KR20200116168A - 잔주름 치료를 위한 진피 필러 조성물 - Google Patents
잔주름 치료를 위한 진피 필러 조성물 Download PDFInfo
- Publication number
- KR20200116168A KR20200116168A KR1020207027527A KR20207027527A KR20200116168A KR 20200116168 A KR20200116168 A KR 20200116168A KR 1020207027527 A KR1020207027527 A KR 1020207027527A KR 20207027527 A KR20207027527 A KR 20207027527A KR 20200116168 A KR20200116168 A KR 20200116168A
- Authority
- KR
- South Korea
- Prior art keywords
- gel
- composition
- months
- vitamin
- skin
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 373
- 239000000945 filler Substances 0.000 title claims abstract description 88
- 230000002500 effect on skin Effects 0.000 title claims abstract description 87
- 229920002674 hyaluronan Polymers 0.000 claims abstract description 244
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 claims abstract description 197
- 229960003160 hyaluronic acid Drugs 0.000 claims abstract description 194
- 230000037303 wrinkles Effects 0.000 claims abstract description 45
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 claims description 111
- MLSJBGYKDYSOAE-DCWMUDTNSA-N L-Ascorbic acid-2-glucoside Chemical group OC[C@H](O)[C@H]1OC(=O)C(O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)O)=C1O MLSJBGYKDYSOAE-DCWMUDTNSA-N 0.000 claims description 93
- 238000000034 method Methods 0.000 claims description 70
- 230000021615 conjugation Effects 0.000 claims description 61
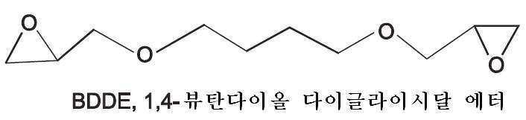
- SHKUUQIDMUMQQK-UHFFFAOYSA-N 2-[4-(oxiran-2-ylmethoxy)butoxymethyl]oxirane Chemical compound C1OC1COCCCCOCC1CO1 SHKUUQIDMUMQQK-UHFFFAOYSA-N 0.000 claims description 40
- -1 ascorbyl 3-aminopropyl phosphate Chemical compound 0.000 claims description 29
- 150000003700 vitamin C derivatives Chemical class 0.000 claims description 29
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 claims description 28
- 229930003268 Vitamin C Natural products 0.000 claims description 28
- 235000019154 vitamin C Nutrition 0.000 claims description 28
- 239000011718 vitamin C Substances 0.000 claims description 28
- 210000004872 soft tissue Anatomy 0.000 claims description 25
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical group CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 claims description 20
- 229960004194 lidocaine Drugs 0.000 claims description 20
- YRWWOAFMPXPHEJ-OFBPEYICSA-K sodium L-ascorbic acid 2-phosphate Chemical compound [Na+].[Na+].[Na+].OC[C@H](O)[C@H]1OC(=O)C(OP([O-])([O-])=O)=C1[O-] YRWWOAFMPXPHEJ-OFBPEYICSA-K 0.000 claims description 20
- 239000003193 general anesthetic agent Substances 0.000 claims description 15
- 150000002118 epoxides Chemical class 0.000 claims description 13
- 229940048058 sodium ascorbyl phosphate Drugs 0.000 claims description 11
- 238000001727 in vivo Methods 0.000 claims description 7
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 4
- 239000004472 Lysine Substances 0.000 claims description 4
- 230000003444 anaesthetic effect Effects 0.000 claims description 4
- PLDLPVSQYMQDBL-UHFFFAOYSA-N 2-[[3-(oxiran-2-ylmethoxy)-2,2-bis(oxiran-2-ylmethoxymethyl)propoxy]methyl]oxirane Chemical compound C1OC1COCC(COCC1OC1)(COCC1OC1)COCC1CO1 PLDLPVSQYMQDBL-UHFFFAOYSA-N 0.000 claims description 3
- 208000032544 Cicatrix Diseases 0.000 claims description 3
- 231100000241 scar Toxicity 0.000 claims description 3
- 230000037387 scars Effects 0.000 claims description 3
- XPOWTVGPJVIVSX-UHFFFAOYSA-N 2-(3-aminopropoxymethyl)-2-(hydroxymethyl)propane-1,3-diol Chemical compound NCCCOCC(CO)(CO)CO XPOWTVGPJVIVSX-UHFFFAOYSA-N 0.000 claims description 2
- 238000003776 cleavage reaction Methods 0.000 claims description 2
- 230000002255 enzymatic effect Effects 0.000 claims description 2
- 230000007017 scission Effects 0.000 claims description 2
- 239000000017 hydrogel Substances 0.000 abstract description 157
- 230000005923 long-lasting effect Effects 0.000 abstract description 3
- 239000000499 gel Substances 0.000 description 223
- 229920000642 polymer Polymers 0.000 description 146
- 210000003491 skin Anatomy 0.000 description 106
- 229920002683 Glycosaminoglycan Polymers 0.000 description 71
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 60
- 239000000243 solution Substances 0.000 description 53
- 239000002953 phosphate buffered saline Substances 0.000 description 52
- KIUKXJAPPMFGSW-MNSSHETKSA-N hyaluronan Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)C1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H](C(O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-MNSSHETKSA-N 0.000 description 50
- 229940099552 hyaluronan Drugs 0.000 description 50
- 230000000694 effects Effects 0.000 description 45
- 206010040954 Skin wrinkling Diseases 0.000 description 44
- 239000000872 buffer Substances 0.000 description 42
- 239000000654 additive Substances 0.000 description 40
- 238000000502 dialysis Methods 0.000 description 37
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 33
- 238000004132 cross linking Methods 0.000 description 30
- 238000002347 injection Methods 0.000 description 29
- 239000007924 injection Substances 0.000 description 29
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 28
- 229960005070 ascorbic acid Drugs 0.000 description 27
- 239000003795 chemical substances by application Substances 0.000 description 27
- 235000010323 ascorbic acid Nutrition 0.000 description 26
- 239000011668 ascorbic acid Substances 0.000 description 26
- 239000003431 cross linking reagent Substances 0.000 description 26
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Substances CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 23
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 22
- NAQMVNRVTILPCV-UHFFFAOYSA-N hexane-1,6-diamine Chemical compound NCCCCCCN NAQMVNRVTILPCV-UHFFFAOYSA-N 0.000 description 22
- MFCMBWRHOUCXEZ-CAHLUQPWSA-N 3-aminopropyl [(2r)-2-[(1s)-1,2-dihydroxyethyl]-3-hydroxy-5-oxo-2h-furan-4-yl] hydrogen phosphate Chemical compound NCCCOP(O)(=O)OC1=C(O)[C@@H]([C@@H](O)CO)OC1=O MFCMBWRHOUCXEZ-CAHLUQPWSA-N 0.000 description 21
- 238000001125 extrusion Methods 0.000 description 20
- 238000006243 chemical reaction Methods 0.000 description 19
- 210000004207 dermis Anatomy 0.000 description 19
- 150000003839 salts Chemical class 0.000 description 19
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 18
- 230000000996 additive effect Effects 0.000 description 18
- 238000009472 formulation Methods 0.000 description 18
- 230000002829 reductive effect Effects 0.000 description 18
- KXCLCNHUUKTANI-RBIYJLQWSA-N keratan Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@H](COS(O)(=O)=O)O[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@H](O[C@@H](O[C@H]3[C@H]([C@@H](COS(O)(=O)=O)O[C@@H](O)[C@@H]3O)O)[C@H](NC(C)=O)[C@H]2O)COS(O)(=O)=O)O[C@H](COS(O)(=O)=O)[C@@H]1O KXCLCNHUUKTANI-RBIYJLQWSA-N 0.000 description 17
- 230000007547 defect Effects 0.000 description 16
- 238000010438 heat treatment Methods 0.000 description 16
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 15
- 229920001287 Chondroitin sulfate Polymers 0.000 description 15
- 229940059329 chondroitin sulfate Drugs 0.000 description 15
- 238000002845 discoloration Methods 0.000 description 15
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 14
- 229920000288 Keratan sulfate Polymers 0.000 description 14
- 201000010099 disease Diseases 0.000 description 14
- 208000035475 disorder Diseases 0.000 description 14
- 150000004676 glycans Chemical class 0.000 description 14
- 229920001282 polysaccharide Polymers 0.000 description 14
- 239000005017 polysaccharide Substances 0.000 description 14
- 239000007987 MES buffer Substances 0.000 description 13
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 13
