical Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Inorganic Chemistry (AREA)
- Manufacture And Refinement Of Metals (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN992DEN2012 IN2012DN00992A (zh) | 2009-08-21 | 2010-08-12 | |
| CN2010800364934A CN102471822B (zh) | 2009-08-21 | 2010-08-12 | 非烧成含碳团矿及其制造方法 |
| KR1020127004077A KR101444562B1 (ko) | 2009-08-21 | 2010-08-12 | 비소성 함탄 괴성광 및 그 제조 방법 |
| BR112012003768-4A BR112012003768B1 (pt) | 2009-08-21 | 2010-08-12 | Aglomerados compósitos de carbono ligados a frio para altos fornos e o método para a sua fabricação |
| JP2010545716A JP4842403B2 (ja) | 2009-08-21 | 2010-08-12 | 非焼成含炭塊成鉱の製造方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009-192273 | 2009-08-21 | ||
| JP2009192273 | 2009-08-21 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011021560A1 true WO2011021560A1 (ja) | 2011-02-24 |
Family
ID=43607015
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2010/063685 WO2011021560A1 (ja) | 2009-08-21 | 2010-08-12 | 非焼成含炭塊成鉱およびその製造方法 |
Country Status (6)
| Country | Link |
|---|---|
| JP (1) | JP4842403B2 (zh) |
| KR (1) | KR101444562B1 (zh) |
| CN (1) | CN102471822B (zh) |
| BR (1) | BR112012003768B1 (zh) |
| IN (1) | IN2012DN00992A (zh) |
| WO (1) | WO2011021560A1 (zh) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013104108A (ja) * | 2011-11-15 | 2013-05-30 | Nippon Steel & Sumitomo Metal Corp | 非焼成含炭塊成鉱の製造方法 |
| JP2017193742A (ja) * | 2016-04-20 | 2017-10-26 | Jfeスチール株式会社 | 炭素内装鉱の製造方法 |
| WO2019033187A1 (pt) * | 2017-08-16 | 2019-02-21 | Travassos Da Rosa Costa Daniel | Processo de pelotização a frio de finos de minério de ferro com flexibilidade de misturas |
| CZ308005B6 (cs) * | 2017-12-19 | 2019-10-16 | Martin Gajdzica | Briketa či peleta pro vsázku do metalurgických agregátů |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006257479A (ja) * | 2005-03-16 | 2006-09-28 | Jfe Steel Kk | 還元鉄の製造方法 |
| JP2008095177A (ja) * | 2006-09-11 | 2008-04-24 | Nippon Steel Corp | 高炉用含炭非焼成ペレットの製造方法 |
| JP2009161791A (ja) * | 2007-12-28 | 2009-07-23 | Nippon Steel Corp | 高炉用含炭非焼成ペレットの製造方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS513310A (zh) * | 1974-06-29 | 1976-01-12 | Sumitomo Metal Ind | |
| JP3732136B2 (ja) * | 2000-10-18 | 2006-01-05 | 新日本製鐵株式会社 | 還元鉄の製造方法および還元鉄の冷却装置 |
| JP3732132B2 (ja) * | 2000-10-18 | 2006-01-05 | 新日本製鐵株式会社 | 回転炉床式還元炉の操業方法 |
| DE60138725D1 (de) * | 2000-10-30 | 2009-06-25 | Nippon Steel Corp | METALLOXID ENTHALTENDES GRÜNPELLET FÜR REDUKTIONSOFEN und VERFAHREN ZU SEINER HERSTELLUNG, VERFAHREN ZU SEINER REDUKTION |
| JP4118604B2 (ja) * | 2002-05-28 | 2008-07-16 | 株式会社 テツゲン | 高炉用のカーボン内装非焼成塊成鉱およびその製造方法 |
| JP4438297B2 (ja) * | 2003-03-10 | 2010-03-24 | 株式会社神戸製鋼所 | 還元金属の製造方法および炭材内装塊成物 |
-
2010
- 2010-08-12 BR BR112012003768-4A patent/BR112012003768B1/pt active IP Right Grant
- 2010-08-12 KR KR1020127004077A patent/KR101444562B1/ko active IP Right Grant
- 2010-08-12 CN CN2010800364934A patent/CN102471822B/zh active Active
- 2010-08-12 WO PCT/JP2010/063685 patent/WO2011021560A1/ja active Application Filing
