RU2485107C2 - Антималярийные соединения с гибкими боковыми цепями - Google Patents
Антималярийные соединения с гибкими боковыми цепями Download PDFInfo
- Publication number
- RU2485107C2 RU2485107C2 RU2010117193/04A RU2010117193A RU2485107C2 RU 2485107 C2 RU2485107 C2 RU 2485107C2 RU 2010117193/04 A RU2010117193/04 A RU 2010117193/04A RU 2010117193 A RU2010117193 A RU 2010117193A RU 2485107 C2 RU2485107 C2 RU 2485107C2
- Authority
- RU
- Russia
- Prior art keywords
- substituted
- ethyl
- diamino
- propoxy
- pyrimidine
- Prior art date
Links
- 239000003430 antimalarial agent Substances 0.000 title 1
- 150000001875 compounds Chemical class 0.000 claims abstract 21
- 125000003118 aryl group Chemical group 0.000 claims abstract 8
- 108010022394 Threonine synthase Proteins 0.000 claims abstract 7
- 102000004419 dihydrofolate reductase Human genes 0.000 claims abstract 7
- -1 nitro, carboxyl Chemical group 0.000 claims abstract 7
- 125000000217 alkyl group Chemical group 0.000 claims abstract 6
- 150000003839 salts Chemical class 0.000 claims abstract 6
- 201000004792 malaria Diseases 0.000 claims abstract 5
- 125000003545 alkoxy group Chemical group 0.000 claims abstract 4
- 125000004104 aryloxy group Chemical group 0.000 claims abstract 4
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 claims abstract 4
- 125000000753 cycloalkyl group Chemical group 0.000 claims abstract 4
- 229910052736 halogen Inorganic materials 0.000 claims abstract 4
- 150000002367 halogens Chemical class 0.000 claims abstract 4
- 125000001072 heteroaryl group Chemical group 0.000 claims abstract 4
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims abstract 4
- 125000005346 substituted cycloalkyl group Chemical group 0.000 claims abstract 4
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims abstract 4
- 239000003814 drug Substances 0.000 claims abstract 3
- 125000001624 naphthyl group Chemical group 0.000 claims abstract 3
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims abstract 3
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 claims abstract 3
- 125000003107 substituted aryl group Chemical group 0.000 claims abstract 3
- 125000002252 acyl group Chemical group 0.000 claims abstract 2
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 claims abstract 2
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract 2
- 239000001257 hydrogen Substances 0.000 claims abstract 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims abstract 2
- 125000003387 indolinyl group Chemical group N1(CCC2=CC=CC=C12)* 0.000 claims abstract 2
- 125000001041 indolyl group Chemical group 0.000 claims abstract 2
- 239000003112 inhibitor Substances 0.000 claims abstract 2
- 230000002401 inhibitory effect Effects 0.000 claims abstract 2
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 claims abstract 2
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims abstract 2
- 125000004076 pyridyl group Chemical group 0.000 claims abstract 2
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 claims abstract 2
- 125000001567 quinoxalinyl group Chemical group N1=C(C=NC2=CC=CC=C12)* 0.000 claims abstract 2
- 125000000547 substituted alkyl group Chemical group 0.000 claims abstract 2
- 125000003831 tetrazolyl group Chemical group 0.000 claims abstract 2
