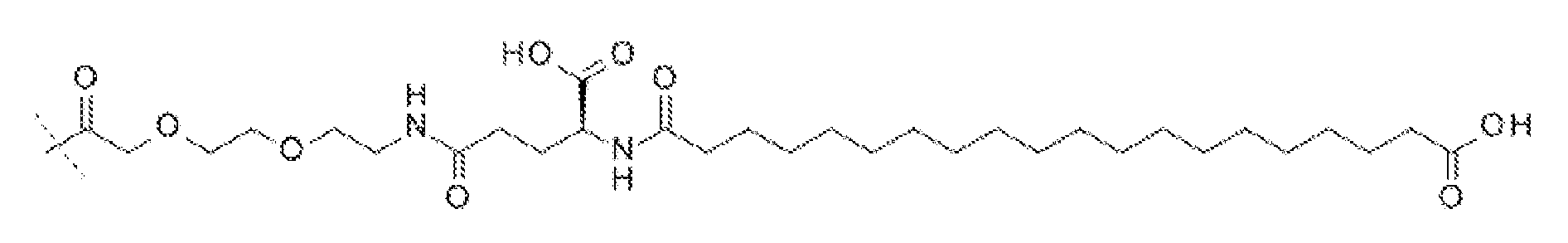GIP/GLP1 공효능제 화합물
본 발명은 인간 글루코스-의존성 인슐린분비자극(insulinotropic) 폴리펩티드 (GIP) 및 글루카곤-유사 펩티드-1 (GLP-1) 수용체 둘 다에서 활성을 갖는 화합물에 관한 것이다. 본 발명은 또한 이들 수용체 각각에서 연장된 작용 지속기간을 갖는 화합물에 관한 것이다. 더욱이, 본 발명은 경구 투여될 수 있는 화합물에 관한 것이다. 화합물은 제2형 진성 당뇨병 ("T2DM")의 치료에서 유용할 수 있다. 또한, 화합물은 비만의 치료에서 유용할 수 있다.
지난 수십 년에 걸쳐, 당뇨병의 유병률은 계속 증가하였다. T2DM은 모든 당뇨병의 대략 90%를 차지하는 가장 흔한 형태의 당뇨병이다. T2DM은 주로 인슐린 저항성과 연관된 높은 혈당 수준을 특징으로 한다. T2DM에 대한 현재 치료 표준은 식이 요법과 운동, 경구 의약 치료, 및 GLP-1 수용체 효능제와 같은 인크레틴-기반 요법을 포함한, 주사가능한 글루코스 저하 약물을 포함한다. 비록 현재 시판되고 있는 GLP-1 수용체 효능제는 일반적으로 메스꺼움 및 구토와 같은 위장 부작용에 의해 용량이 제한되긴 하지만, 여러 가지의 GLP-1 수용체 효능제는 현재 T2DM의 치료에 이용가능하다. 피하 주사는 이용가능한 GLP-1 수용체 효능제의 전형적인 투여 경로이다. 경구 의약 치료와 인크레틴-기반 요법이 불충분한 경우, 인슐린 치료를 고려한다. 오늘날 이용가능한 치료의 발전에도 불구하고, T2DM을 가진 많은 환자는 그의 혈당 제어 목표에 도달할 수 없다. 제어되지 않은 당뇨병은 환자의 이환율 및 사망률 증가와 연관된 몇몇 병태를 야기한다. T2DM을 가진 더 많은 환자가 그의 혈당 치료 목표에 도달할 수 있도록 하는 치료법이 필요하다.
비만은 지방 조직 덩어리의 과도한 축적을 초래하는 복잡한 의학적 장애이다. 오늘날 비만은 원치 않는 건강 결과 및 이환율과 연관된 세계적인 공중 보건 문제이다. 비만을 가진 환자가 원하는 치료법은 과체중을 줄이고, 비만-관련 동반이환을 개선하며, 장기적인 체중 감소를 유지하기 위해 매진한다. 이용가능한 비만 치료법은 중증 비만을 가진 환자에게 특히 불만족스럽다. 이러한 치료를 필요로 하는 환자에서 치료적 체중 감소를 유도하기 위한 대안적 치료 옵션이 필요하다.
WO2016/111971은 GLP-1 및 GIP 활성을 갖는 것으로 언급된 펩티드를 기재한다. WO2013/164483은 또한 GLP-1 및 GIP 활성을 갖는 것으로 언급된 화합물을 개시한다.
이러한 치료를 필요로 하는 환자의 더 많은 부분에 대해 효과적인 글루코스 제어를 제공할 수 있는 T2DM 치료법이 필요하다. 효과적인 글루코스 제어와 유리한 부작용 프로필을 제공할 수 있는 T2D 치료법에 대한 추가 필요가 있다. 이러한 치료를 필요로 하는 환자에서 치료적 체중 감소를 제공하기 위한 대안적 치료 옵션이 필요하다. 중증 비만 치료를 필요로 하는 환자를 위한 대안적 치료 옵션이 필요하다.
경구 투여에 적합한 GIP 및 GLP-1 수용체에서 효능제 활성을 갖는 화합물에 대한 요구가 있다. 각각의 GIP 및 GLP-1 수용체에서 연장된 작용 지속기간을 갖는 화합물은 화합물의 덜 빈번한 투여를 허용하기 위해 바람직하다
따라서, 본 발명은 화학식 I의 화합물; 또는 그의 제약상 허용되는 염을 제공한다:
R1X1X2X3GTX6TSDX10X11X12X13X14DX16X17AX19X20X21X22X23X24X25X26X27
X28X29X30X31 (서열식별번호: 3)
여기서,
R1은 N-말단 아미노 기의 변형이고 여기서 변형이 Ac 및 부재로 이루어진 군으로부터 선택되고;
X1은 Y, H, D-Tyr, F, desH, 및 desY로 이루어진 군으로부터 선택되고,
X2는 Aib, αMeP, A, P, 및 D-Ala로 이루어진 군으로부터 선택되거나;
X1 및 X2가 합하여 desH-ψ[NHCO]-Aib를 형성하고;
X3은 E, N, Aad, 및 cTA로 이루어진 군으로부터 선택되고;
X6은 F, αMeF, 및 αMeF(2F)로 이루어진 군으로부터 선택되고;
X10은 A, L, H, 3Pal, 4Pal, V, Y, E, αMeF, αMeF(2F), I, αMeY, Q, D-His, D-Tyr, cTA, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X11은 S, αMeS, 및 D-Ser로 이루어진 군으로부터 선택되고;
X12는 I, S, D-Ile, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X13은 Nle, Aib, L, αMeL, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X14는 L 및 K로 이루어진 군으로부터 선택되고, 여기서 K는 C16-C22 지방산에 접합되고 여기서 상기 지방산은 링커를 통해 상기 K에 임의로 접합되고;
X16은 K, E, Orn, Dab, Dap, S, T, H, Aib, αMeK, R, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X17은 K, Q, I, 및 C16-C22 지방산에 접합된 아미노산 (여기서 상기 지방산은 링커를 통해 상기 아미노산에 임의로 접합된다)으로 이루어진 군으로부터 선택되고;
X19는 Q, A, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X20은 Aib, Q, H, R, K, αMeK, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X21은 H, Aad, D, Aib, T, A, E, I, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X22는 F 및 αMeF로 이루어진 군으로부터 선택되고;
X23은 I, L, A, G, F, H, E, V, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X24는 S, Aad, D-Glu, E, Aib, H, V, A, Q, D, P, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X25는 Y 및 αMeY로 이루어진 군으로부터 선택되고;
X26은 L, αMeL, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X27은 L, I, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X28은 E, A, S, D-Glu, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X29는 Aib, G, A, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X30은 C, G, G-R2 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H로 이루어진 군으로부터 선택되고;
X31은 부재하거나 PX32X33X34-R2 (서열식별번호: 4), PX32X33X34X35X36X37X38X39-R2 (서열식별번호: 5), PX32X33X34X35X36X37X38X39X40-R2 (서열식별번호: 6), K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H] X32X33X34-R2 (서열식별번호: 7), K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H] X32X33X34X35X36X37X38X39-R2 (서열식별번호: 8), 및 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H] X32X33X34X35X36X37X38X39X40-R2 (서열식별번호: 9)로 이루어진 군으로부터 선택되고;
여기서,
X32는 S 또는 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H]이고;
X33은 S 또는 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H]이고;
X34는 G, C, 및 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H]로 이루어진 군으로부터 선택되고;
X35는 A 또는 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H]로 이루어진 군으로부터 선택되고;
X36은 P 또는 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H]이고;
X37은 P 또는 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H]이고;
X38은 P 또는 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H]이고;
X39는 C, S, 및 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H]로 이루어진 군으로부터 선택되고;
X40은 C 및 K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H]로 이루어진 군으로부터 선택되고;
q는 14, 15, 16, 17, 18, 19, 및 20으로 이루어진 군으로부터 선택되고;
R2는 C-말단 기의 변형이고, 여기서 변형은 NH2이거나 또는 부재하고;
여기서 X30이 G-R2이면, X31은 부재하고;
여기서 X10, X12, X13, X14, X16, X17, X19, X20, X21, X23, X24, X26, X27, X28, X29, X30, X31, X32, X33, X34, X35, X36, X37, X38, X39, 및 X40 중 1개 이하는 지방산을 함유하는 치환기일 수 있고;
여기서 X30, X34, X39, 및 X40 중 1개 이하는 C일 수 있고;
여기서 X30, X34, X39, 및 X40 중 1개가 C이면, X10, X12, X13, X14, X16, X17, X19, X20, X21, X23, X24, X26, X27, X28, X29, X30, X31, X32, X33, X34, X35, X36, X37, X38, X39, 및 X40 중 어떤 것도 지방산을 함유하는 치환기가 아니다.
일 실시양태에서 q가 16인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X31이 서열식별번호: 5 및 서열식별번호: 8로 이루어진 군으로부터 선택된 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 지방산에 접합된 X17 아미노산이 천연 아미노산인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X17이 K, Q 및 I로 이루어진 군으로부터 선택된 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 K가 C16-C22 지방산에 접합되고 여기서 상기 지방산은 링커를 통해 상기 K에 임의로 접합된 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X14 또는 X17이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)16-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)18-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)14-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-CO-(CH2)18-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)-(γ-Glu)-(Trx)-CO-(CH2)18-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)-(Trx)-(γ-Glu)-CO-(CH2)18-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)-(εK)-(γ-Glu)-CO-(CH2)18-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)-(εK)-(εK)-CO-(CH2)18-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)2-CO-(CH2)18-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-CO-(CH2)18-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(εK)-CO-(CH2)16-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(εK)- CO-(CH2)14-CO2H, 및 KDab-(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)-Dab-(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)-CO-(CH2)18-CO2H로 이루어진 군으로부터 선택된 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X14 또는 X17이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)16-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)18-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)14-CO2H, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-CO-(CH2)18-CO2H로 이루어진 군으로부터 선택된 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X14 또는 X17이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)16-CO2H, K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)18-CO2H, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)14-CO2H로 이루어진 군으로부터 선택된 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X14 또는 X17이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)18-CO2H 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)16-CO2H로 이루어진 군으로부터 선택된 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X14 또는 X17이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)a-(γ-Glu)b-CO-(CH2)q-CO2H이고, 여기서 a는 2이고, b는 1이고, q는 18 및 20으로 이루어진 군으로부터 선택된 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X14 또는 X17이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)a-(γ-Glu)b-CO-(CH2)q-CO2H이고, 여기서 a는 2이고, b는 1이고, q는 18인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X14 또는 X17이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)a-(γ-Glu)b-CO-(CH2)q-CO2H이고, 여기서 a는 2이고, b는 1이고, q는 20인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X1 및 X2가 합하여 desH-ψ[NHCO]-Aib를 형성하지 않는 것 (이후로 "화학식 II" 화합물)인 화학식 I 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X17이 C16-C22 지방산에 접합된 아미노산이고 여기서 상기 지방산은 링커를 통해 상기 아미노산에 임의로 접합되고;
X30이 G-R2 및 G로 이루어진 군으로부터 선택되고;
X30이 G이면, X31이 PX32X33X34-R2 (서열식별번호: 4) (여기서 X32는 S이고, X33는 S이고 X34는 G이다) (서열식별번호: 297), 및 PX32X33X34X35X36X37X38X39-R2 (서열식별번호: 5) (여기서 X32는 S이고, X33은 S이고, X34는 G이고, X35는 A이고, X36은 P이고, X37은 P이고, X38은 P이고 X39는 S이다) (서열식별번호: 298)로 이루어진 군으로부터 선택된 것 (이후로 "화학식 III" 화합물)인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X17이 아미노산 링커를 통해 지방산에 접합된 것 (이후로 "화학식 IIIa" 화합물)인 화학식 III의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서
X10이 A, L, H, 3Pal, 4Pal, V, Y, E, αMeF, αMeF(2F), I, αMeY, Q, D-His, D-Tyr, 및 cTA로 이루어진 군으로부터 선택되고;
X12가 I, S, 및 D-Ile로 이루어진 군으로부터 선택되고;
X13이 Nle, Aib, L, 및 αMeL로 이루어진 군으로부터 선택되고;
X14가 L 및 K로 이루어진 군으로부터 선택되고;
X16이 K, E, Orn, Dab, Dap, S, T, H, Aib, αMeK, 및 R로 이루어진 군으로부터 선택되고;
X19가 Q, 및 A로 이루어진 군으로부터 선택되고;
X20이 Aib, Q, H, R, K, 및 αMeK로 이루어진 군으로부터 선택되고;
X21이 H, Aad, D, Aib, T, A, E, 및 I로 이루어진 군으로부터 선택되고;
X23이 I, L, A, G, F, H, E, 및 V로 이루어진 군으로부터 선택되고;
X24가 S, Aad, D-Glu, E, Aib, H, V, A, Q, D, 및 P로 이루어진 군으로부터 선택되고;
X26이 L, 및 αMeL로 이루어진 군으로부터 선택되고;
X27이 L, 및 I로 이루어진 군으로부터 선택되고;
X28이 E, A, S, 및 D-Glu로 이루어진 군으로부터 선택되고;
X29가 Aib, G, 및 A로 이루어진 군으로부터 선택되고;
X30이 G 및 G-R2로 이루어진 군으로부터 선택되고;
X30이 G이면; X31이 PX32X33X34-R2 (서열식별번호: 4) (여기서 X32는 S이고, X33은 S이고 X34는 G이다) (서열식별번호: 297) 및 PX32X33X34X35X36X37X38X39-R2 (서열식별번호: 5) (여기서 X32는 S이고, X33은 S이고, X34는 G이고, X35는 A이고, X36은 P이고, X37은 P이고, X38은 P이고 X39은 S이다) (서열식별번호: 298)로 이루어진 군으로부터 선택된 것인 (이후로 "화학식 IIIb" 화합물)인
화학식 III 및 IIIa의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서, 링커가 1 내지 2개의 아미노산인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있고, 이들 특정한 화학식 III, IIIa 및 IIIb 화합물의 추가 실시양태에서 링커 아미노산이 Glu 및 γ-Glu로 이루어진 군으로부터 독립적으로 선택된 것들이 있다. 또 다른 실시양태에서 링커가 1 또는 2개의 (2-[2-(2-아미노-에톡시)-에톡시]-아세틸) 모이어티를 포함하는 것인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있고, 이들 특정한 화학식 III, IIIa 및 IIIb 화합물의 추가 실시양태에서 링커가 (2-[2-(2-아미노-에톡시)-에톡시]-아세틸)a-(γ-Glu)b이고, 여기서 a가 1 또는 2로 이루어진 군으로부터 선택되고; b가 1 또는 2로 이루어진 군으로부터 선택된 것이 있다.
일 실시양태에서 X17이 C16-C22 지방산에 접합된 아미노산이고, 여기서 아미노산이 K이고 여기서 상기 지방산은 링커를 통해 상기 아미노산에 임의로 접합된 것인
화학식 III의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서
R1이 부재하고;
X1 및 X2가 합하여 desH-ψ[NHCO]-Aib를 형성하지 않고;
X17이 C16-C22 지방산에 접합된 K이고 여기서 상기 지방산은 링커를 통해 상기 아미노산에 임의로 접합된 것인 화학식 III의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서
X1이 Y이고;
X2가 Aib이고;
X3이 E이고;
X10이 A, L, H, 3Pal, 4Pal, V, 및 Y로 이루어진 군으로부터 선택되고;
X11이 S이고;
X12가 I이고;
X14가 L이고;
X16이 K, E, Orn, Dab, 및 Dap로 이루어진 군으로부터 선택되고;
X17이 C16-C22 지방산에 접합된 K이고 여기서 상기 지방산은 링커를 통해 상기 아미노산에 임의로 접합되고;
X19가 Q이고;
X20이 Aib이고;
X21이 H, Aad, D, Aib, T, A, 및 E로 이루어진 군으로부터 선택되고;
X22가 F이고;
X23이 I이고;
X24가 S, Aad, D-Glu, 및 E로 이루어진 군으로부터 선택되고;
X26이 L이고;
X28이 E 및 A로 이루어진 군으로부터 선택된 것인 화학식 III의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서
X1이 Y이고;
X2가 Aib이고;
X3이 E이고;
X6이 αMeF(2F)이고;
X10이 Y, 4-PaI, 및 V로 이루어진 군으로부터 선택되고;
X11이 S이고;
X12가 I이고;
X13이 L, Aib, 및 αMeL로 이루어진 군으로부터 선택되고;
X14가 L이고;
X16이 E, K, 및 Orn으로 이루어진 군으로부터 선택되고;
X17이 C16-C22 지방산에 접합된 K이고 여기서 상기 지방산은 링커를 통해 상기 아미노산에 임의로 접합되고;
X19가 Q이고;
X20이 Aib이고
X21이 E, A, 및 T로 이루어진 군으로부터 선택되고;
X22가 F이고;
X23이 I이고;
X24가 D-Glu이고;
X25가 Y 및 αMeY로 이루어진 군으로부터 선택되고;
X26이 L이고;
X27이 I이고;
X28이 E이고;
X29가 G이고;
X30이 G이고;
X31이 PX32X33X34X35X36X37X38X39-R2 (서열식별번호: 5) (여기서 X32는 S이고, X33은 S이고, X34는 G이고, X35는 A이고, X36은 P이고, X37은 P이고, X38은 P이고, X39는 S이다) (서열식별번호: 298)인
화학식 III의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 R2가 부재하는 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 R2가 NH2인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X13이αMeL인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X25가 Y이고 X13이 αMeL인인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X17이 K 측쇄의 엡실론-아미노 기에 링커를 통해 지방산에 접합된 K이며, 여기서 상기 지방산 및 링커가 하기 화학식을 갖는 것인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다:
(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)a-(γ-Glu)b-CO-(CH2)q-CO2H (여기서 a는 1 또는 2이고; b는 1 또는 2이고; q는 14 내지 20으로 이루어진 군으로부터 선택된다).
일 실시양태에서 X16이 Orn이고, X13이 αMeL이고, X25가 Y인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X16이 E이고, X13이 αMeL이고, X25가 Y인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서, X16이 E이고, X13이 αMeL이고, X10이 Y이고, X25가 αMeY인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X16이 Orn이고, X13이 αMeL이고, X10이 4Pal이고, X25가 Y인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X16이 Orn이고, X13이 αMeL이고, X10이 V이고, X25가 Y인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X16이 E이고, X13이 αMeL이고, X25가 Y이고, X17이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)a-(γ-Glu)b-CO-(CH2)q-CO2H이고, 여기서 a가 2이고; b가 1이고; q가 14 내지 20으로 이루어진 군으로부터 선택된 것인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X16이 E이고, X13이 αMeL이고, X10이 Y이고, X25가 Y이고, X17이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)a-(γ-Glu)b-CO-(CH2)q-CO2H이고, 여기서 a가 2이고; b가 1이고; q가 16 내지 20으로 이루어진 군으로부터 선택된 것인 화학식 III, IIIa 및 IIIb의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 서열식별번호: 10, 서열식별번호: 11, 서열식별번호: 12, 서열식별번호: 13, 및 서열식별번호: 14로 이루어진 군으로부터 선택된 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 10인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 11인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 12인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 13인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 14인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X1이 Y, F, 및 D-Tyr로 이루어진 군으로부터 선택되고; X6이 F이고; X13이 Aib, L, 및 αMeL로 이루어진 군으로부터 선택된 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서, R1이 부재하고; X1이 Y, F, 및 D-Tyr로 이루어진 군으로부터 선택되고; X6이 F이고; X13이 Aib, L, 및 αMeL로 이루어진 군으로부터 선택되고; X2가 Aib이고; X3이 E이고; X10이 Y이고; X11이 S이고; X12가 I이고; X14가 L이고; X16이 K, E, Orn, Dab, Dap, S, T, H, Aib, αMeK, 및 R로 이루어진 군으로부터 선택되고; X17이 C16-C22 지방산에 접합된 아미노산이고 여기서 상기 지방산은 링커를 통해 상기 아미노산에 임의로 접합되고; X19가 Q이고; X20이 Aib, Q, H, 및 K로 이루어진 군으로부터 선택되고; X21이 H, D, T, A, 및 E로 이루어진 군으로부터 선택되고; X22가 F이고; X23이 I이고; X24가 D-Glu 및 E로 이루어진 군으로부터 선택되고; X26이 L이고; X27이 I이고; X28이 E, A, S, 및 D-Glu로 이루어진 군으로부터 선택되고; X29가 Aib, G, 및 A로 이루어진 군으로부터 선택되고; X30이 C, G, 및 G-R2로 이루어진 군으로부터 선택되고; X31이 부재하거나 PX32X33X34-R2 (서열식별번호: 4), PX32X33X34X35X36X37X38X39-R2 (서열식별번호: 5), 및 PX32X33X34X35X36X37X38X39X40-R2 (서열식별번호: 6)로 이루어진 군으로부터 선택되고; 여기서, X32가 S이고; X33이 S이고; X34가 G 및 C로 이루어진 군으로부터 선택되고; X35가 A이고; X36이 P이고; X37이 P이고; X38이 P이고; X39가 C 및 S로 이루어진 군으로부터 선택되고; X40이 C인 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서, X1이 Y, F, 및 D-Tyr로 이루어진 군으로부터 선택되고; X6이 F이고; X13이 Aib, L, 및 αMeL로 이루어진 군으로부터 선택되고; X28이 A이고; X29가 G이고; X30이 G이고; X31이 PX32X33X34X35X36X37X38X39-R2 (서열식별번호: 5)이고; X34가 G이고; X39가 S인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서, X1이 Y 및 D-Tyr로 이루어진 군으로부터 선택되고; X13이 αMeL인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
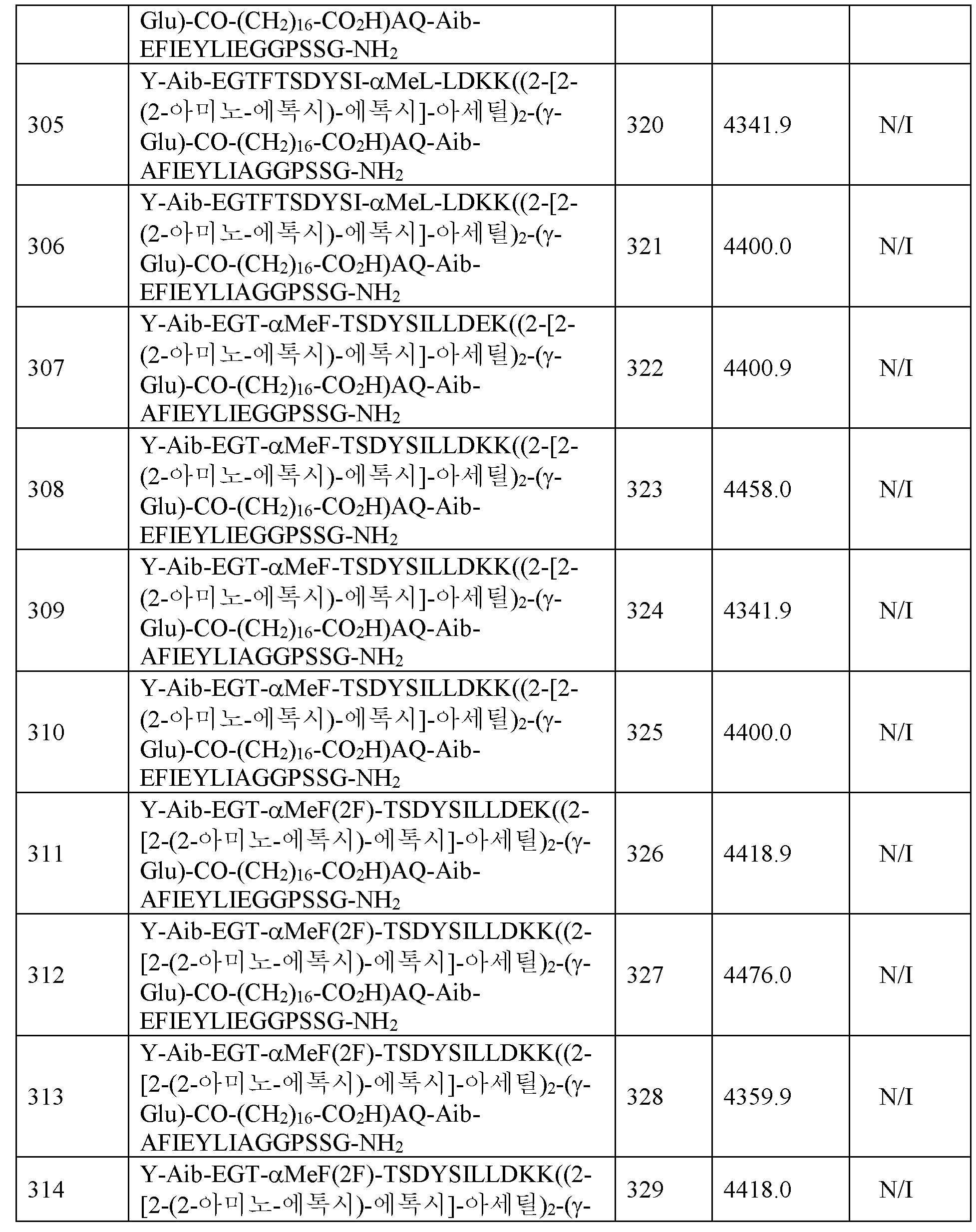
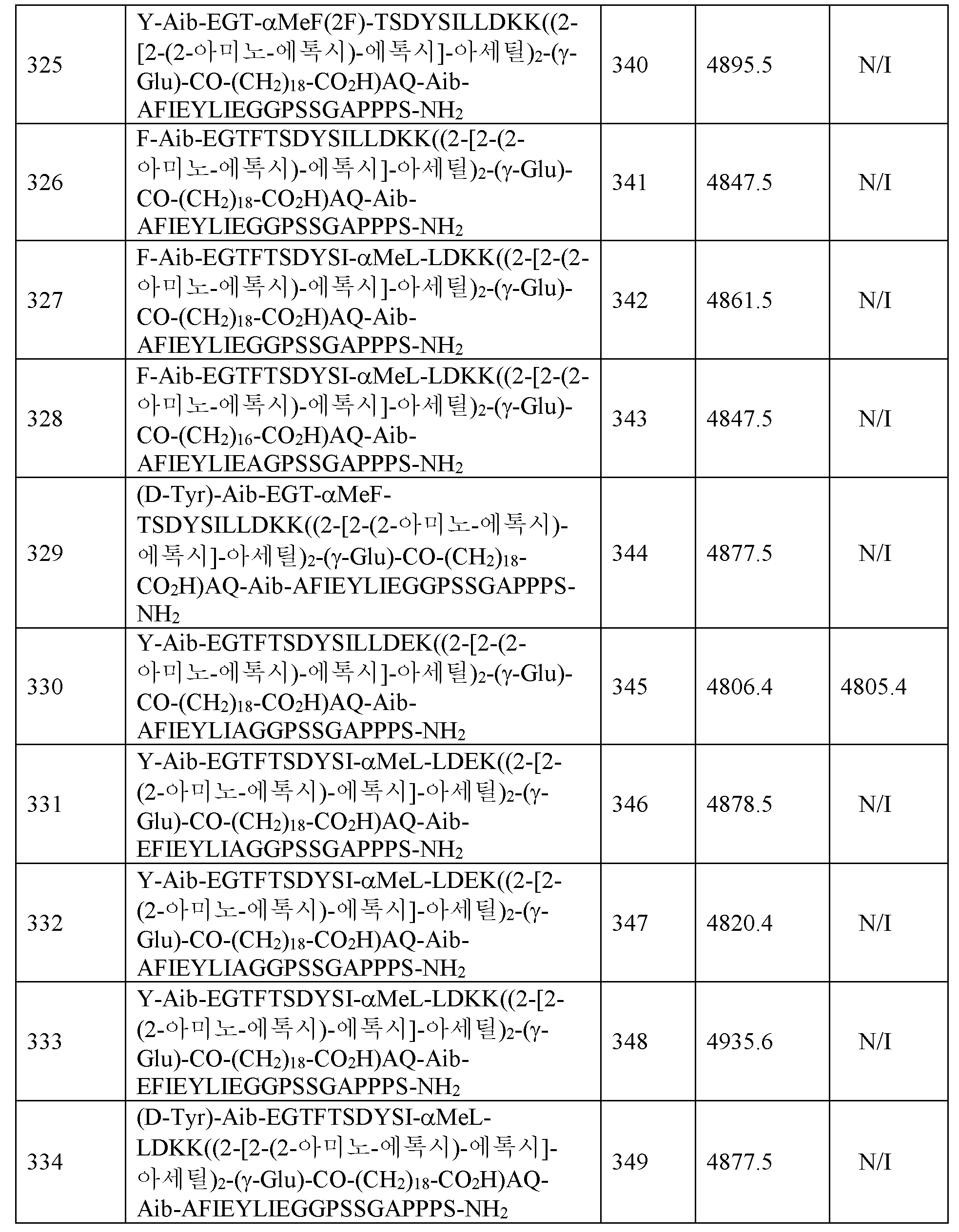
일 실시양태에서 서열식별번호: 303, 서열식별번호: 304, 서열식별번호: 305, 서열식별번호: 306, 서열식별번호: 307, 및 서열식별번호: 308로 이루어진 군으로부터 선택된 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 303인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 304인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 305인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 306인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 307인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 308인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 서열식별번호: 386인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서
X10이 A, L, H, 3Pal, 4Pal, V, Y, αMeF, αMeF(2F), I, αMeY, Q, D-His, E, cTA, 및 D-Tyr로 이루어진 군으로부터 선택되고;
X12가 I, D-Ile, 및 S로 이루어진 군으로부터 선택되고;
X13이 Nle, Aib, L, 및 αMeL로 이루어진 군으로부터 선택되고;
X14가 L이고;
X16이 K, E, Orn, Dab, Dap, S, T, H, Aib, αMeK, 및 R로 이루어진 군으로부터 선택되고;
X17이 K, Q, 및 I로 이루어진 군으로부터 선택되고;
X19가 Q 및 A로 이루어진 군으로부터 선택되고;
X20이 Aib, Q, H, R, K, 및 αMeK로 이루어진 군으로부터 선택되고;
X21이 H, Aad, D, Aib, T, A, E, 및 I로 이루어진 군으로부터 선택되고;
X23이 I, L, A, G, F, H, E, 및 V로 이루어진 군으로부터 선택되고;
X24가 S, Aad, D-Glu, E, Aib, H, V, A, Q, D, 및 P로 이루어진 군으로부터 선택되고;
X26이 L 및 αMeL로 이루어진 군으로부터 선택되고;
X27이 L 및 I로 이루어진 군으로부터 선택되고;
X28이 E, A, S, 및 D-Glu로 이루어진 군으로부터 선택되고;
X29가 Aib, G, 및 A로 이루어진 군으로부터 선택된 것인 (이후로 "화학식 IV" 화합물)인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X39가 C인 화학식 IV의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X40이 C인 화학식 IV의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X30, X34, X39, 및 X40 중 1개, 및 단지 1개가 C인 화학식 IV의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X30, X34, X39, 및 X40 중 1개, 및 단지 1개가 시간-연장 기술을 사용하여 변형된 C인 화학식 IV의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 C가 시간-연장 기술을 사용하여 변형되며 여기서 시간-연장 기술이 XTEN인 화학식 IV의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 C가 시간-연장 기술을 사용하여 변형되며 여기서 시간-연장 기술이 (Glu)m 비오틴 (여기서 m은 0, 1, 2, 또는 3이다)인 화학식 IV의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서
X1이 Y이고;
X2가 Aib이고;
X3이 E이고;
X10이 A, L, H, 3Pal, 4Pal, V, 및 Y로 이루어진 군으로부터 선택되고;
X11이 S이고;
X12가 I이고;
X16이 K, E, Orn, Dab, 및 Dap로 이루어진 군으로부터 선택되고;
X19가 Q이고;
X20이 Aib 및 K로 이루어진 군으로부터 선택되고;
X21이 H, Aad, D, Aib, T, A, 및 E로 이루어진 군으로부터 선택되고;
X22가 F이고;
X23이 I이고;
X24가 S, Aad, D-Glu, 및 E로 이루어진 군으로부터 선택되고;
X26이 L이고;
X28이 E 및 A로 이루어진 군으로부터 선택된 것인
화학식 IV의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서
X1이 Y이고;
X2가 Aib이고;
X3이 E이고;
X10이 A, L, H, 3Pal, 4Pal, V, 및 Y로 이루어진 군으로부터 선택되고;
X11이 S이고;
X12가 I이고;
X16이 K, E, Orn, Dab, 및 Dap로 이루어진 군으로부터 선택되고;
X20이 Aib이고;
X21이 H, Aad, D, Aib, T, A, 및 E로 이루어진 군으로부터 선택되고;
X22가 F이고;
X24가 S, Aad, D-Glu, 및 E로 이루어진 군으로부터 선택되고;
X27이 I이고;
X28이 E 및 A로 이루어진 군으로부터 선택된 것인
화학식 IV의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X14가 L이고;
X17이 K, Q, 및 I로 이루어진 군으로부터 선택되고;
X30이 G-R2 및 G로 이루어진 군으로부터 선택되고;
q가 16, 18, 및 20로 이루어진 군으로부터 선택되고;
X30이 G이면, X31이 다음으로 이루어진 군으로부터 선택되고:
PX32X33X34-R2 (서열식별번호: 4) (여기서,
X32가 S이고, X33이 S이고, X34가 G이고 R2가 부재 (서열식별번호: 299)하거나
X32가 S이고, X33이 S이고, X34가 G이고 R2가 NH2 (서열식별번호: 300)이다);
및
PX32X33X34X35X36X37X38X39-R2 (서열식별번호: 5) (여기서,
X32가 S이고, X33이 S이고, X34가 G이고, X35가 A이고, X36이 P이고, X37이 P이고, X38이 P이고, X39가 S이고 R2가 부재 (서열식별번호: 301)하거나
X32가 S이고, X33이 S이고, X34가 G이고, X35가 A이고, X36이 P이고, X37이 P이고, X38이 P이고, X39가 S이고 R2가 NH2 (서열식별번호: 302)이다);
여기서 X10, X12, X13, X14, X16, X19, X20, X21, X23, X24, X26, X27, X28, 및 X29 중 1개가 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-γGlu-CO-(CH2)qCO2H (이후로 "화학식 V" 화합물)인
화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서
X1이 Y이고;
X2가 Aib이고;
X3이 E이고;
X10이 A, L, H, 3Pal, 4Pal, V, Y, E, cTA, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X11이 S이고;
X12가 I, D-Ile, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X16이 K, E, Orn, Dab, Dap, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X17이 K 및 I로 이루어진 군으로부터 선택되고;
X19가 Q 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X20이 Aib 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X21이 H, Aad, D, Aib, T, A, E, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X22가 F이고;
X24가 S, Aad, D-Glu, E, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X26이 L 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택되고;
X27이 L 및 I로 이루어진 군으로부터 선택되고;
X28이 E, A, 및 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H로 이루어진 군으로부터 선택된 것인 화학식 V의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태에서 X20이 K(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)qCO2H이고, 여기서 q가 16 또는 18인 화학식 V의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X31이 서열식별번호: 301 또는 서열식별번호: 302인 화학식 V의 화합물, 또는 그의 제약상 허용되는 염이 있다.