- 230000008859 change Effects 0.000 description 13
- 230000001815 facial effect Effects 0.000 description 13
- 238000003860 storage Methods 0.000 description 13
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 12
- 239000000463 material Substances 0.000 description 12
- 238000002360 preparation method Methods 0.000 description 12
- ZPUCINDJVBIVPJ-LJISPDSOSA-N cocaine Chemical compound O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-LJISPDSOSA-N 0.000 description 11
- 210000002615 epidermis Anatomy 0.000 description 11
- 238000012360 testing method Methods 0.000 description 11
- 229920002971 Heparan sulfate Polymers 0.000 description 10
- 239000012530 fluid Substances 0.000 description 10
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 9
- 239000002537 cosmetic Substances 0.000 description 9
- AVJBPWGFOQAPRH-FWMKGIEWSA-L dermatan sulfate Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](C([O-])=O)O1 AVJBPWGFOQAPRH-FWMKGIEWSA-L 0.000 description 9
- 229920001223 polyethylene glycol Polymers 0.000 description 9
- 230000001954 sterilising effect Effects 0.000 description 9
- 238000004659 sterilization and disinfection Methods 0.000 description 9
- 229920000045 Dermatan sulfate Polymers 0.000 description 8
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 8
- 239000003589 local anesthetic agent Substances 0.000 description 8
- 238000002156 mixing Methods 0.000 description 8
- FPIPGXGPPPQFEQ-UHFFFAOYSA-N 13-cis retinol Natural products OCC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-UHFFFAOYSA-N 0.000 description 7
- 238000010586 diagram Methods 0.000 description 7
- 150000002016 disaccharides Chemical group 0.000 description 7
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 7
- 239000000126 substance Substances 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- 206010013786 Dry skin Diseases 0.000 description 6
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 6
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 6
- 229940051593 dermatan sulfate Drugs 0.000 description 6
- 238000002513 implantation Methods 0.000 description 6
- 239000010410 layer Substances 0.000 description 6
- 230000007774 longterm Effects 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 6
- 229960003471 retinol Drugs 0.000 description 6
- 235000020944 retinol Nutrition 0.000 description 6
- 239000011607 retinol Substances 0.000 description 6
- 230000035882 stress Effects 0.000 description 6
- 208000024891 symptom Diseases 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 229960001727 tretinoin Drugs 0.000 description 6
- 230000000007 visual effect Effects 0.000 description 6
- 108010035532 Collagen Proteins 0.000 description 5
- 102000008186 Collagen Human genes 0.000 description 5
- 108010014258 Elastin Proteins 0.000 description 5
- 102000016942 Elastin Human genes 0.000 description 5
- 102100024295 Maltase-glucoamylase Human genes 0.000 description 5
- 208000031439 Striae Distensae Diseases 0.000 description 5
- 210000000577 adipose tissue Anatomy 0.000 description 5
- 108010028144 alpha-Glucosidases Proteins 0.000 description 5
- 210000004204 blood vessel Anatomy 0.000 description 5
- 210000000481 breast Anatomy 0.000 description 5
- 210000004027 cell Anatomy 0.000 description 5
- 229920001436 collagen Polymers 0.000 description 5
- 229920002549 elastin Polymers 0.000 description 5
- 238000011156 evaluation Methods 0.000 description 5
- 210000001061 forehead Anatomy 0.000 description 5
- 230000036571 hydration Effects 0.000 description 5
- 238000006703 hydration reaction Methods 0.000 description 5
- 239000007943 implant Substances 0.000 description 5
- 239000004615 ingredient Substances 0.000 description 5
- 239000011159 matrix material Substances 0.000 description 5
- 239000000178 monomer Substances 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 5
- 230000002688 persistence Effects 0.000 description 5
- 229960004919 procaine Drugs 0.000 description 5
- 108090000765 processed proteins & peptides Proteins 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 230000002459 sustained effect Effects 0.000 description 5
- MSWZFWKMSRAUBD-UHFFFAOYSA-N 2-Amino-2-Deoxy-Hexose Chemical compound NC1C(O)OC(CO)C(O)C1O MSWZFWKMSRAUBD-UHFFFAOYSA-N 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 description 4
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 4
- 229920001954 Restylane Polymers 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- AEMOLEFTQBMNLQ-WAXACMCWSA-N alpha-D-glucuronic acid Chemical compound O[C@H]1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-WAXACMCWSA-N 0.000 description 4
- 230000009286 beneficial effect Effects 0.000 description 4
- 229920002678 cellulose Polymers 0.000 description 4
- 210000002808 connective tissue Anatomy 0.000 description 4
- 230000008030 elimination Effects 0.000 description 4
- 238000003379 elimination reaction Methods 0.000 description 4
- 210000002950 fibroblast Anatomy 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 125000000524 functional group Chemical group 0.000 description 4
- YECIFGHRMFEPJK-UHFFFAOYSA-N lidocaine hydrochloride monohydrate Chemical compound O.[Cl-].CC[NH+](CC)CC(=O)NC1=C(C)C=CC=C1C YECIFGHRMFEPJK-UHFFFAOYSA-N 0.000 description 4
- 229960005015 local anesthetics Drugs 0.000 description 4
- 239000011777 magnesium Substances 0.000 description 4
- 238000002595 magnetic resonance imaging Methods 0.000 description 4
- 230000003204 osmotic effect Effects 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 239000011148 porous material Substances 0.000 description 4
- 239000011591 potassium Substances 0.000 description 4
- 229910052700 potassium Inorganic materials 0.000 description 4
- 230000035755 proliferation Effects 0.000 description 4
- 230000035484 reaction time Effects 0.000 description 4
- 229930002330 retinoic acid Natural products 0.000 description 4
- 238000007665 sagging Methods 0.000 description 4
- 230000036558 skin tension Effects 0.000 description 4
- 229940088594 vitamin Drugs 0.000 description 4
- 229930003231 vitamin Natural products 0.000 description 4
- 235000013343 vitamin Nutrition 0.000 description 4
- 239000011782 vitamin Substances 0.000 description 4
- LEBVLXFERQHONN-UHFFFAOYSA-N 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide Chemical compound CCCCN1CCCCC1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-UHFFFAOYSA-N 0.000 description 3
- QTGIAADRBBLJGA-UHFFFAOYSA-N Articaine Chemical compound CCCNC(C)C(=O)NC=1C(C)=CSC=1C(=O)OC QTGIAADRBBLJGA-UHFFFAOYSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- YQKAVWCGQQXBGW-UHFFFAOYSA-N Piperocaine Chemical compound CC1CCCCN1CCCOC(=O)C1=CC=CC=C1 YQKAVWCGQQXBGW-UHFFFAOYSA-N 0.000 description 3
- KCLANYCVBBTKTO-UHFFFAOYSA-N Proparacaine Chemical compound CCCOC1=CC=C(C(=O)OCCN(CC)CC)C=C1N KCLANYCVBBTKTO-UHFFFAOYSA-N 0.000 description 3
- CAJIGINSTLKQMM-UHFFFAOYSA-N Propoxycaine Chemical compound CCCOC1=CC(N)=CC=C1C(=O)OCCN(CC)CC CAJIGINSTLKQMM-UHFFFAOYSA-N 0.000 description 3
- 241000700159 Rattus Species 0.000 description 3
- 206010040925 Skin striae Diseases 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 230000032683 aging Effects 0.000 description 3
- 230000009435 amidation Effects 0.000 description 3
- 238000007112 amidation reaction Methods 0.000 description 3
- 229960003831 articaine Drugs 0.000 description 3
- 230000003416 augmentation Effects 0.000 description 3
- 229960003150 bupivacaine Drugs 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 159000000007 calcium salts Chemical class 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 229960002023 chloroprocaine Drugs 0.000 description 3
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 3
