RU2542500C2 - Фармацевтические композиции, содержащие глюкагон-подобный пептид 1 (glp-1) - Google Patents
Фармацевтические композиции, содержащие глюкагон-подобный пептид 1 (glp-1) Download PDFInfo
- Publication number
- RU2542500C2 RU2542500C2 RU2010137392/15A RU2010137392A RU2542500C2 RU 2542500 C2 RU2542500 C2 RU 2542500C2 RU 2010137392/15 A RU2010137392/15 A RU 2010137392/15A RU 2010137392 A RU2010137392 A RU 2010137392A RU 2542500 C2 RU2542500 C2 RU 2542500C2
- Authority
- RU
- Russia
- Prior art keywords
- glp
- fdkp
- diketopiperazine
- dose
- peptide
- Prior art date
Links
- GCYXWQUSHADNBF-AAEALURTSA-N preproglucagon 78-108 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 GCYXWQUSHADNBF-AAEALURTSA-N 0.000 title description 8
- 239000008194 pharmaceutical composition Substances 0.000 title description 2
- BXRNXXXXHLBUKK-UHFFFAOYSA-N piperazine-2,5-dione Chemical compound O=C1CNC(=O)CN1 BXRNXXXXHLBUKK-UHFFFAOYSA-N 0.000 claims abstract description 131
- 230000000694 effects Effects 0.000 claims abstract description 86
- 238000000034 method Methods 0.000 claims abstract description 74
- 239000000203 mixture Substances 0.000 claims abstract description 46
- 150000003839 salts Chemical class 0.000 claims abstract description 42
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 19
- 206010012601 diabetes mellitus Diseases 0.000 claims abstract description 18
- 201000010099 disease Diseases 0.000 claims abstract description 18
- 230000002685 pulmonary effect Effects 0.000 claims abstract description 18
- 239000011859 microparticle Substances 0.000 claims abstract description 17
- 208000008589 Obesity Diseases 0.000 claims abstract description 14
- 235000020824 obesity Nutrition 0.000 claims abstract description 14
- DTHNMHAUYICORS-KTKZVXAJSA-N Glucagon-like peptide 1 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 DTHNMHAUYICORS-KTKZVXAJSA-N 0.000 claims description 866
- 239000000843 powder Substances 0.000 claims description 52
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 52
- 230000001965 increasing effect Effects 0.000 claims description 32
- -1 glutaryl Chemical group 0.000 claims description 17
- 239000002904 solvent Substances 0.000 claims description 16
- 230000001851 biosynthetic effect Effects 0.000 claims description 15
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 claims description 14
- 238000004519 manufacturing process Methods 0.000 claims description 13
- 239000011734 sodium Substances 0.000 claims description 10
- 101000930822 Giardia intestinalis Dipeptidyl-peptidase 4 Proteins 0.000 claims description 9
- 210000004153 islets of langerhan Anatomy 0.000 claims description 8
- 230000009471 action Effects 0.000 claims description 7
- 238000004108 freeze drying Methods 0.000 claims description 7
- 201000001421 hyperglycemia Diseases 0.000 claims description 5
- 238000002513 implantation Methods 0.000 claims description 5
- 229910052708 sodium Inorganic materials 0.000 claims description 5
- 238000001694 spray drying Methods 0.000 claims description 5
- 208000004476 Acute Coronary Syndrome Diseases 0.000 claims description 4
- 208000032781 Diabetic cardiomyopathy Diseases 0.000 claims description 4
- 208000032928 Dyslipidaemia Diseases 0.000 claims description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 4
- 208000017170 Lipid metabolism disease Diseases 0.000 claims description 4
- 208000006011 Stroke Diseases 0.000 claims description 4
- 230000001925 catabolic effect Effects 0.000 claims description 4
- 208000002551 irritable bowel syndrome Diseases 0.000 claims description 4
- 208000028867 ischemia Diseases 0.000 claims description 4
- 208000010125 myocardial infarction Diseases 0.000 claims description 4
- 208000015122 neurodegenerative disease Diseases 0.000 claims description 4
- 230000001172 regenerating effect Effects 0.000 claims description 4
- 238000001356 surgical procedure Methods 0.000 claims description 4
- 208000028698 Cognitive impairment Diseases 0.000 claims description 3
- 208000010877 cognitive disease Diseases 0.000 claims description 3
- 206010027175 memory impairment Diseases 0.000 claims description 3
- 125000002730 succinyl group Chemical group C(CCC(=O)*)(=O)* 0.000 claims description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 claims description 2
- 102000003779 Dipeptidyl-peptidases and tripeptidyl-peptidases Human genes 0.000 claims description 2
- 108090000194 Dipeptidyl-peptidases and tripeptidyl-peptidases Proteins 0.000 claims description 2
- 206010063837 Reperfusion injury Diseases 0.000 claims description 2
- 229910052791 calcium Inorganic materials 0.000 claims description 2
- 229910052744 lithium Inorganic materials 0.000 claims description 2
- 229910052749 magnesium Inorganic materials 0.000 claims description 2
- 229910052700 potassium Inorganic materials 0.000 claims description 2
- 125000005208 trialkylammonium group Chemical group 0.000 claims description 2
- 101710198884 GATA-type zinc finger protein 1 Proteins 0.000 claims 36
- 102100025101 GATA-type zinc finger protein 1 Human genes 0.000 claims 36
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims 1
- 102000016622 Dipeptidyl Peptidase 4 Human genes 0.000 claims 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 claims 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 claims 1
- KZVODIUXIJWGJF-UHFFFAOYSA-N N1CCNCC1.[Na].[Na] Chemical compound N1CCNCC1.[Na].[Na] KZVODIUXIJWGJF-UHFFFAOYSA-N 0.000 claims 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims 1
- 239000011575 calcium Substances 0.000 claims 1
- 108010063245 glucagon-like peptide 1 (7-36)amide Proteins 0.000 claims 1
- 239000011777 magnesium Substances 0.000 claims 1
- 239000011591 potassium Substances 0.000 claims 1
- 239000003814 drug Substances 0.000 abstract description 34
- 239000000126 substance Substances 0.000 abstract description 19
- 238000010521 absorption reaction Methods 0.000 abstract description 6
- 206010028980 Neoplasm Diseases 0.000 abstract description 5
- 201000011510 cancer Diseases 0.000 abstract description 4
- 230000007774 longterm Effects 0.000 abstract description 3
- 239000000825 pharmaceutical preparation Substances 0.000 abstract description 3
- 108010088406 Glucagon-Like Peptides Proteins 0.000 abstract 2
- 101800000224 Glucagon-like peptide 1 Proteins 0.000 description 841
- 102100040918 Pro-glucagon Human genes 0.000 description 819
- BBNKIRVUCQNAQR-FETIZUMASA-N (e)-4-[4-[(2s,5s)-5-[4-[[(e)-3-carboxyprop-2-enoyl]amino]butyl]-3,6-dioxopiperazin-2-yl]butylamino]-4-oxobut-2-enoic acid Chemical compound OC(=O)\C=C\C(=O)NCCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)\C=C\C(O)=O)NC1=O BBNKIRVUCQNAQR-FETIZUMASA-N 0.000 description 338
- 229940094417 fumaryl diketopiperazine Drugs 0.000 description 332
- 239000002245 particle Substances 0.000 description 128
- 238000002360 preparation method Methods 0.000 description 90
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 76
- 241001465754 Metazoa Species 0.000 description 73
- 239000000243 solution Substances 0.000 description 70
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 50
- 241000700159 Rattus Species 0.000 description 48
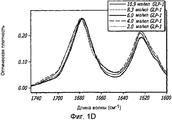
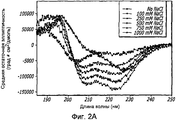
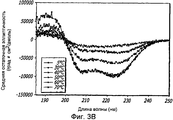
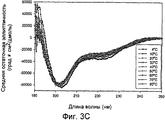
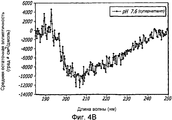
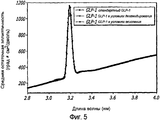
- 238000002983 circular dichroism Methods 0.000 description 42
- 210000004369 blood Anatomy 0.000 description 38
- 239000008280 blood Substances 0.000 description 38
- 239000011780 sodium chloride Substances 0.000 description 38
- 210000004072 lung Anatomy 0.000 description 33
- 210000004027 cell Anatomy 0.000 description 31
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 30
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 30
- 239000008103 glucose Substances 0.000 description 30
- 241000282693 Cercopithecidae Species 0.000 description 29
- 238000001179 sorption measurement Methods 0.000 description 29
- 239000000725 suspension Substances 0.000 description 29
- 229940079593 drug Drugs 0.000 description 26
- 238000002474 experimental method Methods 0.000 description 26
- 102000004877 Insulin Human genes 0.000 description 25
- 108090001061 Insulin Proteins 0.000 description 25
- 150000001875 compounds Chemical class 0.000 description 25
- 229940125396 insulin Drugs 0.000 description 25