일 실시양태는 T2DM, 비만, 비알콜성 지방간 질환 (NAFLD), 비알콜성 지방간염 (NASH), 이상지질혈증 및 대사 증후군으로 이루어진 군으로부터 선택된 병태의 치료를 필요로 하는 대상체에게, 유효량의 화학식 I의 화합물 또는 그의 제약상 허용되는 염을 투여하는 것을 포함하는, T2DM, 비만, 비알콜성 지방간 질환 (NAFLD), 비알콜성 지방간염 (NASH), 이상지질혈증 및 대사 증후군으로 이루어진 군으로부터 선택된 병태를 치료하는 방법을 제공한다. 일 실시양태는 치료적 체중 감소의 제공을 필요로 하는 대상체에게, 유효량의 화학식 I의 화합물, 또는 그의 제약상 허용되는 염을 투여하는 것을 포함하는, 치료적 체중 감소를 제공하는 방법을 제공한다. 일 실시양태에서, 병태는 NAFLD이다. 일 실시양태에서, 병태는 NASH이다.
일 실시양태는 요법에서 사용하기 위한 화학식 I의 화합물, 또는 그의 제약상 허용되는 염을 제공한다. 일 실시양태는 T2DM, 비만, NAFLD, NASH, 이상지질혈증 및 대사 증후군으로 이루어진 군으로부터 선택된 병태를 치료하기 위한 요법에서 사용하기 위한 화학식 I의 화합물, 또는 그의 제약상 허용되는 염을 제공한다. 일 실시양태에서, 병태는 T2DM이다. 일 실시양태에서, 병태는 비만이다. 일 실시양태에서, 병태는 NAFLD이다. 일 실시양태에서, 병태는 NASH이다. 일 실시양태에서, 병태는 대사 증후군이다.
화학식 I의 화합물, 또는 그의 제약상 허용되는 염은, 여러 가지의 증상 또는 장애의 치료에서 유용할 수 있다. 예를 들어, 특정 실시양태는, T2DM의 치료를 필요로 하는 대상체에게 유효량의 화학식 I의 화합물, 또는 그의 제약상 허용되는 염을 투여하는 것을 포함하는, 환자에서 T2DM의 치료 방법을 제공한다. 일 실시양태에서, 비만의 치료를 필요로 하는 대상체에게 유효량의 화학식 I의 화합물, 또는 그의 제약상 허용되는 염을 투여하는 것을 포함하는, 환자에서 비만의 치료 방법이 있다. 일 실시양태에서, 방법은 비치료적 체중 감소의 유도 치료를 필요로 하는 대상체에게 유효량의 화학식 I의 화합물, 또는 그의 제약상 허용되는 염을 투여하는 것을 포함하는, 대상체에서 비치료적 체중 감소를 유도하는 것이다.
특정 실시양태에서, 본 발명은 대사 증후군의 치료를 필요로 하는 대상체에게 유효량의 화학식 I의 화합물, 또는 그의 제약상 허용되는 염을 투여하는 것을 포함하는 환자에서 대사 증후군의 치료 방법을 제공한다. 일 실시양태에서, 방법은 NASH의 치료를 필요로 하는 대상체에게 유효량의 화학식 I의 화합물, 또는 그의 제약상 허용되는 염을 투여하는 것을 포함하는, NASH의 치료이다.
또한, T2DM, 비만, NAFLD, NASH, 이상지질혈증 및 대사 증후군으로 이루어진 군으로부터 선택된 병태의 치료에서, 본 발명의 화합물과 동시에, 개별적으로 및 순차적으로 조합하여 사용하기 위한 메트포르민, 티아졸리딘디온, 술포닐우레아, 디펩티딜 펩티다제 4 억제제, 소듐 글루코스 공수송체, SGLT-2 억제제, 성장 분화 인자 15 조정제 ("GDF15"), 펩티드 티로신 티로신 조정제 ("PYY"), 변형된 인슐린, 아밀린, 이중 아밀린 칼시토닌 수용체 효능제, 및 옥신토모듈린 효능제 ("OXM")로부터 선택된 1종 이상의 작용제가 본원에 제공된다. 일 실시양태에서, 본 발명의 화합물은 메트포르민, 티아졸리딘디온, 술포닐우레아, 디펩티딜 펩티다제 4 억제제, 소듐 글루코스 공수송체, SGLT-2 억제제, GDF15, PYY, 변형된 인슐린, 아밀린, 이중 아밀린 칼시토닌 수용체 효능제, 및 OXM으로부터 선택된 1종 이상의 작용제와 고정 용량 조합으로 제공된다. 일 실시양태에서 T2DM 및 비만으로 이루어진 군으로부터 선택된 병태의 치료에서 메트포르민, 티아졸리딘디온, 술포닐우레아, 디펩티딜 펩티다제 4 억제제, 소듐 글루코스 공수송체, SGLT-2 억제제, GDF15, PYY, 변형된 인슐린, 아밀린, 이중 아밀린 칼시토닌 수용체 효능제, 및 OXM으로부터 선택된 1종 이상의 작용제와 동시에, 개별적으로 및 순차적으로 조합하여 사용하기 위한 본 발명의 화합물이 있다. 일 실시양태에서 T2DM 및 비만으로 이루어진 군으로부터 선택된 병태의 치료에서 메트포르민, 티아졸리딘디온, 술포닐우레아, 디펩티딜 펩티다제 4 억제제, 소듐 글루코스 공수송체, 및 SGLT-2 억제제로부터 선택된 1종 이상의 작용제와 동시에, 개별적으로 및 순차적으로 조합하여 사용하기 위한 본 발명의 화합물이 있다.
다른 실시양태에서, 화합물, 또는 그의 제약상 허용되는 염은 골 강도의 개선을 필요로 하는 대상체에서 골 강도를 개선시키는 데 유용할 수 있다. 본 발명의 화합물, 또는 그의 제약상 허용되는 염은, 다른 장애 예컨대 파킨슨병 또는 알츠하이머병의 치료에서 유용할 수 있다. GIP, GLP-1 및/또는 글루카곤 수용체 중 하나 이상에서 활성을 갖는 인크레틴 및 인크레틴 유사체는 예를 들어 비만, NAFLD 및 NASH, 이상지질혈증, 대사 증후군, 골 관련 장애, 알츠하이머병, 및 파킨슨병을 포함한, 다수의 다른 질환 또는 병태에서 치료학적 가치를 가질 가능성을 갖는 것으로서 기재되어 있다. 예를 들어, 문헌 [Jall S., et. al,
Monomeric GLP-1/GIP/글루카곤 triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice, Mol. Metab. 6(5):440-446 (March 2017)]; [Carbone L.J., et. al.,
Incretin -based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J.Gastroenterol. Hepatol., 31(1):23-31 (Jan. 2016)]; [B. Finan, et. al,
Reappraisal of GIP Pharmacology for Metabolic Diseases. Trends Mol. Med., 22(5):359-76 (May 2016)]; [Choi, I.Y., et al.,
Potent body weight loss and efficacy in a NASH animal model by a novel long-acting GLP-1/Glucagon/GIP triple-agonist (HM15211), ADA 2017 Poster 1139-P]; [Ding, K.H.,
Impact of glucose-dependent insulinotropic peptide on age-induced bone loss, J. Bone Miner. Res., 23(4):536-43 (2008)]; [Tai, J. et. al,
Neuroprotective effects of a triple GLP-1/GIP/glucagon receptor agonist in the APP/PS1 transgenic mouse model of Alzheimer's disease, Brain Res. 1678, 64-74 (2018)]; [T.D. M
ller et al.,
The New Biology and Pharmacology of Glucagon, Physiol. Rev. 97: 721-766 (2017)]; [Finan, B. et. al,
Unimolecular Dual Incretins Maximize Metabolic Benefits in Rodents, Monkeys, and Humans, Sci. Transl. Med., 5:209 (October 2013)]; [Holscher C,
Insulin, incretins and other growth factors as potential novel treatments for Alzheimer's 및 Parkinson's diseases. Biochem. Soc. Trans. 42(2):593-0 (Apr. 2014)]을 참조한다.
또 다른 실시양태는 T2DM, 비만, NAFLD, NASH, 이상지질혈증 및 대사 증후군으로 이루어진 군으로부터 선택된 병태 치료용 의약의 제조에서, 본 발명의 화합물, 또는 그의 제약상 허용되는 염의 용도를 제공한다. 일 실시양태에서, 의약은 T2DM의 치료를 위한 것이다. 일 실시양태에서, 의약은 비만의 치료를 위한 것이다. 일 실시양태에서, 의약은 NAFLD의 치료를 위한 것이다. 일 실시양태에서, 의약은 NASH의 치료를 위한 것이다.
또 다른 실시양태는 화학식 I의 화합물, 또는 그의 제약상 허용되는 염, 및 담체, 희석제, 및 부형제로 이루어진 군으로부터 선택된 적어도 1종을 포함하는 제약 조성물을 제공한다.
일 실시양태에서 화학식 I의 화합물, 또는 그의 제약상 허용되는 염, 적어도 1종의 침투 증진제 및 적어도 1종의 프로테아제 억제제를 포함하는 제약 조성물이 있다. 일 실시양태에서, 화학식 I의 화합물, 또는 그의 제약상 허용되는 염, 적어도 1종의 침투 증진제, 및 담체, 희석제, 및 부형제로 이루어진 군으로부터 선택된 적어도 1종을 포함하는 제약 조성물이 있다.
일 실시양태에서 화학식 I의 화합물, 또는 그의 제약상 허용되는 염, 침투 증진제, 프로테아제 억제제, 및 담체, 희석제, 및 부형제로 이루어진 군으로부터 선택된 적어도 1종을 포함하는 제약 조성물이 있다. 일 실시양태에서 화학식 I의 화합물, 또는 그의 제약상 허용되는 염, 및 침투 증진제를 포함하는 제약 조성물이 있다. 일 실시양태에서 본 발명의 화합물, 또는 그의 제약상 허용되는 염, 및 침투 증진제를 포함하는 제약 조성물이 있다. 일 실시양태에서 침투 증진제는 소듐 데카노에이트 ("C10"), 소듐 타우로데옥시콜레이트 ("NaTDC"), 라우로일 카르니틴 ("LC"), 도데실 말토시드 ("C12-말토시드"), 도데실 포스파티딜콜린 ("DPC"), 소듐 N-[8-(2-히드록시벤조일) 아미노] 카프릴레이트 ("SNAC") 및 람노리피드(Rhamnolipid)로 이루어진 군으로부터 선택된다. 일 실시양태에서 침투 증진제는 C10 및 LC로 이루어진 군으로부터 선택된다. 일 실시양태에서 프로테아제 억제제는 대두 트립신 억제제 ("SBTI"), 대두 트립신-키모트립신 억제제 ("SBTCI"), 에코틴, 해바라기 트립신 억제제 ("SFTI"), 류펩틴, 시트르산, 에틸렌디아민테트라아세트산 ("EDTA"), 소듐 글리코콜레이트 및 4-(2-아미노에틸) 벤젠술포닐 플루오라이드 히드로클로라이드 ("AEBSF")로 이루어진 군으로부터 선택된다. 일 실시양태에서, 프로테아제 억제제는 SBTI, SBTCI, 및 SFTI로 이루어진 군으로부터 선택된다. 일 실시양태에서, 프로테아제 억제제는 SBTI이다.
본원에 사용된 바와 같이, 용어 "치료하는" 또는 "치료하기 위해"는 증상, 병태, 또는 장애의 진행 또는 중증도를 저지시키거나, 둔화시키거나, 정지시키거나, 역전시키는 것을 포함한다.
본 발명의 특정 화합물은 일반적으로 넓은 투여량 범위에 걸쳐 효과적이다. 예를 들어, 주 1회 비경구 투여를 위한 투여량은 1인당 주당 0.05 mg 내지 약 30 mg 범위에 속할 수 있다.
본 발명의 화합물은 각각의 GLP-1 및 GIP 수용체에 대해 친화도를 갖는 신규 아미노산 서열을 포함하며, 이들 수용체 각각에서 원하는 효능을 갖는다. GLP-1은 36개 아미노산 펩티드이며, 이의 주요 생물학적 활성 단편은 30-아미노산, C-말단 아미드화 펩티드 (GLP-17-36) (서열식별번호: 2)로서 생성된다.
GIP는 GLP-1과 마찬가지로 인크레틴으로도 공지된 42개 아미노산 펩티드 (서열식별번호: 1)이며, 글루코스 존재 하에 췌장 베타 세포로부터 인슐린 분비를 자극함으로써 글루코스 항상성에서 생리학적 역할을 한다.
상기 화합물은 GIP 및 GLP-1 수용체 각각에서 원하는 효능을 제공한다. 일 실시양태에서, 화합물은 경구 투여에 적합하다. 일 실시양태에서, 화합물은 바람직한 GIP 및 GLP 수용체 연장된 시간 작용을 갖는다. 일 실시양태에서, 화합물은 바람직한 GIP 및 GLP 수용체 활성을 가지며, 여기서 GIP 효능제 효능은 하기 본원에 기재된 카제인 cAMP 검정에 의해 측정된 바와 같이 GLP1 수용체 효능의 2.5 내지 5 배이고, 여기서 효능은 검정이 시행되는 당일에 천연 GIP 및 GLP에 대해 정규화된다. 일 실시양태에서, 화합물은 바람직한 GIP 및 GLP 수용체 활성을 가지며, 여기서 GIP 효능제 효능은 카제인 cAMP 검정에 의해 측정된 바와 같이 GLP1 수용체 효능의 2.5 내지 10 배이고, 여기서 효능은 검정이 시행되는 당일에 천연 GIP 및 GLP에 대해 정규화된다.
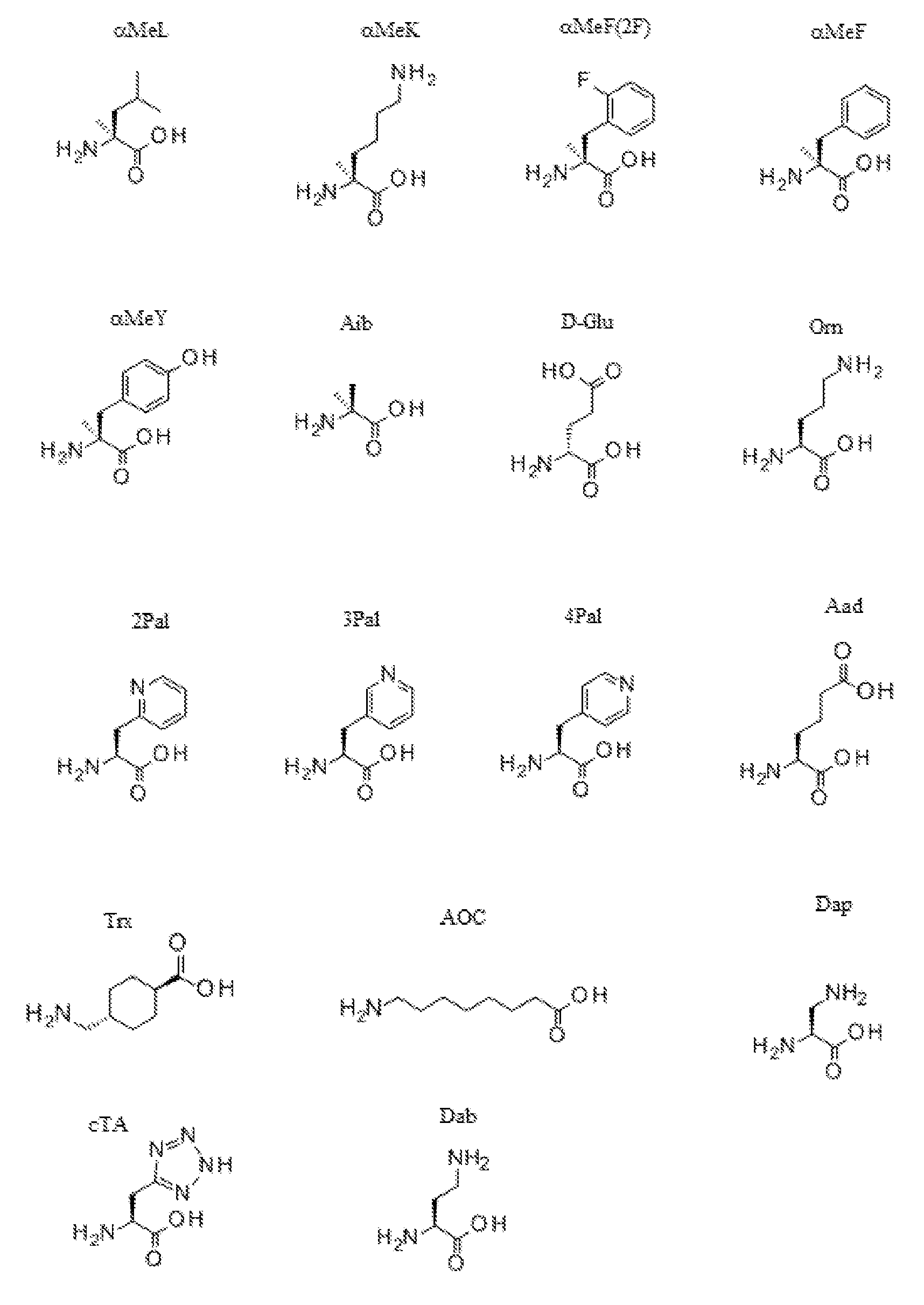
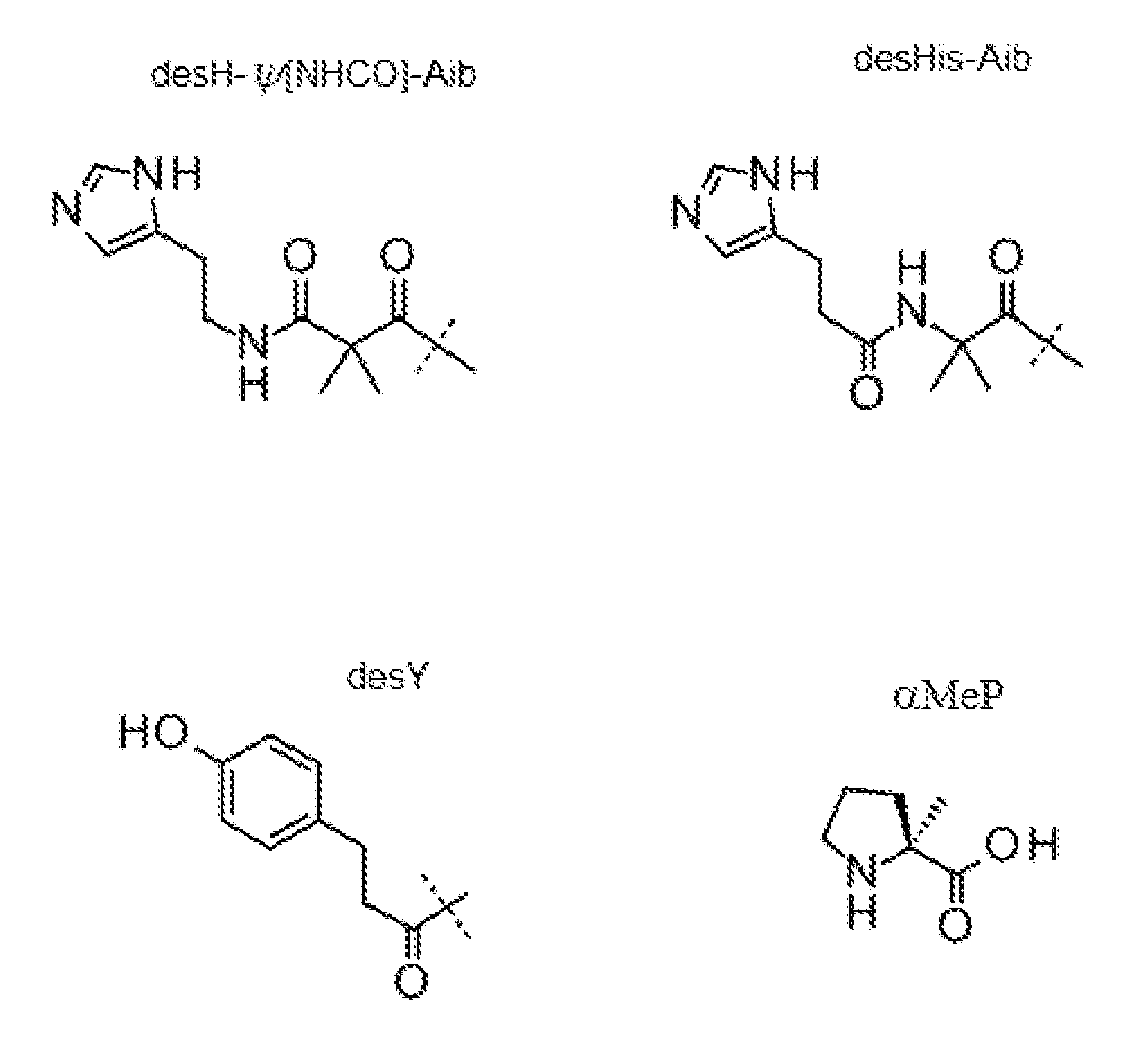
본원에 사용된 바와 같이 용어 "아미노산"은 자연 발생 아미노산 및 비천연 아미노산 둘 다를 의미한다. 아미노산은 전형적으로 표준 하나의 문자 코드 (예를 들어, L = 류신), 뿐만 아니라 천연 아미노산의 알파-메틸 치환 잔기 (예를 들어, α-메틸 류신, 또는 αMeL 및 α-메틸 리신, 또는 αMeK), 및 특정 기타 비천연 아미노산, 예컨대 알파 아미노 이소부티르산, 또는 "Aib", "4Pal", "Orn" 등을 사용하여 표시된다. 이들 아미노산의 구조는 다음과 같다:
본원에 사용된 바와 같이 "Orn"은 오르니틴을 의미한다. 본원에 사용된 바와 같이 "4Pal"은 3-(4-피리딜)-L-알라닌을 의미한다. 본원에 사용된 바와 같이 "αMeF(2F)"는 알파-메틸 2-F-페닐알라닌을 의미한다. 본원에 사용된 바와 같이 "αMeY," "αMeK," 및 "αMeL"은 알파 메틸 티로신, 알파 메틸 리신, 및 알파 메틸 류신 각각을 의미한다. 본원에 사용된 바와 같이, "e" 및 "D-Glu"은 D-글루탐산을 의미한다. 본원에 사용된 바와 같이 "D-His" 및 "h"는 각각 D-히스티딘을 의미한다. 본원에 사용된 바와 같이 "D-Tyr" 및 "y"는 각각 D-티로신을 의미한다. 본원에 사용된 바와 같이 "D-Ser" 및 "s"는 D-세린을 의미한다. 본원에 사용된 바와 같이 "D-Ala" 및 "a"는 각각은 D-알라닌을 의미한다. 본원에 사용된 바와 같이, "αMeF(2F)"는 알파-메틸-F(2F) 및 알파-메틸-Phe(2F)를 의미한다. 본원에 사용된 바와 같이, "αMeF"는 알파-메틸-F 및 알파-메틸-Phe를 의미한다. 본원에 사용된 바와 같이, "αMeY"는 알파-메틸-Tyr을 의미한다. 본원에 사용된 바와 같이 "αMeK"는 알파-메틸-Lys를 의미한다. 본원에 사용된 바와 같이, "αMeL"은 알파-메틸-Leu를 의미한다. 본원에 사용된 바와 같이, "αMeS"는 알파-메틸-세린 및 알파-메틸-Ser을 의미한다. 본원에 사용된 바와 같이 "αMeP"는 알파-메틸-프롤린 및 알파-메틸-Pro를 의미한다. 본원에 사용된 바와 같이, "desH"는 desHis를 의미한다. 본원에 사용된 바와 같이, "desY"는 desTyr을 의미한다.
X1이 DesH이고 X2가 Aib인 경우, DesH와 Aib가 합하여 상기에 예시한 바와 같이 기, DesH-ψ[NHCO]-Aib를 형성할 수 있다.
본원에서 사용될 때, 용어 "C16-C22 지방산에 접합된 아미노산"은 지방산에 대한 공유 결합에 의해 또는, 바람직하게는, 링커에 의해 지방산에 접합되도록 화학적으로 변형된 관능기를 가진 임의의 천연 또는 비천연 아미노산을 지칭한다. 이러한 관능기의 예는 아미노, 카르복실, 클로로, 브로모, 아이오도, 아지도, 알키닐, 알케닐, 및 티올 기를 포함한다. 이러한 관능기를 포함하는 천연 아미노산의 예는 K (아미노), C (티올), E (카르복실) 및 D (카르복실)를 포함한다. 일 실시양태에서 접합된 아미노산은 K이다.
상기에 언급된 바와 같이, 화학식 I, II, III, IV, 및 V의 화합물의 실시양태에서 지방산 모이어티가 링커 또는 직접 결합을 통해 접합된 본 발명의 화합물이 있다. 일 실시양태에서, 본 발명의 화합물은, 바람직하게는 링커를 통해, 위치 14 또는 17에서 K에 접합된 지방산 모이어티를 포함한다. 일 실시양태에서, 접합은 아실화이다. 일 실시양태에서, 접합은 K 측쇄의 엡실론-아미노 기에 대한 것이다. 본 발명의 화합물의 일 실시양태에서 위치 17에서 K에, 링커를 통해, 접합된 지방산 모이어티를 포함한다.
일 실시양태에서, 본 발명의 화합물은 접합에 이용가능한 관능기를 가진 천연 또는 비천연 아미노산에 링커 없이 직접 접합된 지방산 모이어티를 포함한다. 특정 바람직한 실시양태에서 접합된 아미노산은 K, C, E 및 D로 이루어진 군으로부터 선택된다. 특히 바람직한 실시양태에서 접합된 아미노산은 K이다. 이러일 실시양태에서 접합은 K 측쇄의 엡실론-아미노 기에 대한 것이다.
일 실시양태에서, 링커는 1 내지 4 개의 아미노산, 아미노 폴리에틸렌 글리콜 카르복실레이트, 또는 그의 혼합물을 포함한다. 일 실시양태에서, 아미노 폴리에틸렌 글리콜 카르복실레이트는 하기 화학식을 갖는다 :
H-{NH-CH2-CH2-[O-CH2-CH2]p-O-(CH2)z-CO}r-OH, (여기서 p는 1 내지 12의 임의의 정수이고, z는 1 내지 20의 임의의 정수이며, r은 1 또는 2이다).
일 실시양태에서 링커를 통해 지방산에 접합된 아미노산을 포함하는 화학식 I의 화합물이 있으며, 여기서 링커는 Glu 및 γ-Glu로 이루어진 군으로부터 선택된 1 내지 2개의 아미노산이다. 일 실시양태에서 링커는 1 내지 2개의 (2-[2-(2-아미노-에톡시)-에톡시]-아세틸) 모이어티이다. 본 발명의 화합물은 직접 결합 또는 링커에 의해 아미노산의 관능기에 화학적으로 접합된 C16-C22 지방산을 이용한다. 일 실시양태에서, 지방산 모이어티는 리신과 지방산 사이의 링커를 통해 위치 17에서 리신에 접합된다. 한 실시양태에서, 지방산 모이어티는 리신과 지방산 사이의 링커를 통해 위치 20에서 리신에 접합된다. 일 실시양태에서, 지방산 쇄는 임의의 단일 쇄 C16-C22 지방산이다.
