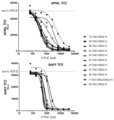
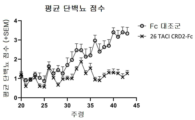
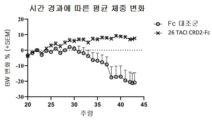
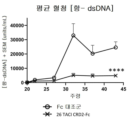
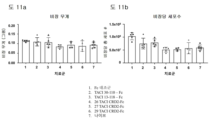
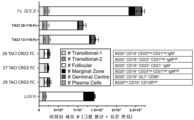
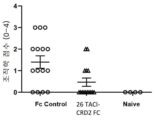
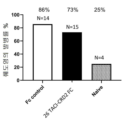
KR20230029622A - April 및 baff 억제 면역조절 단백질 및 사용 방법 - Google Patents
April 및 baff 억제 면역조절 단백질 및 사용 방법 Download PDFInfo
- Publication number
- KR20230029622A KR20230029622A KR1020227043140A KR20227043140A KR20230029622A KR 20230029622 A KR20230029622 A KR 20230029622A KR 1020227043140 A KR1020227043140 A KR 1020227043140A KR 20227043140 A KR20227043140 A KR 20227043140A KR 20230029622 A KR20230029622 A KR 20230029622A
- Authority
- KR
- South Korea
- Prior art keywords
- taci
- amino acid
- seq
- immunomodulatory protein
- polypeptide
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70578—NGF-receptor/TNF-receptor superfamily, e.g. CD27, CD30, CD40, CD95
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
- A61K38/1774—Immunoglobulin superfamily (e.g. CD2, CD4, CD8, ICAM molecules, B7 molecules, Fc-receptors, MHC-molecules)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
- A61K38/1793—Receptors; Cell surface antigens; Cell surface determinants for cytokines; for lymphokines; for interferons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/6425—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent the peptide or protein in the drug conjugate being a receptor, e.g. CD4, a cell surface antigen, i.e. not a peptide ligand targeting the antigen, or a cell surface determinant, i.e. a part of the surface of a cell
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/715—Receptors; Cell surface antigens; Cell surface determinants for cytokines; for lymphokines; for interferons
- C07K14/7151—Receptors; Cell surface antigens; Cell surface determinants for cytokines; for lymphokines; for interferons for tumor necrosis factor [TNF], for lymphotoxin [LT]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/31—Fusion polypeptide fusions, other than Fc, for prolonged plasma life, e.g. albumin
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Cell Biology (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Molecular Biology (AREA)
- Epidemiology (AREA)
- Toxicology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Transplantation (AREA)
- Urology & Nephrology (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202063022373P | 2020-05-08 | 2020-05-08 | |
| US63/022,373 | 2020-05-08 | ||
| US202063034361P | 2020-06-03 | 2020-06-03 | |
| US63/034,361 | 2020-06-03 | ||
| US202063080643P | 2020-09-18 | 2020-09-18 | |
| US63/080,643 | 2020-09-18 | ||
| PCT/US2021/031430 WO2021226551A1 (en) | 2020-05-08 | 2021-05-07 | April and baff inhibitory immunomodulatory proteins and methods of use thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20230029622A true KR20230029622A (ko) | 2023-03-03 |
Family
ID=76270049
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227043140A Pending KR20230029622A (ko) | 2020-05-08 | 2021-05-07 | April 및 baff 억제 면역조절 단백질 및 사용 방법 |
| KR1020227043139A Pending KR20230029621A (ko) | 2020-05-08 | 2021-05-07 | T 세포 억제 단백질을 포함하거나 포함하지 않는 april 및 baff 억제 면역조절 단백질 및 이의 사용 방법 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227043139A Pending KR20230029621A (ko) | 2020-05-08 | 2021-05-07 | T 세포 억제 단백질을 포함하거나 포함하지 않는 april 및 baff 억제 면역조절 단백질 및 이의 사용 방법 |
Country Status (15)
| Country | Link |
|---|---|
| US (7) | US12202882B2 (enExample) |
| EP (2) | EP4146684A2 (enExample) |
| JP (3) | JP7748393B2 (enExample) |
| KR (2) | KR20230029622A (enExample) |
| CN (5) | CN117736297A (enExample) |
| AU (3) | AU2021268033A1 (enExample) |
| BR (2) | BR112022022433A2 (enExample) |
| CA (2) | CA3178882A1 (enExample) |
| CL (1) | CL2022003104A1 (enExample) |
| CO (1) | CO2022017780A2 (enExample) |
| IL (2) | IL297981A (enExample) |
| MX (2) | MX2022013998A (enExample) |
| PE (1) | PE20230494A1 (enExample) |