- 229960003920 cocaine Drugs 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- AFOSIXZFDONLBT-UHFFFAOYSA-N divinyl sulfone Chemical compound C=CS(=O)(=O)C=C AFOSIXZFDONLBT-UHFFFAOYSA-N 0.000 description 3
- 230000032050 esterification Effects 0.000 description 3
- 238000005886 esterification reaction Methods 0.000 description 3
- 239000000835 fiber Substances 0.000 description 3
- 230000008014 freezing Effects 0.000 description 3
- 238000007710 freezing Methods 0.000 description 3
- 239000003102 growth factor Substances 0.000 description 3
- XPXMKIXDFWLRAA-UHFFFAOYSA-N hydrazinide Chemical compound [NH-]N XPXMKIXDFWLRAA-UHFFFAOYSA-N 0.000 description 3
- 229960004716 idoxuridine Drugs 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 229910052749 magnesium Inorganic materials 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 229960002409 mepivacaine Drugs 0.000 description 3
- INWLQCZOYSRPNW-UHFFFAOYSA-N mepivacaine Chemical compound CN1CCCCC1C(=O)NC1=C(C)C=CC=C1C INWLQCZOYSRPNW-UHFFFAOYSA-N 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 210000002445 nipple Anatomy 0.000 description 3
- 230000037311 normal skin Effects 0.000 description 3
- 230000000704 physical effect Effects 0.000 description 3
- 229960001045 piperocaine Drugs 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 102000004196 processed proteins & peptides Human genes 0.000 description 3
- OVARTBFNCCXQKS-UHFFFAOYSA-N propan-2-one;hydrate Chemical compound O.CC(C)=O OVARTBFNCCXQKS-UHFFFAOYSA-N 0.000 description 3
- 229960003981 proparacaine Drugs 0.000 description 3
- 229950003255 propoxycaine Drugs 0.000 description 3
- 208000017520 skin disease Diseases 0.000 description 3
- 230000037394 skin elasticity Effects 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 238000013268 sustained release Methods 0.000 description 3
- 239000012730 sustained-release form Substances 0.000 description 3
- 229960002372 tetracaine Drugs 0.000 description 3
- GKCBAIGFKIBETG-UHFFFAOYSA-N tetracaine Chemical compound CCCCNC1=CC=C(C(=O)OCCN(C)C)C=C1 GKCBAIGFKIBETG-UHFFFAOYSA-N 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- GOZBHBFUQHMKQB-UHFFFAOYSA-N trimecaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=C(C)C=C1C GOZBHBFUQHMKQB-UHFFFAOYSA-N 0.000 description 3
- 229950002569 trimecaine Drugs 0.000 description 3
- OEANUJAFZLQYOD-CXAZCLJRSA-N (2r,3s,4r,5r,6r)-6-[(2r,3r,4r,5r,6r)-5-acetamido-3-hydroxy-2-(hydroxymethyl)-6-methoxyoxan-4-yl]oxy-4,5-dihydroxy-3-methoxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](OC)O[C@H](CO)[C@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](OC)[C@H](C(O)=O)O1 OEANUJAFZLQYOD-CXAZCLJRSA-N 0.000 description 2
- IAJILQKETJEXLJ-KLVWXMOXSA-N (2s,3r,4r,5r)-2,3,4,5-tetrahydroxy-6-oxohexanoic acid Chemical compound O=C[C@H](O)[C@H](O)[C@@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-KLVWXMOXSA-N 0.000 description 2
- ZKMNUMMKYBVTFN-HNNXBMFYSA-N (S)-ropivacaine Chemical compound CCCN1CCCC[C@H]1C(=O)NC1=C(C)C=CC=C1C ZKMNUMMKYBVTFN-HNNXBMFYSA-N 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- WZSPWMATVLBWRS-UHFFFAOYSA-N 2-(diethylamino)-n-(2,6-dimethylphenyl)acetamide;n-(2-methylphenyl)-2-(propylamino)propanamide Chemical compound CCCNC(C)C(=O)NC1=CC=CC=C1C.CCN(CC)CC(=O)NC1=C(C)C=CC=C1C WZSPWMATVLBWRS-UHFFFAOYSA-N 0.000 description 2
- 229920000936 Agarose Polymers 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 206010003694 Atrophy Diseases 0.000 description 2
- 102000016938 Catalase Human genes 0.000 description 2
- 108010053835 Catalase Proteins 0.000 description 2
- 239000004971 Cross linker Substances 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- VTUSIVBDOCDNHS-UHFFFAOYSA-N Etidocaine Chemical compound CCCN(CC)C(CC)C(=O)NC1=C(C)C=CC=C1C VTUSIVBDOCDNHS-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- IWYRWIUNAVNFPE-UHFFFAOYSA-N Glycidaldehyde Chemical compound O=CC1CO1 IWYRWIUNAVNFPE-UHFFFAOYSA-N 0.000 description 2
- 101000934372 Homo sapiens Macrosialin Proteins 0.000 description 2
- 206010021639 Incontinence Diseases 0.000 description 2
- XDBMXUKHMOFBPJ-ZAFYKAAXSA-N L-ascorbic acid 2-sulfate Chemical compound OC[C@H](O)[C@H]1OC(=O)C(OS(O)(=O)=O)=C1O XDBMXUKHMOFBPJ-ZAFYKAAXSA-N 0.000 description 2
- QAQJMLQRFWZOBN-LAUBAEHRSA-N L-ascorbyl-6-palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](O)[C@H]1OC(=O)C(O)=C1O QAQJMLQRFWZOBN-LAUBAEHRSA-N 0.000 description 2
- 239000011786 L-ascorbyl-6-palmitate Substances 0.000 description 2
- 102100025136 Macrosialin Human genes 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- OVRNDRQMDRJTHS-CBQIKETKSA-N N-Acetyl-D-Galactosamine Chemical compound CC(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-CBQIKETKSA-N 0.000 description 2
- UEEJHVSXFDXPFK-UHFFFAOYSA-N N-dimethylaminoethanol Chemical compound CN(C)CCO UEEJHVSXFDXPFK-UHFFFAOYSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- 229920002385 Sodium hyaluronate Polymers 0.000 description 2
- 208000016247 Soft tissue disease Diseases 0.000 description 2
- 102000009618 Transforming Growth Factors Human genes 0.000 description 2
- 108010009583 Transforming Growth Factors Proteins 0.000 description 2
- 206010046543 Urinary incontinence Diseases 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 150000001412 amines Chemical group 0.000 description 2
- 229940035674 anesthetics Drugs 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 235000006708 antioxidants Nutrition 0.000 description 2
- 235000010385 ascorbyl palmitate Nutrition 0.000 description 2
- 230000037444 atrophy Effects 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- BLFLLBZGZJTVJG-UHFFFAOYSA-N benzocaine Chemical compound CCOC(=O)C1=CC=C(N)C=C1 BLFLLBZGZJTVJG-UHFFFAOYSA-N 0.000 description 2
- 229920000249 biocompatible polymer Polymers 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- 210000000170 cell membrane Anatomy 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 229960001747 cinchocaine Drugs 0.000 description 2
- PUFQVTATUTYEAL-UHFFFAOYSA-N cinchocaine Chemical compound C1=CC=CC2=NC(OCCCC)=CC(C(=O)NCCN(CC)CC)=C21 PUFQVTATUTYEAL-UHFFFAOYSA-N 0.000 description 2
- 230000007012 clinical effect Effects 0.000 description 2
- 231100000263 cytotoxicity test Toxicity 0.000 description 2
- 229960002887 deanol Drugs 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000018044 dehydration Effects 0.000 description 2
- 238000006297 dehydration reaction Methods 0.000 description 2
- 230000002939 deleterious effect Effects 0.000 description 2
- OWQIUQKMMPDHQQ-UHFFFAOYSA-N dimethocaine Chemical compound CCN(CC)CC(C)(C)COC(=O)C1=CC=C(N)C=C1 OWQIUQKMMPDHQQ-UHFFFAOYSA-N 0.000 description 2
- 229950010160 dimethocaine Drugs 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 125000003700 epoxy group Chemical group 0.000 description 2
- 238000006266 etherification reaction Methods 0.000 description 2
- 229960003976 etidocaine Drugs 0.000 description 2
- 210000000744 eyelid Anatomy 0.000 description 2
- 208000021302 gastroesophageal reflux disease Diseases 0.000 description 2
- 229930182470 glycoside Natural products 0.000 description 2
- 238000007490 hematoxylin and eosin (H&E) staining Methods 0.000 description 2
- 150000002402 hexoses Chemical class 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- AEMOLEFTQBMNLQ-CLQWQSTFSA-N l-iduronic acid Chemical compound O[C@H]1O[C@H](C(O)=O)[C@H](O)[C@@H](O)[C@@H]1O AEMOLEFTQBMNLQ-CLQWQSTFSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 210000002414 leg Anatomy 0.000 description 2
- 229960004288 levobupivacaine Drugs 0.000 description 2
- LEBVLXFERQHONN-INIZCTEOSA-N levobupivacaine Chemical compound CCCCN1CCCC[C@H]1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-INIZCTEOSA-N 0.000 description 2
