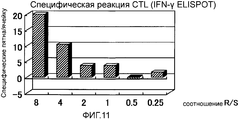
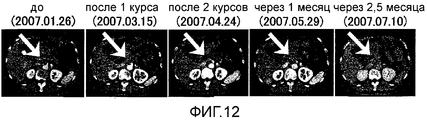
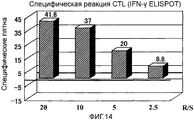
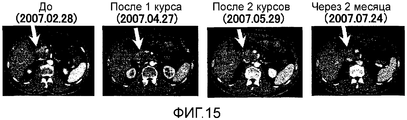
RU2472522C2 - Комбинированная терапия рака поджелудочной железы с использованием антигенного пептида и химиотерапевтического средства - Google Patents
Комбинированная терапия рака поджелудочной железы с использованием антигенного пептида и химиотерапевтического средства Download PDFInfo
- Publication number
- RU2472522C2 RU2472522C2 RU2010111139/15A RU2010111139A RU2472522C2 RU 2472522 C2 RU2472522 C2 RU 2472522C2 RU 2010111139/15 A RU2010111139/15 A RU 2010111139/15A RU 2010111139 A RU2010111139 A RU 2010111139A RU 2472522 C2 RU2472522 C2 RU 2472522C2
- Authority
- RU
- Russia
- Prior art keywords
- seq
- amino acid
- substituted
- acid sequence
- peptides
- Prior art date
Links
- 206010061902 Pancreatic neoplasm Diseases 0.000 title claims abstract description 69
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 title claims abstract description 67
- 208000008443 pancreatic carcinoma Diseases 0.000 title claims abstract description 67
- 201000002528 pancreatic cancer Diseases 0.000 title claims abstract description 66
- 239000002246 antineoplastic agent Substances 0.000 title claims abstract description 39
- 229940127089 cytotoxic agent Drugs 0.000 title claims abstract description 27
- 108090000765 processed proteins & peptides Proteins 0.000 title claims description 203
- 102000004196 processed proteins & peptides Human genes 0.000 title claims description 134
- 108091007433 antigens Proteins 0.000 title abstract description 18
- 239000000427 antigen Substances 0.000 title abstract description 17
- 102000036639 antigens Human genes 0.000 title abstract description 17
- 238000002648 combination therapy Methods 0.000 title abstract description 5
- 229920001184 polypeptide Polymers 0.000 title description 7
- 229960005277 gemcitabine Drugs 0.000 claims abstract description 87
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 claims abstract description 85
- 238000000034 method Methods 0.000 claims abstract description 41
- 230000001939 inductive effect Effects 0.000 claims abstract description 27
- 230000001225 therapeutic effect Effects 0.000 claims abstract description 25
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 17
- 239000003814 drug Substances 0.000 claims abstract description 10
- 239000003795 chemical substances by application Substances 0.000 claims abstract description 7
- 206010028980 Neoplasm Diseases 0.000 claims description 132
- 150000001413 amino acids Chemical group 0.000 claims description 81
- 201000011510 cancer Diseases 0.000 claims description 63
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 claims description 43
- 229940024606 amino acid Drugs 0.000 claims description 41
- 238000011282 treatment Methods 0.000 claims description 34
- 238000006467 substitution reaction Methods 0.000 claims description 32
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Chemical group CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 28
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical group CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 claims description 27
- 229930182817 methionine Chemical group 0.000 claims description 27
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical group CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 19
- 150000003839 salts Chemical class 0.000 claims description 19
- 125000001433 C-terminal amino-acid group Chemical group 0.000 claims description 18
- 108010013476 HLA-A24 Antigen Proteins 0.000 claims description 18
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical group C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 claims description 18
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Chemical group C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 claims description 18
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 18
- COLNVLDHVKWLRT-QMMMGPOBSA-N phenylalanine group Chemical group N[C@@H](CC1=CC=CC=C1)C(=O)O COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 18
- 229940002612 prodrug Drugs 0.000 claims description 17
- 239000000651 prodrug Substances 0.000 claims description 17
- 108010081641 arginyl-phenylalanyl-valyl-prolyl-aspartyl-glycyl-asparagyl-arginyl-isoleucine Proteins 0.000 claims description 13
- -1 respectively Substances 0.000 claims description 12
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 11
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical group CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 claims description 9
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical group OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 claims description 9
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 claims description 9
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Chemical group CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 claims description 9
- 229960000310 isoleucine Drugs 0.000 claims description 9
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 9
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Chemical group OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 claims description 9
- 239000004474 valine Substances 0.000 claims description 9
- 239000004480 active ingredient Substances 0.000 claims description 8
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 8
- 230000002708 enhancing effect Effects 0.000 claims description 7
- 239000003937 drug carrier Substances 0.000 claims description 3
- 229940124597 therapeutic agent Drugs 0.000 claims description 2
- GOJUJUVQIVIZAV-UHFFFAOYSA-N 2-amino-4,6-dichloropyrimidine-5-carbaldehyde Chemical group NC1=NC(Cl)=C(C=O)C(Cl)=N1 GOJUJUVQIVIZAV-UHFFFAOYSA-N 0.000 claims 28
- 101000851007 Homo sapiens Vascular endothelial growth factor receptor 2 Proteins 0.000 abstract description 42
- 230000000694 effects Effects 0.000 abstract description 26
- 231100000433 cytotoxic Toxicity 0.000 abstract description 2
- 230000001472 cytotoxic effect Effects 0.000 abstract description 2
- 238000011275 oncology therapy Methods 0.000 abstract description 2
- 239000000126 substance Substances 0.000 abstract description 2
- 230000001093 anti-cancer Effects 0.000 abstract 1
- 230000003389 potentiating effect Effects 0.000 abstract 1
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 description 52
- 210000004027 cell Anatomy 0.000 description 32
- 229960005486 vaccine Drugs 0.000 description 31
- 230000000259 anti-tumor effect Effects 0.000 description 29
- 238000002255 vaccination Methods 0.000 description 27
- 230000000890 antigenic effect Effects 0.000 description 22
- 210000001744 T-lymphocyte Anatomy 0.000 description 21
- 125000003275 alpha amino acid group Chemical group 0.000 description 21
- 238000006243 chemical reaction Methods 0.000 description 21
- 230000002411 adverse Effects 0.000 description 20
- 108090000623 proteins and genes Proteins 0.000 description 19
- 230000004044 response Effects 0.000 description 19