일 실시양태에서 지방산이 링커로 접합되고, 링커가 1개 이상의 (2-[2-(2-아미노-에톡시)-에톡시]-아세틸) 모이어티를, 0개 또는 1 내지 4 개의 아미노산과 조합하여 포함하는 것인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서, 링커는 1 내지 4 개의 Glu 또는 γ-Glu 아미노산 잔기를 포함할 수 있다. 일 실시양태에서, 링커는 1 또는 2 개의 Glu 또는 γ-Glu 아미노산 잔기를 포함할 수 있다. 일 실시양태에서 화학식 I의 화합물, 또는 그의 제약상 허용되는 염은, 링커를 통해 접합된 지방산을 포함하며, 여기서 링커는 1 또는 2 개의 γ-Glu 아미노산 잔기를 포함한다. 일 실시양태에서 화학식 I의 화합물, 또는 그의 제약상 허용되는 염은, 링커를 통해 접합된 지방산을 포함하며 여기서 링커 최대 36개의 (2-[2-(2-아미노-에톡시)-에톡시]-아세틸) 모이어티와 조합하여 사용된 1 내지 4 개의 아미노산 잔기 (예컨대, 예를 들어 Glu 및 γ-Glu 아미노산)을 포함할 수 있다. 구체적으로, 일 실시양태에서 링커를 통해 접합된 지방산을 포함하는 화학식 I 화합물, 또는 그의 제약상 허용되는 염이 있으며, 여기서 링커는 1 내지 4개의 Glu 및 γ-Glu 아미노산과 1 내지 4개의 (2-[2-(2-아미노-에톡시)-에톡시]-아세틸) 모이어티의 조합을 구성한다. 일 실시양태에서 링커를 통해 접합된 지방산을 포함하는 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있으며, 여기서 링커는 1 또는 2개의 γ-Glu 아미노산과 1 또는 2개의 (2-[2-(2-아미노-에톡시)-에톡시]-아세틸) 모이어티의 조합으로 구성된다. 일 실시양태에서 링커를 통해 접합된 지방산을 포함하는 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있으며, 여기서 링커 및 지방산 성분은 하기 화학식을 갖는다:
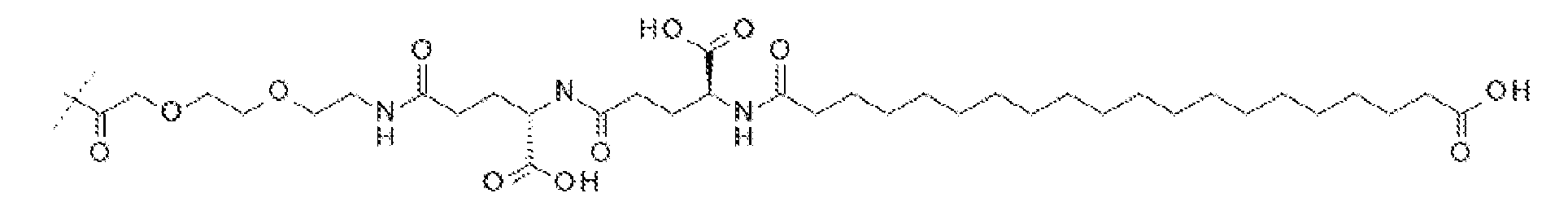
(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)a-(γ-Glu)b-CO-(CH2)q-CO2H, (여기서 a는 1 또는 2이고, b는 1 또는 2이고, q는 16 또는 18이다). 일 실시양태에서, a는 2이고, b는 1이고, q는 18이고; 구조는 다음과 같다:
일 실시양태에서, a는 1이고, b는 2이고 q는 18이고; 구조는 다음과 같다:
일 실시양태에서 a는 1이고, b는 1이고, q는 18이고; 구조는 다음과 같다:
본원에 사용된 바와 같은 용어 "C16-C22 지방산"은 16 내지 22개의 탄소 원자를 가진 카르복실산을 의미한다. 일 실시양태에서, 본원에서 사용하기에 적합한 C16-C22 지방산은 포화 이산일 수 있다. 일 실시양태에서, 지방산은 C20-C22이다. 일 실시양태에서 q는 14, 16, 18, 및 20으로 이루어진 군으로부터 선택된다. 일 실시양태에서 q는 18 및 20으로부터 선택된다. 일 실시양태에서 q는 18이다. 일 실시양태에서 q는 20이다.
일 실시양태에서, 본원에 개시된 그의 화합물 및 용도에 적합한 구체적 포화 C16-C22 지방산은 그의 분지형 및 치환 유도체를 포함하여, 헥사데칸디오산 (C16 이산), 헵타데칸디오산 (C17 이산), 옥타데칸디오산 (C18 이산), 노나데칸디오산 (C19 이산), 에이코산디오산 (C20 이산), 헨에이코산디오산 (C21 이산), 도코산디오산 (C22 이산)을 포함하나, 이에 제한되지는 않는다.
일 실시양태에서, C16-C22 지방산은 포화 C18 이산, 포화 C19 이산, 포화 C20 이산, 및 그의 분지형 및 치환 유도체로 이루어진 군으로부터 선택된다. 일 실시양태에서, C16-C22 지방산은 스테아르산, 아라카드산 및 에이코산디오산으로 이루어진 군으로부터 선택된다. 일 실시양태에서, C16-C22 지방산은 아라카드산이다.
하기 실시예 1 내지 5의 화학 구조에 나타난 바와 같이, 일 실시양태에서 상기에 기재된 링커-지방산 모이어티는 리신 측쇄의 엡실론-아미노 기에 연결된다.
일 실시양태에서, X30, X31, X32, X33, X34, X35, X36, X37, X38, X39, 및 X40 중 어느 것도 C가 아니거나 지방산을 함유하는 치환기인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서, X10, X12, X13, X14, X16, X17, X19, X20, X21, X23, X24, X26, X27, X28, X29, X30, X31, X32, X33, X34, X35, X36, X37, X38, X39, 및 X40 중 어느 것도 지방산을 함유하는 치환기가 아니며; X30, X34, X39, 및 X40 중 어느 것도 C가 아닌 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 X10, X12, X13, X14, X16, X17, X19, X20, X21, X23, X24, X26, X27, X28, X29, X30, X31, X32, X33, X34, X35, X36, X37, X38, X39, 및 X40 중 어느 것도 지방산을 함유하는 치환기가 아닌 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다.
본원에 사용된 바와 같이 "시간-연장 기술(time-extension technology)은 펩티드 시간-연장 기술 예를 들어, 재조합 인간 혈청 알부민 ("rHSA"), 제약상 허용되는 중합체에 대한 펩티드 접합, 예컨대 아미노산의 중합체 서열 ("XTEN"), 비황산화 헤파린-유사 탄수화물 중합체 ("HEP"), 히드록실 에틸 전분 ("HES"), 라마 중쇄 항체 단편 ("VHH"), 페길화, Fc 접합, 소 혈청 알부민 ("BSA")을 의미한다 (Sleep, D. Epert Opin Drug Del (2015) 12, 793-812; Podust VN et al. J Control. Release, 2015; ePUB; Hey, T. et al. in: R. Kontermann (Ed.), Therapeutic Proteins: Strategies to Modulate their Plasma Half-Lives, Wiley-VCH Verlag Gmbh & Co. KGaA, Weinheim, Germany, 2012, pp117-140; DeAngelis, PL, Drug Dev Delivery (2013) January, 12/31/2012). 일 실시양태에서 시간-연장 기술은 링커 기를 사용하여 적용된다. 일 실시양태에서, 시간-연장 기술은 링커로서 0, 1, 2, 또는 3개 아미노산을 사용하여 적용된다.
일 실시양태에서 시간-연장 기술 또는 지방산 없이, 화학식 I의 화합물, 또는 그의 제약상 허용되는 염 (즉, X10, X12, X13, X14, X16, X17, X19, X20, X21, X23, X24, X26, X27, X28, X29, X30, X31, X32, X33, X34, X35, X36, X37, X38, X39, 및 X40 중 어느 것도 지방산을 함유하는 치환기가 아닌 화합물)을 경피 또는 주입 투여 방법을 통해 이를 필요로 하는 환자에게 투여할 수 있다. 추가로, 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이, 지방산 없이, 펩티드 시간-연장 기술 예를 들어, 재조합 인간 혈청 알부민 ("rHSA"), 제약상 허용되는 중합체에 대한 펩티드 접합, 예컨대 아미노산의 중합체 서열 ("XTEN"), 비황산화 헤파린-유사 탄수화물 중합체 ("HEP"), 및 히드록실 에틸 전분 ("HES")을 사용하여 추가로 변형될 수 있다. 일 실시양태에서, 통상의 기술자에게 공지된 절차를 사용하여, 지방산 없이, 화학식 I 화합물의 시스테인 아미노산, 또는 그의 제약상 허용되는 염을 사용하여 시간-연장 기술이 적용된다. 일 실시양태에서, 화학식 I 화합물의 한 아미노산, 또는 그의 제약상 허용되는 염에, 지방산 없이, 시간-연장 기술이 적용된다. 화학식 I 화합물, 또는 그의 제약상 허용되는 염에, 지방산 없이, 시간-연장 기술이 적용되는 일 실시양태에서, X17은 I, K 및 Q로 이루어진 군으로부터 선택된다. 화학식 I 화합물, 또는 그의 제약상 허용되는 염에, 지방산 없이, 시간-연장 기술이 적용되는 일 실시양태에서, X30은 C이다. 화학식 I 화합물, 또는 그의 제약상 허용되는 염에, 지방산 없이, 시간-연장 기술이 적용되는 일 실시양태에서, X34는 C이다. 화학식 I 화합물, 또는 그의 제약상 허용되는 염에, 지방산 없이, 시간-연장 기술이 적용되는 일 실시양태에서, X39는 C이다. 화학식 I 화합물, 또는 그의 제약상 허용되는 염에, 지방산 없이, 시간-연장 기술이 적용되는 일 실시양태에서 X40은 C이다.
하나 이상의 GIP 또는 GLP-1 수용체와 관련하여 본원에서 사용될 때, 용어 "활성", "활성화하다" "활성화하는" 등은 관련 기술분야에 공지된 검정, 예컨대 하기 기재된 시험관내 검정을 사용하여 측정된 바와 같이, 수용체(들)에 결합하고 상기 수용체에서 반응을 유도하는 화합물, 또는 그의 제약상 허용되는 염의 능력을 지칭한다.
GIP 및 GLP-1 수용체 각각에 대한 본 발명의, 화합물, 또는 그의 제약상 허용되는 염의 친화도는, 예를 들어 하기 실시예에 기재된 것들을 포함하고, 통상 Ki 값으로서 표시되는, 관련 기술분야에서 수용체 결합 수준을 측정하기 위해 공지된 기술을 사용하여 측정될 수 있다. 각각의 수용체에서 본 발명의 화합물의 활성은 또한 예를 들어 하기에 기재된 시험관내 활성 검정을 포함하고, 통상 EC50 값으로서 표시되는, 관련 기술분야에서 공지된 기술을 사용하여 측정될 수 있으며, 이는 용량 반응 곡선에서 최대 절반의 시뮬레이션을 야기하는 화합물의 농도이다.
일 실시양태에서, 화학식 I의 화합물의 제약 조성물은 비경구 경로 (예를 들어, 피하, 정맥내, 복강내, 근육내, 또는 경피)에 의한 투여에 적합하다. 일 실시양태에서, 화학식 I의 화합물의 제약 조성물은 경구 투여 (예를 들어, 정제, 캡슐)에 적합하다. 일부 제약 조성물 및 이를 제조하는 방법은 관련 기술분야에 널리 공지되어 있다. (예를 들어, 문헌 [Remington: The Science and Practice of Pharmacy (D.B. Troy, Editor, 21st Edition, Lippincott, Williams & Wilkins, 2006] 참조). 위장관의 해부학적 및 생리학적 특색과 더불어 펩티드의 물리화학적 특성은 펩티드의 효율적인 경구 전달에 도전과제를 제공할 수 있다. 일 실시양태에서 경구 투여용 제약 조성물은 본 발명의 화합물, 및 침투 증진제를 포함한다. 일 실시양태에서, 경구 투여용 제약 조성물은 화학식 I의 화합물 또는 그의 제약상 허용되는 염, 침투 증진제, 및 프로테아제 억제제를 포함한다. 일 실시양태에서, 경구 투여용 제약 조성물은 화학식 I의 화합물, 또는 그의 제약상 허용되는 염, 및 침투 증진제를 포함한다,
본 발명의 화합물에 대한 일체형(monolithic) 및 다중-미립자 투여 형태가 고려된다. 일 실시양태에서, 화학식 I의 화합물은 일체형 조성물로서 제공된다. 일체형 조성물은 단일 위치에서 모든 성분의 방출을 위한 것이다. 다중-미립자 조성물은 위로부터 장으로의 빠른 이동을 달성하고 소장의 넓은 표면에 걸쳐 조성물 성분의 분포를 허용하기 위한 것이다. 일체형 및 다중-미립자 투여 조성물에 대해 화합물 및 기능적 부형제의 동시 방출이 바람직하다. 일 실시양태에서, 화학식 I의 화합물, 또는 그의 제약상 허용되는 염의 일체형 조성물은 장용성 캡슐, 장용성 코팅 캡슐 또는 장용성 코팅 정제로서 제제화된다. 이러한 다중-미립자 조성물은 장용성 코팅 미니정제, 또는 장용성 코팅 과립으로서 제제화될 수 있으며, 여기서 코팅은 일반적으로 낮은 pH에서 위에서 무손상이며 장의 더 높은 pH에서 용해된다. 2 가지 유형의 코팅 미니정제 또는 코팅 과립은 pH 5.5 초과 용해에 의해 근위 소장으로 전달되거나 pH 7 내지 7.2 초과 용해에 의해 원위 소장으로 전달되도록 제제화될 수 있다. 원위 소장 방출을 위한 코팅 시스템은 원위 소장 전달이 필요한 경우 일체형 캡슐 또는 정제에 또한 적용될 수 있다. 미니정제는 표준 코팅되지 않은 캡슐에 채울 수 있다.
본원에 사용된 바와 같이 용어 "침투 증진제"는 본 발명의 화합물의 경구 흡수를 증진시키는 침투 증진제를 의미한다. 본원에 사용된 바와 같이, 침투 증진제는 침투 증진제, 예컨대 소듐 데카노에이트, 소듐 타우로데옥시콜레이트, 라우로일 카르니틴, 도데실 말토시드, 도데실 포스파티딜콜린, SNAC, 람노리피드, 및 문헌에 보고된 침투 증진제, 예컨대 예를 들어, 포스파타제의 침투 억제제, PIP-250 및 PIP-640을 의미한다. 문헌 [Pharmaceutics. 2019 Jan; 11(1): 41]을 참조, (문헌 [See Biomaterials. 2012; 33: 3464-3474] 참조), ZOT (폐쇄띠 독소(zonula occludens toxin)), ΔG (ZOT의 단편) (문헌 [Int. J. Pharm. 2009; 365, 121-130] 참조). 일 실시양태에서, 침투 증진제는 소듐 데카노에이트, 소듐 타우로데옥시콜레이트, 및 라우로일 카르니틴으로 이루어진 군으로부터 선택된다. 일 실시양태에서, 침투 증진제는 C10, LC, 및 NaTDC로 이루어진 군으로부터 선택된다. 일 실시양태에서 침투 증진제는 C10이다.
본원에 사용된 바와 같이 용어 "프로테아제 억제제"는 단백질 기반, 펩티드 기반, 및 소분자 기반으로 이루어진 군으로부터 선택될 수 있는 프로테아제 억제제를 의미한다. 프로테아제 억제제는 널리 공지되어 있으며, 예를 들어, 대두 트립신 억제제 ("SBTI"), 대두 트립신-키모트립신 억제제 ("SBTCI"), 에코틴, 해바라기 트립신 억제제 ("SFTI"), 류펩틴, 시트르산, 에틸렌디아민테트라아세트산 ("EDTA"), 소듐 글리코콜레이트 및 4-(2-아미노에틸) 벤젠술포닐 플루오라이드 히드로클로라이드 ("AEBSF")를 포함할 수 있다. 일 실시양태에서 프로테아제 억제제는 SBTI, SBTCI 및 SFTI로 이루어진 군으로부터 선택된다. 일 실시양태에서, 프로테아제 억제제는 SBTI이다.
일 실시양태에서 세포막 구아노신 5'-(감마-티오) 트리포스페이트-[35S] (GTPγS) 결합 검정에 의해 나타난 바와 같이 GLP-1R에 대한 부분 효능제, 및 β-아레스틴-2 동원 검정에 의해 나타난 바와 같이 GLP-1R에 대한 부분 효능제인 강력한 GIPR/GLP-1R 이중 효능제인 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서 GLP-1R HEK293 세포막 구아노신 5'-(감마-티오) 트리포스페이트-[35S] (GTPγS) 결합 검정에서 Gαs의 GLP-1R 유도된 활성화를 자극하는, 화학식 I의 화합물, 또는 그의 제약상 허용되는 염이 있다. 일 실시양태에서, GLP-1R HEK293 세포막 구아노신 5'-(감마-티오) 트리포스페이트-[35S] (GTPγS) 결합 검정에서 75% 이하, 및 GLP-CHO 세포 β-아레스틴.동원 검정에서 35% 이하의 부분 효능작용을 나타내는 화합물이 있다.
일 실시양태에서 GLP-1R HEK293 세포막 구아노신 5'-(감마-티오) 트리포스페이트-[35S] (GTPγS) 결합 검정에서 75% 이하의 부분 효능작용을 나타내는 화합물의 유효량, 및 GIP 효능제인 화합물의 유효량을 투여하는 것을 포함하는, 당뇨병을 치료하는 방법이 있다. 일 실시양태에서, GLP-1R HEK293 세포막 구아노신 5'-(감마-티오) 트리포스페이트-[35S] (GTPγS) 결합 검정에서 부분 효능작용을 나타내는 화합물은 GIP 효능제 활성을 갖는 화합물과 공동 투여된다. 일 실시양태에서, GLP-1R HEK293 세포막 구아노신 5'-(감마-티오) 트리포스페이트-[35S] (GTPγS) 결합 검정에서 부분 효능작용을 나타내는 화합물은 GIP 효능제 활성을 갖는 화합물 전후 1주 이내에 활성제로서 투여된다. 일 실시양태에서, 당뇨병을 치료하는 방법은 GLP-CHO 세포 β-아레스틴.동원 검정에서 35% 이하를 나타내는 화합물의 유효량을 투여하는 것 및 GLP-1R HEK293 세포막 구아노신 5'-(감마-티오) 트리포스페이트-[35S] (GTPγS) 결합 검정에서 75% 이하의 부분 효능작용을 나타내는 화합물의 유효량을 투여하는 것을 포함한다.
본 발명의 화합물은 다수의 무기 및 유기 산/염기 중 임의의 것과 반응하여 제약상 허용되는 산/염기 부가 염을 형성할 수 있다. 제약상 허용되는 염 및 이들을 제조하기 위한 통상의 방법은 관련 기술분야에 널리 공지되어 있다 (예를 들어, 문헌 [P. Stahl, et al. Handbook of Pharmaceutical Salts: Properties, Selection and Use, 2nd Revised Edition (Wiley-VCH, 2011)] 참조). 본 발명의 제약상 허용되는 염은 소듐, 트리플루오로아세테이트, 히드로클로라이드, 암모늄, 및 아세테이트 염을 포함하나, 이에 제한되지는 않는다. 일 실시양태에서, 그의 제약상 허용되는 염은 소듐, 히드로클로라이드, 및 아세테이트 염으로 이루어진 군으로부터 선택된다.
본 발명은 또한 본 발명의 화합물, 또는 그의 제약상 허용되는 염의 합성에 유용한 신규 중간체 및 방법을 포함한다. 본 발명의 중간체 및 화합물은 관련 기술분야에 공지된 여러 가지의 절차에 의해 제조될 수 있다. 특히, 하기 실시예는 화학 합성을 사용하는 공정을 기재한다. 기재된 경로 각각에 대한 구체적 합성 단계는 본 발명의 화합물을 제조하기 위해 상이한 방식으로 조합될 수 있다. 시약 및 출발 물질은 관련 기술분야의 통상의 기술자가 쉽게 이용할 수 있다.
본원에서 사용될 때, 용어 "유효량"은 본 발명의 화합물, 또는 그의 제약상 허용되는 염의 양 또는 용량을 지칭하며, 이는 환자에게 단일 또는 다중 용량 투여 직후, 진단 또는 치료 중인 환자에게 원하는 효과를 제공한다. 유효량은 공지된 기술을 사용하고 유사한 상황 하에 수득된 결과를 관찰함으로써 관련 기술분야의 통상의 기술자에 의해 결정될 수 있다. 대상체에 대한 유효량을 결정할 때, 포유동물의 종; 그의 크기, 연령, 및 일반적 건강; 관련된 구체적 질환 또는 장애; 질환 또는 장애의 정도 또는 관여 또는 중증도; 개별 환자의 반응; 투여되는 특정한 화합물; 투여 방식; 투여된 제제의 생체이용률 특성; 선택된 용량 요법; 수반되는 의약의 사용; 및 기타 관련 상황을 포함하나, 이에 제한되지는 않는 다수의 인자가 고려된다.
본원에서 사용될 때, 용어 "이를 필요로 하는 대상체"는, 예를 들어 이전 단락에 열거된 것들을 포함하여, 치료 또는 요법을 필요로 하는 질환 또는 병태를 가진, 포유동물, 바람직하게는 인간을 지칭한다.
본원에 사용된 바와 같이 "EDTA"는 에틸렌디아민테트라아세트산을 의미한다. 본원에 사용된 바와 같이 "DMSO"는 디메틸 술폭시드를 의미한다. 본원에 사용된 바와 같이 "CPM"은 분당 카운트를 의미한다. 본원에 사용된 바와 같이 "IBMX"는 3-이소부틸-1-메틸크산틴을 의미한다. 본원에 사용된 바와 같이 "LC/MS"는 액체 크로마토그래피/질량 분석법을 의미한다. 본원에 사용된 바와 같이 "HTRF"는 균질한 시간-분해 형광을 의미한다. 본원에 사용된 바와 같이 "BSA"는 소 혈청 알부민을 의미한다.
본 발명은 하기 실시예에 의해 추가로 설명되며, 이는 제한하는 것으로 해석되지 않아야 한다.
펩티드 합성
실시예 1
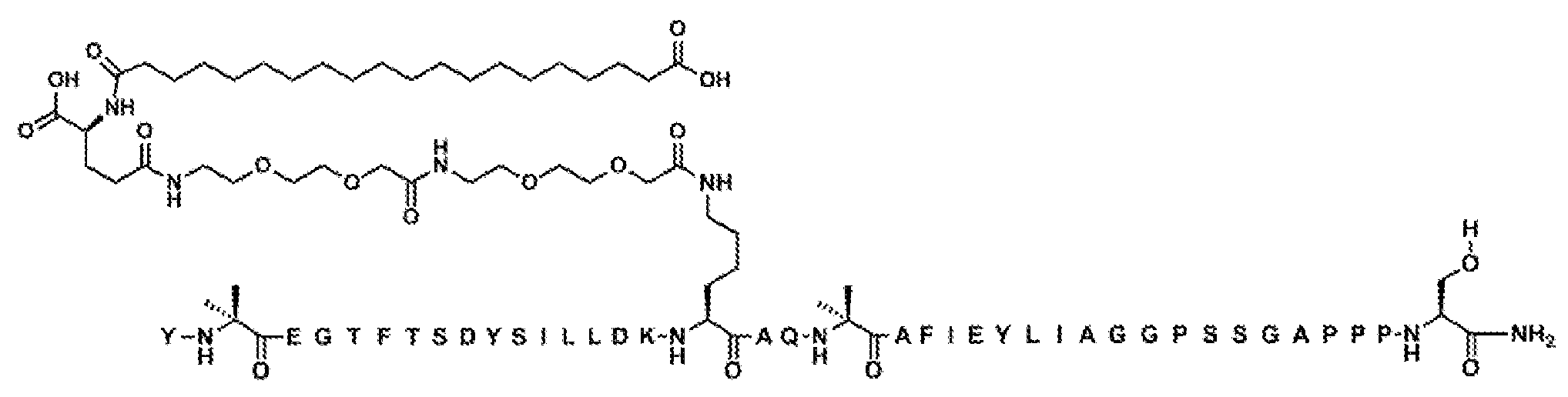
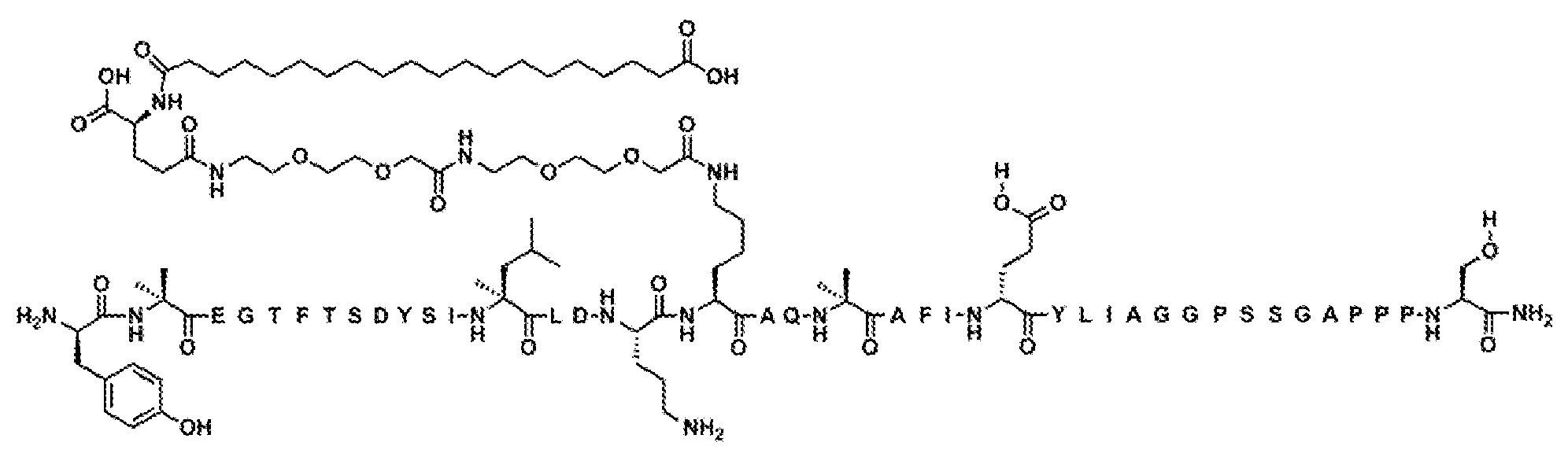
Y-Aib-EGT-αMeF(2F)-TSDYSI-αMeL-LDEK((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
18
-CO
2
H)AQ-Aib-EFI-(D-Glu)-YLIEGGPSSGAPPPS-NH
2
(서열식별번호: 10).
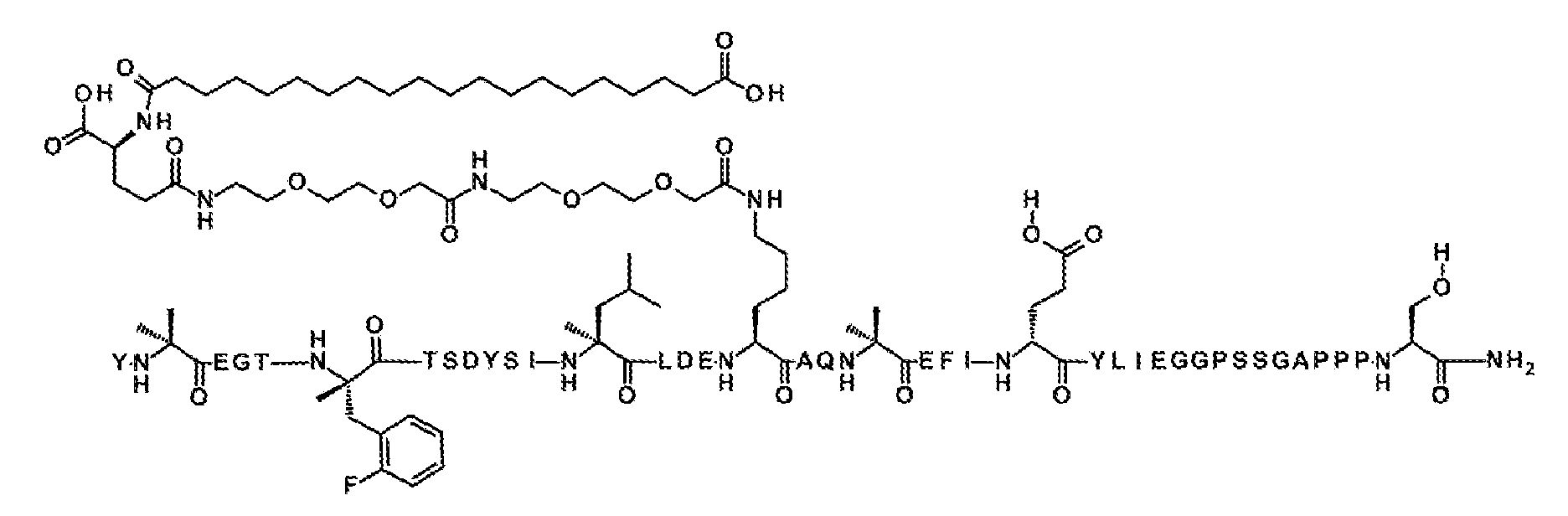
서열식별번호: 10의 구조는 Aib2, αMeF(2F)6, αMeL13, K17, Aib20, D-Glu24, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며 여기서 이들 아미노산 잔기의 구조가 확장되었다:
실시예 1의 펩티드 백본은 심포니(Symphony) X 펩티드 합성기 (기로스 프로테인 테크놀로지즈(Gyros Protein Technologies). 애리조나주 투손)에서 플루오레닐메틸옥시카르보닐 (Fmoc)/tert-부틸 (t-Bu) 화학을 사용하여 합성하였다.
수지는 0.3-0.4 meq/g의 치환으로 1% DVB 가교-연결된 폴리스티렌 (Fmoc-Rink-MBHA 낮은 로딩(Low Loading) 수지, 100-200 메시, EMD 밀리포어(Millipore))으로 이루어졌다. 표준 측쇄 보호기를 사용하였다. Fmoc-Lys(Mtt)-OH는 위치 17의 리신에 사용되고 Boc-Tyr(tBu)-OH)는 위치 1의 티로신에 사용되었다. Fmoc 기는 DMF에서 20% 피페리딘을 사용하여 각각의 커플링 단계 전에 (2 x 7분) 제거하였다. 모든 표준 아미노산 커플링은 이론적 펩티드 로딩에 비해 9배 몰 과량으로, Fmoc 아미노산 (0.3 mM), 디이소프로필카르보디이미드 (0.9 mM) 및 옥시마(Oxyma) (0.9 mM)의 동일한 몰비를 사용하여, 1급 아민에 1시간 및 2급 아민에 3시간 동안 수행하였다. 예외는 3시간 동안 커플링되는, Cα-메틸화 아미노산에 대한 커플링이다. 펩티드 백본의 합성 완료 후, 수지를 DCM으로 6회 철저히 세척하여 잔류 DMF를 제거하였다. 위치 17의 리신에 있는 Mtt 보호기는 DCM에서 30% 헥사플루오로이소프로판올 (오우크우드 케미컬즈(Oakwood Chemicals))의 2회 처리 (2 x 40 분 처리)를 사용하여 펩티드 수지로부터 선택적으로 제거하였다.
지방산-링커 모이어티의 후속 부착은 2-[2-(2-Fmoc-아미노-에톡시)-에톡시]-아세트산 (Fmoc-AEEA-OH, 켐펩, 인크.(ChemPep, Inc.)), Fmoc-글루탐산 α-t-부틸 에스테르 (Fmoc-Glu-OtBu, 아르크 팜, 인크.(Ark Pharm, Inc.)), 모노-OtBu-에이코산디오산 (우시 앱텍(WuXi AppTec), 중국 상하이)의 커플링에 의해 완수하였다. 3배 과량의 시약 (AA: PyAOP: DIPEA=1: 1 :1 mol/mol)을 1시간 길이의 각각의 커플링에 사용하였다.
합성이 완료된 후, 펩티드 수지를 DCM으로 세척한 다음에, 완전히 공기-건조시켰다. 건조 수지를 실온에서 2시간 동안 10 mL의 절단 칵테일 (트리플루오로아세트산: 물: 트리이소프로필실란, 95:2.5:2.5 v/v)로 처리하였다. 수지를 여과 제거하고 2 mL의 순수 T'FA로 각각 2회 세척하고, 합해진 여액을 5배 과량의 냉 디에틸 에테르 (-20℃)로 처리하여 조 펩티드를 침전시켰다. 이어서 펩티드/에테르 현탁액을 3500 rpm에서 2분 동안 원심분리하여 고체 펠릿을 형성하고, 상청액을 따라 내고, 고체 펠릿을 에테르로 추가로 2회 연화처리하고 진공 중에 건조시켰다. 조 펩티드를 20% 아세토니트릴/20% 아세트산/60% 물에 가용화시키고 100% 아세토니트릴 및 0.1% TFA/물 완충 시스템 (60분 내에 30-50% 아세토니트릴)의 선형 구배로 루나(Luna) 5 μm 페닐-헥실 분취용 컬럼 (21 x 250 mm, 페노멕스(Phenomenex)) 상에서 RP-HPLC에 의해 정제하였다. 분석용 RP-HPLC를 사용하여 펩티드의 순도를 평가하고 풀링 기준은 >95%이었다. 화합물 1의 주요 풀 순도는 98.0%인 것으로 밝혀졌다. 최종 주요 생성물 풀의 후속 동결건조는 동결건조된 펩티드 TFA 염을 산출하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1657.2; 계산치 M+3 =1657.0).
