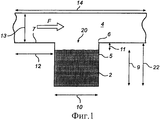
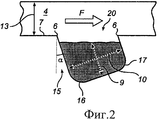
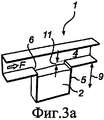
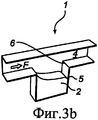
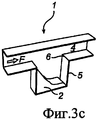
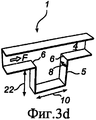
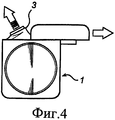
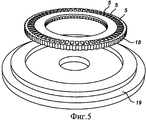
RU2484852C2 - Устройство и способ дезагрегации порошкообразного средства 854 - Google Patents
Устройство и способ дезагрегации порошкообразного средства 854 Download PDFInfo
- Publication number
- RU2484852C2 RU2484852C2 RU2010126836/14A RU2010126836A RU2484852C2 RU 2484852 C2 RU2484852 C2 RU 2484852C2 RU 2010126836/14 A RU2010126836/14 A RU 2010126836/14A RU 2010126836 A RU2010126836 A RU 2010126836A RU 2484852 C2 RU2484852 C2 RU 2484852C2
- Authority
- RU
- Russia
- Prior art keywords
- cavity
- flow passage
- flow
- inhaler
- powdery
- Prior art date
Links
- 239000000843 powder Substances 0.000 title claims abstract description 22
- 238000000034 method Methods 0.000 title claims abstract description 14
- 239000003814 drug Substances 0.000 claims abstract description 32
- 229940079593 drug Drugs 0.000 claims description 27
- 239000003795 chemical substances by application Substances 0.000 claims description 6
- 238000009826 distribution Methods 0.000 claims description 5
- 238000010276 construction Methods 0.000 claims description 2
- 230000000694 effects Effects 0.000 abstract description 6
- 239000000126 substance Substances 0.000 abstract description 5
- 239000002245 particle Substances 0.000 description 18
- 239000002775 capsule Substances 0.000 description 10
- 239000002585 base Substances 0.000 description 7
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 6
- 229960004436 budesonide Drugs 0.000 description 6
- 229960002848 formoterol Drugs 0.000 description 6
- BPZSYCZIITTYBL-UHFFFAOYSA-N formoterol Chemical compound C1=CC(OC)=CC=C1CC(C)NCC(O)C1=CC=C(O)C(NC=O)=C1 BPZSYCZIITTYBL-UHFFFAOYSA-N 0.000 description 6
- LERNTVKEWCAPOY-DZZGSBJMSA-N tiotropium Chemical compound O([C@H]1C[C@@H]2[N+]([C@H](C1)[C@@H]1[C@H]2O1)(C)C)C(=O)C(O)(C=1SC=CC=1)C1=CC=CS1 LERNTVKEWCAPOY-DZZGSBJMSA-N 0.000 description 6
- 238000011144 upstream manufacturing Methods 0.000 description 6
- 229940110309 tiotropium Drugs 0.000 description 5
- UCHDWCPVSPXUMX-TZIWLTJVSA-N Montelukast Chemical compound CC(C)(O)C1=CC=CC=C1CC[C@H](C=1C=C(\C=C\C=2N=C3C=C(Cl)C=CC3=CC=2)C=CC=1)SCC1(CC(O)=O)CC1 UCHDWCPVSPXUMX-TZIWLTJVSA-N 0.000 description 3
- YEEZWCHGZNKEEK-UHFFFAOYSA-N Zafirlukast Chemical compound COC1=CC(C(=O)NS(=O)(=O)C=2C(=CC=CC=2)C)=CC=C1CC(C1=C2)=CN(C)C1=CC=C2NC(=O)OC1CCCC1 YEEZWCHGZNKEEK-UHFFFAOYSA-N 0.000 description 3
- 239000002246 antineoplastic agent Substances 0.000 description 3
- 229940041181 antineoplastic drug Drugs 0.000 description 3
- 229960002714 fluticasone Drugs 0.000 description 3
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 description 3
- 210000004072 lung Anatomy 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 229960001664 mometasone Drugs 0.000 description 3
- QLIIKPVHVRXHRI-CXSFZGCWSA-N mometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)C[C@@H]2O QLIIKPVHVRXHRI-CXSFZGCWSA-N 0.000 description 3
- 229960005127 montelukast Drugs 0.000 description 3
- 229960004764 zafirlukast Drugs 0.000 description 3
- PJFHZKIDENOSJB-UHFFFAOYSA-N Budesonide/formoterol Chemical compound C1=CC(OC)=CC=C1CC(C)NCC(O)C1=CC=C(O)C(NC=O)=C1.C1CC2=CC(=O)C=CC2(C)C2C1C1CC3OC(CCC)OC3(C(=O)CO)C1(C)CC2O PJFHZKIDENOSJB-UHFFFAOYSA-N 0.000 description 2
- 239000012867 bioactive agent Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 229940112141 dry powder inhaler Drugs 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 239000003199 leukotriene receptor blocking agent Substances 0.000 description 2
