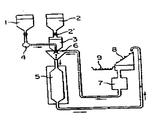
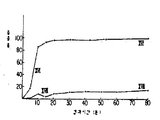
KR880000970B1 - 생물학적 유용성이 높은 경구용 약학적 조성물의 제조방법 - Google Patents
생물학적 유용성이 높은 경구용 약학적 조성물의 제조방법 Download PDFInfo
- Publication number
- KR880000970B1 KR880000970B1 KR8203134A KR820003134A KR880000970B1 KR 880000970 B1 KR880000970 B1 KR 880000970B1 KR 8203134 A KR8203134 A KR 8203134A KR 820003134 A KR820003134 A KR 820003134A KR 880000970 B1 KR880000970 B1 KR 880000970B1
- Authority
- KR
- South Korea
- Prior art keywords
- water
- drug
- oil
- unsaturated fatty
- fatty acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/21—Esters, e.g. nitroglycerine, selenocyanates
- A61K31/215—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids
- A61K31/22—Esters, e.g. nitroglycerine, selenocyanates of carboxylic acids of acyclic acids, e.g. pravastatin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/14—Esters of carboxylic acids, e.g. fatty acid monoglycerides, medium-chain triglycerides, parabens or PEG fatty acid esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Emergency Medicine (AREA)
- Dispersion Chemistry (AREA)
- Medicinal Preparation (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP10977781A JPS5813508A (ja) | 1981-07-14 | 1981-07-14 | ポリグリセロール飽和脂肪酸エステルを含む経口薬剤組成物 |
| JP109777/81 | 1981-07-14 | ||
| JP109777/81? | 1981-07-14 | ||
| JP175168/81 | 1981-11-01 | ||
| JP17516881A JPS5877810A (ja) | 1981-11-01 | 1981-11-01 | ポリグリセロ−ル不飽和脂肪酸エステルを含む経口薬剤組成物 |
| JP175168/81? | 1981-11-11 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR840000233A KR840000233A (ko) | 1984-02-18 |
| KR880000970B1 true KR880000970B1 (ko) | 1988-06-07 |
Family
ID=26449492
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR8203134A Expired KR880000970B1 (ko) | 1981-07-14 | 1982-07-14 | 생물학적 유용성이 높은 경구용 약학적 조성물의 제조방법 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US4751241A (OSRAM) |
| KR (1) | KR880000970B1 (OSRAM) |
| CH (1) | CH652307A5 (OSRAM) |
| DE (1) | DE3224619A1 (OSRAM) |
Families Citing this family (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5656620A (en) * | 1984-01-28 | 1997-08-12 | Ismail; Roshdy | Method for treating pain |
| DE3588023D1 (de) * | 1984-01-28 | 1995-06-29 | Roshdy Ismail | Mittel zur Behandlung von Herzerkrankungen. |
| US5229164A (en) * | 1985-12-19 | 1993-07-20 | Capsoid Pharma Gmbh | Process for producing individually dosed administration forms |
| SE457693B (sv) * | 1986-07-01 | 1989-01-23 | Drilletten Ab | Komposition med reglerad frigoering vari ett biologiskt material aer loest eller dispergerat i en l2-fas |
| JP2643217B2 (ja) * | 1988-01-22 | 1997-08-20 | エーザイ株式会社 | 脂溶性物質水性液 |
| AU645003B2 (en) * | 1988-11-08 | 1994-01-06 | Takeda Chemical Industries Ltd. | Sustained release preparations |
| JP2893191B2 (ja) * | 1988-11-08 | 1999-05-17 | 武田薬品工業株式会社 | 放出制御性マトリックス剤 |
| JPH02188523A (ja) * | 1989-01-13 | 1990-07-24 | M S C:Kk | 睡眠時無呼吸症治療薬 |
| DE69008534T2 (de) * | 1989-08-03 | 1994-08-18 | Hisamitsu Pharmaceutical Co | Hautcremezubereitung zur äusserlichen verwendung. |
| IE65045B1 (en) * | 1990-04-28 | 1995-10-04 | Takeda Chemical Industries Ltd | Granulated preparations and method of producing the same |