- 102000004169 proteins and genes Human genes 0.000 description 18
- 238000004458 analytical method Methods 0.000 description 14
- 230000003247 decreasing effect Effects 0.000 description 14
- 238000002560 therapeutic procedure Methods 0.000 description 14
- 108010053099 Vascular Endothelial Growth Factor Receptor-2 Proteins 0.000 description 13
- 210000002889 endothelial cell Anatomy 0.000 description 13
- 201000010099 disease Diseases 0.000 description 12
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 12
- 239000012636 effector Substances 0.000 description 12
- 210000003289 regulatory T cell Anatomy 0.000 description 12
- 230000009885 systemic effect Effects 0.000 description 12
- 241000282414 Homo sapiens Species 0.000 description 11
- 238000002512 chemotherapy Methods 0.000 description 11
- 230000001900 immune effect Effects 0.000 description 10
- 238000002347 injection Methods 0.000 description 10
- 239000007924 injection Substances 0.000 description 10
- 238000004519 manufacturing process Methods 0.000 description 10
- 238000012544 monitoring process Methods 0.000 description 10
- 230000004083 survival effect Effects 0.000 description 10
- 230000028993 immune response Effects 0.000 description 8
- 239000000203 mixture Substances 0.000 description 8
- 208000004235 neutropenia Diseases 0.000 description 8
- 125000000539 amino acid group Chemical group 0.000 description 7
- 210000000612 antigen-presenting cell Anatomy 0.000 description 7
- 210000003162 effector t lymphocyte Anatomy 0.000 description 7
- 239000012634 fragment Substances 0.000 description 7
- 210000001519 tissue Anatomy 0.000 description 7
- 208000008771 Lymphadenopathy Diseases 0.000 description 6
- 239000002671 adjuvant Substances 0.000 description 6
- 238000002591 computed tomography Methods 0.000 description 6
- 210000001808 exosome Anatomy 0.000 description 6
- 208000018555 lymphatic system disease Diseases 0.000 description 6
- 108091005601 modified peptides Proteins 0.000 description 6
- 239000000825 pharmaceutical preparation Substances 0.000 description 6
- 238000010186 staining Methods 0.000 description 6
- 239000000439 tumor marker Substances 0.000 description 6
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 5
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 5
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 description 5
- 206010027457 Metastases to liver Diseases 0.000 description 5
- 210000004204 blood vessel Anatomy 0.000 description 5
- 238000009566 cancer vaccine Methods 0.000 description 5
- 229940022399 cancer vaccine Drugs 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 150000007523 nucleic acids Chemical class 0.000 description 5
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 4
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 238000005056 compaction Methods 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- OKKDEIYWILRZIA-OSZBKLCCSA-N gemcitabine hydrochloride Chemical class [H+].[Cl-].O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 OKKDEIYWILRZIA-OSZBKLCCSA-N 0.000 description 4
- 238000009169 immunotherapy Methods 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 210000004698 lymphocyte Anatomy 0.000 description 4
- 108020004707 nucleic acids Proteins 0.000 description 4
- 102000039446 nucleic acids Human genes 0.000 description 4
- 229940023041 peptide vaccine Drugs 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 238000002271 resection Methods 0.000 description 4
- 230000000638 stimulation Effects 0.000 description 4
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 4
- 210000004881 tumor cell Anatomy 0.000 description 4
- 206010060933 Adverse event Diseases 0.000 description 3
- 206010006187 Breast cancer Diseases 0.000 description 3
- 208000026310 Breast neoplasm Diseases 0.000 description 3
- 102100027207 CD27 antigen Human genes 0.000 description 3
- 101000914511 Homo sapiens CD27 antigen Proteins 0.000 description 3
- 102100037850 Interferon gamma Human genes 0.000 description 3
- 108010074328 Interferon-gamma Proteins 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 3
- 206010029113 Neovascularisation Diseases 0.000 description 3
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 3
- 108091008605 VEGF receptors Proteins 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 239000012472 biological sample Substances 0.000 description 3
- 230000007012 clinical effect Effects 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 210000004443 dendritic cell Anatomy 0.000 description 3
- ZAJHBCDPAUKEPU-GIKXZWSFSA-N elpamotide Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCN=C(N)N)CC1=CC=CC=C1 ZAJHBCDPAUKEPU-GIKXZWSFSA-N 0.000 description 3
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 229960002949 fluorouracil Drugs 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 231100000226 haematotoxicity Toxicity 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 208000019423 liver disease Diseases 0.000 description 3
- 230000005976 liver dysfunction Effects 0.000 description 3
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 3
- 238000011321 prophylaxis Methods 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 230000008961 swelling Effects 0.000 description 3
- 238000007910 systemic administration Methods 0.000 description 3
- 238000013519 translation Methods 0.000 description 3
- 230000005747 tumor angiogenesis Effects 0.000 description 3
- INZOTETZQBPBCE-NYLDSJSYSA-N 3-sialyl lewis Chemical compound O[C@H]1[C@H](O)[C@H](O)[C@H](C)O[C@H]1O[C@H]([C@H](O)CO)[C@@H]([C@@H](NC(C)=O)C=O)O[C@H]1[C@H](O)[C@@H](O[C@]2(O[C@H]([C@H](NC(C)=O)[C@@H](O)C2)[C@H](O)[C@H](O)CO)C(O)=O)[C@@H](O)[C@@H](CO)O1 INZOTETZQBPBCE-NYLDSJSYSA-N 0.000 description 2
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 2
- 101710186708 Agglutinin Proteins 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 2
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 2
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 2
- 108010092160 Dactinomycin Proteins 0.000 description 2
- 229940124226 Farnesyltransferase inhibitor Drugs 0.000 description 2
- 102000005720 Glutathione transferase Human genes 0.000 description 2
- 108010070675 Glutathione transferase Proteins 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101710146024 Horcolin Proteins 0.000 description 2
- 101710189395 Lectin Proteins 0.000 description 2
- 101710175625 Maltose/maltodextrin-binding periplasmic protein Proteins 0.000 description 2
- 101710179758 Mannose-specific lectin Proteins 0.000 description 2
- 101710150763 Mannose-specific lectin 1 Proteins 0.000 description 2
- 101710150745 Mannose-specific lectin 2 Proteins 0.000 description 2
- 206010030113 Oedema Diseases 0.000 description 2
- 229930012538 Paclitaxel Natural products 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- 102000016549 Vascular Endothelial Growth Factor Receptor-2 Human genes 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 239000000910 agglutinin Substances 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000000711 cancerogenic effect Effects 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 239000000470 constituent Substances 0.000 description 2