- 229940119319 lidocaine / prilocaine Drugs 0.000 description 2
- AGBQKNBQESQNJD-UHFFFAOYSA-N lipoic acid Chemical compound OC(=O)CCCCC1CCSS1 AGBQKNBQESQNJD-UHFFFAOYSA-N 0.000 description 2
- 159000000003 magnesium salts Chemical class 0.000 description 2
- 238000000694 mesotherapy Methods 0.000 description 2
- VMGAPWLDMVPYIA-HIDZBRGKSA-N n'-amino-n-iminomethanimidamide Chemical compound N\N=C\N=N VMGAPWLDMVPYIA-HIDZBRGKSA-N 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 206010033675 panniculitis Diseases 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 2
- 159000000001 potassium salts Chemical class 0.000 description 2
- 229960001807 prilocaine Drugs 0.000 description 2
- MVFGUOIZUNYYSO-UHFFFAOYSA-N prilocaine Chemical compound CCCNC(C)C(=O)NC1=CC=CC=C1C MVFGUOIZUNYYSO-UHFFFAOYSA-N 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 108090000623 proteins and genes Proteins 0.000 description 2
- 238000004445 quantitative analysis Methods 0.000 description 2
- BOLDJAUMGUJJKM-LSDHHAIUSA-N renifolin D Natural products CC(=C)[C@@H]1Cc2c(O)c(O)ccc2[C@H]1CC(=O)c3ccc(O)cc3O BOLDJAUMGUJJKM-LSDHHAIUSA-N 0.000 description 2
- 229960001549 ropivacaine Drugs 0.000 description 2
- 210000001732 sebaceous gland Anatomy 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 229940010747 sodium hyaluronate Drugs 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- YWIVKILSMZOHHF-QJZPQSOGSA-N sodium;(2s,3s,4s,5r,6r)-6-[(2s,3r,4r,5s,6r)-3-acetamido-2-[(2s,3s,4r,5r,6r)-6-[(2r,3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2- Chemical compound [Na+].CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 YWIVKILSMZOHHF-QJZPQSOGSA-N 0.000 description 2
- 238000004611 spectroscopical analysis Methods 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 210000004304 subcutaneous tissue Anatomy 0.000 description 2
- 210000000106 sweat gland Anatomy 0.000 description 2
- 230000008961 swelling Effects 0.000 description 2
- 230000008719 thickening Effects 0.000 description 2
- 229960001295 tocopherol Drugs 0.000 description 2
- 239000011732 tocopherol Substances 0.000 description 2
- 239000008181 tonicity modifier Substances 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 2
- 239000008154 viscoelastic solution Substances 0.000 description 2
- 150000003722 vitamin derivatives Chemical class 0.000 description 2
- WCDDVEOXEIYWFB-VXORFPGASA-N (2s,3s,4r,5r,6r)-3-[(2s,3r,5s,6r)-3-acetamido-5-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4,5,6-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@@H]1C[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](C(O)=O)O[C@@H](O)[C@H](O)[C@H]1O WCDDVEOXEIYWFB-VXORFPGASA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- LFKLPJRVSHJZPL-UHFFFAOYSA-N 1,2:7,8-diepoxyoctane Chemical compound C1OC1CCCCC1CO1 LFKLPJRVSHJZPL-UHFFFAOYSA-N 0.000 description 1
- WPRAXAOJIODQJR-UHFFFAOYSA-N 1-(3,4-dimethylphenyl)ethanone Chemical compound CC(=O)C1=CC=C(C)C(C)=C1 WPRAXAOJIODQJR-UHFFFAOYSA-N 0.000 description 1
- SXGZJKUKBWWHRA-UHFFFAOYSA-N 2-(N-morpholiniumyl)ethanesulfonate Chemical compound [O-]S(=O)(=O)CC[NH+]1CCOCC1 SXGZJKUKBWWHRA-UHFFFAOYSA-N 0.000 description 1
- ZLMQPGUWYWFPEG-UHFFFAOYSA-N 2-(diethylamino)ethyl 4-amino-2-butoxybenzoate Chemical compound CCCCOC1=CC(N)=CC=C1C(=O)OCCN(CC)CC ZLMQPGUWYWFPEG-UHFFFAOYSA-N 0.000 description 1
- QNIUOGIMJWORNZ-UHFFFAOYSA-N 2-(diethylamino)ethyl 4-butoxybenzoate Chemical compound CCCCOC1=CC=C(C(=O)OCCN(CC)CC)C=C1 QNIUOGIMJWORNZ-UHFFFAOYSA-N 0.000 description 1
- PGMKGZOHRBZSSQ-UHFFFAOYSA-N 2-[2-(oxiran-2-ylmethoxy)ethenoxymethyl]oxirane Chemical group C1OC1COC=COCC1CO1 PGMKGZOHRBZSSQ-UHFFFAOYSA-N 0.000 description 1
- MSWZFWKMSRAUBD-GASJEMHNSA-N 2-amino-2-deoxy-D-galactopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@H](O)[C@@H]1O MSWZFWKMSRAUBD-GASJEMHNSA-N 0.000 description 1
- MSWZFWKMSRAUBD-IVMDWMLBSA-N 2-amino-2-deoxy-D-glucopyranose Chemical compound N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O MSWZFWKMSRAUBD-IVMDWMLBSA-N 0.000 description 1
- PUYOAVGNCWPANW-UHFFFAOYSA-N 2-methylpropyl 4-aminobenzoate Chemical compound CC(C)COC(=O)C1=CC=C(N)C=C1 PUYOAVGNCWPANW-UHFFFAOYSA-N 0.000 description 1
- IOZLWTIARGQJEJ-UHFFFAOYSA-N 3-[3-(3-aminopropoxy)-2,2-bis(3-aminopropoxymethyl)propoxy]propan-1-amine Chemical compound NCCCOCC(COCCCN)(COCCCN)COCCCN IOZLWTIARGQJEJ-UHFFFAOYSA-N 0.000 description 1
- HQFWVSGBVLEQGA-UHFFFAOYSA-N 4-aminobenzoic acid 3-(dibutylamino)propyl ester Chemical compound CCCCN(CCCC)CCCOC(=O)C1=CC=C(N)C=C1 HQFWVSGBVLEQGA-UHFFFAOYSA-N 0.000 description 1
- XOBTWQWSFMZPNQ-UHFFFAOYSA-N 5-(oxiran-2-ylmethyl)-7-oxabicyclo[4.1.0]heptane Chemical compound C1CCC2OC2C1CC1CO1 XOBTWQWSFMZPNQ-UHFFFAOYSA-N 0.000 description 1
- LITUBCVUXPBCGA-WMZHIEFXSA-N Ascorbyl stearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](O)[C@H]1OC(=O)C(O)=C1O LITUBCVUXPBCGA-WMZHIEFXSA-N 0.000 description 1
- 239000004261 Ascorbyl stearate Substances 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 239000004255 Butylated hydroxyanisole Substances 0.000 description 1
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 1
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 1
- 208000035484 Cellulite Diseases 0.000 description 1
- 229920002101 Chitin Polymers 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 101710088194 Dehydrogenase Proteins 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 102400001368 Epidermal growth factor Human genes 0.000 description 1
- 101800003838 Epidermal growth factor Proteins 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 206010064503 Excessive skin Diseases 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 208000034347 Faecal incontinence Diseases 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- DKLKMKYDWHYZTD-UHFFFAOYSA-N Hexylcaine Chemical compound C=1C=CC=CC=1C(=O)OC(C)CNC1CCCCC1 DKLKMKYDWHYZTD-UHFFFAOYSA-N 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 108010003272 Hyaluronate lyase Proteins 0.000 description 1
- 102000001974 Hyaluronidases Human genes 0.000 description 1
- PWKSKIMOESPYIA-BYPYZUCNSA-N L-N-acetyl-Cysteine Chemical compound CC(=O)N[C@@H](CS)C(O)=O PWKSKIMOESPYIA-BYPYZUCNSA-N 0.000 description 1
- 239000002211 L-ascorbic acid Substances 0.000 description 1
- 235000000069 L-ascorbic acid Nutrition 0.000 description 1
- MIJPAVRNWPDMOR-ZAFYKAAXSA-N L-ascorbic acid 2-phosphate Chemical compound OC[C@H](O)[C@H]1OC(=O)C(OP(O)(O)=O)=C1O MIJPAVRNWPDMOR-ZAFYKAAXSA-N 0.000 description 1
- 150000000996 L-ascorbic acids Chemical class 0.000 description 1
- AEMOLEFTQBMNLQ-HNFCZKTMSA-N L-idopyranuronic acid Chemical compound OC1O[C@@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-HNFCZKTMSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- 108010085895 Laminin Proteins 0.000 description 1
- 102000007547 Laminin Human genes 0.000 description 1
- 231100000002 MTT assay Toxicity 0.000 description 1
- 238000000134 MTT assay Methods 0.000 description 1
- 239000012901 Milli-Q water Substances 0.000 description 1
- QPCDCPDFJACHGM-UHFFFAOYSA-N N,N-bis{2-[bis(carboxymethyl)amino]ethyl}glycine Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(=O)O)CCN(CC(O)=O)CC(O)=O QPCDCPDFJACHGM-UHFFFAOYSA-N 0.000 description 1
- SQVRNKJHWKZAKO-PFQGKNLYSA-N N-acetyl-beta-neuraminic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@](O)(C(O)=O)O[C@H]1[C@H](O)[C@H](O)CO SQVRNKJHWKZAKO-PFQGKNLYSA-N 0.000 description 1
- 241000208125 Nicotiana Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- VNQABZCSYCTZMS-UHFFFAOYSA-N Orthoform Chemical compound COC(=O)C1=CC=C(O)C(N)=C1 VNQABZCSYCTZMS-UHFFFAOYSA-N 0.000 description 1
- 206010049752 Peau d'orange Diseases 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 208000019222 Poland syndrome Diseases 0.000 description 1