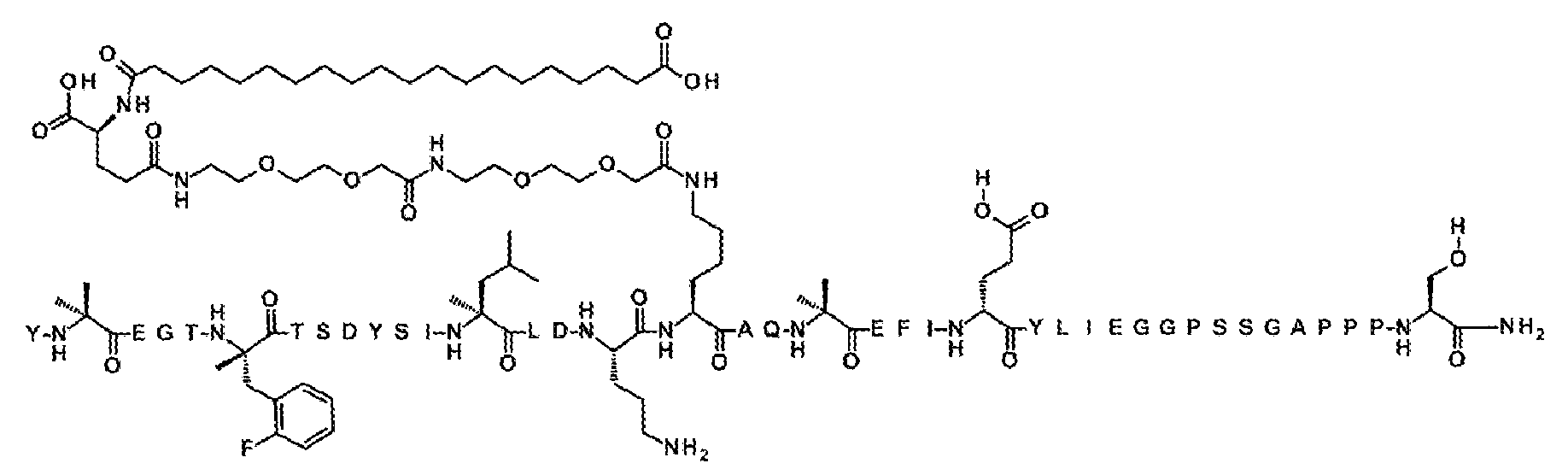
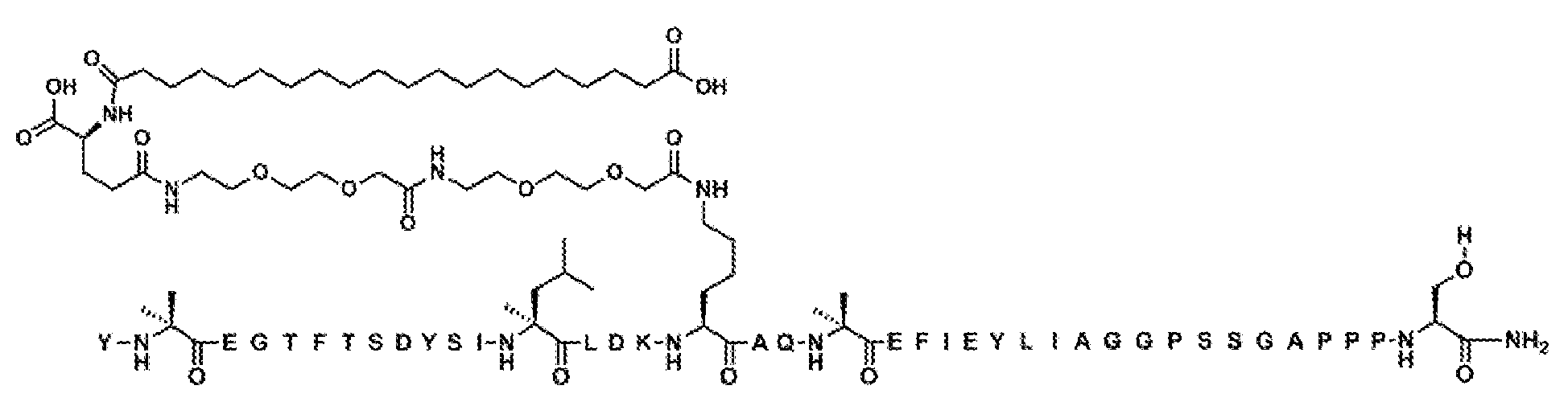
실시예 2
Y-Aib-EGT-αMeF(2F)-TSDYSI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
16
-CO
2
H)AQ-Aib-EFI-(D-Glu)-YLIEGGPSSGAPPPS-NH
2
(서열식별번호: 11)
서열식별번호: 11의 구조는 Aib2, αMeF(2F)6, αMeL13, Orn16, K17, Aib20 D-Glu24, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 11에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1642.6; 계산치 M+3 =1642.8).
실시예 3
실시예 3은 하기 기재로 표시되는 화합물이다.
Y-Aib-EGT-αMeF(2F)-TSDYSI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
18
-CO
2
H)AQ-Aib-EFI-(D-Glu)-YLIEGGPSSGAPPPS-NH
2
(서열식별번호: 12)
서열식별번호: 12의 구조는 Aib2, αMeF(2F)6, αMeL13, Orn16, K17, Aib20, D-Glu24, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며, 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 12에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1651.8; 계산치 M+3 =1652.2).
실시예 4
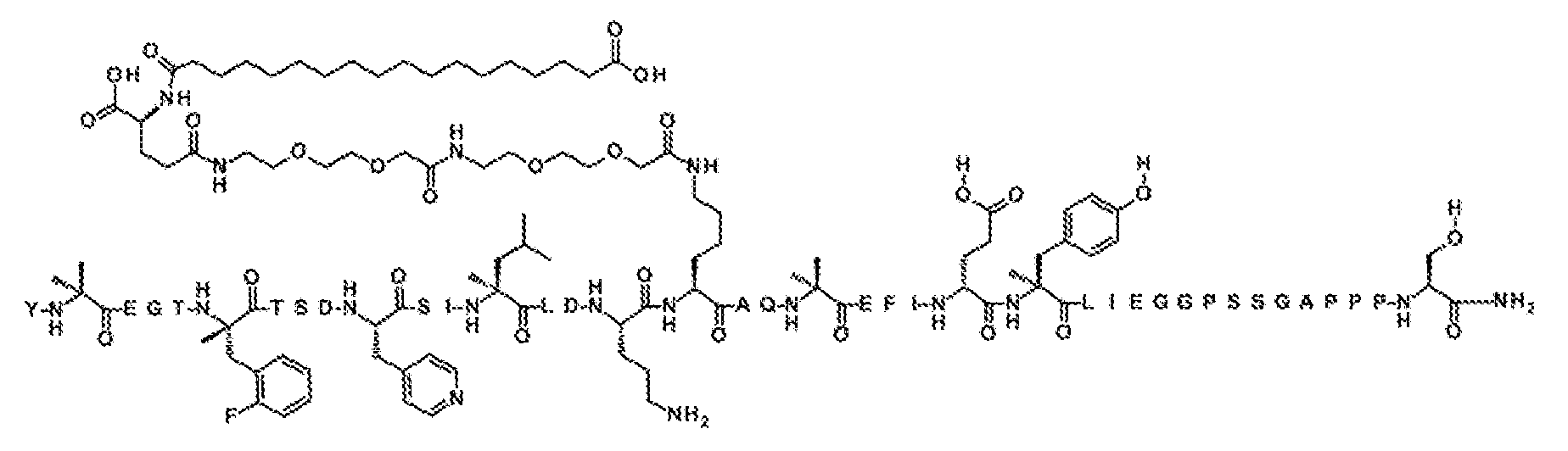
Y-Aib-EGT-αMeF(2F)-TSD-4Pal-SI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
16
-CO
2
H)AQ-Aib-EFI-(D-Glu)-αMeY-LIEGGPSSGAPPPS-NH
2
(서열식별번호: 13)
서열식별번호: 13의 구조는 Aib2, αMeF(2F)6, 4Pal10, αMeL13, Orn16, K17, Aib20, D-Glu24 αMeY25, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며, 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 13에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1642.5; 계산치 M+3 =1642.1).
실시예 5
Y-Aib-EGT-αMeF(2F)-TSDVSI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
16
-CO
2
H)AQ-Aib-EFI-(D-Glu)-αMeY-LIEGGPSSGAPPPS-NH
2
(서열식별번호: 14)
서열식별번호: 14의 구조는 Aib2, αMeF(2F)6, αMeL13, Orn16, K17, Aib20, D-Glu24,αMeY25, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며, 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 14에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1626.1; 계산치 M+3 =1626.1).
실시예 6 내지 실시예 287
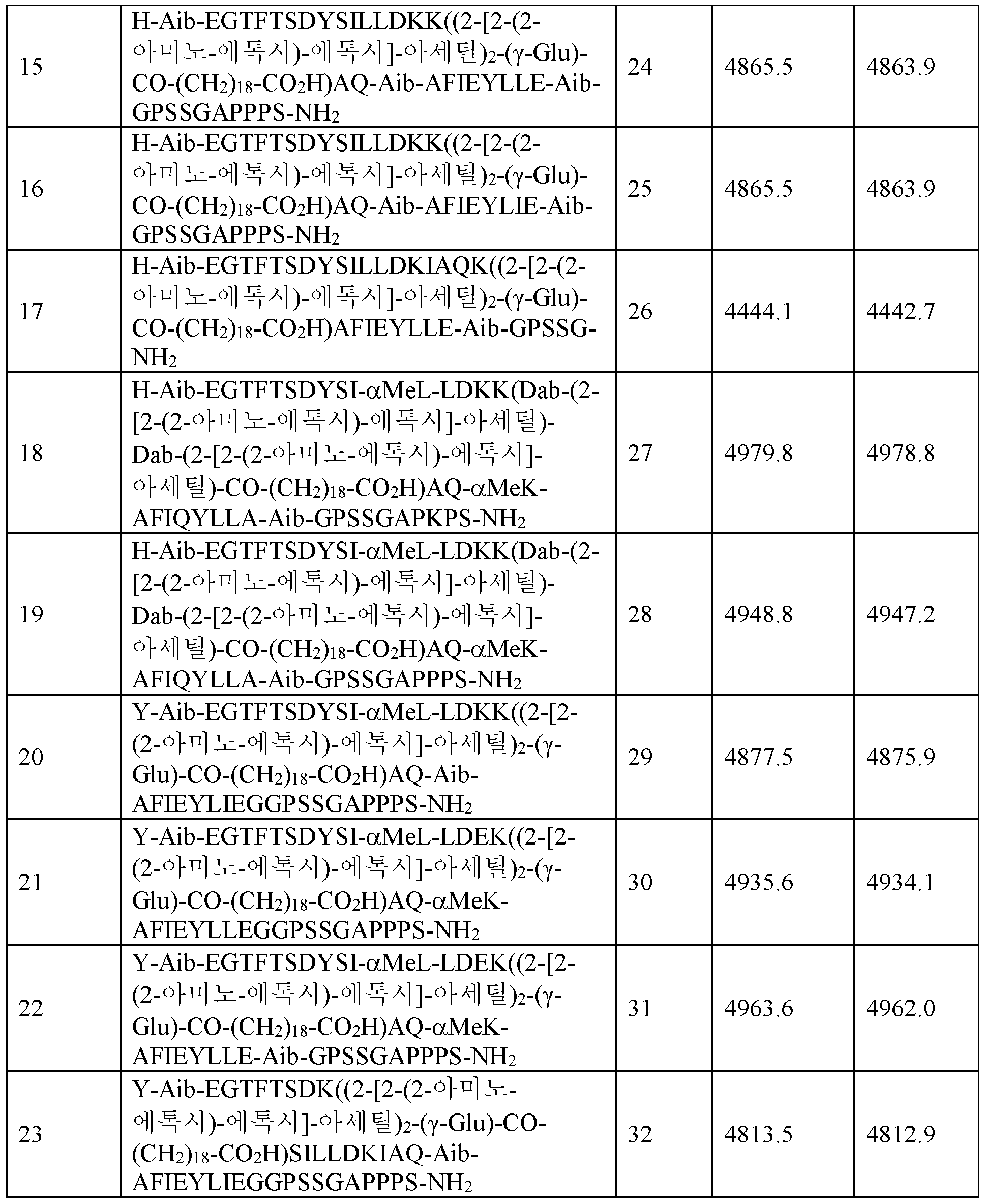
실시예 6 (서열식별번호: 15) 내지 실시예 287 (서열식별번호: 296)에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다.
N/I는 포함되지 않음을 의미한다
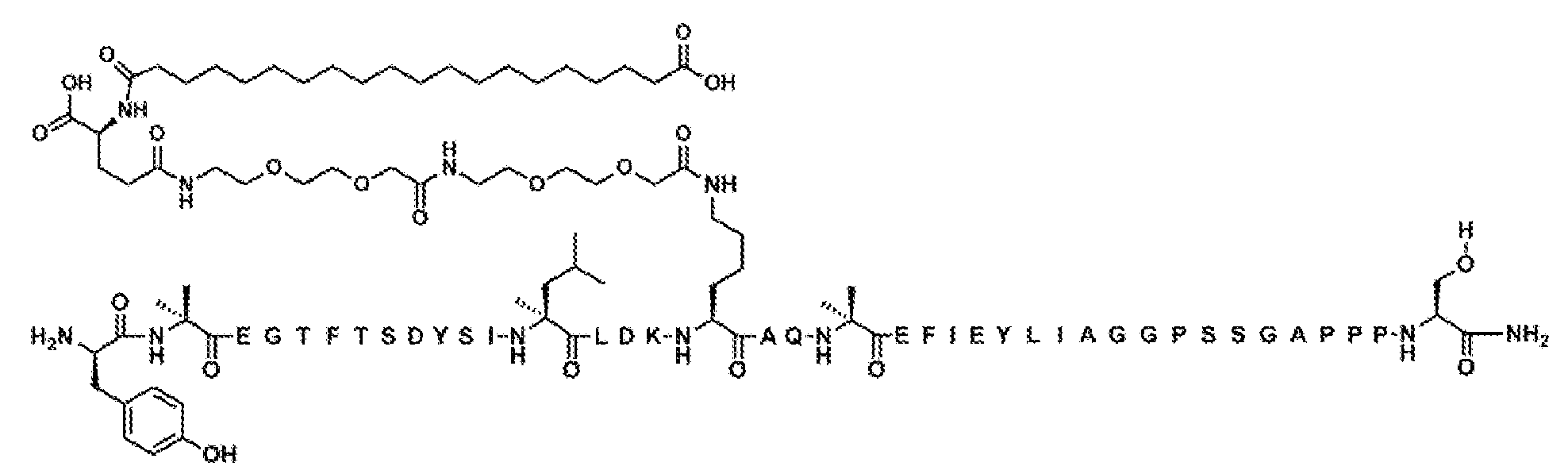
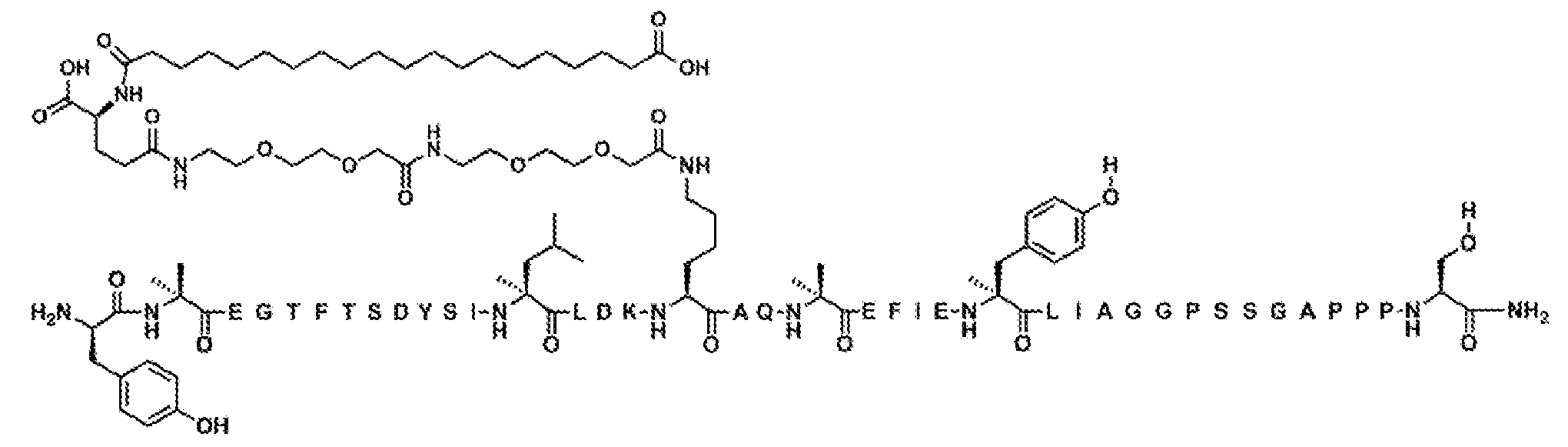
실시예 288
Y-Aib-EGTFTSDYSILLDKK((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
18
-CO
2
H)AQ-Aib-AFIEYLIAGGPSSGAPPPS-NH
2
(서열식별번호: 303)
서열식별번호: 303의 구조는 Aib2, K17, Aib20, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며, 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 303에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1602.5; 계산치 M+3 =1602.8).
실시예 289
Y-Aib-EGTFTSDYSI-αMeL-LDKK((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
18
-CO
2
H)AQ-Aib-EFIEYLIAGGPSSGAPPPS-NH
2
(서열식별번호: 304)
서열식별번호: 304의 구조는 Aib2, αMeL13, K17, Aib20, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며, 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 304에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1626.8; 계산치 M+3 =1626.8).
실시예 290
(D-Tyr)-Aib-EGTFTSDYSI-αMeL-LDKK((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
18
-CO
2
H)AQ-Aib-EFIEYLIAGGPSSGAPPPS-NH
2
(서열식별번호: 305)
서열식별번호: 305의 구조는 D-Tyr1, Aib2, αMeL13, K17, Aib20, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며, 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 305에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1626.6; 계산치 M+3 =1626.8).
실시예 291
(D-Tyr)-Aib-EGTFTSDYSI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
18
-CO
2
H)AQ-Aib-AFI-(D-Glu)-YLIAGGPSSGAPPPS-NH
2
(서열식별번호: 306)
서열식별번호: 306의 구조는 D-Tyr1, Aib2, αMeL13, Orn16, K17, Aib20, D-Glu24, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며, 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 306에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1602.4; 계산치 M+3 =1602.8).
실시예 292
(D-Tyr)-Aib-EGTFTSDYSI-αMeL-LDKK((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
18
-CO
2
H)AQ-Aib-EFIE-αMeY-LIAGGPSSGAPPPS-NH
2
(서열식별번호: 307)
서열식별번호: 307의 구조는 D-Tyr1, Aib2, αMeL13, K17, Aib20, αMeY25, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며, 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 307에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1631.3; 계산치 M+3 =1631.5).
실시예 293
(D-Tyr)-Aib-EGTFTSDYSI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)
2
-(γ-Glu)-CO-(CH
2
)
18
-CO
2
H)AQ-Aib-EFIE-αMeY-LIAGGPSSGAPPPS-NH
2
(서열식별번호: 308)
서열식별번호: 308의 구조는 D-Tyr1, Aib2, αMeL13, Orn16, K17, Aib20, αMeY25, 및 Ser39 잔기를 제외하고 표준 단일 문자 아미노산 코드를 사용하여 하기에 도시하였으며, 여기서 이들 아미노산 잔기의 구조가 확장되었다:
서열식별번호: 308 에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다. 분자량은 LC-MS에 의해 결정하였다 (관찰치: M+3 =1626.5; 계산치 M+3 =1626.8).
실시예 294 내지 실시예 381
실시예 294 (서열식별번호: 309) 내지 실시예 381 (서열식별번호: 396)에 따른 화합물은 실질적으로 실시예 1의 절차에 의해 기재된 바와 같이 제조하였다.
N/I는 포함되지 않음을 의미한다
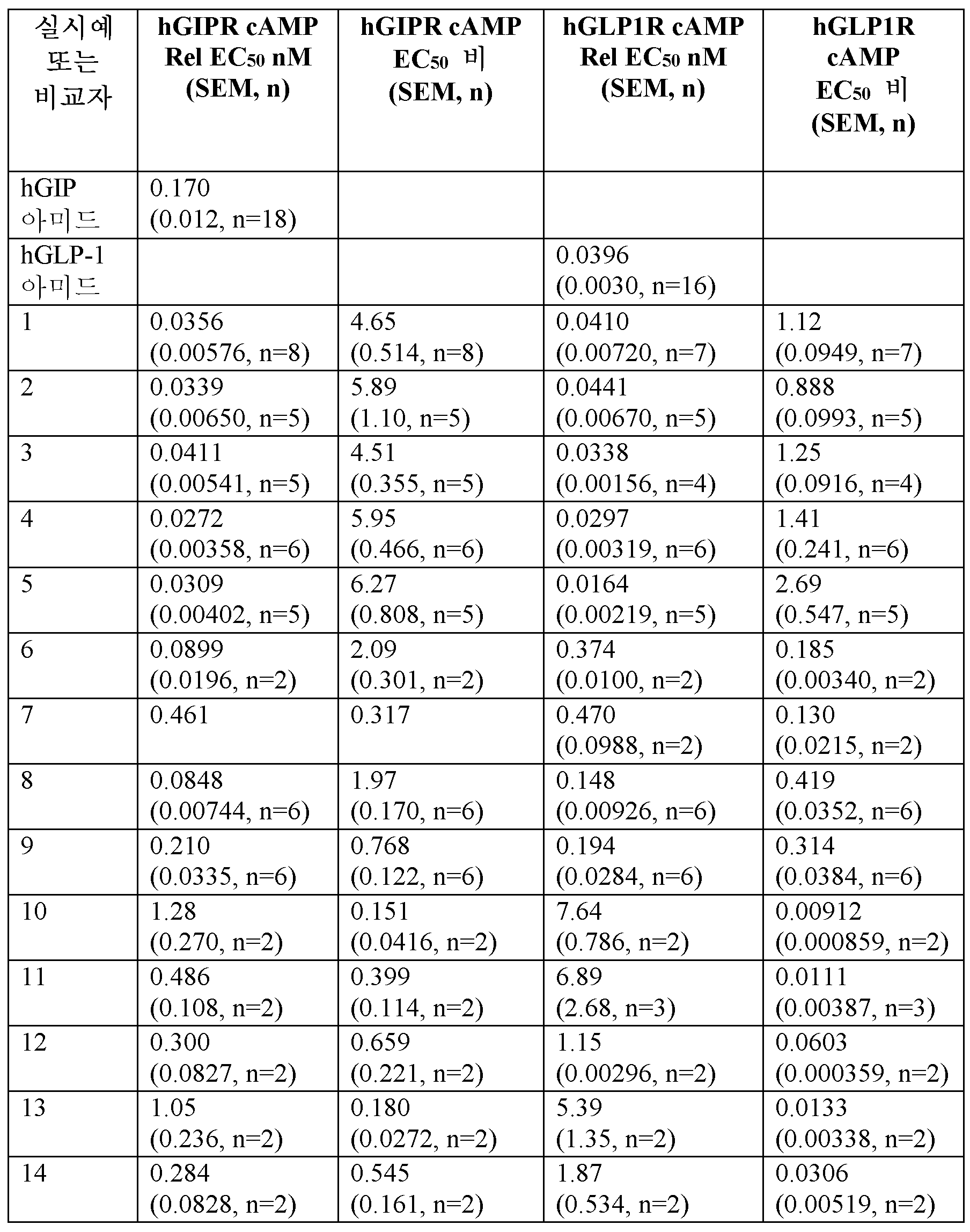
결합 검정
글루카곤 (Gcg라고 함)은 일라이 릴리 앤드 캄파니(Eli Lilly and Company)에서 제조한 참조 표준이다. GLP-1, 7-36-NH2 (GLP-1이라고 함)는 CPC 사이언티픽(CPC Scientific)으로부터 입수하였다 (선베일(Sunnyvale), CA, 97.2% 순도, 100% DMSO 중 100 μM 분취액). GIP 1-42 (GIP라고 함)는 상기에 기재된 바와 같이 펩티드 합성 및 HPLC 크로마토그래피를 사용하여 릴리 리서치 래보러토리즈(Lilly Research Laboratories)에서 제조하였다 (> 80% 순도, 100% DMSO 중 100 μM 분취액). [125I]-방사선표지된 Gcg, GLP-1, 또는 GIP는 [125I]-락토페록시다제를 사용하여 제조되고 퍼킨 엘머(Perkin Elmer) (매사추세츠주 보스턴)로부터 입수하였다.
안정적으로 형질감염된 세포주는 수용체 cDNA를 pcDNA3 발현 플라스미드로 서브클로닝하여 제조하고 인간 배아 신장 (HEK) 293 (hGcgR 및 hGLP-1R) 또는 차이니즈 햄스터 난소 (CHO) (hGIPR) 세포로 형질감염시킨 후 제네티신(Geneticin) (hGLP-1R 및 hGIPR) 또는 히그로마이신 B (hGcgR)로 선택하였다
조 세포막의 제조에 두 가지 방법을 사용하였다.
방법 1: 동결된 세포 펠릿을 50 mM Tris HCl, pH 7.5 ,및 EDTA를 가진 로슈 콤플리트(Roche Complete)™ 프로테아제 억제제를 함유하는 저장성 완충제에서 얼음 위에 용해시켰다. 세포 현탁액은 25회 스트로크 동안 테플론(Teflon)® 막자가 장착된 유리 포토-엘베이햄(Potter-Elvehjem) 균질화기를 사용하여 파괴하였다. 균질화물은 4℃에서 1100 x g에서 10분 동안 원심분리하였다. 상청액을 수집하고 얼음에 저장하며 한편 펠릿은 균질화 완충제에 재현탁시키고 상기에 기재된 바와 같이 재균질화시켰다. 균질화물은 1100 x g에서 10분 동안 원심분리시켰다. 제2 상청액을 제1 상청액과 합하고 4℃에서 1시간 동안 35000 x g에서 원심분리시켰다. 생성된 막 펠릿을 대략 1 내지 3 mg/mL의 프로테아제 억제제가 함유된 균질화 완충제에 재현탁하고, 액체 질소에서 빠르게 동결시키고 사용할 때까지 -80℃ 냉동고에 분취액으로서 저장하였다.
방법 2: 동결된 세포 펠릿을 50 mM Tris HCl, pH 7.5, 1 mM MgCl2, 로슈 콤플리트™ EDTA-미함유 프로테아제 억제제 및 25 단위/ml DNAse I (인비트로겐(Invitrogen))를 함유하는 저장성 완충제에서 얼음 위에 용해시켰다. 세포 현탁액은 20회 내지 25회 스트로크 동안 테플론® 막자가 장착된 유리 포토-엘베이햄 균질화기를 사용하여 파괴하였다. 균질화물은 4℃에서 1800 x g에서 15분 동안 원심분리하였다. 상청액을 수집하고 얼음에 저장하며 한편 펠릿은 균질화 완충제 (DNAse I 무함유)에 재현탁시키고 상기에 기재된 바와 같이 재균질화시켰다. 균질화물은 1800 x g에서 15분 동안 원심분리시켰다. 제2 상청액을 제1 상청액과 합하고 15분 동안 1800 x g에서 추가 시간 원심분리시켰다. 이어서 전반적인 상청액을 4℃에서 30분 동안 25000 x g에서 원심분리하였다. 생성된 막 펠릿을 대략 1 내지 3 mg/mL의 프로테아제 억제제를 함유하는 균질화 완충제 (DNAse I 무함유)에 재현탁시키고, 사용할 때까지 -80℃ 냉동고에 분취액으로서 저장하였다.
결합 결정 방법
다양한 수용체/방사성리간드 상호작용에 대한 평형 결합 해리 상수 (Kd)는 [125I] 스톡 물질 중 높은 프로판올 함량으로 인한 포화 결합 대신에 상동 경쟁 결합 분석으로부터 결정하였다. 수용체 제제에 대해 결정된 Kd 값은 다음과 같았다: hGcgR (3.9 nM), hGLP-1R (1.2 nM) 및 hGIPR (0.14 nM).
[
125
I]-글루카곤 결합
인간 Gcg 수용체 결합 검정은 밀 배아 응집소 (WGA) 비드 (퍼킨 엘머)를 이용하여 섬광 근접 검정법 (SPA) 형식을 사용하여 수행하였다. 결합 완충제는 25 mM 4-(2-히드록시에틸)-1-피페라진에탄술폰산 (HEPES), pH 7.4, 2.5 mM CaCl2, 1 mM MgCl2, 0.1% (w/v) 바시트라신 (리서치 프로덕츠(Research Products)), 0.003% (w/v) 폴리옥시에틸렌소르비탄 모노라우레이트 (TWEEN®-20), 및 EDTA 무함유 로슈 콤플리트™ 프로테아제 억제제를 함유하였다. 펩티드와 Gcg를 해동시키고 100% DMSO (10점 농도 반응 곡선)에서 연속적으로 3배 희석하였다. 다음으로, 5 μL 연속 희석된 화합물 또는 DMSO를 45 μL 검정 결합 완충제 또는 표지되지 않은 Gcg 대조군 (최종 1 μM에서 비특이적 결합 또는 NSB)을 함유하는 코닝(Corning)® 3632 투명 바닥 검정 플레이트로 옮겼다. 이어서, 50 μL [125I]-Gcg (0.15 nM 최종), 50 μL 인간 GcgR 막 (1.5 μg/웰) 및 50 μL의 WGA SPA 비드 (80 내지 150 μg/웰)를 바이오텍 멀티플로 디스펜서(Biotek Multiflo dispenser)로 첨가하였다. 플레이트를 밀봉하고 플레이트 진탕기 (설정 6) 상에서 1분 동안 혼합하고 실온에서 12시간의 인큐베이션/침강 시간 후에 퍼킨엘머 트리룩스 마이크로베타(Trilux MicroBeta)® 섬광 계수기로 판독하였다. 반응 곡선에서 시험된 펩티드의 최종 검정 농도 범위는 전형적으로 1150 nM 내지 0.058 nM이고 대조군 Gcg의 경우 1000 nM 내지 0.05 nM이었다.
[
125
I]-GLP-1 결합
인간 GLP-1 수용체 결합 검정은 WGA 비드를 이용하여 SPA 형식을 사용하여 수행하였다. 결합 완충제는 25 mM HEPES, pH 7.4, 2.5 mM CaCl2, 1 mM MgCl2, 0.1% (w/v) 바시트라신, 0.003% (w/v) TWEEN®-20, 및 EDTA 무함유 로슈 콤플리트™ 프로테아제 억제제를 함유하였다. 펩티드와 GLP-1을 해동시키고 100% DMSO (10점 농도 반응 곡선)에서 연속적으로 3배 희석하였다. 다음으로, 5 μL 연속 희석된 화합물 또는 DMSO를 45 μL 검정 결합 완충제 또는 표지되지 않은 GLP-1 대조군 (0.25 μM 최종에서 비특이적 결합 또는 NSB)을 함유하는 코닝® 3632 투명 바닥 검정 플레이트로 옮겼다. 이어서, 50 μL [125I]-GLP-1 (0.15 nM 최종), 50 μL 인간 GLP-1R 막 (0.5 μg/웰 및 50 μL의 WGA SPA 비드 (100 내지 150 μg/웰)를 바이오텍 멀티플로 디스펜서로 첨가하였다. 플레이트를 밀봉하고 플레이트 진탕기 (설정 6) 상에서 1분 동안 혼합하고 실온에서 5 내지 12시간의 인큐베이션/침강 시간 후에 퍼킨엘머 트리룩스 마이크로베타® 섬광 계수기로 판독하였다. 반응 곡선에서 시험된 펩티드의 최종 검정 농도 범위는 전형적으로 1150 nM 내지 0.058 nM이고 대조군 GLP-1의 경우, 250 nM 내지 0.013 nM이었다.
[125I]-GIP 결합
인간 GIP 수용체 결합 검정은 WGA 비드를 이용하여 SPA 형식을 사용하여 수행하였다. 결합 완충제는 25 mM HEPES, pH 7.4, 2.5 mM CaCl2, 1 mM MgCl2, 0.1% (w/v) 바시트라신, 0.003% (w/v) TWEEN®-20, 및 EDTA 무함유 로슈 콤플리트™ 프로테아제 억제제를 함유하였다. 펩티드와 GIP를 해동시키고 100% DMSO (10점 농도 반응 곡선)에서 연속적으로 3배 희석하였다. 다음으로, 5 μL 연속 희석된 화합물 또는 DMSO를 45 μL 검정 결합 완충제 또는 표지되지 않은 GIP 대조군 (0.25 μM 최종에서 비특이적 결합 또는 NSB)을 함유하는 코닝® 3632 투명 바닥 검정 플레이트로 옮겼다. 이어서, 50 μL [125I]-GIP (0.075-0.15 nM 최종), 50 μL 인간 GIPR 막 (3 μg/웰) 및 50 μL의 WGA SPA 비드 (100 내지 150 μg/웰)를 바이오텍 멀티플로 디스펜서로 첨가하였다. 플레이트를 밀봉하고 플레이트 진탕기 (설정 6) 상에서 1분 동안 혼합하고 실온에서 2.5 내지 12시간의 인큐베이션/침강 시간 후에 퍼킨엘머 트리룩스 마이크로베타® 섬광 계수기로 판독하였다. 반응 곡선에서 시험된 펩티드의 최종 검정 농도 범위는 전형적으로 1150 nM 내지 0.058 nM 또는 115 nM 내지 0.0058 nM이고 대조군 GIP의 경우, 250 nM 내지 0.013 nM이었다.
