RU2685416C2 - Устройство для диагностики in vitro и его применения - Google Patents
Устройство для диагностики in vitro и его применения Download PDFInfo
- Publication number
- RU2685416C2 RU2685416C2 RU2015100233A RU2015100233A RU2685416C2 RU 2685416 C2 RU2685416 C2 RU 2685416C2 RU 2015100233 A RU2015100233 A RU 2015100233A RU 2015100233 A RU2015100233 A RU 2015100233A RU 2685416 C2 RU2685416 C2 RU 2685416C2
- Authority
- RU
- Russia
- Prior art keywords
- reaction
- reaction area
- red
- sample
- porous membrane
- Prior art date
Links
- 238000000338 in vitro Methods 0.000 title claims abstract description 10
- 238000003745 diagnosis Methods 0.000 title claims abstract 14
- 238000006243 chemical reaction Methods 0.000 claims abstract description 146
- 239000012528 membrane Substances 0.000 claims abstract description 130
- 239000000427 antigen Substances 0.000 claims abstract description 66
- 102000036639 antigens Human genes 0.000 claims abstract description 66
- 108091007433 antigens Proteins 0.000 claims abstract description 66
- 210000003743 erythrocyte Anatomy 0.000 claims abstract description 49
- 210000004369 blood Anatomy 0.000 claims abstract description 40
- 239000008280 blood Substances 0.000 claims abstract description 40
- 238000012360 testing method Methods 0.000 claims abstract description 32
- 238000000034 method Methods 0.000 claims abstract description 31
- 230000002209 hydrophobic effect Effects 0.000 claims abstract description 28
- 239000000758 substrate Substances 0.000 claims abstract description 21
- 239000000126 substance Substances 0.000 claims abstract description 13
- 239000000910 agglutinin Substances 0.000 claims abstract description 10
- 101710186708 Agglutinin Proteins 0.000 claims abstract description 9
- 101710146024 Horcolin Proteins 0.000 claims abstract description 9
- 101710189395 Lectin Proteins 0.000 claims abstract description 9
- 101710179758 Mannose-specific lectin Proteins 0.000 claims abstract description 9
- 101710150763 Mannose-specific lectin 1 Proteins 0.000 claims abstract description 9
- 101710150745 Mannose-specific lectin 2 Proteins 0.000 claims abstract description 9
- 239000011148 porous material Substances 0.000 claims abstract description 7
- 238000004519 manufacturing process Methods 0.000 claims abstract description 5
- 239000003795 chemical substances by application Substances 0.000 claims description 106
- 210000004027 cell Anatomy 0.000 claims description 71
- 239000000243 solution Substances 0.000 claims description 70
- 238000000151 deposition Methods 0.000 claims description 39
- 239000000872 buffer Substances 0.000 claims description 36
- 239000003599 detergent Substances 0.000 claims description 35
- 238000005406 washing Methods 0.000 claims description 27
- 239000012491 analyte Substances 0.000 claims description 26
- 230000008021 deposition Effects 0.000 claims description 20
- 239000000203 mixture Substances 0.000 claims description 15
- 230000004931 aggregating effect Effects 0.000 claims description 14
- 210000002966 serum Anatomy 0.000 claims description 11
- 239000003153 chemical reaction reagent Substances 0.000 claims description 9
- 239000007853 buffer solution Substances 0.000 claims description 7
- 238000001514 detection method Methods 0.000 claims description 6
- 238000011533 pre-incubation Methods 0.000 claims description 6
- 230000002391 anti-complement effect Effects 0.000 claims description 5
- 108010008730 anticomplement Proteins 0.000 claims description 5
- 230000006870 function Effects 0.000 claims description 5
- 238000011534 incubation Methods 0.000 claims description 5
- 238000010790 dilution Methods 0.000 claims description 4
- 239000012895 dilution Substances 0.000 claims description 4
- 238000001035 drying Methods 0.000 claims description 4
- 230000036571 hydration Effects 0.000 claims description 4
- 238000006703 hydration reaction Methods 0.000 claims description 4
- 238000011835 investigation Methods 0.000 claims description 4
- 229920002851 polycationic polymer Polymers 0.000 claims description 4
- 102000001554 Hemoglobins Human genes 0.000 claims description 3