| TW (1) | TW202208414A (enExample) |
| WO (2) | WO2021226553A2 (enExample) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102813968B1 (ko) | 2017-10-10 | 2025-05-29 | 알파인 이뮨 사이언시즈, 인코포레이티드 | Ctla-4 변이체 면역조절 단백질 및 이의 용도 |
| US12297253B2 (en) | 2018-01-03 | 2025-05-13 | Alpine Immune Sciences, Inc. | Multi-domain immunomodulatory proteins and methods of use thereof |
| CN113573732A (zh) * | 2019-12-24 | 2021-10-29 | 荣昌生物制药(烟台)股份有限公司 | TACI-Fc融合蛋白及其用途 |
| IL297981A (en) | 2020-05-08 | 2023-01-01 | Alpine Immune Sciences Inc | April and baff inhibitory immunomodulatory proteins with and without a t cell inhibitory protein and methods of use thereof |
| CN116897159A (zh) * | 2021-03-31 | 2023-10-17 | 江苏恒瑞医药股份有限公司 | 截短的taci多肽及其融合蛋白和用途 |
| CA3216795A1 (en) * | 2021-05-07 | 2022-11-10 | Alpine Immune Sciences, Inc. | Methods of dosing and treatment with a taci-fc fusion immunomodulatory protein |
| CA3228678A1 (en) * | 2021-08-11 | 2023-02-16 | Amato J. Giaccia | Methods of reducing production of iga, igm and/or igg using sbcma variants and fc fusion proteins thereof |
| IL312791A (en) * | 2021-11-17 | 2024-07-01 | Aurinia Pharmaceuticals Inc | B cell activating factor (baff)-a proliferation inducing ligand (april) dual inhibitors |
| CN118475598A (zh) * | 2022-06-08 | 2024-08-09 | 荣昌生物制药(烟台)股份有限公司 | 用TACI-Fc融合蛋白治疗重症肌无力的方法 |
| WO2024044721A1 (en) * | 2022-08-25 | 2024-02-29 | University Of Washington | Injectable recombinant protein-based hydrogels for therapeutic delivery |
| WO2024077018A2 (en) * | 2022-10-04 | 2024-04-11 | Alpine Immune Sciences, Inc. | Methods and uses of taci-fc fusion immunomodulatory protein |
| JP2025540182A (ja) * | 2022-12-05 | 2025-12-11 | アレクシオン ファーマシューティカルズ, インコーポレイテッド | Baff、april、及び新生児fc受容体の多機能阻害のためのtaci-fc融合タンパク質 |
| TWI882569B (zh) * | 2022-12-07 | 2025-05-01 | 大陸商榮昌生物製藥(煙臺)股份有限公司 | TACI-Fc融合蛋白液體藥物製劑 |
| TW202525323A (zh) * | 2023-07-06 | 2025-07-01 | 大陸商榮昌生物製藥(煙臺)股份有限公司 | 用TACI-Fc融合蛋白治療ANCA相關性血管炎的方法 |
| WO2025240392A1 (en) * | 2024-05-13 | 2025-11-20 | Paragon Therapeutics, Inc. | Fusion proteins |
Family Cites Families (173)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7045313B1 (en) | 1982-11-30 | 2006-05-16 | The United States Of America As Represented By The Department Of Health And Human Services | Recombinant vaccinia virus containing a chimeric gene having foreign DNA flanked by vaccinia regulatory DNA |
| US5168062A (en) | 1985-01-30 | 1992-12-01 | University Of Iowa Research Foundation | Transfer vectors and microorganisms containing human cytomegalovirus immediate-early promoter-regulatory DNA sequence |
| US4751180A (en) | 1985-03-28 | 1988-06-14 | Chiron Corporation | Expression using fused genes providing for protein product |
| US4935233A (en) | 1985-12-02 | 1990-06-19 | G. D. Searle And Company | Covalently linked polypeptide cell modulators |
| IL85035A0 (en) | 1987-01-08 | 1988-06-30 | Int Genetic Eng | Polynucleotide molecule,a chimeric antibody with specificity for human b cell surface antigen,a process for the preparation and methods utilizing the same |
| EP0307434B2 (en) | 1987-03-18 | 1998-07-29 | Scotgen Biopharmaceuticals, Inc. | Altered antibodies |
| US5443964A (en) | 1987-08-10 | 1995-08-22 | Duke University | Poxvirus insertion/expression vector |
| US5283173A (en) | 1990-01-24 | 1994-02-01 | The Research Foundation Of State University Of New York | System to detect protein-protein interactions |
| US6410010B1 (en) | 1992-10-13 | 2002-06-25 | Board Of Regents, The University Of Texas System | Recombinant P53 adenovirus compositions |
| CA2102623C (en) | 1991-05-06 | 2003-04-22 | Jeffrey Schlom | Recombinant virus expressing carcinoembryonic antigen and methods of use thereof |
| DE122004000008I1 (de) | 1991-06-14 | 2005-06-09 | Genentech Inc | Humanisierter Heregulin Antikörper. |
| US5262522A (en) | 1991-11-22 | 1993-11-16 | Immunex Corporation | Receptor for oncostatin M and leukemia inhibitory factor |
| EP0714409A1 (en) | 1993-06-16 | 1996-06-05 | Celltech Therapeutics Limited | Antibodies |