결합 검정 데이터 분석
펩티드, Gcg, GLP-1, 또는 GIP의 농도 곡선에 대한 원시 CPM 데이터는 개별 CPM 값으로부터 비특이적 결합 (각각 과잉 표지되지 않은 Gcg, GLP-1, 또는 GIP의 존재 하에 결합)을 빼고 총 결합 신호로 나눔으로써 퍼센트 억제로 전환시키고, 또한 비특이적 결합을 빼서 보정하였다. 데이터는 4-파라미터 (곡선 최대, 곡선 최소, IC50, 언덕 경사) 비선형 회귀 작업 (진데이터 스크리너, 버전 12.0.4, 진데이터 아게, 스위스 바젤)을 사용하여 분석하였다. 친화도 상수 (Ki)는 방정식 Ki = IC50/(1 + D/Kd)을 기반으로 하여 절대 IC50 값으로부터 계산하며 여기서 D = 실험에서 사용된 방사성리간드의 농도이고, IC50은 결합을 50% 억제하는 농도이고 Kd는 방사성리간드의 평형 결합 해리 상수이다 (상기에 기재됨). Ki 값은 기하 평균으로 보고되며, 오차는 평균의 표준 오차 (SEM)로 표시되고 n은 독립 반복실험 횟수와 동일하다 (상이한 날에 수행된 분석에서 결정됨). 기하 평균은 다음과 같이 계산된다:
기하 평균 = 10(Log Ki 값의 산술 평균))
각각의 수용체 및 각각의 종에서 Ki 비 (천연 대조군 펩티드의 경우 Ki/시험 화합물의 경우 Ki)가 계산되었다. Ki 비는 천연 대조군 펩티드와 비교하여 펩티드의 겉보기 친화도를 빠르게 나타낸다. Ki 비 < 1은 시험 펩티드가 천연 펩티드보다 수용체에 대해 더 낮은 친화도 (더 높은 Ki 값)를 가짐을 나타내며, 한편 Ki 비 >1은 시험 펩티드가 천연 펩티드보다 수용체에 대해 더 높은 친화도 (더 낮은 Ki 값)를 가짐을 나타낸다.
n=1/x는 총 반복실험 횟수 (x) 중 단지 1개를 평균을 표시하는 데 사용됨을 의미한다. SEM은 n=2 이상의 비적격 결과가 존재하는 경우에만 계산된다. 평균은 평균의 표준 오차 (SEM)와 반복실험 횟수 (n)가 괄호 안에 표시된 기하 평균으로서 표시하였다
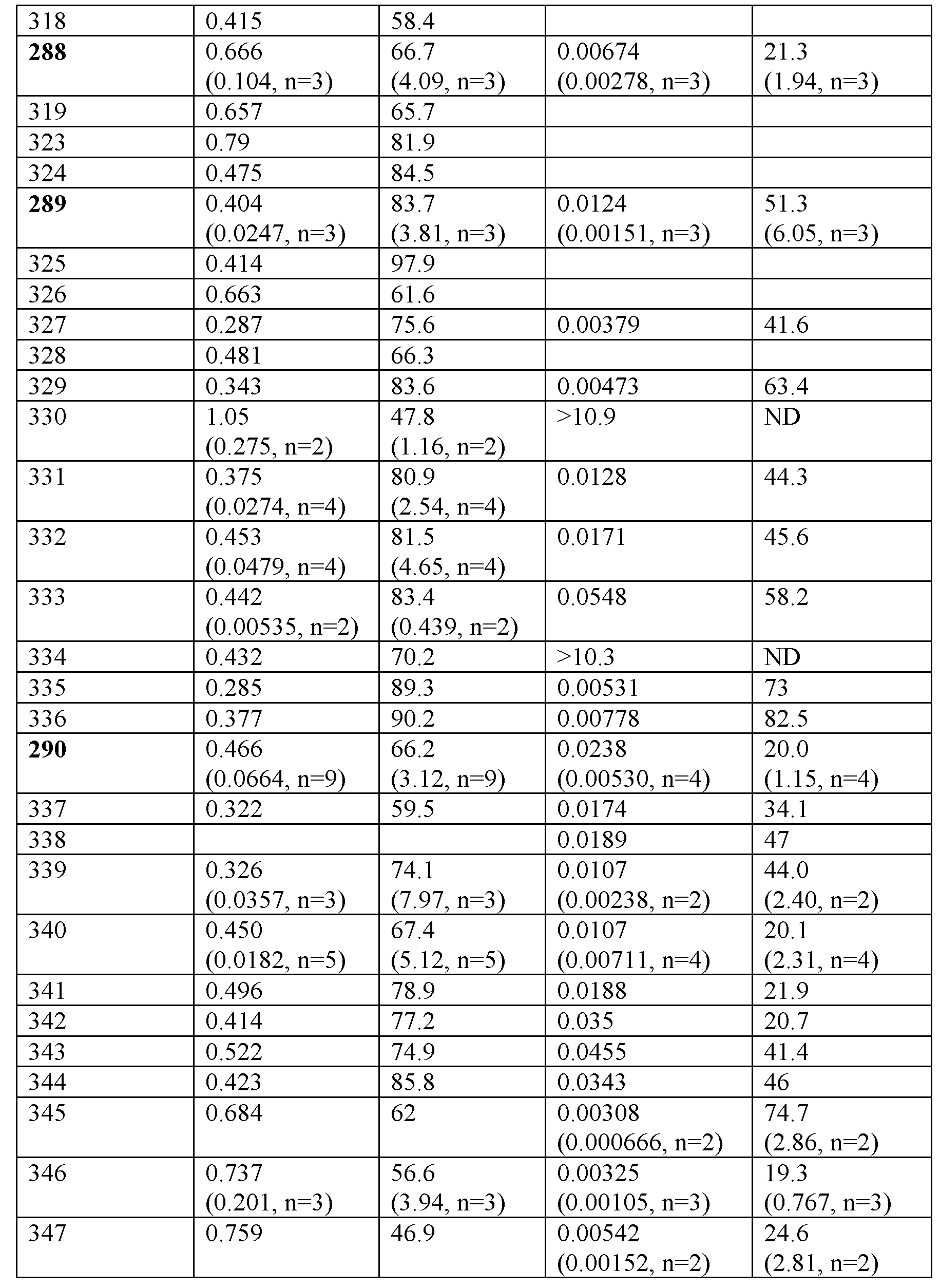
<표 1>
인간 GLP-1R, GcgR 및 GIPR에 대한 명시된 실시예 및 비교자 분자의 시험관내 결합 친화도 (Ki).
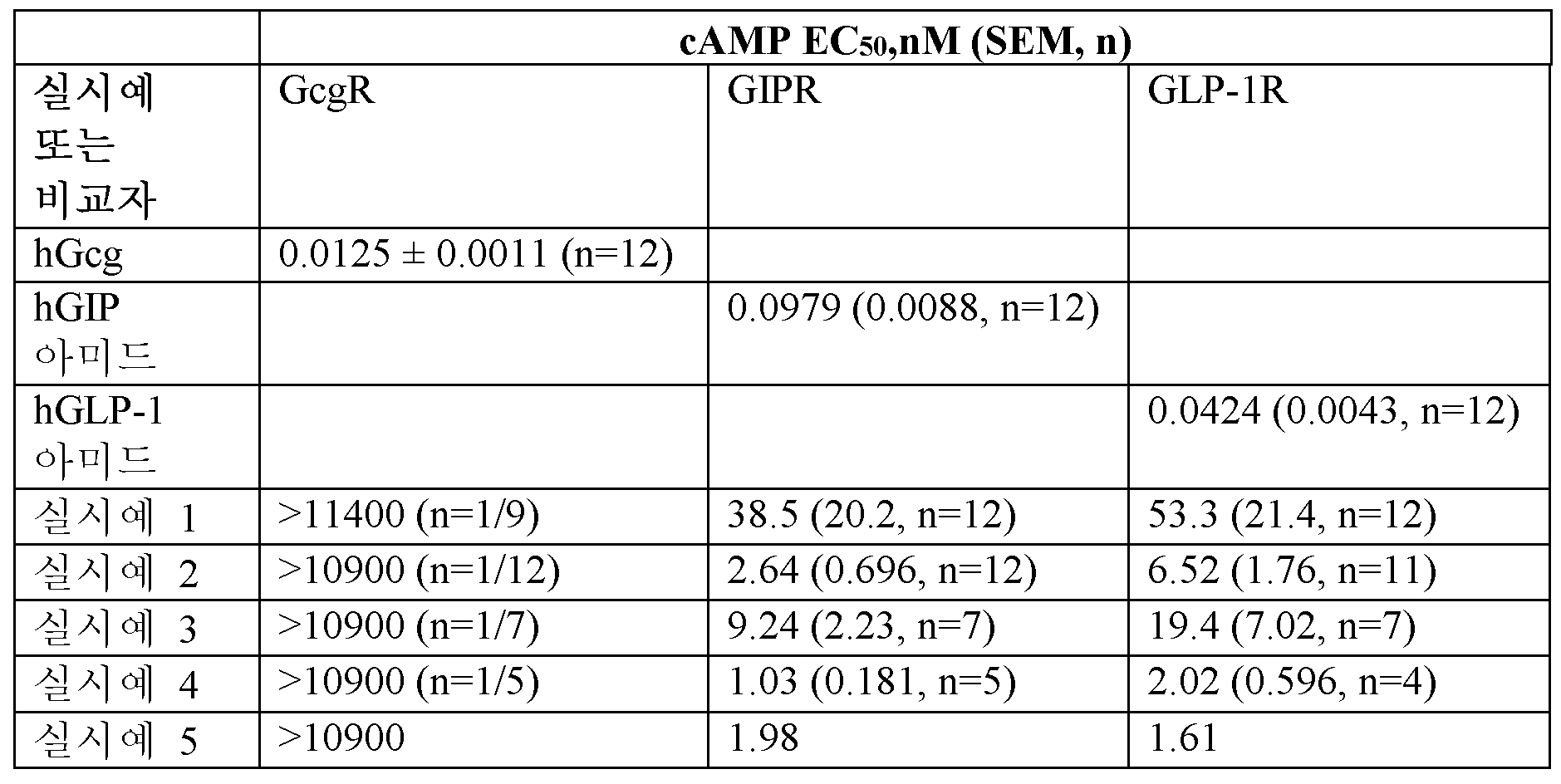
기능적 활성 (BSA 사용)
기능적 활성은 HEK-293 클로날 세포주를 발현하는 hGLP-1R, hGcgR 및 hGIP-R에서 결정하였다. 각각의 수용체 과발현 세포주를 20 μl 검정 부피로 1X GlutaMAX™ 보충제 (L-알라닐-L-글루타민 디펩티드 깁코®), 0.25% FBS (우 태아 혈청), 0.05% 분획 V BSA (소 혈청 알부민), 250 μM 3-이소부틸-1-메틸크산틴 (IBMX) 및 20 mM 4-(2-히드록시에틸)-1-피페라진에탄술폰산 (HEPES)이 보충된 DMEM (깁코 카탈로그 번호 31053) 중 펩티드 (20점 CRC, 2.75배 랩사이트(Labcyte) 에코(Echo) 직접 희석)로 처리하였다.
실온에서 60분 인큐베이션한 후, 시스비오(CisBio) cAMP 다이내믹(Dynamic) 2 균질 시간-분해 형광(HTRF) 검정 키트를 사용하여 세포내 cAMP의 결과적인 증가를 정량적으로 결정하였다. 세포 용해 완충제 중 cAMP-d2 접합체를 첨가한 후 또한 세포 용해 완충제 중 항체 항-cAMP-Eu3+-크립테이트를 첨가하여 세포내 cAMP 수준을 검출하였다. 생성된 경쟁적 검정을 실온에서 적어도 60분 동안 인큐베이션한 다음에, 320 nm에서 여기 및 665 nm 및 620 nm에서 방출을 가진 기기를 사용하여 검출하였다. 엔비젼 단위 (665nm/620nm에서의 방출*10,000)는 존재하는 cAMP의 양에 반비례하며 cAMP 표준 곡선을 사용하여 웰당 nM cAMP로 전환시켰다.
각각의 웰에서 생성된 cAMP의 양 (nM)은 인간 GLP-1(7-36)NH2, 인간 Gcg, 또는 인간 GIP(1-42)NH2에서 관찰된 최대 반응의 백분율로 전환시켰다. 상대 EC50 값은 4-파라미터 로지스틱 방정식에 맞춰 첨가된 펩티드의 농도에 비해 최대 반응 백분율을 사용하는 비선형 회귀 분석에 의해 유래된다.
인간 GLP-1R에서 인간 GLP-1(7-36)NH2, 인간 GcgR에서 인간 Gcg, 및 인간 GIP-R에서 인간 GIP(1-42)NH2의 EC50 결정: 펩티드 농도 범위는 448 pM 내지 99.5 nM이었다. 인간 GLP-1R, 인간 GcgR, 및 인간 GIP-R에서 실시예의 EC50 결정: 펩티드 농도 범위는 51.5 fM - 11.4 μM이었다.
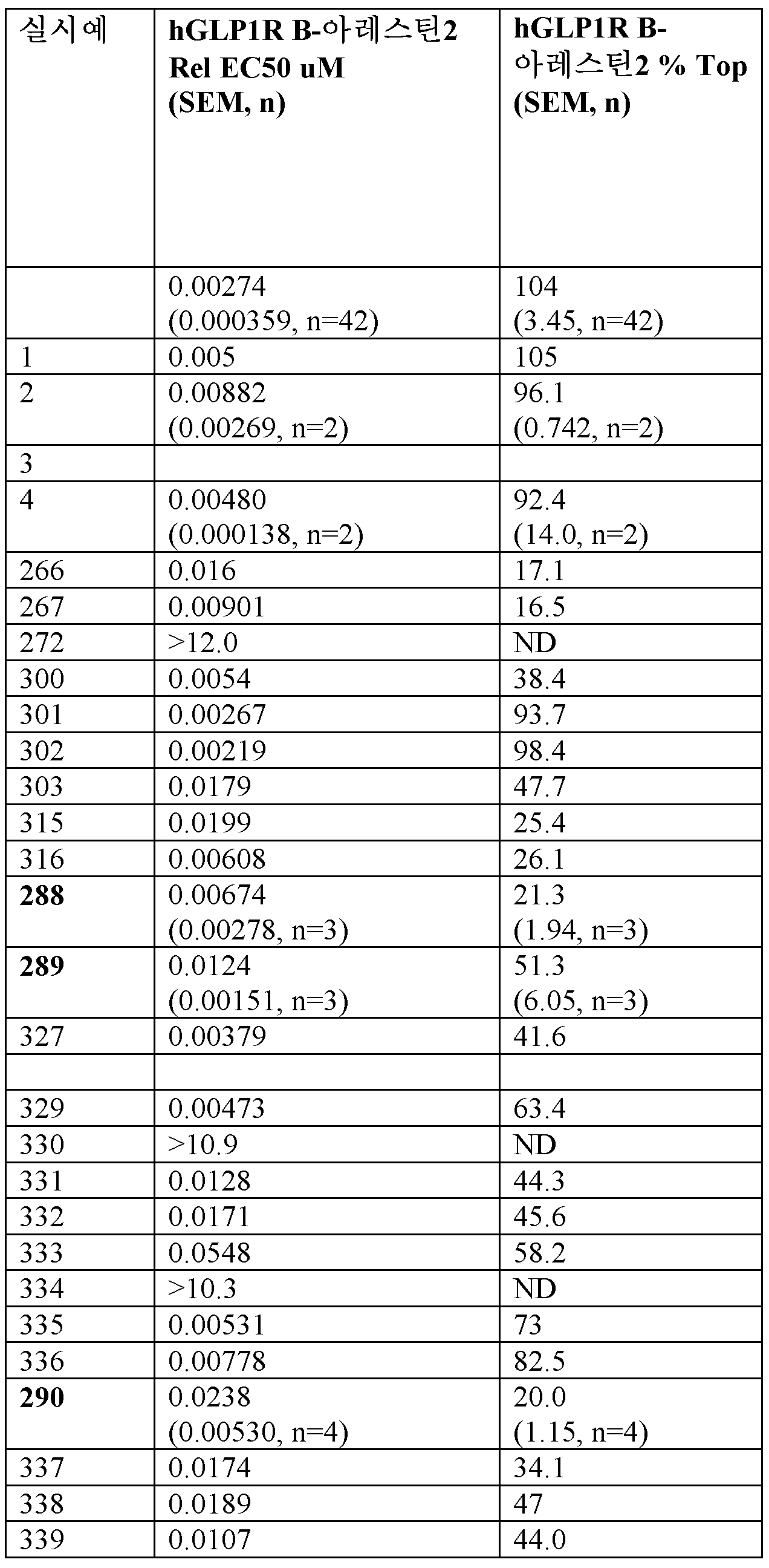
<표 2>
FBS의 존재 하에 실시예 및 비교자 펩티드 (hGcg, hGIP 아미드, 및 hGLP-1 아미드)에 대한 기능적 cAMP 효능 (EC50).
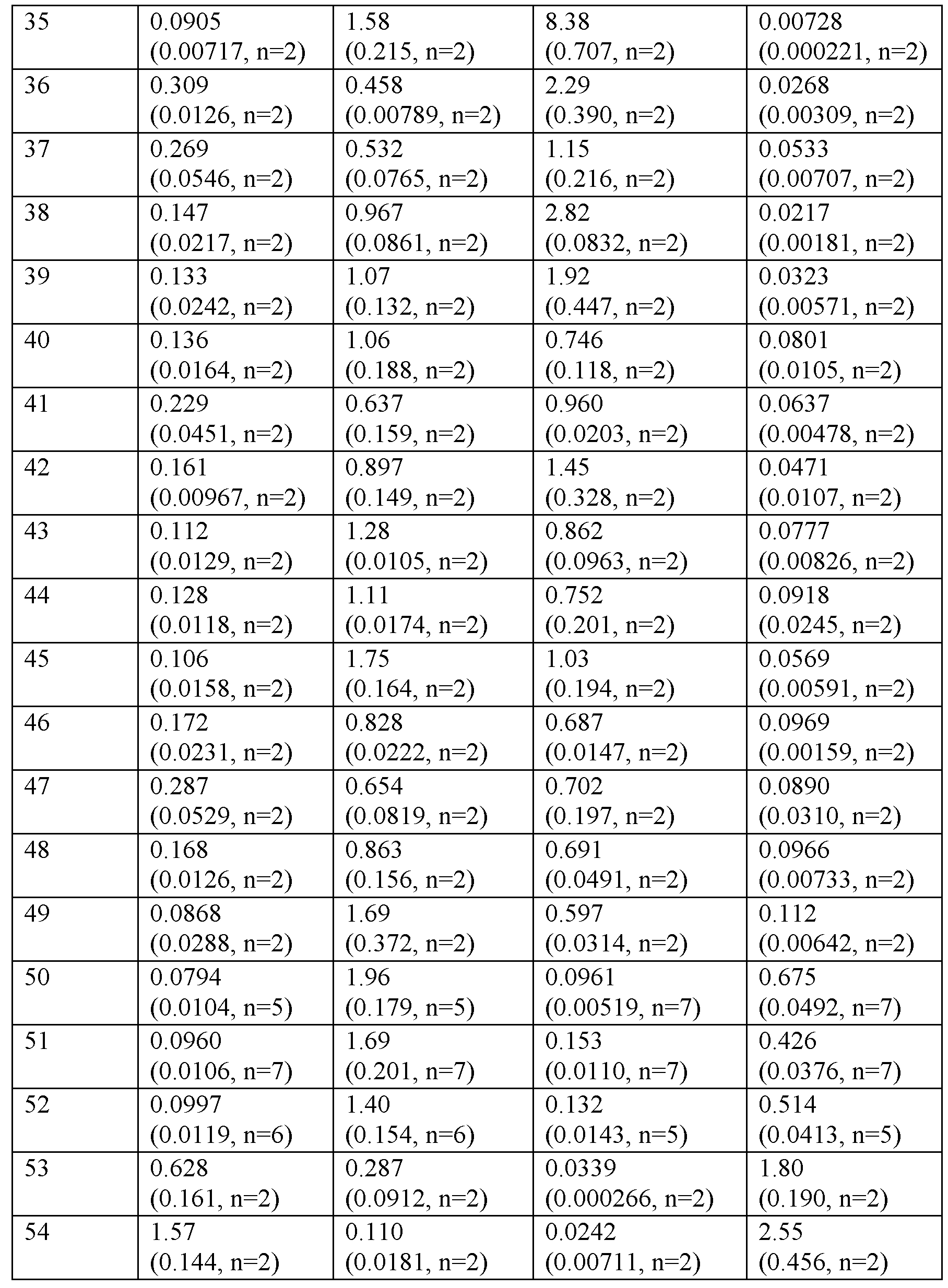
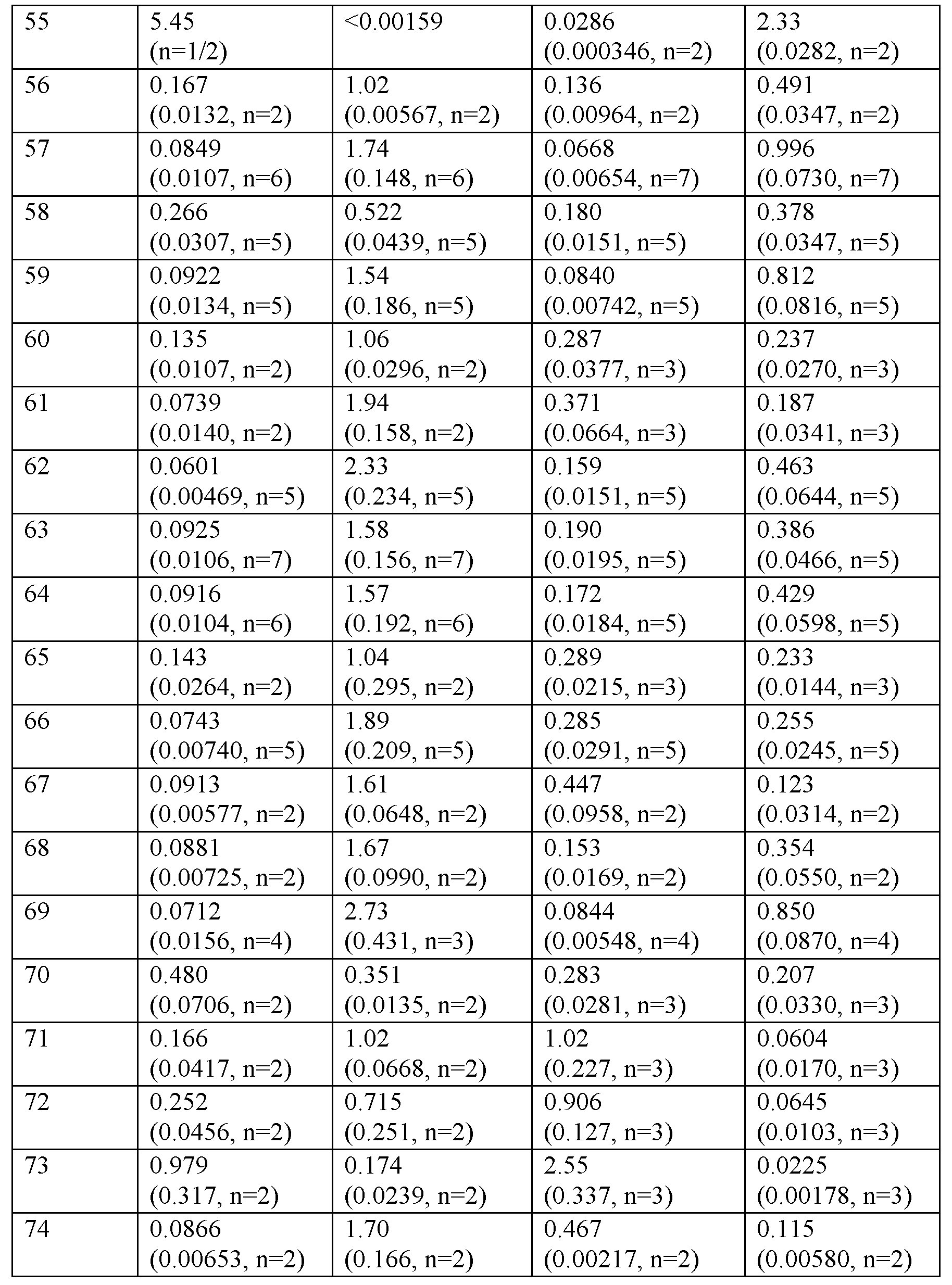
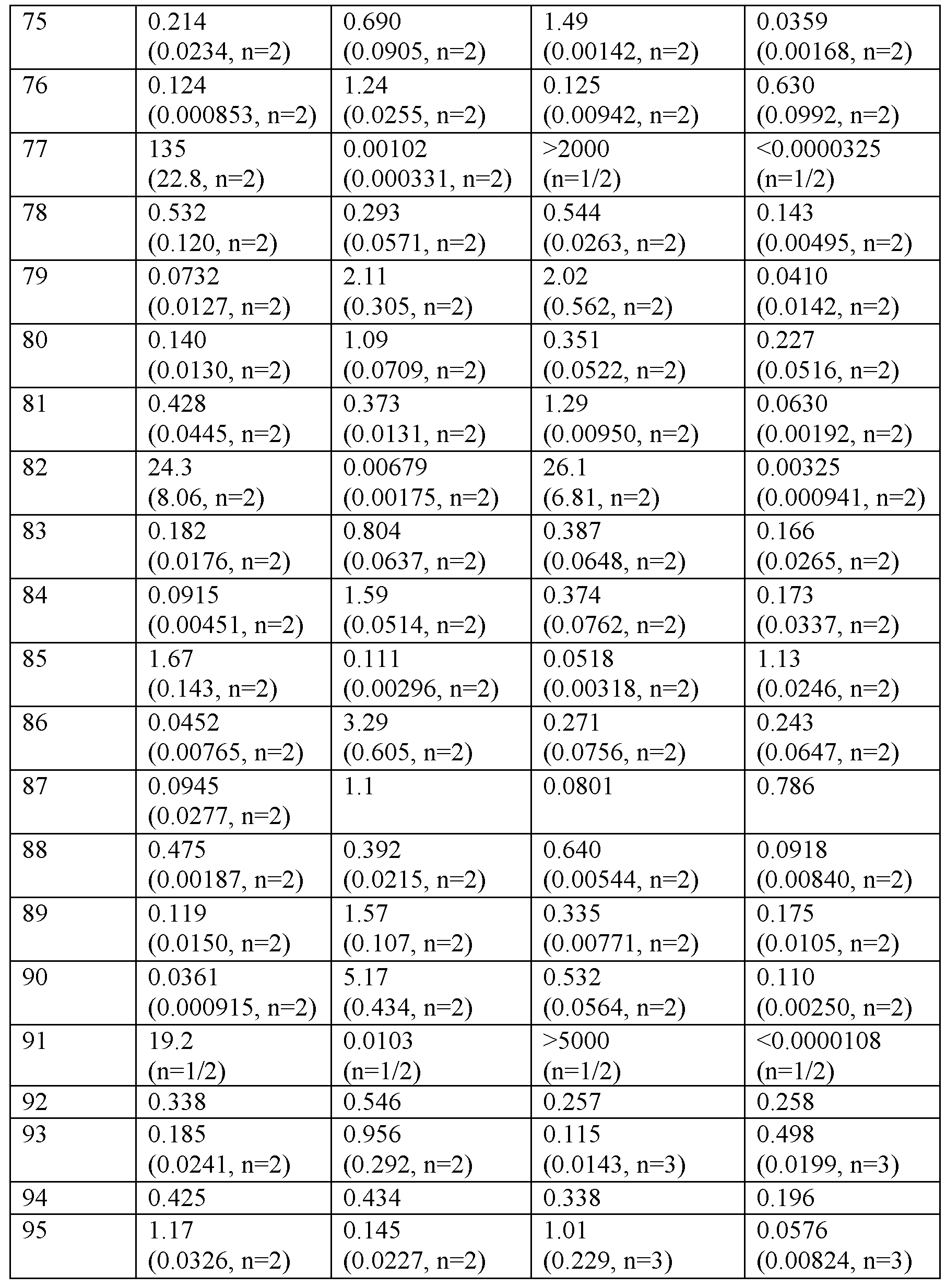
카제인의 존재 하애 cAMP 약리학적 기능적 검정
추가 세트의 cAMP 검정은 인간 GLP-1 수용체 (GLP-1R), 위 억제 펩티드 수용체 (GIPR), 글루카곤 수용체 (GcgR)를 발현하는 HEK293 세포에서 수행하였다. hGLP1R/GIPR 펩티드의 약리학적 활성은 인간 GLP-1 수용체 (GLP-1R), 위 억제 펩티드 수용체 (GIPR), 또는 GLP-2 수용체 (GLP-2R)를 안정적으로 발현하는 HEK293 세포에서 결정하였다. 각각의 수용체 과발현 세포주 (20 μl)를 20 μl 검정 부피로 0.1% 카제인 (시그마(Sigma) 카탈로그 번호 C4765), 250 μM IBMX, 1X GlutaMAXTM (깁코(Gibco) 카탈로그 번호 35050), 및 20 mM HEPES (히클론(HyClone) 카탈로그 번호 SH30237.01)가 보충된 DMEM (깁코 카탈로그 번호 31053) 중 시험 펩티드로 처리하였다. 실온에서 60분 인큐베이션한 후, 시스비오(CisBio) cAMP 다이내믹(Dynamic) 2 HTRF 검정 키트 (62AM4PEJ)를 사용하여 세포내 cAMP의 결과적인 증가를 정량적으로 결정하였다. cAMP-d2 접합체 (20 μl) 및 항체 항-cAMP-Eu3+-크립테이트 (20 μl)를 포함하는 용해 완충제를 첨가하여 cAMP 수준을 결정하였다. 실온에서 1 h-인큐베이션 후, 엔비젼(Envision) 2104 플레이트 판독기 (퍼킨엘머)로 HTRF 신호를 검출하였다. 620 nm 및 665 nm에서 형광 방출을 측정하고 620 nm와 665 nm 사이의 비를 계산한 다음에 cAMP 표준 곡선을 사용하여 웰당 nM cAMP로 전환시켰다. 화합물의 용량 반응 곡선은 최소 (완충제만) 및 최대 (각각의 대조군 리간드의 최대 농도) 값으로 정규화된 자극의 백분율로서 플롯팅하고 가변 기울기 (진데이터 스크리너 13)가 있는 4 파라미터 비선형 회귀 피팅을 사용하여 분석하였다. EC50은 용량 반응 곡선에서 최대 절반의 시뮬레이션을 야기하는 화합물의 농도이다. 상대 EC50 값은 4-파라미터 로지스틱 방정식에 맞춰 첨가된 펩티드의 농도에 비해 최대 반응 백분율을 사용하는 비선형 회귀 분석에 의해 유래된다.
균질한 시간 분해 형광법을 사용하여, 분석된 분자의 지방산 모이어티와 상호작용하지 않는, 비특이적 차단제로서 카제인 (혈청 알부민 대신)의 존재 하에 수행된 비교자 분자와 실시예의 고유 효능을 결정하기 위해 검정을 수행하였다.
세포내 cAMP 수준을 표준 곡선을 사용한 외삽법에 의해 결정하였다. 화합물의 용량 반응 곡선은 최소 (완충제만) 및 최대 (각각의 대조군 리간드의 최대 농도) 값으로 정규화된 자극의 백분율로서 플롯팅하고 가변 기울기 (진데이터 스크리너 13)가 있는 4 파라미터 비선형 회귀 피팅을 사용하여 분석하였다. EC50은 용량 반응 곡선에서 최대 절반의 시뮬레이션을 야기하는 화합물의 농도이다. 기하 평균 계산을 위한 각각의 상대 EC50 값은 곡선 피팅으로부터 결정하였다.
화합물의 용량 반응 곡선은 최소 (완충제만) 및 최대 (각각의 대조군 리간드의 최대 농도) 값으로 정규화된 자극의 백분율로서 플롯팅하고 가변 기울기 (진데이터 스크리너 13)가 있는 4 파라미터 비선형 회귀 피팅을 사용하여 분석하였다. EC50은 용량 반응 곡선에서 최대 절반의 시뮬레이션을 야기하는 화합물의 농도이다.
EC50 요약 통계는 다음과 같이 계산하였다:
기하 평균:
GM = 10^(log10 변환된 EC50 값의 산술 평균).