- 239000003433 contraceptive agent Substances 0.000 description 2
- 230000002254 contraceptive effect Effects 0.000 description 2
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 2
- 229960004397 cyclophosphamide Drugs 0.000 description 2
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- VLCYCQAOQCDTCN-UHFFFAOYSA-N eflornithine Chemical compound NCCCC(N)(C(F)F)C(O)=O VLCYCQAOQCDTCN-UHFFFAOYSA-N 0.000 description 2
- 230000007717 exclusion Effects 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- CHPZKNULDCNCBW-UHFFFAOYSA-N gallium nitrate Chemical compound [Ga+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O CHPZKNULDCNCBW-UHFFFAOYSA-N 0.000 description 2
- 210000001035 gastrointestinal tract Anatomy 0.000 description 2
- 229940020967 gemzar Drugs 0.000 description 2
- 210000002767 hepatic artery Anatomy 0.000 description 2
- 229960001101 ifosfamide Drugs 0.000 description 2
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- 229960001428 mercaptopurine Drugs 0.000 description 2
- 230000001394 metastastic effect Effects 0.000 description 2
- 206010061289 metastatic neoplasm Diseases 0.000 description 2
- 229960004857 mitomycin Drugs 0.000 description 2
- 229960001156 mitoxantrone Drugs 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 229960001592 paclitaxel Drugs 0.000 description 2
- 210000005259 peripheral blood Anatomy 0.000 description 2
- 239000011886 peripheral blood Substances 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 238000010837 poor prognosis Methods 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 238000004393 prognosis Methods 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 239000003528 protein farnesyltransferase inhibitor Substances 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- PVYJZLYGTZKPJE-UHFFFAOYSA-N streptonigrin Chemical compound C=1C=C2C(=O)C(OC)=C(N)C(=O)C2=NC=1C(C=1N)=NC(C(O)=O)=C(C)C=1C1=CC=C(OC)C(OC)=C1O PVYJZLYGTZKPJE-UHFFFAOYSA-N 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 230000008685 targeting Effects 0.000 description 2
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 2
- 229960001196 thiotepa Drugs 0.000 description 2
- 229960003087 tioguanine Drugs 0.000 description 2
- 241000712461 unidentified influenza virus Species 0.000 description 2
- 229960002066 vinorelbine Drugs 0.000 description 2
- GBABOYUKABKIAF-IELIFDKJSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-IELIFDKJSA-N 0.000 description 2
- NNJPGOLRFBJNIW-HNNXBMFYSA-N (-)-demecolcine Chemical compound C1=C(OC)C(=O)C=C2[C@@H](NC)CCC3=CC(OC)=C(OC)C(OC)=C3C2=C1 NNJPGOLRFBJNIW-HNNXBMFYSA-N 0.000 description 1
- FLWWDYNPWOSLEO-HQVZTVAUSA-N (2s)-2-[[4-[1-(2-amino-4-oxo-1h-pteridin-6-yl)ethyl-methylamino]benzoyl]amino]pentanedioic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1C(C)N(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FLWWDYNPWOSLEO-HQVZTVAUSA-N 0.000 description 1
- CGMTUJFWROPELF-YPAAEMCBSA-N (3E,5S)-5-[(2S)-butan-2-yl]-3-(1-hydroxyethylidene)pyrrolidine-2,4-dione Chemical compound CC[C@H](C)[C@@H]1NC(=O)\C(=C(/C)O)C1=O CGMTUJFWROPELF-YPAAEMCBSA-N 0.000 description 1
- XRBSKUSTLXISAB-XVVDYKMHSA-N (5r,6r,7r,8r)-8-hydroxy-7-(hydroxymethyl)-5-(3,4,5-trimethoxyphenyl)-5,6,7,8-tetrahydrobenzo[f][1,3]benzodioxole-6-carboxylic acid Chemical compound COC1=C(OC)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@H](O)[C@@H](CO)[C@@H]2C(O)=O)=C1 XRBSKUSTLXISAB-XVVDYKMHSA-N 0.000 description 1
- AESVUZLWRXEGEX-DKCAWCKPSA-N (7S,9R)-7-[(2S,4R,5R,6R)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione iron(3+) Chemical compound [Fe+3].COc1cccc2C(=O)c3c(O)c4C[C@@](O)(C[C@H](O[C@@H]5C[C@@H](N)[C@@H](O)[C@@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO AESVUZLWRXEGEX-DKCAWCKPSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 1
- MYBLAOJMRYYKMS-RTRLPJTCSA-N 1-(2-chloroethyl)-1-nitroso-3-[(3r,4r,5s,6r)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]urea Chemical compound OC[C@H]1OC(O)[C@H](NC(=O)N(CCCl)N=O)[C@@H](O)[C@@H]1O MYBLAOJMRYYKMS-RTRLPJTCSA-N 0.000 description 1
- BTOTXLJHDSNXMW-POYBYMJQSA-N 2,3-dideoxyuridine Chemical compound O1[C@H](CO)CC[C@@H]1N1C(=O)NC(=O)C=C1 BTOTXLJHDSNXMW-POYBYMJQSA-N 0.000 description 1
- BOMZMNZEXMAQQW-UHFFFAOYSA-N 2,5,11-trimethyl-6h-pyrido[4,3-b]carbazol-2-ium-9-ol;acetate Chemical compound CC([O-])=O.C[N+]1=CC=C2C(C)=C(NC=3C4=CC(O)=CC=3)C4=C(C)C2=C1 BOMZMNZEXMAQQW-UHFFFAOYSA-N 0.000 description 1
- QCXJFISCRQIYID-IAEPZHFASA-N 2-amino-1-n-[(3s,6s,7r,10s,16s)-3-[(2s)-butan-2-yl]-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-10-propan-2-yl-8-oxa-1,4,11,14-tetrazabicyclo[14.3.0]nonadecan-6-yl]-4,6-dimethyl-3-oxo-9-n-[(3s,6s,7r,10s,16s)-7,11,14-trimethyl-2,5,9,12,15-pentaoxo-3,10-di(propa Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N=C2C(C(=O)N[C@@H]3C(=O)N[C@H](C(N4CCC[C@H]4C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]3C)=O)[C@@H](C)CC)=C(N)C(=O)C(C)=C2O2)C2=C(C)C=C1 QCXJFISCRQIYID-IAEPZHFASA-N 0.000 description 1
- FDAYLTPAFBGXAB-UHFFFAOYSA-N 2-chloro-n,n-bis(2-chloroethyl)ethanamine Chemical compound ClCCN(CCCl)CCCl FDAYLTPAFBGXAB-UHFFFAOYSA-N 0.000 description 1
- ZOOGRGPOEVQQDX-UUOKFMHZSA-N 3',5'-cyclic GMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-UUOKFMHZSA-N 0.000 description 1
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 1
- PWMYMKOUNYTVQN-UHFFFAOYSA-N 3-(8,8-diethyl-2-aza-8-germaspiro[4.5]decan-2-yl)-n,n-dimethylpropan-1-amine Chemical compound C1C[Ge](CC)(CC)CCC11CN(CCCN(C)C)CC1 PWMYMKOUNYTVQN-UHFFFAOYSA-N 0.000 description 1
- DODQJNMQWMSYGS-QPLCGJKRSA-N 4-[(z)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-1-phenylbut-1-en-2-yl]phenol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 DODQJNMQWMSYGS-QPLCGJKRSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- IDPUKCWIGUEADI-UHFFFAOYSA-N 5-[bis(2-chloroethyl)amino]uracil Chemical compound ClCCN(CCCl)C1=CNC(=O)NC1=O IDPUKCWIGUEADI-UHFFFAOYSA-N 0.000 description 1
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 1
- WYXSYVWAUAUWLD-SHUUEZRQSA-N 6-azauridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=N1 WYXSYVWAUAUWLD-SHUUEZRQSA-N 0.000 description 1
- VVIAGPKUTFNRDU-UHFFFAOYSA-N 6S-folinic acid Natural products C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-UHFFFAOYSA-N 0.000 description 1
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical compound NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 description 1
- HDZZVAMISRMYHH-UHFFFAOYSA-N 9beta-Ribofuranosyl-7-deazaadenin Natural products C1=CC=2C(N)=NC=NC=2N1C1OC(CO)C(O)C1O HDZZVAMISRMYHH-UHFFFAOYSA-N 0.000 description 1
- ZOZKYEHVNDEUCO-DKXJUACHSA-N Aceglatone Chemical compound O1C(=O)[C@H](OC(C)=O)[C@H]2OC(=O)[C@@H](OC(=O)C)[C@H]21 ZOZKYEHVNDEUCO-DKXJUACHSA-N 0.000 description 1
- CEIZFXOZIQNICU-UHFFFAOYSA-N Alternaria alternata Crofton-weed toxin Natural products CCC(C)C1NC(=O)C(C(C)=O)=C1O CEIZFXOZIQNICU-UHFFFAOYSA-N 0.000 description 1
- 206010002199 Anaphylactic shock Diseases 0.000 description 1
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 1