| US5457035A (en) | 1993-07-23 | 1995-10-10 | Immunex Corporation | Cytokine which is a ligand for OX40 |
| JP4418965B2 (ja) | 1994-09-23 | 2010-02-24 | タップイミューン・インコーポレイテッド | 内在性ペプチドを有するmhcクラスi分子の発現を増強する方法 |
| DK0784483T3 (da) | 1994-10-03 | 2001-03-26 | Us Gov Health & Human Serv | Præparat indeholdende et rekombinant virus, der udtrykker et antigen, og et rekombinant virus, der udtrykker et immunstimul |
| US5731168A (en) | 1995-03-01 | 1998-03-24 | Genentech, Inc. | Method for making heteromultimeric polypeptides |
| US5641870A (en) | 1995-04-20 | 1997-06-24 | Genentech, Inc. | Low pH hydrophobic interaction chromatography for antibody purification |
| CA2266439C (en) | 1996-10-25 | 2009-06-16 | Human Genome Sciences, Inc. | Neutrokine .alpha. |
| US5969102A (en) | 1997-03-03 | 1999-10-19 | St. Jude Children's Research Hospital | Lymphocyte surface receptor that binds CAML, nucleic acids encoding the same and methods of use thereof |
| AU751659B2 (en) | 1997-05-02 | 2002-08-22 | Genentech Inc. | A method for making multispecific antibodies having heteromultimeric and common components |
| GB9801930D0 (en) | 1998-01-29 | 1998-03-25 | Univ London | Mutant herpes simplex viruses and uses thereof |
| DE69937291T2 (de) | 1998-04-02 | 2008-07-10 | Genentech, Inc., South San Francisco | Antikörpervarianten und fragmente davon |
| US6194551B1 (en) | 1998-04-02 | 2001-02-27 | Genentech, Inc. | Polypeptide variants |
| GB2337755B (en) | 1998-05-29 | 2003-10-29 | Secr Defence | Virus vaccine |
| CN1348463A (zh) | 1998-08-07 | 2002-05-08 | 华盛顿大学 | 免疫学单纯疱疹病毒抗原及其使用方法 |
| PL209535B1 (pl) * | 1999-01-07 | 2011-09-30 | Zymogenetics Inc | Zastosowanie białka fuzyjnego |
| US7833529B1 (en) | 1999-01-07 | 2010-11-16 | Zymogenetics, Inc. | Methods for inhibiting B lymphocyte proliferation with soluble ztnf4 receptor |
| US20060067933A1 (en) | 1999-01-07 | 2006-03-30 | Gross Jane A | Soluble receptor BR43x2 and methods of using |
| KR101155191B1 (ko) | 1999-01-15 | 2012-06-13 | 제넨테크, 인크. | 효과기 기능이 변화된 폴리펩티드 변이체 |
| US6737056B1 (en) | 1999-01-15 | 2004-05-18 | Genentech, Inc. | Polypeptide variants with altered effector function |
| US20030095967A1 (en) | 1999-01-25 | 2003-05-22 | Mackay Fabienne | BAFF, inhibitors thereof and their use in the modulation of B-cell response and treatment of autoimmune disorders |
| US20030022233A1 (en) | 1999-04-30 | 2003-01-30 | Raymond G. Goodwin | Methods of use of the taci/taci-l interaction |
| WO2001011034A2 (en) | 1999-08-09 | 2001-02-15 | Targeted Genetics Corporation | Enhancement of expression of a single-stranded, heterologous nucleotide sequence from recombinant viral vectors by designing the sequence such that it forms intrastrand base pairs |
| US7329728B1 (en) | 1999-10-25 | 2008-02-12 | The Scripps Research Institute | Ligand activated transcriptional regulator proteins |
| EP1666052B1 (en) | 2000-02-16 | 2011-06-08 | Genentech, Inc. | Anti-APRIL monoclonal antibody and its use for the treatment of an immune related disease or cancer |
| US20040013674A1 (en) | 2001-04-27 | 2004-01-22 | Christine Ambrose | Taci as an anti-tumor agent |
| EP1746106A3 (en) | 2000-04-27 | 2007-03-14 | Biogen Idec MA Inc. | Use of TACI as an anti-tumor agent |
| NZ521629A (en) | 2000-04-27 | 2004-05-28 | Biogen Inc | TACI as an anti-tumor agent |
| EP1280826B1 (en) | 2000-05-12 | 2007-05-02 | Amgen Inc. | Polypeptides for inhibiting april-mediated b- and t-cell proliferation. |
| EP1294949A4 (en) | 2000-06-15 | 2004-08-25 | Human Genome Sciences Inc | HUMAN TUMORNESCROSE FACTOR DELTA AND EPSILON |
| DK1294769T3 (da) | 2000-06-16 | 2011-04-26 | Human Genome Sciences Inc | Antistoffer der immunspecifikt binder til BLyS |
| US7879328B2 (en) | 2000-06-16 | 2011-02-01 | Human Genome Sciences, Inc. | Antibodies that immunospecifically bind to B lymphocyte stimulator |
| US7220840B2 (en) | 2000-06-16 | 2007-05-22 | Human Genome Sciences, Inc. | Antibodies that immunospecifically bind to B lymphocyte stimulator protein |