평균의 표준 오차가 보고된다:
SEM = 기하 평균 x (log10 변환된 EC50 값의 표준 편차/시행 횟수(#)의 제곱근) x 10의 loge.
로그 변환은 산술 척도라기 보다는, 곱셈에 속하는 EC50 값을 설명한다.
검정이 시행되는 매일, 시험 펩티드에 더하여 천연 리간드 GIP 및 GLP-1 및 기준선 (최소)으로서만 완충제가 시행되고, 각각의 GIP 및 GLP-1 표준의 최고 농도가 계산을 위해 최대 값으로서 사용되었다. 예시를 위해, 실시예 1에 나타낸 바와 같이, 시험 펩티드는 검정의 8회 시행에서 시험하였다. 의심의 여지를 없애기 위해, 표 3의 hGIP 아미드 및 hGLP-1 아미드 EC50은 일련의 18개 검정 값으로부터의 기하 평균 값을 예시하며, 값은 완충제 미함유와 비교하여 매일 달라질 것이다. 따라서, 각각의 실시예는 실시예 검정 시행을 정규화하기 위해 이들 값의 기하 평균을 사용할 것이다.
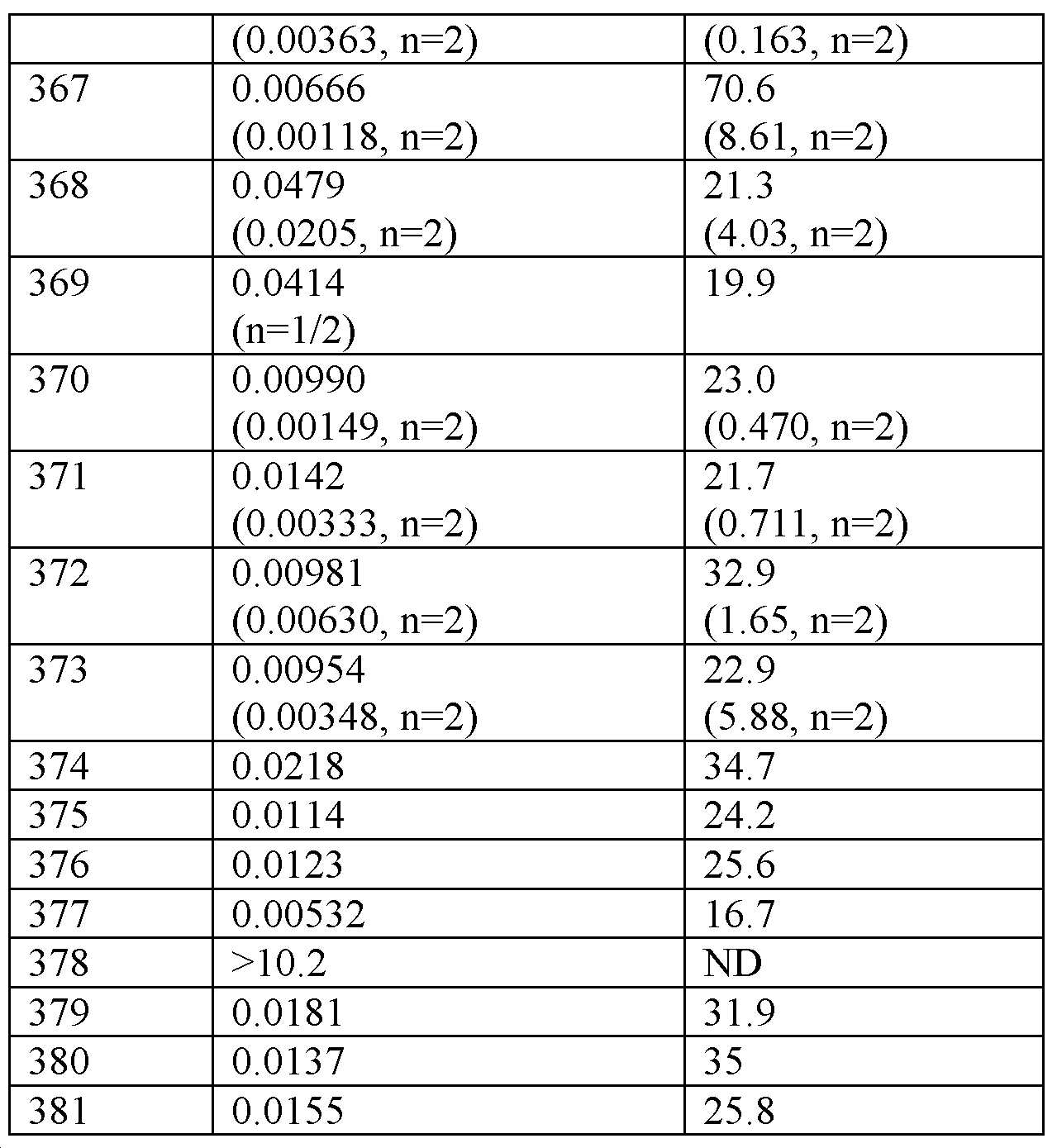
<표 3>
0.1% 카제인의 존재 하에 hGLP-1R, hGIPR, hGcgR의 기능적 활성화.
표 3의 데이터에 의해 입증된 바와 같이, 실시예 화합물은 0.1% 카제인의 존재 하에 인간 GLP-1R 및 GIPR로부터 cAMP를 자극한다.
생체내 연구
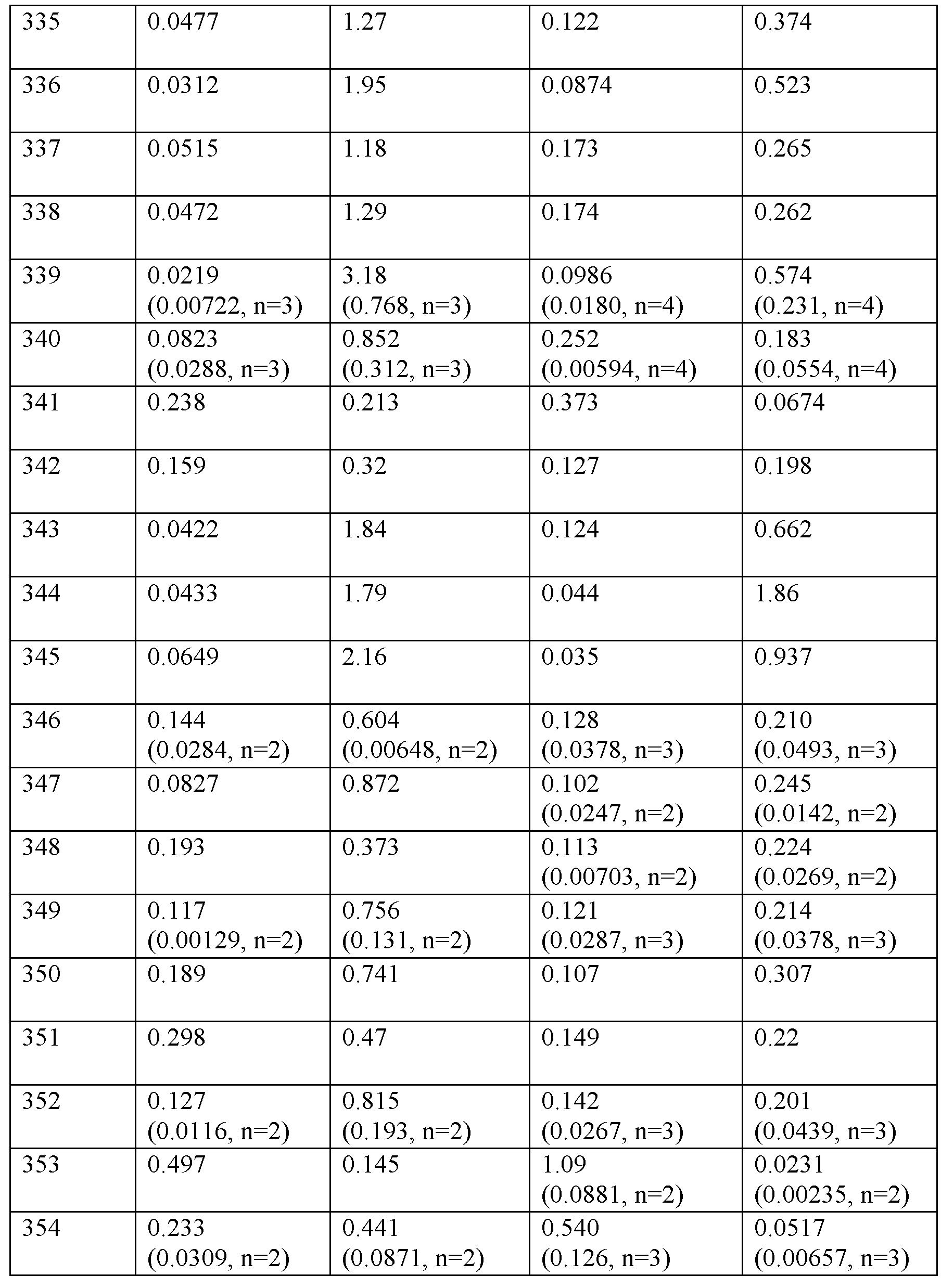
수컷 CD-1 마우스에서의 약물동태학
선택된 실시예의 약물동태학을 수컷 CD-1 마우스에게 200 nMol/kg의 단일 피하 투여 후에 평가하였다. 혈액 샘플을 168시간에 걸쳐 수집하며 생성된 개별 혈장 농도를 사용하여 약물동태학적 파라미터를 계산하였다. 혈장 (K3 EDTA) 농도를 실시예의 무손상 매스를 측정하는 적격 LC/MS 방법을 사용하여 결정하였다. 항-GIP/GLP1 항체를 사용한 면역친화도 기반 침전을 사용하여 100% 마우스 혈장으로부터 각각의 실시예 및 내부 표준물질로서 유사체를 추출하였다. LC/MS 검출을 위해 기기를 조합하였다. 평균 약물동태학적 파라미터를 표 4에 나타냈다.
<표 4>
수컷 CD-1 마우스에게 200 nMol/kg의 단일 피하 투여 후의 펩티드의 평균 약물동태학적 파라미터 (N=2/시점 비연속 샘플링).
약어: T1/2 = 반감기, Tmax = 최대 농도까지의 시간, Cmax = 최대 혈장 농도, AUCINF_D_obs = 용량으로 나눈 AUCinf, CL/F = 청소율/생체이용률. 주: 데이터는 평균이며, 여기서 n=2/시점/군이다.
시험된 실시예에 대한 이 연구로부터의 결과는 연장된 약물동태학적 프로파일과 일치한다.
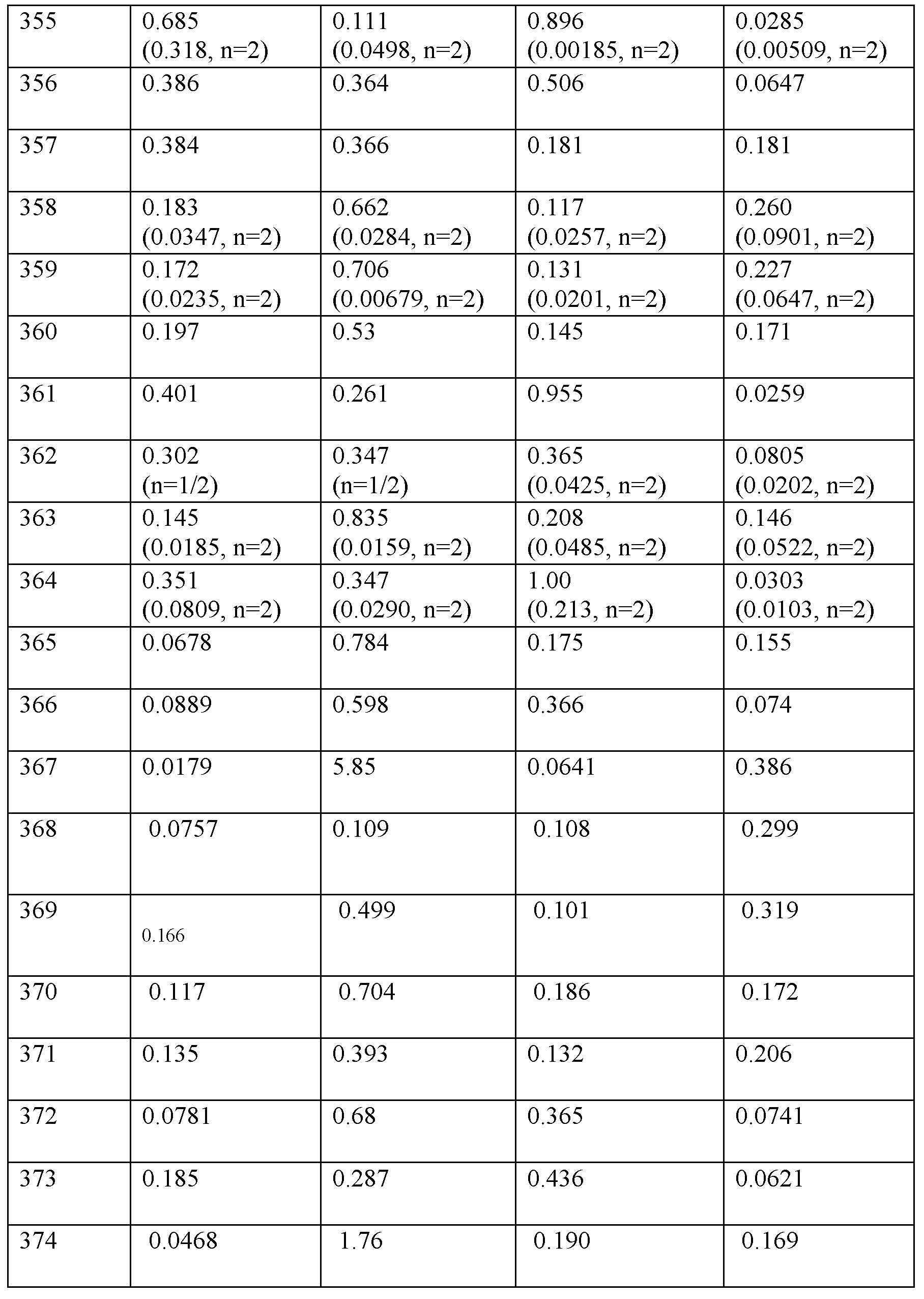
수컷 시노몰구스(Cynomolgus) 원숭이에서의 약물동태학
선택된 실시예의 약물동태학을 수컷 시노몰구스 원숭이에게 50 nMol/kg의 단일 피하 투여 후에 평가하였다. 혈액 샘플을 336시간에 걸쳐 수집하며 생성된 개별 혈장 농도를 사용하여 약물동태학적 파라미터를 계산하였다. 펩티드 혈장 (K3 EDTA) 농도를 화합물의 무손상 매스를 측정한 적격 LC/MS 방법을 사용하여 결정하였다. 항-GIP/GLG1 항체를 사용한 면역친화도 기반 침전을 사용하여 100% 시노몰구스 원숭이 혈장으로부터 각각의 펩티드 및 내부 표준물질로서 유사체를 추출하였다. LC/MS 검출을 위해 기기를 조합하였다. 평균 약물동태학적 파라미터를 표 5에 나타냈다.
<표 5>
수컷 시노몰구스 원숭이에게 50 nMol/kg의 단일 피하 투여 후의 펩티드의 평균 약물동태학적 파라미터.
약어: T1/2 = 반감기, Tmax = 최대 농도까지의 시간, Cmax = 최대 혈장 농도, AUCINF_D_obs = 용량으로 나눈 AUCinf, CL/F = 청소율/생체이용률. 주: 데이터는 평균이며, 여기서 n=2/군이다. 주: 데이터는 평균이며, 여기서 n=2/군이다. 표 5에 나타난 바와 같이, 시험된 펩티드에 대한 이 연구로부터의 결과는 연장된 약물동태학적 프로파일과 일치한다.
피하 또는 공장내 투여 후의 수컷 스프래그 다울리(Sprague Dawley) 래트에서의 약물동태학
선택된 실시예의 약물동태학을 50 nMol/kg (PBS에 용해, pH 7.4에 용해됨)의 단일 피하 (SC) 투여 또는 단일 1 μmol/kg (250 mM 소듐 데카노에이트 ("C10") 및 12 mg/mL 대두 트립신 억제제 (SBTI)과 혼합됨)의 수컷 스프래그 다울리 래트에 대한 공장내 (IJ) 투여 후에 평가하였다. 혈액 샘플을 SC 투여 후 168 시간 및 IJ 투여 후 72시간에 걸쳐 수집하였다. 개별 혈장 농도를 사용하여 약물동태학적 파라미터를 계산하였다. 실시예의 무손상 매스를 측정하는 적격 LC/MS 방법을 사용하여 혈장 (K3 EDTA) 농도를 결정하였다. 각각의 실시예를 내부 표준으로서 유사체 펩티드와 함께 시험하였다. 항-GIP/GLP1 항체를 사용한 면역친화도 기반 침전을 사용하여 각각의 시험 펩티드 및 유사체를 추출하였다. 상기 실시예에 대한 평균 약물동태학적 파라미터를 표 6 및 표 7에 나타냈다.
<표 6>
수컷 스프래그 다울리 래트에게 50 nMol/kg의 단일 피하 투여 후의 펩티드의 평균 (+/- SD) 약물동태학적 파라미터.
약어: T1/2 = 반감기, Tmax = 최대 농도까지의 시간, Cmax = 최대 혈장 농도, AUCINF_D_obs = 용량으로 나눈 AUCinf, CL/F = 청소율/생체이용률. 주: 데이터는 평균이며, 여기서 n=3/군이다 (표 6). 표 6에 나타난 바와 같이, 이들 실시예 펩티드를 사용한 이 연구로부터의 결과는 연장된 약물동태학적 프로파일과 일치한다.
<표 7>
수컷 스프래그 다울리 래트에게 1 μmol/kg의 단일 공장내 투여 후의 펩티드의 평균 (+/- SD) 약물동태학적 파라미터.
데이터는 평균이며, 여기서 n=3/군 n=6/군이다 (표 7).
표 7의 결과에 의해 예시된 바와 같이, 이들 실시예는 공장내 투여 후의 노출과 일치한다. 이 검정에서 공장내 노출은 실시예가 경구 제제 및 투여에 적합할 수 있음을 뒷받침한다.
수컷 위스타(Wistar) 래트에서의 인슐린 분비에 대한 생체내 효과
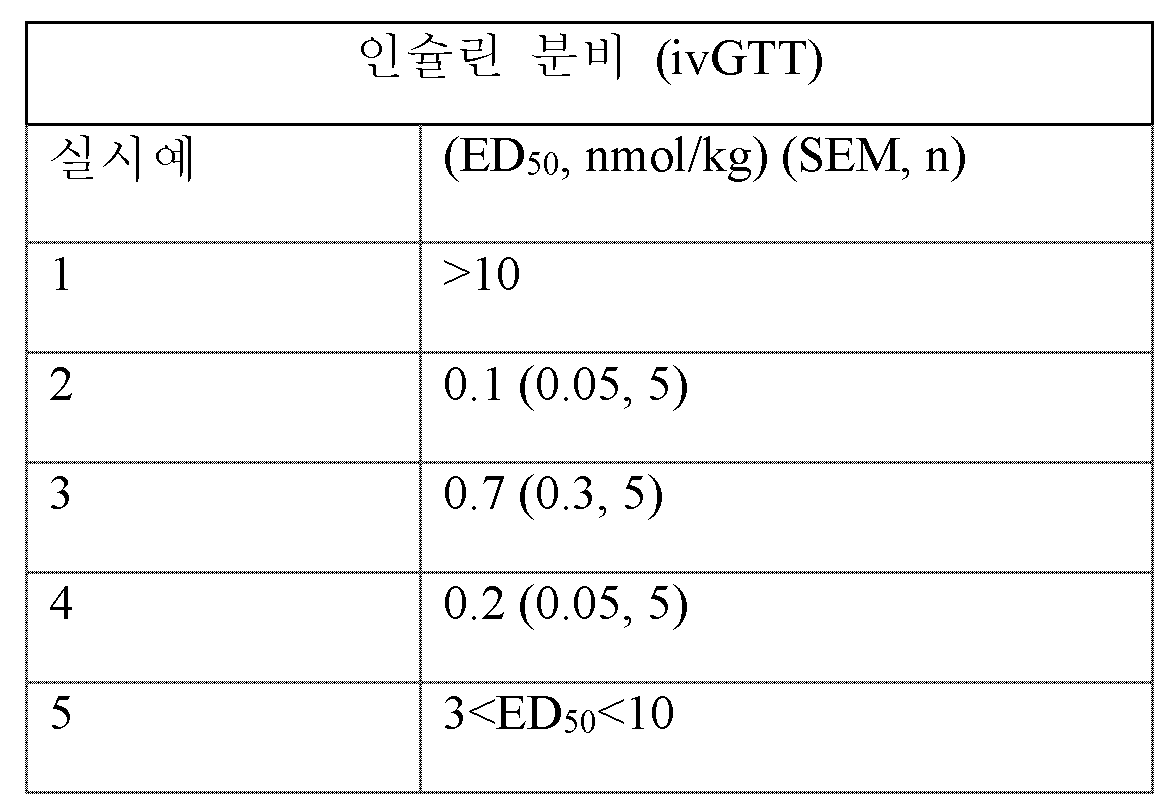
대퇴 동맥 및 대퇴 정맥 캐뉼라 (엔비고(Envigo), 인디애나주 인디애나폴리스)를 가진 수컷 위스타 래트 (280- 320 그램)를 필터 상단을 가진 폴리카르보네이트 케이지에 단독-수용하였다. 래트는 21℃에서 12:12시간 명암 주기 (오전 6:00에 점등)를 유지하였고 사료과 탈염수를 무제한으로 받았다. 래트는 체중에 따라 무작위화하고 글루코스 투여 16시간 전에 0.04, 0.1, 0.3, 1, 3 및 10 nmol/kg의 용량으로 1.5 ml/kg을 투여한 다음에 금식시켰다. 동물의 체중을 측정하고 소듐 펜토바르비탈을 복강내 (65 mg/kg, 30 mg/ml) 투여하여 마취시켰다. 시간 0에 혈액 샘플을 EDTA 튜브 내로 수집한 후, 글루코스를 정맥내 (0.5 mg/kg, 5 ml/kg) 투여하였다. 혈액 샘플을 글루코스의 정맥내 투여 후 2, 4, 6, 10, 20, 및 30분째에 글루코스 및 인슐린 수준에 대해 수집하였다. 혈장 글루코스 수준은 임상 화학 분석기를 사용하여 결정하였다. 전기화학발광 검정 (메조 스케일(Meso Scale), 메릴랜드주 게이더스버그)을 사용하여 혈장 인슐린을 결정하였다. 글루코스 및 인슐린 AUC는 군당 n = 5 마리 동물로 비히클 대조군과 비교하여 검사하였다. 결과를 제시하였다 (SEM) (N).
<표 8>
정맥내 글루코스 내성 시험 동안에 인슐린 분비에 대한 실시예 화합물의 효과.
표 8에 제공된 데이터는 인슐린 분비의 용량 의존적 증가를 입증한다.
<표 9>
하기 데이터로 나타낸 ivGTT 인슐린 분비:
표 9에 제공된 데이터는 인슐린 분비의 용량 의존적 증가를 입증한다.
식이-유도 비만 C57/B16 마우스에서의 연구
체중이 41-50 g인 C57/Bl6 식이-유도 비만 (DIO) 수컷 마우스 (타코닉(Taconic), 뉴욕주 저먼타운)를 사용하였다. 동물은 12시간의 명/암 광주기 (오전 10:00시에 소등하고 오후 10:00시에 점등)로, 사료 및 물을 무제한으로 이용할 수 있는, 온도-제어 (24℃) 시설에 개별적으로 수용하였다. 시설에 2주 순응 후, 각각의 군이 유사한 출발 평균 체중을 갖도록 마우스를 체중에 기초하여 처리군 (n=6/군)으로 무작위화하였다.
마우스는 비히클 (pH 8.0에서 40 mM Tris-HCl) 또는 0.03 nmol/kg 내지 10 nmol/kg의 용량 범위 사이의 몇몇 펩티드로 처리하였다. 14일 동안 매일 암주기 (QD)가 시작되기 30-90 분 전에 무제한으로 공급된 DIO 마우스에게 치료제를 피하 투여하였다. 연구 과정 동안에, 체중 및 사료 섭취량을 매일 모니터링하였다.
모든 데이터는 군당 5-6 마리 래트의 평균 ± SEM으로서 표시하였다. 통계 분석은 일원 ANOVA에 이어서 두넷(Dunnett)의 다중 비교 검정에 의해 평가하여 처리군을 비히클군과 또는 서로 비교하였다. 유의한 차이는 p<0.05에서 확인하였다.
체중 백분율 = 14일 처리 후 체중 x 100
처리 시작 전 체중
"0" 용량 군은 각각의 연구 동안에 비히클-처리된 마우스를 나타낸다. 모든 데이터는 군당 5-6 마리 마우스의 평균 ± SEM으로서 표시하였다. 통계 분석은 일원 ANOVA에 이어서 두넷 다중 비교 검정을 통해 처리군을 '0' 용량 (비히클)과 비교하였다. *유의한 차이는 p<0.05에서 확인된다. 15일 후 실시예 화합물로 처리한 후 체중 변화. "비히클로부터의 Δ"는 시험군과 비히클군 간의 15일째 체중 차이를 지칭한다. "% 변화"는 시험군에서 1일과 15일 사이에 체중 감소율 (%)을 지칭한다. 비히클을 받은 동물의 체중 감소율(%)이 기록되며, 각각의 연구에서 약 1% 미만이다. 비히클 및 % 변화 데이터로부터의 Δ는 시험된 모든 용량에서 모든 실시예에 대한 대조군과 통계적으로 유의하게 상이하다 (p<0.05).
<표 10>
14일의 치료 후의 식이-유도 비만 마우스에서 체중 (%)에 대한 GIP/GLP-1 수용체 공효능제의 효과.
상기 표 10에 제공된 데이터에 의해 예시된 바와 같이, 검정에서 시험된 실시예 화합물은 기재된 연구에서 용량-의존적으로 체중을 감소시킨다.
단백질분해 안정성 검정
단백질분해 안정성 검정은 펩티드의 경구 전달 가능성을 평가하는 데 유용한다. 펩티드의 안정성은 1% 래트 소장액 (rSIF)에서 비교하였다. 단백질분해 안정성을 평가하기 위해 0, 3, 15, 및 30분에 샘플 펩티드에 대해 무손상 펩티드의 양을 측정하였다. 단백질분해 안정성을 평가하기 위해 샘플 펩티드에 대한 무손상 펩티드의 양을 90% 돼지 소장액 (pSIF)에서 0분, 30분, 45분 및 60분에 측정하였다.
래트 소장액 (rSIF)을 사용한 경우의 샘플 제조:
펩티드를 50 mM Tris pH8.0에서 0.4 mg/mL로 제조하였다. 래트 소장액은 1% (v/v)의 비로 첨가하였다. 혼합물을 37℃에서 150 rpm으로 인큐베이션하였다. rSIF를 첨가하기 전과 3분, 15분, 및 60분에 30 μL의 각각의 샘플을 제거하고 새로운 튜브에 넣었다. 각각의 시점에서, 반응은 1:1에서 50% ACN에서 1% TFA에 의해 켄칭하였다. 샘플을 희석 완충제 (50% ACN에서 1% TFA의 1:1: 50 mM Tris pH8)를 사용하여 100배 희석하고 질량 분석법 (MS)을 사용하여 분석할 준비를 하였다.
돼지 소장액 (pSIF)을 사용한 경우의 샘플 제조:
펩티드를 90% 돼지 소장액에서 0.4 mg/mL의 농도로 희석하였다. 혼합 후, 20 μL를 즉시 제거하였다 (사전-인큐베이션 시점의 경우 시간 0). 이어서 혼합물을 150 rpm에서 37℃에서 인큐베이션하였다. 20 μL의 각각의 샘플을 제거하고 30, 45 및 60분에 새 튜브에 넣었다. 각각의 시점 (0, 30, 45, 60)에서 반응은 1:1에서 50% ACN에서 1% TFA에 의해 켄칭하였다. 샘플은 4℃에서 20분 동안 20,000xg에서 원심분리하였다. 상청액을 희석 완충제 (50% ACN에서 1% TFA의 1:1: 50 mM Tris pH 8)를 사용하여 100배 희석하고 질량 분석법 (MS)을 사용하여 분석할 준비를 하였다.
MS 조건: 액체 크로마토그래피 분리는 이동상 A (물 중 0.1% 포름산) 및 B (아세토니트릴 중 0.1% 포름산) 및 액쿼티(ACQUITY UPLC) 프로테인 BEH C4 컬럼 (300Å, 1.7 μm, 1 mm X 50 mm)을 40℃에서 사용하여 워터스 액쿼티(Waters Acquity) UPLC에서 수행하였다. 구배는 0-1.5 동안 5%의 B, 1.5-1.8 동안 5-90%의 B, 1.8-3.0 동안 90-95%의 B, 3.0-3.5 동안 95-95%의 B, 3.5-4.0 동안 95-5%의 B, 및 4.0-5.0 동안 5-5%의 B였다. MS 분석은 워터스(Waters) 크세보(Xevo) G2-XS QTOF에서 수행하였다. 데이터는 포지티브 및 감도 모드에서 50-2000 m/z 범위의 MSe 컨티넘(Continuum)을 사용하여 획득하였다. 데이터 분석은 매스링크스(MassLynx)를 사용하여 수행하였다.
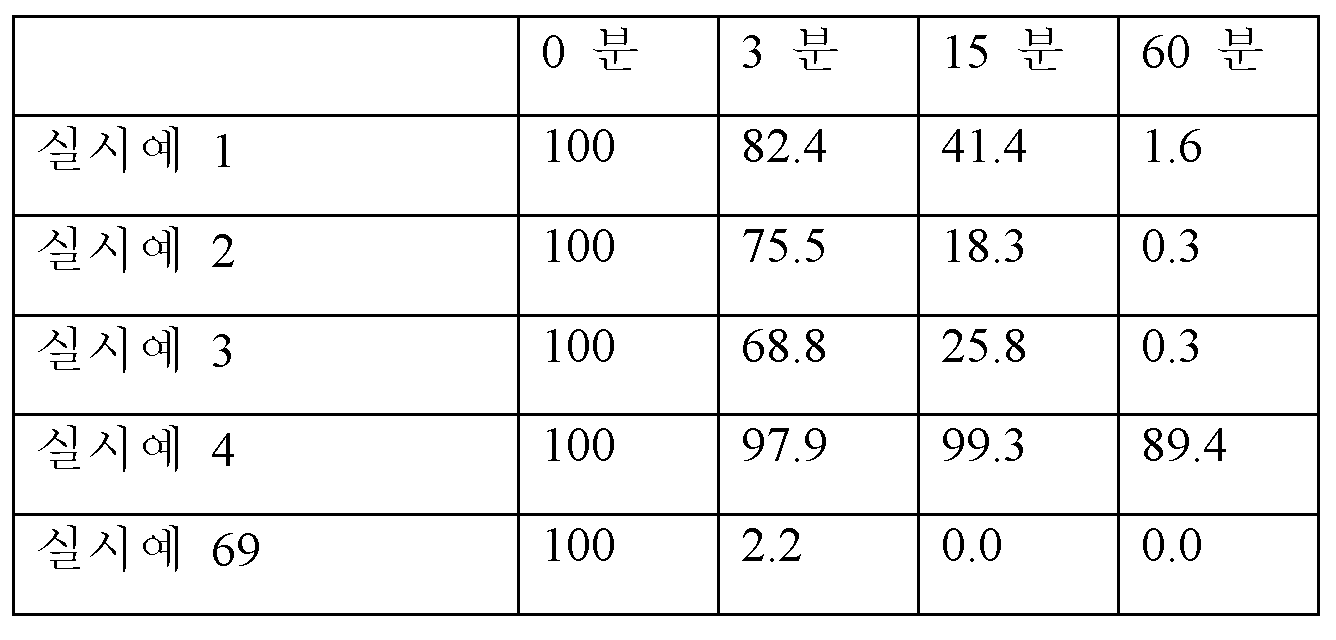
<표 11>
rSIF를 사용하여 상이한 시점에서 절단되지 않은 각각의 펩티드의 백분율.
표 11에 제공된 단백질분해 펩티드 결과는 실시예 4의 펩티드가 경구 제제 및 전달에 적합할 수 있음을 시사한다.
<표 12>
pSIF를 사용하여 상이한 시점에서 절단되지 않은 각각의 펩티드의 백분율.