- VGGGPCQERPFHOB-MCIONIFRSA-N Bestatin Chemical compound CC(C)C[C@H](C(O)=O)NC(=O)[C@@H](O)[C@H](N)CC1=CC=CC=C1 VGGGPCQERPFHOB-MCIONIFRSA-N 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 101100180402 Caenorhabditis elegans jun-1 gene Proteins 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 150000008574 D-amino acids Chemical class 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- OBMLHUPNRURLOK-XGRAFVIBSA-N Epitiostanol Chemical compound C1[C@@H]2S[C@@H]2C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@H]21 OBMLHUPNRURLOK-XGRAFVIBSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- JOYRKODLDBILNP-UHFFFAOYSA-N Ethyl urethane Chemical compound CCOC(N)=O JOYRKODLDBILNP-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 108090000386 Fibroblast Growth Factor 1 Proteins 0.000 description 1
- 102100031706 Fibroblast growth factor 1 Human genes 0.000 description 1
- 208000010188 Folic Acid Deficiency Diseases 0.000 description 1
- MPJKWIXIYCLVCU-UHFFFAOYSA-N Folinic acid Natural products NC1=NC2=C(N(C=O)C(CNc3ccc(cc3)C(=O)NC(CCC(=O)O)CC(=O)O)CN2)C(=O)N1 MPJKWIXIYCLVCU-UHFFFAOYSA-N 0.000 description 1
- 208000012671 Gastrointestinal haemorrhages Diseases 0.000 description 1
- BLCLNMBMMGCOAS-URPVMXJPSA-N Goserelin Chemical compound C([C@@H](C(=O)N[C@H](COC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(=O)NNC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 BLCLNMBMMGCOAS-URPVMXJPSA-N 0.000 description 1
- 108010069236 Goserelin Proteins 0.000 description 1
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 102100028972 HLA class I histocompatibility antigen, A alpha chain Human genes 0.000 description 1
- 108010075704 HLA-A Antigens Proteins 0.000 description 1
- 108010088729 HLA-A*02:01 antigen Proteins 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 101000599940 Homo sapiens Interferon gamma Proteins 0.000 description 1
- 101001030211 Homo sapiens Myc proto-oncogene protein Proteins 0.000 description 1
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- VSNHCAURESNICA-UHFFFAOYSA-N Hydroxyurea Chemical compound NC(=O)NO VSNHCAURESNICA-UHFFFAOYSA-N 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 208000029523 Interstitial Lung disease Diseases 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- 229920001491 Lentinan Polymers 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- 206010067125 Liver injury Diseases 0.000 description 1
- 206010024769 Local reaction Diseases 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 208000007433 Lymphatic Metastasis Diseases 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- VJRAUFKOOPNFIQ-UHFFFAOYSA-N Marcellomycin Natural products C12=C(O)C=3C(=O)C4=C(O)C=CC(O)=C4C(=O)C=3C=C2C(C(=O)OC)C(CC)(O)CC1OC(OC1C)CC(N(C)C)C1OC(OC1C)CC(O)C1OC1CC(O)C(O)C(C)O1 VJRAUFKOOPNFIQ-UHFFFAOYSA-N 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 206010027452 Metastases to bone Diseases 0.000 description 1
- 206010027459 Metastases to lymph nodes Diseases 0.000 description 1
- 206010051676 Metastases to peritoneum Diseases 0.000 description 1
- VFKZTMPDYBFSTM-KVTDHHQDSA-N Mitobronitol Chemical compound BrC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-KVTDHHQDSA-N 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 206010028817 Nausea and vomiting symptoms Diseases 0.000 description 1
- 206010061309 Neoplasm progression Diseases 0.000 description 1
- KGTDRFCXGRULNK-UHFFFAOYSA-N Nogalamycin Natural products COC1C(OC)(C)C(OC)C(C)OC1OC1C2=C(O)C(C(=O)C3=C(O)C=C4C5(C)OC(C(C(C5O)N(C)C)O)OC4=C3C3=O)=C3C=C2C(C(=O)OC)C(C)(O)C1 KGTDRFCXGRULNK-UHFFFAOYSA-N 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 229930187135 Olivomycin Natural products 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 102100035593 POU domain, class 2, transcription factor 1 Human genes 0.000 description 1
- 101710084414 POU domain, class 2, transcription factor 1 Proteins 0.000 description 1
- 206010033799 Paralysis Diseases 0.000 description 1
- 108010057150 Peplomycin Proteins 0.000 description 1
- KHGNFPUMBJSZSM-UHFFFAOYSA-N Perforine Natural products COC1=C2CCC(O)C(CCC(C)(C)O)(OC)C2=NC2=C1C=CO2 KHGNFPUMBJSZSM-UHFFFAOYSA-N 0.000 description 1
- HRHKSTOGXBBQCB-UHFFFAOYSA-N Porfiromycine Chemical compound O=C1C(N)=C(C)C(=O)C2=C1C(COC(N)=O)C1(OC)C3N(C)C3CN12 HRHKSTOGXBBQCB-UHFFFAOYSA-N 0.000 description 1
- HFVNWDWLWUCIHC-GUPDPFMOSA-N Prednimustine Chemical compound O=C([C@@]1(O)CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)[C@@H](O)C[C@@]21C)COC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 HFVNWDWLWUCIHC-GUPDPFMOSA-N 0.000 description 1
- 101800004937 Protein C Proteins 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- AHHFEZNOXOZZQA-ZEBDFXRSSA-N Ranimustine Chemical compound CO[C@H]1O[C@H](CNC(=O)N(CCCl)N=O)[C@@H](O)[C@H](O)[C@H]1O AHHFEZNOXOZZQA-ZEBDFXRSSA-N 0.000 description 1
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 102100036546 Salivary acidic proline-rich phosphoprotein 1/2 Human genes 0.000 description 1
- 101800001700 Saposin-D Proteins 0.000 description 1
- 208000007271 Substance Withdrawal Syndrome Diseases 0.000 description 1
- CGMTUJFWROPELF-UHFFFAOYSA-N Tenuazonic acid Natural products CCC(C)C1NC(=O)C(=C(C)/O)C1=O CGMTUJFWROPELF-UHFFFAOYSA-N 0.000 description 1
- IWEQQRMGNVVKQW-OQKDUQJOSA-N Toremifene citrate Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 IWEQQRMGNVVKQW-OQKDUQJOSA-N 0.000 description 1
- UMILHIMHKXVDGH-UHFFFAOYSA-N Triethylene glycol diglycidyl ether Chemical compound C1OC1COCCOCCOCCOCC1CO1 UMILHIMHKXVDGH-UHFFFAOYSA-N 0.000 description 1
- FYAMXEPQQLNQDM-UHFFFAOYSA-N Tris(1-aziridinyl)phosphine oxide Chemical compound C1CN1P(N1CC1)(=O)N1CC1 FYAMXEPQQLNQDM-UHFFFAOYSA-N 0.000 description 1
- 102000004243 Tubulin Human genes 0.000 description 1
- 108090000704 Tubulin Proteins 0.000 description 1
- 206010049750 Tumour haemorrhage Diseases 0.000 description 1
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 description 1
- 108010034265 Vascular Endothelial Growth Factor Receptors Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- IFJUINDAXYAPTO-UUBSBJJBSA-N [(8r,9s,13s,14s,17s)-17-[2-[4-[4-[bis(2-chloroethyl)amino]phenyl]butanoyloxy]acetyl]oxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-3-yl] benzoate Chemical compound C([C@@H]1[C@@H](C2=CC=3)CC[C@]4([C@H]1CC[C@@H]4OC(=O)COC(=O)CCCC=1C=CC(=CC=1)N(CCCl)CCCl)C)CC2=CC=3OC(=O)C1=CC=CC=C1 IFJUINDAXYAPTO-UUBSBJJBSA-N 0.000 description 1
- IHGLINDYFMDHJG-UHFFFAOYSA-N [2-(4-methoxyphenyl)-3,4-dihydronaphthalen-1-yl]-[4-(2-pyrrolidin-1-ylethoxy)phenyl]methanone Chemical compound C1=CC(OC)=CC=C1C(CCC1=CC=CC=C11)=C1C(=O)C(C=C1)=CC=C1OCCN1CCCC1 IHGLINDYFMDHJG-UHFFFAOYSA-N 0.000 description 1
- XZSRRNFBEIOBDA-CFNBKWCHSA-N [2-[(2s,4s)-4-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-3,4-dihydro-1h-tetracen-2-yl]-2-oxoethyl] 2,2-diethoxyacetate Chemical compound O([C@H]1C[C@](CC2=C(O)C=3C(=O)C4=CC=CC(OC)=C4C(=O)C=3C(O)=C21)(O)C(=O)COC(=O)C(OCC)OCC)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 XZSRRNFBEIOBDA-CFNBKWCHSA-N 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000001919 adrenal effect Effects 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229940045714 alkyl sulfonate alkylating agent Drugs 0.000 description 1
- 150000008052 alkyl sulfonates Chemical class 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 229960000473 altretamine Drugs 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229960002749 aminolevulinic acid Drugs 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- 210000004381 amniotic fluid Anatomy 0.000 description 1
- 229960001220 amsacrine Drugs 0.000 description 1
- XCPGHVQEEXUHNC-UHFFFAOYSA-N amsacrine Chemical compound COC1=CC(NS(C)(=O)=O)=CC=C1NC1=C(C=CC=C2)C2=NC2=CC=CC=C12 XCPGHVQEEXUHNC-UHFFFAOYSA-N 0.000 description 1
- 208000003455 anaphylaxis Diseases 0.000 description 1
- BBDAGFIXKZCXAH-CCXZUQQUSA-N ancitabine Chemical compound N=C1C=CN2[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3OC2=N1 BBDAGFIXKZCXAH-CCXZUQQUSA-N 0.000 description 1
- 229950000242 ancitabine Drugs 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 229940030486 androgens Drugs 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000002280 anti-androgenic effect Effects 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000006023 anti-tumor response Effects 0.000 description 1
- 239000000051 antiandrogen Substances 0.000 description 1
- 229940030495 antiandrogen sex hormone and modulator of the genital system Drugs 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000013059 antihormonal agent Substances 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940045686 antimetabolites antineoplastic purine analogs Drugs 0.000 description 1
- 229940045687 antimetabolites folic acid analogs Drugs 0.000 description 1
- 229940045719 antineoplastic alkylating agent nitrosoureas Drugs 0.000 description 1
- 229940045713 antineoplastic alkylating drug ethylene imines Drugs 0.000 description 1
- 230000005975 antitumor immune response Effects 0.000 description 1
- 238000011398 antitumor immunotherapy Methods 0.000 description 1
- 210000000702 aorta abdominal Anatomy 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 150000008209 arabinosides Chemical class 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 229940046844 aromatase inhibitors Drugs 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 1
- 150000001541 aziridines Chemical class 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 229960000997 bicalutamide Drugs 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- 108700002839 cactinomycin Proteins 0.000 description 1
- 229950009908 cactinomycin Drugs 0.000 description 1
- 229930195731 calicheamicin Natural products 0.000 description 1
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 description 1
- IVFYLRMMHVYGJH-PVPPCFLZSA-N calusterone Chemical compound C1C[C@]2(C)[C@](O)(C)CC[C@H]2[C@@H]2[C@@H](C)CC3=CC(=O)CC[C@]3(C)[C@H]21 IVFYLRMMHVYGJH-PVPPCFLZSA-N 0.000 description 1
- 229950009823 calusterone Drugs 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 231100000315 carcinogenic Toxicity 0.000 description 1
- 229930188550 carminomycin Natural products 0.000 description 1
- XREUEWVEMYWFFA-CSKJXFQVSA-N carminomycin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XREUEWVEMYWFFA-CSKJXFQVSA-N 0.000 description 1
- XREUEWVEMYWFFA-UHFFFAOYSA-N carminomycin I Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=C(O)C=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XREUEWVEMYWFFA-UHFFFAOYSA-N 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 229950001725 carubicin Drugs 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 230000000973 chemotherapeutic effect Effects 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 238000013170 computed tomography imaging Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 230000002435 cytoreductive effect Effects 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- ATWWYGQDYGSWQA-UHFFFAOYSA-N demecolceine Natural products C1=C(O)C(=O)C=C2C(NC)CCC3=CC(OC)=C(OC)C(OC)=C3C2=C1 ATWWYGQDYGSWQA-UHFFFAOYSA-N 0.000 description 1
- 229960005052 demecolcine Drugs 0.000 description 1
- 229950003913 detorubicin Drugs 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- WVYXNIXAMZOZFK-UHFFFAOYSA-N diaziquone Chemical compound O=C1C(NC(=O)OCC)=C(N2CC2)C(=O)C(NC(=O)OCC)=C1N1CC1 WVYXNIXAMZOZFK-UHFFFAOYSA-N 0.000 description 1
- 230000003467 diminishing effect Effects 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- NOTIQUSPUUHHEH-UXOVVSIBSA-N dromostanolone propionate Chemical compound C([C@@H]1CC2)C(=O)[C@H](C)C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H](OC(=O)CC)[C@@]2(C)CC1 NOTIQUSPUUHHEH-UXOVVSIBSA-N 0.000 description 1
- 229950004683 drostanolone propionate Drugs 0.000 description 1
- 238000013399 early diagnosis Methods 0.000 description 1
- 230000002497 edematous effect Effects 0.000 description 1
- 229960002759 eflornithine Drugs 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 229950000549 elliptinium acetate Drugs 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 229950002973 epitiostanol Drugs 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- QSRLNKCNOLVZIR-KRWDZBQOSA-N ethyl (2s)-2-[[2-[4-[bis(2-chloroethyl)amino]phenyl]acetyl]amino]-4-methylsulfanylbutanoate Chemical compound CCOC(=O)[C@H](CCSC)NC(=O)CC1=CC=C(N(CCCl)CCCl)C=C1 QSRLNKCNOLVZIR-KRWDZBQOSA-N 0.000 description 1
- 229960005237 etoglucid Drugs 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 229940043168 fareston Drugs 0.000 description 1
- 230000006126 farnesylation Effects 0.000 description 1
- 210000004700 fetal blood Anatomy 0.000 description 1
- 239000012997 ficoll-paque Substances 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- 150000002224 folic acids Chemical class 0.000 description 1
- VVIAGPKUTFNRDU-ABLWVSNPSA-N folinic acid Chemical compound C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-ABLWVSNPSA-N 0.000 description 1
- 235000008191 folinic acid Nutrition 0.000 description 1
- 239000011672 folinic acid Substances 0.000 description 1
- 210000000245 forearm Anatomy 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 229960004783 fotemustine Drugs 0.000 description 1
- YAKWPXVTIGTRJH-UHFFFAOYSA-N fotemustine Chemical compound CCOP(=O)(OCC)C(C)NC(=O)N(CCCl)N=O YAKWPXVTIGTRJH-UHFFFAOYSA-N 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 210000000232 gallbladder Anatomy 0.000 description 1
- 229940044658 gallium nitrate Drugs 0.000 description 1
- 208000030304 gastrointestinal bleeding Diseases 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 229960002913 goserelin Drugs 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 231100000234 hepatic damage Toxicity 0.000 description 1
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 1
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 102000053563 human MYC Human genes 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 229960001330 hydroxycarbamide Drugs 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 229960002411 imatinib Drugs 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 229960003444 immunosuppressant agent Drugs 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- DBIGHPPNXATHOF-UHFFFAOYSA-N improsulfan Chemical compound CS(=O)(=O)OCCCNCCCOS(C)(=O)=O DBIGHPPNXATHOF-UHFFFAOYSA-N 0.000 description 1
- 229950008097 improsulfan Drugs 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 230000003907 kidney function Effects 0.000 description 1
- 229940115286 lentinan Drugs 0.000 description 1
- 229960001691 leucovorin Drugs 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 230000008818 liver damage Effects 0.000 description 1
- 230000003908 liver function Effects 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 229960003538 lonidamine Drugs 0.000 description 1
- WDRYRZXSPDWGEB-UHFFFAOYSA-N lonidamine Chemical compound C12=CC=CC=C2C(C(=O)O)=NN1CC1=CC=C(Cl)C=C1Cl WDRYRZXSPDWGEB-UHFFFAOYSA-N 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 230000001926 lymphatic effect Effects 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- MQXVYODZCMMZEM-ZYUZMQFOSA-N mannomustine Chemical compound ClCCNC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CNCCCl MQXVYODZCMMZEM-ZYUZMQFOSA-N 0.000 description 1
- 229950008612 mannomustine Drugs 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- 210000001363 mesenteric artery superior Anatomy 0.000 description 1
- 210000001758 mesenteric vein Anatomy 0.000 description 1
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 1
- VJRAUFKOOPNFIQ-TVEKBUMESA-N methyl (1r,2r,4s)-4-[(2r,4s,5s,6s)-5-[(2s,4s,5s,6s)-5-[(2s,4s,5s,6s)-4,5-dihydroxy-6-methyloxan-2-yl]oxy-4-hydroxy-6-methyloxan-2-yl]oxy-4-(dimethylamino)-6-methyloxan-2-yl]oxy-2-ethyl-2,5,7,10-tetrahydroxy-6,11-dioxo-3,4-dihydro-1h-tetracene-1-carboxylat Chemical compound O([C@H]1[C@@H](O)C[C@@H](O[C@H]1C)O[C@H]1[C@H](C[C@@H](O[C@H]1C)O[C@H]1C[C@]([C@@H](C2=CC=3C(=O)C4=C(O)C=CC(O)=C4C(=O)C=3C(O)=C21)C(=O)OC)(O)CC)N(C)C)[C@H]1C[C@H](O)[C@H](O)[C@H](C)O1 VJRAUFKOOPNFIQ-TVEKBUMESA-N 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 229960005485 mitobronitol Drugs 0.000 description 1
- 229960003539 mitoguazone Drugs 0.000 description 1
- MXWHMTNPTTVWDM-NXOFHUPFSA-N mitoguazone Chemical compound NC(N)=N\N=C(/C)\C=N\N=C(N)N MXWHMTNPTTVWDM-NXOFHUPFSA-N 0.000 description 1
- VFKZTMPDYBFSTM-GUCUJZIJSA-N mitolactol Chemical compound BrC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-GUCUJZIJSA-N 0.000 description 1
- 229950010913 mitolactol Drugs 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- FOYWNSCCNCUEPU-UHFFFAOYSA-N mopidamol Chemical compound C12=NC(N(CCO)CCO)=NC=C2N=C(N(CCO)CCO)N=C1N1CCCCC1 FOYWNSCCNCUEPU-UHFFFAOYSA-N 0.000 description 1
- 229950010718 mopidamol Drugs 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- 238000011328 necessary treatment Methods 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- QZGIWPZCWHMVQL-UIYAJPBUSA-N neocarzinostatin chromophore Chemical compound O1[C@H](C)[C@H](O)[C@H](O)[C@@H](NC)[C@H]1O[C@@H]1C/2=C/C#C[C@H]3O[C@@]3([C@@H]3OC(=O)OC3)C#CC\2=C[C@H]1OC(=O)C1=C(O)C=CC2=C(C)C=C(OC)C=C12 QZGIWPZCWHMVQL-UIYAJPBUSA-N 0.000 description 1
- XWXYUMMDTVBTOU-UHFFFAOYSA-N nilutamide Chemical compound O=C1C(C)(C)NC(=O)N1C1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 XWXYUMMDTVBTOU-UHFFFAOYSA-N 0.000 description 1
- 229960002653 nilutamide Drugs 0.000 description 1
- 229960001420 nimustine Drugs 0.000 description 1
- VFEDRRNHLBGPNN-UHFFFAOYSA-N nimustine Chemical compound CC1=NC=C(CNC(=O)N(CCCl)N=O)C(N)=N1 VFEDRRNHLBGPNN-UHFFFAOYSA-N 0.000 description 1
- YMVWGSQGCWCDGW-UHFFFAOYSA-N nitracrine Chemical compound C1=CC([N+]([O-])=O)=C2C(NCCCN(C)C)=C(C=CC=C3)C3=NC2=C1 YMVWGSQGCWCDGW-UHFFFAOYSA-N 0.000 description 1
- 229950008607 nitracrine Drugs 0.000 description 1
- 229950009266 nogalamycin Drugs 0.000 description 1
- KGTDRFCXGRULNK-JYOBTZKQSA-N nogalamycin Chemical compound CO[C@@H]1[C@@](OC)(C)[C@@H](OC)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=C(O)C=C4[C@@]5(C)O[C@H]([C@H]([C@@H]([C@H]5O)N(C)C)O)OC4=C3C3=O)=C3C=C2[C@@H](C(=O)OC)[C@@](C)(O)C1 KGTDRFCXGRULNK-JYOBTZKQSA-N 0.000 description 1
- 239000003865 nucleic acid synthesis inhibitor Substances 0.000 description 1
- 230000000474 nursing effect Effects 0.000 description 1
- CZDBNBLGZNWKMC-MWQNXGTOSA-N olivomycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1)O[C@H]1O[C@@H](C)[C@H](O)[C@@H](OC2O[C@@H](C)[C@H](O)[C@@H](O)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@H](O)[C@H](OC)[C@H](C)O1 CZDBNBLGZNWKMC-MWQNXGTOSA-N 0.000 description 1
- 229950005848 olivomycin Drugs 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- QIMGFXOHTOXMQP-GFAGFCTOSA-N peplomycin Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCCN[C@@H](C)C=1C=CC=CC=1)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1NC=NC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C QIMGFXOHTOXMQP-GFAGFCTOSA-N 0.000 description 1
- 229950003180 peplomycin Drugs 0.000 description 1
- 229930192851 perforin Natural products 0.000 description 1
- 239000008177 pharmaceutical agent Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- NUKCGLDCWQXYOQ-UHFFFAOYSA-N piposulfan Chemical compound CS(=O)(=O)OCCC(=O)N1CCN(C(=O)CCOS(C)(=O)=O)CC1 NUKCGLDCWQXYOQ-UHFFFAOYSA-N 0.000 description 1
- 229950001100 piposulfan Drugs 0.000 description 1
- 150000003057 platinum Chemical class 0.000 description 1
- 210000003240 portal vein Anatomy 0.000 description 1
- 230000001124 posttranscriptional effect Effects 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 229960004694 prednimustine Drugs 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 1
- 229960000624 procarbazine Drugs 0.000 description 1
- 229960000856 protein c Drugs 0.000 description 1
- WOLQREOUPKZMEX-UHFFFAOYSA-N pteroyltriglutamic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(=O)NC(CCC(=O)NC(CCC(O)=O)C(O)=O)C(O)=O)C(O)=O)C=C1 WOLQREOUPKZMEX-UHFFFAOYSA-N 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 238000003908 quality control method Methods 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 1
- 229960002185 ranimustine Drugs 0.000 description 1
- BMKDZUISNHGIBY-UHFFFAOYSA-N razoxane Chemical compound C1C(=O)NC(=O)CN1C(C)CN1CC(=O)NC(=O)C1 BMKDZUISNHGIBY-UHFFFAOYSA-N 0.000 description 1
- 229960000460 razoxane Drugs 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- VHXNKPBCCMUMSW-FQEVSTJZSA-N rubitecan Chemical compound C1=CC([N+]([O-])=O)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VHXNKPBCCMUMSW-FQEVSTJZSA-N 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 210000000582 semen Anatomy 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 229950006315 spirogermanium Drugs 0.000 description 1
- 238000011255 standard chemotherapy Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000011272 standard treatment Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 210000001179 synovial fluid Anatomy 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- BPEWUONYVDABNZ-DZBHQSCQSA-N testolactone Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(OC(=O)CC4)[C@@H]4[C@@H]3CCC2=C1 BPEWUONYVDABNZ-DZBHQSCQSA-N 0.000 description 1