- 108010054147 Hemoglobins Proteins 0.000 claims description 3
- 230000002093 peripheral effect Effects 0.000 claims description 3
- 239000002736 nonionic surfactant Substances 0.000 claims description 2
- 229920003023 plastic Polymers 0.000 claims description 2
- 239000004033 plastic Substances 0.000 claims description 2
- 239000007787 solid Substances 0.000 claims description 2
- 230000000694 effects Effects 0.000 abstract description 6
- 230000035945 sensitivity Effects 0.000 abstract description 4
- 238000004458 analytical method Methods 0.000 abstract description 3
- 239000003814 drug Substances 0.000 abstract 1
- 239000000523 sample Substances 0.000 description 48
- 229920001213 Polysorbate 20 Polymers 0.000 description 15
- 239000004365 Protease Substances 0.000 description 15
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 15
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 15
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 15
- -1 for example Polymers 0.000 description 11
- 230000003993 interaction Effects 0.000 description 11
- 230000002708 enhancing effect Effects 0.000 description 10
- 238000009738 saturating Methods 0.000 description 10
- 239000011780 sodium chloride Substances 0.000 description 8
- 229920004890 Triton X-100 Polymers 0.000 description 7
- 239000004094 surface-active agent Substances 0.000 description 7
- 229920000209 Hexadimethrine bromide Polymers 0.000 description 6
- 108010039918 Polylysine Proteins 0.000 description 6
- 239000012530 fluid Substances 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 229920000656 polylysine Polymers 0.000 description 6
- 108010004032 Bromelains Proteins 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 5
- 108090000790 Enzymes Proteins 0.000 description 5
- 108090000526 Papain Proteins 0.000 description 5
- 108091005804 Peptidases Proteins 0.000 description 5
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 5
- 230000004520 agglutination Effects 0.000 description 5
- 239000007864 aqueous solution Substances 0.000 description 5
- 235000019835 bromelain Nutrition 0.000 description 5
- 229940088598 enzyme Drugs 0.000 description 5
- 235000019834 papain Nutrition 0.000 description 5
- 229940055729 papain Drugs 0.000 description 5
- 235000019419 proteases Nutrition 0.000 description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 230000002776 aggregation Effects 0.000 description 4
- 238000004220 aggregation Methods 0.000 description 4
- 230000002349 favourable effect Effects 0.000 description 4
- 239000012634 fragment Substances 0.000 description 4
- 230000001788 irregular Effects 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 229930188929 simonin Natural products 0.000 description 4
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 3
- 239000013504 Triton X-100 Substances 0.000 description 3
- 239000002250 absorbent Substances 0.000 description 3
- 230000002745 absorbent Effects 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 230000000295 complement effect Effects 0.000 description 3
- 238000009792 diffusion process Methods 0.000 description 3
- 229920000136 polysorbate Polymers 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 230000002441 reversible effect Effects 0.000 description 3
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 108060003951 Immunoglobulin Proteins 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 229920002873 Polyethylenimine Polymers 0.000 description 2
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 2
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 238000002405 diagnostic procedure Methods 0.000 description 2
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 2
- 229920001903 high density polyethylene Polymers 0.000 description 2
- 239000004700 high-density polyethylene Substances 0.000 description 2
- 230000000887 hydrating effect Effects 0.000 description 2
- 102000018358 immunoglobulin Human genes 0.000 description 2
- 229940072221 immunoglobulins Drugs 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 230000003204 osmotic effect Effects 0.000 description 2