- 229960003088 loratadine Drugs 0.000 description 2
- JCCNYMKQOSZNPW-UHFFFAOYSA-N loratadine Chemical compound C1CN(C(=O)OCC)CCC1=C1C2=NC=CC=C2CCC2=CC(Cl)=CC=C21 JCCNYMKQOSZNPW-UHFFFAOYSA-N 0.000 description 2
- 239000003380 propellant Substances 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 230000029058 respiratory gaseous exchange Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- XWTYSIMOBUGWOL-UHFFFAOYSA-N (+-)-Terbutaline Chemical compound CC(C)(C)NCC(O)C1=CC(O)=CC(O)=C1 XWTYSIMOBUGWOL-UHFFFAOYSA-N 0.000 description 1
- RATSWNOMCHFQGJ-TUYNVFRMSA-N (e)-but-2-enedioic acid;n-[2-hydroxy-5-[(1s)-1-hydroxy-2-[[(2s)-1-(4-methoxyphenyl)propan-2-yl]amino]ethyl]phenyl]formamide;dihydrate Chemical compound O.O.OC(=O)\C=C\C(O)=O.C1=CC(OC)=CC=C1C[C@H](C)NC[C@@H](O)C1=CC=C(O)C(NC=O)=C1.C1=CC(OC)=CC=C1C[C@H](C)NC[C@@H](O)C1=CC=C(O)C(NC=O)=C1 RATSWNOMCHFQGJ-TUYNVFRMSA-N 0.000 description 1
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- GIIZNNXWQWCKIB-UHFFFAOYSA-N Serevent Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1 GIIZNNXWQWCKIB-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 229940124599 anti-inflammatory drug Drugs 0.000 description 1
- 239000000043 antiallergic agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940065524 anticholinergics inhalants for obstructive airway diseases Drugs 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 229950000210 beclometasone dipropionate Drugs 0.000 description 1
- 239000004044 bronchoconstricting agent Substances 0.000 description 1
- 230000003435 bronchoconstrictive effect Effects 0.000 description 1
- 229940124630 bronchodilator Drugs 0.000 description 1
- 239000000168 bronchodilator agent Substances 0.000 description 1
- 239000002327 cardiovascular agent Substances 0.000 description 1
- 229940125692 cardiovascular agent Drugs 0.000 description 1
- 239000000812 cholinergic antagonist Substances 0.000 description 1
- 229960001117 clenbuterol Drugs 0.000 description 1
- STJMRWALKKWQGH-UHFFFAOYSA-N clenbuterol Chemical compound CC(C)(C)NCC(O)C1=CC(Cl)=C(N)C(Cl)=C1 STJMRWALKKWQGH-UHFFFAOYSA-N 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 239000000032 diagnostic agent Substances 0.000 description 1
- 229940039227 diagnostic agent Drugs 0.000 description 1
- 229940126534 drug product Drugs 0.000 description 1
- 125000000031 ethylamino group Chemical group [H]C([H])([H])C([H])([H])N([H])[*] 0.000 description 1
- 229960001022 fenoterol Drugs 0.000 description 1
- LSLYOANBFKQKPT-UHFFFAOYSA-N fenoterol Chemical compound C=1C(O)=CC(O)=CC=1C(O)CNC(C)CC1=CC=C(O)C=C1 LSLYOANBFKQKPT-UHFFFAOYSA-N 0.000 description 1
- 229960000289 fluticasone propionate Drugs 0.000 description 1
- WMWTYOKRWGGJOA-CENSZEJFSA-N fluticasone propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O WMWTYOKRWGGJOA-CENSZEJFSA-N 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 229940050287 formoterol / mometasone Drugs 0.000 description 1
- 229960003610 formoterol fumarate dihydrate Drugs 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 229960001361 ipratropium bromide Drugs 0.000 description 1
- KEWHKYJURDBRMN-ZEODDXGYSA-M ipratropium bromide hydrate Chemical compound O.[Br-].O([C@H]1C[C@H]2CC[C@@H](C1)[N@@+]2(C)C(C)C)C(=O)C(CO)C1=CC=CC=C1 KEWHKYJURDBRMN-ZEODDXGYSA-M 0.000 description 1
- 239000003580 lung surfactant Substances 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000001902 propagating effect Effects 0.000 description 1
- 229960002720 reproterol Drugs 0.000 description 1