- 229920002845 Poly(methacrylic acid) Polymers 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 206010063493 Premature ageing Diseases 0.000 description 1
- 208000032038 Premature aging Diseases 0.000 description 1
- 108010050808 Procollagen Proteins 0.000 description 1
- 208000037534 Progressive hemifacial atrophy Diseases 0.000 description 1
- 208000003251 Pruritus Diseases 0.000 description 1
- 206010040829 Skin discolouration Diseases 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229920001492 Tholin Polymers 0.000 description 1
- FPIPGXGPPPQFEQ-BOOMUCAASA-N Vitamin A Natural products OC/C=C(/C)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-BOOMUCAASA-N 0.000 description 1
- 229930003270 Vitamin B Natural products 0.000 description 1
- 229930003316 Vitamin D Natural products 0.000 description 1
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- RFPVXZWXDPIKSD-UHFFFAOYSA-N [2-(diethylamino)-4-methylpentyl] 4-aminobenzoate;methanesulfonic acid Chemical compound CS(O)(=O)=O.CCN(CC)C(CC(C)C)COC(=O)C1=CC=C(N)C=C1 RFPVXZWXDPIKSD-UHFFFAOYSA-N 0.000 description 1
- VPRGXNLHFBBDFS-UHFFFAOYSA-N [3-(diethylamino)-1-phenylpropyl] benzoate Chemical compound C=1C=CC=CC=1C(CCN(CC)CC)OC(=O)C1=CC=CC=C1 VPRGXNLHFBBDFS-UHFFFAOYSA-N 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- 229960004308 acetylcysteine Drugs 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 210000001789 adipocyte Anatomy 0.000 description 1
- IAJILQKETJEXLJ-QTBDOELSSA-N aldehydo-D-glucuronic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-QTBDOELSSA-N 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229950008211 ambucaine Drugs 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000000058 anti acne agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940124340 antiacne agent Drugs 0.000 description 1
- 229940030225 antihemorrhagics Drugs 0.000 description 1
- 210000000040 apocrine gland Anatomy 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- 235000019276 ascorbyl stearate Nutrition 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000003796 beauty Effects 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 229960005274 benzocaine Drugs 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-FPRJBGLDSA-N beta-D-galactose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-FPRJBGLDSA-N 0.000 description 1
- SQVRNKJHWKZAKO-UHFFFAOYSA-N beta-N-Acetyl-D-neuraminic acid Natural products CC(=O)NC1C(O)CC(O)(C(O)=O)OC1C(O)C(O)CO SQVRNKJHWKZAKO-UHFFFAOYSA-N 0.000 description 1
- 229950005028 betoxycaine Drugs 0.000 description 1
- CXYOBRKOFHQONJ-UHFFFAOYSA-N betoxycaine Chemical compound CCCCOC1=CC=C(C(=O)OCCOCCN(CC)CC)C=C1N CXYOBRKOFHQONJ-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 238000012925 biological evaluation Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- VYLDEYYOISNGST-UHFFFAOYSA-N bissulfosuccinimidyl suberate Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)CCCCCCC(=O)ON1C(=O)C(S(O)(=O)=O)CC1=O VYLDEYYOISNGST-UHFFFAOYSA-N 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 229960003369 butacaine Drugs 0.000 description 1
- IUWVALYLNVXWKX-UHFFFAOYSA-N butamben Chemical compound CCCCOC(=O)C1=CC=C(N)C=C1 IUWVALYLNVXWKX-UHFFFAOYSA-N 0.000 description 1
- 229960000400 butamben Drugs 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 229960001290 butanilicaine Drugs 0.000 description 1
- VWYQKFLLGRBICZ-UHFFFAOYSA-N butanilicaine Chemical compound CCCCNCC(=O)NC1=C(C)C=CC=C1Cl VWYQKFLLGRBICZ-UHFFFAOYSA-N 0.000 description 1
- 229960002463 butoxycaine Drugs 0.000 description 1
- 210000001217 buttock Anatomy 0.000 description 1
- 235000019282 butylated hydroxyanisole Nutrition 0.000 description 1
- 229940043253 butylated hydroxyanisole Drugs 0.000 description 1
- CZBZUDVBLSSABA-UHFFFAOYSA-N butylated hydroxyanisole Chemical compound COC1=CC=C(O)C(C(C)(C)C)=C1.COC1=CC=C(O)C=C1C(C)(C)C CZBZUDVBLSSABA-UHFFFAOYSA-N 0.000 description 1
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 1
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 1
- BHRQIJRLOVHRKH-UHFFFAOYSA-L calcium;2-[bis[2-[bis(carboxylatomethyl)amino]ethyl]amino]acetate;hydron Chemical compound [Ca+2].OC(=O)CN(CC(O)=O)CCN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O BHRQIJRLOVHRKH-UHFFFAOYSA-L 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- 238000011088 calibration curve Methods 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 229940105657 catalase Drugs 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000007541 cellular toxicity Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- NEHMKBQYUWJMIP-NJFSPNSNSA-N chloro(114C)methane Chemical compound [14CH3]Cl NEHMKBQYUWJMIP-NJFSPNSNSA-N 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- KXKPYJOVDUMHGS-OSRGNVMNSA-N chondroitin sulfate Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](OS(O)(=O)=O)[C@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](C(O)=O)O1 KXKPYJOVDUMHGS-OSRGNVMNSA-N 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007596 consolidation process Methods 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 239000011243 crosslinked material Substances 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 238000013016 damping Methods 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- GYZLOYUZLJXAJU-UHFFFAOYSA-N diglycidyl ether Chemical compound C1OC1COCC1CO1 GYZLOYUZLJXAJU-UHFFFAOYSA-N 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- AMQDHYXCJCIBQJ-YCWPWOODSA-L disodium;[(2r)-2-[(1s)-1,2-dihydroxyethyl]-3-oxido-5-oxo-2h-furan-4-yl] sulfate Chemical compound [Na+].[Na+].OC[C@H](O)[C@H]1OC(=O)C(OS([O-])(=O)=O)=C1[O-] AMQDHYXCJCIBQJ-YCWPWOODSA-L 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 229940116977 epidermal growth factor Drugs 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 208000002980 facial hemiatrophy Diseases 0.000 description 1
- 229950003499 fibrin Drugs 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 210000004392 genitalia Anatomy 0.000 description 1
- 229960002442 glucosamine Drugs 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 239000002874 hemostatic agent Substances 0.000 description 1
- 229960005388 hexylcaine Drugs 0.000 description 1
- 229920001903 high density polyethylene Polymers 0.000 description 1
- 239000004700 high-density polyethylene Substances 0.000 description 1
- 229940014041 hyaluronate Drugs 0.000 description 1
- 229960002773 hyaluronidase Drugs 0.000 description 1
- DHCUQNSUUYMFGX-UHFFFAOYSA-N hydroxytetracaine Chemical compound CCCCNC1=CC=C(C(=O)OCCN(C)C)C(O)=C1 DHCUQNSUUYMFGX-UHFFFAOYSA-N 0.000 description 1
- 229950000638 hydroxytetracaine Drugs 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 210000004969 inflammatory cell Anatomy 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 239000012212 insulator Substances 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 239000002085 irritant Substances 0.000 description 1
- 210000002510 keratinocyte Anatomy 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 210000001821 langerhans cell Anatomy 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 235000019136 lipoic acid Nutrition 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000002690 local anesthesia Methods 0.000 description 1
- 150000004668 long chain fatty acids Chemical class 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 206010025135 lupus erythematosus Diseases 0.000 description 1
- 210000001365 lymphatic vessel Anatomy 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 229940078752 magnesium ascorbyl phosphate Drugs 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 210000002752 melanocyte Anatomy 0.000 description 1