- 229960005353 testolactone Drugs 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 229960005026 toremifene Drugs 0.000 description 1
- XFCLJVABOIYOMF-QPLCGJKRSA-N toremifene Chemical compound C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 XFCLJVABOIYOMF-QPLCGJKRSA-N 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 229960000575 trastuzumab Drugs 0.000 description 1
- 230000008736 traumatic injury Effects 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 229950001353 tretamine Drugs 0.000 description 1
- IUCJMVBFZDHPDX-UHFFFAOYSA-N tretamine Chemical compound C1CN1C1=NC(N2CC2)=NC(N2CC2)=N1 IUCJMVBFZDHPDX-UHFFFAOYSA-N 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- 229960004560 triaziquone Drugs 0.000 description 1
- PXSOHRWMIRDKMP-UHFFFAOYSA-N triaziquone Chemical compound O=C1C(N2CC2)=C(N2CC2)C(=O)C=C1N1CC1 PXSOHRWMIRDKMP-UHFFFAOYSA-N 0.000 description 1
- 229960001670 trilostane Drugs 0.000 description 1
- KVJXBPDAXMEYOA-CXANFOAXSA-N trilostane Chemical compound OC1=C(C#N)C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CC[C@@]32O[C@@H]31 KVJXBPDAXMEYOA-CXANFOAXSA-N 0.000 description 1
- 229950000212 trioxifene Drugs 0.000 description 1
- 229960000875 trofosfamide Drugs 0.000 description 1
- UMKFEPPTGMDVMI-UHFFFAOYSA-N trofosfamide Chemical compound ClCCN(CCCl)P1(=O)OCCCN1CCCl UMKFEPPTGMDVMI-UHFFFAOYSA-N 0.000 description 1
- HDZZVAMISRMYHH-LITAXDCLSA-N tubercidin Chemical compound C1=CC=2C(N)=NC=NC=2N1[C@@H]1O[C@@H](CO)[C@H](O)[C@H]1O HDZZVAMISRMYHH-LITAXDCLSA-N 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 230000005751 tumor progression Effects 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 description 1
- 229950009811 ubenimex Drugs 0.000 description 1
- 238000012285 ultrasound imaging Methods 0.000 description 1
- 210000003606 umbilical vein Anatomy 0.000 description 1
- 229960001055 uracil mustard Drugs 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 210000003556 vascular endothelial cell Anatomy 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 229960004355 vindesine Drugs 0.000 description 1
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 229940053867 xeloda Drugs 0.000 description 1
- 229950009268 zinostatin Drugs 0.000 description 1
- FBTUMDXHSRTGRV-ALTNURHMSA-N zorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(\C)=N\NC(=O)C=1C=CC=CC=1)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 FBTUMDXHSRTGRV-ALTNURHMSA-N 0.000 description 1
- 229960000641 zorubicin Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/08—Peptides having 5 to 11 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/10—Peptides having 12 to 20 amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55566—Emulsions, e.g. Freund's adjuvant, MF59
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/80—Vaccine for a specifically defined cancer
- A61K2039/852—Pancreas
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Oncology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Molecular Biology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US95792307P | 2007-08-24 | 2007-08-24 | |
| US60/957,923 | 2007-08-24 | ||
| PCT/JP2008/002232 WO2009028150A1 (en) | 2007-08-24 | 2008-08-19 | Combination therapy for pancreatic cancer using an antigenic peptide and chemotherapeutic agent |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2010111139A RU2010111139A (ru) | 2011-09-27 |
| RU2472522C2 true RU2472522C2 (ru) | 2013-01-20 |
Family
ID=40386893
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010111139/15A RU2472522C2 (ru) | 2007-08-24 | 2008-08-19 | Комбинированная терапия рака поджелудочной железы с использованием антигенного пептида и химиотерапевтического средства |
Country Status (19)
| Country | Link |
|---|---|
| US (1) | US8703713B2 (enExample) |
| EP (1) | EP2195003A4 (enExample) |
| JP (1) | JP5417667B2 (enExample) |
| KR (1) | KR20100047899A (enExample) |
| CN (1) | CN101883577A (enExample) |
| AR (1) | AR068020A1 (enExample) |
| AU (1) | AU2008292966C1 (enExample) |
| BR (1) | BRPI0815726A2 (enExample) |
| CA (1) | CA2697501A1 (enExample) |
| IL (1) | IL204143A (enExample) |
| MX (1) | MX2010002178A (enExample) |
| MY (1) | MY160406A (enExample) |
| NZ (1) | NZ583578A (enExample) |
| RU (1) | RU2472522C2 (enExample) |
| SG (1) | SG183770A1 (enExample) |
| TW (1) | TWI436775B (enExample) |
| UA (1) | UA100702C2 (enExample) |
| WO (1) | WO2009028150A1 (enExample) |
| ZA (1) | ZA201001580B (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2670147C1 (ru) * | 2014-10-09 | 2018-10-18 | Ямагути Юниверсити | Вектор экспрессии car и car-экспрессирующие т-клетки |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SI1548032T1 (sl) | 2002-09-12 | 2009-10-31 | Oncotherapy Science Inc | KDR peptidi in cepiva, ki obsegajo le-te |
| US20080034017A1 (en) * | 2006-08-04 | 2008-02-07 | Dominic Giampaolo | Links to a common item in a data structure |
| TW201109029A (en) * | 2009-06-11 | 2011-03-16 | Oncotherapy Science Inc | Vaccine therapy for choroidal neovascularization |
| EP2578233B1 (en) | 2010-05-28 | 2017-04-26 | National Cancer Center | Therapeutic agent for pancreatic cancer |
| DE102013214023B4 (de) * | 2013-07-17 | 2015-02-12 | Siemens Aktiengesellschaft | Verfahren zu einem Auswerten und Vergleichen von zeitlich aufeinander folgenden kombinierten medizinischen Bildgebungsuntersuchungen sowie ein medizinisches Bildgebungssystem, das zu einem Ausführen des erfindungsgemäßen Verfahrens ausgelegt ist |
| KR20230004907A (ko) * | 2014-10-14 | 2023-01-06 | 립타이드 바이오사이언스, 인코포레이티드 | 항-염증 특성을 갖는 펩타이드 |
| US20200147194A1 (en) * | 2017-05-19 | 2020-05-14 | Keio University | Peptide vaccine and peptide vaccine composition for cranial nerve disease |
| US10413584B1 (en) | 2018-08-29 | 2019-09-17 | Riptide Bioscience, Inc. | Peptides having immunomodulatory properties |
| US10548944B1 (en) | 2018-10-19 | 2020-02-04 | Riptide Bioscience, Inc. | Antimicrobial peptides and methods of using the same |
| CN114959031A (zh) * | 2022-05-20 | 2022-08-30 | 上海交通大学医学院附属瑞金医院 | 胰腺腺癌预后评估的标志物组合及其应用 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004024766A1 (ja) * | 2002-09-12 | 2004-03-25 | Oncotherapy Science, Inc. | Kdrペプチド及びこれを含むワクチン |
| US20060216288A1 (en) * | 2005-03-22 | 2006-09-28 | Amgen Inc | Combinations for the treatment of cancer |
| RU2376373C2 (ru) * | 2003-06-30 | 2009-12-20 | Ридженерон Фармасьютикалз, Инк. | Изолированная молекула нуклеиновой кислоты, кодирующая слитый полипептид, способный связывать фактор роста эндотелиальных клеток сосудов (vegf), слитый полипептид, реплицируемый экспрессионный вектор, способ получения слитого полипептида, ловушка vegf, фармацевтическая композиция, способ лечения и набор для лечения vegf-опосредованного заболевания или состояния |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB213645A (en) | 1922-12-22 | 1924-03-24 | Frank Lincoln Morse | Improvements in and relating to chain and sprocket wheel gearing |
| US3265572A (en) | 1961-09-20 | 1966-08-09 | S B Penick And Company | Process for preparing tyrociding and product produced thereby |