- WVLAAKXASPCBGT-UHFFFAOYSA-N reproterol Chemical compound C1=2C(=O)N(C)C(=O)N(C)C=2N=CN1CCCNCC(O)C1=CC(O)=CC(O)=C1 WVLAAKXASPCBGT-UHFFFAOYSA-N 0.000 description 1
- 229950004432 rofleponide Drugs 0.000 description 1
- IXTCZMJQGGONPY-XJAYAHQCSA-N rofleponide Chemical compound C1([C@@H](F)C2)=CC(=O)CC[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3O[C@@H](CCC)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O IXTCZMJQGGONPY-XJAYAHQCSA-N 0.000 description 1
- -1 rrofleponide Chemical compound 0.000 description 1
- 229960002052 salbutamol Drugs 0.000 description 1
- 229960004017 salmeterol Drugs 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 229940035073 symbicort Drugs 0.000 description 1
- PJFHZKIDENOSJB-JIVDDGRNSA-N symbicort inhalation aerosol Chemical compound C1=CC(OC)=CC=C1C[C@H](C)NC[C@@H](O)C1=CC=C(O)C(NC=O)=C1.C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O PJFHZKIDENOSJB-JIVDDGRNSA-N 0.000 description 1
- 229960000195 terbutaline Drugs 0.000 description 1
- 229960005105 terbutaline sulfate Drugs 0.000 description 1
- KFVSLSTULZVNPG-UHFFFAOYSA-N terbutaline sulfate Chemical compound [O-]S([O-])(=O)=O.CC(C)(C)[NH2+]CC(O)C1=CC(O)=CC(O)=C1.CC(C)(C)[NH2+]CC(O)C1=CC(O)=CC(O)=C1 KFVSLSTULZVNPG-UHFFFAOYSA-N 0.000 description 1
- IMCGHZIGRANKHV-AJNGGQMLSA-N tert-butyl (3s,5s)-2-oxo-5-[(2s,4s)-5-oxo-4-propan-2-yloxolan-2-yl]-3-propan-2-ylpyrrolidine-1-carboxylate Chemical compound O1C(=O)[C@H](C(C)C)C[C@H]1[C@H]1N(C(=O)OC(C)(C)C)C(=O)[C@H](C(C)C)C1 IMCGHZIGRANKHV-AJNGGQMLSA-N 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
- A61M15/0025—Mouthpieces therefor with caps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0046—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier
- A61M15/0048—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier the dosages being arranged in a plane, e.g. on diskettes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2206/00—Characteristics of a physical parameter; associated device therefor
- A61M2206/10—Flow characteristics
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Anesthesiology (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Animal Behavior & Ethology (AREA)
- Pulmonology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biophysics (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US1538307P | 2007-12-20 | 2007-12-20 | |
| US61/015,383 | 2007-12-20 | ||
| PCT/SE2008/051488 WO2009082341A1 (en) | 2007-12-20 | 2008-12-18 | Device and method for deaggregating powder 854 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2010126836A RU2010126836A (ru) | 2012-01-27 |
| RU2484852C2 true RU2484852C2 (ru) | 2013-06-20 |
Family
ID=40801454
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010126836/14A RU2484852C2 (ru) | 2007-12-20 | 2008-12-18 | Устройство и способ дезагрегации порошкообразного средства 854 |
| RU2010126838/14A RU2492876C2 (ru) | 2007-12-20 | 2008-12-18 | Устройство раздачи и способ захвата порошкообразного средства в потоке воздуха 537 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010126838/14A RU2492876C2 (ru) | 2007-12-20 | 2008-12-18 | Устройство раздачи и способ захвата порошкообразного средства в потоке воздуха 537 |
Country Status (12)
| Country | Link |
|---|---|
| US (5) | US9283337B2 (enExample) |
| EP (2) | EP2224984A4 (enExample) |
| JP (3) | JP5646341B2 (enExample) |
| KR (2) | KR20100103834A (enExample) |
| CN (2) | CN101965208B (enExample) |
| AU (3) | AU2008341217B2 (enExample) |
| BR (2) | BRPI0821648A2 (enExample) |
| CA (2) | CA2710274A1 (enExample) |
| MX (2) | MX2010006657A (enExample) |
| NZ (2) | NZ586105A (enExample) |
| RU (2) | RU2484852C2 (enExample) |
| WO (2) | WO2009082343A1 (enExample) |