| US5179122A (en) * | 1991-02-11 | 1993-01-12 | Eastman Kodak Company | Nutritional supplement containing vitamin e |
| EP0573978B1 (en) * | 1992-06-12 | 1999-04-21 | Kao Corporation | Bath additive composition comprising surfactant-containing seamless capsules and method for producing the capsules. |
| IT1256386B (it) * | 1992-11-13 | 1995-12-04 | Luigi Boltri | Composizioni farmaceutiche comprendenti un farmaco,una sostanza polimerica reticolata,un olio ed un agente tensioattivo |
| WO1994015595A1 (en) * | 1993-01-06 | 1994-07-21 | Jemo-Pharm A/S | Medium comprising a pharmacological/biological active substance |
| DE19647352C2 (de) * | 1996-11-15 | 2000-06-29 | Aqua Nova Getraenketechnologie | Nicht alkoholisches Getränk mit einem Gehalt an Q 10 |
| DE19722405A1 (de) * | 1997-05-28 | 1998-12-03 | Ifac Inst Fuer Angewandte Coll | Einkapseln von wasserunlöslichen Wirkstoffen mit amphiphilem Charakter |
| WO1999022719A1 (fr) * | 1997-10-30 | 1999-05-14 | Morishita Jintan Co., Ltd. | Preparation de capsule contenant un acide gras insature ou un derive de celui-ci, et procede de fabrication |
| US6280770B1 (en) | 1998-08-13 | 2001-08-28 | Cima Labs Inc. | Microemulsions as solid dosage forms for oral administration |
| IT1304406B1 (it) * | 1998-10-21 | 2001-03-19 | Danital Italia S R L | Preparazione per la veicolazione di principi attivi basata su acidigrassi polinsaturi del gruppo omega 3. |
| CZ20022047A3 (cs) | 1999-12-23 | 2003-09-17 | Pfizer Products Inc. | Farmaceutické kompozice poskytující zvýšenou koncentraci léčiva |
| US6692771B2 (en) * | 2001-02-23 | 2004-02-17 | Cima Labs Inc. | Emulsions as solid dosage forms for oral administration |
| DE10133305B4 (de) * | 2001-07-12 | 2004-06-03 | Aquanova German Solubilisate Technologies (Agt) Gmbh | Ubichinon Konzentrat |
| JP3549197B2 (ja) * | 2001-08-10 | 2004-08-04 | 日清ファルマ株式会社 | ユビキノン含有製剤 |
| TW200302056A (en) * | 2002-01-18 | 2003-08-01 | Kaneka Corp | Method for stabilizing reduced coenzyme Q10 and composition therefor |
| CA2513276C (en) * | 2003-01-17 | 2012-09-04 | Taiyo Kagaku Co., Ltd. | Compositions containing coenzyme q10 |
| CA2520660C (en) * | 2003-03-28 | 2013-08-20 | Sigmoid Biotechnologies Limited | Solid oral dosage form containing seamless microcapsules |
| US20080020018A1 (en) * | 2004-09-27 | 2008-01-24 | Joey Moodley | Combination Products |
| US8480797B2 (en) | 2005-09-12 | 2013-07-09 | Abela Pharmaceuticals, Inc. | Activated carbon systems for facilitating use of dimethyl sulfoxide (DMSO) by removal of same, related compounds, or associated odors |
| AU2006291134C1 (en) | 2005-09-12 | 2013-08-15 | Abela Pharmaceuticals, Inc. | Systems for removing dimethyl sulfoxide (DMSO) or related compounds, or odors associated with same |
| WO2007033180A1 (en) | 2005-09-12 | 2007-03-22 | Abela Pharmaceuticals, Inc. | Materials for facilitating administration of dimethyl sulfoxide (dmso) and related compounds |
| EP1937286B1 (en) | 2005-09-12 | 2016-03-09 | Abela Pharmaceuticals, Inc. | Compositions comprising dimethyl sulfoxide (dmso) |
| US8506956B2 (en) * | 2005-10-31 | 2013-08-13 | Kaneka Corporation | Method for stabilizing reduced coenzyme Q10 |