- 125000001425 triazolyl group Chemical group 0.000 claims abstract 2
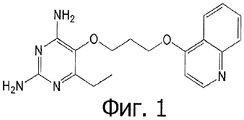
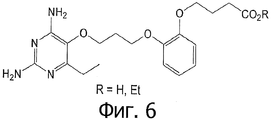
- VDGXZSSDCDPCRF-UHFFFAOYSA-N 3-[2-[3-(2,4-diamino-6-ethylpyrimidin-5-yl)oxypropoxy]phenyl]propanoic acid Chemical compound CCC1=NC(N)=NC(N)=C1OCCCOC1=CC=CC=C1CCC(O)=O VDGXZSSDCDPCRF-UHFFFAOYSA-N 0.000 claims 3
- SZPWXAOBLNYOHY-UHFFFAOYSA-N [C]1=CC=NC2=CC=CC=C12 Chemical group [C]1=CC=NC2=CC=CC=C12 SZPWXAOBLNYOHY-UHFFFAOYSA-N 0.000 claims 3
- QMNFFXRFOJIOKZ-UHFFFAOYSA-N cycloguanil Chemical compound CC1(C)N=C(N)N=C(N)N1C1=CC=C(Cl)C=C1 QMNFFXRFOJIOKZ-UHFFFAOYSA-N 0.000 claims 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims 2
- 239000004052 folic acid antagonist Substances 0.000 claims 2
- 125000001424 substituent group Chemical group 0.000 claims 2
- BVRPZXMKYBDROK-UHFFFAOYSA-N 3-[2-[3-(2,4-diamino-6-ethylpyrimidin-5-yl)oxypropoxy]phenyl]propanoic acid;hydrochloride Chemical compound Cl.CCC1=NC(N)=NC(N)=C1OCCCOC1=CC=CC=C1CCC(O)=O BVRPZXMKYBDROK-UHFFFAOYSA-N 0.000 claims 1
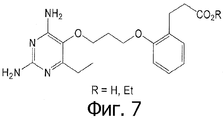
- GHOFVMYEOYDYBA-UHFFFAOYSA-N 4-[2-[3-(2,4-diamino-6-ethylpyrimidin-5-yl)oxypropoxy]phenoxy]butanoic acid Chemical compound CCC1=NC(N)=NC(N)=C1OCCCOC1=CC=CC=C1OCCCC(O)=O GHOFVMYEOYDYBA-UHFFFAOYSA-N 0.000 claims 1
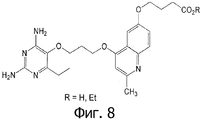
- YMMSABUVXVSZAP-UHFFFAOYSA-N 4-[4-[3-(2,4-diamino-6-ethylpyrimidin-5-yl)oxypropoxy]-2-methylquinolin-6-yl]oxybutanoic acid Chemical compound CCC1=NC(N)=NC(N)=C1OCCCOC1=CC(C)=NC2=CC=C(OCCCC(O)=O)C=C12 YMMSABUVXVSZAP-UHFFFAOYSA-N 0.000 claims 1
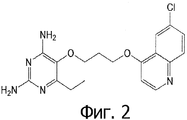
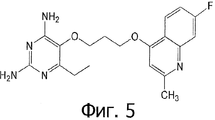
- QFXATCAZIFUIJR-UHFFFAOYSA-N 5-[3-(6-chloroquinolin-4-yl)oxypropoxy]-6-ethylpyrimidine-2,4-diamine Chemical compound CCC1=NC(N)=NC(N)=C1OCCCOC1=CC=NC2=CC=C(Cl)C=C12 QFXATCAZIFUIJR-UHFFFAOYSA-N 0.000 claims 1
- XLXPFGVHLQXPOE-UHFFFAOYSA-N 6-ethyl-5-(3-quinolin-4-yloxypropoxy)pyrimidine-2,4-diamine Chemical compound CCC1=NC(N)=NC(N)=C1OCCCOC1=CC=NC2=CC=CC=C12 XLXPFGVHLQXPOE-UHFFFAOYSA-N 0.000 claims 1
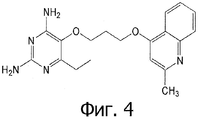
- PZIKABAOIAWTKV-UHFFFAOYSA-N 6-ethyl-5-[3-(2-methylquinolin-4-yl)oxypropoxy]pyrimidine-2,4-diamine Chemical compound CCC1=NC(N)=NC(N)=C1OCCCOC1=CC(C)=NC2=CC=CC=C12 PZIKABAOIAWTKV-UHFFFAOYSA-N 0.000 claims 1
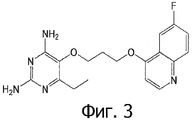
- FNNDLIDZIPMLHG-UHFFFAOYSA-N 6-ethyl-5-[3-(6-fluoroquinolin-4-yl)oxypropoxy]pyrimidine-2,4-diamine Chemical compound CCC1=NC(N)=NC(N)=C1OCCCOC1=CC=NC2=CC=C(F)C=C12 FNNDLIDZIPMLHG-UHFFFAOYSA-N 0.000 claims 1
- DAISISXXOKACIQ-UHFFFAOYSA-N 6-ethyl-5-[3-(7-fluoro-2-methylquinolin-4-yl)oxypropoxy]pyrimidine-2,4-diamine Chemical compound CCC1=NC(N)=NC(N)=C1OCCCOC1=CC(C)=NC2=CC(F)=CC=C12 DAISISXXOKACIQ-UHFFFAOYSA-N 0.000 claims 1
- 229950004734 cycloguanil Drugs 0.000 claims 1
- 229940079593 drug Drugs 0.000 claims 1
- 125000004494 ethyl ester group Chemical group 0.000 claims 1
- 239000000546 pharmaceutical excipient Substances 0.000 claims 1
- WKSAUQYGYAYLPV-UHFFFAOYSA-N pyrimethamine Chemical compound CCC1=NC(N)=NC(N)=C1C1=CC=C(Cl)C=C1 WKSAUQYGYAYLPV-UHFFFAOYSA-N 0.000 claims 1
- 229960000611 pyrimethamine Drugs 0.000 claims 1
- 241000224016 Plasmodium Species 0.000 abstract 1
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 abstract 1
- 239000012964 benzotriazole Substances 0.000 abstract 1
- 230000000694 effects Effects 0.000 abstract 1
- 239000000126 substance Substances 0.000 abstract 1
- 0 CCc(nc(N)nc1N)c1OCCCCOc1ccccc1CCC(O*)=O Chemical compound CCc(nc(N)nc1N)c1OCCCCOc1ccccc1CCC(O*)=O 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
- A61P33/06—Antimalarials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D239/00—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings
- C07D239/02—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings
- C07D239/24—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members
- C07D239/28—Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings not condensed with other rings having three or more double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to ring carbon atoms
- C07D239/46—Two or more oxygen, sulphur or nitrogen atoms
- C07D239/48—Two nitrogen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a chain containing hetero atoms as chain links
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Tropical Medicine & Parasitology (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US97837507P | 2007-10-08 | 2007-10-08 | |
| US60/978,375 | 2007-10-08 | ||
| PCT/US2008/079210 WO2009048957A1 (en) | 2007-10-08 | 2008-10-08 | Antimalarial compounds with flexible side-chains |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2010117193A RU2010117193A (ru) | 2011-11-20 |
| RU2485107C2 true RU2485107C2 (ru) | 2013-06-20 |
Family
ID=40534843
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010117193/04A RU2485107C2 (ru) | 2007-10-08 | 2008-10-08 | Антималярийные соединения с гибкими боковыми цепями |
Country Status (22)
| Country | Link |
|---|---|
| US (1) | US8530491B2 (enExample) |
| EP (2) | EP2194985B1 (enExample) |
| JP (1) | JP5603775B2 (enExample) |
| KR (1) | KR101578949B1 (enExample) |
| CN (1) | CN101820879B (enExample) |
| AU (1) | AU2008310898B2 (enExample) |
| BR (1) | BRPI0818618B8 (enExample) |
| CA (1) | CA2700880C (enExample) |
| CY (1) | CY1121598T1 (enExample) |
| DK (1) | DK2194985T3 (enExample) |
| ES (1) | ES2727015T3 (enExample) |
| HR (1) | HRP20190589T1 (enExample) |
| HU (1) | HUE044398T2 (enExample) |
| LT (1) | LT2194985T (enExample) |
| MX (1) | MX2010003739A (enExample) |
| PL (1) | PL2194985T3 (enExample) |
| PT (1) | PT2194985T (enExample) |
| RU (1) | RU2485107C2 (enExample) |
| SI (1) | SI2194985T1 (enExample) |
| TR (1) | TR201904913T4 (enExample) |
| UA (1) | UA105759C2 (enExample) |
| WO (1) | WO2009048957A1 (enExample) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2012108845A1 (en) * | 2011-02-09 | 2012-08-16 | National Science And Technology Development Agency | A bacterial surrogate for testing of antimalarials: thya knockout and fola knockout bacteria for testing of inhibition of malarial dihydrofolate reductase-thymidylate synthase |
| WO2012121682A2 (en) * | 2011-03-10 | 2012-09-13 | National Science And Technology Development Agency | Anti-folate antimalarials with dual-binding modes and their preparation |
| WO2015047199A2 (en) | 2013-09-30 | 2015-04-02 | National Science And Technology Development Agency | A bacterial surrogate for testing of antimalarials: thya knockout, fola knockout, folp knockout, and folk knockout bacteria for testing of inhibition of antifolate pathway |
| CN104593406A (zh) * | 2015-01-08 | 2015-05-06 | 山西医科大学 | pIRES/TgDHFR-TS真核表达重组质粒及其构建和应用 |
| WO2017052479A1 (en) * | 2015-09-23 | 2017-03-30 | National Science And Technology Development Agency | 2,4-diamino-6-ethylpyrimidine derivatives with antimalarial activities against plasmodium falciparum |
| CN106083742A (zh) * | 2016-05-31 | 2016-11-09 | 广东工业大学 | 一种2,4‑二胺基喹唑啉衍生物及其制备方法和应用 |
| WO2021155748A1 (en) * | 2020-02-04 | 2021-08-12 | Tsinghua University | Inhibitors of malarial and plasmodium falciparum hexose transporter and uses thereof |
| CN112961106A (zh) * | 2021-03-13 | 2021-06-15 | 德州学院 | 一种4-羟基-6氯喹啉合成新工艺 |
| EP4438596A1 (en) * | 2023-03-28 | 2024-10-02 | Université de Lille | Diaminopyrimidine derivatives as hdac and dhfr dual-targeting inhibitors and use thereof in preventing and treating malaria |
| WO2025053796A1 (en) * | 2023-09-04 | 2025-03-13 | National Science And Technology Development Agency | Hybrid molecules between pyrimidine derivatives and idasanutlin |
| CN117263804A (zh) * | 2023-09-07 | 2023-12-22 | 中国烟草总公司郑州烟草研究院 | 邻羟基苯丙酸酯及其制备方法和应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4033962A (en) * | 1975-06-26 | 1977-07-05 | Hoffman-La Roche Inc. | 2,4-Diamino-pyrimidine derivatives and processes |
| US4232023A (en) * | 1977-07-11 | 1980-11-04 | Dick Pierre R G | Novel soluble derivatives of 2,4-diamino pyrimidine |
| RU2002124136A (ru) * | 2000-02-11 | 2004-01-10 | Домпе С.П.А. (It) | (r)-2-арилпропионамиды, полезные при ингибировании ил-8-индуцированного хемотаксиса нейтрофилов |
| US20040180913A1 (en) * | 2003-03-13 | 2004-09-16 | National Science And Technology Development Agency | Antimalarial pyrimidine derivatives and methods of making and using them |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1546937A (en) | 1976-07-29 | 1979-05-31 | Beecham Group Ltd | 2,4-diaminopyrimidine derivatives |
| OA07362A (fr) * | 1980-10-27 | 1984-06-30 | May & Baker Ltd | Dérivés de la pyrimidine, leur procédé de préparation et les compositions les contenant. |
| GB2086386B (en) | 1980-10-27 | 1984-01-18 | May & Baker Ltd | Pyrimidine derivatives |
| US5322858A (en) | 1992-02-14 | 1994-06-21 | Jacobus Pharmaceutical Co. Inc. | N,N'-substituted imidodicarbonimidic diamides derived from hydroxylamines |
| RU95113597A (ru) * | 1992-12-02 | 1997-06-10 | ФМК Корпорейшн (US) | Инсектицидная композиция, способы борьбы с насекомыми |
| PL352159A1 (en) | 1999-05-24 | 2003-07-28 | Sankyo Co | Pharmaceutical compositions containing antibody against the fas |
-
2008
- 2008-10-08 WO PCT/US2008/079210 patent/WO2009048957A1/en not_active Ceased
- 2008-10-08 US US12/247,953 patent/US8530491B2/en active Active
- 2008-10-08 TR TR2019/04913T patent/TR201904913T4/tr unknown
- 2008-10-08 RU RU2010117193/04A patent/RU2485107C2/ru active
- 2008-10-08 MX MX2010003739A patent/MX2010003739A/es active IP Right Grant
- 2008-10-08 UA UAA201004117A patent/UA105759C2/uk unknown
- 2008-10-08 JP JP2010528223A patent/JP5603775B2/ja active Active
- 2008-10-08 BR BRPI0818618A patent/BRPI0818618B8/pt active IP Right Grant
- 2008-10-08 AU AU2008310898A patent/AU2008310898B2/en active Active
- 2008-10-08 CN CN2008801106805A patent/CN101820879B/zh active Active
- 2008-10-08 SI SI200832053T patent/SI2194985T1/sl unknown
- 2008-10-08 HR HRP20190589TT patent/HRP20190589T1/hr unknown
- 2008-10-08 KR KR1020107010151A patent/KR101578949B1/ko active Active
- 2008-10-08 EP EP08837766.8A patent/EP2194985B1/en active Active
- 2008-10-08 ES ES08837766T patent/ES2727015T3/es active Active
- 2008-10-08 PL PL08837766T patent/PL2194985T3/pl unknown
- 2008-10-08 EP EP19157867.3A patent/EP3546452A1/en not_active Withdrawn
- 2008-10-08 HU HUE08837766 patent/HUE044398T2/hu unknown
- 2008-10-08 LT LTEP08837766.8T patent/LT2194985T/lt unknown
- 2008-10-08 PT PT08837766T patent/PT2194985T/pt unknown
- 2008-10-08 DK DK08837766.8T patent/DK2194985T3/da active
- 2008-10-08 CA CA2700880A patent/CA2700880C/en active Active
-
2019
- 2019-04-19 CY CY20191100432T patent/CY1121598T1/el unknown
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4033962A (en) * | 1975-06-26 | 1977-07-05 | Hoffman-La Roche Inc. | 2,4-Diamino-pyrimidine derivatives and processes |
| US4232023A (en) * | 1977-07-11 | 1980-11-04 | Dick Pierre R G | Novel soluble derivatives of 2,4-diamino pyrimidine |
| RU2002124136A (ru) * | 2000-02-11 | 2004-01-10 | Домпе С.П.А. (It) | (r)-2-арилпропионамиды, полезные при ингибировании ил-8-индуцированного хемотаксиса нейтрофилов |
| US20040180913A1 (en) * | 2003-03-13 | 2004-09-16 | National Science And Technology Development Agency | Antimalarial pyrimidine derivatives and methods of making and using them |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2485107C2 (ru) | Антималярийные соединения с гибкими боковыми цепями | |
| JP2010540666A5 (enExample) | ||
| RU2489148C2 (ru) | Ингибитор активации stat3/5 | |
| JP2010501478A5 (enExample) | ||
| JP2011527334A5 (enExample) | ||
| RU2010110829A (ru) | Производные бензилбензола и способы их применения | |
| JP2020521740A5 (enExample) | ||
| NZ547615A (en) | Quinoline derivatives and use thereof as mycobacterial inhibitors | |
| RU2502730C2 (ru) | Сульфонамидные соединения и их применение | |
| NZ587547A (en) | Modulators of ATP-Binding Cassette Transporters | |
| CA2690226A1 (en) | Novel malonic acid sulfonamide derivative and pharmaceutical use thereof | |
| BRPI0519262A2 (pt) | derivados de pirazol fundidos e mÉtodos de tratamento de distérbios metabàlicos-relacionados destes | |
| RU2009141522A (ru) | Дифенилдигидроимидазопиридиноны | |
| NO20055305D0 (no) | Pyrrolopyridin-2-karboksylsyreamid-inhibitorer av glykogenfosforylase | |
| PE20090216A1 (es) | Compuestos triazolil aminopirimidina | |
| CA2475619A1 (en) | Piperidine derivatives | |
| CA2617991A1 (en) | N-phenyl-2-pyrimidine-amine derivatives and process for the preparation thereof | |
| RU2007130802A (ru) | Пуриновые производные в качестве агонистов рецептора а2а | |
| PE20060589A1 (es) | FENILAMINOTIAZOLES SUSTITUIDOS COMO AGONISTAS DE ADENOSINA A1 Y A2b | |
| RU2005111590A (ru) | 2,4-замещенные индолы и их использование в качестве модуляторов 5-гт6 | |
| NO20032261D0 (no) | Medisinske preparater | |
| CY1107519T1 (el) | Yποκατεστημενες χαλκονες ως θepαπευτικες ενωσεις | |
| JP2004525183A5 (enExample) | ||
| BRPI0415631A (pt) | derivados de tetrazol e métodos de tratamento de distúrbios metabólico-relacionados dos mesmos | |
| CA2626402A1 (en) | Potassium channel inhibitors |