Families Citing this family (43)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| AU2003220125B2 (en) | 2002-03-20 | 2006-06-15 | Mannkind Corporation | Inhalation apparatus |
| PL1786784T3 (pl) | 2004-08-20 | 2011-04-29 | Mannkind Corp | Kataliza syntezy diketopiperazyn |
| EP2314298B1 (en) | 2004-08-23 | 2015-05-27 | MannKind Corporation | Microparticles comprising diketopiperazine salts for drug delivery |
| AU2006290870B2 (en) | 2005-09-14 | 2013-02-28 | Mannkind Corporation | Method of drug formulation based on increasing the affinity of active agents for crystalline microparticle surfaces |
| DK1986679T3 (da) | 2006-02-22 | 2017-11-20 | Mannkind Corp | Fremgangsmåde til forbedring af mikropartiklers farmaceutiske egenskaber omfattende diketopiperazin og et aktivt indholdsstof |
| TW200833670A (en) * | 2006-12-20 | 2008-08-16 | Astrazeneca Ab | Novel compounds 569 |
| WO2009079078A1 (en) | 2007-12-14 | 2009-06-25 | Labogroup S.A.S. | Delivering aerosolizable food products |
| MX2010006657A (es) | 2007-12-20 | 2010-10-05 | Astrazeneca Ab | Dispositivo y metodo para desagregar polvo 854. |
| CN103252007B (zh) | 2008-06-13 | 2016-06-22 | 曼金德公司 | 干粉吸入器和用于药物输送的系统 |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| JP5479465B2 (ja) | 2008-06-20 | 2014-04-23 | マンカインド コーポレイション | 吸入努力をリアルタイムにプロファイルする対話式機器および方法 |
| TWI614024B (zh) | 2008-08-11 | 2018-02-11 | 曼凱公司 | 超快起作用胰島素之用途 |
| US8314106B2 (en) | 2008-12-29 | 2012-11-20 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| EP2676695A3 (en) | 2009-03-11 | 2017-03-01 | MannKind Corporation | Apparatus, system and method for measuring resistance of an inhaler |
| KR101875969B1 (ko) | 2009-06-12 | 2018-07-06 | 맨카인드 코포레이션 | 한정된 비표면적을 갖는 디케토피페라진 마이크로입자 |
| JP2012531961A (ja) * | 2009-07-01 | 2012-12-13 | アストラゼネカ・アクチエボラーグ | 粉末を気流に引き込むためのディスペンサーおよび方法 |
| WO2011056889A1 (en) | 2009-11-03 | 2011-05-12 | Mannkind Corporation | An apparatus and method for simulating inhalation efforts |
| RU2531455C2 (ru) | 2010-06-21 | 2014-10-20 | Маннкайнд Корпорейшн | Системы и способы доставки сухих порошковых лекарств |
| WO2012010877A2 (en) | 2010-07-21 | 2012-01-26 | Astrazeneca Ab | New device |
| ES2535701T3 (es) | 2010-07-21 | 2015-05-14 | Astrazeneca Ab | Inhalador |
| CN105667994B (zh) * | 2011-04-01 | 2018-04-06 | 曼金德公司 | 用于药物药盒的泡罩包装 |
| WO2012174472A1 (en) | 2011-06-17 | 2012-12-20 | Mannkind Corporation | High capacity diketopiperazine microparticles |
| WO2013063160A1 (en) | 2011-10-24 | 2013-05-02 | Mannkind Corporation | Methods and compositions for treating pain |
| US9854839B2 (en) | 2012-01-31 | 2018-01-02 | Altria Client Services Llc | Electronic vaping device and method |
| ITBO20120185A1 (it) * | 2012-04-06 | 2013-10-07 | Emodial S R L | Medicazione antimicrobica, composizione antimicrobica e relativo uso |
| SG11201500218VA (en) * | 2012-07-12 | 2015-03-30 | Mannkind Corp | Dry powder drug delivery systems and methods |
| EP2911690A1 (en) | 2012-10-26 | 2015-09-02 | MannKind Corporation | Inhalable influenza vaccine compositions and methods |
| EP2970149B1 (en) | 2013-03-15 | 2019-08-21 | MannKind Corporation | Microcrystalline diketopiperazine compositions and methods |
| CN105451716A (zh) | 2013-07-18 | 2016-03-30 | 曼金德公司 | 热稳定性干粉药物组合物和方法 |
| WO2015021064A1 (en) | 2013-08-05 | 2015-02-12 | Mannkind Corporation | Insufflation apparatus and methods |
| EP3041555A4 (en) * | 2013-09-04 | 2017-03-08 | 3M Innovative Properties Company | Dry-powder inhaler and method |
| PL3104853T3 (pl) | 2014-02-10 | 2020-05-18 | Respivant Sciences Gmbh | Leczenie stabilizatorami komórek tucznych zaburzeń ogólnoustrojowych |
| CA2938996A1 (en) | 2014-02-10 | 2015-08-13 | Patara Pharma, LLC | Methods for the treatment of lung diseases with mast cell stabilizers |