| US4526988A (en) | 1983-03-10 | 1985-07-02 | Eli Lilly And Company | Difluoro antivirals and intermediate therefor |
| DK0694042T3 (da) | 1993-03-25 | 2005-02-14 | Merck & Co Inc | Inhibitor af vaskulær endothelcellevækstfaktor |
| US6080728A (en) | 1996-07-16 | 2000-06-27 | Mixson; A. James | Carrier: DNA complexes containing DNA encoding anti-angiogenic peptides and their use in gene therapy |
| DE19638745C2 (de) | 1996-09-11 | 2001-05-10 | Schering Ag | Monoklonale Antikörper gegen die extrazelluläre Domäne des menschlichen VEGF - Rezeptorproteins (KDR) |
| WO1998031794A1 (fr) | 1997-01-17 | 1998-07-23 | Toa Gosei Co., Ltd. | Polypeptide liant le facteur vegf |
| AU2299099A (en) | 1998-02-04 | 1999-08-23 | Kyowa Hakko Kogyo Co. Ltd. | Antibodies against human vegf receptor kdr |
| GB9804121D0 (en) | 1998-02-26 | 1998-04-22 | Cancer Res Campaign Tech | Anti-angiogenic vaccines: materials and methods relating thereto |
| EP1086705A4 (en) | 1998-05-20 | 2002-02-06 | Kyowa Hakko Kogyo Kk | INHIBITORS OF THE ACTIVITY OF THE VASCULAR ENDOTHELIAL GROWTH FACTOR |
| US7264810B2 (en) | 2001-01-19 | 2007-09-04 | Cytos Biotechnology Ag | Molecular antigen array |
| CU23178A1 (es) * | 2002-04-15 | 2006-09-22 | Ct Ingenieria Genetica Biotech | INMUNOTERAPIA ACTIVA ANTIANGIOGéNICA |
| AU2003291726A1 (en) | 2002-11-04 | 2004-06-07 | Xenoport, Inc. | Gemcitabine prodrugs, pharmaceutical compositions and uses thereof |
| WO2005087808A2 (en) * | 2004-03-05 | 2005-09-22 | Ludwig Institute For Cancer Research | Growth factor binding constructs materials and methods |
| RU2006146619A (ru) * | 2004-06-03 | 2008-07-20 | Ф.Хоффманн-Ля Рош Аг (Ch) | Лечение гемцитабином и ингибитором рецептора эпидермального фактора роста(egfr) |
| ES2310860T3 (es) | 2004-12-17 | 2009-01-16 | Eli Lilly And Company | Profarmaco amida de gemcitabina, composiciones y uso del mismo. |
| PL2119447T3 (pl) | 2007-02-16 | 2013-08-30 | Oncotherapy Science Inc | Terapia szczepionkowa przeciwko neowaskularyzacji naczyniówkowej |
-
2008
- 2008-08-13 TW TW097130789A patent/TWI436775B/zh not_active IP Right Cessation
- 2008-08-19 BR BRPI0815726A patent/BRPI0815726A2/pt not_active IP Right Cessation
- 2008-08-19 EP EP08828542A patent/EP2195003A4/en not_active Withdrawn
- 2008-08-19 MY MYPI2010000779A patent/MY160406A/en unknown
- 2008-08-19 KR KR1020107005967A patent/KR20100047899A/ko not_active Ceased
- 2008-08-19 CN CN2008801133893A patent/CN101883577A/zh active Pending
- 2008-08-19 JP JP2010506728A patent/JP5417667B2/ja not_active Expired - Fee Related
- 2008-08-19 UA UAA201002881A patent/UA100702C2/ru unknown
- 2008-08-19 WO PCT/JP2008/002232 patent/WO2009028150A1/en not_active Ceased
- 2008-08-19 RU RU2010111139/15A patent/RU2472522C2/ru not_active IP Right Cessation
- 2008-08-19 NZ NZ583578A patent/NZ583578A/en not_active IP Right Cessation
- 2008-08-19 US US12/674,754 patent/US8703713B2/en not_active Expired - Fee Related
- 2008-08-19 AU AU2008292966A patent/AU2008292966C1/en not_active Ceased
- 2008-08-19 SG SG2012063046A patent/SG183770A1/en unknown
- 2008-08-19 MX MX2010002178A patent/MX2010002178A/es active IP Right Grant
- 2008-08-19 CA CA2697501A patent/CA2697501A1/en not_active Abandoned
- 2008-08-22 AR ARP080103679A patent/AR068020A1/es unknown
-
2010
- 2010-02-24 IL IL204143A patent/IL204143A/en not_active IP Right Cessation
- 2010-03-04 ZA ZA2010/01580A patent/ZA201001580B/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004024766A1 (ja) * | 2002-09-12 | 2004-03-25 | Oncotherapy Science, Inc. | Kdrペプチド及びこれを含むワクチン |
| RU2376373C2 (ru) * | 2003-06-30 | 2009-12-20 | Ридженерон Фармасьютикалз, Инк. | Изолированная молекула нуклеиновой кислоты, кодирующая слитый полипептид, способный связывать фактор роста эндотелиальных клеток сосудов (vegf), слитый полипептид, реплицируемый экспрессионный вектор, способ получения слитого полипептида, ловушка vegf, фармацевтическая композиция, способ лечения и набор для лечения vegf-опосредованного заболевания или состояния |
| US20060216288A1 (en) * | 2005-03-22 | 2006-09-28 | Amgen Inc | Combinations for the treatment of cancer |
Non-Patent Citations (2)
| Title |
|---|
| BRUNS СJ et al "Effect of the vascular endothelial growth factor receptor-2 antibody DC101 plus gemcitabine on growth, metastasis and angiogenesis of human pancreatic cancer growing orthotopically in nude mice. Int. J.Cancer, 2002, Nov.10, 102(2); 101-8, PMID: 12385004 реферат. * |
| WADA S et al. Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor2.CancerRes.2005, Jun 1, 65(11):4939-46, PMID: 15930316. * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2670147C1 (ru) * | 2014-10-09 | 2018-10-18 | Ямагути Юниверсити | Вектор экспрессии car и car-экспрессирующие т-клетки |
Also Published As
| Publication number | Publication date |
|---|---|
| IL204143A (en) | 2013-04-30 |
| MX2010002178A (es) | 2010-03-18 |
| MY160406A (en) | 2017-03-15 |
| TW200914037A (en) | 2009-04-01 |
| AU2008292966C1 (en) | 2013-03-14 |
| ZA201001580B (en) | 2011-02-23 |
| SG183770A1 (en) | 2012-09-27 |
| JP5417667B2 (ja) | 2014-02-19 |
| US20110082088A1 (en) | 2011-04-07 |
| KR20100047899A (ko) | 2010-05-10 |
| NZ583578A (en) | 2012-07-27 |
| CN101883577A (zh) | 2010-11-10 |
| BRPI0815726A2 (pt) | 2016-01-05 |
| CA2697501A1 (en) | 2009-03-05 |
| AR068020A1 (es) | 2009-10-28 |
| US8703713B2 (en) | 2014-04-22 |
| WO2009028150A1 (en) | 2009-03-05 |
| AU2008292966A1 (en) | 2009-03-05 |
| EP2195003A1 (en) | 2010-06-16 |
| UA100702C2 (ru) | 2013-01-25 |
| JP2010536714A (ja) | 2010-12-02 |
| EP2195003A4 (en) | 2011-07-06 |
| AU2008292966B2 (en) | 2012-07-19 |
| RU2010111139A (ru) | 2011-09-27 |
| TWI436775B (zh) | 2014-05-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2472522C2 (ru) | Комбинированная терапия рака поджелудочной железы с использованием антигенного пептида и химиотерапевтического средства | |
| RU2700929C2 (ru) | Пептидная вакцина, включающая пептид ras, содержащий мутации, и химиотерапевтический агент | |
| CN105979961A (zh) | 用于治疗癌症和感染性疾病的方法和组合物 | |
| BRPI0912462B1 (pt) | Uso de um agente indutor de imunidade, uso de um polipeptídeo e uso de um vetor recombinante | |
| JP6112171B2 (ja) | 免疫誘導剤 | |
| WO2017177207A1 (en) | Construction and methods of use of a therapeutic cancer vaccine library comprising fusion-specific vaccines | |
| CN105435218A (zh) | 预防乳腺癌复发的疫苗 | |
| JP5435938B2 (ja) | ワクチン接種のための天然ペプチド及びそれらの最適化された誘導体の使用 | |
| Parizadeh et al. | Personalized peptide-based vaccination for treatment of colorectal cancer: rational and progress | |
| US20250302976A1 (en) | New vaccinal strategy | |
| Durrant et al. | Cancer vaccines entering Phase III clinical trials | |
| RU2614386C2 (ru) | Средство, индуцирующее иммунитет | |
| US11478539B2 (en) | Immunity-inducing agent | |
| RU2733841C2 (ru) | Иммуноиндуцирующее средство | |
| US11045534B2 (en) | Immunity-inducing agent | |
| JP5589274B2 (ja) | 免疫誘導剤 | |
| BR112017021957B1 (pt) | Agente indutor da imunidade, métodos de preparação de células apresentadoras de antígeno e de células t citotóxicas e uso do agente indutor da imunidade |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20150820 |