| EP1360290A2 (en) | 2000-06-23 | 2003-11-12 | Maxygen, Inc. | Co-stimulatory molecules |
| AU2002238052A1 (en) | 2001-02-20 | 2002-09-04 | Zymogenetics, Inc. | Antibodies that bind both bcma and taci |
| DE60234202D1 (de) | 2001-05-24 | 2009-12-10 | Zymogenetics Inc | Taci-immunoglobulin-fusionsproteine |
| EP1401870A4 (en) | 2001-05-24 | 2006-04-19 | Human Genome Sciences | ANTIBODIES AGAINST TUMOR NECROSIS FACTOR DELTA (APRIL) |
| PL377119A1 (pl) | 2001-08-03 | 2006-01-23 | Genentech, Inc. | Peptydy TACIs i BR3 i ich zastosowanie |
| AU2002364954A1 (en) | 2001-11-16 | 2003-07-15 | Human Genome Sciences, Inc. | ANTIBODIES THAT IMMUNOSPECIFICALLY BIND TO BLyS |
| DK1461073T3 (da) | 2001-11-30 | 2010-03-29 | Us Gov Health & Human Serv | Peptidagonister til prostataspecifikt antigen og anvendelser heraf |
| US8188231B2 (en) | 2002-09-27 | 2012-05-29 | Xencor, Inc. | Optimized FC variants |
| MXPA05000940A (es) | 2002-07-25 | 2005-05-16 | Genentech Inc | Anticuerpos taci y su uso. |
| JP2006502715A (ja) | 2002-10-11 | 2006-01-26 | ザイモジェネティクス,インコーポレイティド | ホモ3量体融合タンパク質の製造 |
| US7361740B2 (en) | 2002-10-15 | 2008-04-22 | Pdl Biopharma, Inc. | Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis |
| DE60332957D1 (de) | 2002-12-16 | 2010-07-22 | Genentech Inc | Immunoglobulinvarianten und deren verwendungen |
| AU2003299971A1 (en) | 2002-12-30 | 2004-07-29 | Amgen Inc. | Combination therapy with co-stimulatory factors |
| US20050163775A1 (en) | 2003-06-05 | 2005-07-28 | Genentech, Inc. | Combination therapy for B cell disorders |
| CA2528434A1 (en) | 2003-06-05 | 2005-01-06 | Genentech, Inc. | Combination therapy for b cell disorders |
| AU2004285455A1 (en) | 2003-10-20 | 2005-05-12 | Biogen Idec Ma Inc. | Therapeutic regimens for BAFF antagonists |
| ATE554107T1 (de) | 2003-12-19 | 2012-05-15 | Genentech Inc | Als therapeutika geeignete monovalente antikörperfragmente |
| PL1737891T3 (pl) | 2004-04-13 | 2013-08-30 | Hoffmann La Roche | Przeciwciała przeciw selektynie p |
| EP2940043A1 (en) | 2004-07-15 | 2015-11-04 | Xencor, Inc. | Optimized fc variants |
| TWI380996B (zh) | 2004-09-17 | 2013-01-01 | Hoffmann La Roche | 抗ox40l抗體 |
| JP4958555B2 (ja) | 2004-09-22 | 2012-06-20 | 協和発酵キリン株式会社 | 安定化されたヒトIgG4抗体 |
| AU2005305182A1 (en) | 2004-11-04 | 2006-05-18 | Genentech, Inc. | Polypeptides that bind BAFF and/or APRIL |
| EP1819823A2 (en) | 2004-12-01 | 2007-08-22 | Bayer Schering Pharma Aktiengesellschaft | Generation of replication competent viruses for therapeutic use |
| MX2007008017A (es) * | 2004-12-31 | 2007-09-12 | Genentech Inc | Polipeptidos que se ligan a br3 y usos de los mismos. |
| DK3050963T3 (da) | 2005-03-31 | 2019-12-09 | Chugai Pharmaceutical Co Ltd | Fremgangsmåde til fremstilling af polypeptid ved regulering af arrangement |
| RS54271B1 (sr) | 2005-07-01 | 2016-02-29 | E. R. Squibb & Sons, L.L.C. | Humana monoklonska antitela za ligand programirane smrti 1 (pd-l1) |
| AU2006278227B2 (en) | 2005-08-09 | 2011-10-20 | Ares Trading S.A. | Methods for the treatment and prevention of abnormal cell proliferation using TACI-fusion molecules |
| MX2008001661A (es) | 2005-08-09 | 2008-04-07 | Ares Trading Sa | Metodos para tratar afecciones malignas de celulas b que utilizan una molecula de fusion del activador de transmembrana o interactor del ligando de ciclofilina de modulacion de calcio-ig. |
| AU2006281978A1 (en) | 2005-08-12 | 2007-02-22 | Garvan Institute Of Medical Research | Phrophylactic and/or therapeutic method for treatment of autoimmune disease |
| JP2007103345A (ja) | 2005-09-07 | 2007-04-19 | Toyota Motor Corp | チューブ状固体高分子型燃料電池、及びチューブ状固体高分子型燃料電池の製造方法 |
| WO2007030803A2 (en) | 2005-09-09 | 2007-03-15 | Zymogenetics, Inc. | Method for preparing trimeric proteins |
| US9168286B2 (en) | 2005-10-13 | 2015-10-27 | Human Genome Sciences, Inc. | Methods and compositions for use in treatment of patients with autoantibody positive disease |
| EP1933873A4 (en) | 2005-10-13 | 2009-12-02 | Human Genome Sciences Inc | METHODS AND COMPOSITIONS FOR THE TREATMENT OF PATIENTS WITH POSITIVE DISEASES FOR SELF-ANTIBODIES |