| US10307464B2 (en) | 2014-03-28 | 2019-06-04 | Mannkind Corporation | Use of ultrarapid acting insulin |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| DE102014017409B4 (de) * | 2014-11-26 | 2016-06-09 | Klaus Dieter Beller | Einzeldosis-Pulverinhalator und Verfahren zu dessen Herstellung |
| SE539111C2 (en) * | 2015-06-03 | 2017-04-11 | Iconovo Ab | Single dose dry powder inhaler |
| EP3331522A1 (en) | 2015-08-07 | 2018-06-13 | Patara Pharma LLC | Methods for the treatment of mast cell related disorders with mast cell stabilizers |
| US10265296B2 (en) | 2015-08-07 | 2019-04-23 | Respivant Sciences Gmbh | Methods for the treatment of systemic disorders treatable with mast cell stabilizers, including mast cell related disorders |
| AU2017321495A1 (en) | 2016-08-31 | 2019-03-21 | Respivant Sciences Gmbh | Cromolyn compositions for treatment of chronic cough due to idiopathic pulmonary fibrosis |
| AU2017339366A1 (en) | 2016-10-07 | 2019-04-11 | Respivant Sciences Gmbh | Cromolyn compositions for treatment of pulmonary fibrosis |
| CN111550475B (zh) * | 2020-03-27 | 2021-12-07 | 中国航天空气动力技术研究院 | 一种用于边界层转捩控制的“⊥”型凹腔结构 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU651910B2 (en) * | 1991-08-15 | 1994-08-04 | Franco Del Bon | Inhaler |
| US20030192539A1 (en) * | 2002-04-12 | 2003-10-16 | Mattias Myrman | De-aggregating and dispersing dry medicament powder into air |
| US20040069303A1 (en) * | 2000-10-27 | 2004-04-15 | David Brown | Dry powder inhaler |
| RU2275900C2 (ru) * | 2000-06-09 | 2006-05-10 | Адвансд Инхалейшен Рисёч, Инк. | Способ высокоэффективной доставки аэрозоля с большой терапевтической массой |
Family Cites Families (88)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2214032A (en) * | 1939-06-23 | 1940-09-10 | Walter B Stewart | Apparatus for administering powdered aluminum |
| DE2346914C3 (de) | 1973-09-18 | 1980-10-16 | Paul Ritzau Pari-Werk, Gmbh & Co, 8130 Starnberg | Inhalator für pulverförmige Substanzen |
| US3872970A (en) | 1974-01-11 | 1975-03-25 | Lilly Co Eli | Child-resistant blister package |
| IT1017153B (it) | 1974-07-15 | 1977-07-20 | Isf Spa | Apparecchio per inalazioni |
| US4014336A (en) | 1975-01-13 | 1977-03-29 | Syntex Puerto Rico, Inc. | Inhalation device |
| US3948264A (en) | 1975-05-21 | 1976-04-06 | Mead Johnson & Company | Inhalation device |
| GB1521000A (en) | 1975-06-13 | 1978-08-09 | Syntex Puerto Rico Inc | Inhalation device |
| GB1598081A (en) | 1977-02-10 | 1981-09-16 | Allen & Hanburys Ltd | Inhaler device for dispensing medicaments |
| CH656311A5 (de) | 1979-10-30 | 1986-06-30 | Riker Laboratories Inc | Durch atemluft betaetigte vorrichtung zum oralen inhalieren eines medikamentes in pulverform. |
| ES8206980A1 (es) | 1980-10-30 | 1982-09-01 | Riker Laboratories Inc | Un dispositivo para facilitar la inhalacion oral de medica- mentos en forma de polvo |
| US4849606A (en) | 1987-12-23 | 1989-07-18 | S. C. Johnson & Son, Inc. | Tamper-resistant container utilizing a flexible seal |
| US4946038A (en) | 1989-12-20 | 1990-08-07 | Rolland Eaton | Medicine container and cover therefor |
| GB9004781D0 (en) | 1990-03-02 | 1990-04-25 | Glaxo Group Ltd | Device |
| SE9002895D0 (sv) * | 1990-09-12 | 1990-09-12 | Astra Ab | Inhalation devices for dispensing powders i |
| GB9021433D0 (en) * | 1990-10-02 | 1990-11-14 | Atomic Energy Authority Uk | Power inhaler |
| US5042472A (en) | 1990-10-15 | 1991-08-27 | Merck & Co., Inc. | Powder inhaler device |
| US5469843A (en) | 1991-11-12 | 1995-11-28 | Minnesota Mining And Manufacturing Company | Inhalation device |
| GB2264237A (en) | 1992-02-05 | 1993-08-25 | Robert Edward Newell | An inhaler |