표 12에 제공된 단백질분해 펩티드 결과는 실시예 4 및 5의 펩티드 둘 다가 경구 제제 및 전달에 적합할 수 있음을 시사한다.
생체내 연구
이 연구의 목적은 화합물의 임상 면역원성에 대한 상대적 가능성을 결정하는 것이다.
방법:
CD8+ T 세포 고갈된 말초 혈액 단핵 세포를 준비하고 10명의 건강한 공여자의 코호트로부터 카르복시플루오레세인 디아세테이트 숙신이미딜 에스테르 (CFSE, 인비트로겐)로 표지하였다. 샘플을 2.0 mL 배지 대조군, 키홀 림펫 헤모시아닌 ( "KLH") (0.33 μM), 항-케모카인 수용체 유형 4 ("CD4+") (0.33 μM), 및 실시예 1, 2, 및 3의 화합물 (10 μM)로 삼중으로 시험하였다. 배양물을 5% CO2로 37℃에서 7일 동안 인큐베이션하였다. 제7일에, 고 처리량 샘플러(High Throughput Sampler) (HTS)를 사용하여 유세포 분석법에 의해 샘플을 분석하였다. 데이터는 플로우조(FlowJo)® 소프트웨어 (플오루조, 엘엘씨(FlowJo, LLC), 트리스타(TreeStar))를 사용하여 분석하였다.
결과 및 논의
모든 공여자는 KLH (100%)에 대해 양성 T 세포 반응을 초래하였다. 실시예 화합물에 대한 CD4+ T 세포 반응의 빈도 및 크기 분석을 표 13에 나타냈다.
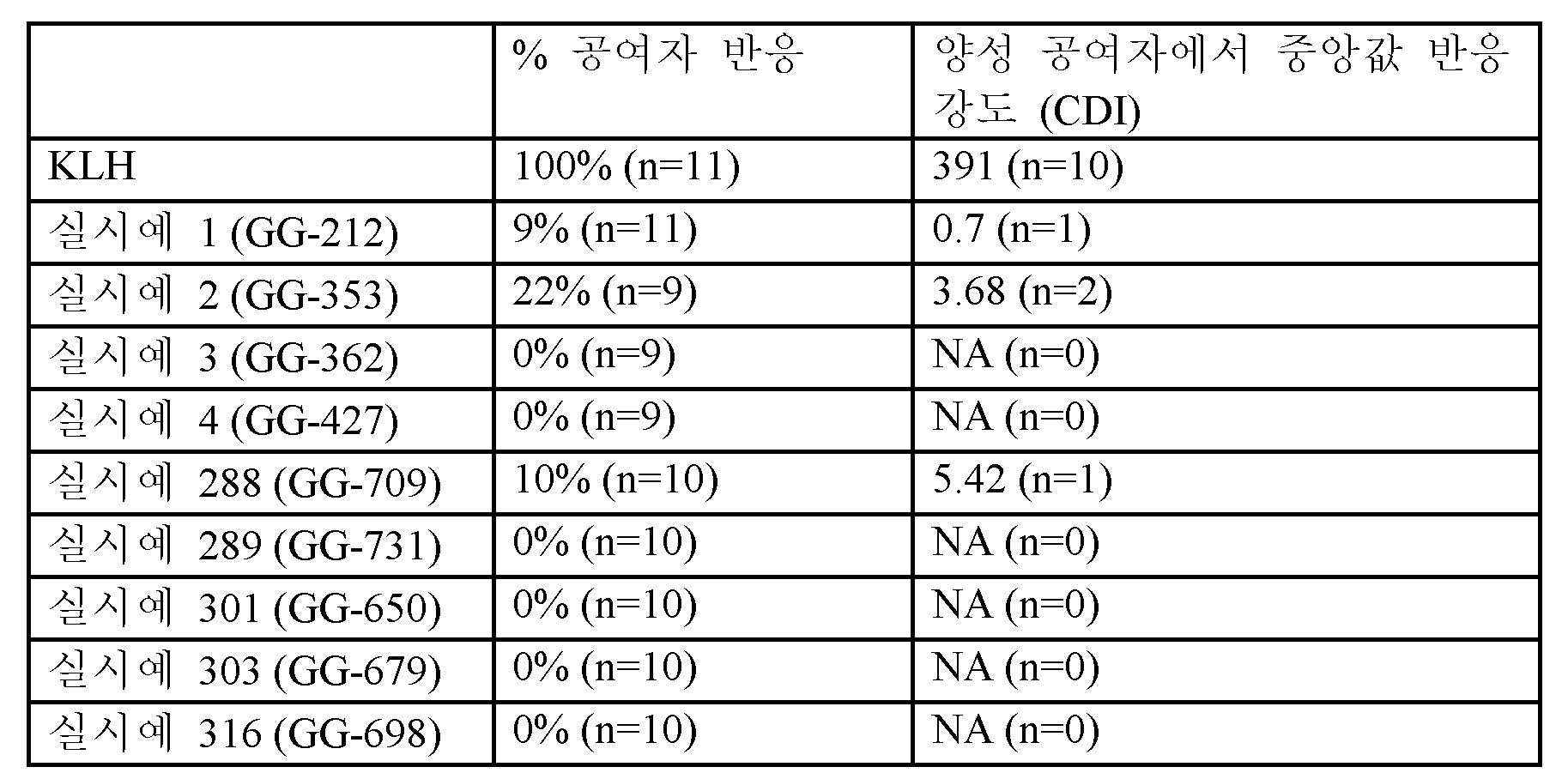
<표 13>
실시예 화합물 및 양성 대조군 (KLH)에 대한 CD4+ T 세포 반응.
세포 분열 지수 ("CDI"): 자극되지 않은 샘플에 비해 자극된 샘플에서 CD4+ T 세포의 총 수에 대한 분할된 CD4+ T 세포의 비율.
이들 데이터는 실시예 1, 2, 3, 4, 288, 289, 301, 303 및 316의 화합물에 대해 양성 CD+ T 세포 반응의 빈도 (CDI> 2.5)가 낮았고, 소수의 양성 공여자에서의 반응의 크기가 낮았으며 (CDI <6), 이는 CD4+ T 세포 검정을 사용한 면역원성의 낮은 위험을 나타낸다.
GLP-1R HEK293 세포막 [
35
S]GTPγS 결합 검정
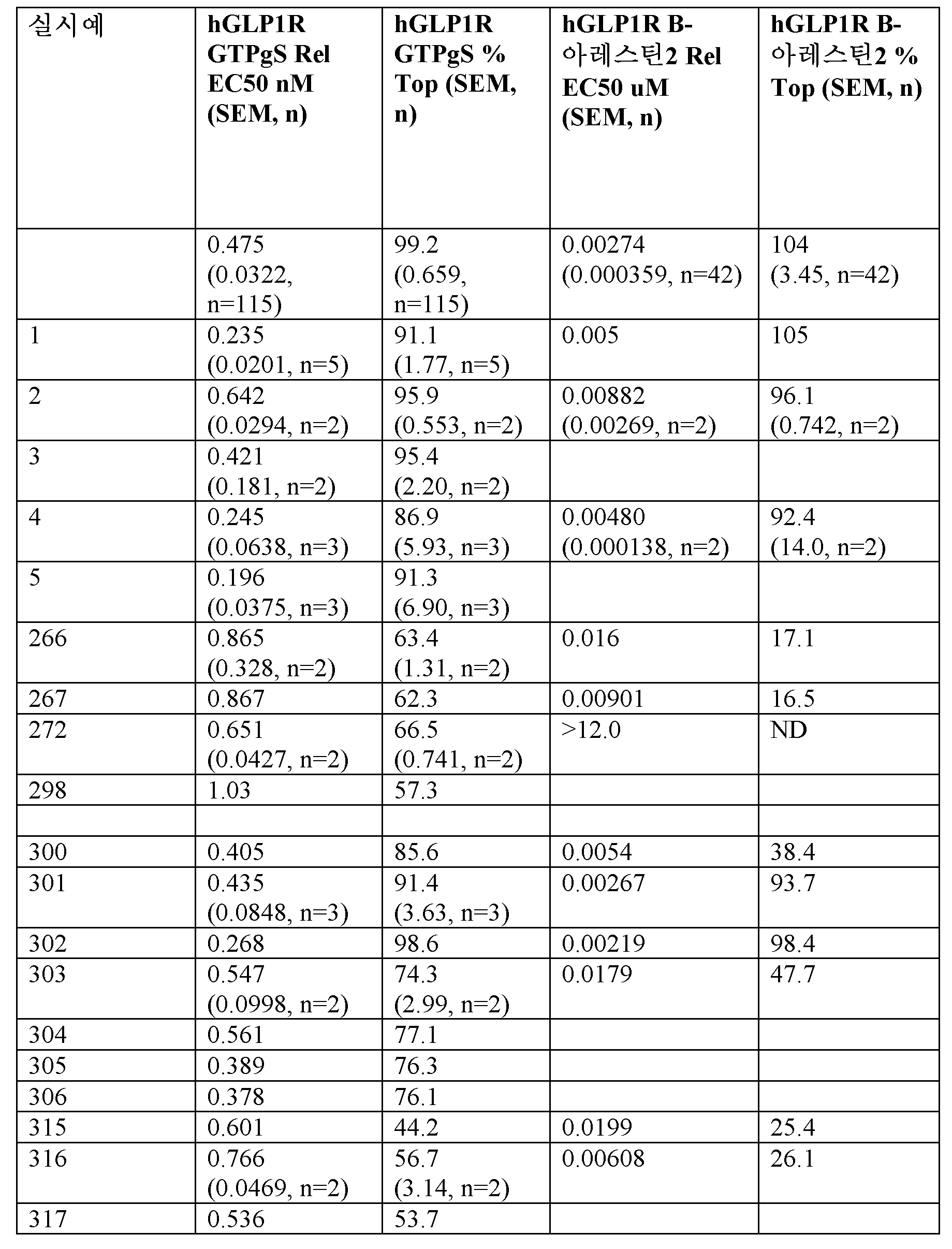
GLP-1 수용체는 리간드 유도 수용체 활성화시 GTP-결합 Gαs를 증가시키는 G-단백질 커플링된 수용체이다. Gαs의 GLP-1R 유도 활성화를 자극하는 펩티드의 효능은 인간 GLP-1R을 발현하는 HEK293 세포로부터 정제된 막의 제조를 사용하여 결정하였다. 검정은 이전에 기재된 바와 유사하게 수행된다 (Bueno et al., J. Biol. Chem., (2016) 291, 10700 및 Willard et al., Mol. Pharmacol. (2012) 82,1066). 시험 펩티드는 DMSO에 가용화시키고 20 mM HEPES pH 7.4, 50 mM NaCl, 5 mM MgCl2, 40 μg/ml 사포닌, 0.1% BSA, 및 500 pM 35S-표지된 GTPγS에 5 μg의 막을 함유하는 반응 완충제로 실온에서 30분 동안 희석하였다. 토끼 항-Gαs 폴리클로날 항체 및 0.5 mg의 항-토끼 폴리비닐톨루엔 비드를 함유하는 0.2% 노니뎃(Nonidet) P-40 세정제를 첨가함으로써 반응을 종결시켰다. 혼합물을 30분 동안 현상하고, 80 x g에서 10분 동안 원심분리하고, 마이크로베타 트리룩스 기기를 사용하여 1분/웰로 계수하였다. 펩티드 농도-반응 곡선을 EC50으로서 효능을 계산하기 위해 4-파라미터 로지스틱 모델에 피팅하였다. % 자극에 대한 데이터 정규화는 수용체에 대한 최소 및 최대 대조군으로서 DMSO 및 GLP-1 (7-36)을 사용하여 수행하였다 (Campbell et al., Assay Guidance Manual 2017). Gαs의 GIPR 유도 활성화를 자극하는 샘플 펩티드의 효능을 표 14에 보고하였다. 검정 결과는 Gαs의 GLP-1R 유도된 활성화와 관련하여 GLP-1R에 대한 부분 효능제인 펩티드를 확인하였다.
GLP-1R CHO 세포 β-아레스틴 동원 검정
활성화된 G-단백질 커플링된 수용체는 신호전달 단백질의 β-아레스틴 계열과 상호작용할 수 있다. GLP-1R 유도된 아레스틴 동원을 위한 펩티드의 효능은 문헌 (von Degenfeld et al., FASEB J., 2007 (14):3819-26 및 Hamdouchi et al., J. Med Chem., 2016 59(24):10891-10916)에 기재된 바와 같이 실질적으로 패스헌터(PathHunter) 효소 단편 보완 접근법을 사용하여 결정하였다. Pro-Link 태그부착된 인간 GLP-1R 및 효소-수용체-태그부착된 β-아레스틴-2를 발현하는 CHO-K1 세포는 디스커버엑스(DiscoveRx)로부터 입수할 수 있으며 검정-준비된 동결된 세포로서 제조할 수 있다. 시험 펩티드를 DMSO에 가용화시키고 에코(Echo) 음향 분배기 (랩사이트(LabCyte))를 사용하여 연속 희석을 수행하였다. 검정 배지는 0.1% w/v 가수분해된 카제인 (시그마)을 함유하는 팬트헌터 세포 검정 완충제 (디스커버엑스)였다. 100 nl의 펩티드를 384 웰 플레이트에서 10 μl의 검정 배지에 분배한 다음에 검정 배지에서의 10 μl의 세포를 첨가하여 웰당 5000개 세포를 제공하였다. 플레이트를 37℃/5% CO2 인큐베이터에서 90분 동안 인큐베이션하고 10 μl의 패스헌터 검출 시약 (디스커버엑스)을 첨가하고 플레이트를 실온에서 60분 동안 인큐베이션하였다. 발광 신호를 측정하였다. 펩티드 농도-반응 곡선을 EC50으로서 효능을 계산하기 위해 4-파라미터 로지스틱 모델에 피팅하였다. % 자극에 대한 데이터 정규화는 수용체에 대한 최소 및 최대 대조군으로서 DMSO 및 GLP-1 (7-36)을 사용하여 수행하였다 (Campbell et al., Assay Guidance Manual 2017). GLP-1R 유도된 β-아레스틴 동원을 자극하는 샘플 펩티드의 효능을 표 14에 보고하였다. 검정 결과는 β-아레스틴-2 동원과 관련하여 GLP-1R에 대한 부분 효능제인 펩티드를 확인하였다.
<표 14>
경구 투여용 조성물
펩티드를 Tris 완충제 (pH 8.0, 50 mM)에 용해시켰다. 침투 증진제 ("PE")를 다음과 같이 제조하였다: C10을 Tris 완충제 (pH 8.0, 50 mM)에 용해시키고, LC, DPC, C12-말토시드 및 람노리피드를 각각 인산염 완충 식염수 ("PBS") (1X, pH 7.2)에 용해시켰다. 펩티드, PE, 및 프로테아제 억제제의 용액을 혼합하여 300 uM의 최종 펩티드 농도, 100 mM의 PE (람노리피드의 경우 5% w/v) 및 프로테아제 억제제의 경우 1% (v/v)에 도달하였다.
펩티드는 1% (v/v) 래트 소장액 또는 50% (v/v) 돼지 소장액에서 펩티다제 억제제 유무에 관계없이 37℃에서 인큐베이션하였다. 상이한 시점에서, 샘플을 채취해 낸 후에, 50% ACN/물에 1% TFA로 켄칭하여 효소 활성을 중지시켰다. 상이한 시점에서 무손상 펩티드는 자외선 (UV) 검출기 또는 LC-MS/MS가 장착된 고성능 액체 크로마토그래피 (HPLC)에 의해 분석하고 효소 용액과 혼합하기 전에 펩티드의 양으로 정규화하였다. 실시예 2의 페티드 및 실시예 4의 펩티드를 사용한 연구를 표 15에 보고하였다.
<표 15>
표 15의 결과는 실시예 4의 펩티드용 경구 제제 조성물이 PI 없이 PE를 사용하여 제조될 수 있음을 뒷받침한다.
경구 제제 조성물
본 발명의 펩티드용 제제 조성물의 예를 표 16에 제공하였다. 본 발명의 펩티드용 제제 조성물은 제공된 실시예에 의해 결코 제한되지 않는다.
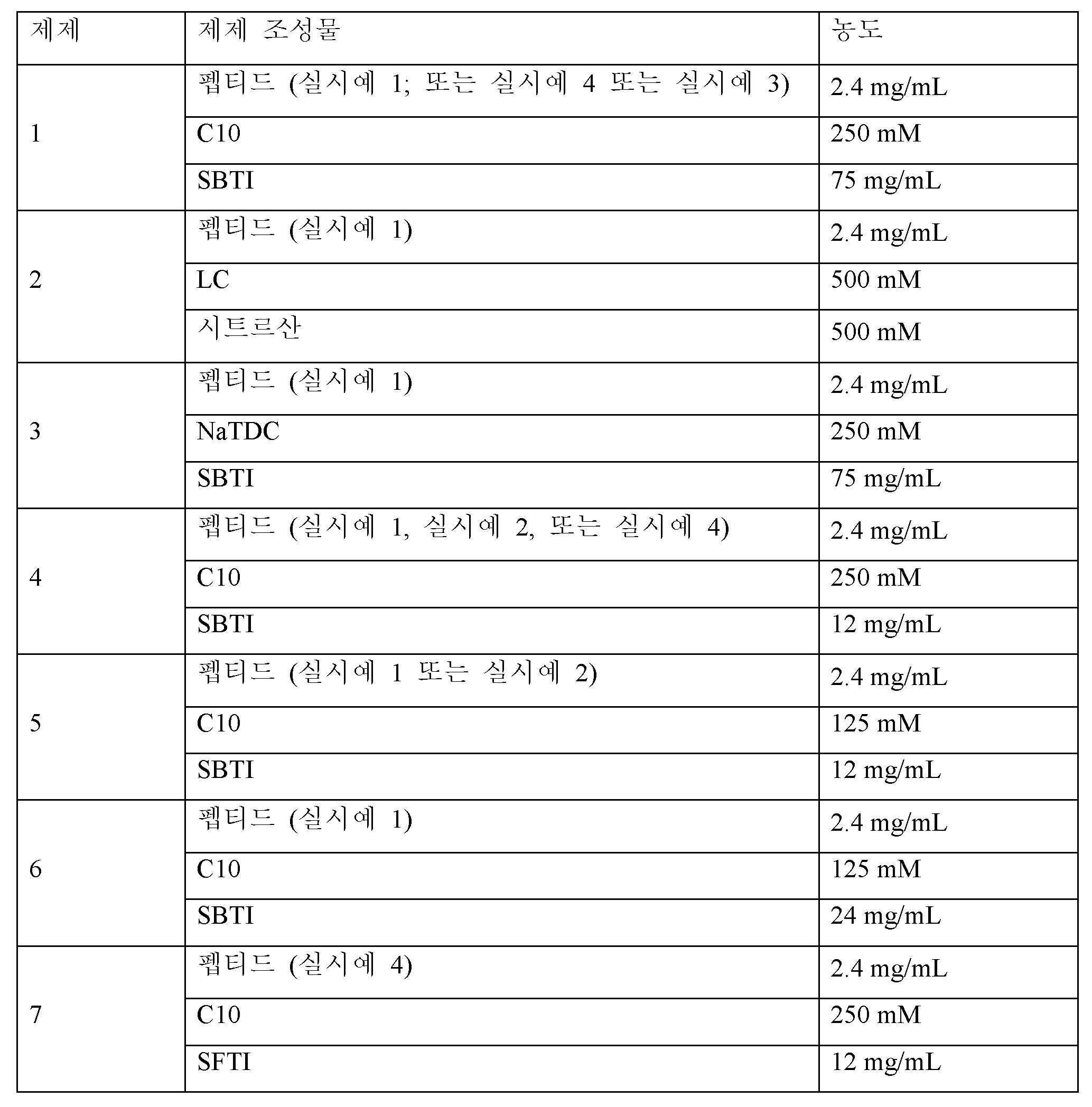
<표 16>
펩티드 노출에 대한 제제 조성물의 효과는 액체 제제를 사용하여 공장내 (IJ) 투여를 통해 래트에서 평가하였다. 래트 IJ 투여를 위한 액체 제제를 제조하기 위해, 펩티드, C10 또는 NaTDC 및 SBTI를 50 mM Tris 완충제 pH 8.0에 용해시키고 혼합하여 최종 원하는 농도를 달성하였다. LC/시트르산 제제의 경우, LC 및 시트르산을 물에 용해시키고 Tris 완충제에 용해된 펩티드와 혼합하였다. 표 16에 제공된 제제 조성물은 경구 조성물로서 투여될 수 있다.
장용성 캡슐
장용성 캡슐 조성물은 본 발명의 특정 펩티드에 대해 바람직할 수 있고, 예를 들어 표 17에 제시된 방법을 사용하여 제조될 수 있다. 장용성 조성물은 성분을 함께 블렌딩하고 블렌드를 장용성 캡슐에 충전함으로써 제조될 수 있다.
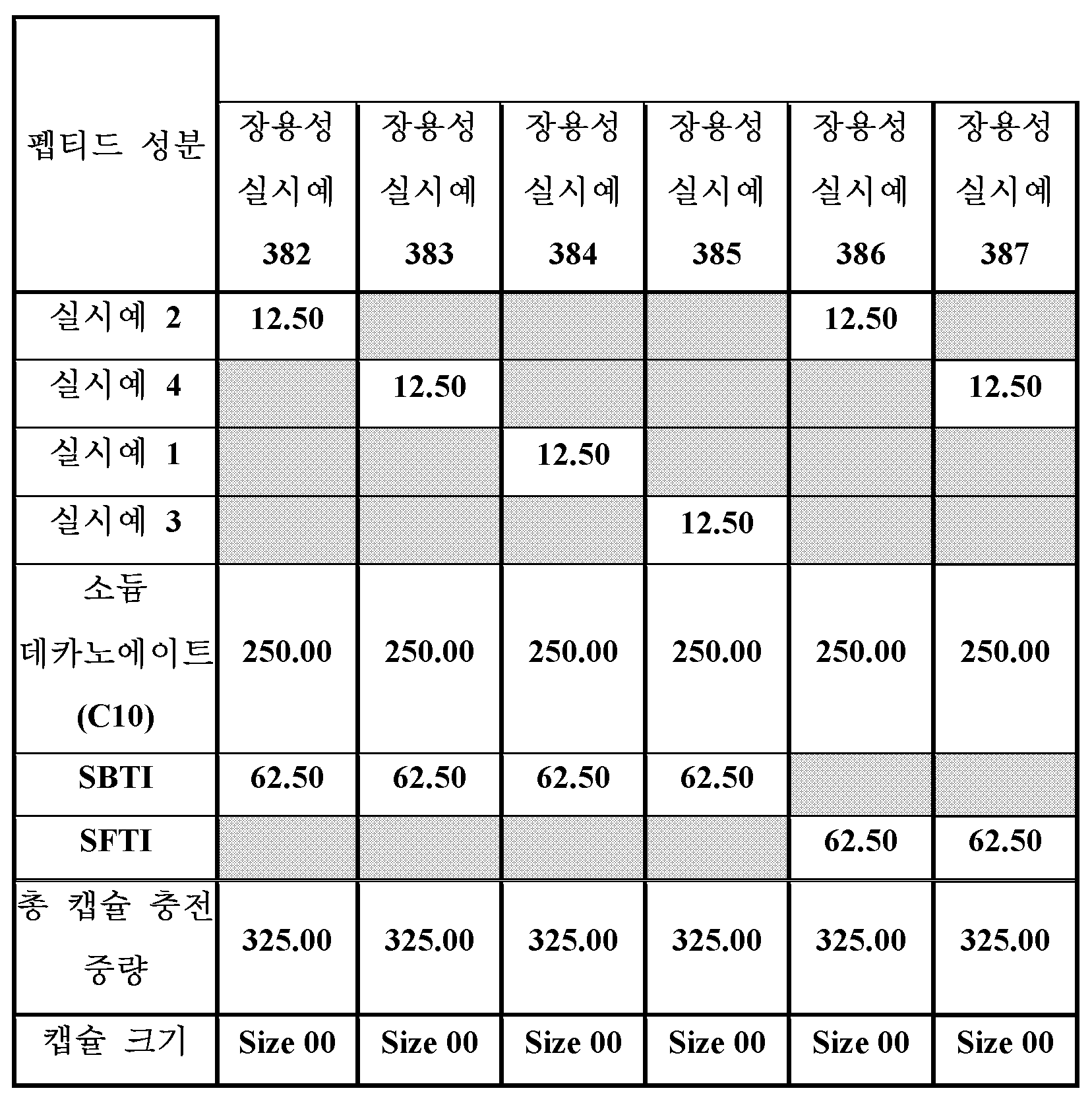
표 17의 장용성 조성물은 명시된 양의 소듐 데카노에이트의 절반을 막자사발에 첨가하여 제조하였다. 표 17에 나타낸 바와 같이, SBTI (실시예 382-385의 경우) 또는 SFTI (실시예 386 및 387의 경우), 및 펩티드 (실시예 1-4의 펩티드). 남은 절반의 소듐 데카노에이트를 첨가하였다. 혼합물을 막자, 및 주걱을 사용하여 함께 부드럽게 블렌딩하였다. 원하는 경우, 막자를 사용하여 추가 혼합하여 균일한 혼합을 제공하였다. 필요한 양의 블렌드를 개별적으로 계량하고, 캡슐을 채우고, 캡슐 캡을 캡슐 본체에 단단히 닫음으로써 캡슐을 수동으로 채울 수 있다.
단일 캡슐의 용해 시험은 공지된 방법을 사용하여 완료하였다. 본 발명의 펩티드는 장용성 경구 조성물로서 제제화될 수 있다.
<표 17>
제제를 위한 개별 장용성 캡슐의 조성물
아미노산 서열
서열식별번호: 1
GIP (인간)
YAEGTFISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ
서열식별번호: 2
GLP-1 (7-36) (인간)
HAEGTFTSDVSSYLEGQAAKEFIAWLVKGR-NH2
서열식별번호: 3
R1X1 X2 X3GT X6TSD X10 X11 X12 X13 X14D X16X17AX19 X20 X21 X22X23 X24 X25 X26 X27 X28 X29 X30X31
서열식별번호: 4
PX32 X33 X34-R2
서열식별번호: 5
PX32X33X34X35X36X37X38X39-R2
서열식별번호: 6
PX32 X33 X34 X35X36 X37 X38 X39 X40-R2
서열식별번호: 7
K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H] X32 X33 X34-R2
서열식별번호: 8
K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H] X32 X33 X34 X35X36 X37 X38 X39-R2
서열식별번호: 9
K[(2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)q-CO2H] X32 X33 X34 X35X36 X37 X38 X39 X40-R2
서열식별번호: 10
실시예 1
Y-Aib-EGT-αMeF(2F)-TSDYSI-αMeL-LDEK((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)18-CO2H)AQ-Aib-EFI-(D-Glu)-YLIEGGPSSGAPPPS-NH2
서열식별번호: 11
실시예 2
Y-Aib-EGT-αMeF(2F)-TSDYSI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)16-CO2H)AQ-Aib-EFI-(D-Glu)-YLIEGGPSSGAPPPS-NH2
서열식별번호: 12
실시예 3
Y-Aib-EGT-αMeF(2F)-TSDYSI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)18-CO2H)AQ-Aib-EFI-(D-Glu)-YLIEGGPSSGAPPPS-NH2
서열식별번호: 13
실시예 4
Y-Aib-EGT-αMeF(2F)-TSD-4Pal-SI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)16-CO2H)AQ-Aib-EFI-(D-Glu)-αMeY-LIEGGPSSGAPPPS-NH2
서열식별번호: 14
실시예 5
Y-Aib-EGT-αMeF(2F)-TSDVSI-αMeL-LD-Orn-K((2-[2-(2-아미노-에톡시)-에톡시]-아세틸)2-(γ-Glu)-CO-(CH2)16-CO2H)AQ-Aib-EFI-(D-Glu)-αMeY-LIEGGPSSGAPPPS-NH2
서열식별번호: 297
PSSG-R2
서열식별번호: 298
PSSGAPPPS-R2
서열식별번호: 299
PSSG
서열식별번호: 300
PSSG-NH2
서열식별번호: 301
PSSGAPPPS
서열식별번호: 302
PSSGAPPPS-NH2
SEQUENCE LISTING
<110> Eli Lilly and Company
<120> GIP/GLP1 CO-AGONIST COMPOUNDS
<130> X21852
<150> US 62/702,072
<151> 2018-07-23
<150> US 62/730,563
<151> 2018-09-13
<150> US 62/740,596
<151> 2018-10-03
<160> 396
<170> PatentIn version 3.5
<210> 1
<211> 42
<212> PRT
<213> Homo sapiens
<400> 1
Tyr Ala Glu Gly Thr Phe Ile Ser Asp Tyr Ser Ile Ala Met Asp Lys
1 5 10 15
Ile His Gln Gln Asp Phe Val Asn Trp Leu Leu Ala Gln Lys Gly Lys
20 25 30
Lys Asn Asp Trp Lys His Asn Ile Thr Gln
35 40
<210> 2
<211> 30
<212> PRT
<213> Homo sapiens
<220>
<221> MOD_RES
<222> (30)..(30)
<223> AMIDATION
<400> 2
His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Ser Tyr Leu Glu Gly
1 5 10 15
Gln Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly Arg
20 25 30
<210> 3
<211> 31
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic construct; Formula I
<220>
<221> VARIANT
<222> (1)..(1)
<223> Xaa at position 1 is selected from the group consisting of Y, H,
D-Tyr, F, desH, and desY, or Xaa at position 1 and Xaa at
position 2 combine to form desH-psi[NHCO]-Aib
<220>
<221> MOD_RES
<222> (1)..(1)
<223> The N-terminus of Xaa at position 1 is modified with R1, wherein
the modification is selected from the group consisting of Ac and
absent.
<220>
<221> VARIANT
<222> (2)..(2)
<223> Xaa at position 2 is selected from the group consisting of Aib,
alpha-MeP, A, P, and D-Ala, or Xaa at position 1 combines with
Xaa at position 2 to form desH-psi[NHCO]-Aib
<220>
<221> VARIANT
<222> (3)..(3)
<223> Xaa at position 3 is selected from the group of E, N, Aad, and
cTA
<220>
<221> VARIANT
<222> (6)..(6)
<223> Xaa at position 6 is selected from the group consisting of F,
alpha-MeF, and alpha-MeF(2F)
<220>
<221> VARIANT
<222> (10)..(10)
<223> Xaa at position 10 is selected from the group consisting of A, L,
H, 3Pal, 4Pal, V, Y, E, alpha-MeF, alpha-MeF(2F), I, alpha-MeY,
Q, D-His, D-Tyr, cTA, and K
<220>
<221> VARIANT
<222> (10)..(10)
<223> when Xaa at position 10 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> MISC_FEATURE
<222> (10)..(31)
<223> When Xaa is
K(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO
2H at positions 10, 12, 13, 14, 16, 17, 19, 20, 21, 23, 24, 26,
27, 28, 29, 30 or 31, q is 14, 15, 16, 17, 18, 19 or 20.