| JP5128801B2 (ja) * | 2006-10-16 | 2013-01-23 | フロイント産業株式会社 | 高水分散性粉末とその製造方法 |
| WO2008122965A2 (en) * | 2007-04-04 | 2008-10-16 | Sigmoid Pharma Limited | Pharmaceutical cyclosporin compositions |
| JP2010527285A (ja) * | 2007-04-26 | 2010-08-12 | シグモイド・ファーマ・リミテッド | 複数のミニカプセルの製造 |
| WO2008132710A2 (en) * | 2007-05-01 | 2008-11-06 | Sigmoid Pharma Limited | Pharmaceutical nimodipine compositions |
| TW200918096A (en) * | 2007-06-22 | 2009-05-01 | Kaneka Corp | Composition comprising bioactive substance |
| EP2186789B1 (en) * | 2007-08-22 | 2013-12-25 | Kaneka Corporation | Method of producing reduced coenzyme q10 and method of stabilizing the same |
| WO2009025372A1 (ja) * | 2007-08-23 | 2009-02-26 | Kaneka Corporation | 還元型補酵素q10含有組成物及びその安定化方法 |
| BRPI0921494A2 (pt) * | 2008-11-03 | 2018-10-30 | Prad Reasearch And Development Ltd | método de planejamento de uma operação de amostragem para uma formação subterrãnea, método de contolar uma operação de amostragem de formação subterrânea, método de controlar uma operação de perfuração para uma formação subterrãnea, e método de realizar uma amostragem durante a operação de perfuração. |
| WO2010133609A2 (en) | 2009-05-18 | 2010-11-25 | Sigmoid Pharma Limited | Composition comprising oil drops |
| US9878036B2 (en) | 2009-08-12 | 2018-01-30 | Sigmoid Pharma Limited | Immunomodulatory compositions comprising a polymer matrix and an oil phase |
| US9855212B2 (en) | 2009-10-30 | 2018-01-02 | Abela Pharmaceuticals, Inc. | Dimethyl sulfoxide (DMSO) or DMSO and methylsulfonylmethane (MSM) formulations to treat infectious diseases |
| GB201020032D0 (en) | 2010-11-25 | 2011-01-12 | Sigmoid Pharma Ltd | Composition |
| GB201212010D0 (en) | 2012-07-05 | 2012-08-22 | Sigmoid Pharma Ltd | Formulations |
| GB201319791D0 (en) | 2013-11-08 | 2013-12-25 | Sigmoid Pharma Ltd | Formulations |
| AU2015217651A1 (en) * | 2014-02-13 | 2016-09-01 | Clariant International Ltd. | Preparation of polyglycerols |
| AU2015341695B2 (en) | 2014-11-07 | 2021-07-08 | Sublimity Therapeutics Limited | Compositions comprising cyclosporin |
| JP7560982B2 (ja) * | 2020-09-18 | 2024-10-03 | 日清ファルマ株式会社 | コエンザイムq10含有水溶性組成物 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3932634A (en) * | 1973-06-28 | 1976-01-13 | Pfizer Inc. | High potency vitamin water dispersible formulations |
| US3966632A (en) * | 1974-06-06 | 1976-06-29 | G. D. Searle & Co. | Vegetable oil emulsion |
| US4073943A (en) * | 1974-09-11 | 1978-02-14 | Apoteksvarucentralen Vitrum Ab | Method of enhancing the administration of pharmalogically active agents |
| US3996355A (en) * | 1975-01-02 | 1976-12-07 | American Home Products Corporation | Permanent suspension pharmaceutical dosage form |
| SE445174B (sv) * | 1978-03-07 | 1986-06-09 | Sandoz Ag | Farmaceutisk komposition innehallande en cyklosporin och en berarsubstans |
| JPS574916A (en) * | 1980-05-28 | 1982-01-11 | Furointo Sangyo Kk | Activation of medical preparation |
| US4327076A (en) * | 1980-11-17 | 1982-04-27 | Life Savers, Inc. | Compressed chewable antacid tablet and method for forming same |
| US4327077A (en) * | 1981-05-29 | 1982-04-27 | Life Savers, Inc. | Compressed chewable antacid tablet and method for forming same |
-
1982
- 1982-07-01 DE DE19823224619 patent/DE3224619A1/de active Granted
- 1982-07-06 CH CH4123/82A patent/CH652307A5/de not_active IP Right Cessation
- 1982-07-14 KR KR8203134A patent/KR880000970B1/ko not_active Expired
-
1985
- 1985-04-19 US US06/724,502 patent/US4751241A/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| DE3224619A1 (de) | 1983-05-19 |
| KR840000233A (ko) | 1984-02-18 |
| CH652307A5 (de) | 1985-11-15 |
| US4751241A (en) | 1988-06-14 |
| DE3224619C2 (OSRAM) | 1990-10-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR880000970B1 (ko) | 생물학적 유용성이 높은 경구용 약학적 조성물의 제조방법 | |
| US5672358A (en) | Controlled release aqueous emulsion | |
| JPS5813508A (ja) | ポリグリセロール飽和脂肪酸エステルを含む経口薬剤組成物 | |
| AU648573B2 (en) | Self-emulsifying glasses | |
| JP2006511536A (ja) | 水貧溶性薬物のバイオアベイラビリティーを改善する自由流動性固形製剤およびその製造方法 | |
| KR100425226B1 (ko) | 아세클로페낙을 함유하는 경제적인 경구용 제제의 조성 및제법 | |
| JPS6230965B2 (OSRAM) | ||
| EP0985411B1 (en) | Sterol esters in tableted solid dosage forms | |
| KR100887034B1 (ko) | 부틸프탈라이드 자가 에멀젼화 약물 전달 시스템, 그 제조방법 및 응용 | |
| CN112353845B (zh) | 含中药挥发油的热熔挤出组合物及其制备方法和药物制剂 | |
| US5189066A (en) | Pharmaceutical compositions of tebufelone | |
| EP0431659B1 (en) | Pharmaceutical compositions of tebufelone | |
| TW202128150A (zh) | 3'—[(2z)—[1—(3,4—二甲基苯基)—1,5—二氫—3—甲基—5—側氧基—4h—吡唑—4—亞基]肼基]—2'—羥基—[1,1'—聯苯基]—3—甲酸及其鹽配製物 | |
| MXPA99008044A (en) | Sterol esters in solid dose forms as table |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0109 | Patent application |
St.27 status event code: A-0-1-A10-A12-nap-PA0109 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| PG1605 | Publication of application before grant of patent |
St.27 status event code: A-2-2-Q10-Q13-nap-PG1605 |
|
| PE0701 | Decision of registration |
St.27 status event code: A-1-2-D10-D22-exm-PE0701 |
|
| PR0701 | Registration of establishment |
St.27 status event code: A-2-4-F10-F11-exm-PR0701 |
|
| PR1002 | Payment of registration fee |
St.27 status event code: A-2-2-U10-U11-oth-PR1002 Fee payment year number: 1 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 4 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 5 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 6 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 7 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 8 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 9 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 10 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 11 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 12 |
|
| PC1903 | Unpaid annual fee |
St.27 status event code: A-4-4-U10-U13-oth-PC1903 Not in force date: 20000608 Payment event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE |
|
| PC1903 | Unpaid annual fee |
St.27 status event code: N-4-6-H10-H13-oth-PC1903 Ip right cessation event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE Not in force date: 20000608 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-5-5-R10-R18-oth-X000 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-5-5-R10-R18-oth-X000 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-5-5-R10-R18-oth-X000 |