- 210000000716 merkel cell Anatomy 0.000 description 1
- LJQWYEFHNLTPBZ-UHFFFAOYSA-N metabutoxycaine Chemical compound CCCCOC1=C(N)C=CC=C1C(=O)OCCN(CC)CC LJQWYEFHNLTPBZ-UHFFFAOYSA-N 0.000 description 1
- 229950004316 metabutoxycaine Drugs 0.000 description 1
- ZPUCINDJVBIVPJ-XGUBFFRZSA-N methyl (1s,3s,4s,5r)-3-benzoyloxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate Chemical compound O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-XGUBFFRZSA-N 0.000 description 1
- KPNBUPJZFJCCIQ-LURJTMIESA-N methyl L-lysinate Chemical compound COC(=O)[C@@H](N)CCCCN KPNBUPJZFJCCIQ-LURJTMIESA-N 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 239000003226 mitogen Substances 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 230000003020 moisturizing effect Effects 0.000 description 1
- ACXCKRZOISAYHH-UHFFFAOYSA-N molecular chlorine hydrate Chemical compound O.ClCl ACXCKRZOISAYHH-UHFFFAOYSA-N 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 229950006780 n-acetylglucosamine Drugs 0.000 description 1
- IYFWKMMMFIDAQJ-UHFFFAOYSA-N n-ethyl-n'-[6-(ethyliminomethylideneamino)hexyl]methanediimine Chemical compound CCN=C=NCCCCCCN=C=NCC IYFWKMMMFIDAQJ-UHFFFAOYSA-N 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 210000001640 nerve ending Anatomy 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 229940053973 novocaine Drugs 0.000 description 1
- HKOURKRGAFKVFP-UHFFFAOYSA-N octacaine Chemical compound CCN(CC)C(C)CC(=O)NC1=CC=CC=C1 HKOURKRGAFKVFP-UHFFFAOYSA-N 0.000 description 1
- 229950009333 octacaine Drugs 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 229950006098 orthocaine Drugs 0.000 description 1
- 230000000065 osmolyte Effects 0.000 description 1
- 125000005430 oxychloro group Chemical group 0.000 description 1
- 230000036407 pain Effects 0.000 description 1
- OWWVHQUOYSPNNE-UHFFFAOYSA-N parethoxycaine Chemical compound CCOC1=CC=C(C(=O)OCCN(CC)CC)C=C1 OWWVHQUOYSPNNE-UHFFFAOYSA-N 0.000 description 1
- 229960003899 parethoxycaine Drugs 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- XEBWQGVWTUSTLN-UHFFFAOYSA-M phenylmercury acetate Chemical compound CC(=O)O[Hg]C1=CC=CC=C1 XEBWQGVWTUSTLN-UHFFFAOYSA-M 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 239000008055 phosphate buffer solution Substances 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 230000019612 pigmentation Effects 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920000083 poly(allylamine) Polymers 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920000768 polyamine Polymers 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 150000004804 polysaccharides Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000001103 potassium chloride Substances 0.000 description 1
- 235000011164 potassium chloride Nutrition 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 229960001896 pramocaine Drugs 0.000 description 1
- DQKXQSGTHWVTAD-UHFFFAOYSA-N pramocaine Chemical compound C1=CC(OCCCC)=CC=C1OCCCN1CCOCC1 DQKXQSGTHWVTAD-UHFFFAOYSA-N 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 229950008865 propanocaine Drugs 0.000 description 1
- 239000003586 protic polar solvent Substances 0.000 description 1
- 238000003908 quality control method Methods 0.000 description 1
- 239000002516 radical scavenger Substances 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 125000002523 retinol group Chemical group 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- CQRYARSYNCAZFO-UHFFFAOYSA-N salicyl alcohol Chemical compound OCC1=CC=CC=C1O CQRYARSYNCAZFO-UHFFFAOYSA-N 0.000 description 1
- 230000020341 sensory perception of pain Effects 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 238000004513 sizing Methods 0.000 description 1
- 230000009759 skin aging Effects 0.000 description 1
- 210000004927 skin cell Anatomy 0.000 description 1
- 230000037370 skin discoloration Effects 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 1
- 229940001584 sodium metabisulfite Drugs 0.000 description 1
- 235000010262 sodium metabisulphite Nutrition 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 229940001474 sodium thiosulfate Drugs 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 210000005070 sphincter Anatomy 0.000 description 1
- 238000013223 sprague-dawley female rat Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 210000004003 subcutaneous fat Anatomy 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- 230000036561 sun exposure Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 125000003831 tetrazolyl group Chemical group 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 229960002663 thioctic acid Drugs 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 239000012780 transparent material Substances 0.000 description 1
- HTJNEBVCZXHBNJ-XCTPRCOBSA-H trimagnesium;(2r)-2-[(1s)-1,2-dihydroxyethyl]-3,4-dihydroxy-2h-furan-5-one;diphosphate Chemical compound [Mg+2].[Mg+2].[Mg+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.OC[C@H](O)[C@H]1OC(=O)C(O)=C1O HTJNEBVCZXHBNJ-XCTPRCOBSA-H 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 239000005526 vasoconstrictor agent Substances 0.000 description 1
- 229940124549 vasodilator Drugs 0.000 description 1
- 239000003071 vasodilator agent Substances 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 235000019155 vitamin A Nutrition 0.000 description 1
- 239000011719 vitamin A Substances 0.000 description 1
- 239000011720 vitamin B Substances 0.000 description 1
- 235000019156 vitamin B Nutrition 0.000 description 1
- 235000019166 vitamin D Nutrition 0.000 description 1
- 239000011710 vitamin D Substances 0.000 description 1
- 150000003710 vitamin D derivatives Chemical class 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 229940045997 vitamin a Drugs 0.000 description 1
- 229940046008 vitamin d Drugs 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229960003434 xenysalate Drugs 0.000 description 1
- HLDCSYXMVXILQC-UHFFFAOYSA-N xenysalate Chemical compound CCN(CC)CCOC(=O)C1=CC=CC(C=2C=CC=CC=2)=C1O HLDCSYXMVXILQC-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/20—Polysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/54—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic compound
- A61K47/545—Heterocyclic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/67—Vitamins
- A61K8/676—Ascorbic acid, i.e. vitamin C
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/73—Polysaccharides
- A61K8/735—Mucopolysaccharides, e.g. hyaluronic acid; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/505—Stabilizers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/54—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/08—Anti-ageing preparations
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08B—POLYSACCHARIDES; DERIVATIVES THEREOF
- C08B37/00—Preparation of polysaccharides not provided for in groups C08B1/00 - C08B35/00; Derivatives thereof
- C08B37/006—Heteroglycans, i.e. polysaccharides having more than one sugar residue in the main chain in either alternating or less regular sequence; Gellans; Succinoglycans; Arabinogalactans; Tragacanth or gum tragacanth or traganth from Astragalus; Gum Karaya from Sterculia urens; Gum Ghatti from Anogeissus latifolia; Derivatives thereof
- C08B37/0063—Glycosaminoglycans or mucopolysaccharides, e.g. keratan sulfate; Derivatives thereof, e.g. fucoidan
- C08B37/0072—Hyaluronic acid, i.e. HA or hyaluronan; Derivatives thereof, e.g. crosslinked hyaluronic acid (hylan) or hyaluronates
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J3/00—Processes of treating or compounding macromolecular substances
- C08J3/24—Crosslinking, e.g. vulcanising, of macromolecules
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L5/00—Compositions of polysaccharides or of their derivatives not provided for in groups C08L1/00 or C08L3/00