- 2010-08-12 JP JP2010545716A patent/JP4842403B2/ja active Active
- 2010-08-12 IN IN992DEN2012 patent/IN2012DN00992A/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006257479A (ja) * | 2005-03-16 | 2006-09-28 | Jfe Steel Kk | 還元鉄の製造方法 |
| JP2008095177A (ja) * | 2006-09-11 | 2008-04-24 | Nippon Steel Corp | 高炉用含炭非焼成ペレットの製造方法 |
| JP2009161791A (ja) * | 2007-12-28 | 2009-07-23 | Nippon Steel Corp | 高炉用含炭非焼成ペレットの製造方法 |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013104108A (ja) * | 2011-11-15 | 2013-05-30 | Nippon Steel & Sumitomo Metal Corp | 非焼成含炭塊成鉱の製造方法 |
| JP2017193742A (ja) * | 2016-04-20 | 2017-10-26 | Jfeスチール株式会社 | 炭素内装鉱の製造方法 |
| WO2019033187A1 (pt) * | 2017-08-16 | 2019-02-21 | Travassos Da Rosa Costa Daniel | Processo de pelotização a frio de finos de minério de ferro com flexibilidade de misturas |
| CZ308005B6 (cs) * | 2017-12-19 | 2019-10-16 | Martin Gajdzica | Briketa či peleta pro vsázku do metalurgických agregátů |
Also Published As
| Publication number | Publication date |
|---|---|
| BR112012003768B1 (pt) | 2018-06-05 |
| JP4842403B2 (ja) | 2011-12-21 |
| JPWO2011021560A1 (ja) | 2013-01-24 |
| IN2012DN00992A (zh) | 2015-04-10 |
| BR112012003768A2 (pt) | 2016-04-12 |
| KR101444562B1 (ko) | 2014-09-24 |
| KR20120047948A (ko) | 2012-05-14 |
| CN102471822B (zh) | 2013-12-25 |
| CN102471822A (zh) | 2012-05-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5000402B2 (ja) | 高炉用含炭非焼成ペレットの製造方法 | |
| KR101054136B1 (ko) | 핫 브리켓 아이언 및 그 제조 방법 | |
| JP4118604B2 (ja) | 高炉用のカーボン内装非焼成塊成鉱およびその製造方法 | |
| JP4842403B2 (ja) | 非焼成含炭塊成鉱の製造方法 | |
| JP5803540B2 (ja) | 非焼成含炭塊成鉱の製造方法 | |
| JP6228101B2 (ja) | 炭材内装鉱の製造方法 | |
| JP5114742B2 (ja) | 高炉用含炭非焼成ペレットの製造方法 | |
| TWI649429B (zh) | 燒結礦的製造方法 | |
| JP5786668B2 (ja) | 非焼成含炭塊成鉱の製造方法 | |
| JP5498919B2 (ja) | 還元鉄の製造方法 | |
| JP6326074B2 (ja) | 炭材内装鉱およびその製造方法 | |
| JP5454505B2 (ja) | 高炉用非焼成含炭塊成鉱の製造方法 | |
| JP5825180B2 (ja) | 石炭チャーを使用した高炉用非焼成含炭塊成鉱の製造方法 | |
| TWI632241B (zh) | Method for manufacturing sinter ore in carbon material | |
| JP4867394B2 (ja) | 製鉄用非焼成塊成鉱 | |
| JPS5926651B2 (ja) | 非焼成塊成鉱の製造方法 | |
| JP5835144B2 (ja) | 高炉用非焼成含炭塊成鉱の製造方法 | |
| JP5447410B2 (ja) | 高炉用非焼成含炭塊成鉱の製造方法 | |
| JP2003027150A (ja) | 耐粉化特性に優れた非焼成塊成鉱の製造方法および非焼成塊成鉱 | |
| JP2005290525A (ja) | 高炉用冷間塊成鉱の製造方法 | |
| JPS5831043A (ja) | コ−ルドボンド鉱の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201080036493.4 Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2010545716 Country of ref document: JP |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10809905 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 992/DELNP/2012 Country of ref document: IN |
|
| ENP | Entry into the national phase |
Ref document number: 20127004077 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10809905 Country of ref document: EP Kind code of ref document: A1 |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112012003768 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112012003768 Country of ref document: BR Kind code of ref document: A2 Effective date: 20120217 |