| WO2007056288A2 (en) | 2005-11-08 | 2007-05-18 | Biogen Idec Ma Inc. | Methods of evaluating baff |
| US8784812B2 (en) | 2006-05-15 | 2014-07-22 | Zymogenetics, Inc. | Methods for treating autoimmune diseases using a TACI-Ig fusion molecule |
| BRPI0714495B8 (pt) | 2006-07-21 | 2021-05-25 | California Inst Of Techn | lentivírus deficiente para replicação recombinante pseudotipado |
| JP2010501622A (ja) | 2006-08-28 | 2010-01-21 | アレス トレーディング ソシエテ アノニム | Fc−融合タンパク質の精製法 |
| PL2061803T5 (pl) | 2006-08-28 | 2023-03-27 | Ares Trading S.A. | Proces oczyszczania białek zawierających fc |
| CN101541825B (zh) | 2006-08-28 | 2013-08-14 | 阿雷斯贸易股份有限公司 | Fc融合蛋白的纯化方法 |
| WO2008092117A2 (en) | 2007-01-25 | 2008-07-31 | Xencor, Inc. | Immunoglobulins with modifications in the fcr binding region |
| ATE509107T1 (de) | 2007-01-26 | 2011-05-15 | Merck Serono Sa | Aufreinigung von fc-tact-fusionsproteinen unter verwendung der ölkörper-technik |
| MX2009010125A (es) | 2007-03-27 | 2010-02-17 | Zymogenetics Inc | Combinacion de inhibicion de estimulador de linfocitos b (blys) y/o inhibicion de ligando inductor de proliferacion (april) e inmunosupresores para tratamiento de enfermedad autoinmunitaria. |
| PT2167038T (pt) | 2007-06-13 | 2018-06-01 | Zymogenetics Inc | Utilização de proteína de fusão taci-ig, tal como atacicept para o fabrico de um medicamento para tratar lúpus eritematoso |
| CN101323643B (zh) | 2007-06-15 | 2010-12-01 | 烟台荣昌生物工程有限公司 | 优化的TACI-Fc融合蛋白 |
| PL2173890T3 (pl) | 2007-06-21 | 2011-07-29 | Univ Muenchen Tech | Białka czynne biologicznie o zwiększonej stabilności in vivo i (lub) in vitro |
| CA2693707A1 (en) | 2007-07-13 | 2009-03-05 | The Johns Hopkins University | B7-dc variants |
| DK2233149T3 (en) | 2007-10-16 | 2016-05-17 | Zymogenetics Inc | COMBINATION OF TRANSMEMBRANAKTIVATOR AND CALCIUM MODULATOR AND cyclophilin-LIGAND INTERAKTOR (TACI) AND ANTI-CD20 MEANS FOR TREATMENT OF AUTO-IMMUNE DISEASE |
| AU2008314689A1 (en) | 2007-10-22 | 2009-04-30 | Merck Serono S.A. | Method for purifying an Fc-containing protein |
| WO2009062916A1 (en) | 2007-11-12 | 2009-05-22 | Ares Trading S.A. | Taci-immunoglobulin fusion proteins for treatment of optic neuritis |
| JP2011503036A (ja) | 2007-11-12 | 2011-01-27 | アレス トレーディング ソシエテ アノニム | 再発性多発性硬化症治療のためのtaci−免疫グロブリン融合タンパク質 |
| AU2008322930B2 (en) | 2007-11-12 | 2014-05-15 | Ares Trading S.A. | Formulations for TACI-immunoglobulin fusion proteins |
| DK2222861T3 (en) | 2007-12-11 | 2018-02-05 | Univ North Carolina Chapel Hill | POLYPURIN-TRACT MODIFIED RETROVIRAL VECTORS |
| CA2720682A1 (en) | 2008-04-25 | 2009-10-29 | Zymogenetics, Inc. | Levels of bcma protein expression on b cells and use in diagnostic methods |
| US8003335B2 (en) | 2008-04-30 | 2011-08-23 | Universite Paris-SUD11 | Levels of APRIL in serum and use in diagnostic methods |
| PL2291657T3 (pl) | 2008-05-01 | 2016-09-30 | Stężenie heterotrimerów blys/april w surowicy oraz zastosowanie w sposobach diagnostycznych | |
| EP2310409A2 (en) | 2008-06-17 | 2011-04-20 | Apogenix GmbH | Multimeric tnf receptors |
| CN102203125A (zh) | 2008-08-25 | 2011-09-28 | 安普利穆尼股份有限公司 | Pd-1拮抗剂及其使用方法 |
| HRP20170908T1 (hr) | 2008-12-09 | 2017-09-22 | F. Hoffmann - La Roche Ag | Protutijela anti-pd-l1 i njihova uporaba za poboljšanje funkcije t-stanice |
| EP2396035A4 (en) | 2009-02-12 | 2012-09-12 | Human Genome Sciences Inc | USE OF ANTAGONISTS OF PROTEIN STIMULATING LYMPHOCYTES B TO PROMOTE GRAFT TOLERANCE |
| JP2012527875A (ja) | 2009-05-28 | 2012-11-12 | グラクソ グループ リミテッド | 抗原結合タンパク質 |
| WO2011109280A1 (en) | 2010-03-05 | 2011-09-09 | Lerner Research Institute | Methods and compositions to treat immune-mediated disorders |
| CN101851278B (zh) | 2010-05-26 | 2013-03-13 | 石药集团中奇制药技术(石家庄)有限公司 | B细胞激活因子拮抗剂及其制备方法与用途 |
| JP2013530188A (ja) | 2010-06-18 | 2013-07-25 | ヒューマン ゲノム サイエンシズ,インコーポレイテッド | 喘息並びに呼吸器系の他のアレルギー性及び炎症性症状を治療するためのbリンパ球刺激タンパク質アンタゴニストの使用 |
| EP2614078B1 (en) | 2010-09-10 | 2018-11-07 | TiGenix, S.A.U. | Stem cell culture media and methods |
| CN102085368B (zh) | 2011-01-19 | 2013-06-12 | 烟台荣昌生物工程有限公司 | 优化的TACI-Fc融合蛋白用于制备治疗系统性红斑狼疮药物的应用 |
| AU2012229048B2 (en) | 2011-03-16 | 2016-01-21 | Amgen Inc | Fc variants |
| AU2012243039B2 (en) | 2011-04-08 | 2017-07-13 | Immune Design Corp. | Immunogenic compositions and methods of using the compositions for inducing humoral and cellular immune responses |
| SI2697257T1 (sl) | 2011-04-13 | 2017-02-28 | Bristol-Myers Squibb Company | FC fuzijski proteini, ki vsebujejo nove linkerje ali razmestitve |
| WO2012149364A1 (en) | 2011-04-28 | 2012-11-01 | Diamond Don J | Tumor associated vaccines and compositions for disrupting tumor-derived immunosuppression for use in combination cancer immunotherapy |
| WO2013041029A1 (en) * | 2011-09-23 | 2013-03-28 | Igenimed Pharmaceuticals Inc. | Novel soluble ctla4 variants |
| TWI476001B (zh) | 2011-12-26 | 2015-03-11 | Ind Tech Res Inst | 三倍體Fc融合蛋白及其用途 |
| KR102057356B1 (ko) | 2012-02-27 | 2019-12-18 | 아뮤닉스 파마슈티컬스, 인크. | Xten 콘주게이트 조성물 및 그의 제조 방법 |
| CN102585016B (zh) * | 2012-03-06 | 2014-06-04 | 江苏健德生物药业有限公司 | 一种可同时抑制t、b淋巴细胞功能的免疫融合蛋白、其制备方法及其应用 |
| EP3480316B1 (en) | 2012-03-30 | 2022-09-14 | Immune Design Corp. | Lentiviral vector particles having improved transduction efficiency for cells expressing dc-sign |
| HK1204950A1 (en) | 2012-06-06 | 2015-12-11 | 昂考梅德药品有限公司 | Binding agents that modulate the hippo pathway and uses thereof |
| CN103232542B (zh) * | 2013-02-01 | 2015-05-13 | 殷勇 | 一种双靶标嵌合蛋白 |
| ES2751735T3 (es) | 2013-02-12 | 2020-04-01 | Bristol Myers Squibb Co | Métodos de replegado de proteínas a elevado pH |
| EP3744728A1 (en) | 2013-02-12 | 2020-12-02 | Bristol-Myers Squibb Company | Tangential flow filtration based protein refolding methods |
| AU2014236986A1 (en) | 2013-03-15 | 2015-09-03 | Biogen Ma Inc. | Treatment and prevention of acute kidney injury using anti-alpha v beta 5 antibodies |
| CN105899534B (zh) | 2014-01-15 | 2020-01-07 | 豪夫迈·罗氏有限公司 | 具有修饰的FCRN和保持的蛋白A结合性质的Fc区变体 |
| WO2015172305A1 (zh) | 2014-05-12 | 2015-11-19 | 上海康岱生物医药技术股份有限公司 | 抑制taci-baff复合物形成的融合蛋白及其制法和用途 |
| CA2947687C (en) | 2014-05-30 | 2021-07-06 | Ventana Medical Systems, Inc. | Multiplex assay for improved scoring of tumor tissues stained for pd-l1 |
| CA2955015A1 (en) | 2014-07-15 | 2016-01-21 | Immune Design Corp. | Prime-boost regimens with a tlr4 agonist adjuvant and a lentiviral vector |
| WO2016023001A1 (en) | 2014-08-08 | 2016-02-11 | The Board Of Trustees Of The Leland Stanford Junior University | Multispecific high affinity pd-1 agents and methods of use |
| NL2014108B1 (en) | 2015-01-09 | 2016-09-30 | Aduro Biotech Holdings Europe B V | Altered april binding antibodies. |
| TW202444897A (zh) | 2015-06-25 | 2024-11-16 | 美商生物細胞基因治療有限公司 | 嵌合抗原受體(car)、組合物及其使用方法 |
| MA42895A (fr) | 2015-07-15 | 2018-05-23 | Juno Therapeutics Inc | Cellules modifiées pour thérapie cellulaire adoptive |
| SMT202100527T1 (it) | 2015-07-30 | 2021-11-12 | Macrogenics Inc | Molecole di legame a pd-1 e lag-3 e loro metodi d'uso |
| HUE064791T2 (hu) | 2015-11-25 | 2024-04-28 | Visterra Inc | APRIL ellenes antitest molekulák és alkalmazásaik |
| SG11201804839WA (en) * | 2015-12-14 | 2018-07-30 | Macrogenics Inc | Bispecific molecules having immunoreactivity with pd-1 and ctla-4, and methods of use thereof |
| ES2898329T3 (es) | 2016-01-12 | 2022-03-07 | Oncotracker Inc | Métodos mejorados para supervisar el estado inmunitario de un sujeto |
| AU2017248830B2 (en) | 2016-04-15 | 2023-03-09 | Alpine Immune Sciences, Inc. | CD80 variant immunomodulatory proteins and uses thereof |
| CA3019199A1 (en) | 2016-04-15 | 2017-10-19 | Alpine Immune Sciences, Inc. | Icos ligand variant immunomodulatory proteins and uses thereof |
| US11078247B2 (en) | 2016-05-04 | 2021-08-03 | Curevac Ag | RNA encoding a therapeutic protein |
| WO2017201040A1 (en) | 2016-05-16 | 2017-11-23 | Berenson James Richard | Improved methods for monitoring immune status of a subject |
| CA3029197A1 (en) | 2016-06-24 | 2017-12-28 | Icell Gene Therapeutics Llc | Chimeric antigen receptors (cars), compositions and methods thereof |
| EP3491013A1 (en) | 2016-07-28 | 2019-06-05 | Alpine Immune Sciences, Inc. | Cd155 variant immunomodulatory proteins and uses thereof |
| US11834490B2 (en) | 2016-07-28 | 2023-12-05 | Alpine Immune Sciences, Inc. | CD112 variant immunomodulatory proteins and uses thereof |
| AU2017345764A1 (en) | 2016-10-20 | 2019-05-02 | Alpine Immune Sciences, Inc. | Secretable variant immunomodulatory proteins and engineered cell therapy |
| IL319966A (en) | 2017-03-16 | 2025-05-01 | Alpine Immune Sciences Inc | CD80 Variant Immune Modulator Proteins and Uses Thereof |
| CA3053812A1 (en) | 2017-03-16 | 2018-09-20 | Alpine Immune Sciences, Inc. | Pd-l2 variant immunomodulatory proteins and uses thereof |
| IL320149A (en) | 2017-03-16 | 2025-06-01 | Alpine Immune Sciences Inc | Pd-l1 variant immunomodulatory proteins and uses thereof |
| EP3642243A4 (en) | 2017-06-20 | 2021-04-28 | Dana-Farber Cancer Institute, Inc. | PROCESSES FOR MODULATION OF REGULATORY T-LYMPHOCYTES, REGULATORY B-LYMPHOCYTES AND IMMUNE RESPONSES USING MODULATORS OF THE AVRIL-TACI INTERACTION |
| CA3070468A1 (en) | 2017-09-01 | 2019-03-07 | Dana-Farber Cancer Institute, Inc. | Immunogenic peptides specific to bcma and taci antigens for treatment of cancer |
| KR102813968B1 (ko) | 2017-10-10 | 2025-05-29 | 알파인 이뮨 사이언시즈, 인코포레이티드 | Ctla-4 변이체 면역조절 단백질 및 이의 용도 |
| TW202500579A (zh) | 2017-10-18 | 2025-01-01 | 美商艾爾潘免疫科學有限公司 | 變異型icos 配位體免疫調節蛋白及相關組合物及方法 |
| GB201720426D0 (en) | 2017-12-07 | 2018-01-24 | Hummingbird Bioscience Pte Ltd | CD47 and BCMA antigen-binding molecules |
| US12297253B2 (en) | 2018-01-03 | 2025-05-13 | Alpine Immune Sciences, Inc. | Multi-domain immunomodulatory proteins and methods of use thereof |
| IL317065A (en) | 2018-03-02 | 2025-01-01 | Allogene Therapeutics Inc | Chimeric inducible cytokine receptors |
| US20210238295A1 (en) | 2018-04-26 | 2021-08-05 | University Of Virginia Patent Foundation | Compositions and methods for treating abdominal aortic aneurysm |
| CN110522908A (zh) | 2018-05-25 | 2019-12-03 | 荣昌生物制药(烟台)有限公司 | TACI-Fc融合蛋白用于制备治疗视神经脊髓炎谱系疾病和多发性硬化之药物中的应用 |
| WO2019241758A1 (en) | 2018-06-15 | 2019-12-19 | Alpine Immune Sciences, Inc. | Pd-1 variant immunomodulatory proteins and uses thereof |
| WO2020028572A2 (en) | 2018-08-01 | 2020-02-06 | Unum Therapeutics Inc. | ANTIBODY-COUPLED T CELL RECEPTORS (ACTRs) IN COMBINATION WITH TRANS CO-STIMULATORY MOLECULES AND THERAPEUTIC USES THEREOF |
| WO2020047329A1 (en) | 2018-08-29 | 2020-03-05 | Shattuck Labs, Inc. | Chimeric proteins comprising extracellular domains and uses thereof |
| US20220177587A1 (en) | 2018-09-19 | 2022-06-09 | Alpine Immune Sciences, Inc. | Methods and uses of variant cd80 fusion proteins and related constructs |
| US12263190B2 (en) | 2018-11-08 | 2025-04-01 | Juno Therapeutics, Inc. | Methods and combinations for treatment and T cell modulation |
| WO2020113141A2 (en) | 2018-11-30 | 2020-06-04 | Alpine Immune Sciences, Inc. | Cd86 variant immunomodulatory proteins and uses thereof |
| WO2020214867A1 (en) | 2019-04-17 | 2020-10-22 | Alpine Immune Sciences, Inc. | Methods and uses of variant icos ligand (icosl) fusion proteins |
| CN114728040A (zh) | 2019-06-14 | 2022-07-08 | 科优基因公司 | 新型白介素-2变体及其双功能融合分子 |
| US20220340642A1 (en) | 2019-09-13 | 2022-10-27 | Kyowa Kirin Co., Ltd. | Dcr3 variant |
| EP4074337B1 (en) | 2019-12-10 | 2025-05-07 | RemeGen Co., Ltd. | Pharmaceutical taci-fc fusion protein formulation |
| CN113573732A (zh) | 2019-12-24 | 2021-10-29 | 荣昌生物制药(烟台)股份有限公司 | TACI-Fc融合蛋白及其用途 |
| IL297981A (en) | 2020-05-08 | 2023-01-01 | Alpine Immune Sciences Inc | April and baff inhibitory immunomodulatory proteins with and without a t cell inhibitory protein and methods of use thereof |
| TW202210095A (zh) | 2020-06-02 | 2022-03-16 | 德商馬克專利公司 | 與a型免疫球蛋白腎病變之治療有關之方法 |
| CA3216795A1 (en) | 2021-05-07 | 2022-11-10 | Alpine Immune Sciences, Inc. | Methods of dosing and treatment with a taci-fc fusion immunomodulatory protein |