- C08L5/08—Chitin; Chondroitin sulfate; Hyaluronic acid; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/80—Process related aspects concerning the preparation of the cosmetic composition or the storage or application thereof
- A61K2800/91—Injection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/402—Anaestetics, analgesics, e.g. lidocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/428—Vitamins, e.g. tocopherol, riboflavin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
- A61L2400/06—Flowable or injectable implant compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/34—Materials or treatment for tissue regeneration for soft tissue reconstruction
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2305/00—Characterised by the use of polysaccharides or of their derivatives not provided for in groups C08J2301/00 or C08J2303/00
- C08J2305/08—Chitin; Chondroitin sulfate; Hyaluronic acid; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J3/00—Processes of treating or compounding macromolecular substances
- C08J3/02—Making solutions, dispersions, lattices or gels by other methods than by solution, emulsion or suspension polymerisation techniques
- C08J3/03—Making solutions, dispersions, lattices or gels by other methods than by solution, emulsion or suspension polymerisation techniques in aqueous media
- C08J3/075—Macromolecular gels
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Organic Chemistry (AREA)
- Polymers & Plastics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Birds (AREA)
- Engineering & Computer Science (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Molecular Biology (AREA)
- Gerontology & Geriatric Medicine (AREA)
- Biochemistry (AREA)
- Materials Engineering (AREA)
- Biomedical Technology (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Materials For Medical Uses (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020217032224A KR20210125119A (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161534780P | 2011-09-14 | 2011-09-14 | |
| US61/534,780 | 2011-09-14 | ||
| USPCT/US2012/052125 | 2012-08-23 | ||
| PCT/US2012/052125 WO2013028904A2 (en) | 2011-08-25 | 2012-08-23 | Dermal filler compositions including antioxidants |
| PCT/US2012/055211 WO2013040242A2 (en) | 2011-09-14 | 2012-09-13 | Dermal filler compositions for fine line treatment |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020147009782A Division KR102161861B1 (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020217032224A Division KR20210125119A (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200116168A true KR20200116168A (ko) | 2020-10-08 |
Family
ID=47883970
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207027527A Ceased KR20200116168A (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
| KR1020147009782A Expired - Fee Related KR102161861B1 (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
| KR1020217032224A Abandoned KR20210125119A (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
| KR1020227001920A Withdrawn KR20220013588A (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020147009782A Expired - Fee Related KR102161861B1 (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
| KR1020217032224A Abandoned KR20210125119A (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
| KR1020227001920A Withdrawn KR20220013588A (ko) | 2011-09-14 | 2012-09-13 | 잔주름 치료를 위한 진피 필러 조성물 |
Country Status (9)
Families Citing this family (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2861734B1 (fr) | 2003-04-10 | 2006-04-14 | Corneal Ind | Reticulation de polysaccharides de faible et forte masse moleculaire; preparation d'hydrogels monophasiques injectables; polysaccharides et hydrogels obtenus |
| EP2484387A1 (en) | 2011-02-03 | 2012-08-08 | Q-Med AB | Hyaluronic acid composition |
| AU2012262047A1 (en) | 2011-06-03 | 2014-01-16 | Allergan, Inc. | Dermal filler compositions including antioxidants |
| US20130096081A1 (en) | 2011-06-03 | 2013-04-18 | Allergan, Inc. | Dermal filler compositions |
| US9393263B2 (en) | 2011-06-03 | 2016-07-19 | Allergan, Inc. | Dermal filler compositions including antioxidants |
| US9408797B2 (en) | 2011-06-03 | 2016-08-09 | Allergan, Inc. | Dermal filler compositions for fine line treatment |
| AU2012308503B2 (en) * | 2011-09-14 | 2015-08-13 | Allergan Industrie, Sas | Dermal filler compositions for fine line treatment |
| KR102168028B1 (ko) * | 2011-12-08 | 2020-10-20 | 알러간 인더스트리 에스에이에스 | 진피 필러 조성물 |
| US20140315828A1 (en) * | 2013-04-22 | 2014-10-23 | Allergan, Inc. | Cross-linked silk-hyaluronic acid compositions |
| EP2988755A4 (en) * | 2013-04-25 | 2016-11-30 | Aluron Biopharma Inc | RETICULATED HYALURONIC ACID COMPOSITIONS |
| WO2016128783A1 (en) * | 2015-02-09 | 2016-08-18 | Allergan Industrie Sas | Compositions and methods for improving skin appearance |
| CN107223061A (zh) * | 2015-02-13 | 2017-09-29 | 阿勒根工业有限公司 | 用于雕塑、填充或矫正面部特征例如下巴的植入物 |
| WO2017220622A1 (en) | 2016-06-23 | 2017-12-28 | Galderma S.A. | Cyclodextrin-grafted cross-linked hyaluronic acid complexed with active drug substances and uses thereof |
| AU2017315431B2 (en) | 2016-08-24 | 2020-06-25 | Allergan, Inc. | Co-crosslinked hyaluronic acid-silk fibroin hydrogels for improving tissue graft viability and for soft tissue augmentation |
| US11389563B2 (en) | 2017-06-12 | 2022-07-19 | Advanced Aesthetic Technologies, Inc. | Dermal filler |
| CN111263646A (zh) * | 2017-06-26 | 2020-06-09 | 自然进化公司 | 基于丝-透明质酸的组织填充物及其使用方法 |
| US20230190997A1 (en) | 2017-06-26 | 2023-06-22 | Evolved By Nature, Inc. | Silk-hyaluronic acid based tissue filers and methods of using the same |
| EP3654920A1 (en) * | 2017-07-19 | 2020-05-27 | Dentsply Sirona Inc. | Water-soluble hydrogel-based dental composition and methods of making and using same |
| KR102532037B1 (ko) * | 2017-12-29 | 2023-05-12 | 마텍스 랩 에스.피.에이 | 히알루론산 염기를 가지는 필러를 제조하는 방법 |
| KR101967153B1 (ko) | 2018-04-04 | 2019-04-09 | 윤성준 | 주름과 피부 결손 및 위축성 흉터 개선을 위한 필러 배합 조성물 |
| US20210259943A1 (en) * | 2018-06-15 | 2021-08-26 | Croma-Pharma Gmbh | Stabilized hyaluronic acid |
| CA3124196A1 (en) | 2018-12-19 | 2020-06-25 | Evolved By Nature, Inc. | Silk-hyaluronic acid tissue fillers and methods of making and using the same |
| IT201900006929A1 (it) * | 2019-05-16 | 2020-11-16 | Giuliani Spa | Kit per il trattamento estetico della pelle |
| CN110467689A (zh) * | 2019-09-09 | 2019-11-19 | 山东众山生物科技有限公司 | 一种透明质酸衍生物及其制备方法 |
| CN110907370A (zh) * | 2019-12-04 | 2020-03-24 | 桂林理工大学 | 一种普适性的超灵敏化学与生物比色传感方法 |
| US20230040919A1 (en) * | 2019-12-26 | 2023-02-09 | Allergan, Inc. | Crosslinked ha-collagen hydrogels as dermal fillers |
| CN111249172A (zh) * | 2020-01-15 | 2020-06-09 | 陈勇 | 一种美容注射凝胶及其制备方法 |
| AR128245A1 (es) | 2022-01-11 | 2024-04-10 | Gpq S R L | Nuevos derivados de ácido hialurónico como rellenos innovadores |
| WO2024055132A1 (es) * | 2022-09-12 | 2024-03-21 | Universidad De Talca | Método para la síntesis de un copolímero ramificado de ácido hialurónico y ácido poli(láctico-co-glicólico) |
| KR102782287B1 (ko) | 2022-12-19 | 2025-03-18 | 주식회사 코루파마 | 히알루론산과 아가 또는 아가로오스 복합화 하이드로겔 제조방법, 이로부터 제조된 히알루론산과 아가 또는 아가로오스를 포함하는 하이드로겔 및 이를 포함하는 필러 조성물 |
| EP4450062A1 (en) * | 2023-04-20 | 2024-10-23 | Abiogen Pharma S.p.A. | Composition for the treatment of osteoarthrosis |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110171286A1 (en) | 2010-01-13 | 2011-07-14 | Allergan, Inc. | Hyaluronic acid compositions for dermatological use |
| US20110171311A1 (en) | 2010-01-13 | 2011-07-14 | Allergan Industrie, Sas | Stable hydrogel compositions including additives |