- 238000004458 analytical method Methods 0.000 description 24
- 210000000227 basophil cell of anterior lobe of hypophysis Anatomy 0.000 description 24
- 238000011068 loading method Methods 0.000 description 24
- 239000003795 chemical substances by application Substances 0.000 description 23
- 108010011459 Exenatide Proteins 0.000 description 22
- 150000002500 ions Chemical class 0.000 description 22
- 230000015572 biosynthetic process Effects 0.000 description 21
- HKSZLNNOFSGOKW-UHFFFAOYSA-N ent-staurosporine Natural products C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1C1CC(NC)C(OC)C4(C)O1 HKSZLNNOFSGOKW-UHFFFAOYSA-N 0.000 description 20
- HKSZLNNOFSGOKW-FYTWVXJKSA-N staurosporine Chemical compound C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1[C@H]1C[C@@H](NC)[C@@H](OC)[C@]4(C)O1 HKSZLNNOFSGOKW-FYTWVXJKSA-N 0.000 description 20
- 235000001014 amino acid Nutrition 0.000 description 19
- 230000037396 body weight Effects 0.000 description 19
- 230000007423 decrease Effects 0.000 description 19
- 235000012631 food intake Nutrition 0.000 description 19
- 239000004094 surface-active agent Substances 0.000 description 19
- 150000001413 amino acids Chemical class 0.000 description 18
- JUFFVKRROAPVBI-PVOYSMBESA-N chembl1210015 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(=O)N[C@H]1[C@@H]([C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO[C@]3(O[C@@H](C[C@H](O)[C@H](O)CO)[C@H](NC(C)=O)[C@@H](O)C3)C(O)=O)O2)O)[C@@H](CO)O1)NC(C)=O)C(=O)NCC(=O)NCC(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 JUFFVKRROAPVBI-PVOYSMBESA-N 0.000 description 18
- 229960001519 exenatide Drugs 0.000 description 18
- 230000037406 food intake Effects 0.000 description 18
- 229940024606 amino acid Drugs 0.000 description 17
- 230000002354 daily effect Effects 0.000 description 17
- 230000000065 osmolyte Effects 0.000 description 17
- 210000002966 serum Anatomy 0.000 description 17
- 230000027455 binding Effects 0.000 description 16
- 230000006907 apoptotic process Effects 0.000 description 15
- 238000004128 high performance liquid chromatography Methods 0.000 description 15
- 238000012360 testing method Methods 0.000 description 15
- 230000003285 pharmacodynamic effect Effects 0.000 description 14
- 238000001556 precipitation Methods 0.000 description 13
- 102000004196 processed proteins & peptides Human genes 0.000 description 13
- 108090000623 proteins and genes Proteins 0.000 description 13
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 12
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 12
- 238000000354 decomposition reaction Methods 0.000 description 12
- 230000003247 decreasing effect Effects 0.000 description 12
- 230000006870 function Effects 0.000 description 12
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 12
- 238000001727 in vivo Methods 0.000 description 12
- 230000036470 plasma concentration Effects 0.000 description 12
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 11
- 108010086246 Glucagon-Like Peptide-1 Receptor Proteins 0.000 description 11
- 102100032882 Glucagon-like peptide 1 receptor Human genes 0.000 description 11
- 230000008859 change Effects 0.000 description 11
- 238000001990 intravenous administration Methods 0.000 description 11
- 102000004169 proteins and genes Human genes 0.000 description 11
- 231100000607 toxicokinetics Toxicity 0.000 description 11
- 230000005764 inhibitory process Effects 0.000 description 10
- 230000003993 interaction Effects 0.000 description 10
- 235000018102 proteins Nutrition 0.000 description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- BYEAHWXPCBROCE-UHFFFAOYSA-N 1,1,1,3,3,3-hexafluoropropan-2-ol Chemical compound FC(F)(F)C(O)C(F)(F)F BYEAHWXPCBROCE-UHFFFAOYSA-N 0.000 description 9
- 102100025012 Dipeptidyl peptidase 4 Human genes 0.000 description 9
- 239000013543 active substance Substances 0.000 description 9
- 239000000872 buffer Substances 0.000 description 9
- 230000030833 cell death Effects 0.000 description 9
- 238000004949 mass spectrometry Methods 0.000 description 9
- SVTBMSDMJJWYQN-UHFFFAOYSA-N 2-methylpentane-2,4-diol Chemical compound CC(O)CC(C)(C)O SVTBMSDMJJWYQN-UHFFFAOYSA-N 0.000 description 8
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 8
- 239000002253 acid Substances 0.000 description 8
- 150000001298 alcohols Chemical class 0.000 description 8
- 238000003556 assay Methods 0.000 description 8
- 230000006240 deamidation Effects 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 230000018109 developmental process Effects 0.000 description 8
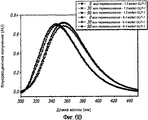
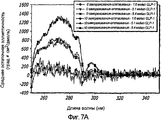
- 230000005284 excitation Effects 0.000 description 8
- 239000012530 fluid Substances 0.000 description 8
- 238000001802 infusion Methods 0.000 description 8
- 230000003914 insulin secretion Effects 0.000 description 8
- 238000002156 mixing Methods 0.000 description 8
- 230000003647 oxidation Effects 0.000 description 8
- 238000007254 oxidation reaction Methods 0.000 description 8
- SCVFZCLFOSHCOH-UHFFFAOYSA-M potassium acetate Chemical compound [K+].CC([O-])=O SCVFZCLFOSHCOH-UHFFFAOYSA-M 0.000 description 8
- 230000005855 radiation Effects 0.000 description 8
- 239000003381 stabilizer Substances 0.000 description 8
- 238000007920 subcutaneous administration Methods 0.000 description 8
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 8
- 101100337060 Caenorhabditis elegans glp-1 gene Proteins 0.000 description 7
- RHQDFWAXVIIEBN-UHFFFAOYSA-N Trifluoroethanol Chemical compound OCC(F)(F)F RHQDFWAXVIIEBN-UHFFFAOYSA-N 0.000 description 7
- 239000000443 aerosol Substances 0.000 description 7
- 238000010253 intravenous injection Methods 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 210000000056 organ Anatomy 0.000 description 7
- 238000001338 self-assembly Methods 0.000 description 7
- 230000035882 stress Effects 0.000 description 7
- 230000001225 therapeutic effect Effects 0.000 description 7
- 108010039627 Aprotinin Proteins 0.000 description 6
- 229960004405 aprotinin Drugs 0.000 description 6
- 230000004071 biological effect Effects 0.000 description 6
- 230000015556 catabolic process Effects 0.000 description 6
- 230000003833 cell viability Effects 0.000 description 6
- 238000006731 degradation reaction Methods 0.000 description 6
- 230000001419 dependent effect Effects 0.000 description 6
- 230000008021 deposition Effects 0.000 description 6
- 238000010790 dilution Methods 0.000 description 6
- 239000012895 dilution Substances 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 229910052739 hydrogen Inorganic materials 0.000 description 6
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 6
- 210000000496 pancreas Anatomy 0.000 description 6
- 230000007170 pathology Effects 0.000 description 6
- 239000000816 peptidomimetic Substances 0.000 description 6
- 239000000546 pharmaceutical excipient Substances 0.000 description 6
- 230000035755 proliferation Effects 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- FVAUCKIRQBBSSJ-UHFFFAOYSA-M sodium iodide Chemical compound [Na+].[I-] FVAUCKIRQBBSSJ-UHFFFAOYSA-M 0.000 description 6
- CGPUWJWCVCFERF-UHFFFAOYSA-N staurosporine Natural products C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1C1CC(NC)C(OC)C4(OC)O1 CGPUWJWCVCFERF-UHFFFAOYSA-N 0.000 description 6
- 238000012916 structural analysis Methods 0.000 description 6
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 5
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 5
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 5
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 5
- NYHBQMYGNKIUIF-UUOKFMHZSA-N Guanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O NYHBQMYGNKIUIF-UUOKFMHZSA-N 0.000 description 5
- 101000788682 Homo sapiens GATA-type zinc finger protein 1 Proteins 0.000 description 5
- 102000003855 L-lactate dehydrogenase Human genes 0.000 description 5
- 108700023483 L-lactate dehydrogenases Proteins 0.000 description 5
- 229910019142 PO4 Inorganic materials 0.000 description 5
- 210000004556 brain Anatomy 0.000 description 5
- 238000001142 circular dichroism spectrum Methods 0.000 description 5
- 238000003776 cleavage reaction Methods 0.000 description 5
- 239000003112 inhibitor Substances 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 230000000144 pharmacologic effect Effects 0.000 description 5
- 239000010452 phosphate Substances 0.000 description 5
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 5