| DE4208880A1 (de) * | 1992-03-19 | 1993-09-23 | Boehringer Ingelheim Kg | Separator fuer pulverinhalatoren |
| DE59303719D1 (de) * | 1992-06-05 | 1996-10-17 | Hoechst Ag | Inhalator |
| US5437271A (en) * | 1993-04-06 | 1995-08-01 | Minnesota Mining And Manufacturing Company | Deagglomerators for dry powder inhalers |
| US5921237A (en) | 1995-04-24 | 1999-07-13 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| SE9502800D0 (sv) | 1995-08-10 | 1995-08-10 | Astra Ab | Disposable inhaler |
| US6065472A (en) | 1996-01-03 | 2000-05-23 | Glaxo Wellcome Inc. | Multidose powder inhalation device |
| US5694920A (en) | 1996-01-25 | 1997-12-09 | Abrams; Andrew L. | Inhalation device |
| US5699789A (en) * | 1996-03-11 | 1997-12-23 | Hendricks; Mark R. | Dry powder inhaler |
| SE9700421D0 (sv) * | 1997-02-07 | 1997-02-07 | Astra Ab | Single dose inhaler I |
| SE9700423D0 (sv) * | 1997-02-07 | 1997-02-07 | Astra Ab | Disposable inhaler |
| US6006747A (en) | 1997-03-20 | 1999-12-28 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| CA2212430A1 (en) * | 1997-08-07 | 1999-02-07 | George Volgyesi | Inhalation device |
| NL1008031C2 (nl) | 1998-01-15 | 1999-07-21 | Pharmachemie Bv | Inrichting voor het inhaleren van medicament. |
| US7371254B2 (en) * | 1998-01-23 | 2008-05-13 | Innercool Therapies, Inc. | Medical procedure |
| US6234169B1 (en) | 1998-08-14 | 2001-05-22 | Arthur Slutsky | Inhaler |
| GB9821791D0 (en) * | 1998-10-06 | 1998-12-02 | Sgs Thomson Microelectronics | Data transfer |
| GB9905538D0 (en) * | 1999-03-10 | 1999-05-05 | Glaxo Group Ltd | A device |
| GB9909357D0 (en) * | 1999-04-24 | 1999-06-16 | Glaxo Group Ltd | Medicament carrier |
| SE9904706D0 (sv) | 1999-12-21 | 1999-12-21 | Astra Ab | An inhalation device |
| US6810872B1 (en) | 1999-12-10 | 2004-11-02 | Unisia Jecs Corporation | Inhalant medicator |
| US6427688B1 (en) | 2000-02-01 | 2002-08-06 | Dura Pharmaceuticals, Icn. | Dry powder inhaler |
| PL201275B1 (pl) * | 2000-03-10 | 2009-03-31 | Univ North Carolina | Inhalator suchego proszku |
| US6971383B2 (en) * | 2001-01-24 | 2005-12-06 | University Of North Carolina At Chapel Hill | Dry powder inhaler devices, multi-dose dry powder drug packages, control systems, and associated methods |
| US6948494B1 (en) | 2000-05-10 | 2005-09-27 | Innovative Devices, Llc. | Medicament container with same side airflow inlet and outlet and method of use |
| AR028746A1 (es) | 2000-06-23 | 2003-05-21 | Norton Health Care Ltd | Cartucho de dosis previamente medidas para inhalador de polvo seco accionado por la respiracion, el inhalador y un metodo de provision de dosis previamente medidas de polvo seco |
| US7080644B2 (en) | 2000-06-28 | 2006-07-25 | Microdose Technologies, Inc. | Packaging and delivery of pharmaceuticals and drugs |
| AUPR020400A0 (en) | 2000-09-19 | 2000-10-12 | Glaxo Wellcome Australia Ltd | Inhalation device |
| DE10057832C1 (de) * | 2000-11-21 | 2002-02-21 | Hartmann Paul Ag | Blutanalysegerät |
| US6722364B2 (en) * | 2001-01-12 | 2004-04-20 | Becton, Dickinson And Company | Medicament inhalation delivery devices and methods for using the same |
| EG24184A (en) * | 2001-06-15 | 2008-10-08 | Otsuka Pharma Co Ltd | Dry powder inhalation system for transpulmonary |
| US6681768B2 (en) * | 2001-06-22 | 2004-01-27 | Sofotec Gmbh & Co. Kg | Powder formulation disintegrating system and method for dry powder inhalers |
| WO2003024514A1 (en) | 2001-09-19 | 2003-03-27 | Advent Pharmaceuticals Pty Ltd | An inhaler |
| SE0104251D0 (sv) | 2001-12-14 | 2001-12-14 | Astrazeneca Ab | Novel compounds |
| SE525027C2 (sv) * | 2002-04-12 | 2004-11-16 | Microdrug Ag | Anordning utgörande en pulverlufthyvel |
| AU2003267809A1 (en) | 2002-06-07 | 2003-12-22 | Sun Pharmaceutical Industries Limited | Powder inhaler |
| US6874647B2 (en) * | 2002-08-12 | 2005-04-05 | Owens-Illinois Closure Inc. | Plastic closure, closure and container package, and method of manufacture |
| BR0316924A (pt) * | 2002-12-02 | 2005-10-18 | Univ Alberta | Dispositivo e método para desaglomeração de pó para inalação |
| GB2401548B (en) | 2003-05-13 | 2005-07-13 | Boots Healthcare Int Ltd | Improvements relating to vaporisers |
| EP1488819A1 (en) | 2003-06-16 | 2004-12-22 | Rijksuniversiteit te Groningen | Dry powder inhaler and method for pulmonary inhalation of dry powder |
| GB0315509D0 (en) | 2003-07-02 | 2003-08-06 | Meridica Ltd | Dispensing device |
| UA90997C2 (ru) | 2003-07-09 | 2010-06-25 | Ціпла Лімітед | Устройство для ингаляции |
| GB0322544D0 (en) | 2003-09-26 | 2003-10-29 | Innovata Biomed Ltd | Apparatus |
| GB2407042B (en) | 2003-10-17 | 2007-10-24 | Vectura Ltd | Inhaler |
| MXPA06009516A (es) | 2004-02-24 | 2007-03-26 | Microdose Technologies Inc | Inhalador con sensor de flujo direccional. |
| SE0401654D0 (sv) * | 2004-06-24 | 2004-06-24 | Astrazeneca Ab | A support structure for a medicament |
| AU2005266789B2 (en) * | 2004-07-26 | 2010-11-25 | 1355540 Ontario Inc. | Powder inhaler featuring reduced compaction inhaler |
| CN101048407A (zh) | 2004-09-03 | 2007-10-03 | 普莱希科公司 | 双环杂芳基pde4b抑制剂 |
| GB0427028D0 (en) | 2004-12-09 | 2005-01-12 | Cambridge Consultants | Dry powder inhalers |
| GB0427856D0 (en) | 2004-12-20 | 2005-01-19 | Glaxo Group Ltd | Maniflod for use in medicament dispenser |
| JP2008540264A (ja) | 2005-05-02 | 2008-11-20 | アストラゼネカ・アクチエボラーグ | キャビティを開口するための構成と方法、薬剤パッケージ及び配布デバイス |
| US8763605B2 (en) | 2005-07-20 | 2014-07-01 | Manta Devices, Llc | Inhalation device |
| DE102005046645B3 (de) | 2005-09-29 | 2006-07-20 | Braunform Gmbh | Pulverinhalator |
| CA2626708A1 (en) | 2005-11-07 | 2007-05-31 | Alkermes, Inc. | Receptacle packaging with inhaler-accommodating geometry |
| EP1844806A1 (de) | 2006-04-13 | 2007-10-17 | Boehringer Ingelheim Pharma GmbH | Medikamenten-Ausgabevorrichtung, Medikamentenmagazin dafür, und Verfahren zur Entnahme eines Medikaments aus einer Medikamentenkammer |
| DE102006043637A1 (de) | 2006-05-18 | 2007-11-22 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Zerstäuber |
| GB0611656D0 (en) | 2006-06-13 | 2006-07-19 | Cambridge Consultants | Dry powder inhalers |
| US20090250058A1 (en) | 2006-07-14 | 2009-10-08 | Astrazeneca Ab | Inhalation System and Delivery Device for the Administration of a Drug in the Form of Dry Powder |
| KR20090030347A (ko) | 2006-07-19 | 2009-03-24 | 아스트라제네카 아베 | 신규 트리시클릭 스피로피페리딘 화합물, 이들의 합성 및 이들의 케모킨 수용체 활성의 조절제로서의 용도 |
| GB0704928D0 (en) | 2007-03-14 | 2007-04-25 | Cambridge Consultants | Dry powder inhalers |
| EP3453418A1 (en) | 2007-07-06 | 2019-03-13 | Manta Devices, LLC | Delivery device and related methods |
| WO2009008832A1 (en) | 2007-07-12 | 2009-01-15 | Astrazeneca Ab | Medicament containing structure, method and inhalation device comprising such structure |
| WO2009046072A1 (en) | 2007-10-02 | 2009-04-09 | Baxter International Inc | Dry powder inhaler |
| MX2010006657A (es) | 2007-12-20 | 2010-10-05 | Astrazeneca Ab | Dispositivo y metodo para desagregar polvo 854. |
| CA2711907A1 (en) | 2008-01-23 | 2009-07-30 | Astrazeneca Ab | A medicament -containing dispenser provided with a display for presenting indicia to a user |
| CN103252007B (zh) | 2008-06-13 | 2016-06-22 | 曼金德公司 | 干粉吸入器和用于药物输送的系统 |
| BRPI0920671A2 (pt) | 2008-10-08 | 2019-07-09 | Astrazeneca Ab | inalador com meio indicador audível |
| EP2341967A4 (en) | 2008-10-08 | 2016-03-09 | Astrazeneca Ab | INHALER WITH INDEXATION BASED ON MOVEMENT OF COVERAGE |
| JP2012531961A (ja) | 2009-07-01 | 2012-12-13 | アストラゼネカ・アクチエボラーグ | 粉末を気流に引き込むためのディスペンサーおよび方法 |
| WO2012010877A2 (en) | 2010-07-21 | 2012-01-26 | Astrazeneca Ab | New device |
| ES2535701T3 (es) | 2010-07-21 | 2015-05-14 | Astrazeneca Ab | Inhalador |
-
2008
- 2008-12-18 MX MX2010006657A patent/MX2010006657A/es not_active Application Discontinuation
- 2008-12-18 NZ NZ586105A patent/NZ586105A/xx not_active IP Right Cessation
- 2008-12-18 CA CA2710274A patent/CA2710274A1/en not_active Abandoned
- 2008-12-18 KR KR1020107016028A patent/KR20100103834A/ko not_active Abandoned
- 2008-12-18 RU RU2010126836/14A patent/RU2484852C2/ru not_active IP Right Cessation
- 2008-12-18 MX MX2010006658A patent/MX2010006658A/es not_active Application Discontinuation
- 2008-12-18 EP EP08864819.1A patent/EP2224984A4/en not_active Withdrawn
- 2008-12-18 KR KR1020107016071A patent/KR101503560B1/ko not_active Expired - Fee Related
- 2008-12-18 JP JP2010539382A patent/JP5646341B2/ja not_active Expired - Fee Related
- 2008-12-18 WO PCT/SE2008/051490 patent/WO2009082343A1/en not_active Ceased
- 2008-12-18 EP EP08864465.3A patent/EP2224983B1/en not_active Not-in-force
- 2008-12-18 NZ NZ586106A patent/NZ586106A/xx not_active IP Right Cessation
- 2008-12-18 RU RU2010126838/14A patent/RU2492876C2/ru not_active IP Right Cessation
- 2008-12-18 BR BRPI0821648-7A patent/BRPI0821648A2/pt not_active IP Right Cessation
- 2008-12-18 US US12/809,155 patent/US9283337B2/en not_active Expired - Fee Related
- 2008-12-18 AU AU2008341217A patent/AU2008341217B2/en not_active Ceased
- 2008-12-18 CN CN2008801262201A patent/CN101965208B/zh not_active Expired - Fee Related
- 2008-12-18 JP JP2010539380A patent/JP2011507593A/ja active Pending
- 2008-12-18 CN CN200880126221.6A patent/CN101939040B/zh not_active Expired - Fee Related
- 2008-12-18 BR BRPI0821726-2A patent/BRPI0821726A2/pt not_active IP Right Cessation
- 2008-12-18 CA CA2708190A patent/CA2708190A1/en not_active Abandoned
- 2008-12-18 US US12/809,157 patent/US8479729B2/en not_active Expired - Fee Related
- 2008-12-18 WO PCT/SE2008/051488 patent/WO2009082341A1/en not_active Ceased
- 2008-12-18 AU AU2008341216A patent/AU2008341216B2/en not_active Ceased
-
2009
- 2009-07-01 US US12/496,525 patent/US8578933B2/en not_active Expired - Fee Related
-
2010
- 2010-11-05 US US12/940,683 patent/US8151793B2/en not_active Expired - Fee Related
-
2011
- 2011-04-05 AU AU2011201528A patent/AU2011201528B2/en not_active Ceased
-
2013
- 2013-10-11 US US14/051,975 patent/US20140096771A1/en not_active Abandoned
-
2014
- 2014-04-24 JP JP2014090216A patent/JP2014158950A/ja active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU651910B2 (en) * | 1991-08-15 | 1994-08-04 | Franco Del Bon | Inhaler |
| RU2275900C2 (ru) * | 2000-06-09 | 2006-05-10 | Адвансд Инхалейшен Рисёч, Инк. | Способ высокоэффективной доставки аэрозоля с большой терапевтической массой |
| US20040069303A1 (en) * | 2000-10-27 | 2004-04-15 | David Brown | Dry powder inhaler |
| US20030192539A1 (en) * | 2002-04-12 | 2003-10-16 | Mattias Myrman | De-aggregating and dispersing dry medicament powder into air |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2484852C2 (ru) | Устройство и способ дезагрегации порошкообразного средства 854 | |
| JP5651233B2 (ja) | 簡易なカプセル・ベースの吸入器 | |
| US8109267B2 (en) | Simple inhaler | |
| TWI517868B (zh) | 吸入器(二) | |
| EP3099364B1 (en) | Powder compartment for high dosage drug delivery | |
| JP2016530030A (ja) | ドライパウダー吸入器及び方法 | |
| MX2011013368A (es) | Dispensador y metodo para arrastrar polvo en un flujo de aire. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20151219 |