<220>
<221> VARIANT
<222> (11)..(11)
<223> Xaa at position 11 is selected from the group consisting of S,
alpha-MeS, or D-Ser
<220>
<221> VARIANT
<222> (12)..(12)
<223> Xaa at position 12 is selected from the group consisting of I, S,
D-Ile, and K
<220>
<221> MOD_RES
<222> (12)..(12)
<223> when Xaa at position 12 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (13)..(13)
<223> Xaa at position 13 is selected from the group consisting of Nle,
Aib, L, alpha-MeL, and K
<220>
<221> MOD_RES
<222> (13)..(13)
<223> when Xaa at position 13 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (14)..(14)
<223> Xaa at position 14 is selected from the group consisting of L and
K, wherein K is conjugated to a C16-C22 fatty acid wherein said
fatty acid is optionally conjugated to said K via a linker
<220>
<221> VARIANT
<222> (16)..(16)
<223> Xaa at position 16 is selected from the group consisting of E,
Orn, Dab, Dap, S, T, H, Aib, alpha-MeK, R, and K
<220>
<221> MOD_RES
<222> (16)..(16)
<223> when Xaa at position 16 is K, then K is optionally chemically
modified by conjugation of the epsilon-amino group of the K side
chain with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> VARIANT
<222> (17)..(17)
<223> Xaa at position 17 is selected from the group consisting of K, Q,
I, and an amino acid conjugated to a C16-C22 fatty acid wherein
said fatty acid is optionally conjugated to said amino acid via a
linker
<220>
<221> VARIANT
<222> (19)..(19)
<223> Xaa at position 19 is selected from the group consisting of Q, A,
and K
<220>
<221> MOD_RES
<222> (19)..(19)
<223> when Xaa at position 19 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> VARIANT
<222> (20)..(20)
<223> Xaa at position 20 is selected from the group consisting of Aib,
Q, H, R, K, and alpha-MeK
<220>
<221> MOD_RES
<222> (20)..(20)
<223> when Xaa at position 20 is K, then K is optionally chemically
modified by conjugation of the epsilon-amino group of the K side
chain with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> VARIANT
<222> (21)..(21)
<223> Xaa at position 21 is selected from the group consisting of H,
Aad, D, Aib, T, A, E, I, and K
<220>
<221> MOD_RES
<222> (21)..(21)
<223> when Xaa at position 21 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
K(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO
2H
<220>
<221> VARIANT
<222> (22)..(22)
<223> Xaa at position 22 is selected from the group consisting of F and
alpha-MeF
<220>
<221> VARIANT
<222> (23)..(23)
<223> Xaa at position 23 is selected from the group consisting of I, L,
A, G, F, H, E, V, and K
<220>
<221> MOD_RES
<222> (23)..(23)
<223> when Xaa at position 23 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
K(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO
2H
<220>
<221> VARIANT
<222> (24)..(24)
<223> Xaa at position 24 is selected from the group consisting of S,
Aad, D-Glu, E, Aib, H, V, A, Q, D, P, and K
<220>
<221> MOD_RES
<222> (24)..(24)
<223> when Xaa at position 24 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
K(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO
2H
<220>
<221> VARIANT
<222> (25)..(25)
<223> Xaa at position 25 is selected from the group consisting of Y or
alpha-MeY
<220>
<221> VARIANT
<222> (26)..(26)
<223> Xaa at position 26 is selected from the group consisting of L,
alpha-MeL, and K
<220>
<221> MOD_RES
<222> (26)..(26)
<223> when Xaa at position 26 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (27)..(27)
<223> Xaa at position 27 is selected from the group consisting of L, I,
and K
<220>
<221> MOD_RES
<222> (27)..(27)
<223> when Xaa at position 27 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (28)..(28)
<223> Xaa at position 28 is selected from the group consisting of E, A,
S, D-Glu, and K
<220>
<221> MOD_RES
<222> (28)..(28)
<223> when Xaa at position 28 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (29)..(29)
<223> Xaa at position 29 is selected from the group consisting of Aib,
G, A, and K
<220>
<221> MOD_RES
<222> (29)..(29)
<223> when Xaa at position 29 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (30)..(30)
<223> Xaa at position 30 is selected from the group consisting of C, G,
G-R2, and K, wherein R2 is a modification of the C-terminal
group, wherein the modification is NH2 or absent, wherein if X30
is G-R2, then X31 is absent.
<220>
<221> MOD_RES
<222> (30)..(30)
<223> when Xaa at position 30 is K, then K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (31)..(31)
<223> Xaa at position 31 is absent or is selected from the group
consisting of SEQ ID NO:4, SEQ ID NO:5, SEQ ID:6, SEQ ID NO:7,
SEQ ID NO:8, and SEQ ID NO:9
<220>
<221> MISC_FEATURE
<222> (31)..(31)
<223> wherein no more than one of X10, X12, X13, X14, X16, X17, X19,
X20, X21, X23, X24, X26, X27, X28, X29, X30, X31, X32, X33, X34,
X35, X36, X37, X38, X39, and X40 may be a substituent that
contains a fatty acid
<220>
<221> MISC_FEATURE
<222> (31)..(31)
<223> wherein no more than one of X30, X34, X39, and X40 may be C
<220>
<221> MISC_FEATURE
<222> (31)..(31)
<223> wherein if one of X30, X34, X39, and X40 is C, then none of X10,
X12, X13, X14, X16, X17, X19, X20, X21, X23, X24, X26, X27, X28,
X29, X30, X31, X32, X33, X34, X35, X36, X37, X38, X39, and X40 is
a substituent that contains a fatty acid
<220>
<221> MOD_RES
<222> (31)..(31)
<223> Xaa at position 31 is modified with R2, wherein the modification
is NH2 to form a C-terminal amide or absent
<400> 3
Xaa Xaa Xaa Gly Thr Xaa Thr Ser Asp Xaa Xaa Xaa Xaa Xaa Asp Xaa
1 5 10 15
Xaa Ala Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
20 25 30
<210> 4
<211> 4
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic construct; SEQ ID NO:4 is PX32X33X34
<220>
<221> VARIANT
<222> (2)..(2)
<223> Xaa at position 2 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> MISC_FEATURE
<222> (2)..(4)
<223> wherein q is selected from the group consisting of 14, 15, 16,
17, 18, 19, and 20.
<220>
<221> VARIANT
<222> (3)..(3)
<223> Xaa at position 3 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (4)..(4)
<223> Xaa at position 4 is G, C or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> MOD_RES
<222> (4)..(4)
<223> The C-terminal group of Xaa at position 4 is modified with R2,
wherein the modification is NH2 to form a C-terminal amide or
absent
<400> 4
Pro Xaa Xaa Xaa
1
<210> 5
<211> 9
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct; SEQ ID NO:5 is PX32X33X34X35X36X37X38X39
<220>
<221> VARIANT
<222> (2)..(2)
<223> Xaa at position 2 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> MISC_FEATURE
<222> (2)..(9)
<223> wherein q is selected from the group consisting of 14, 15, 16,
17, 18, 19, and 20.
<220>
<221> VARIANT
<222> (3)..(3)
<223> Xaa at position 3 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (4)..(4)
<223> Xaa at position 4 is G, C or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> VARIANT
<222> (5)..(5)
<223> Xaa at position 5 is A or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (6)..(6)
<223> Xaa at position 6 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (7)..(7)
<223> Xaa at position 7 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (8)..(8)
<223> Xaa at position 8 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (9)..(9)
<223> Xaa at position 9 is C, S or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> MOD_RES
<222> (9)..(9)
<223> The C-terminal group of Xaa at position 9 is modified with R2,
wherein the modification is NH2 to from a C-terminal amide or
absent
<400> 5
Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
1 5
<210> 6
<211> 10
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct; SEQ ID NO:6 is PX32X33X34X35X36X37X38X39X40
<220>
<221> VARIANT
<222> (2)..(2)
<223> Xaa at position 2 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> MISC_FEATURE
<222> (2)..(10)
<223> wherein q is selected from the group consisting of 14, 15, 16,
17, 18, 19, and 20.
<220>
<221> VARIANT
<222> (3)..(3)
<223> Xaa at position 3 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (4)..(4)
<223> Xaa at position 4 is G, C or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> VARIANT
<222> (5)..(5)
<223> Xaa at position 5 is A or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (6)..(6)
<223> Xaa at position 6 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (7)..(7)
<223> Xaa at position 7 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
K(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO
2H
<220>
<221> VARIANT
<222> (8)..(8)
<223> Xaa at position 8 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
K(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO
2H
<220>
<221> VARIANT
<222> (9)..(9)
<223> Xaa at position 9 is C, S or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> VARIANT
<222> (10)..(10)
<223> Xaa at position 10 is C or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> MOD_RES
<222> (10)..(10)
<223> The C-terminal group of Xaa at position 10 is modified with R2,
wherein the modification is NH2 to form a C-terminal amide or
absent
<400> 6
Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
1 5 10
<210> 7
<211> 4
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct; SEQ ID NO:7 is
K[(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-C
O2 H]X32X33X34
<220>
<221> MOD_RES
<222> (1)..(1)
<223> K is chemically modified by conjugation of the epsilon-amino
group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> wherein q is selected from the group consisting of 14, 15, 16,
17, 18, 19, and 20
<220>
<221> VARIANT
<222> (2)..(2)
<223> Xaa at position 2 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (3)..(3)
<223> Xaa at position 3 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (4)..(4)
<223> Xaa at position 4 is G, C or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> MOD_RES
<222> (4)..(4)
<223> The C-terminal group of Xaa at position 4 is modified with R2,
wherein the modification is NH2 to form a C-terminal amide or
absent
<400> 7
Lys Xaa Xaa Xaa
1
<210> 8
<211> 9
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct; SEQ ID NO:8 is
K[(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-C
O2H]X32X33X34X35X36X37X38X39
<220>
<221> MOD_RES
<222> (1)..(1)
<223> K is chemically modified by conjugation of the epsilon-amino
group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> wherein q is selected from the group consisting of 14, 15, 16,
17, 18, 19, and 20
<220>
<221> VARIANT
<222> (2)..(2)
<223> Xaa at position 2 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (3)..(3)
<223> Xaa at position 3 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (4)..(4)
<223> Xaa at position 4 is G, C or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> VARIANT
<222> (5)..(5)
<223> Xaa at position 5 is A or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (6)..(6)
<223> Xaa at position 6 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (7)..(7)
<223> Xaa at position 7 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (8)..(8)
<223> Xaa at position 8 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (9)..(9)
<223> Xaa at position 9 is C, S or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
<220>
<221> MOD_RES
<222> (9)..(9)
<223> The C-terminal group of Xaa at position 9 is modified with R2,
wherein the modification is NH2 to form a C-terminal amide or
absent
<400> 8
Lys Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
1 5
<210> 9
<211> 10
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct; SEQ ID NO:9 is
K[(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-C
O2H]X32X33X34X35X36X37X38X39X40
<220>
<221> MOD_RES
<222> (1)..(1)
<223> K is chemically modified by conjugation of the epsilon-amino
group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> wherein q is selected from the group consisting of 14, 15, 16,
17, 18, 19, and 20
<220>
<221> VARIANT
<222> (2)..(2)
<223> Xaa at position 2 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (3)..(3)
<223> Xaa at position 3 is S or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (4)..(4)
<223> Xaa at position 4 is G, C or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> VARIANT
<222> (5)..(5)
<223> Xaa at position 5 is A or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (6)..(6)
<223> Xaa at position 6 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (7)..(7)
<223> Xaa at position 7 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (8)..(8)
<223> Xaa at position 8 is P or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)q-CO2
H
<220>
<221> VARIANT
<222> (9)..(9)
<223> Xaa at position 9 is C, S or K, wherein K is chemically modified
by conjugation of the epsilon-amino group of the K side chain
with (2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> VARIANT
<222> (10)..(10)
<223> Xaa at position 10 is C or K, wherein K is chemically modified by
conjugation of the epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2
-(gamma-Glu)-CO-(CH2)q-CO2H
<220>
<221> MOD_RES
<222> (10)..(10)
<223> The C-terminal group of Xaa at position 10 is modified with R2,
wherein the modification is NH2 to form a C-terminal amide or
absent
<400> 9
Lys Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
1 5 10
<210> 10
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-MeF(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-MeL
<220>
<221> MOD_RES
<222> (17)..(17)
<223> K at position 17 is chemically modified by conjugation of the
epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> The Serine at position 39 is amidated
<400> 10
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 11
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-MeF(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-MeL
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> K at position 17 is chemically modified by conjugation of the
epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> The Serine at position 39 is amidated
<400> 11
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 12
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-MeF(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-MeL
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> K at position 17 is chemically modified by conjugation of the
epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (39)..(39)
<223> The Serine at position 39 is amidated
<400> 12
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 13
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-MeF(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-MeL
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> K at position 17 is chemically modified by conjugation of the
epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-MeY
<220>
<221> MOD_RES
<222> (39)..(39)
<223> The Serine at position 39 is amidated
<400> 13
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 14
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-MeF(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-MeL
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> K at position 17 is chemically modified by conjugation of the
epsilon-amino group of the K side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-MeY
<220>
<221> MOD_RES
<222> (39)..(39)
<223> The Serine at position 39 is amidated
<400> 14
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Val Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 15
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 15
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Leu Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 16
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 16
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Ser
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Leu Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 17
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 17
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 18
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 18
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Ser
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 19
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (24)..(24)
<223> Lys at position 24 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 19
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Ser
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Lys Tyr Leu Leu Ala Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 20
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (28)..(28)
<223> Lys at position 28 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 20
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Ser
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Leu Lys Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 21
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (20)..(20)
<223> Lys at position 20 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 21
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Lys Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 22
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Aib
<220>
<221> MOD_RES
<222> (20)..(20)
<223> Lys at position 20 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 22
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Xaa
1 5 10 15
Ile Ala Gln Lys Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 23
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (20)..(20)
<223> Lys at position 20 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 23
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Lys Glu Phe Ile Gln Tyr Leu Leu Glu Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 24
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 24
His Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Leu Glu Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 25
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 25
His Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 26
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (20)..(20)
<223> Lys at position 20 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Ser at position 34 is amidated
<400> 26
His Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Lys Ala Phe Ile Glu Tyr Leu Leu Glu Xaa Gly Pro Ser
20 25 30
Ser Gly
<210> 27
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-L
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
Dab-(2-[2-(2-Amino-ethoxy)-ethoxy]-acetyl)-Dab-(2-[2-(2-Amino-eth
oxy)-ethoxy]-acetyl)-CO-(CH2)18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is alpha-methyl-Lys
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 27
His Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Gln Tyr Leu Leu Ala Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Lys Pro Ser
35
<210> 28
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
Dab-(2-[2-(2-Amino-ethoxy)-ethoxy]-acetyl)-Dab-(2-[2-(2-Amino-eth
oxy)-ethoxy]-acetyl)-CO-(CH2)18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is alpha-methyl-Lys
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 28
His Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Gln Tyr Leu Leu Ala Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 29
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 29
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 30
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is alpha-methyl-Lys
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 30
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Leu Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 31
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is alpha-methyl-Lys
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 31
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Leu Glu Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 32
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (10)..(10)
<223> Lys at position 10 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 32
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Lys Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 33
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (11)..(11)
<223> Lys at position 11 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 33
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Lys Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 34
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (12)..(12)
<223> Lys at position 12 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 34
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Lys Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 35
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (13)..(13)
<223> Lys at position 13 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 35
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Lys Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 36
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (14)..(14)
<223> Lys at position 14 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 36
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Lys Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 37
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (15)..(15)
<223> Lys at position 15 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 37
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Lys Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 38
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (16)..(16)
<223> Lys at position 16 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 38
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 39
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (18)..(18)
<223> Lys at position 18 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 39
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Lys Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 40
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (19)..(19)
<223> Lys at position 19 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 40
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Lys Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 41
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (21)..(21)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 41
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Lys Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 42
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (23)..(23)
<223> Lys at position 23 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 42
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Lys Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 43
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (24)..(24)
<223> Lys at position 24 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 43
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Lys Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 44
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (26)..(26)
<223> Lys at position 26 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 44
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Lys Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 45
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (27)..(27)
<223> Lys at position 27 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 45
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Lys Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 46
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (28)..(28)
<223> Lys at position 28 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 46
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Lys Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 47
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (29)..(29)
<223> Lys at position 29 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 47
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Lys Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 48
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Lys at position 30 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 48
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Lys Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 49
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (31)..(31)
<223> Lys at position 31 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 49
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Lys Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 50
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (32)..(32)
<223> Lys at position 32 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 50
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Lys
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 51
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (33)..(33)
<223> Lys at position 33 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 51
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Lys Gly Ala Pro Pro Pro Ser
35
<210> 52
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Lys at position 34 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 52
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Lys Ala Pro Pro Pro Ser
35
<210> 53
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (35)..(35)
<223> Lys at position 35 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 53
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Lys Pro Pro Pro Ser
35
<210> 54
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (36)..(36)
<223> Lys at position 36 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 54
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Lys Pro Pro Ser
35
<210> 55
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (37)..(37)
<223> Lys at position 37 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 55
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Lys Pro Ser
35
<210> 56
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (38)..(38)
<223> Lys at position 38 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 56
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Lys Ser
35
<210> 57
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Lys at position 39 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Lys at position 39 is amidated
<400> 57
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Lys
35
<210> 58
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (40)..(40)
<223> Lys at position 40 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (40)..(40)
<223> Lys at position 40 is amidated
<400> 58
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser Lys
35 40
<210> 59
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 59
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 60
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 60
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 61
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 61
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Ser
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 62
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 62
His Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 63
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 63
His Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln His Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 64
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 64
His Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln His Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 65
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 65
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln His Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 66
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 66
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 67
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 67
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ser Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 68
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 68
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 69
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 69
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 70
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 70
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Ser
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Leu Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 71
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 71
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 72
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 72
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 73
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 73
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Ser
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 74
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 74
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Thr
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 75
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 75
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Leu Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 76
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (22)..(22)
<223> Xaa at position 22 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 76
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Xaa Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 77
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (11)..(11)
<223> Xaa at position 11 is alpha-methyl-Ser
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 77
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Xaa Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 78
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 78
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 79
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (10)..(10)
<223> Lys at position 10 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 79
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Lys Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 80
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (14)..(14)
<223> Lys at position 14 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 80
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Lys Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 81
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (21)..(21)
<223> Lys at position 21 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 81
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Lys Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 82
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (28)..(28)
<223> Lys at position 28 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 82
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Lys Gly Gly
20 25 30
<210> 83
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (22)..(22)
<223> Xaa at position 22 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 83
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Xaa Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 84
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (26)..(26)
<223> Xaa at position 26 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 84
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Xaa Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 85
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (22)..(22)
<223> Xaa at position 22 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 85
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Xaa Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 86
<211> 38
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (19)..(19)
<223> Xaa at position 19 is Aib
<220>
<221> MOD_RES
<222> (37)..(37)
<223> Lys at position 37 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (38)..(38)
<223> Ser at position 38 is amidated
<400> 86
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Lys Ile
1 5 10 15
Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser Ser
20 25 30
Gly Ala Pro Pro Lys Ser
35
<210> 87
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 87
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 88
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MOD_RES
<222> (1)..(1)
<223> The N-terminal amino is acetylated
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 88
Xaa Ala Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 89
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is D-Ala
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 89
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 90
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (11)..(11)
<223> Xaa at position 11 is D-Ser
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 90
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Xaa Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 91
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (12)..(12)
<223> Xaa at position 12 is D-Ile
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 91
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Xaa Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 92
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (28)..(28)
<223> Xaa at position 28 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 92
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Xaa Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 93
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 93
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 94
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 94
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Ala Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 95
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 95
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Ala Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 96
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is alpha-methyl-Pro
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 96
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 97
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 97
Tyr Pro Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 98
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (3)..(3)
<223> Xaa at position 3 is Aad
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 98
Tyr Xaa Xaa Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 99
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 99
Tyr Xaa Asn Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 100
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (3)..(3)
<223> Xaa at position 3 is gamma-Glu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 100
Tyr Xaa Xaa Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 101
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (10)..(10)
<223> Lys at position 10 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 101
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Lys Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 102
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (14)..(14)
<223> Lys at position 14 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 102
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Lys Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 103
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (21)..(21)
<223> Lys at position 21 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 103
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Lys Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 104
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (28)..(28)
<223> Lys at position 28 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 104
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Lys Gly Gly
20 25 30
<210> 105
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (10)..(10)
<223> Lys at position 10 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 105
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Lys Ser Ile Xaa Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 106
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (28)..(28)
<223> Lys at position 28 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 106
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Ile Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Lys Gly Gly
20 25 30
<210> 107
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 13 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 107
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 108
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 108
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 109
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 109
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 110
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 110
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 111
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 111
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 112
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 112
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 113
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 113
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 114
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(2)
<223> Xaa at position 1 combines with Xaa at position 2 to form
desHis-psi[NHCO]-Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 114
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 115
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(2)
<223> Xaa at position 1 combines with Xaa at position 2 to form
desHis-Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 115
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 116
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is Des-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 116
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 117
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-AOC-(gamma-Glu)-CO-(CH2)18
-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 117
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 118
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
AOC-(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(gamma-Glu)-CO-(CH2)18
-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 118
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 119
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(gamma-Glu)-(Trx)-CO-(CH2)
18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 119
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 120
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(Trx)-(gamma-Glu)-CO-(CH2)
18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 120
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 121
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(epsilon-Lys)-(gamma-Glu)-
CO-(CH2)18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 121
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 122
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(epsilon-Lys)-CO-(CH2)18-
CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 122
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 123
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(epsilon-Lys)-(epsilon-Lys
)-CO-(CH2)18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 123
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 124
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 124
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 125
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 125
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 126
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 126
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 127
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 127
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 128
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 128
Phe Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 129
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (3)..(3)
<223> Xaa at position 3 is cTA
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 129
Tyr Xaa Xaa Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 130
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 130
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 131
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> Xaa at position 29 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 131
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 132
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 132
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 133
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 133
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 134
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)2-CO-(CH2)18-C
O2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 134
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 135
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 135
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Ala Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 136
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 136
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Ile Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 137
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 137
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 138
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 138
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Leu Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 139
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 139
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Glu Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 140
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 140
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 141
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 3Pal
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 141
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 142
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is DesTyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 142
Xaa Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 143
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is DesTyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 143
Xaa Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 144
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 144
His Xaa Asn Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 145
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 145
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Ala Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Ala Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 146
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (21)..(21)
<223> Xaa at position 21 is Aad
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 146
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Xaa Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 147
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 147
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ser Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 148
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 148
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 149
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 149
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Asp Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 150
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 150
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ile Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 151
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 151
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa His Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 152
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (21)..(21)
<223> Xaa at position 21 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 152
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Xaa Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 153
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (21)..(21)
<223> Xaa at position 21 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 153
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln His Xaa Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 154
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 154
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Ala Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 155
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 155
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Gln Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 156
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is Aad
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 156
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 157
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 157
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Ala Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 158
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 158
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Val Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 159
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 159
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Ser Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 160
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 160
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Pro Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 161
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 161
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 162
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 162
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile His Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 163
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 163
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 164
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is cTA
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 164
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 165
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 2Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 165
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 166
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 3Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 166
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 167
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 167
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 168
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 168
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 169
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 169
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 170
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)2-CO-(CH2)18-C
O2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 170
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 171
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)2-CO-(CH2)18-C
O2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 171
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa His Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 172
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 172
His Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 173
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (28)..(28)
<223> Xaa at position 28 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 173
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Xaa Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 174
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (28)..(28)
<223> Xaa at position 28 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 174
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Xaa Leu Ile Xaa Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 175
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (30)..(30)
<400> 175
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 176
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 176
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 177
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 177
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 178
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 178
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 179
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)14-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 179
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 180
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 180
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 181
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dab
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is a Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 181
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 182
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dap
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 182
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 183
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-CO-(CH2)18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 183
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 184
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(epsilon-Lys)-CO-(CH2)18-
CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 184
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 185
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(epsilon-Lys)-CO-(CH2)16-
CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 185
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 186
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 186
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa His Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 187
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)14-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 187
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa His Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 188
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 188
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa His Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 189
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 189
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp His Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa His Phe Ile Xaa Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 190
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 3Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 190
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 191
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 191
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 192
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 192
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Leu Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 193
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 193
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 194
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is D-His
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 194
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 195
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is alpha-methyl-Tyr
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 195
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 196
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 196
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Gln Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 197
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 3Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 197
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 198
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 198
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 199
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 3Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 199
His Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 200
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 200
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Val Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 201
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 201
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Ala Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 202
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is alpha-methyl-Pro
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 202
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 203
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is alpha-methyl-Pro
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 203
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln His Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 204
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is alpha-methyl-Pro
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 204
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Arg
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 205
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is alpha-methyl-Pro
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 205
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Arg
1 5 10 15
Lys Ala Gln His Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 206
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is alpha-methyl-Pro
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 206
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 207
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is alpha-methyl-Pro
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 207
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 208
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is DesTyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 208
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 209
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is DesTyr
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 209
Xaa Ala Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 210
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is DesHis
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is alpha-methyl-Pro
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 210
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 211
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-Amino-ethoxy)-ethoxy]-acetyl)-(gamma-Glu)-(2-[2-(2-Amino
-ethoxy)-ethoxy]-acetyl)-CO-(CH2)18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 211
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 212
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 212
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 213
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 213
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 214
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-CO-(CH2)16-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 214
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 215
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-CO-(CH2)18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 215
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 216
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-Amino-ethoxy)-ethoxy]-acetyl)2-(epsilon-Lys)-CO-(CH2)18-
CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 216
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 217
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)14-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 217
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 218
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dab
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 218
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 219
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dap
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 219
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 220
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-Amino-ethoxy)-ethoxy]-acetyl)-(gamma-Glu)-(2-[2-(2-Amino
-ethoxy)-ethoxy]-acetyl)-CO-(CH2)18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 220
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 221
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 221
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 222
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 222
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 223
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-CO-(CH2)16-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 223
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 224
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-CO-(CH2)18-CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 224
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 225
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(epsilon-Lys)-CO-(CH2)18-
CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 225
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 226
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dab
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 226
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 227
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dap
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 227
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 228
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 228
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 229
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 229
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 230
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 230
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 231
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 231
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 232
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 232
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 233
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 233
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 234
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 234
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 235
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 235
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 236
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 236
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 237
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 237
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 238
<211> 38
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (12)..(12)
<223> Xaa at position 12 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (16)..(16)
<223> Lys at position 16 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (19)..(19)
<223> Xaa at position 19 is Aib
<220>
<221> MISC_FEATURE
<222> (23)..(23)
<223> Xaa at position 23 is D-Glu
<220>
<221> MOD_RES
<222> (38)..(38)
<223> Ser at position 39 is amidated
<400> 238
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Xaa Leu Asp Glu Lys
1 5 10 15
Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser Ser
20 25 30
Gly Ala Pro Pro Pro Ser
35
<210> 239
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 239
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp His
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 240
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 240
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 241
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 241
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 242
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dab
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 242
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 243
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dab
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 243
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Leu Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 244
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dab
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 244
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 245
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 245
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 246
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 246
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Ala Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 247
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 247
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Leu Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 248
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 248
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 249
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 249
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 250
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 250
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Val Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 251
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 251
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Val Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 252
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 252
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Leu Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 253
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 253
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Leu Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 254
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 254
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Ala Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 255
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 255
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Ala Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 256
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is Aib
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 256
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 257
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 257
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 258
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is Nle
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 258
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 259
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is Aib
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 259
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 260
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(epsilon-Lys)-CO-(CH2)18-
CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 260
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 261
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(epsilon-Lys)-CO-(CH2)18-
CO2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 261
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 262
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 262
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Ala Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 263
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 263
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 264
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 264
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln His Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 265
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 265
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Lys Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 266
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 266
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Arg Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 267
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 267
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Lys Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 268
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 268
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 269
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 269
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp His Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 270
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 270
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Glu Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 271
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 271
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Thr Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 272
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 272
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Ser Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 273
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 273
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 274
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 13 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 274
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly
20 25 30
<210> 275
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 275
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 276
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 276
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 277
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 277
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 278
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 278
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 279
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 279
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 280
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 280
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 281
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 281
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 282
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 282
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly
20 25 30
<210> 283
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 283
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Pro Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 284
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 284
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Pro Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 285
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 285
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 286
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 286
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Gln Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 287
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (40)..(40)
<223> Cys at position 40 is amidated
<400> 287
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser Cys
35 40
<210> 288
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (40)..(40)
<223> Cys at position 40 is amidated
<400> 288
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Gln Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser Cys
35 40
<210> 289
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 289
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 290
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 290
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Gln Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 291
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (40)..(40)
<223> Cys at position 40 is amidated
<400> 291
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser Cys
35 40
<210> 292
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (40)..(40)
<223> Cys at position 40 is amidated
<400> 292
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Gln Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser Cys
35 40
<210> 293
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 293
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 294
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> mod
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 294
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Gln Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 295
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (40)..(40)
<223> Cys at position 40 is amidated
<400> 295
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser Cys
35 40
<210> 296
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (10)..(10)
<223> Xaa at position 10 is 4Pal
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (40)..(40)
<223> Cys at position 40 is amidated
<400> 296
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Xaa Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Gln Ala Gln Xaa Glu Phe Ile Xaa Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser Cys
35 40
<210> 297
<211> 4
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MOD_RES
<222> (4)..(4)
<223> The C-terminal Gly is modified with R2 to provide PSSG-R2,
wherein the modification is NH2 or absent.
<400> 297
Pro Ser Ser Gly
1
<210> 298
<211> 9
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MOD_RES
<222> (9)..(9)
<223> The C-terminal Ser is modified with R2 to provide PSSGAPPPS-R2,
wherein the modification is NH2 or absent.
<400> 298
Pro Ser Ser Gly Ala Pro Pro Pro Ser
1 5
<210> 299
<211> 4
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<400> 299
Pro Ser Ser Gly
1
<210> 300
<211> 4
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MOD_RES
<222> (4)..(4)
<223> Gly at position 4 is amidated
<400> 300
Pro Ser Ser Gly
1
<210> 301
<211> 9
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<400> 301
Pro Ser Ser Gly Ala Pro Pro Pro Ser
1 5
<210> 302
<211> 9
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MOD_RES
<222> (9)..(9)
<223> Ser at position 9 is amidated
<400> 302
Pro Ser Ser Gly Ala Pro Pro Pro Ser
1 5
<210> 303
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 303
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 304
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 304
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 305
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 305
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 306
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 306
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 307
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 307
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 308
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 308
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 309
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 309
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Gln Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 310
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 310
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln His Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly
20 25 30
<210> 311
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 311
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Lys Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 312
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (30)..(30)
<223> Gly at position 30 is amidated
<400> 312
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly
20 25 30
<210> 313
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 313
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Val Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 314
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 314
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Leu Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 315
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 315
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 316
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 316
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 317
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 317
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 318
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 318
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 319
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 319
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 320
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 320
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 321
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 321
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 322
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 322
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 323
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 323
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 324
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 324
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 325
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 325
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 326
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 326
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 327
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 327
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 328
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 328
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 329
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 329
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 330
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 330
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 331
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 331
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 332
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 332
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 333
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 333
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 334
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 334
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 335
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 335
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Asp Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 336
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 336
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Thr Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 337
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 337
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa His Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 338
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 338
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 339
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 339
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 340
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 340
Tyr Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 341
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 341
Phe Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 342
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 342
Phe Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 343
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 343
Phe Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Ala Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 344
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 344
Xaa Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Leu Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 345
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 345
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Leu Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 346
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 346
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 347
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 2 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 347
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 348
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 348
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 349
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 349
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 350
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 350
Xaa Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 351
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe(2F)
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 351
Xaa Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 352
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 352
Phe Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 353
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 353
Phe Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 354
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(gamma-Glu)-CO-(CH2)18-CO2
H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 354
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 355
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 355
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 356
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 356
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 357
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 357
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 358
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 358
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 359
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 359
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Asp Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 360
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (6)..(6)
<223> Xaa at position 6 is alpha-methyl-Phe
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 360
Xaa Xaa Glu Gly Thr Xaa Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 361
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 361
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 362
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dab
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 362
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 363
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dap
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 363
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 364
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 364
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 365
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dab
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 365
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 366
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Dap
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 366
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 367
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 367
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 368
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 368
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 369
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 369
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 370
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 370
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 371
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 371
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 372
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 372
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 373
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 373
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Val Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 374
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 374
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Val Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 375
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 375
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Val Glu Tyr Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 376
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 376
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Val Xaa Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 377
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 377
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Val Xaa Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 378
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 378
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Xaa Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 379
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (24)..(24)
<223> Xaa at position 24 is D-Glu
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 379
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Val Xaa Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 380
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 380
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 381
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 381
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 382
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 382
Tyr Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 383
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 383
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 384
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 384
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Asp Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 385
<211> 34
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (34)..(34)
<223> Gly at position 34 is amidated
<400> 385
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly
<210> 386
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(gamma-Glu)-CO-(CH2)18-CO2
H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 386
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 387
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(gamma-Glu)-CO-(CH2)18-CO2
H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 387
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 388
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(gamma-Glu)-CO-(CH2)18-CO2
H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 388
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 389
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 389
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 390
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 390
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 391
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 391
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 392
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)16-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 392
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 393
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)-(gamma-Glu)-CO-(CH2)18-CO2
H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 393
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Tyr Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 394
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 394
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Lys
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 395
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MISC_FEATURE
<222> (16)..(16)
<223> Xaa at position 16 is Orn
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 395
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Xaa
1 5 10 15
Lys Ala Gln Xaa Glu Phe Ile Glu Xaa Leu Ile Glu Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35
<210> 396
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Construct
<220>
<221> MISC_FEATURE
<222> (1)..(1)
<223> Xaa at position 1 is D-Tyr
<220>
<221> MISC_FEATURE
<222> (2)..(2)
<223> Xaa at position 2 is Aib
<220>
<221> MISC_FEATURE
<222> (13)..(13)
<223> Xaa at position 13 is alpha-methyl-Leu
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Lys at position 17 is chemically modified by conjugation of the
epsilon-amino group of the Lys side chain with
(2-[2-(2-amino-ethoxy)-ethoxy]-acetyl)2-(gamma-Glu)-CO-(CH2)18-CO
2H
<220>
<221> MISC_FEATURE
<222> (20)..(20)
<223> Xaa at position 20 is Aib
<220>
<221> MISC_FEATURE
<222> (25)..(25)
<223> Xaa at position 25 is alpha-methyl-Tyr
<220>
<221> MOD_RES
<222> (39)..(39)
<223> Ser at position 39 is amidated
<400> 396
Xaa Xaa Glu Gly Thr Phe Thr Ser Asp Tyr Ser Ile Xaa Leu Asp Glu
1 5 10 15
Lys Ala Gln Xaa Ala Phe Ile Glu Xaa Leu Ile Ala Gly Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
35