- 229920001184 polypeptide Polymers 0.000 description 5
- 230000002441 reversible effect Effects 0.000 description 5
- 230000007017 scission Effects 0.000 description 5
- 238000010254 subcutaneous injection Methods 0.000 description 5
- 239000007929 subcutaneous injection Substances 0.000 description 5
- 238000013293 zucker diabetic fatty rat Methods 0.000 description 5
- WSEVKKHALHSUMB-RYVRVIGHSA-N (4S)-4-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2R)-5-amino-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-carboxypropanoyl]amino]-4-methylpentanoyl]amino]-3-hydroxypropanoyl]amino]hexanoyl]amino]-5-oxopentanoyl]amino]-4-methylsulfanylbutanoyl]amino]-4-carboxybutanoyl]amino]-4-carboxybutanoyl]amino]-5-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-4-amino-1-[[2-[[2-[(2S)-2-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[(2S)-2-[(2S)-2-[(2S)-2-[[(2S)-1-amino-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidine-1-carbonyl]pyrrolidine-1-carbonyl]pyrrolidin-1-yl]-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-2-oxoethyl]amino]-2-oxoethyl]amino]-1,4-dioxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-5-oxopentanoic acid Chemical compound CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(N)=O WSEVKKHALHSUMB-RYVRVIGHSA-N 0.000 description 4
- 108090000672 Annexin A5 Proteins 0.000 description 4
- 102000004121 Annexin A5 Human genes 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 102000051325 Glucagon Human genes 0.000 description 4
- 108060003199 Glucagon Proteins 0.000 description 4
- 229940089838 Glucagon-like peptide 1 receptor agonist Drugs 0.000 description 4
- 239000004471 Glycine Substances 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- MKUXAQIIEYXACX-UHFFFAOYSA-N aciclovir Chemical compound N1C(N)=NC(=O)C2=C1N(COCCO)C=N2 MKUXAQIIEYXACX-UHFFFAOYSA-N 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- 239000013060 biological fluid Substances 0.000 description 4
- 238000010241 blood sampling Methods 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 230000004069 differentiation Effects 0.000 description 4
- 238000002189 fluorescence spectrum Methods 0.000 description 4
- 239000012634 fragment Substances 0.000 description 4
- MASNOZXLGMXCHN-ZLPAWPGGSA-N glucagon Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)C(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=CC=C1 MASNOZXLGMXCHN-ZLPAWPGGSA-N 0.000 description 4
- 229960004666 glucagon Drugs 0.000 description 4
- 238000007446 glucose tolerance test Methods 0.000 description 4
- 238000004442 gravimetric analysis Methods 0.000 description 4
- 229940051250 hexylene glycol Drugs 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 238000000338 in vitro Methods 0.000 description 4
- 210000004185 liver Anatomy 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 230000003278 mimic effect Effects 0.000 description 4
- 239000003960 organic solvent Substances 0.000 description 4
- 235000011056 potassium acetate Nutrition 0.000 description 4
- 229910000160 potassium phosphate Inorganic materials 0.000 description 4
- 235000011009 potassium phosphates Nutrition 0.000 description 4
- 239000002244 precipitate Substances 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 238000005185 salting out Methods 0.000 description 4
- 238000001228 spectrum Methods 0.000 description 4
- 238000013223 sprague-dawley female rat Methods 0.000 description 4
- 230000004936 stimulating effect Effects 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- 208000011580 syndromic disease Diseases 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- 230000001988 toxicity Effects 0.000 description 4
- 210000002237 B-cell of pancreatic islet Anatomy 0.000 description 3
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 208000013016 Hypoglycemia Diseases 0.000 description 3
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 3
- 229930195725 Mannitol Natural products 0.000 description 3
- 206010028813 Nausea Diseases 0.000 description 3
- 241000283973 Oryctolagus cuniculus Species 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 3
- 102000012479 Serine Proteases Human genes 0.000 description 3
- 108010022999 Serine Proteases Proteins 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 206010047700 Vomiting Diseases 0.000 description 3
- 229960004150 aciclovir Drugs 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 239000000556 agonist Substances 0.000 description 3
- 150000001408 amides Chemical class 0.000 description 3
- 239000005557 antagonist Substances 0.000 description 3
- 230000000840 anti-viral effect Effects 0.000 description 3
- 230000006399 behavior Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 150000001576 beta-amino acids Chemical class 0.000 description 3
- 238000005460 biophysical method Methods 0.000 description 3
- AIYUHDOJVYHVIT-UHFFFAOYSA-M caesium chloride Chemical compound [Cl-].[Cs+] AIYUHDOJVYHVIT-UHFFFAOYSA-M 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 238000012054 celltiter-glo Methods 0.000 description 3
- 210000003169 central nervous system Anatomy 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 238000012937 correction Methods 0.000 description 3
- 230000034994 death Effects 0.000 description 3
- 231100000517 death Toxicity 0.000 description 3
- 229940124447 delivery agent Drugs 0.000 description 3
- 231100000673 dose–response relationship Toxicity 0.000 description 3
- 238000012377 drug delivery Methods 0.000 description 3
- 230000002124 endocrine Effects 0.000 description 3
- 230000002349 favourable effect Effects 0.000 description 3
- 230000035558 fertility Effects 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- 235000013305 food Nutrition 0.000 description 3
- 230000030136 gastric emptying Effects 0.000 description 3
- 210000001035 gastrointestinal tract Anatomy 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- 230000002218 hypoglycaemic effect Effects 0.000 description 3
- MGXWVYUBJRZYPE-YUGYIWNOSA-N incretin Chemical class C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1C=CC(O)=CC=1)[C@@H](C)O)[C@@H](C)CC)C1=CC=C(O)C=C1 MGXWVYUBJRZYPE-YUGYIWNOSA-N 0.000 description 3
- 239000000859 incretin Substances 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 230000002473 insulinotropic effect Effects 0.000 description 3
- 230000014759 maintenance of location Effects 0.000 description 3
- 239000000594 mannitol Substances 0.000 description 3
- 235000010355 mannitol Nutrition 0.000 description 3
- 210000004379 membrane Anatomy 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 238000007431 microscopic evaluation Methods 0.000 description 3
- 239000012452 mother liquor Substances 0.000 description 3
- 230000008693 nausea Effects 0.000 description 3
- 230000007935 neutral effect Effects 0.000 description 3
- 230000036542 oxidative stress Effects 0.000 description 3
- 239000008177 pharmaceutical agent Substances 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 231100000191 repeated dose toxicity Toxicity 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 230000000241 respiratory effect Effects 0.000 description 3
- 210000002345 respiratory system Anatomy 0.000 description 3
- 230000000717 retained effect Effects 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 231100000161 signs of toxicity Toxicity 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 238000004611 spectroscopical analysis Methods 0.000 description 3
- 238000010186 staining Methods 0.000 description 3
- 125000001424 substituent group Chemical group 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 230000004083 survival effect Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 231100001072 toxicokinetic profile Toxicity 0.000 description 3
- UYPYRKYUKCHHIB-UHFFFAOYSA-N trimethylamine N-oxide Chemical compound C[N+](C)(C)[O-] UYPYRKYUKCHHIB-UHFFFAOYSA-N 0.000 description 3
- 210000002700 urine Anatomy 0.000 description 3
- 230000008673 vomiting Effects 0.000 description 3
- 238000005303 weighing Methods 0.000 description 3
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 2
- NGJOFQZEYQGZMB-KTKZVXAJSA-N (4S)-5-[[2-[[(2S,3R)-1-[[(2S)-1-[[(2S,3R)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[2-[[(1S)-4-carbamimidamido-1-carboxybutyl]amino]-2-oxoethyl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-2-oxoethyl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-4-[[(2S)-2-[[(2S)-2-amino-3-(1H-imidazol-4-yl)propanoyl]amino]propanoyl]amino]-5-oxopentanoic acid Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 NGJOFQZEYQGZMB-KTKZVXAJSA-N 0.000 description 2
- 125000000349 (Z)-3-carboxyprop-2-enoyl group Chemical group O=C([*])/C([H])=C([H])\C(O[H])=O 0.000 description 2
- JKXYOQDLERSFPT-UHFFFAOYSA-N 2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-octadecoxyethoxy)ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethoxy]ethanol Chemical compound CCCCCCCCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO JKXYOQDLERSFPT-UHFFFAOYSA-N 0.000 description 2
- NUYHTXOPSQBZKC-UHFFFAOYSA-N 3,6-bis(4-aminobutyl)piperazine-2,5-dione Chemical compound NCCCCC1NC(=O)C(CCCCN)NC1=O NUYHTXOPSQBZKC-UHFFFAOYSA-N 0.000 description 2
- UMCMPZBLKLEWAF-BCTGSCMUSA-N 3-[(3-cholamidopropyl)dimethylammonio]propane-1-sulfonate Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCC[N+](C)(C)CCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 UMCMPZBLKLEWAF-BCTGSCMUSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- LZZYPRNAOMGNLH-UHFFFAOYSA-M Cetrimonium bromide Chemical compound [Br-].CCCCCCCCCCCCCCCC[N+](C)(C)C LZZYPRNAOMGNLH-UHFFFAOYSA-M 0.000 description 2
- MIKUYHXYGGJMLM-GIMIYPNGSA-N Crotonoside Natural products C1=NC2=C(N)NC(=O)N=C2N1[C@H]1O[C@@H](CO)[C@H](O)[C@@H]1O MIKUYHXYGGJMLM-GIMIYPNGSA-N 0.000 description 2
- NYHBQMYGNKIUIF-UHFFFAOYSA-N D-guanosine Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1OC(CO)C(O)C1O NYHBQMYGNKIUIF-UHFFFAOYSA-N 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- HTQBXNHDCUEHJF-XWLPCZSASA-N Exenatide Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NCC(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 HTQBXNHDCUEHJF-XWLPCZSASA-N 0.000 description 2
- KRHYYFGTRYWZRS-UHFFFAOYSA-M Fluoride anion Chemical compound [F-] KRHYYFGTRYWZRS-UHFFFAOYSA-M 0.000 description 2
- 101800004295 Glucagon-like peptide 1(7-36) Proteins 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 2
- 208000026139 Memory disease Diseases 0.000 description 2
- 108010058003 Proglucagon Proteins 0.000 description 2
- 108010029485 Protein Isoforms Proteins 0.000 description 2
- 102000001708 Protein Isoforms Human genes 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 229960000583 acetic acid Drugs 0.000 description 2
- 238000007792 addition Methods 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 2
- 150000001450 anions Chemical class 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- 230000005540 biological transmission Effects 0.000 description 2
- HQABUPZFAYXKJW-UHFFFAOYSA-N butan-1-amine Chemical compound CCCCN HQABUPZFAYXKJW-UHFFFAOYSA-N 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 210000000748 cardiovascular system Anatomy 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 229960002798 cetrimide Drugs 0.000 description 2
- 230000018044 dehydration Effects 0.000 description 2
- 238000006297 dehydration reaction Methods 0.000 description 2
- 239000013583 drug formulation Substances 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- 210000002919 epithelial cell Anatomy 0.000 description 2
- 108010015174 exendin 3 Proteins 0.000 description 2
- LMHMJYMCGJNXRS-IOPUOMRJSA-N exendin-3 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)NCC(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@H](C)O)[C@H](C)O)C(C)C)C1=CC=CC=C1 LMHMJYMCGJNXRS-IOPUOMRJSA-N 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 238000009615 fourier-transform spectroscopy Methods 0.000 description 2
- 238000007710 freezing Methods 0.000 description 2
- 230000008014 freezing Effects 0.000 description 2
- 108020001507 fusion proteins Proteins 0.000 description 2
- 102000037865 fusion proteins Human genes 0.000 description 2
- IRSCQMHQWWYFCW-UHFFFAOYSA-N ganciclovir Chemical compound O=C1NC(N)=NC2=C1N=CN2COC(CO)CO IRSCQMHQWWYFCW-UHFFFAOYSA-N 0.000 description 2
- 229960002963 ganciclovir Drugs 0.000 description 2
- 231100000025 genetic toxicology Toxicity 0.000 description 2
- 229960001031 glucose Drugs 0.000 description 2
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 2
- 229940029575 guanosine Drugs 0.000 description 2
- 230000002489 hematologic effect Effects 0.000 description 2
- 238000002991 immunohistochemical analysis Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000009533 lab test Methods 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 2
- 230000002535 lyotropic effect Effects 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000004060 metabolic process Effects 0.000 description 2
- 229930182817 methionine Natural products 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 238000012544 monitoring process Methods 0.000 description 2
- 231100000243 mutagenic effect Toxicity 0.000 description 2
- 230000006654 negative regulation of apoptotic process Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 231100000062 no-observed-adverse-effect level Toxicity 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 210000004923 pancreatic tissue Anatomy 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 229940124531 pharmaceutical excipient Drugs 0.000 description 2
- 150000003904 phospholipids Chemical class 0.000 description 2
- 231100000683 possible toxicity Toxicity 0.000 description 2
- 230000007542 postnatal development Effects 0.000 description 2
- 230000000291 postprandial effect Effects 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 229930010796 primary metabolite Natural products 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 229940002612 prodrug Drugs 0.000 description 2
- 239000000651 prodrug Chemical class 0.000 description 2
- 230000010410 reperfusion Effects 0.000 description 2
- 230000036186 satiety Effects 0.000 description 2
- 235000019627 satiety Nutrition 0.000 description 2
- 238000004626 scanning electron microscopy Methods 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 229930000044 secondary metabolite Natural products 0.000 description 2
- MFFMDFFZMYYVKS-SECBINFHSA-N sitagliptin Chemical compound C([C@H](CC(=O)N1CC=2N(C(=NN=2)C(F)(F)F)CC1)N)C1=CC(F)=C(F)C=C1F MFFMDFFZMYYVKS-SECBINFHSA-N 0.000 description 2
- 210000000813 small intestine Anatomy 0.000 description 2
- SUKJFIGYRHOWBL-UHFFFAOYSA-N sodium hypochlorite Chemical compound [Na+].Cl[O-] SUKJFIGYRHOWBL-UHFFFAOYSA-N 0.000 description 2
- 235000009518 sodium iodide Nutrition 0.000 description 2
- VGTPCRGMBIAPIM-UHFFFAOYSA-M sodium thiocyanate Chemical compound [Na+].[S-]C#N VGTPCRGMBIAPIM-UHFFFAOYSA-M 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- 210000002784 stomach Anatomy 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000010257 thawing Methods 0.000 description 2
- 231100000827 tissue damage Toxicity 0.000 description 2
- 230000000451 tissue damage Effects 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- SYOKIDBDQMKNDQ-XWTIBIIYSA-N vildagliptin Chemical compound C1C(O)(C2)CC(C3)CC1CC32NCC(=O)N1CCC[C@H]1C#N SYOKIDBDQMKNDQ-XWTIBIIYSA-N 0.000 description 2
- 229960001254 vildagliptin Drugs 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- FDKWRPBBCBCIGA-REOHCLBHSA-N (2r)-2-azaniumyl-3-$l^{1}-selanylpropanoate Chemical compound [Se]C[C@H](N)C(O)=O FDKWRPBBCBCIGA-REOHCLBHSA-N 0.000 description 1
- LBIRTXMKYFJJHM-SNAWJCMRSA-N (e)-4-[4-(3,6-dioxopiperazin-2-yl)butylamino]-4-oxobut-2-enoic acid Chemical compound OC(=O)\C=C\C(=O)NCCCCC1NC(=O)CNC1=O LBIRTXMKYFJJHM-SNAWJCMRSA-N 0.000 description 1
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 1
- VOUAQYXWVJDEQY-QENPJCQMSA-N 33017-11-7 Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)NCC(=O)NCC(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N1[C@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O)CCC1 VOUAQYXWVJDEQY-QENPJCQMSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- HSFWRNGVRCDJHI-UHFFFAOYSA-N Acetylene Chemical compound C#C HSFWRNGVRCDJHI-UHFFFAOYSA-N 0.000 description 1
- 238000010953 Ames test Methods 0.000 description 1
- 231100000039 Ames test Toxicity 0.000 description 1
- ATRRKUHOCOJYRX-UHFFFAOYSA-N Ammonium bicarbonate Chemical compound [NH4+].OC([O-])=O ATRRKUHOCOJYRX-UHFFFAOYSA-N 0.000 description 1
- 229910000013 Ammonium bicarbonate Inorganic materials 0.000 description 1
- 239000004382 Amylase Substances 0.000 description 1
- 102000013142 Amylases Human genes 0.000 description 1
- 108010065511 Amylases Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108010075254 C-Peptide Proteins 0.000 description 1
- 102000004007 Calpain-10 Human genes 0.000 description 1
- 108090000451 Calpain-10 Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 208000031404 Chromosome Aberrations Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 206010011224 Cough Diseases 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- FDKWRPBBCBCIGA-UWTATZPHSA-N D-Selenocysteine Natural products [Se]C[C@@H](N)C(O)=O FDKWRPBBCBCIGA-UWTATZPHSA-N 0.000 description 1
- 230000006820 DNA synthesis Effects 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- 241001649081 Dina Species 0.000 description 1
- 108010016626 Dipeptides Proteins 0.000 description 1
- 229940124213 Dipeptidyl peptidase 4 (DPP IV) inhibitor Drugs 0.000 description 1
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical class [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 1
- 102400000921 Gastrin Human genes 0.000 description 1
- 108010052343 Gastrins Proteins 0.000 description 1
- 241000699694 Gerbillinae Species 0.000 description 1
- 101800004266 Glucagon-like peptide 1(7-37) Proteins 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 229920002971 Heparan sulfate Polymers 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 101001033280 Homo sapiens Cytokine receptor common subunit beta Proteins 0.000 description 1
- 101500028774 Homo sapiens Glucagon-like peptide 1 Proteins 0.000 description 1
- 101001047090 Homo sapiens Potassium voltage-gated channel subfamily H member 2 Proteins 0.000 description 1
- 101000684208 Homo sapiens Prolyl endopeptidase FAP Proteins 0.000 description 1
- NNIVFXCKHNRSRL-VKHMYHEASA-N Hydroxyglutamic acid Chemical compound ON[C@H](C(O)=O)CCC(O)=O NNIVFXCKHNRSRL-VKHMYHEASA-N 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 241000282596 Hylobatidae Species 0.000 description 1
- 102000036770 Islet Amyloid Polypeptide Human genes 0.000 description 1
- 108010041872 Islet Amyloid Polypeptide Proteins 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- RHGKLRLOHDJJDR-BYPYZUCNSA-N L-citrulline Chemical compound NC(=O)NCCC[C@H]([NH3+])C([O-])=O RHGKLRLOHDJJDR-BYPYZUCNSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- QEFRNWWLZKMPFJ-ZXPFJRLXSA-N L-methionine (R)-S-oxide Chemical compound C[S@@](=O)CC[C@H]([NH3+])C([O-])=O QEFRNWWLZKMPFJ-ZXPFJRLXSA-N 0.000 description 1
- QEFRNWWLZKMPFJ-UHFFFAOYSA-N L-methionine sulphoxide Natural products CS(=O)CCC(N)C(O)=O QEFRNWWLZKMPFJ-UHFFFAOYSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- YSDQQAXHVYUZIW-QCIJIYAXSA-N Liraglutide Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCC)C(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=C(O)C=C1 YSDQQAXHVYUZIW-QCIJIYAXSA-N 0.000 description 1
- 108010019598 Liraglutide Proteins 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 208000001145 Metabolic Syndrome Diseases 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 1
- 241001460678 Napo <wasp> Species 0.000 description 1
- RHGKLRLOHDJJDR-UHFFFAOYSA-N Ndelta-carbamoyl-DL-ornithine Natural products OC(=O)C(N)CCCNC(N)=O RHGKLRLOHDJJDR-UHFFFAOYSA-N 0.000 description 1
- 241000282579 Pan Species 0.000 description 1
- 241000282520 Papio Species 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 102000004257 Potassium Channel Human genes 0.000 description 1
- 102100022807 Potassium voltage-gated channel subfamily H member 2 Human genes 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 101710081551 Pyrolysin Proteins 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 206010074268 Reproductive toxicity Diseases 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 102000019027 Ryanodine Receptor Calcium Release Channel Human genes 0.000 description 1
- 108010012219 Ryanodine Receptor Calcium Release Channel Proteins 0.000 description 1
- 241000555745 Sciuridae Species 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 229940122055 Serine protease inhibitor Drugs 0.000 description 1
- 101710102218 Serine protease inhibitor Proteins 0.000 description 1
- 241000270295 Serpentes Species 0.000 description 1
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 1
- 244000171468 Solanum jamaicense Species 0.000 description 1
- 102000005157 Somatostatin Human genes 0.000 description 1
- 108010056088 Somatostatin Proteins 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 229940100389 Sulfonylurea Drugs 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 238000012288 TUNEL assay Methods 0.000 description 1
- 206010043275 Teratogenicity Diseases 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 229940122618 Trypsin inhibitor Drugs 0.000 description 1
- 101710162629 Trypsin inhibitor Proteins 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 208000021017 Weight Gain Diseases 0.000 description 1
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 238000007259 addition reaction Methods 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 150000001371 alpha-amino acids Chemical class 0.000 description 1
- 235000008206 alpha-amino acids Nutrition 0.000 description 1
- 230000009435 amidation Effects 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- 150000003862 amino acid derivatives Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 235000012538 ammonium bicarbonate Nutrition 0.000 description 1
- 239000001099 ammonium carbonate Substances 0.000 description 1
- 235000019418 amylase Nutrition 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000002424 anti-apoptotic effect Effects 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 229940127219 anticoagulant drug Drugs 0.000 description 1
- 239000003472 antidiabetic agent Substances 0.000 description 1
- 229940125708 antidiabetic agent Drugs 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 230000005775 apoptotic pathway Effects 0.000 description 1
- 230000036528 appetite Effects 0.000 description 1
- 235000019789 appetite Nutrition 0.000 description 1
- 239000002830 appetite depressant Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 235000009697 arginine Nutrition 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 239000012131 assay buffer Substances 0.000 description 1
- 238000011888 autopsy Methods 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 238000012742 biochemical analysis Methods 0.000 description 1
- 238000010876 biochemical test Methods 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 230000003925 brain function Effects 0.000 description 1
- 210000000621 bronchi Anatomy 0.000 description 1
- 229940084891 byetta Drugs 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 230000006652 catabolic pathway Effects 0.000 description 1
- 210000001159 caudate nucleus Anatomy 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 238000003570 cell viability assay Methods 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 230000003196 chaotropic effect Effects 0.000 description 1
- AOXOCDRNSPFDPE-UKEONUMOSA-N chembl413654 Chemical compound C([C@H](C(=O)NCC(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@H](CCSC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](C)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H]1N(CCC1)C(=O)CNC(=O)[C@@H](N)CCC(O)=O)C1=CC=C(O)C=C1 AOXOCDRNSPFDPE-UKEONUMOSA-N 0.000 description 1
- 231100000005 chromosome aberration Toxicity 0.000 description 1
- 235000013477 citrulline Nutrition 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- 238000004581 coalescence Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000003930 cognitive ability Effects 0.000 description 1
- 230000009137 competitive binding Effects 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 239000013068 control sample Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 238000006074 cyclodimerization reaction Methods 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000002716 delivery method Methods 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 230000001687 destabilization Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 239000012470 diluted sample Substances 0.000 description 1
- 239000003603 dipeptidyl peptidase IV inhibitor Substances 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 231100000371 dose-limiting toxicity Toxicity 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 1
- 230000013020 embryo development Effects 0.000 description 1
- 230000009228 embryo fetal development Effects 0.000 description 1
- 210000002257 embryonic structure Anatomy 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 230000006862 enzymatic digestion Effects 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- HQPMKSGTIOYHJT-UHFFFAOYSA-N ethane-1,2-diol;propane-1,2-diol Chemical compound OCCO.CC(O)CO HQPMKSGTIOYHJT-UHFFFAOYSA-N 0.000 description 1
- 230000003203 everyday effect Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 108010024703 exendin (9-39) Proteins 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 235000021050 feed intake Nutrition 0.000 description 1
- 210000003191 femoral vein Anatomy 0.000 description 1
- 230000008175 fetal development Effects 0.000 description 1
- 235000019525 fullness Nutrition 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 230000005176 gastrointestinal motility Effects 0.000 description 1
- 235000021474 generally recognized As safe (food) Nutrition 0.000 description 1
- 235000021473 generally recognized as safe (food ingredients) Nutrition 0.000 description 1
- 230000007674 genetic toxicity Effects 0.000 description 1
- 230000001738 genotoxic effect Effects 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 230000004153 glucose metabolism Effects 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 235000004554 glutamine Nutrition 0.000 description 1
- KZNQNBZMBZJQJO-YFKPBYRVSA-N glyclproline Chemical group NCC(=O)N1CCC[C@H]1C(O)=O KZNQNBZMBZJQJO-YFKPBYRVSA-N 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 210000001308 heart ventricle Anatomy 0.000 description 1
- 108010069764 helospectin I Proteins 0.000 description 1
- HTMVMVKJOPFRMK-OYZAELBCSA-N helospectin i Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)O)C1=CC=C(O)C=C1 HTMVMVKJOPFRMK-OYZAELBCSA-N 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 230000036732 histological change Effects 0.000 description 1
- 102000055647 human CSF2RB Human genes 0.000 description 1
- 235000003642 hunger Nutrition 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 150000002431 hydrogen Chemical group 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 208000036260 idiopathic disease Diseases 0.000 description 1
- 210000003405 ileum Anatomy 0.000 description 1
- 238000012744 immunostaining Methods 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000005462 in vivo assay Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 210000002660 insulin-secreting cell Anatomy 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 238000007917 intracranial administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- 229940090473 januvia Drugs 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 150000002605 large molecules Chemical class 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000012669 liquid formulation Substances 0.000 description 1
- 229960002701 liraglutide Drugs 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 208000004731 long QT syndrome Diseases 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 230000004199 lung function Effects 0.000 description 1
- 238000013123 lung function test Methods 0.000 description 1
- 210000002751 lymph Anatomy 0.000 description 1
- 238000007433 macroscopic evaluation Methods 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 235000012054 meals Nutrition 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229940126601 medicinal product Drugs 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- LSDPWZHWYPCBBB-UHFFFAOYSA-O methylsulfide anion Chemical compound [SH2+]C LSDPWZHWYPCBBB-UHFFFAOYSA-O 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000004001 molecular interaction Effects 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- 230000003505 mutagenic effect Effects 0.000 description 1
- 231100000150 mutagenicity / genotoxicity testing Toxicity 0.000 description 1
- 239000006199 nebulizer Substances 0.000 description 1
- 230000009707 neogenesis Effects 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 229940127073 nucleoside analogue Drugs 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 229920002113 octoxynol Polymers 0.000 description 1
- 238000006384 oligomerization reaction Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 229940126701 oral medication Drugs 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 230000001151 other effect Effects 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 210000001711 oxyntic cell Anatomy 0.000 description 1
- 239000006174 pH buffer Substances 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 239000000813 peptide hormone Substances 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920001993 poloxamer 188 Polymers 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 108020001213 potassium channel Proteins 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 230000009237 prenatal development Effects 0.000 description 1
- 230000007425 progressive decline Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 239000012460 protein solution Substances 0.000 description 1
- 229940124272 protein stabilizer Drugs 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 150000003222 pyridines Chemical class 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 230000021419 recognition of apoptotic cell Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000003893 regulation of appetite Effects 0.000 description 1
- 230000009892 regulation of energy homeostasis Effects 0.000 description 1
- 230000029964 regulation of glucose metabolic process Effects 0.000 description 1
- 230000002336 repolarization Effects 0.000 description 1
- 230000033458 reproduction Effects 0.000 description 1
- 230000007696 reproductive toxicity Effects 0.000 description 1
- 231100000372 reproductive toxicity Toxicity 0.000 description 1
- 230000029054 response to nutrient Effects 0.000 description 1
- 238000004007 reversed phase HPLC Methods 0.000 description 1
- 230000033764 rhythmic process Effects 0.000 description 1
- 238000007363 ring formation reaction Methods 0.000 description 1
- 238000011076 safety test Methods 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000009738 saturating Methods 0.000 description 1
- 238000007423 screening assay Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 235000016491 selenocysteine Nutrition 0.000 description 1
- ZKZBPNGNEQAJSX-UHFFFAOYSA-N selenocysteine Natural products [SeH]CC(N)C(O)=O ZKZBPNGNEQAJSX-UHFFFAOYSA-N 0.000 description 1
- 229940055619 selenocysteine Drugs 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 239000003001 serine protease inhibitor Substances 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 208000013220 shortness of breath Diseases 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 229960004034 sitagliptin Drugs 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 238000005063 solubilization Methods 0.000 description 1
- 230000007928 solubilization Effects 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- NHXLMOGPVYXJNR-ATOGVRKGSA-N somatostatin Chemical compound C([C@H]1C(=O)N[C@H](C(N[C@@H](CO)C(=O)N[C@@H](CSSC[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(=O)N1)[C@@H](C)O)NC(=O)CNC(=O)[C@H](C)N)C(O)=O)=O)[C@H](O)C)C1=CC=CC=C1 NHXLMOGPVYXJNR-ATOGVRKGSA-N 0.000 description 1
- 229960000553 somatostatin Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000012453 sprague-dawley rat model Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- YROXIXLRRCOBKF-UHFFFAOYSA-N sulfonylurea Chemical class OC(=N)N=S(=O)=O YROXIXLRRCOBKF-UHFFFAOYSA-N 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 231100000211 teratogenicity Toxicity 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 231100000440 toxicity profile Toxicity 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000723 toxicological property Toxicity 0.000 description 1
- 231100000041 toxicology testing Toxicity 0.000 description 1
- 230000008359 toxicosis Effects 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 1
- 239000002753 trypsin inhibitor Substances 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 238000002211 ultraviolet spectrum Methods 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 239000002435 venom Substances 0.000 description 1
- 210000001048 venom Anatomy 0.000 description 1
- 231100000611 venom Toxicity 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/19—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles lyophilised, i.e. freeze-dried, solutions or dispersions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene or sparfloxacin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/26—Glucagons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
- A61K9/0073—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
- A61K9/0073—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy
- A61K9/0075—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy for inhalation via a dry powder inhaler [DPI], e.g. comprising micronized drug mixed with lactose carrier particles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/08—Solutions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/143—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/145—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1611—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1682—Processes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
Landscapes
- Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Diabetes (AREA)
- Immunology (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Endocrinology (AREA)
- Otolaryngology (AREA)
- Pulmonology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Inorganic Chemistry (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Transplantation (AREA)
- Hospice & Palliative Care (AREA)
- Vascular Medicine (AREA)
- Urology & Nephrology (AREA)
- Emergency Medicine (AREA)
- Dermatology (AREA)
- Child & Adolescent Psychology (AREA)
- Psychiatry (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US74488206P | 2006-04-14 | 2006-04-14 | |
| US60/744,882 | 2006-04-14 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2008144965/15A Division RU2409349C2 (ru) | 2006-04-14 | 2007-04-16 | Фармацевтические композиции, содержащие глюкагонподобный пептид(glp-1) |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2010137392A RU2010137392A (ru) | 2012-03-20 |
| RU2542500C2 true RU2542500C2 (ru) | 2015-02-20 |
Family
ID=38567214
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010137392/15A RU2542500C2 (ru) | 2006-04-14 | 2007-04-16 | Фармацевтические композиции, содержащие глюкагон-подобный пептид 1 (glp-1) |
| RU2008144965/15A RU2409349C2 (ru) | 2006-04-14 | 2007-04-16 | Фармацевтические композиции, содержащие глюкагонподобный пептид(glp-1) |
| RU2014152320A RU2014152320A (ru) | 2006-04-14 | 2014-12-23 | Фармацевтические композиции, содержащие глюкагон-подобный пептид 1 (glp-1) |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2008144965/15A RU2409349C2 (ru) | 2006-04-14 | 2007-04-16 | Фармацевтические композиции, содержащие глюкагонподобный пептид(glp-1) |
| RU2014152320A RU2014152320A (ru) | 2006-04-14 | 2014-12-23 | Фармацевтические композиции, содержащие глюкагон-подобный пептид 1 (glp-1) |
Country Status (11)
| Country | Link |
|---|---|
| EP (1) | EP2010155A2 (cg-RX-API-DMAC7.html) |
| JP (3) | JP5415938B2 (cg-RX-API-DMAC7.html) |
| KR (3) | KR20150042304A (cg-RX-API-DMAC7.html) |
| CN (2) | CN104288756A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2007238000B2 (cg-RX-API-DMAC7.html) |
| BR (1) | BRPI0709964A2 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2646400A1 (cg-RX-API-DMAC7.html) |
| HK (1) | HK1206241A1 (cg-RX-API-DMAC7.html) |
| MX (1) | MX2008013216A (cg-RX-API-DMAC7.html) |
| RU (3) | RU2542500C2 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2007121411A2 (cg-RX-API-DMAC7.html) |
Families Citing this family (68)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2377204C (en) | 1999-06-29 | 2015-01-20 | Pharmaceutical Discovery Corporation | Purification and stabilization of peptide and protein pharmaceutical agents |
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| WO2003080149A2 (en) | 2002-03-20 | 2003-10-02 | Mannkind Corporation | Inhalation apparatus |
| US8921311B2 (en) | 2003-08-01 | 2014-12-30 | Mannkind Corporation | Method for treating hyperglycemia |
| EP1786784B1 (en) | 2004-08-20 | 2010-10-27 | MannKind Corporation | Catalysis of diketopiperazine synthesis |
| CN104436170B (zh) | 2004-08-23 | 2018-02-23 | 曼金德公司 | 用于药物输送的二酮哌嗪盐 |
| IN2014DN09128A (cg-RX-API-DMAC7.html) | 2005-09-14 | 2015-07-10 | Mannkind Corp | |
| CN101389348A (zh) | 2006-02-22 | 2009-03-18 | 曼金德公司 | 用于改善包含二酮哌嗪和活性剂的微粒的药物性质的方法 |
| KR20150042304A (ko) * | 2006-04-14 | 2015-04-20 | 맨카인드 코포레이션 | 글루카곤 유사 펩타이드 1 (glp-1) 약제학적 제제 |
| AU2008316636B2 (en) | 2007-10-24 | 2014-02-06 | Mannkind Corporation | Delivery of active agents |
| HUE025485T2 (en) | 2007-10-24 | 2016-02-29 | Mannkind Corp | Respiratory dry powder formulation containing GLP-1 for use in the treatment of hyperglycemia and diabetes by pulmonary administration |
| US8785396B2 (en) | 2007-10-24 | 2014-07-22 | Mannkind Corporation | Method and composition for treating migraines |
| JP5856843B2 (ja) | 2008-05-27 | 2016-02-10 | アンピオ ファーマシューティカルズ,インコーポレイテッド | ジケトピペラジンを用いた医薬組成物 |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| US9358352B2 (en) | 2008-06-13 | 2016-06-07 | Mannkind Corporation | Dry powder drug delivery system and methods |
| CN109568740B (zh) | 2008-06-13 | 2022-05-27 | 曼金德公司 | 干粉吸入器和用于药物输送的系统 |
| BR122020002400B1 (pt) | 2008-06-20 | 2021-12-07 | Mannkind Corporation | Sistema de detecção de inalação de pó seco e sistema de monitoramento acoplável para um inalador de pó seco |
| TWI614024B (zh) | 2008-08-11 | 2018-02-11 | 曼凱公司 | 超快起作用胰島素之用途 |
| US8314106B2 (en) | 2008-12-29 | 2012-11-20 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| JP5918539B2 (ja) | 2009-01-08 | 2016-05-18 | マンカインド コーポレイション | Glp−1を用いる高血糖症の治療方法 |
| KR101733816B1 (ko) * | 2009-03-04 | 2017-05-08 | 맨카인드 코포레이션 | 개선된 건조 분말 약물 전달 시스템 |
| PL2405963T3 (pl) | 2009-03-11 | 2014-04-30 | Mannkind Corp | Urządzenie, układ i sposób pomiaru oporu inhalatora |
| CA2764505C (en) | 2009-06-12 | 2018-09-25 | Mannkind Corporation | Diketopiperazine microparticles with defined specific surface areas |
| ES2880102T3 (es) | 2009-06-12 | 2021-11-23 | Mannkind Corp | Micropartículas de dicetopiperazina con contenido de isómeros definido |
| WO2011017554A2 (en) * | 2009-08-07 | 2011-02-10 | Mannkind Corporation | Val (8) glp-1 composition and method for treating functional dyspepsia and/or irritable bowel syndrome |
| EP2496295A1 (en) | 2009-11-03 | 2012-09-12 | MannKind Corporation | An apparatus and method for simulating inhalation efforts |
| WO2012033792A2 (en) | 2010-09-07 | 2012-03-15 | Dmi Acquisition Corp. | Treatment of diseases |
| JP6133270B2 (ja) | 2011-04-01 | 2017-05-24 | マンカインド コーポレイション | 薬剤カートリッジのためのブリスター包装 |
| WO2012174472A1 (en) | 2011-06-17 | 2012-12-20 | Mannkind Corporation | High capacity diketopiperazine microparticles |
| EA028343B1 (ru) | 2011-10-10 | 2017-11-30 | Ампио Фармасьютикалз, Инк. | Лечение дегенеративного заболевания сустава |
| PH12014500686A1 (en) | 2011-10-10 | 2020-12-18 | Ampio Pharmaceuticals Inc | Implantable medical devices with increased immune tolerance, and methods for making and implanting |
| HK1201475A1 (en) | 2011-10-24 | 2015-09-04 | Mannkind Corporation | Methods and compositions for treating pain |
| MX355446B (es) | 2011-10-28 | 2018-04-18 | Ampio Pharmaceuticals Inc | Tratamiento de rinitis. |
| JP6059802B2 (ja) | 2012-07-01 | 2017-01-11 | ノヴォ ノルディスク アー/エス | 長時間作用型glp−1ペプチドの使用 |
| CA2878457C (en) | 2012-07-12 | 2021-01-19 | Mannkind Corporation | Dry powder drug delivery systems and methods |
| JP2015526523A (ja) * | 2012-08-29 | 2015-09-10 | マンカインド コーポレイション | 高血糖症の治療のための方法および組成物 |
| UA116217C2 (uk) | 2012-10-09 | 2018-02-26 | Санофі | Пептидна сполука як подвійний агоніст рецепторів glp1-1 та глюкагону |
| EP2911690A1 (en) | 2012-10-26 | 2015-09-02 | MannKind Corporation | Inhalable influenza vaccine compositions and methods |
| RS57531B1 (sr) | 2012-12-21 | 2018-10-31 | Sanofi Sa | Derivati eksendina-4 kao dualni glp1/gip- ili trigonalni glp1/gip/glukagon agonisti |
| MX392016B (es) * | 2013-03-15 | 2025-03-21 | Mannkind Corp | Composiciones de dicetopiperazina microcristalina y métodos. |
| US9808454B2 (en) | 2013-03-15 | 2017-11-07 | Ampio Pharmaceuticals, Inc. | Compositions for the mobilization, homing, expansion and differentiation of stem cells and methods of using the same |
| EP3021834A1 (en) | 2013-07-18 | 2016-05-25 | MannKind Corporation | Heat-stable dry powder pharmaceutical compositions and methods |
| JP2016530930A (ja) | 2013-08-05 | 2016-10-06 | マンカインド コーポレイション | 通気装置及び方法 |
| EP3035952B1 (en) | 2013-08-22 | 2018-12-26 | ZP Opco, Inc. | Stable glucagon peptide formulations |
| US9782344B2 (en) | 2013-08-22 | 2017-10-10 | Zp Opco, Inc. | Stable glucagon peptide formulations |
| EP3080150B1 (en) | 2013-12-13 | 2018-08-01 | Sanofi | Exendin-4 peptide analogues as dual glp-1/gip receptor agonists |
| TW201609796A (zh) | 2013-12-13 | 2016-03-16 | 賽諾菲公司 | 非醯化之艾塞那肽-4(exendin-4)胜肽類似物 |
| WO2015086733A1 (en) | 2013-12-13 | 2015-06-18 | Sanofi | Dual glp-1/glucagon receptor agonists |
| WO2015086729A1 (en) | 2013-12-13 | 2015-06-18 | Sanofi | Dual glp-1/gip receptor agonists |
| EA034620B1 (ru) | 2014-03-18 | 2020-02-27 | Апептико Форшунг Унд Энтвиклунг Гмбх | Пептидный медикамент в виде сухого порошка |
| US10307464B2 (en) | 2014-03-28 | 2019-06-04 | Mannkind Corporation | Use of ultrarapid acting insulin |
| TW201625668A (zh) | 2014-04-07 | 2016-07-16 | 賽諾菲公司 | 作為胜肽性雙重glp-1/昇糖素受體激動劑之艾塞那肽-4衍生物 |
| TW201625670A (zh) | 2014-04-07 | 2016-07-16 | 賽諾菲公司 | 衍生自exendin-4之雙重glp-1/升糖素受體促效劑 |
| TW201625669A (zh) | 2014-04-07 | 2016-07-16 | 賽諾菲公司 | 衍生自艾塞那肽-4(Exendin-4)之肽類雙重GLP-1/升糖素受體促效劑 |
| US9932381B2 (en) | 2014-06-18 | 2018-04-03 | Sanofi | Exendin-4 derivatives as selective glucagon receptor agonists |
| EP3183240A4 (en) | 2014-08-18 | 2018-06-27 | Ampio Pharmaceuticals, Inc. | Treatment of joint conditions |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| AR105319A1 (es) | 2015-06-05 | 2017-09-27 | Sanofi Sa | Profármacos que comprenden un conjugado agonista dual de glp-1 / glucagón conector ácido hialurónico |
| EP3310375A4 (en) | 2015-06-22 | 2019-02-20 | Ampio Pharmaceuticals, Inc. | USE OF LOW-MOLECULAR FRACTIONS OF HUMAN SERUMALBUMINE IN THE TREATMENT OF ILLNESSES |
| TW201706291A (zh) | 2015-07-10 | 2017-02-16 | 賽諾菲公司 | 作為選擇性肽雙重glp-1/升糖素受體促效劑之新毒蜥外泌肽(exendin-4)衍生物 |
| CN108066762B (zh) * | 2016-11-07 | 2022-04-01 | 赫兰 | Glp-1受体激动剂类药物的用途 |
| BR112020006246A2 (pt) | 2017-10-12 | 2021-03-30 | Novo Nordisk A/S | Método para controle do peso de um sujeito em necessidade |
| TWI705820B (zh) * | 2018-06-22 | 2020-10-01 | 美商美國禮來大藥廠 | Gip/glp1促效劑組合物 |
| CN109827875A (zh) * | 2019-04-10 | 2019-05-31 | 上海市食品药品检验所 | 一种用于测定吸入制剂溶出度的装置和方法 |
| KR20210062509A (ko) | 2019-11-21 | 2021-05-31 | 대한민국(전북기계공업고등학교장) | 필터리스 다이나믹 미세먼지 공기 청정기 |
| CN115192554A (zh) * | 2022-08-08 | 2022-10-18 | 浙江仙琚萃泽医药科技有限公司 | 无抛射剂的含肽吸入溶液剂及其制备方法 |
| WO2025159538A1 (ko) * | 2024-01-26 | 2025-07-31 | 주식회사 아울바이오 | Glp-1 ra 기반 계열 펩타이드를 포함하는 미립구, 이의 제조방법, 및 이를 포함하는 약학적 조성물 |
| WO2025220834A1 (ko) | 2024-04-16 | 2025-10-23 | 한미약품 주식회사 | 흡입제 조성물 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993018754A1 (en) * | 1992-03-11 | 1993-09-30 | Pharmaceutical Discovery Corporation | Self-assembling diketopiperazine drug delivery system |
| WO2002098348A2 (en) * | 2001-06-01 | 2002-12-12 | Eli Lilly And Company | Glp-1 formulations with protracted time action |
| WO2006023943A1 (en) * | 2004-08-23 | 2006-03-02 | Mannkind Corporation | Diketopiperazine salts, diketomorpholine salts or diketodioxane salts for drug delivery |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE4242863A1 (de) * | 1992-12-18 | 1994-06-23 | Boehringer Mannheim Gmbh | Stabile lyophilisierte pharmazeutische Zubereitungen von G-CSF |
| EP1012188B1 (de) * | 1997-09-12 | 2004-08-18 | Pharis Biotec GmbH | Zusammensetzung zur therapie von diabetes mellitus und fettsucht |
| CA2377204C (en) * | 1999-06-29 | 2015-01-20 | Pharmaceutical Discovery Corporation | Purification and stabilization of peptide and protein pharmaceutical agents |
| JP4417113B2 (ja) * | 2002-02-20 | 2010-02-17 | エミスフェアー・テクノロジーズ・インク | Glp−1分子の投与方法 |
| WO2003075834A2 (en) * | 2002-03-08 | 2003-09-18 | Eli Lilly And Company | Activated protein c formulations |
| WO2004012672A2 (en) * | 2002-08-01 | 2004-02-12 | Mannkind Corporation | Cell transport compositions and uses thereof |
| US20040038865A1 (en) * | 2002-08-01 | 2004-02-26 | Mannkind Corporation | Cell transport compositions and uses thereof |
| PL1631264T5 (pl) * | 2003-06-02 | 2018-09-28 | Glaxosmithkline Biologicals Sa | Kompozycje immunogenne oparte na biodegradowalnych mikrocząstkach zawierających toksoid błonicy i tężca |
| IN2014DN09128A (cg-RX-API-DMAC7.html) * | 2005-09-14 | 2015-07-10 | Mannkind Corp | |
| CN101389348A (zh) * | 2006-02-22 | 2009-03-18 | 曼金德公司 | 用于改善包含二酮哌嗪和活性剂的微粒的药物性质的方法 |
| KR20150042304A (ko) * | 2006-04-14 | 2015-04-20 | 맨카인드 코포레이션 | 글루카곤 유사 펩타이드 1 (glp-1) 약제학적 제제 |
-
2007
- 2007-04-16 KR KR1020157008571A patent/KR20150042304A/ko not_active Ceased
- 2007-04-16 JP JP2009505655A patent/JP5415938B2/ja not_active Expired - Fee Related
- 2007-04-16 BR BRPI0709964-9A patent/BRPI0709964A2/pt not_active IP Right Cessation
- 2007-04-16 MX MX2008013216A patent/MX2008013216A/es active IP Right Grant
- 2007-04-16 EP EP20070760728 patent/EP2010155A2/en not_active Withdrawn
- 2007-04-16 AU AU2007238000A patent/AU2007238000B2/en not_active Ceased
- 2007-04-16 CN CN201410514033.8A patent/CN104288756A/zh active Pending
- 2007-04-16 RU RU2010137392/15A patent/RU2542500C2/ru not_active IP Right Cessation
- 2007-04-16 RU RU2008144965/15A patent/RU2409349C2/ru not_active IP Right Cessation
- 2007-04-16 KR KR1020147011316A patent/KR101558829B1/ko active Active
- 2007-04-16 KR KR1020087027774A patent/KR101438839B1/ko active Active
- 2007-04-16 CN CNA200780013424XA patent/CN101453988A/zh active Pending
- 2007-04-16 WO PCT/US2007/066728 patent/WO2007121411A2/en not_active Ceased
- 2007-04-16 CA CA002646400A patent/CA2646400A1/en active Pending
-
2013
- 2013-09-17 JP JP2013191549A patent/JP5898156B2/ja active Active
-
2014
- 2014-12-23 RU RU2014152320A patent/RU2014152320A/ru not_active Application Discontinuation
-
2015
- 2015-07-16 HK HK15106772.4A patent/HK1206241A1/xx unknown
- 2015-11-30 JP JP2015233125A patent/JP2016104736A/ja active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993018754A1 (en) * | 1992-03-11 | 1993-09-30 | Pharmaceutical Discovery Corporation | Self-assembling diketopiperazine drug delivery system |
| WO2002098348A2 (en) * | 2001-06-01 | 2002-12-12 | Eli Lilly And Company | Glp-1 formulations with protracted time action |
| WO2006023943A1 (en) * | 2004-08-23 | 2006-03-02 | Mannkind Corporation | Diketopiperazine salts, diketomorpholine salts or diketodioxane salts for drug delivery |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20080111533A (ko) | 2008-12-23 |
| BRPI0709964A2 (pt) | 2011-08-02 |
| RU2010137392A (ru) | 2012-03-20 |
| JP2009533476A (ja) | 2009-09-17 |
| JP5415938B2 (ja) | 2014-02-12 |
| EP2010155A2 (en) | 2009-01-07 |
| KR101438839B1 (ko) | 2014-10-02 |
| RU2008144965A (ru) | 2010-05-20 |
| CN101453988A (zh) | 2009-06-10 |
| CN104288756A (zh) | 2015-01-21 |
| HK1206241A1 (en) | 2016-01-08 |
| AU2007238000B2 (en) | 2013-01-17 |
| KR20150042304A (ko) | 2015-04-20 |
| WO2007121411A2 (en) | 2007-10-25 |
| RU2014152320A (ru) | 2016-07-20 |
| JP2016104736A (ja) | 2016-06-09 |
| KR101558829B1 (ko) | 2015-10-08 |
| MX2008013216A (es) | 2008-10-27 |
| KR20140072138A (ko) | 2014-06-12 |
| JP2014043445A (ja) | 2014-03-13 |
| JP5898156B2 (ja) | 2016-04-06 |
| WO2007121411A3 (en) | 2007-12-13 |
| RU2409349C2 (ru) | 2011-01-20 |
| AU2007238000A1 (en) | 2007-10-25 |
| CA2646400A1 (en) | 2007-10-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2542500C2 (ru) | Фармацевтические композиции, содержащие глюкагон-подобный пептид 1 (glp-1) | |
| US20080260838A1 (en) | Glucagon-like peptide 1 (glp-1) pharmaceutical formulations | |
| US8642548B2 (en) | Val (8) GLP-1 composition and method for treating functional dyspepsia and/or irritable bowel syndrome | |
| KR101996739B1 (ko) | 글루카곤 및 glp-1 수용체의 공-효능제 | |
| EP2934567B1 (en) | Exendin-4 derivatives as dual glp1/gip- or trigonal glp1/gip/glucagon agonists | |
| JP5918539B2 (ja) | Glp−1を用いる高血糖症の治療方法 | |
| TW201609795A (zh) | 作為雙重glp-1/gip受體促效劑的艾塞那肽-4(exendin-4)胜肽類似物 | |
| TW201625669A (zh) | 衍生自艾塞那肽-4(Exendin-4)之肽類雙重GLP-1/升糖素受體促效劑 | |
| TW201609800A (zh) | 做為雙重glp-1/升糖素受體促效劑之艾塞那肽-4胜肽類似物 | |
| AU2013201640A1 (en) | Glucagon-like peptide 1(GLP-1) pharmaceutical formulations | |
| HK1133383A (en) | Glucagon-like peptide 1(glp-1) pharmaceutical formulations | |
| HK1262784A1 (en) | Functionalized exendin-4 derivatives | |
| HK1211233B (en) | Exendin-4 derivatives as dual glp1/gip- or trigonal glp1/gip/glucagon agonists | |
| HK1183045B (en) | Peptide conjugates of glp-1 receptor agonists and gastrin and their use | |
| HK1183045A (en) | Peptide conjugates of glp-1 receptor agonists and gastrin and their use | |
| HK1209766A1 (en) | Exendin-4 derivatives as dual glp1/glucagon agonists | |
| HK1209766B (en) | Exendin-4 derivatives as dual glp1/glucagon agonists |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20170417 |