| WO2023016444A1 (zh) | 2021-08-10 | 2023-02-16 | 荣昌生物制药(烟台)股份有限公司 | 用TACI-Fc融合蛋白治疗IgA肾病的方法 |
| CN117203242A (zh) | 2021-09-30 | 2023-12-08 | 荣昌生物制药(烟台)股份有限公司 | 用TACI-Fc融合蛋白治疗干燥综合征的方法 |
| CN118475598A (zh) | 2022-06-08 | 2024-08-09 | 荣昌生物制药(烟台)股份有限公司 | 用TACI-Fc融合蛋白治疗重症肌无力的方法 |
| WO2024077018A2 (en) | 2022-10-04 | 2024-04-11 | Alpine Immune Sciences, Inc. | Methods and uses of taci-fc fusion immunomodulatory protein |
-
2021
- 2021-05-07 IL IL297981A patent/IL297981A/en unknown
- 2021-05-07 WO PCT/US2021/031432 patent/WO2021226553A2/en not_active Ceased
- 2021-05-07 MX MX2022013998A patent/MX2022013998A/es unknown
- 2021-05-07 MX MX2022013999A patent/MX2022013999A/es unknown
- 2021-05-07 CN CN202311249622.3A patent/CN117736297A/zh active Pending
- 2021-05-07 CN CN202180047593.5A patent/CN116194467A/zh active Pending
- 2021-05-07 US US17/315,168 patent/US12202882B2/en active Active
- 2021-05-07 BR BR112022022433A patent/BR112022022433A2/pt unknown
- 2021-05-07 TW TW110116616A patent/TW202208414A/zh unknown
- 2021-05-07 PE PE2022002607A patent/PE20230494A1/es unknown
- 2021-05-07 WO PCT/US2021/031430 patent/WO2021226551A1/en not_active Ceased
- 2021-05-07 CN CN202180047833.1A patent/CN115812077A/zh active Pending
- 2021-05-07 CN CN202411219401.6A patent/CN119258195A/zh active Pending
- 2021-05-07 AU AU2021268033A patent/AU2021268033A1/en active Pending
- 2021-05-07 BR BR112022022524A patent/BR112022022524A2/pt unknown
- 2021-05-07 KR KR1020227043140A patent/KR20230029622A/ko active Pending
- 2021-05-07 CA CA3178882A patent/CA3178882A1/en active Pending
- 2021-05-07 JP JP2022567473A patent/JP7748393B2/ja active Active
- 2021-05-07 US US17/923,208 patent/US20230241168A1/en active Pending
- 2021-05-07 EP EP21730004.5A patent/EP4146684A2/en active Pending
- 2021-05-07 CN CN202310582491.4A patent/CN116903727A/zh active Pending
- 2021-05-07 JP JP2022567472A patent/JP2023525032A/ja active Pending
- 2021-05-07 EP EP21730003.7A patent/EP4146683A1/en active Pending
- 2021-05-07 KR KR1020227043139A patent/KR20230029621A/ko active Pending
- 2021-05-07 AU AU2021267276A patent/AU2021267276B2/en active Active
- 2021-05-07 IL IL297980A patent/IL297980A/en unknown
- 2021-05-07 CA CA3178885A patent/CA3178885A1/en active Pending
- 2021-06-11 US US17/346,095 patent/US11274140B2/en active Active
-
2022
- 2022-11-08 CL CL2022003104A patent/CL2022003104A1/es unknown
- 2022-12-07 CO CONC2022/0017780A patent/CO2022017780A2/es unknown
-
2023
- 2023-03-17 US US18/186,098 patent/US20240218045A1/en active Pending
-
2024
- 2024-08-16 US US18/807,825 patent/US12304943B2/en active Active
- 2024-09-24 US US18/895,187 patent/US20250019412A1/en active Pending
- 2024-09-24 US US18/895,259 patent/US20250019413A1/en active Pending
-
2025
- 2025-01-22 AU AU2025200430A patent/AU2025200430A1/en active Pending
- 2025-06-19 JP JP2025103686A patent/JP2025134866A/ja active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12304943B2 (en) | April and BAFF inhibitory immunomodulatory proteins and methods of use thereof | |
| US20240279310A1 (en) | Methods of dosing and treatment with a taci-fc fusion immunomodulatory protein | |
| KR20250020681A (ko) | Ctla-4 변이체 면역조절 단백질 및 이의 용도 | |
| JP2025535041A (ja) | 自己抗体介在疾患の治療における使用のための変異taci-fc融合タンパク質 | |
| HK40090097A (zh) | April和baff抑制性免疫调节蛋白及其使用方法 | |
| CN117915937A (zh) | 使用TACI-Fc融合免疫调节蛋白的给药和治疗方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| A201 | Request for examination | ||
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| D16 | Fast track examination requested |
Free format text: ST27 STATUS EVENT CODE: A-1-2-D10-D16-EXM-PA0302 (AS PROVIDED BY THE NATIONAL OFFICE) |
|
| P11 | Amendment of application requested |
Free format text: ST27 STATUS EVENT CODE: A-2-2-P10-P11-NAP-X000 (AS PROVIDED BY THE NATIONAL OFFICE) |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| PA0302 | Request for accelerated examination |
St.27 status event code: A-1-2-D10-D16-exm-PA0302 |