- 239000002953 phosphate buffered saline Substances 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- XOAAWQZATWQOTB-UHFFFAOYSA-N taurine Chemical compound NCCS(O)(=O)=O XOAAWQZATWQOTB-UHFFFAOYSA-N 0.000 description 2
- 239000003656 tris buffered saline Substances 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 108010044091 Globulins Proteins 0.000 description 1
- 102000006395 Globulins Human genes 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 208000035388 Ring chromosome 22 syndrome Diseases 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 108010008038 Synthetic Vaccines Proteins 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- XECAHXYUAAWDEL-UHFFFAOYSA-N acrylonitrile butadiene styrene Chemical compound C=CC=C.C=CC#N.C=CC1=CC=CC=C1 XECAHXYUAAWDEL-UHFFFAOYSA-N 0.000 description 1
- 239000004676 acrylonitrile butadiene styrene Substances 0.000 description 1
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 230000033558 biomineral tissue development Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 108700021073 cold agglutinins Proteins 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 239000013578 denaturing buffer Substances 0.000 description 1
- 230000001687 destabilization Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 239000012470 diluted sample Substances 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 229920002313 fluoropolymer Polymers 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 239000000076 hypertonic saline solution Substances 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 239000002523 lectin Substances 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 238000003754 machining Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 230000002906 microbiologic effect Effects 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 230000000065 osmolyte Effects 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 229920000193 polymethacrylate Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 239000001397 quillaja saponaria molina bark Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 229930182490 saponin Natural products 0.000 description 1
- 150000007949 saponins Chemical class 0.000 description 1
- 230000011218 segmentation Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 229960003080 taurine Drugs 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5023—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures with a sample being transported to, and subsequently stored in an absorbent for analysis
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/508—Containers for the purpose of retaining a material to be analysed, e.g. test tubes rigid containers not provided for above
- B01L3/5085—Containers for the purpose of retaining a material to be analysed, e.g. test tubes rigid containers not provided for above for multiple samples, e.g. microtitration plates
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/52—Use of compounds or compositions for colorimetric, spectrophotometric or fluorometric investigation, e.g. use of reagent paper and including single- and multilayer analytical elements
- G01N33/525—Multi-layer analytical elements
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54393—Improving reaction conditions or stability, e.g. by coating or irradiation of surface, by reduction of non-specific binding, by promotion of specific binding
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6854—Immunoglobulins
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/80—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving blood groups or blood types or red blood cells
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/06—Auxiliary integrated devices, integrated components
- B01L2300/0627—Sensor or part of a sensor is integrated
- B01L2300/0663—Whole sensors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/06—Auxiliary integrated devices, integrated components
- B01L2300/069—Absorbents; Gels to retain a fluid
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0809—Geometry, shape and general structure rectangular shaped
- B01L2300/0829—Multi-well plates; Microtitration plates
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/16—Surface properties and coatings
- B01L2300/161—Control and use of surface tension forces, e.g. hydrophobic, hydrophilic
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/22—Haematology
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T29/00—Metal working
- Y10T29/49—Method of mechanical manufacture
- Y10T29/49826—Assembling or joining
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- General Health & Medical Sciences (AREA)
- Analytical Chemistry (AREA)
- Food Science & Technology (AREA)
- General Physics & Mathematics (AREA)
- Medicinal Chemistry (AREA)
- Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Pathology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Clinical Laboratory Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR1255452 | 2012-06-11 | ||
| FR1255452A FR2991689B1 (fr) | 2012-06-11 | 2012-06-11 | Dispositif de diagnostic immuno-hematologique et utilisations |
| PCT/FR2013/051358 WO2013186482A1 (fr) | 2012-06-11 | 2013-06-11 | Dispositif de diagnostic in vitro et utilisations |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2015100233A RU2015100233A (ru) | 2016-08-10 |
| RU2685416C2 true RU2685416C2 (ru) | 2019-04-18 |
Family
ID=48914324
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015100233A RU2685416C2 (ru) | 2012-06-11 | 2013-06-11 | Устройство для диагностики in vitro и его применения |
Country Status (14)
| Country | Link |
|---|---|
| US (2) | US10591494B2 (enExample) |
| EP (1) | EP2859344B1 (enExample) |
| JP (1) | JP6339562B2 (enExample) |
| KR (1) | KR102034915B1 (enExample) |
| CN (1) | CN104769429B (enExample) |
| BR (1) | BR112014030953B8 (enExample) |
| ES (1) | ES2681972T3 (enExample) |
| FR (1) | FR2991689B1 (enExample) |
| IL (1) | IL236062A0 (enExample) |
| IN (1) | IN2015DN00170A (enExample) |
| MA (1) | MA37744B1 (enExample) |
| PL (1) | PL2859344T3 (enExample) |
| RU (1) | RU2685416C2 (enExample) |
| WO (1) | WO2013186482A1 (enExample) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107666961B (zh) * | 2015-01-14 | 2021-08-10 | 伯乐实验室公司 | 血液分析系统和方法 |
| WO2018176262A1 (zh) * | 2017-03-27 | 2018-10-04 | 武汉优视科技有限公司 | 一种免疫印迹的自动检测系统 |
| CN106932596A (zh) * | 2017-03-27 | 2017-07-07 | 武汉优视科技有限公司 | 一种免疫印迹薄膜及其制备方法 |
| CN107045066A (zh) * | 2017-03-27 | 2017-08-15 | 武汉优视科技有限公司 | 一种免疫印迹卷轴 |
| EP3749452B1 (en) | 2018-02-05 | 2022-08-10 | Bio-Rad Laboratories, Inc. | Microfluidic probe head with barrier projections |
| EP3749451B1 (en) | 2018-02-05 | 2024-05-29 | Bio-Rad Laboratories, Inc. | Microfluidic probe head with aspiration posts |
| WO2019158726A1 (en) | 2018-02-16 | 2019-08-22 | Diagast | In vitro diagnosis device comprising beads and uses thereof |
| CN108333033B (zh) * | 2018-05-07 | 2024-09-24 | 皖南医学院第一附属医院(皖南医学院弋矶山医院) | 血型鉴定和交叉配血用防红细胞冷凝集热湿化装置 |
| CN110286238B (zh) * | 2019-07-25 | 2023-01-06 | 深圳市爱康试剂有限公司 | ABO/Rh(D/C/E)血型检测卡及其制备方法 |
| CN112345773A (zh) * | 2020-09-24 | 2021-02-09 | 北京健乃喜生物技术有限公司 | 体外即时测定人ABO和/或Rh血型的免疫测定装置 |
| EP4392786A4 (en) * | 2021-08-25 | 2024-12-18 | Dia Pro Tibbi Urunler Sanayi Ve Ticaret A.s. | Blood grouping test |
Citations (4)
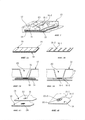
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0367468A1 (en) * | 1988-11-04 | 1990-05-09 | Immucor, Inc. | Method for drying mammalian cells for use in solid phase immunoassays and articles incorporating same |
| EP0408378A2 (en) * | 1989-07-12 | 1991-01-16 | W.L. GORE & ASSOCIATES (UK) LTD | Hydrophilic semi-permeable PTFE membranes and their manufacture |
| WO2009007649A2 (fr) * | 2007-07-02 | 2009-01-15 | Abo Diag | Dispositif et procede d'identification et de determination de groupes sanguins |
| RU2398235C2 (ru) * | 2005-08-31 | 2010-08-27 | Эгомедикаль Технологиз Аг | Тест-система с использованием элементов неэнзиматического распознавания анализируемого вещества |
Family Cites Families (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS61152700A (ja) | 1984-12-26 | 1986-07-11 | Susumu Kogyo Kk | タンパク質固定用膜担体およびその製造法 |
| US4797259A (en) | 1986-12-15 | 1989-01-10 | Pall Corporation | Well-type diagnostic plate device |
| JPH079087Y2 (ja) * | 1987-04-13 | 1995-03-06 | 大日本印刷株式会社 | 検査シ−ト |
| US4902481A (en) * | 1987-12-11 | 1990-02-20 | Millipore Corporation | Multi-well filtration test apparatus |
| DE68915194T2 (de) | 1988-03-23 | 1994-08-18 | Becton Dickinson Co | Vorrichtung zur Abgabe einer flüssigen Probe auf ein diagnostisches Gerät in reguliertem Ausmass. |
| IE903118A1 (en) * | 1989-09-21 | 1991-03-27 | Becton Dickinson Co | Test device including flow control means |
| US5185127A (en) * | 1989-09-21 | 1993-02-09 | Becton, Dickinson And Company | Test device including flow control means |
| JP3097768B2 (ja) * | 1991-04-19 | 2000-10-10 | 株式会社ニッショー | 診断用容器 |
| CN2169850Y (zh) * | 1993-06-17 | 1994-06-22 | 朱烨峰 | A、b、o血型系统诊断试剂卡 |
| JPH0954091A (ja) * | 1995-08-09 | 1997-02-25 | Nippon Zoki Pharmaceut Co Ltd | 抗赤血球抗体の測定法 |
| JPH09257794A (ja) * | 1996-03-26 | 1997-10-03 | Kikkoman Corp | 多重測定法 |
| US5968765A (en) * | 1996-11-08 | 1999-10-19 | Mercury Diagnostics, Inc. | Opaque reaction matrix for the analysis of the whole blood |
| AU4101697A (en) * | 1996-11-22 | 1998-05-28 | Robert A. Levine | Process for enhancing the aggregation and/or agglutination of erythrocytes prior to centrifugation |
| US6045899A (en) * | 1996-12-12 | 2000-04-04 | Usf Filtration & Separations Group, Inc. | Highly assymetric, hydrophilic, microfiltration membranes having large pore diameters |
| JP3808304B2 (ja) * | 2000-10-30 | 2006-08-09 | 三菱電機株式会社 | 自動変速機 |
| EP1845375A3 (en) * | 2000-12-22 | 2008-05-21 | Bio A.R.T. BVBA | Flow through assay device, diagnostic kit comprising said device and use in the detection of an analyte in a sample |
| US7605004B2 (en) * | 2001-07-18 | 2009-10-20 | Relia Diagnostic Systems Llc | Test strip for a lateral flow assay for a sample containing whole cells |
| WO2003016902A1 (en) | 2001-08-20 | 2003-02-27 | Proteome Systems Intellectual Property Pty Ltd | Diagnostic testing process and apparatus |
| AU2002331408B2 (en) * | 2001-08-20 | 2008-05-08 | Proteome Systems Ltd | Diagnostic testing process and apparatus |
| DE60219207T2 (de) * | 2001-12-12 | 2008-01-24 | Proteome Systems Intellectual Property Pty. Ltd., North Ryde | Diagnostisches testverfahren |
| US7781172B2 (en) | 2003-11-21 | 2010-08-24 | Kimberly-Clark Worldwide, Inc. | Method for extending the dynamic detection range of assay devices |
| US20080318342A1 (en) | 2004-06-04 | 2008-12-25 | Durack Matthew C A | Diagnostic Testing Process and Apparatus Incorporating Controlled Sample Flow |
| CA2588071A1 (en) * | 2004-11-15 | 2006-05-18 | Inverness Medical Switzerland Gmbh | Blood type method system and device |
| DE602005026588D1 (de) | 2005-08-31 | 2011-04-07 | Egomedical Technologies Ag | Analytentestsystem unter verwendung nichtenzymatischer analytenerkennungselemente |
| CN100425990C (zh) * | 2005-10-17 | 2008-10-15 | 中国人民解放军第三军医大学第一附属医院 | 血液检测微阵列制备方法 |
| FR2892820B1 (fr) * | 2005-11-03 | 2008-02-01 | Diagast Soc Par Actions Simpli | Procede magnetique d'immunodiagnostic pour la mise en evidence de complexe anticorps/antigene, en particulier de groupe sanguin |
| WO2007064462A1 (en) * | 2005-11-29 | 2007-06-07 | The Trustees Of The University Of Pennsylvania | Phage particle diagnostic reagents |
| EP2076775B1 (en) * | 2006-10-18 | 2015-08-19 | President and Fellows of Harvard College | Lateral flow and flow-through bioassay based on patterned porous media, methods of making same, and methods of using same |
| WO2008133880A1 (en) | 2007-04-23 | 2008-11-06 | Clavina Diagnostics, Inc. | Method of detecting red cell antigen-antibody reactions |
| FR2917174B1 (fr) * | 2007-06-08 | 2021-02-12 | Bio Rad Pasteur | Analyse multiple d'echantillons sanguins |
| DE202008017854U1 (de) * | 2007-08-14 | 2010-08-19 | Millipore Corp., Billerica | Mittel zur Ionenaustausch-Membranchromatographie auf der Basis von primären polymerischen Aminen, Sorptionsvorrichtung mit diesem Mittel, Chromatographieverfahren und Reinigungsverfahren unter dessen Verwendung |
| CN101603967B (zh) | 2008-06-10 | 2015-02-18 | 英科新创(厦门)科技有限公司 | 快速检测人ABO/Rh/MN血型的方法及试剂盒 |
| WO2010056889A2 (en) * | 2008-11-12 | 2010-05-20 | The Trustees Of The University Of Pennsylvania | Use of an antibody and a rare-earth based crystal |
| FR2963108B1 (fr) * | 2010-07-21 | 2017-06-23 | Diagast | Procede magnetique d'immunodiagnostic et kit pour la mise en evidence de complexe anticorps/antigene de groupe/phenotype sanguin |
-
2012
- 2012-06-11 FR FR1255452A patent/FR2991689B1/fr active Active
-
2013
- 2013-06-11 BR BR112014030953A patent/BR112014030953B8/pt not_active IP Right Cessation
- 2013-06-11 JP JP2015515577A patent/JP6339562B2/ja active Active
- 2013-06-11 KR KR1020157000619A patent/KR102034915B1/ko active Active
- 2013-06-11 CN CN201380042301.4A patent/CN104769429B/zh active Active
- 2013-06-11 RU RU2015100233A patent/RU2685416C2/ru active
- 2013-06-11 PL PL13744587T patent/PL2859344T3/pl unknown
- 2013-06-11 US US14/406,539 patent/US10591494B2/en active Active
- 2013-06-11 EP EP13744587.0A patent/EP2859344B1/fr active Active
- 2013-06-11 MA MA37744A patent/MA37744B1/fr unknown
- 2013-06-11 ES ES13744587.0T patent/ES2681972T3/es active Active
- 2013-06-11 WO PCT/FR2013/051358 patent/WO2013186482A1/fr not_active Ceased
- 2013-06-11 IN IN170DEN2015 patent/IN2015DN00170A/en unknown
-
2014
- 2014-12-04 IL IL236062A patent/IL236062A0/en active IP Right Grant
-
2019
- 2019-08-21 US US16/546,493 patent/US20190376986A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0367468A1 (en) * | 1988-11-04 | 1990-05-09 | Immucor, Inc. | Method for drying mammalian cells for use in solid phase immunoassays and articles incorporating same |
| EP0408378A2 (en) * | 1989-07-12 | 1991-01-16 | W.L. GORE & ASSOCIATES (UK) LTD | Hydrophilic semi-permeable PTFE membranes and their manufacture |
| RU2398235C2 (ru) * | 2005-08-31 | 2010-08-27 | Эгомедикаль Технологиз Аг | Тест-система с использованием элементов неэнзиматического распознавания анализируемого вещества |
| WO2009007649A2 (fr) * | 2007-07-02 | 2009-01-15 | Abo Diag | Dispositif et procede d'identification et de determination de groupes sanguins |
Also Published As
| Publication number | Publication date |
|---|---|
| FR2991689A1 (fr) | 2013-12-13 |
| IL236062A0 (en) | 2015-01-29 |
| US20150140579A1 (en) | 2015-05-21 |
| EP2859344A1 (fr) | 2015-04-15 |
| ES2681972T3 (es) | 2018-09-17 |
| MA37744A1 (fr) | 2016-05-31 |
| EP2859344B1 (fr) | 2018-05-02 |
| BR112014030953A2 (pt) | 2017-06-27 |
| WO2013186482A1 (fr) | 2013-12-19 |
| CN104769429A (zh) | 2015-07-08 |
| CN104769429B (zh) | 2018-02-16 |
| US20190376986A1 (en) | 2019-12-12 |
| PL2859344T3 (pl) | 2018-10-31 |
| KR20150040269A (ko) | 2015-04-14 |
| KR102034915B1 (ko) | 2019-10-21 |
| JP2015518969A (ja) | 2015-07-06 |
| MA37744B1 (fr) | 2017-10-31 |
| US10591494B2 (en) | 2020-03-17 |
| JP6339562B2 (ja) | 2018-06-06 |
| BR112014030953B1 (pt) | 2021-01-19 |
| FR2991689B1 (fr) | 2018-04-20 |
| BR112014030953B8 (pt) | 2021-02-17 |
| IN2015DN00170A (enExample) | 2015-06-12 |
| RU2015100233A (ru) | 2016-08-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2685416C2 (ru) | Устройство для диагностики in vitro и его применения | |
| JP5181058B2 (ja) | ヒトabo/rh/mn血液型を迅速に判定する方法及びキット | |
| US5096809A (en) | Whole blood assays using porous membrane support devices | |
| US7713708B2 (en) | Immunological assay system and method | |
| KR102651297B1 (ko) | 미생물 항원의 회수법 | |
| JPH11230963A (ja) | 血漿または血清試料の測定用装置 | |
| JP2012008136A (ja) | 制御流れアッセイ装置及び方法 | |
| US8546084B2 (en) | Device and method for identifying and determining blood groups | |
| KR102825182B1 (ko) | 비드를 포함하는 시험관내 진단 장치 및 그 용도 | |
| AU2010246531A1 (en) | Immunological assay system and method | |
| CA2266747A1 (en) | Diagnostic test devices with improved fluid movement and resistance to interferences | |
| JP6154126B2 (ja) | 検査キットおよび検査方法 | |
| CN213121338U (zh) | 一种全血检测装置 | |
| CN111829863A (zh) | 一种全血分离装置及其分离方法 | |
| RU2818259C2 (ru) | Устройство, содержащее шарики, для диагностики in vitro и его применения | |
| JPH06331625A (ja) | 便潜血判定装置 | |
| KR910001313B1 (ko) | 면역 분석 방법 및 장치 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| HZ9A | Changing address for correspondence with an applicant |