Family Cites Families (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6174999B1 (en) * | 1987-09-18 | 2001-01-16 | Genzyme Corporation | Water insoluble derivatives of polyanionic polysaccharides |
| KR100721752B1 (ko) * | 2000-01-24 | 2007-05-25 | 쿠라레 메디카루 가부시키가이샤 | 수팽윤성 고분자 겔 및 그 제조법 |
| IT1317091B1 (it) * | 2000-02-08 | 2003-05-26 | S F I R Societa Fondaria Ind R | Gel di acido ialuronico cross-linked con l-amminoacidi ol-amminoesteri bifunzionali. |
| FR2811996B1 (fr) | 2000-07-19 | 2003-08-08 | Corneal Ind | Reticulation de polysaccharide(s), preparation d'hydrogel(s) ; polysaccharide(s) et hydrogel(s) obtenus,leurs utilisations |
| WO2004067575A1 (en) * | 2003-01-31 | 2004-08-12 | Biosphere S.P.A. | Water soluble and biocompatible gels of hyaluronic acid cross-linked with bi-functional l-aminoacids or l-aminoesters |
| AU2003206922A1 (en) | 2003-02-19 | 2004-09-09 | Aventis Pharmaceuticals Holdings Inc. | Composition and method for intradermal soft tissue augmentation |
| FR2861734B1 (fr) | 2003-04-10 | 2006-04-14 | Corneal Ind | Reticulation de polysaccharides de faible et forte masse moleculaire; preparation d'hydrogels monophasiques injectables; polysaccharides et hydrogels obtenus |
| US8124120B2 (en) * | 2003-12-22 | 2012-02-28 | Anika Therapeutics, Inc. | Crosslinked hyaluronic acid compositions for tissue augmentation |
| EP1701981B1 (en) * | 2003-12-30 | 2017-06-07 | Genzyme Corporation | Cohesive gels from cross-linked hyaluronan and/or hylan,their preparation and use |
| FR2865737B1 (fr) * | 2004-02-03 | 2006-03-31 | Anteis Sa | Gel reticule biocompatible |
| CA2567532C (en) * | 2004-05-20 | 2013-10-01 | Mentor Corporation | Methods for making injectable polymer hydrogels |
| FR2873379B1 (fr) * | 2004-07-23 | 2008-05-16 | Jerome Asius | Procede de preparation d'acide hyaluronique reticule, acide hyaluronique reticule susceptible d'etre obtenu par ledit procede, implant contenant ledit acide hyaluronique reticule, et son utilisation |
| FR2878444B1 (fr) | 2004-11-30 | 2008-04-25 | Corneal Ind Soc Par Actions Si | Solutions viscoelastiques renfermant du hyaluronate de sodiu et de l'hydroxypropylmethylcellulose, preparation et utilisations |
| AU2006292752A1 (en) * | 2005-09-15 | 2007-03-29 | University Of Utah Research Foundation | Polymeric compositions and methods of making and using thereof |
| US20100273734A1 (en) * | 2006-02-28 | 2010-10-28 | Novozymes Biopolymer A/S | Derivatives of Hyaluronic Acids |
| CN1837265A (zh) * | 2006-04-25 | 2006-09-27 | 江南大学 | 一种透明质酸-羧甲基纤维素复合改性的方法 |
| CN100417666C (zh) * | 2006-09-07 | 2008-09-10 | 重庆大学 | 赖氨酸-壳聚糖树脂及其制备方法 |
| FR2908415B1 (fr) * | 2006-11-10 | 2009-01-23 | Abr Dev Sarl | Acide hyaluronique reticule et son procede de preparation |
| FR2909560B1 (fr) * | 2006-12-06 | 2012-12-28 | Fabre Pierre Dermo Cosmetique | Gel d'acide hyaluronique pour injection intradermique |
| FR2918377B1 (fr) * | 2007-07-05 | 2010-10-08 | Estelle Piron | Gel co-reticule de polysaccharides |
| US8394782B2 (en) * | 2007-11-30 | 2013-03-12 | Allergan, Inc. | Polysaccharide gel formulation having increased longevity |
| CN101918032A (zh) * | 2007-12-21 | 2010-12-15 | 恩希瑟有限公司 | 含有活性物质的交联水凝胶 |
| KR20090110090A (ko) * | 2008-04-17 | 2009-10-21 | 엘지전자 주식회사 | 초음파를 이용한 인듐 셀레나이드 나노화합물의 제조방법및 이를 포함하는 화합물 반도체 태양전지 |
| US8357795B2 (en) | 2008-08-04 | 2013-01-22 | Allergan, Inc. | Hyaluronic acid-based gels including lidocaine |
| CN101961344A (zh) * | 2009-07-23 | 2011-02-02 | 山东省生物药物研究院 | 药物组合物及其应用 |
| IT1395392B1 (it) * | 2009-08-27 | 2012-09-14 | Fidia Farmaceutici | Geli viscoelastici come nuovi filler |
| EP2504025A4 (en) * | 2009-11-23 | 2013-05-01 | Stephen F Olmstead | COMPOSITIONS AND METHODS USING SERRATIA PEPTIDASE FOR INHIBITING AND TREATING BIOFILMS ASSOCIATED WITH PARTICULAR SUFFERING |
| US20110172180A1 (en) * | 2010-01-13 | 2011-07-14 | Allergan Industrie. Sas | Heat stable hyaluronic acid compositions for dermatological use |
| AU2012262047A1 (en) * | 2011-06-03 | 2014-01-16 | Allergan, Inc. | Dermal filler compositions including antioxidants |
| AU2012308503B2 (en) * | 2011-09-14 | 2015-08-13 | Allergan Industrie, Sas | Dermal filler compositions for fine line treatment |
-
2012
- 2012-09-13 AU AU2012308503A patent/AU2012308503B2/en not_active Ceased
- 2012-09-13 JP JP2014530798A patent/JP6125509B2/ja not_active Expired - Fee Related
- 2012-09-13 KR KR1020207027527A patent/KR20200116168A/ko not_active Ceased
- 2012-09-13 KR KR1020147009782A patent/KR102161861B1/ko not_active Expired - Fee Related
- 2012-09-13 KR KR1020217032224A patent/KR20210125119A/ko not_active Abandoned
- 2012-09-13 CN CN201810249654.6A patent/CN108379112A/zh active Pending
- 2012-09-13 EP EP12769798.5A patent/EP2755630A2/en not_active Withdrawn
- 2012-09-13 CN CN201280055657.7A patent/CN104105474B/zh not_active Expired - Fee Related
- 2012-09-13 HK HK14111615.6A patent/HK1198124A1/xx unknown
- 2012-09-13 KR KR1020227001920A patent/KR20220013588A/ko not_active Withdrawn
- 2012-09-13 CA CA2848833A patent/CA2848833C/en active Active
- 2012-09-13 RU RU2014113663A patent/RU2626513C2/ru active
- 2012-09-13 WO PCT/US2012/055211 patent/WO2013040242A2/en unknown
-
2017
- 2017-04-05 JP JP2017075224A patent/JP2017140433A/ja not_active Withdrawn
-
2018
- 2018-11-22 JP JP2018219395A patent/JP2019058692A/ja not_active Withdrawn
-
2021
- 2021-03-23 JP JP2021048584A patent/JP2021100603A/ja active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110171286A1 (en) | 2010-01-13 | 2011-07-14 | Allergan, Inc. | Hyaluronic acid compositions for dermatological use |
| US20110171311A1 (en) | 2010-01-13 | 2011-07-14 | Allergan Industrie, Sas | Stable hydrogel compositions including additives |
Non-Patent Citations (1)
| Title |
|---|
| Park, D.J. et al. 2010, Orthopaedic Proceedings, Vol. 92-B, No. SUPP I |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019058692A (ja) | 2019-04-18 |
| CA2848833C (en) | 2017-04-04 |
| AU2012308503A1 (en) | 2014-04-03 |
| CN104105474A (zh) | 2014-10-15 |
| KR20140096028A (ko) | 2014-08-04 |
| JP2017140433A (ja) | 2017-08-17 |
| KR20220013588A (ko) | 2022-02-04 |
| RU2626513C2 (ru) | 2017-07-28 |
| EP2755630A2 (en) | 2014-07-23 |
| KR20210125119A (ko) | 2021-10-15 |
| WO2013040242A2 (en) | 2013-03-21 |
| AU2012308503B2 (en) | 2015-08-13 |
| CN108379112A (zh) | 2018-08-10 |
| CA2848833A1 (en) | 2013-03-21 |
| JP2021100603A (ja) | 2021-07-08 |
| CN104105474B (zh) | 2018-04-06 |
| HK1198124A1 (en) | 2015-03-13 |
| JP6125509B2 (ja) | 2017-05-10 |
| JP2014526342A (ja) | 2014-10-06 |
| WO2013040242A3 (en) | 2014-03-13 |
| RU2014113663A (ru) | 2015-10-20 |
| KR102161861B1 (ko) | 2020-10-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102161861B1 (ko) | 잔주름 치료를 위한 진피 필러 조성물 | |
| US10994049B2 (en) | Dermal filler compositions for fine line treatment | |
| KR102238406B1 (ko) | 항산화제를 포함하는 피부 충전제 조성물 | |
| KR102168028B1 (ko) | 진피 필러 조성물 | |
| AU2021200516B2 (en) | Dermal filler compositions for fine line treatment |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20200923 Application number text: 1020147009782 Filing date: 20140414 |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20201222 Patent event code: PE09021S01D |
|
| E90F | Notification of reason for final refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Final Notice of Reason for Refusal Patent event date: 20210812 Patent event code: PE09021S02D |
|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20211007 Application number text: 1020147009782 Filing date: 20140414 |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20211019 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20210812 Comment text: Final Notice of Reason for Refusal Patent event code: PE06011S02I Patent event date: 20201222 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |