KR20180098222A - 활성화된 지방산의 치료학적 유효량을 이용한 질환의 예방, 치료 및 역행 - Google Patents
활성화된 지방산의 치료학적 유효량을 이용한 질환의 예방, 치료 및 역행 Download PDFInfo
- Publication number
- KR20180098222A KR20180098222A KR1020187012337A KR20187012337A KR20180098222A KR 20180098222 A KR20180098222 A KR 20180098222A KR 1020187012337 A KR1020187012337 A KR 1020187012337A KR 20187012337 A KR20187012337 A KR 20187012337A KR 20180098222 A KR20180098222 A KR 20180098222A
- Authority
- KR
- South Korea
- Prior art keywords
- nitro
- octadec
- enoic acid
- disease
- milligrams
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 title claims abstract description 55
- 201000010099 disease Diseases 0.000 title claims abstract description 43
- 235000014113 dietary fatty acids Nutrition 0.000 title abstract description 201
- 239000000194 fatty acid Substances 0.000 title abstract description 201
- 229930195729 fatty acid Natural products 0.000 title abstract description 201
- 150000004665 fatty acids Chemical class 0.000 title abstract description 187
- 238000011282 treatment Methods 0.000 title abstract description 75
- 230000002265 prevention Effects 0.000 title description 4
- WRADPCFZZWXOTI-BMRADRMJSA-N (9E)-10-nitrooctadecenoic acid Chemical compound CCCCCCCC\C([N+]([O-])=O)=C/CCCCCCCC(O)=O WRADPCFZZWXOTI-BMRADRMJSA-N 0.000 claims abstract description 265
- 238000000034 method Methods 0.000 claims abstract description 52
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 34
- 201000005206 focal segmental glomerulosclerosis Diseases 0.000 claims abstract description 11
- 231100000854 focal segmental glomerulosclerosis Toxicity 0.000 claims abstract description 11
- 230000000694 effects Effects 0.000 claims description 60
- 206010057190 Respiratory tract infections Diseases 0.000 claims description 39
- 239000002253 acid Substances 0.000 claims description 29
- 208000020832 chronic kidney disease Diseases 0.000 claims description 20
- 208000017169 kidney disease Diseases 0.000 claims description 16
- 206010016654 Fibrosis Diseases 0.000 claims description 15
- 208000009304 Acute Kidney Injury Diseases 0.000 claims description 14
- 206010064911 Pulmonary arterial hypertension Diseases 0.000 claims description 14
- 230000002757 inflammatory effect Effects 0.000 claims description 13
- 206010029164 Nephrotic syndrome Diseases 0.000 claims description 12
- 230000000302 ischemic effect Effects 0.000 claims description 12
- 208000033626 Renal failure acute Diseases 0.000 claims description 11
- 201000011040 acute kidney failure Diseases 0.000 claims description 11
- 230000004761 fibrosis Effects 0.000 claims description 11
- 208000008589 Obesity Diseases 0.000 claims description 10
- 206010061481 Renal injury Diseases 0.000 claims description 10
- 235000020824 obesity Nutrition 0.000 claims description 10
- 230000001684 chronic effect Effects 0.000 claims description 8
- 208000037806 kidney injury Diseases 0.000 claims description 8
- 201000009794 Idiopathic Pulmonary Fibrosis Diseases 0.000 claims description 7
- 206010002026 amyotrophic lateral sclerosis Diseases 0.000 claims description 7
- 208000036971 interstitial lung disease 2 Diseases 0.000 claims description 7
- 208000008338 non-alcoholic fatty liver disease Diseases 0.000 claims description 7
- 206010053219 non-alcoholic steatohepatitis Diseases 0.000 claims description 7
- 208000007342 Diabetic Nephropathies Diseases 0.000 claims description 6
- 208000007056 sickle cell anemia Diseases 0.000 claims description 6
- 208000024172 Cardiovascular disease Diseases 0.000 claims description 5
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 5
- 208000001145 Metabolic Syndrome Diseases 0.000 claims description 5
- 208000001647 Renal Insufficiency Diseases 0.000 claims description 5
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 claims description 5
- 208000033679 diabetic kidney disease Diseases 0.000 claims description 5
- 208000010706 fatty liver disease Diseases 0.000 claims description 5
- 208000027866 inflammatory disease Diseases 0.000 claims description 5
- 238000001990 intravenous administration Methods 0.000 claims description 5
- 201000006370 kidney failure Diseases 0.000 claims description 5
- 230000002438 mitochondrial effect Effects 0.000 claims description 5
- 230000004770 neurodegeneration Effects 0.000 claims description 5
- 208000015122 neurodegenerative disease Diseases 0.000 claims description 5
- 201000001119 neuropathy Diseases 0.000 claims description 5
- 230000007823 neuropathy Effects 0.000 claims description 5
- 208000033808 peripheral neuropathy Diseases 0.000 claims description 5
- 208000005069 pulmonary fibrosis Diseases 0.000 claims description 5
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims description 5
- 206010018364 Glomerulonephritis Diseases 0.000 claims description 4
- 206010009887 colitis Diseases 0.000 claims description 4
- 208000010643 digestive system disease Diseases 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 4
- 238000007920 subcutaneous administration Methods 0.000 claims description 4
- 208000010693 Charcot-Marie-Tooth Disease Diseases 0.000 claims description 2
- 208000018522 Gastrointestinal disease Diseases 0.000 claims description 2
- 206010023421 Kidney fibrosis Diseases 0.000 claims description 2
- 206010040642 Sickle cell anaemia with crisis Diseases 0.000 claims description 2
- 208000028004 allergic respiratory disease Diseases 0.000 claims description 2
- 230000000390 anti-adipogenic effect Effects 0.000 claims description 2
- 208000018685 gastrointestinal system disease Diseases 0.000 claims description 2
- 229940038384 octadecane Drugs 0.000 claims 1
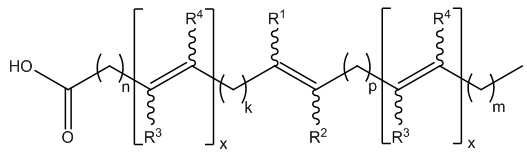
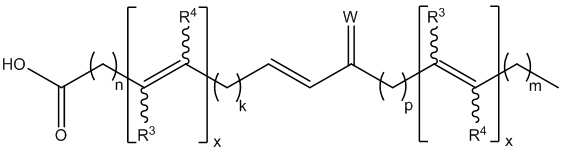
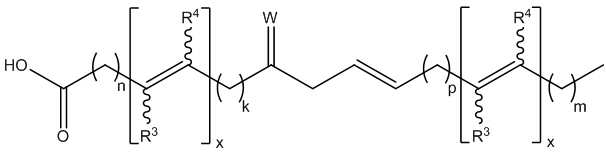
- -1 keto fatty acids Chemical class 0.000 abstract description 72
- 239000000203 mixture Substances 0.000 abstract description 22
- 125000000217 alkyl group Chemical group 0.000 abstract description 19
- 229930194542 Keto Natural products 0.000 abstract description 3
- 208000002815 pulmonary hypertension Diseases 0.000 abstract 1
- 150000001875 compounds Chemical class 0.000 description 71
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 70
- 229910052799 carbon Inorganic materials 0.000 description 60
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 50
- TUZYXOIXSAXUGO-UHFFFAOYSA-N Pravastatin Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(O)C=C21 TUZYXOIXSAXUGO-UHFFFAOYSA-N 0.000 description 38
- 229960002965 pravastatin Drugs 0.000 description 38
- 150000003839 salts Chemical class 0.000 description 37
- TUZYXOIXSAXUGO-PZAWKZKUSA-N pravastatin Chemical compound C1=C[C@H](C)[C@H](CC[C@@H](O)C[C@@H](O)CC(O)=O)[C@H]2[C@@H](OC(=O)[C@@H](C)CC)C[C@H](O)C=C21 TUZYXOIXSAXUGO-PZAWKZKUSA-N 0.000 description 36
- 210000002966 serum Anatomy 0.000 description 36
- 239000003814 drug Substances 0.000 description 35
- PNAMDJVUJCJOIX-IUNFJCKHSA-N [(1s,3r,7s,8s,8ar)-8-[2-[(2r,4r)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate;(3r,4s)-1-(4-fluorophenyl)-3-[(3s)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)C(C)(C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1.N1([C@@H]([C@H](C1=O)CC[C@H](O)C=1C=CC(F)=CC=1)C=1C=CC(O)=CC=1)C1=CC=C(F)C=C1 PNAMDJVUJCJOIX-IUNFJCKHSA-N 0.000 description 33
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 33
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 33
- 108010061435 Enalapril Proteins 0.000 description 32
- GBXSMTUPTTWBMN-XIRDDKMYSA-N enalapril Chemical compound C([C@@H](C(=O)OCC)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(O)=O)CC1=CC=CC=C1 GBXSMTUPTTWBMN-XIRDDKMYSA-N 0.000 description 32
- 229960000873 enalapril Drugs 0.000 description 32
- 229940068196 placebo Drugs 0.000 description 27
- 239000000902 placebo Substances 0.000 description 27
- 238000006243 chemical reaction Methods 0.000 description 26
- 229940079593 drug Drugs 0.000 description 26
- 239000003981 vehicle Substances 0.000 description 26
- 229940109239 creatinine Drugs 0.000 description 25
- 210000002700 urine Anatomy 0.000 description 23
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 22
- 210000003734 kidney Anatomy 0.000 description 22
- 229920001223 polyethylene glycol Polymers 0.000 description 21
- 230000002829 reductive effect Effects 0.000 description 21
- 101710155857 C-C motif chemokine 2 Proteins 0.000 description 20
- 150000001338 aliphatic hydrocarbons Chemical group 0.000 description 19
- DNIAPMSPPWPWGF-UHFFFAOYSA-N monopropylene glycol Natural products CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 19
- 102100021943 C-C motif chemokine 2 Human genes 0.000 description 18
- 238000011156 evaluation Methods 0.000 description 18
- 239000002207 metabolite Substances 0.000 description 18
- 230000001225 therapeutic effect Effects 0.000 description 18
- 229940009349 vytorin Drugs 0.000 description 18
- OTOSPSFTSJOWAM-XBIODWJFSA-N (e)-octadec-9-enoic acid Chemical compound CCCCCCCC\C=C\CCCCCCCC(O)=O.CCCCCCCC\C=C\CCCCCCCC(O)=O.CCCCCCCC\C=C\CCCCCCCC(O)=O OTOSPSFTSJOWAM-XBIODWJFSA-N 0.000 description 17
- 239000000090 biomarker Substances 0.000 description 16
- 239000004359 castor oil Substances 0.000 description 16
- 235000019438 castor oil Nutrition 0.000 description 16
- 230000006378 damage Effects 0.000 description 16
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 15
- 235000020777 polyunsaturated fatty acids Nutrition 0.000 description 15
- 230000009467 reduction Effects 0.000 description 15
- 230000014509 gene expression Effects 0.000 description 14
- 239000000523 sample Substances 0.000 description 14
- 206010012735 Diarrhoea Diseases 0.000 description 13
- 241000699670 Mus sp. Species 0.000 description 13
- 239000002202 Polyethylene glycol Substances 0.000 description 13
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 13
- 210000004027 cell Anatomy 0.000 description 13
- 239000003085 diluting agent Substances 0.000 description 13
- 125000001183 hydrocarbyl group Chemical group 0.000 description 13
- 229940074790 simvastatin and ezetimibe Drugs 0.000 description 13
- 208000035475 disorder Diseases 0.000 description 12
- 125000000524 functional group Chemical group 0.000 description 12
- 210000001519 tissue Anatomy 0.000 description 12
- 241001465754 Metazoa Species 0.000 description 11
- RYMZZMVNJRMUDD-UHFFFAOYSA-N SJ000286063 Natural products C12C(OC(=O)C(C)(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 RYMZZMVNJRMUDD-UHFFFAOYSA-N 0.000 description 11
- 230000008859 change Effects 0.000 description 11
- OLNTVTPDXPETLC-XPWALMASSA-N ezetimibe Chemical compound N1([C@@H]([C@H](C1=O)CC[C@H](O)C=1C=CC(F)=CC=1)C=1C=CC(O)=CC=1)C1=CC=C(F)C=C1 OLNTVTPDXPETLC-XPWALMASSA-N 0.000 description 11
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 11
- 108090000623 proteins and genes Proteins 0.000 description 11
- RYMZZMVNJRMUDD-HGQWONQESA-N simvastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)C(C)(C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 RYMZZMVNJRMUDD-HGQWONQESA-N 0.000 description 11
- 229960002855 simvastatin Drugs 0.000 description 11
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 description 11
- 102000016267 Leptin Human genes 0.000 description 10
- 108010092277 Leptin Proteins 0.000 description 10
- 125000003342 alkenyl group Chemical group 0.000 description 10
- 230000001174 ascending effect Effects 0.000 description 10
- 210000004369 blood Anatomy 0.000 description 10
- 239000008280 blood Substances 0.000 description 10
- 235000012000 cholesterol Nutrition 0.000 description 10
- 125000006575 electron-withdrawing group Chemical group 0.000 description 10
- 230000029142 excretion Effects 0.000 description 10
- 229960000815 ezetimibe Drugs 0.000 description 10
- NRYBAZVQPHGZNS-ZSOCWYAHSA-N leptin Chemical compound O=C([C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(C)C)CCSC)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CS)C(O)=O NRYBAZVQPHGZNS-ZSOCWYAHSA-N 0.000 description 10
- 229940039781 leptin Drugs 0.000 description 10
- 239000000546 pharmaceutical excipient Substances 0.000 description 10
- 125000001424 substituent group Chemical group 0.000 description 10
- 235000015112 vegetable and seed oil Nutrition 0.000 description 10
- 239000008158 vegetable oil Substances 0.000 description 10
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 9
- 229930182558 Sterol Natural products 0.000 description 9
- 150000001336 alkenes Chemical class 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 9
- 125000001246 bromo group Chemical group Br* 0.000 description 9
- 125000004432 carbon atom Chemical group C* 0.000 description 9
- 238000013461 design Methods 0.000 description 9
- 230000003285 pharmacodynamic effect Effects 0.000 description 9
- 150000003432 sterols Chemical class 0.000 description 9
- 235000003702 sterols Nutrition 0.000 description 9
- 235000021122 unsaturated fatty acids Nutrition 0.000 description 9
- 101000588302 Homo sapiens Nuclear factor erythroid 2-related factor 2 Proteins 0.000 description 8
- 102000004889 Interleukin-6 Human genes 0.000 description 8
- 108090001005 Interleukin-6 Proteins 0.000 description 8
- 206010028813 Nausea Diseases 0.000 description 8
- 102100031701 Nuclear factor erythroid 2-related factor 2 Human genes 0.000 description 8
- 229920002472 Starch Polymers 0.000 description 8
- 150000001412 amines Chemical class 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 8
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 8
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 8
- 239000007787 solid Substances 0.000 description 8
- 239000000243 solution Substances 0.000 description 8
- 235000019698 starch Nutrition 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- 206010001580 Albuminuria Diseases 0.000 description 7
- 102000004420 Creatine Kinase Human genes 0.000 description 7
- 108010042126 Creatine kinase Proteins 0.000 description 7
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 7
- 241000700159 Rattus Species 0.000 description 7
- 125000001931 aliphatic group Chemical group 0.000 description 7
- 125000003118 aryl group Chemical group 0.000 description 7
- 230000036772 blood pressure Effects 0.000 description 7
- 230000037396 body weight Effects 0.000 description 7
- 235000013305 food Nutrition 0.000 description 7
- 206010061989 glomerulosclerosis Diseases 0.000 description 7
- 125000000623 heterocyclic group Chemical group 0.000 description 7
- 230000001976 improved effect Effects 0.000 description 7
- 230000006872 improvement Effects 0.000 description 7
- 239000011777 magnesium Substances 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- 230000008693 nausea Effects 0.000 description 7
- 102000004169 proteins and genes Human genes 0.000 description 7
- 238000005070 sampling Methods 0.000 description 7
- 229920006395 saturated elastomer Polymers 0.000 description 7
- 239000008107 starch Substances 0.000 description 7
- 229940032147 starch Drugs 0.000 description 7
- 208000024891 symptom Diseases 0.000 description 7
- 238000004448 titration Methods 0.000 description 7
- 230000002485 urinary effect Effects 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 108010088751 Albumins Proteins 0.000 description 6
- 102000009027 Albumins Human genes 0.000 description 6
- XFXPMWWXUTWYJX-UHFFFAOYSA-N Cyanide Chemical compound N#[C-] XFXPMWWXUTWYJX-UHFFFAOYSA-N 0.000 description 6
- 206010013710 Drug interaction Diseases 0.000 description 6
- 108060006698 EGF receptor Proteins 0.000 description 6
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 6
- 238000006842 Henry reaction Methods 0.000 description 6
- 206010020880 Hypertrophy Diseases 0.000 description 6
- 108010022233 Plasminogen Activator Inhibitor 1 Proteins 0.000 description 6
- 102100039418 Plasminogen activator inhibitor 1 Human genes 0.000 description 6
- 239000004698 Polyethylene Substances 0.000 description 6
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 6
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- ZQPPMHVWECSIRJ-MDZDMXLPSA-N elaidic acid Chemical compound CCCCCCCC\C=C\CCCCCCCC(O)=O ZQPPMHVWECSIRJ-MDZDMXLPSA-N 0.000 description 6
- 125000001072 heteroaryl group Chemical group 0.000 description 6
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 150000002632 lipids Chemical class 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 238000012544 monitoring process Methods 0.000 description 6
- 235000021315 omega 9 monounsaturated fatty acids Nutrition 0.000 description 6
- 239000008188 pellet Substances 0.000 description 6
- 229920000573 polyethylene Polymers 0.000 description 6
- 235000018102 proteins Nutrition 0.000 description 6
- 201000001474 proteinuria Diseases 0.000 description 6
- 230000004044 response Effects 0.000 description 6
- 238000012552 review Methods 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000003786 synthesis reaction Methods 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 5
- 229920001202 Inulin Polymers 0.000 description 5
- 108010007622 LDL Lipoproteins Proteins 0.000 description 5
- 102000007330 LDL Lipoproteins Human genes 0.000 description 5
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 5
- 229910019142 PO4 Inorganic materials 0.000 description 5
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 5
- 125000004429 atom Chemical group 0.000 description 5
- 230000009286 beneficial effect Effects 0.000 description 5
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 5
- 229940092714 benzenesulfonic acid Drugs 0.000 description 5
- 235000021152 breakfast Nutrition 0.000 description 5
- 150000001720 carbohydrates Chemical class 0.000 description 5
- 238000009833 condensation Methods 0.000 description 5
- 230000005494 condensation Effects 0.000 description 5
- 230000003247 decreasing effect Effects 0.000 description 5
- 150000005690 diesters Chemical class 0.000 description 5
- 231100000673 dose–response relationship Toxicity 0.000 description 5
- 230000001434 glomerular Effects 0.000 description 5
- 125000005843 halogen group Chemical group 0.000 description 5
- JYJIGFIDKWBXDU-MNNPPOADSA-N inulin Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)OC[C@]1(OC[C@]2(OC[C@]3(OC[C@]4(OC[C@]5(OC[C@]6(OC[C@]7(OC[C@]8(OC[C@]9(OC[C@]%10(OC[C@]%11(OC[C@]%12(OC[C@]%13(OC[C@]%14(OC[C@]%15(OC[C@]%16(OC[C@]%17(OC[C@]%18(OC[C@]%19(OC[C@]%20(OC[C@]%21(OC[C@]%22(OC[C@]%23(OC[C@]%24(OC[C@]%25(OC[C@]%26(OC[C@]%27(OC[C@]%28(OC[C@]%29(OC[C@]%30(OC[C@]%31(OC[C@]%32(OC[C@]%33(OC[C@]%34(OC[C@]%35(OC[C@]%36(O[C@@H]%37[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O%37)O)[C@H]([C@H](O)[C@@H](CO)O%36)O)[C@H]([C@H](O)[C@@H](CO)O%35)O)[C@H]([C@H](O)[C@@H](CO)O%34)O)[C@H]([C@H](O)[C@@H](CO)O%33)O)[C@H]([C@H](O)[C@@H](CO)O%32)O)[C@H]([C@H](O)[C@@H](CO)O%31)O)[C@H]([C@H](O)[C@@H](CO)O%30)O)[C@H]([C@H](O)[C@@H](CO)O%29)O)[C@H]([C@H](O)[C@@H](CO)O%28)O)[C@H]([C@H](O)[C@@H](CO)O%27)O)[C@H]([C@H](O)[C@@H](CO)O%26)O)[C@H]([C@H](O)[C@@H](CO)O%25)O)[C@H]([C@H](O)[C@@H](CO)O%24)O)[C@H]([C@H](O)[C@@H](CO)O%23)O)[C@H]([C@H](O)[C@@H](CO)O%22)O)[C@H]([C@H](O)[C@@H](CO)O%21)O)[C@H]([C@H](O)[C@@H](CO)O%20)O)[C@H]([C@H](O)[C@@H](CO)O%19)O)[C@H]([C@H](O)[C@@H](CO)O%18)O)[C@H]([C@H](O)[C@@H](CO)O%17)O)[C@H]([C@H](O)[C@@H](CO)O%16)O)[C@H]([C@H](O)[C@@H](CO)O%15)O)[C@H]([C@H](O)[C@@H](CO)O%14)O)[C@H]([C@H](O)[C@@H](CO)O%13)O)[C@H]([C@H](O)[C@@H](CO)O%12)O)[C@H]([C@H](O)[C@@H](CO)O%11)O)[C@H]([C@H](O)[C@@H](CO)O%10)O)[C@H]([C@H](O)[C@@H](CO)O9)O)[C@H]([C@H](O)[C@@H](CO)O8)O)[C@H]([C@H](O)[C@@H](CO)O7)O)[C@H]([C@H](O)[C@@H](CO)O6)O)[C@H]([C@H](O)[C@@H](CO)O5)O)[C@H]([C@H](O)[C@@H](CO)O4)O)[C@H]([C@H](O)[C@@H](CO)O3)O)[C@H]([C@H](O)[C@@H](CO)O2)O)[C@@H](O)[C@H](O)[C@@H](CO)O1 JYJIGFIDKWBXDU-MNNPPOADSA-N 0.000 description 5
- 229940029339 inulin Drugs 0.000 description 5
- 238000006317 isomerization reaction Methods 0.000 description 5
- 229910052749 magnesium Inorganic materials 0.000 description 5
- 229910021645 metal ion Inorganic materials 0.000 description 5
- 239000002480 mineral oil Substances 0.000 description 5
- 125000002950 monocyclic group Chemical group 0.000 description 5
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 239000003921 oil Substances 0.000 description 5
- 235000019198 oils Nutrition 0.000 description 5
- 235000020660 omega-3 fatty acid Nutrition 0.000 description 5
- 235000020665 omega-6 fatty acid Nutrition 0.000 description 5
- 229940033080 omega-6 fatty acid Drugs 0.000 description 5
- 229910052760 oxygen Inorganic materials 0.000 description 5
- 239000001301 oxygen Substances 0.000 description 5
- 235000021317 phosphate Nutrition 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 210000005084 renal tissue Anatomy 0.000 description 5
- 238000007619 statistical method Methods 0.000 description 5
- 239000000758 substrate Substances 0.000 description 5
- 229910052717 sulfur Inorganic materials 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 229940124597 therapeutic agent Drugs 0.000 description 5
- 150000003573 thiols Chemical class 0.000 description 5
- 150000004670 unsaturated fatty acids Chemical class 0.000 description 5
- CQOAKBVRRVHWKV-SAPNQHFASA-N (9E)-9-nitrooctadecenoic acid Chemical compound CCCCCCCC\C=C([N+]([O-])=O)/CCCCCCCC(O)=O CQOAKBVRRVHWKV-SAPNQHFASA-N 0.000 description 4
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 4
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 4
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 4
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 4
- 102000004127 Cytokines Human genes 0.000 description 4
- 108090000695 Cytokines Proteins 0.000 description 4
- 238000002965 ELISA Methods 0.000 description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 4
- 244000068988 Glycine max Species 0.000 description 4
- 235000010469 Glycine max Nutrition 0.000 description 4
- 108010010234 HDL Lipoproteins Proteins 0.000 description 4
- 102000015779 HDL Lipoproteins Human genes 0.000 description 4
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 4
- 108010018924 Heme Oxygenase-1 Proteins 0.000 description 4
- 102000001554 Hemoglobins Human genes 0.000 description 4
- 108010054147 Hemoglobins Proteins 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 4
- 239000005642 Oleic acid Substances 0.000 description 4
- 102000004264 Osteopontin Human genes 0.000 description 4
- 108010081689 Osteopontin Proteins 0.000 description 4
- 102100024616 Platelet endothelial cell adhesion molecule Human genes 0.000 description 4
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 4
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 4
- 102100022748 Wilms tumor protein Human genes 0.000 description 4
- 208000027418 Wounds and injury Diseases 0.000 description 4
- 238000010306 acid treatment Methods 0.000 description 4
- 125000000304 alkynyl group Chemical group 0.000 description 4
- YZXBAPSDXZZRGB-DOFZRALJSA-N arachidonic acid Chemical compound CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O YZXBAPSDXZZRGB-DOFZRALJSA-N 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 4
- 235000014633 carbohydrates Nutrition 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 238000006297 dehydration reaction Methods 0.000 description 4
- 238000009826 distribution Methods 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 238000007046 ethoxylation reaction Methods 0.000 description 4
- 235000019197 fats Nutrition 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 230000024924 glomerular filtration Effects 0.000 description 4
- 239000008103 glucose Substances 0.000 description 4
- 125000001188 haloalkyl group Chemical group 0.000 description 4
- 230000036541 health Effects 0.000 description 4
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- 208000014674 injury Diseases 0.000 description 4
- 150000002500 ions Chemical class 0.000 description 4
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 230000001404 mediated effect Effects 0.000 description 4
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 4
- 210000000056 organ Anatomy 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 4
- 239000010452 phosphate Substances 0.000 description 4
- 239000008389 polyethoxylated castor oil Substances 0.000 description 4
- 230000008092 positive effect Effects 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 239000000651 prodrug Substances 0.000 description 4
- 229940002612 prodrug Drugs 0.000 description 4
- 201000002793 renal fibrosis Diseases 0.000 description 4
- XWLXKKNPFMNSFA-HGQWONQESA-N simvastatin hydroxy acid Chemical compound C1=C[C@H](C)[C@H](CC[C@@H](O)C[C@@H](O)CC(O)=O)[C@H]2[C@@H](OC(=O)C(C)(C)CC)C[C@@H](C)C=C21 XWLXKKNPFMNSFA-HGQWONQESA-N 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 235000010356 sorbitol Nutrition 0.000 description 4
- 239000011593 sulfur Substances 0.000 description 4
- 239000003826 tablet Substances 0.000 description 4
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 4
- UOFYCFMTERCNEW-ADEYADIWSA-N (2s,3s,4s,5r,6s)-6-[4-[(2s,3r)-1-(4-fluorophenyl)-3-[(3s)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-oxoazetidin-2-yl]phenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound N1([C@@H]([C@H](C1=O)CC[C@H](O)C=1C=CC(F)=CC=1)C=1C=CC(O[C@H]2[C@@H]([C@@H](O)[C@H](O)[C@H](O2)C(O)=O)O)=CC=1)C1=CC=C(F)C=C1 UOFYCFMTERCNEW-ADEYADIWSA-N 0.000 description 3
- KBPLFHHGFOOTCA-UHFFFAOYSA-N 1-Octanol Chemical compound CCCCCCCCO KBPLFHHGFOOTCA-UHFFFAOYSA-N 0.000 description 3
- KWVPFECTOKLOBL-KTKRTIGZSA-N 2-[(z)-octadec-9-enoxy]ethanol Chemical compound CCCCCCCC\C=C/CCCCCCCCOCCO KWVPFECTOKLOBL-KTKRTIGZSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 3
- 102000008186 Collagen Human genes 0.000 description 3
- 108010035532 Collagen Proteins 0.000 description 3
- 102000004328 Cytochrome P-450 CYP3A Human genes 0.000 description 3
- 108010081668 Cytochrome P-450 CYP3A Proteins 0.000 description 3
- VPGRYOFKCNULNK-ACXQXYJUSA-N Deoxycorticosterone acetate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)COC(=O)C)[C@@]1(C)CC2 VPGRYOFKCNULNK-ACXQXYJUSA-N 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical group NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- 101000665882 Homo sapiens Retinol-binding protein 4 Proteins 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical class [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- 206010020772 Hypertension Diseases 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- 108010057466 NF-kappa B Proteins 0.000 description 3
- 102000003945 NF-kappa B Human genes 0.000 description 3
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 3
- 238000011529 RT qPCR Methods 0.000 description 3
- 235000021355 Stearic acid Nutrition 0.000 description 3
- VNJBSPJILLFAIC-BZPMIXESSA-N Tetranor-PGDM Chemical compound O[C@H]1CC(=O)[C@H](CCC(=O)CCCCC(O)=O)[C@H]1CCC(O)=O VNJBSPJILLFAIC-BZPMIXESSA-N 0.000 description 3
- 230000005856 abnormality Effects 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 230000000172 allergic effect Effects 0.000 description 3
- DTOSIQBPPRVQHS-PDBXOOCHSA-N alpha-linolenic acid Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCC(O)=O DTOSIQBPPRVQHS-PDBXOOCHSA-N 0.000 description 3
- 230000003110 anti-inflammatory effect Effects 0.000 description 3
- 229940053200 antiepileptics fatty acid derivative Drugs 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 235000006708 antioxidants Nutrition 0.000 description 3
- 208000010668 atopic eczema Diseases 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 235000001465 calcium Nutrition 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 150000001721 carbon Chemical group 0.000 description 3
- 150000001728 carbonyl compounds Chemical class 0.000 description 3
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 3
- 208000022831 chronic renal failure syndrome Diseases 0.000 description 3
- 229920001436 collagen Polymers 0.000 description 3
- 238000006880 cross-coupling reaction Methods 0.000 description 3
- 125000004093 cyano group Chemical group *C#N 0.000 description 3
- 230000018044 dehydration Effects 0.000 description 3
- 229960004486 desoxycorticosterone acetate Drugs 0.000 description 3
- 230000035487 diastolic blood pressure Effects 0.000 description 3
- 230000003511 endothelial effect Effects 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 210000002744 extracellular matrix Anatomy 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 125000005456 glyceride group Chemical group 0.000 description 3
- 125000005842 heteroatom Chemical group 0.000 description 3
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 3
- 238000003364 immunohistochemistry Methods 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 239000003701 inert diluent Substances 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- 125000005956 isoquinolyl group Chemical group 0.000 description 3
- 125000005647 linker group Chemical group 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 238000007449 liver function test Methods 0.000 description 3
- 229960003511 macrogol Drugs 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 235000012054 meals Nutrition 0.000 description 3
- 230000004060 metabolic process Effects 0.000 description 3
- 235000010446 mineral oil Nutrition 0.000 description 3
- 210000003205 muscle Anatomy 0.000 description 3
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 3
- 230000003287 optical effect Effects 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- 229940124531 pharmaceutical excipient Drugs 0.000 description 3
- 230000000144 pharmacologic effect Effects 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- 230000036470 plasma concentration Effects 0.000 description 3
- 229920001451 polypropylene glycol Polymers 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 238000011321 prophylaxis Methods 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 230000036387 respiratory rate Effects 0.000 description 3
- 150000004671 saturated fatty acids Chemical class 0.000 description 3
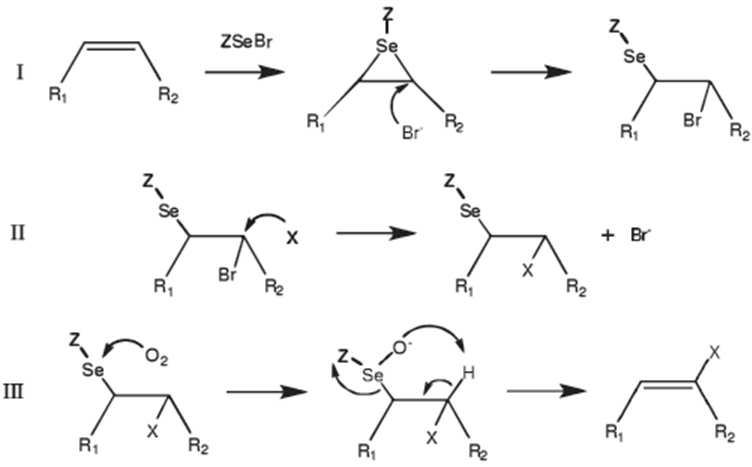
- 229940065287 selenium compound Drugs 0.000 description 3
- 150000003343 selenium compounds Chemical class 0.000 description 3
- 239000008159 sesame oil Substances 0.000 description 3
- 235000011803 sesame oil Nutrition 0.000 description 3
- 230000011664 signaling Effects 0.000 description 3
- 239000008109 sodium starch glycolate Substances 0.000 description 3
- 229920003109 sodium starch glycolate Polymers 0.000 description 3
- 229940079832 sodium starch glycolate Drugs 0.000 description 3
- 239000007909 solid dosage form Substances 0.000 description 3
- 238000013097 stability assessment Methods 0.000 description 3
- 239000008117 stearic acid Substances 0.000 description 3
- 239000000375 suspending agent Substances 0.000 description 3
- 238000010189 synthetic method Methods 0.000 description 3
- 230000009885 systemic effect Effects 0.000 description 3
- 230000035488 systolic blood pressure Effects 0.000 description 3
- 150000003626 triacylglycerols Chemical class 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 description 2
- 125000006648 (C1-C8) haloalkyl group Chemical group 0.000 description 2
- GHOKWGTUZJEAQD-ZETCQYMHSA-N (D)-(+)-Pantothenic acid Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-ZETCQYMHSA-N 0.000 description 2
- PXGPLTODNUVGFL-BRIYLRKRSA-N (E,Z)-(1R,2R,3R,5S)-7-(3,5-Dihydroxy-2-((3S)-(3-hydroxy-1-octenyl))cyclopentyl)-5-heptenoic acid Chemical compound CCCCC[C@H](O)C=C[C@H]1[C@H](O)C[C@H](O)[C@@H]1CC=CCCCC(O)=O PXGPLTODNUVGFL-BRIYLRKRSA-N 0.000 description 2
- CSLMOPHXEZYLSC-DTQAZKPQSA-N (e)-9-methylsulfonylhexadec-9-enoic acid Chemical compound CCCCCC\C=C(S(C)(=O)=O)/CCCCCCCC(O)=O CSLMOPHXEZYLSC-DTQAZKPQSA-N 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- LLVWLCAZSOLOTF-UHFFFAOYSA-N 1-methyl-4-[1,4,4-tris(4-methylphenyl)buta-1,3-dienyl]benzene Chemical group C1=CC(C)=CC=C1C(C=1C=CC(C)=CC=1)=CC=C(C=1C=CC(C)=CC=1)C1=CC=C(C)C=C1 LLVWLCAZSOLOTF-UHFFFAOYSA-N 0.000 description 2
- 125000004206 2,2,2-trifluoroethyl group Chemical group [H]C([H])(*)C(F)(F)F 0.000 description 2
- GJWSUKYXUMVMGX-UHFFFAOYSA-M 3,7-dimethyloct-6-enoate Chemical compound [O-]C(=O)CC(C)CCC=C(C)C GJWSUKYXUMVMGX-UHFFFAOYSA-M 0.000 description 2
- WHBMMWSBFZVSSR-UHFFFAOYSA-N 3-hydroxybutyric acid Chemical compound CC(O)CC(O)=O WHBMMWSBFZVSSR-UHFFFAOYSA-N 0.000 description 2
- KJDSORYAHBAGPP-UHFFFAOYSA-N 4-(3,4-diaminophenyl)benzene-1,2-diamine;hydron;tetrachloride Chemical compound Cl.Cl.Cl.Cl.C1=C(N)C(N)=CC=C1C1=CC=C(N)C(N)=C1 KJDSORYAHBAGPP-UHFFFAOYSA-N 0.000 description 2
- HVBSAKJJOYLTQU-UHFFFAOYSA-N 4-aminobenzenesulfonic acid Chemical compound NC1=CC=C(S(O)(=O)=O)C=C1 HVBSAKJJOYLTQU-UHFFFAOYSA-N 0.000 description 2
- 208000010444 Acidosis Diseases 0.000 description 2
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 2
- 108010082126 Alanine transaminase Proteins 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- 102000015427 Angiotensins Human genes 0.000 description 2
- 108010064733 Angiotensins Proteins 0.000 description 2
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 2
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 2
- 206010006580 Bundle branch block left Diseases 0.000 description 2
- 206010006582 Bundle branch block right Diseases 0.000 description 2
- 206010006578 Bundle-Branch Block Diseases 0.000 description 2
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 2
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 2
- 102100032752 C-reactive protein Human genes 0.000 description 2
- 101100504320 Caenorhabditis elegans mcp-1 gene Proteins 0.000 description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- 206010007572 Cardiac hypertrophy Diseases 0.000 description 2
- 208000006029 Cardiomegaly Diseases 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 229930008398 Citronellate Natural products 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 102000016359 Fibronectins Human genes 0.000 description 2
- 108010067306 Fibronectins Proteins 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 2
- 108020004206 Gamma-glutamyltransferase Proteins 0.000 description 2
- 102100033398 Glutamate-cysteine ligase regulatory subunit Human genes 0.000 description 2
- 229930186217 Glycolipid Natural products 0.000 description 2
- 102100034459 Hepatitis A virus cellular receptor 1 Human genes 0.000 description 2
- 101710185991 Hepatitis A virus cellular receptor 1 homolog Proteins 0.000 description 2
- 101000870644 Homo sapiens Glutamate-cysteine ligase regulatory subunit Proteins 0.000 description 2
- 241000725303 Human immunodeficiency virus Species 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 102000000589 Interleukin-1 Human genes 0.000 description 2
- 108010002352 Interleukin-1 Proteins 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 101000761736 Naja atra Cytotoxin 10 Proteins 0.000 description 2
- 208000013901 Nephropathies and tubular disease Diseases 0.000 description 2
- 238000003527 Peterson olefination reaction Methods 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- 229920002565 Polyethylene Glycol 400 Polymers 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 108010029485 Protein Isoforms Proteins 0.000 description 2
- 102000001708 Protein Isoforms Human genes 0.000 description 2
- LCTONWCANYUPML-UHFFFAOYSA-N Pyruvic acid Chemical compound CC(=O)C(O)=O LCTONWCANYUPML-UHFFFAOYSA-N 0.000 description 2
- 101710149118 Quinone oxidoreductase 1 Proteins 0.000 description 2
- 238000010240 RT-PCR analysis Methods 0.000 description 2
- 206010063837 Reperfusion injury Diseases 0.000 description 2
- 102100038246 Retinol-binding protein 4 Human genes 0.000 description 2
- 108091006735 SLC22A2 Proteins 0.000 description 2
- 238000012300 Sequence Analysis Methods 0.000 description 2
- 108010071390 Serum Albumin Proteins 0.000 description 2
- 102000007562 Serum Albumin Human genes 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 238000000692 Student's t-test Methods 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 2
- 235000009754 Vitis X bourquina Nutrition 0.000 description 2
- 235000012333 Vitis X labruscana Nutrition 0.000 description 2
- 240000006365 Vitis vinifera Species 0.000 description 2
- 235000014787 Vitis vinifera Nutrition 0.000 description 2
- PNNCWTXUWKENPE-UHFFFAOYSA-N [N].NC(N)=O Chemical compound [N].NC(N)=O PNNCWTXUWKENPE-UHFFFAOYSA-N 0.000 description 2
- 206010000059 abdominal discomfort Diseases 0.000 description 2
- 230000007950 acidosis Effects 0.000 description 2
- 208000026545 acidosis disease Diseases 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 208000012998 acute renal failure Diseases 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 229910052783 alkali metal Inorganic materials 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 125000003545 alkoxy group Chemical group 0.000 description 2
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 2
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 239000012491 analyte Substances 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 230000003078 antioxidant effect Effects 0.000 description 2
- 235000021342 arachidonic acid Nutrition 0.000 description 2
- 229940114079 arachidonic acid Drugs 0.000 description 2
- 230000004872 arterial blood pressure Effects 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- JUHORIMYRDESRB-UHFFFAOYSA-N benzathine Chemical compound C=1C=CC=CC=1CNCCNCC1=CC=CC=C1 JUHORIMYRDESRB-UHFFFAOYSA-N 0.000 description 2
- 125000004603 benzisoxazolyl group Chemical group O1N=C(C2=C1C=CC=C2)* 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 125000001164 benzothiazolyl group Chemical group S1C(=NC2=C1C=CC=C2)* 0.000 description 2
- 125000004541 benzoxazolyl group Chemical group O1C(=NC2=C1C=CC=C2)* 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 229910000389 calcium phosphate Inorganic materials 0.000 description 2
- 235000011010 calcium phosphates Nutrition 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 230000000747 cardiac effect Effects 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229960000541 cetyl alcohol Drugs 0.000 description 2
- JQXXHWHPUNPDRT-BQVAUQFYSA-N chembl1523493 Chemical compound O([C@](C1=O)(C)O\C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)/C=C\C=C(C)/C(=O)NC=2C(O)=C3C(O)=C4C)C)OC)C4=C1C3=C(O)C=2C=NN1CCN(C)CC1 JQXXHWHPUNPDRT-BQVAUQFYSA-N 0.000 description 2
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 2
- 229960001231 choline Drugs 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 235000005687 corn oil Nutrition 0.000 description 2
- 239000002285 corn oil Substances 0.000 description 2
- 235000012343 cottonseed oil Nutrition 0.000 description 2
- 239000002385 cottonseed oil Substances 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- 229910003460 diamond Inorganic materials 0.000 description 2
- 239000010432 diamond Substances 0.000 description 2
- 150000001993 dienes Chemical class 0.000 description 2
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 2
- 229940043237 diethanolamine Drugs 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- NOPFSRXAKWQILS-UHFFFAOYSA-N docosan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCCCCCO NOPFSRXAKWQILS-UHFFFAOYSA-N 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 210000003743 erythrocyte Anatomy 0.000 description 2
- 229940012017 ethylenediamine Drugs 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 229940054572 ezetimibe / simvastatin Drugs 0.000 description 2
- 150000002191 fatty alcohols Chemical class 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 229940098330 gamma linoleic acid Drugs 0.000 description 2
- 102000006640 gamma-Glutamyltransferase Human genes 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 239000007943 implant Substances 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 125000001041 indolyl group Chemical group 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 239000007972 injectable composition Substances 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 239000003456 ion exchange resin Substances 0.000 description 2
- 229920003303 ion-exchange polymer Polymers 0.000 description 2
- 208000028867 ischemia Diseases 0.000 description 2
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 2
- 125000002183 isoquinolinyl group Chemical group C1(=NC=CC2=CC=CC=C12)* 0.000 description 2
- 201000008627 kidney hypertrophy Diseases 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 229960004488 linolenic acid Drugs 0.000 description 2
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 2
- 239000008297 liquid dosage form Substances 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 239000006210 lotion Substances 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 229960003194 meglumine Drugs 0.000 description 2
- 150000002730 mercury Chemical class 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- 238000001000 micrograph Methods 0.000 description 2
- 125000001624 naphthyl group Chemical group 0.000 description 2
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 2
- 231100000062 no-observed-adverse-effect level Toxicity 0.000 description 2
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 2
- 239000000346 nonvolatile oil Substances 0.000 description 2
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 235000021354 omega 7 monounsaturated fatty acids Nutrition 0.000 description 2
- 238000003305 oral gavage Methods 0.000 description 2
- KHPXUQMNIQBQEV-UHFFFAOYSA-N oxaloacetic acid Chemical compound OC(=O)CC(=O)C(O)=O KHPXUQMNIQBQEV-UHFFFAOYSA-N 0.000 description 2
- 239000007800 oxidant agent Substances 0.000 description 2
- 230000036542 oxidative stress Effects 0.000 description 2
- SECPZKHBENQXJG-FPLPWBNLSA-N palmitoleic acid Chemical compound CCCCCC\C=C/CCCCCCCC(O)=O SECPZKHBENQXJG-FPLPWBNLSA-N 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 230000007170 pathology Effects 0.000 description 2
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 2
- 150000003904 phospholipids Chemical class 0.000 description 2
- 125000001476 phosphono group Chemical group [H]OP(*)(=O)O[H] 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- 239000006187 pill Substances 0.000 description 2
- 210000000557 podocyte Anatomy 0.000 description 2
- 229940068918 polyethylene glycol 400 Drugs 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 238000004321 preservation Methods 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- 230000001681 protective effect Effects 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 239000000376 reactant Substances 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000036454 renin-angiotensin system Effects 0.000 description 2
- 230000010410 reperfusion Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 229960001225 rifampicin Drugs 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 150000003408 sphingolipids Chemical class 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 230000000707 stereoselective effect Effects 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 2
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 description 2
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 239000008399 tap water Substances 0.000 description 2
- 235000020679 tap water Nutrition 0.000 description 2
- 238000011191 terminal modification Methods 0.000 description 2
- 238000011287 therapeutic dose Methods 0.000 description 2
- 125000001544 thienyl group Chemical group 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 231100000041 toxicology testing Toxicity 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- 125000000876 trifluoromethoxy group Chemical group FC(F)(F)O* 0.000 description 2
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 2
- 238000002562 urinalysis Methods 0.000 description 2
- 230000004584 weight gain Effects 0.000 description 2
- 235000019786 weight gain Nutrition 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- DQJCDTNMLBYVAY-ZXXIYAEKSA-N (2S,5R,10R,13R)-16-{[(2R,3S,4R,5R)-3-{[(2S,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5-(ethylamino)-6-hydroxy-2-(hydroxymethyl)oxan-4-yl]oxy}-5-(4-aminobutyl)-10-carbamoyl-2,13-dimethyl-4,7,12,15-tetraoxo-3,6,11,14-tetraazaheptadecan-1-oic acid Chemical compound NCCCC[C@H](C(=O)N[C@@H](C)C(O)=O)NC(=O)CC[C@H](C(N)=O)NC(=O)[C@@H](C)NC(=O)C(C)O[C@@H]1[C@@H](NCC)C(O)O[C@H](CO)[C@H]1O[C@H]1[C@H](NC(C)=O)[C@@H](O)[C@H](O)[C@@H](CO)O1 DQJCDTNMLBYVAY-ZXXIYAEKSA-N 0.000 description 1
- RUHCWQAFCGVQJX-RVWHZBQESA-N (3s,8s,9s,10r,13r,14s,17r)-3-hydroxy-10,13-dimethyl-17-[(2r)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-1-one Chemical compound C1C=C2C[C@H](O)CC(=O)[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 RUHCWQAFCGVQJX-RVWHZBQESA-N 0.000 description 1
- QYIXCDOBOSTCEI-QCYZZNICSA-N (5alpha)-cholestan-3beta-ol Chemical compound C([C@@H]1CC2)[C@@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@H](C)CCCC(C)C)[C@@]2(C)CC1 QYIXCDOBOSTCEI-QCYZZNICSA-N 0.000 description 1
- YUFFSWGQGVEMMI-JLNKQSITSA-N (7Z,10Z,13Z,16Z,19Z)-docosapentaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCCCC(O)=O YUFFSWGQGVEMMI-JLNKQSITSA-N 0.000 description 1
- ALSTYHKOOCGGFT-KTKRTIGZSA-N (9Z)-octadecen-1-ol Chemical compound CCCCCCCC\C=C/CCCCCCCCO ALSTYHKOOCGGFT-KTKRTIGZSA-N 0.000 description 1
- OYHQOLUKZRVURQ-NTGFUMLPSA-N (9Z,12Z)-9,10,12,13-tetratritiooctadeca-9,12-dienoic acid Chemical compound C(CCCCCCC\C(=C(/C\C(=C(/CCCCC)\[3H])\[3H])\[3H])\[3H])(=O)O OYHQOLUKZRVURQ-NTGFUMLPSA-N 0.000 description 1
- JXNPEDYJTDQORS-HZJYTTRNSA-N (9Z,12Z)-octadecadien-1-ol Chemical compound CCCCC\C=C/C\C=C/CCCCCCCCO JXNPEDYJTDQORS-HZJYTTRNSA-N 0.000 description 1
- IKYKEVDKGZYRMQ-PDBXOOCHSA-N (9Z,12Z,15Z)-octadecatrien-1-ol Chemical compound CC\C=C/C\C=C/C\C=C/CCCCCCCCO IKYKEVDKGZYRMQ-PDBXOOCHSA-N 0.000 description 1
- FDGMTLFOSHMHCC-WYMLVPIESA-N (e)-10-chloropentadec-9-enoic acid Chemical compound CCCCC\C(Cl)=C/CCCCCCCC(O)=O FDGMTLFOSHMHCC-WYMLVPIESA-N 0.000 description 1
- JVZYOCBKVRLCGB-FMIVXFBMSA-N (e)-11-acetyltetradec-9-enoic acid Chemical compound CCCC(C(C)=O)\C=C\CCCCCCCC(O)=O JVZYOCBKVRLCGB-FMIVXFBMSA-N 0.000 description 1
- PMFWYDDKWWOEDU-SAPNQHFASA-N (e)-11-methylsulfonylnonadec-9-enoic acid Chemical compound CCCCCCCCC(S(C)(=O)=O)\C=C\CCCCCCCC(O)=O PMFWYDDKWWOEDU-SAPNQHFASA-N 0.000 description 1
- WXZAXSOOJMFSAV-FMIVXFBMSA-N (e)-11-nitropentadec-9-enoic acid Chemical compound CCCCC([N+]([O-])=O)\C=C\CCCCCCCC(O)=O WXZAXSOOJMFSAV-FMIVXFBMSA-N 0.000 description 1
- MOROIQSUDRJGHA-SDNWHVSQSA-N (e)-8-acetylheptadec-9-enoic acid Chemical compound CCCCCCC\C=C\C(C(C)=O)CCCCCCC(O)=O MOROIQSUDRJGHA-SDNWHVSQSA-N 0.000 description 1
- LXOXVARCMWHDPQ-DHZHZOJOSA-N (e)-8-acetyltetradec-9-enoic acid Chemical compound CCCC\C=C\C(C(C)=O)CCCCCCC(O)=O LXOXVARCMWHDPQ-DHZHZOJOSA-N 0.000 description 1
- MGBRKOWENABGQD-JLHYYAGUSA-N (e)-8-methylsulfonylhexadec-9-enoic acid Chemical compound CCCCCC\C=C\C(S(C)(=O)=O)CCCCCCC(O)=O MGBRKOWENABGQD-JLHYYAGUSA-N 0.000 description 1
- LKEYYVMZBVVFDU-KNTRCKAVSA-N (e)-9-methylsulfonylnonadec-9-enoic acid Chemical compound CCCCCCCCC\C=C(S(C)(=O)=O)/CCCCCCCC(O)=O LKEYYVMZBVVFDU-KNTRCKAVSA-N 0.000 description 1
- AFVMHUCSSSRWSI-SDNWHVSQSA-N (e)-9-nitropentadec-9-enoic acid Chemical compound CCCCC\C=C([N+]([O-])=O)/CCCCCCCC(O)=O AFVMHUCSSSRWSI-SDNWHVSQSA-N 0.000 description 1
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- OKMWKBLSFKFYGZ-UHFFFAOYSA-N 1-behenoylglycerol Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(O)CO OKMWKBLSFKFYGZ-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-N 2-Methylbenzenesulfonic acid Chemical compound CC1=CC=CC=C1S(O)(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-N 0.000 description 1
- GHHURQMJLARIDK-UHFFFAOYSA-N 2-hydroxypropyl octanoate Chemical compound CCCCCCCC(=O)OCC(C)O GHHURQMJLARIDK-UHFFFAOYSA-N 0.000 description 1
- HVCOBJNICQPDBP-UHFFFAOYSA-N 3-[3-[3,5-dihydroxy-6-methyl-4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxydecanoyloxy]decanoic acid;hydrate Chemical compound O.OC1C(OC(CC(=O)OC(CCCCCCC)CC(O)=O)CCCCCCC)OC(C)C(O)C1OC1C(O)C(O)C(O)C(C)O1 HVCOBJNICQPDBP-UHFFFAOYSA-N 0.000 description 1
- HSJKGGMUJITCBW-UHFFFAOYSA-N 3-hydroxybutanal Chemical class CC(O)CC=O HSJKGGMUJITCBW-UHFFFAOYSA-N 0.000 description 1
- 101150059573 AGTR1 gene Proteins 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 101710134784 Agnoprotein Proteins 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 101100248253 Arabidopsis thaliana RH40 gene Proteins 0.000 description 1
- 241001553178 Arachis glabrata Species 0.000 description 1
- 208000008035 Back Pain Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 208000031636 Body Temperature Changes Diseases 0.000 description 1
- DPUOLQHDNGRHBS-UHFFFAOYSA-N Brassidinsaeure Natural products CCCCCCCCC=CCCCCCCCCCCCC(O)=O DPUOLQHDNGRHBS-UHFFFAOYSA-N 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- VYLJAYXZTOTZRR-BTPDVQIOSA-N CC(C)(O)[C@H]1CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2CC[C@@H]2[C@@]3(C)CCCC(C)(C)[C@@H]3[C@@H](O)[C@H](O)[C@@]12C Chemical compound CC(C)(O)[C@H]1CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2CC[C@@H]2[C@@]3(C)CCCC(C)(C)[C@@H]3[C@@H](O)[C@H](O)[C@@]12C VYLJAYXZTOTZRR-BTPDVQIOSA-N 0.000 description 1
- GAWIXWVDTYZWAW-UHFFFAOYSA-N C[CH]O Chemical group C[CH]O GAWIXWVDTYZWAW-UHFFFAOYSA-N 0.000 description 1
- 208000006339 Caliciviridae Infections Diseases 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-N Caprylic acid Natural products CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 1
- 101710163595 Chaperone protein DnaK Proteins 0.000 description 1
- 102000000018 Chemokine CCL2 Human genes 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- GHOKWGTUZJEAQD-UHFFFAOYSA-N Chick antidermatitis factor Natural products OCC(C)(C)C(O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-UHFFFAOYSA-N 0.000 description 1
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 1
- 240000004307 Citrus medica Species 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- 241001440269 Cutina Species 0.000 description 1
- GVOUFPWUYJWQSK-UHFFFAOYSA-N Cyclofenil Chemical group C1=CC(OC(=O)C)=CC=C1C(C=1C=CC(OC(C)=O)=CC=1)=C1CCCCC1 GVOUFPWUYJWQSK-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- AEMOLEFTQBMNLQ-YMDCURPLSA-N D-galactopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-YMDCURPLSA-N 0.000 description 1
- AEMOLEFTQBMNLQ-AQKNRBDQSA-N D-glucopyranuronic acid Chemical compound OC1O[C@H](C(O)=O)[C@@H](O)[C@H](O)[C@H]1O AEMOLEFTQBMNLQ-AQKNRBDQSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 102000016911 Deoxyribonucleases Human genes 0.000 description 1
- 108010053770 Deoxyribonucleases Proteins 0.000 description 1
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical group O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 1
- 102100029715 DnaJ homolog subfamily A member 4 Human genes 0.000 description 1
- 235000021294 Docosapentaenoic acid Nutrition 0.000 description 1
- 238000008157 ELISA kit Methods 0.000 description 1
- 108010067770 Endopeptidase K Proteins 0.000 description 1
- 108020002908 Epoxide hydrolase Proteins 0.000 description 1
- 244000024675 Eruca sativa Species 0.000 description 1
- 235000014755 Eruca sativa Nutrition 0.000 description 1
- URXZXNYJPAJJOQ-UHFFFAOYSA-N Erucic acid Natural products CCCCCCC=CCCCCCCCCCCCC(O)=O URXZXNYJPAJJOQ-UHFFFAOYSA-N 0.000 description 1
- 101001039702 Escherichia coli (strain K12) Methyl-accepting chemotaxis protein I Proteins 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 208000004930 Fatty Liver Diseases 0.000 description 1
- DSLZVSRJTYRBFB-UHFFFAOYSA-N Galactaric acid Natural products OC(=O)C(O)C(O)C(O)C(O)C(O)=O DSLZVSRJTYRBFB-UHFFFAOYSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 102000002068 Glycopeptides Human genes 0.000 description 1
- 108010015899 Glycopeptides Proteins 0.000 description 1
- 108010023302 HDL Cholesterol Proteins 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- 101710178376 Heat shock 70 kDa protein Proteins 0.000 description 1
- 102100028761 Heat shock 70 kDa protein 6 Human genes 0.000 description 1
- 101710152018 Heat shock cognate 70 kDa protein Proteins 0.000 description 1
- 102100023043 Heat shock protein beta-8 Human genes 0.000 description 1
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 1
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 1
- 102000002737 Heme Oxygenase-1 Human genes 0.000 description 1
- 241000711549 Hepacivirus C Species 0.000 description 1
- 206010019708 Hepatic steatosis Diseases 0.000 description 1
- 241000700721 Hepatitis B virus Species 0.000 description 1
- 101000866014 Homo sapiens DnaJ homolog subfamily A member 4 Proteins 0.000 description 1
- 101001078680 Homo sapiens Heat shock 70 kDa protein 6 Proteins 0.000 description 1
- 101001047341 Homo sapiens Heat shock protein beta-8 Proteins 0.000 description 1
- 101000973778 Homo sapiens NAD(P)H dehydrogenase [quinone] 1 Proteins 0.000 description 1
- 101001002197 Homoeomma sp. Mu-theraphotoxin-Hsp1a Proteins 0.000 description 1
- 238000006130 Horner-Wadsworth-Emmons olefination reaction Methods 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 208000035150 Hypercholesterolemia Diseases 0.000 description 1
- 102100023915 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- 238000006908 Julia-Kocienski olefination reaction Methods 0.000 description 1
- 238000012313 Kruskal-Wallis test Methods 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 102100025357 Lipid-phosphate phosphatase Human genes 0.000 description 1
- 239000005913 Maltodextrin Substances 0.000 description 1
- 229920002774 Maltodextrin Polymers 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- 101000836282 Mus musculus Solute carrier organic anion transporter family member 1C1 Proteins 0.000 description 1
- 208000000112 Myalgia Diseases 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- 102100022365 NAD(P)H dehydrogenase [quinone] 1 Human genes 0.000 description 1
- 102000007561 NF-E2-Related Factor 2 Human genes 0.000 description 1
- 108010071382 NF-E2-Related Factor 2 Proteins 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
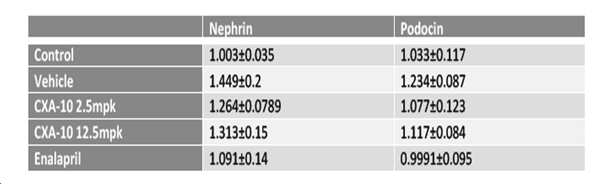
- 102100023195 Nephrin Human genes 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- IOVCWXUNBOPUCH-UHFFFAOYSA-M Nitrite anion Chemical compound [O-]N=O IOVCWXUNBOPUCH-UHFFFAOYSA-M 0.000 description 1
- 101710163270 Nuclease Proteins 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 102000007990 Organic Anion Transporters Human genes 0.000 description 1
- 108010089503 Organic Anion Transporters Proteins 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 102100035591 POU domain, class 2, transcription factor 2 Human genes 0.000 description 1
- 235000019482 Palm oil Nutrition 0.000 description 1
- 235000021319 Palmitoleic acid Nutrition 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 102000013566 Plasminogen Human genes 0.000 description 1
- 108010051456 Plasminogen Proteins 0.000 description 1
- 229920002266 Pluriol® Polymers 0.000 description 1
- 229920000604 Polyethylene Glycol 200 Polymers 0.000 description 1
- 229920002556 Polyethylene Glycol 300 Polymers 0.000 description 1
- 229920002564 Polyethylene Glycol 3500 Polymers 0.000 description 1
- 229920002582 Polyethylene Glycol 600 Polymers 0.000 description 1
- 229920002596 Polyethylene Glycol 900 Polymers 0.000 description 1
- 229920002685 Polyoxyl 35CastorOil Polymers 0.000 description 1
- 229920002690 Polyoxyl 40 HydrogenatedCastorOil Polymers 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 206010036653 Presyncope Diseases 0.000 description 1
- 102000004005 Prostaglandin-endoperoxide synthases Human genes 0.000 description 1
- 108090000459 Prostaglandin-endoperoxide synthases Proteins 0.000 description 1
- 239000013614 RNA sample Substances 0.000 description 1
- 238000003559 RNA-seq method Methods 0.000 description 1
- 238000011530 RNeasy Mini Kit Methods 0.000 description 1
- 208000035977 Rare disease Diseases 0.000 description 1
- 208000030118 Red blood cell disease Diseases 0.000 description 1
- 206010038468 Renal hypertrophy Diseases 0.000 description 1
- 241000220010 Rhode Species 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 235000019485 Safflower oil Nutrition 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- 244000000231 Sesamum indicum Species 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 208000000223 Solitary Kidney Diseases 0.000 description 1
- 102100027233 Solute carrier organic anion transporter family member 1B1 Human genes 0.000 description 1
- FBPFZTCFMRRESA-NQAPHZHOSA-N Sorbitol Chemical compound OCC(O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-NQAPHZHOSA-N 0.000 description 1
- 208000035286 Spontaneous Remission Diseases 0.000 description 1
- 238000006619 Stille reaction Methods 0.000 description 1
- 238000003639 Student–Newman–Keuls (SNK) method Methods 0.000 description 1
- 102000018075 Subfamily B ATP Binding Cassette Transporter Human genes 0.000 description 1
- 108010091105 Subfamily B ATP Binding Cassette Transporter Proteins 0.000 description 1
- 235000019486 Sunflower oil Nutrition 0.000 description 1
- 208000002847 Surgical Wound Diseases 0.000 description 1
- 238000006069 Suzuki reaction reaction Methods 0.000 description 1
- 241001061127 Thione Species 0.000 description 1
- 208000010641 Tooth disease Diseases 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- 208000008383 Wilms tumor Diseases 0.000 description 1
- 208000026448 Wilms tumor 1 Diseases 0.000 description 1
- 101710127857 Wilms tumor protein Proteins 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- PTFCDOFLOPIGGS-UHFFFAOYSA-N Zinc dication Chemical compound [Zn+2] PTFCDOFLOPIGGS-UHFFFAOYSA-N 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 125000002015 acyclic group Chemical group 0.000 description 1
- 125000002252 acyl group Chemical group 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000005882 aldol condensation reaction Methods 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 125000003302 alkenyloxy group Chemical group 0.000 description 1
- 150000001345 alkine derivatives Chemical class 0.000 description 1
- 125000005907 alkyl ester group Chemical group 0.000 description 1
- JAZBEHYOTPTENJ-JLNKQSITSA-N all-cis-5,8,11,14,17-icosapentaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O JAZBEHYOTPTENJ-JLNKQSITSA-N 0.000 description 1
- MBMBGCFOFBJSGT-KUBAVDMBSA-N all-cis-docosa-4,7,10,13,16,19-hexaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O MBMBGCFOFBJSGT-KUBAVDMBSA-N 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- QYIXCDOBOSTCEI-UHFFFAOYSA-N alpha-cholestanol Natural products C1CC2CC(O)CCC2(C)C2C1C1CCC(C(C)CCCC(C)C)C1(C)CC2 QYIXCDOBOSTCEI-UHFFFAOYSA-N 0.000 description 1
- 229910000323 aluminium silicate Inorganic materials 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 238000002399 angioplasty Methods 0.000 description 1
- 229940125364 angiotensin receptor blocker Drugs 0.000 description 1
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 230000004596 appetite loss Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000011914 asymmetric synthesis Methods 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 125000003354 benzotriazolyl group Chemical group N1N=NC2=C1C=CC=C2* 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 125000002619 bicyclic group Chemical group 0.000 description 1
- 210000000941 bile Anatomy 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 238000009530 blood pressure measurement Methods 0.000 description 1
- 238000010241 blood sampling Methods 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 108091006374 cAMP receptor proteins Proteins 0.000 description 1
- 229960005069 calcium Drugs 0.000 description 1
- 235000010410 calcium alginate Nutrition 0.000 description 1
- 239000000648 calcium alginate Substances 0.000 description 1
- 229960002681 calcium alginate Drugs 0.000 description 1
- FNAQSUUGMSOBHW-UHFFFAOYSA-H calcium citrate Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O.[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O FNAQSUUGMSOBHW-UHFFFAOYSA-H 0.000 description 1
- 239000001354 calcium citrate Substances 0.000 description 1
- 239000000378 calcium silicate Substances 0.000 description 1
- 229910052918 calcium silicate Inorganic materials 0.000 description 1
- 235000012241 calcium silicate Nutrition 0.000 description 1
- OKHHGHGGPDJQHR-YMOPUZKJSA-L calcium;(2s,3s,4s,5s,6r)-6-[(2r,3s,4r,5s,6r)-2-carboxy-6-[(2r,3s,4r,5s,6r)-2-carboxylato-4,5,6-trihydroxyoxan-3-yl]oxy-4,5-dihydroxyoxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylate Chemical compound [Ca+2].O[C@@H]1[C@H](O)[C@H](O)O[C@@H](C([O-])=O)[C@H]1O[C@H]1[C@@H](O)[C@@H](O)[C@H](O[C@H]2[C@H]([C@@H](O)[C@H](O)[C@H](O2)C([O-])=O)O)[C@H](C(O)=O)O1 OKHHGHGGPDJQHR-YMOPUZKJSA-L 0.000 description 1
- OYACROKNLOSFPA-UHFFFAOYSA-N calcium;dioxido(oxo)silane Chemical compound [Ca+2].[O-][Si]([O-])=O OYACROKNLOSFPA-UHFFFAOYSA-N 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- 235000013877 carbamide Nutrition 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 230000006315 carbonylation Effects 0.000 description 1
- 238000005810 carbonylation reaction Methods 0.000 description 1
- 229920003064 carboxyethyl cellulose Polymers 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 230000000739 chaotic effect Effects 0.000 description 1
- RCTYPNKXASFOBE-UHFFFAOYSA-M chloromercury Chemical compound [Hg]Cl RCTYPNKXASFOBE-UHFFFAOYSA-M 0.000 description 1
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 1
- 229960002023 chloroprocaine Drugs 0.000 description 1
- 150000001840 cholesterol esters Chemical class 0.000 description 1
- 230000007882 cirrhosis Effects 0.000 description 1
- 208000019425 cirrhosis of liver Diseases 0.000 description 1
- SECPZKHBENQXJG-UHFFFAOYSA-N cis-palmitoleic acid Natural products CCCCCCC=CCCCCCCCC(O)=O SECPZKHBENQXJG-UHFFFAOYSA-N 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 239000013066 combination product Substances 0.000 description 1
- 229940127555 combination product Drugs 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000006482 condensation reaction Methods 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 229960001681 croscarmellose sodium Drugs 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- 229960002944 cyclofenil Drugs 0.000 description 1
- HCAJEUSONLESMK-UHFFFAOYSA-N cyclohexylsulfamic acid Chemical compound OS(=O)(=O)NC1CCCCC1 HCAJEUSONLESMK-UHFFFAOYSA-N 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- DTPCFIHYWYONMD-UHFFFAOYSA-N decaethylene glycol Chemical compound OCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO DTPCFIHYWYONMD-UHFFFAOYSA-N 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 239000003405 delayed action preparation Substances 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- CYQFCXCEBYINGO-IAGOWNOFSA-N delta1-THC Chemical compound C1=C(C)CC[C@H]2C(C)(C)OC3=CC(CCCCC)=CC(O)=C3[C@@H]21 CYQFCXCEBYINGO-IAGOWNOFSA-N 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 150000002005 dihydroxyeicosatrienoic acids Chemical class 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 1
- 239000001177 diphosphate Substances 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 230000009266 disease activity Effects 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 229960000735 docosanol Drugs 0.000 description 1
- LLRANSBEYQZKFY-UHFFFAOYSA-N dodecanoic acid;propane-1,2-diol Chemical compound CC(O)CO.CCCCCCCCCCCC(O)=O LLRANSBEYQZKFY-UHFFFAOYSA-N 0.000 description 1
- 235000020188 drinking water Nutrition 0.000 description 1
- 239000003651 drinking water Substances 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 238000007876 drug discovery Methods 0.000 description 1
- 230000000857 drug effect Effects 0.000 description 1
- 230000036267 drug metabolism Effects 0.000 description 1
- 235000020673 eicosapentaenoic acid Nutrition 0.000 description 1
- 229960005135 eicosapentaenoic acid Drugs 0.000 description 1
- JAZBEHYOTPTENJ-UHFFFAOYSA-N eicosapentaenoic acid Natural products CCC=CCC=CCC=CCC=CCC=CCCCC(O)=O JAZBEHYOTPTENJ-UHFFFAOYSA-N 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 230000001804 emulsifying effect Effects 0.000 description 1
- OYFJQPXVCSSHAI-QFPUQLAESA-N enalapril maleate Chemical compound OC(=O)\C=C/C(O)=O.C([C@@H](C(=O)OCC)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(O)=O)CC1=CC=CC=C1 OYFJQPXVCSSHAI-QFPUQLAESA-N 0.000 description 1
- 238000003821 enantio-separation Methods 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 238000001952 enzyme assay Methods 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- YQGOJNYOYNNSMM-UHFFFAOYSA-N eosin Chemical compound [Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21 YQGOJNYOYNNSMM-UHFFFAOYSA-N 0.000 description 1
- 150000002121 epoxyeicosatrienoic acids Chemical class 0.000 description 1
- DPUOLQHDNGRHBS-KTKRTIGZSA-N erucic acid Chemical compound CCCCCCCC\C=C/CCCCCCCCCCCC(O)=O DPUOLQHDNGRHBS-KTKRTIGZSA-N 0.000 description 1
- 208000019501 erythrocyte disease Diseases 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- IDGUHHHQCWSQLU-UHFFFAOYSA-N ethanol;hydrate Chemical compound O.CCO IDGUHHHQCWSQLU-UHFFFAOYSA-N 0.000 description 1
- AEOCXXJPGCBFJA-UHFFFAOYSA-N ethionamide Chemical compound CCC1=CC(C(N)=S)=CC=N1 AEOCXXJPGCBFJA-UHFFFAOYSA-N 0.000 description 1
- MVPICKVDHDWCJQ-UHFFFAOYSA-N ethyl 3-pyrrolidin-1-ylpropanoate Chemical compound CCOC(=O)CCN1CCCC1 MVPICKVDHDWCJQ-UHFFFAOYSA-N 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 229960004667 ethyl cellulose Drugs 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 125000005469 ethylenyl group Chemical group 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 238000011985 exploratory data analysis Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 244000144992 flock Species 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 235000007983 food acid Nutrition 0.000 description 1
- 235000019138 food restriction Nutrition 0.000 description 1
- 125000002485 formyl group Chemical group [H]C(*)=O 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- DSLZVSRJTYRBFB-DUHBMQHGSA-N galactaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)C(O)=O DSLZVSRJTYRBFB-DUHBMQHGSA-N 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- VZCCETWTMQHEPK-UHFFFAOYSA-N gamma-Linolensaeure Natural products CCCCCC=CCC=CCC=CCCCCC(O)=O VZCCETWTMQHEPK-UHFFFAOYSA-N 0.000 description 1
- VZCCETWTMQHEPK-QNEBEIHSSA-N gamma-linolenic acid Chemical compound CCCCC\C=C/C\C=C/C\C=C/CCCCC(O)=O VZCCETWTMQHEPK-QNEBEIHSSA-N 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 150000002314 glycerols Chemical class 0.000 description 1
- 229940049654 glyceryl behenate Drugs 0.000 description 1
- 125000003827 glycol group Chemical group 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 235000015220 hamburgers Nutrition 0.000 description 1
- 239000007887 hard shell capsule Substances 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 230000002489 hematologic effect Effects 0.000 description 1
- 230000000004 hemodynamic effect Effects 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- FBPFZTCFMRRESA-UHFFFAOYSA-N hexane-1,2,3,4,5,6-hexol Chemical compound OCC(O)C(O)C(O)C(O)CO FBPFZTCFMRRESA-UHFFFAOYSA-N 0.000 description 1
- 125000006038 hexenyl group Chemical group 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- VYLJAYXZTOTZRR-UHFFFAOYSA-N hopane-6alpha,7beta,22-triol Natural products C12CCC3C4(C)CCCC(C)(C)C4C(O)C(O)C3(C)C1(C)CCC1C2(C)CCC1C(C)(O)C VYLJAYXZTOTZRR-UHFFFAOYSA-N 0.000 description 1
- 235000006486 human diet Nutrition 0.000 description 1
- 150000007857 hydrazones Chemical class 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 229940071826 hydroxyethyl cellulose Drugs 0.000 description 1
- 125000004029 hydroxymethyl group Chemical group [H]OC([H])([H])* 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 238000011532 immunohistochemical staining Methods 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 125000003453 indazolyl group Chemical group N1N=C(C2=C1C=CC=C2)* 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229940102223 injectable solution Drugs 0.000 description 1
- 229940102213 injectable suspension Drugs 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 208000037906 ischaemic injury Diseases 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 125000000904 isoindolyl group Chemical group C=1(NC=C2C=CC=CC12)* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 150000002535 isoprostanes Chemical class 0.000 description 1
- 125000001786 isothiazolyl group Chemical group 0.000 description 1
- 125000000842 isoxazolyl group Chemical group 0.000 description 1
- 238000011862 kidney biopsy Methods 0.000 description 1
- 230000003907 kidney function Effects 0.000 description 1
- 238000009533 lab test Methods 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 201000001715 left bundle branch hemiblock Diseases 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- KQQKGWQCNNTQJW-UHFFFAOYSA-N linolenic acid Natural products CC=CCCC=CCC=CCCCCCCCC(O)=O KQQKGWQCNNTQJW-UHFFFAOYSA-N 0.000 description 1
- JXNPEDYJTDQORS-UHFFFAOYSA-N linoleyl alcohol Natural products CCCCCC=CCC=CCCCCCCCCO JXNPEDYJTDQORS-UHFFFAOYSA-N 0.000 description 1
- 230000037356 lipid metabolism Effects 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 208000004731 long QT syndrome Diseases 0.000 description 1
- 208000019017 loss of appetite Diseases 0.000 description 1
- 235000021266 loss of appetite Nutrition 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 206010025482 malaise Diseases 0.000 description 1
- 229940035034 maltodextrin Drugs 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 229960002900 methylcellulose Drugs 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 210000005087 mononuclear cell Anatomy 0.000 description 1
- 150000004712 monophosphates Chemical class 0.000 description 1
- 235000021281 monounsaturated fatty acids Nutrition 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 238000011201 multiple comparisons test Methods 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 230000000869 mutational effect Effects 0.000 description 1
- 125000001421 myristyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N n-hexanoic acid Natural products CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- SNMVRZFUUCLYTO-UHFFFAOYSA-N n-propyl chloride Chemical compound CCCCl SNMVRZFUUCLYTO-UHFFFAOYSA-N 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 201000009240 nasopharyngitis Diseases 0.000 description 1
- 108010027531 nephrin Proteins 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000000926 neurological effect Effects 0.000 description 1
- SFDJOSRHYKHMOK-UHFFFAOYSA-N nitramide Chemical compound N[N+]([O-])=O SFDJOSRHYKHMOK-UHFFFAOYSA-N 0.000 description 1
- 125000004971 nitroalkyl group Chemical group 0.000 description 1
- 125000006501 nitrophenyl group Chemical group 0.000 description 1
- 230000008634 non enzymatic mechanism Effects 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000002889 oleic acids Chemical class 0.000 description 1
- 229940055577 oleyl alcohol Drugs 0.000 description 1
- XMLQWXUVTXCDDL-UHFFFAOYSA-N oleyl alcohol Natural products CCCCCCC=CCCCCCCCCCCO XMLQWXUVTXCDDL-UHFFFAOYSA-N 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 229940012843 omega-3 fatty acid Drugs 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 208000038009 orphan disease Diseases 0.000 description 1
- 125000002971 oxazolyl group Chemical group 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000004783 oxidative metabolism Effects 0.000 description 1
- 150000002923 oximes Chemical class 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 238000007427 paired t-test Methods 0.000 description 1
- 239000002540 palm oil Substances 0.000 description 1
- 125000000913 palmityl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- WLJNZVDCPSBLRP-UHFFFAOYSA-N pamoic acid Chemical compound C1=CC=C2C(CC=3C4=CC=CC=C4C=C(C=3O)C(=O)O)=C(O)C(C(O)=O)=CC2=C1 WLJNZVDCPSBLRP-UHFFFAOYSA-N 0.000 description 1
- 239000011713 pantothenic acid Substances 0.000 description 1
- 229940055726 pantothenic acid Drugs 0.000 description 1
- 235000019161 pantothenic acid Nutrition 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000008807 pathological lesion Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 238000005502 peroxidation Methods 0.000 description 1
- 230000002974 pharmacogenomic effect Effects 0.000 description 1
- 239000003548 pharmacological biomarker Substances 0.000 description 1
- LCEFEIBEOBPPSJ-UHFFFAOYSA-N phenyl selenohypobromite Chemical compound Br[Se]C1=CC=CC=C1 LCEFEIBEOBPPSJ-UHFFFAOYSA-N 0.000 description 1
- WJCXADMLESSGRI-UHFFFAOYSA-N phenyl selenohypochlorite Chemical compound Cl[Se]C1=CC=CC=C1 WJCXADMLESSGRI-UHFFFAOYSA-N 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000003075 phytoestrogen Substances 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920001515 polyalkylene glycol Polymers 0.000 description 1
- 125000003367 polycyclic group Chemical group 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229940113115 polyethylene glycol 200 Drugs 0.000 description 1
- 229940068886 polyethylene glycol 300 Drugs 0.000 description 1
- 229940057847 polyethylene glycol 600 Drugs 0.000 description 1
- 229940094543 polyethylene glycol 900 Drugs 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 230000001323 posttranslational effect Effects 0.000 description 1
- 235000010408 potassium alginate Nutrition 0.000 description 1
- 239000000737 potassium alginate Substances 0.000 description 1
- MZYRDLHIWXQJCQ-YZOKENDUSA-L potassium alginate Chemical compound [K+].[K+].O1[C@@H](C([O-])=O)[C@@H](OC)[C@H](O)[C@H](O)[C@@H]1O[C@@H]1[C@@H](C([O-])=O)O[C@@H](O)[C@@H](O)[C@H]1O MZYRDLHIWXQJCQ-YZOKENDUSA-L 0.000 description 1
- 235000019814 powdered cellulose Nutrition 0.000 description 1
- 229920003124 powdered cellulose Polymers 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 150000003163 prostaglandin D2 derivatives Chemical class 0.000 description 1
- 230000002997 prostaglandinlike Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 125000000561 purinyl group Chemical group N1=C(N=C2N=CNC2=C1)* 0.000 description 1
- 125000003373 pyrazinyl group Chemical group 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 125000000714 pyrimidinyl group Chemical group 0.000 description 1
- 229940107700 pyruvic acid Drugs 0.000 description 1
- 238000000306 qrs interval Methods 0.000 description 1
- 238000011158 quantitative evaluation Methods 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- 125000002294 quinazolinyl group Chemical group N1=C(N=CC2=CC=CC=C12)* 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 125000002943 quinolinyl group Chemical group N1=C(C=CC2=CC=CC=C12)* 0.000 description 1
- 125000005493 quinolyl group Chemical group 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 238000011552 rat model Methods 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 108091000053 retinol binding Proteins 0.000 description 1
- 102000029752 retinol binding Human genes 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 201000007916 right bundle branch block Diseases 0.000 description 1
- 235000005713 safflower oil Nutrition 0.000 description 1
- 239000003813 safflower oil Substances 0.000 description 1
- 235000003441 saturated fatty acids Nutrition 0.000 description 1
- 229930195734 saturated hydrocarbon Natural products 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000012883 sequential measurement Methods 0.000 description 1
- 230000000405 serological effect Effects 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 229940045902 sodium stearyl fumarate Drugs 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 125000003003 spiro group Chemical group 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000011301 standard therapy Methods 0.000 description 1
- 238000011272 standard treatment Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 231100000240 steatosis hepatitis Toxicity 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 125000002328 sterol group Chemical group 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 229950000244 sulfanilic acid Drugs 0.000 description 1
- 125000000475 sulfinyl group Chemical group [*:2]S([*:1])=O 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 239000002600 sunflower oil Substances 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 206010042772 syncope Diseases 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 208000037905 systemic hypertension Diseases 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 125000001712 tetrahydronaphthyl group Chemical group C1(CCCC2=CC=CC=C12)* 0.000 description 1
- 238000011285 therapeutic regimen Methods 0.000 description 1
- 125000001113 thiadiazolyl group Chemical group 0.000 description 1
- 125000000335 thiazolyl group Chemical group 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 125000003944 tolyl group Chemical group 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- LKOVPWSSZFDYPG-WUKNDPDISA-N trans-octadec-2-enoic acid Chemical compound CCCCCCCCCCCCCCC\C=C\C(O)=O LKOVPWSSZFDYPG-WUKNDPDISA-N 0.000 description 1
- 125000004306 triazinyl group Chemical group 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- 235000013337 tricalcium citrate Nutrition 0.000 description 1
- 235000011178 triphosphate Nutrition 0.000 description 1
- 239000001226 triphosphate Substances 0.000 description 1
- UNXRWKVEANCORM-UHFFFAOYSA-N triphosphoric acid Chemical compound OP(O)(=O)OP(O)(=O)OP(O)(O)=O UNXRWKVEANCORM-UHFFFAOYSA-N 0.000 description 1
- 239000003656 tris buffered saline Substances 0.000 description 1
- 210000005239 tubule Anatomy 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 150000003672 ureas Chemical class 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 230000036325 urinary excretion Effects 0.000 description 1
- 238000005353 urine analysis Methods 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/20—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids
- A61K31/201—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids having one or two double bonds, e.g. oleic, linoleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Neurology (AREA)
- Pulmonology (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Neurosurgery (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Urology & Nephrology (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Physical Education & Sports Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Immunology (AREA)
- Gastroenterology & Hepatology (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Child & Adolescent Psychology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
도 2는 DOCA 염 연구에서 얻은 시간 경과에 따른 체중 변화를 나타낸다. 대조군(Ctrl)은 회색 다이아몬드, DOCA는 밝은 회색 사각형, CXA-10 (10-nitro-9(E)-octadec-9-enoic acid) 2.5mpk는 중간 회색 삼각형, CXA-10 (10-nitro-9(E)-octadec-9-enoic acid) 12.5mpk는 밝은 회색 사각형 및 에날라프릴은 어두운 회색 사각형으로 나타낸다.
도 3은 5개 그룹 각각에 대한 DOCA 염 연구에서 얻은 평균 동맥 혈압을 나타낸다: 대조군, 비히클, CXA-10 2.5 (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg), CXA-10 12.5 (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg), 및 Enal (에날라프릴).
도 4는 DOCA 염 연구에서 얻은 혈장(plasma) 콜레스테롤 수준에서의 치료 효과를 나타낸다. 왼쪽에서 오른쪽으로, 첫 번째 막대는 대조군을 나타내고, 두 번째 막대는 비히클이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이고, 마지막 막대는 에날라프릴이다.
도 5는 DOCA 염 연구에서 얻은 신장/바디 무게 및 심장/바디 무게 비율에서의 치료 효과를 나타낸다. 두 그룹(신장/바디 비율 및 심장/바디 비율)에서, 왼쪽에서 오른쪽으로, 첫 번째 막대는 대조군을 나타내고, 두 번째 막대는 비처리군이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이고, 마지막 막대는 에날라프릴이다.
도 6은 DOCA 염 연구에서 얻은 알부민뇨 및 네프린 배설에서의 치료 효과에 대한 시간 경과를 나타낸다. 두 그래프(알부민뇨(Albuminuria) 왼쪽 및 네프린뇨(Nephrinuria) 오른쪽)에서, 대조군은 회색 다이아몬드, 비히클은 중간 회색 작은 사각형, CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)는 중간 회색 삼각형, CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)는 검은색 사각형 및 에날라프릴은 어두운 회색 사각형으로 나타낸다. 왼쪽 그래프에서, *p<0.05 및 **p<0.01이다. 오른쪽 그래프에서, *p<0.01이다.
도 7은 DOCA 염 연구에서 얻은 소변의(urinary) 알부민 및 네프린 배설에서의 치료 효과를 나타낸다. 두 그래프(알부민뇨 왼쪽 및 네프린뇨 오른쪽)에서, 왼쪽에서 오른쪽으로, 첫 번째 막대는 대조군을 나타내고, 두 번째 막대는 비히클이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이고, 마지막 막대는 에날라프릴이다.
도 8은 DOCA 염 연구에서 얻은 소변의 Kim-1에서의 치료 효과를 나타낸다. 왼쪽에서 오른쪽으로, 첫 번째 막대는 대조군을 나타내고, 두 번째 막대는 비히클이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이고, 마지막 막대는 에날라프릴이다.
도 9는 DOCA 염 연구에서 얻은 GFR에서의 치료 효과를 나타낸다.
도 10은 처리 4주 후 DOCA 염 연구에서 얻은 세럼(serum) 크레아티닌 및 BUN 수치에서의 치료 효과를 나타낸다. 두 그래프(세럼 크레아티닌 수치 왼쪽 및 세럼 BUN 수치 오른쪽)에서, 왼쪽에서 오른쪽으로, 첫 번째 막대는 대조군을 나타내고, 두 번째 막대는 비히클이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이고, 마지막 막대는 에날라프릴이다.
도 11은 처리 후 DOCA 염 연구에서 얻은 신장 조직의 조직학적 평가를 나타낸다. 피코시리우스 레드로 염색된 부분의 대표적인 현미경 사진을 나타낸다(x200). 상단 3개의 현미경 사진은 대조군, 비처리군, 및 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이다. 하단 2개의 현미경 사진은 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg) 및 에날라프릴이다.
도 12는 DOCA 염 연구에서 얻은 사구체 경화증(glomerulosclerosis)에서의 치료 효과를 나타낸다. 상단 그래프: 왼쪽에서 오른쪽으로, 첫 번째 막대는 대조군을 나타내고, 두 번째 막대는 비히클이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이고, 마지막 막대는 에날라프릴이다. 하단 그래프: 스코어<1은 솔리드 그늘진 영역으로 표시되고, 스코어 1은 스코어<1보다 높고 수평선으로 표시되고, 스코어 2는 스코어 1보다 높고 수직선으로 표시되고, 스코어 3은 스코어 2보다 높고 대각선으로 표시되고, 스코어 4는 스코어 3보다 높고 다이어몬드선으로 표시된다.
도 13은 DOCA 염 연구에서 처리 후 사구체 비대 및 다리세포(podocyte) 수의 정량화를 나타낸다. 두 그래프(사구체 비대 상단 및 다리세포 수 하단)에서, 왼쪽에서 오른쪽으로, 첫 번째 막대는 정상(대조군이라고도 함)을 나타내고, 두 번째 막대는 비히클이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이고, 마지막 막대는 에날라프릴이다.
도 14는 DOCA 염 연구에서 얻은 신장 조직에서의 CD31+ 염색을 나타낸다. 상단 3개의 이미지는 대조군, 비히클, 및 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이다. 하단 2개의 이미지는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg) 및 에날라프릴이다.
도 15는 DOCA 염 연구에서 얻은 소변의 MCP-1 배설에서의 치료 효과를 나타낸다. 왼쪽에서 오른쪽으로 첫 번째 막대는 대조군을 나타내고, 두 번째 막대는 비히클이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이다.
도 16은 DOCA 염 연구에서 얻은 MCP-1 및 오스테오폰틴 유전자 발현에서의 치료 효과를 나타낸다. 왼쪽에서 오른쪽으로, 첫 번째 막대는 대조군을 나타내고, 두 번째 막대는 비히클이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이고, 마지막 막대는 에날라프릴이다.
도 17은 DOCA 염 연구에서 얻은 섬유증 및 염증성 유전자 발현에서의 치료 효과를 나타낸다. 모든 세 그룹에서(콜라겐 III, 피브로텍틴, PAI-1), 왼쪽에서 오른쪽으로, 첫 번째 막대는 대조군을 나타내고, 두 번째 막대는 비히클이고, 세 번째 막대는 CXA-10 2.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg)이고, 네 번째 막대는 CXA-10 12.5 mpk (10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg)이고, 마지막 막대는 에날라프릴이다.
도 18은 DOCA 염 연구에서 얻은 처리 후 소변의 이소프로스탄의 수치를 나타낸다. 왼쪽에서 오른쪽으로, 각 그래프는 첫 번째로 모의(sham)(대조군이라고도 함), 두 번째로 DOCA, 세 번째로 DOCA + 2.5mg/ml CXA-10 (CTX-10 2.5mpk 또는 10-nitro-9(E)-octadec-9-enoic acid, 2.5 mg이라고도 함); 네 번째로 DOCA + 12.5mg/ml CXA-10 (CTX-10 12.5mpk 또는 10-nitro-9(E)-octadec-9-enoic acid, 12.5 mg이라고도 함); 및 마지막으로 에날라프릴을 묘사한다.
도 19는 질산화 지방산(nitrated fatty acids)을 생산하는 일반적인 합성 방법을 나타낸다.
도 20은 허혈성/재관류 연구에서 래트에서 10-니트로-9(E)-옥타데크-9-에노산 처리 후의 세럼 크레아티닌 수치를 나타낸다. 각 그래프의 0, 24, 48 및 72시간에서, 왼쪽에서 오른쪽으로, 첫 번째 막대는 비히클 + sham을 나타내고, 두 번째 막대는 CXA-10(10-니트로-9(E)-옥타데크-9-에노산) + sham이고, 세 번째 막대는 비히클 + I/R이고, 마지막 막대는 CXA-10(10-니트로-9(E)-옥타데크-9-에노산) + I/R이다.
도 21은 래트 허혈성 재관류 연구에서 12.5 mg/kg 10-니트로-9(E)-옥타데크-9-에노산으로 처리된 래트에서 I/R 손상 후 신장의 조직학적 및 정량적 평가를 나타낸다.
도 22는 1일 및 14일 및 15일 복용으로, 비만 남성에서 10-니트로-9(E)-옥타데크-9-에노산의 복합 상승 용량(multiple ascending dose) 연구에서 모든 세 그룹에 대한 평균 농도-시간 PK 프로파일을 나타낸다. 이 그래프에서 선은 바닥에서 시작하여 구별될 수 있으며, 가장 아래에 있는 열린 원은 1일에 25 mg 처리를 나타내고, 채워진 원은 14일에 25 mg 처리를 나타내고; 이어서 열린 사각형은 1일에 150 mg 처리를 나타내고, 채워진 사각형은 14일에 150 mg 처리를 나타내고; 이어서 열린 삼각형은 1일에 600 mg 처리를 나타내고, 채워진 삼각형은 14일 450 mg 처리를 나타내고; 가장 위에 있는 채워진 삼각형은 15일에 150 mg 처리를 나타낸다.
도 23은 비만 남성에서 10-니트로-9(E)-옥타데크-9-에노산의 복합 상승 용량(multiple ascending dose) 연구에서 처리에 의한 평균 렙틴 농도(A) 및 베이스라인 처리로부터의 퍼센트 변화(B)를 나타낸다. 두 그래프에서 검은 선은 위약(placebo)을 나타내고, 어두운 회색 삼각형은 CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 25 mg을 나타내고, 밝은 회색 사각형은 CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 150 mg을 나타내고, 및 밝은 회색 원은 CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 450 mg을 나타낸다.
도 24는 비만 남성에서 10-니트로-9(E)-옥타데크-9-에노산의 복합 상승 용량(multiple ascending dose) 연구에서 처리에 의한 베이스라인으로부터의 MCP-1 변화를 나타낸다. 파선은 7일-위약이고, 실선은 14일-위약이고, 어두운 회색 삼각형을 갖는 파선은 7일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 25 mg이고, 어두운 회색 삼각형을 갖는 실선은 14일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 25 mg이고, 밝은 회색 사각형을 갖는 파선은 7일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 150 mg이고, 밝은 회색 사각형을 갖는 실선은 14일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 150 mg이고, 밝은 회색 원을 갖는 파선은 7일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 450 mg이고, 밝은 회색 원을 갖는 실선은 14일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 450 mg이다.
도 25는 비만 남성에서 10-니트로-9(E)-옥타데크-9-에노산의 복합 상승 용량(multiple ascending dose) 연구에서 처리에 의한 베이스라인으로부터의 평균 변화 IL-6 농도를 나타낸다. 파선은 7일-위약이고, 실선은 14일-위약이고, 어두운 회색 삼각형을 갖는 파선은 7일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 25 mg이고, 어두운 회색 삼각형을 갖는 실선은 14일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 25 mg이고, 밝은 회색 사각형을 갖는 파선은 7일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 150 mg이고, 밝은 회색 사각형을 갖는 실선은 14일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 150 mg이고, 밝은 회색 원을 갖는 파선은 7일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 450 mg이고, 밝은 회색 원을 갖는 실선은 14일-CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 450 mg이다.
도 26은 비만 남성에서 10-니트로-9(E)-옥타데크-9-에노산의 복합 상승 용량(multiple ascending dose) 연구에서 처리에 의한 베이스라인으로부터의 트리글리세라이드 변화를 나타낸다. 이 그래프에서, 검은 선은 위약을 나타내고, 어두운 회색 삼각형은 CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 25 mg을 나타내고, 밝은 회색 사각형은 CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 150 mg을 나타내고, 및 밝은 회색 원은 CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 450 mg을 나타낸다.
도 27은 비만 남성에서 10-니트로-9(E)-옥타데크-9-에노산의 복합 상승 용량(multiple ascending dose) 연구에서 처리에 의한 베이스라인으로부터의 콜레스테롤 농도 평균 변화를 나타낸다. 이 그래프에서, 검은 선은 위약을 나타내고, 어두운 회색 삼각형은 CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 25 mg을 나타내고, 밝은 회색 사각형은 CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 150 mg을 나타내고, 및 밝은 회색 원은 CXA-10 (10-니트로-9(E)-옥타데크-9-에노산) 450 mg을 나타낸다.
도 28은 건강한 남성 연구에서 프라바스타틴 및 바이토린®(심바스타틴 및 에제티미브)과 정상 상태로 투여된 10-니트로-9(E)-옥타데크-9-에노산의 약물동력학적 상호작용에 대한 연구 설계 및 타임라인을 나타낸다. P는 프라바스타틴이고 V는 바이토린®이다.
도 29는 건강한 남성에서 프라바스타틴 및 바이토린®(심바스타틴 및 에제티미브)과 정상 상태로 투여된 10-니트로-9(E)-옥타데크-9-에노산의 약물동력학적 상호작용 연구에 대한 PK 혈액 샘플링을 위한 시간 및 사건 테이블을 나타낸다.
도 30은 건강한 남성에서 프라바스타틴 및 바이토린®(심바스타틴 및 에제티미브)과 정상 상태로 투여된 10-니트로-9(E)-옥타데크-9-에노산의 약물동력학적 상호작용 연구에서 40 mg의 프라바스타틴 (A) 및 3-알파-하이드록시 프라바스타틴 (B)의 경구 투여 후 평균 (+SD) 혈장 프라바스타틴 농도-시간 프로파일을 나타낸다. Day 1: 프라바스타틴 단독은 음영이 없는 원을 갖는 어두운 회색 선으로 표시되고 Day 11: 프라바스타틴 + CXA-10 (10-니트로-9(E)-옥타데크-9-에노산)은 음영이 없는 사각형을 갖는 밝은 회색 선으로 표시된다.
도 31은 건강한 남성에서 프라바스타틴 및 바이토린®(심바스타틴 및 에제티미브)과 정상 상태로 투여된 10-니트로-9(E)-옥타데크-9-에노산의 약물동력학적 상호작용 연구에서 10/20 mg의 에제티미브의 경구 투여 후 평균 (+SD) 혈장 에제티미브 전체 농도-시간 프로파일을 나타낸다. Day 2: 바이토린® 단독은 음영이 없는 삼각형을 갖는 밝은 회색 선으로 표시되고 Day 12: 바이토린® + CXA-10 (10-니트로-9(E)-옥타데크-9-에노산)은 음영이 없는 사각형을 갖는 어두운 회색 선으로 표시된다.
도 32는 건강한 남성에서 프라바스타틴 및 바이토린®(심바스타틴 및 에제티미브)과 정상 상태로 투여된 10-니트로-9(E)-옥타데크-9-에노산의 약물동력학적 상호작용 연구에서 10/20 mg의 바이토린®의 경구 투여 후 평균 (+SD) 혈장 심바스타틴 및 심바스타틴 하이드록시산(hydroxyl acid) 농도-시간 프로파일을 나타낸다. Day 2: 바이토린® 단독은 음영이 없는 삼각형을 갖는 밝은 회색 선으로 표시되고 Day 12: 바이토린® + CXA-10 (10-니트로-9(E)-옥타데크-9-에노산)은 음영이 없는 사각형을 갖는 어두운 회색 선으로 표시된다.
도 33은 건강한 남성에서 프라바스타틴 및 바이토린®(심바스타틴 및 에제티미브)과 정상 상태로 투여된 10-니트로-9(E)-옥타데크-9-에노산의 약물동력학적 상호작용 연구에서 표준 분석물(단일 제제로 주어진 분석물)에 대한 시험(CXA-10으로 주어진 분석물)의 비교 요약 통계 테이블이다.
도 34는 일차 국소 분절 사구체 경화증(primary focal segmental glomerulosclerosis: FSGS)으로 인한 신 증후군(nephrotic syndrome)을 갖는 환자에서 10-니트로-9(E)-옥타데크-9-에노산의 두 가지 적정 요법(titration regimens)의 3개월 오픈 라벨 무작위 연구에 대한 연구 평가 테이블이다.
도 35는 일차 국소 분절 사구체 경화증(primary focal segmental glomerulosclerosis: FSGS)으로 인한 신 증후군(nephrotic syndrome)을 갖는 환자에서 10-니트로-9(E)-옥타데크-9-에노산의 두 가지 적정 요법(titration regimens)의 3개월 오픈 라벨 무작위 연구에 대한 연구 디자인이다.
| AE | 부작용 사례(Adverse Event) |
| ALT | 알라닌 아미노전이효소(Alanine aminotransferase) |
| AST | 아스파테이트 아미노전이효소(Aspartate aminotransferase) |
| AUC0-∞ | 시간 0에서 무한대까지 혈장 약물 농도 시간 곡선 하의 면적 |
| AUC0-last | 시간 0에서 마지막 측정 가능한 농도의 시간까지 혈장 약물 농도 대 시간 곡선 하의 면적 |
| BHT | 부틸화 하이드록시톨루엔(Butylated hydroxytoluene) |
| BID | 매일 두 번 |
| BMI | 체질량지수(Body mass index) |
| BP | 혈압(Blood pressure) |
| BPM | 분 당 박동수(Beats per minute) |
| BUN | 혈액요소질소(Blood urea nitrogen) |
| CI | 신뢰구간(Confidence Interval) |
| CKD | 만성 신장 질환(Chronic Kidney Disease) |
| CKI | 만성 신장 손상(Chronic Kidney Injury) |
| CL/F | 경구 투여 후 클리어런스(Clearance following oral administration) |
| Cmax | 관찰된 최대 혈장 약물 농도 |
| CPK | 크레아틴 포스포키나아제(Creatine phosphokinase) |
| CRF | 사례 보고 형식(Case report form) |
| CRP | C-반승성 단백질(C-Reactive Protein) |
| DBP | 확장기혈압(Diastolic blood pressure) |
| DHETs | 디하이드록시에이코사트리엔산(Dihydroxyeicosatrienoic acids) |
| DL | 용량 수치(Dose level) |
| DOCA | 데옥시코르티코스테론 아세테이트(Deoxycorticosterone acetate) |
| E/T | 조기 종료(Early termination) |
| ECG | 심전도(Electrocardiogram) |
| EETs | 에폭시에이코사트리엔산(Epoxyeicosatrienoic acids) |
| eGFR | 측정된 사구체여과율(glomerular filtration rate) |
| ELISA | 효소결합면역흡착검사(Enzyme-linked immunosorbent assay) |
| ES | 노출 반응(Exposure response) |
| FBG | 공복혈당(Fasting blood glucose) |
| FDA | 식품의약국(Food and Drug Administration) |
| FIH | 최초 인간(First-in-human) |
| GCLM | 글루타메이트 시스테인 리가아제 변경인자 서브유닛(Glutamate cysteine ligase modifier subunit) |
| GLP | 우수실험실관리기준(Good laboratory practice) |
| GGT | 감마-글루타밀 전이효소(Gamma-glutamyl transferase) |
| HbA1c | 헤모글로빈 A1c(Hemoglobin A1c) |
| HBsAg | B형 간염 바이러스 표면 항원(Hepatitis B virus surface antigen) |
| HCV Ab | C형 간염 바이러스 항체(Hepatitis C virus antibody) |
| HDL | 고밀도지질단백질(High density lipoprotein) |
| HIPAA | 1996년 건강보험 이전 및 책임에 관한 법률(Health Insurance Portability and Accountability Act) |
| HIV | 인간 면역 결핍 바이러스(Human immunodeficiency virus) |
| HO-1 | 헴 산화효소-1(Heme oxygenase-1) |
| HR | 심박수(Heart rate) |
| HSP | 열 충격 단백질(Heat shock proteins) |
| ICH | 의약품국제조화회의(International Conference on Harmonization) |
| IEC | 독립윤리위원회(Independent Ethics Committee) |
| IL-1 | 인터루킨 1(Interleukin 1) |
| IL-6 | 인터루킨 6(Interleukin 6) |
| IRB | 기관감사위원회(Institutional Review Board) |
| IVCD | 심실 내 전도 지연(Intra-ventricular conduction delay) |
| Keap 1 | 켈치-유사 ECH-연관 단백질(Kelch-like ECH-associated protein) |
| Kim-1 | 신장 손상 분자-1(Kidney injury molecule-1) |
| LBBB | 좌각블록(Left bundle branch block) |
| LDL | 저밀도지질단백질(Low density lipoprotein) |
| LFT | 간 기능 검사(Liver function test) |
| LQTS | 긴QT증후군(Long QT syndrome) |
| MCH | 평균적혈구혈색소량(Mean corpuscular hemoglobin) |
| MCHC | 평균적혈구혈색소농도(Mean cell hemoglobin concentration) |
| MCP-1 | 단핵구 화학유인물질 단백질-1(Monocyte chemoattractant protein-1) |
| MCV | 평균 적혈구 용적(Mean cell volume) |
| NCA | 비구획적 분석(Non-compartmental analysis) |
| NF-κB | 뉴클리어 팩터 κB(Nuclear factor κB) |
| NKDEP | 국가 신장 질환 교육 프로그램(National Kidney Disease Education Program) |
| NOAEL | 무독성량(No observed adverse effect level) |
| NQO1 | NAD(P)H 퀴논 산화환원효소 1(NAD(P)H quinone oxidoreductase 1) |
| Nrf2 | 뉴클리어 팩터 E2-관련 팩터 2(Nuclear factor E2-related factor 2) |
| NSAIDs | 비스테로이드 항염증제(Non-steroidal anti-inflammatory drugs) |
| OA-NO2 | 니트로-올레산(Nitro-oleic acid) |
| PAI-1 | 플라스미노겐 활성화제 억제제-1(Plasminogen activator inhibitor-1) |
| PBMCs | 말초혈액단핵구세포(Peripheral blood mononuclear cells) |
| PD | 약력학(Pharmacodynamics) |
| PGx | 약리유전학(Pharmacogenetic) |
| PK | 약물동력학(Pharmacokinetics) |
| qRT-PCR | 정량적 역전사효소중합효소연쇄반응(Quantitative reverse transcriptase-polymerase chain reaction) |
| RAS | 레닌-앤지오텐신계(Renin-angiotensin system) |
| RBBB | 우각블록(Right bundle branch block) |
| RBC | 적혈구(Red blood cell) |
| RBP4 | 레티놀 결합 단백질(Retinol binding protein) |
| RQ | 상대량(Relative Quantity) |
| RR | 호흡수(Respiratory rate) |
| SAE | 중대한 이상 반응(Serious Adverse Event) |
| SAP | 통계분석계획(Statistical Analysis Plan) |
| sEH | 가용성 에폭사이드 가수분해효소(Soluble epoxide hydrolase) |
| SBP | 최대혈압(Systolic blood pressure) |
| SOP | 표준운영절차(Standard Operating Procedure) |
| SPM | 연구절차메뉴얼(Study Procedures Manuals) |
| t½ | 터미널 페이즈 반감기(Terminal phase half-life) |
| Tmax | 최대 혈장 약물 농도에 대한 시간(Time to maximum plasma drug concentration) |
| TNFα | 종양괴사인자알파(Tumor Necrosis Factor alpha) |
| UAE | 요중 알부민 배설(Urinary albumin excretion) |
| ULN | 정상 상한치(Upper limit of normal) |
| WBC | 백혈구(White blood cell) |
| Vd/F | 경구 투여 후 분포 용적(Volume of distribution following oral administration) |
| WNL | 정상범위내(Within normal limits) |
| λz | 터미널 제거율 상수(Terminal elimination rate constant) |
| 코호트 /복용량 |
CXA
-10
N= 피험자 수 |
위약
N=피험자 수 |
| 1 / 25 mg | 10 | 4 |
| 2 / 150 mg | 10 | 4 |
| 3 / 450 mg* | 10 | 5** |
| 코호트 1 | 코호트 2 | 코호트 3 | ||
| 변수 | 25 mg (n=10) | 150 mg (n=10) | 450 mg (n=10) | 위약* (n=13) |
| 평균 나이 (yrs) (범위) |
37.5 (23, 48) |
38.1 (21,54) |
38.3 (26, 54) |
39.3 (19, 57) |
| 성별 | 남성 | 남성 | M남성 | 남성 |
| 인종 흑인/아프리칸 아메리칸 백인 |
50 % 50% |
50% 50% |
60% 40% |
53.8% 46.2% |
| 평균 BMI (kg/m2) (범위) |
30.2 (27, 38.7) |
28.6 (27, 33.2) |
30.1 (27.3, 39.5) |
30.6 (27.6, 37.4) |
|
코호트
1
25 mg (N=10 |
코호트
2
150 mg (n=10) |
코호트
3
450 mg (n=10) |
위약*
(n=13) |
|
| GI | 2 (20%) | 5 (50%) | 9 (90%) | 2 (15.4%) |
| 신경계 시스템 | 0 (0%) | 1 (10%) | 4 (40%) | 3 (23.1%) |
| 피부 및 SQ 장애 | 2 (20%) | 0 (0%) | 1 (10%) | 1 (7.7%) |
| 일반적 장애 | 1 (10%) | 0 (0%) | 3 (30%) | 0 (0%) |
| 근육 및 CT 장애 | 2 (20%) | 0 (0%) | 2 (20%) | 0 (0%) |
| GI 관련 AEs | |
| 위약 | 1 피험자는 구역질 증세가 있었다 |
| 코호트 1 (25 mg) | 1 피험자는 구역질 증세가 있었다 |
| 코호트 2 (150 mg) | 5 피험자는 설사를 보였고: 1 피험자는 매일 투여 ~2hr 후 설사를 보였다. 다른 모든 설사 AEs는 산발적이었고, 구역질을 갖는 피험자는 없었다 |
| 코호트 3 (450 mg) | 9 피험자는 설사를 보였고, 4 피험자는 구역질 증세가 있었다 |
| 코호트 3 (450 mg w food) | 3 피험자는 설사를 보였고, 1명은 구역질 증세가 있었다 |
| 21일 (ng/mL) | 28일 (ng/mL) | |
| 25 mg | (n=1) 0.17 |
0 |
| 150 mg | (n= 7) 0.33 |
(n=5) (0.39 |
| 450 mg | (n=8) 0.38 |
(n=9) 0.30 |
|
코호트
2
150mg (n=10) |
위약
(n=12) |
LS
평균
차이(Mean
Difference)
( pg /mL) (CI) |
|
| 기준선으로부터 % 변화 | |||
| 14일 | -21.5 | +35 | -56.5 (-140.5, 27.5) |
| 시간 |
LS
평균 차이(Mean Difference)
( pg /mL) (CI) |
| 14일 (0-10 hr) | -228.5 (-387.2, -69.77) |
| 시간 |
LS
평균 차이(Mean Difference)
( pg /mL) (CI) |
| 8일 | -45.44 (-87.38, -3.50) |
| 15일 | -59.6 (-102.3, -16.91) |
Claims (20)
- 고형 기관 섬유증(solid organ fibrosis), 염증 질환(inflammatory disease), 심혈관계 질환(cardiovascular disease), 신장 질환(renal disease), 신부전(kidney failure), 허혈성 신장 손상(ischemic kidney injury), 급성 신장 손상(acute kidney injury: AKI), 만성 신장 손상(chronic kidney injury: CKI), 만성 신장 질환(chronic kidney disease: CKD), 비만 관련 만성 신장 질환(obesity associated chronic kidney disease), 당뇨병성 신장 질환(diabetic nephropathy), 신장 섬유증(kidney fibrosis), 국소 분절 사구체 경화증(focal segmental glomerulosclerosis: FSGS), 일차 FSGS(primary FSGS), 이차 FSGS(secondary FSGS), 겸상적혈구병 관련 신장 질환(sickle cell nephropathy), 사구체신염(glomerulonephritis), 신 증후군(nephrotic syndrome), 비알콜성 지방간염(non-alcoholic steatohepatitis: NASH), 지방간 질환(fatty liver disease), 폐동맥 고혈압(pulmonary arterial hypertension: PAH), 폐 섬유증(pulmonary fibrosis), 알러지성 기도 질환(allergic airway disease), 비만(obesity), 항-지방세포화 질환(anti-adipogenic disease), 제2형 당뇨병(type II diabetes), 겸상적혈구병(sickle cell disease), 겸상적혈구증발증(sickle cell crisis), 특발성 폐 섬유증(idiopathic pulmonary fibrosis: IPF), 염증성 소화기계 질환(inflammatory gastrointestinal disease), 대장염(colitis), 염증성 장 질환(inflammatory bowel disease), 신경퇴행성 질환(neurodegenerative disease), 루게릭병(amyotrophic lateral sclerosis: ALS), 대사 증후군(metabolic syndrome), 신경병증(neuropathy), 샤르코마리투스병(Charcot-Marie-Tooth disease) 및 미토콘드리아 관련 질환(mitochondrial related diseases)에서 선택되는 질환의 치료 방법으로, 이를 필요로 하는 대상에게 10-니트로-9(E)-옥타데크-9-에노산의 치료학적 유효량을 투여하는 것을 포함하는 질환의 치료 방법.
- 제 1항에 있어서, 상기 치료학적 유효량은 약 25 밀리그램 내지 약 450 밀리그램 노출에 충분한 양인, 방법.
- 제 1항에 있어서, 상기 치료학적 유효량은 약 100 밀리그램 내지 약 200 밀리그램 노출에 충분한 양인, 방법.
- 제 1항에 있어서, 상기 치료학적 유효량은 약 75 밀리그램 내지 약 300 밀리그램 노출에 충분한 양인, 방법.
- 제 1항에 있어서, 상기 치료학적 유효량은 약 75 밀리그램 내지 약 150 밀리그램 노출에 충분한 양인, 방법.
- 제 1항에 있어서, 상기 치료학적 유효량은 약 150 밀리그램 내지 약 300 밀리그램 노출에 충분한 양인, 방법.
- 제 1항에 있어서, 상기 치료학적 유효량은 약 150 밀리그램 노출에 충분한 양인, 방법.
- 제 1항에 있어서, 상기 10-니트로-9(E)-옥타데크-9-에노산은 약학 조성물에 존재하는, 방법.
- 제 1항에 있어서, 상기 투여는 경구, 피하, 또는 정맥 내 투여인, 방법.
- 제 1항에 있어서, 상기 투여 간격은 1일 1회, 1일 2회, 1일 3회, 또는 1일 4회에서 선택되는, 방법.
- 제 1항에 있어서, 상기 질환은 국소 분절 사구체 경화증(focal segmental glomerulosclerosis) 또는 폐동맥 고혈압(pulmonary arterial hypertension)인, 방법.
- 국소 분절 사구체 경화증(focal segmental glomerulosclerosis) 또는 폐동맥 고혈압(pulmonary arterial hypertension)에서 선택되는 질환의 치료 방법으로 이를 필요로 하는 대상에게 약 150 mg 노출에 충분한 양으로 10-니트로-9(E)-옥타데크-9-에노산을 투여하는 것을 포함하는 방법.
- 제 12항에 있어서, 상기 10-니트로-9(E)-옥타데크-9-에노산은 1일 1회, 1일 2회, 1일 3회, 또는 1일 4회 투여되는, 방법.
- 10-니트로-9(E)-옥타데크-9-에노산의 치료학적 유효량 및 약학적으로 허용되는 담체를 포함하는 약학 조성물.
- 제 14항에 있어서, 상기 치료학적 유효량은 약 25 밀리그램 내지 약 450 밀리그램 노출에 충분한 양인, 약학 조성물.
- 제 14항에 있어서, 상기 치료학적 유효량은 약 100 밀리그램 내지 약 300 밀리그램 노출에 충분한 양인, 약학 조성물.
- 제 14항에 있어서, 상기 치료학적 유효량은 약 75 밀리그램 내지 약 300 밀리그램 노출에 충분한 양인, 약학 조성물.
- 제 14항에 있어서, 상기 치료학적 유효량은 약 75 밀리그램 내지 약 150 밀리그램 노출에 충분한 양인, 약학 조성물.
- 제 14항에 있어서, 상기 치료학적 유효량은 약 150 밀리그램 내지 약 300 밀리그램 노출에 충분한 양인, 약학 조성물.
- 제 14항에 있어서, 상기 치료학적 유효량은 약 150 밀리그램 노출에 충분한 양인, 약학 조성물.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562236702P | 2015-10-02 | 2015-10-02 | |
| US62/236,702 | 2015-10-02 | ||
| PCT/US2016/055206 WO2017059451A1 (en) | 2015-10-02 | 2016-10-03 | Prevention, treatment and reversal of disease using therapeutically effective amounts of activated fatty acids |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180098222A true KR20180098222A (ko) | 2018-09-03 |
Family
ID=58428002
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187012337A Ceased KR20180098222A (ko) | 2015-10-02 | 2016-10-03 | 활성화된 지방산의 치료학적 유효량을 이용한 질환의 예방, 치료 및 역행 |
Country Status (17)
| Country | Link |
|---|---|
| US (3) | US10537541B2 (ko) |
| EP (1) | EP3355879A4 (ko) |
| JP (2) | JP2018529779A (ko) |
| KR (1) | KR20180098222A (ko) |
| CN (2) | CN113440506A (ko) |
| AU (2) | AU2016331314A1 (ko) |
| BR (1) | BR112018006687A2 (ko) |
| CA (1) | CA3000842A1 (ko) |
| CL (1) | CL2018000835A1 (ko) |
| CO (1) | CO2018004576A2 (ko) |
| CR (1) | CR20180246A (ko) |
| EA (1) | EA201890859A1 (ko) |
| HK (1) | HK1259031A1 (ko) |
| IL (2) | IL297844A (ko) |
| MX (1) | MX2018004043A (ko) |
| SG (1) | SG10201913953UA (ko) |
| WO (1) | WO2017059451A1 (ko) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2692291T3 (es) * | 2008-05-01 | 2018-12-03 | Complexa Inc. | Ácidos grasos vinil sustituidos |
| US20140024713A1 (en) | 2008-06-19 | 2014-01-23 | University Of Utah Research Foundation | Use of nitrated lipids for treatment of side effects of toxic medical therapies |
| ES2971969T3 (es) | 2015-07-07 | 2024-06-10 | H Lundbeck As | Inhibidores de PDE9 con cadena principal de imidazopirazinona para el tratamiento de enfermedades periféricas |
| KR20180098222A (ko) * | 2015-10-02 | 2018-09-03 | 컴플렉사, 인코포레이티드 | 활성화된 지방산의 치료학적 유효량을 이용한 질환의 예방, 치료 및 역행 |
| MA52792A (fr) | 2018-05-25 | 2021-04-14 | Imara Inc | Formes cristallines et monohydrate de 6- [(3s,4s)-4-méthyl-1-(pyrimidin-2-ylméthyl) pyrrolidin-3-yl]-3-tétrahydropyran-4-yl-7h-imidazo [1,5-a] pyrazin-8-one |
| KR20250108770A (ko) | 2018-08-31 | 2025-07-15 | 카듀리온 파마슈티칼스, 인크. | 겸상 세포 질환의 치료를 위한 pde9 억제제 |
| CN111229117B (zh) * | 2018-11-29 | 2022-01-04 | 中国石油化工股份有限公司 | 含脂肪酸型表面活性剂的混合体系及其制备方法 |
| CN111229120B (zh) * | 2018-11-29 | 2022-01-07 | 中国石油化工股份有限公司 | 含脂肪酸型表面活性剂的混合体系及其制备方法 |
| FR3092968B1 (fr) * | 2019-02-22 | 2021-05-21 | Microphyt | Complement alimentaire |
| CN110157792A (zh) * | 2019-04-22 | 2019-08-23 | 中山大学孙逸仙纪念医院 | 血清外泌体has_circ_0004771在制备酒精依赖综合征诊断试剂中的应用 |
| WO2021242758A1 (en) * | 2020-05-26 | 2021-12-02 | Imara Inc. | Improved nitro-fatty acid oral dose regimens |
| WO2023014748A1 (en) * | 2021-08-03 | 2023-02-09 | Imara Inc. | Nitrated fatty acids for the treatment of sickle cell disorders |
Family Cites Families (83)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB587992A (en) | 1944-12-11 | 1947-05-12 | Charles William Scaife | Improvements in and relating to the production of organic nitrogen compounds |
| US3578687A (en) | 1968-01-30 | 1971-05-11 | Texaco Inc | Process for producing 4-nitroalkanoic acids |
| US3819561A (en) | 1970-10-23 | 1974-06-25 | Aerojet General Co | Wetting agents for non-aqueous dispersions |
| JPS5313608B2 (ko) | 1972-06-16 | 1978-05-11 | ||
| JPS5318013B2 (ko) | 1973-03-19 | 1978-06-13 | ||
| US4599430A (en) | 1981-12-21 | 1986-07-08 | The Standard Oil Company | Nitrogenation of hydrocarbons, including the production of maleimide |
| JPS62132804A (ja) | 1985-12-05 | 1987-06-16 | Aguro Kanesho Kk | 植物生長調節剤 |
| US5412137A (en) | 1993-06-07 | 1995-05-02 | Sandoz Ltd. | Process for preparing phosphinyloxy propanaminium inner salt derivatives |
| DE69524639T2 (de) | 1994-10-13 | 2002-08-08 | Peptech Ltd., North Ryde | Modifizierte mehrfach ungesättigte fettsäuren |
| US5919816A (en) | 1994-11-14 | 1999-07-06 | Bionumerik Pharmaceuticals, Inc. | Formulations and methods of reducing toxicity of antineoplastic agents |
| US5741211A (en) | 1995-10-26 | 1998-04-21 | Medtronic, Inc. | System and method for continuous monitoring of diabetes-related blood constituents |
| GB9618420D0 (en) | 1996-09-04 | 1996-10-16 | Scotia Holdings Plc | Fatty acid treatment |
| US6187747B1 (en) | 1997-09-08 | 2001-02-13 | Panacea Biotech Limited | Pharmaceutical composition comprising cyclosporin |
| WO1999022719A1 (fr) | 1997-10-30 | 1999-05-14 | Morishita Jintan Co., Ltd. | Preparation de capsule contenant un acide gras insature ou un derive de celui-ci, et procede de fabrication |
| WO2000009075A2 (en) | 1998-08-14 | 2000-02-24 | Galenica Pharmaceuticals, Inc. | Chemically modified saponins and the use thereof as adjuvants |
| IL145712A0 (en) | 1999-04-01 | 2002-07-25 | Esperion Therapeutics Inc | Ether compounds, compositions, and uses thereof |
| WO2001006983A2 (en) | 1999-07-22 | 2001-02-01 | Incell Corporation, Llc | Fatty acids to minimize cancer therapy side effects |
| SE9903028D0 (sv) | 1999-08-27 | 1999-08-27 | Astra Ab | New use |
| AUPQ291499A0 (en) | 1999-09-17 | 1999-10-07 | Women's And Children's Hospital Adelaide | Novel nitro and sulphur containing compounds |
| US6346231B1 (en) | 1999-10-06 | 2002-02-12 | Joar Opheim | Flavored gelatin capsule and method of manufacture |
| US20010037598A1 (en) | 1999-12-14 | 2001-11-08 | Suppes Galen J. | Process for producing cetane improvers from triglycerides |
| CN100357244C (zh) | 2000-02-16 | 2007-12-26 | 布里格姆及妇女医院股份有限公司 | 阿司匹林触发的脂质介体 |
| AR030416A1 (es) | 2000-04-13 | 2003-08-20 | Pharmacia Corp | COMPUESTO DERIVADO HALOGENADO DEL ACIDO 2-AMINO-3,4 HEPTENOICO, COMPOSICION FARMACEUTICA QUE LO COMPRENDE Y SU USO EN LA FABRICACION DE UN MEDICAMENTO uTIL COMO INHIBIDOR DE LA OXIDO NITRICO SINTETASA |
| AR034120A1 (es) | 2000-04-13 | 2004-02-04 | Pharmacia Corp | Compuesto derivado halogenado del acido 2-amino-4,5 heptenoico, composicion farmaceutica que lo comprende y el uso de dicho compuesto y dicha composicion en la fabricacion de un medicamento para inhibir o modular la sintesis de acido nitrico |
| AR032318A1 (es) | 2000-04-13 | 2003-11-05 | Pharmacia Corp | Compuesto derivado halogenado del acido 2-amino-5,6 heptenoico; composicion farmaceutica que lo comprende y su uso en la fabricacion de un medicamento util como inhibidor de la oxido nitrico sintetasa |
| US20040006248A1 (en) | 2000-06-28 | 2004-01-08 | Zambon Group S.P.A | Process for the preparation of nitroalkenes |
| AR031129A1 (es) | 2000-09-15 | 2003-09-10 | Pharmacia Corp | Derivados de los acidos 2-amino-2-alquil-4-hexenoico y -hexinoico utiles como inhibidores de oxido nitrico sintetasa |
| US7745488B2 (en) | 2001-04-18 | 2010-06-29 | Prometic Biosciences, Inc. | Medium-chain length fatty acids, glycerides and analogues as neutrophil survival and activation factors |
| US7105556B2 (en) | 2001-05-30 | 2006-09-12 | Bristol-Myers Squibb Company | Conformationally constrained analogs useful as antidiabetic and antiobesity agents and method |
| JPWO2002102364A1 (ja) | 2001-06-18 | 2004-09-30 | 山田 幸子 | PPARγ作動性医薬組成物 |
| WO2003015704A2 (en) | 2001-08-17 | 2003-02-27 | University Of Pittsburgh | Administration of estradiol metabolites for the treatment or prevention of obesity, metabolic syndrome, diabetes, and vascular and renal disorders |
| GB0123961D0 (en) | 2001-10-05 | 2001-11-28 | Astrazeneca Ab | Process and intermediates |
| CA2465117C (en) | 2001-11-06 | 2012-01-03 | Brigham And Women's Hospital, Inc. | Lipoxins and their stable analogs in the treatment of asthma and inflammatory airway diseases |
| US7759395B2 (en) | 2002-08-12 | 2010-07-20 | The Brigham And Women's Hospital, Inc. | Use of docosatrienes, resolvins and their stable analogs in the treatment of airway diseases and asthma |
| US20060100278A1 (en) | 2002-08-20 | 2006-05-11 | Cooper Garth J S | Dosage forms and related therapies |
| AU2003272738B2 (en) | 2002-09-27 | 2010-04-01 | Martek Biosciences Corporation | Docosahexaenoic acid for improved glycemic control |
| US7166575B2 (en) | 2002-12-17 | 2007-01-23 | Nastech Pharmaceutical Company Inc. | Compositions and methods for enhanced mucosal delivery of peptide YY and methods for treating and preventing obesity |
| WO2005016864A1 (en) | 2003-07-29 | 2005-02-24 | The Arizona Disease Control Research Commission | Conjugated nitro alkene anticancer agents based on isoprenoid metabolism |
| US20050136103A1 (en) | 2003-09-17 | 2005-06-23 | Ben-Sasson Shmuel A. | Compositions capable of facilitating penetration across a biological barrier |
| US7935365B2 (en) | 2003-10-22 | 2011-05-03 | Enzymotec, Ltd. | Glycerophospholipids for the improvement of cognitive functions |
| JP2007522118A (ja) | 2004-01-30 | 2007-08-09 | ペプリン バイオリピッズ ピーティーワイ エルティーディー | 治療用分子および担体分子 |
| WO2006097793A2 (en) | 2004-04-15 | 2006-09-21 | Chiasma, Ltd. | Compositions capable of facilitating penetration across a biological barrier |
| US7776916B2 (en) | 2004-04-28 | 2010-08-17 | The Uab Research Foundation | Nitrated lipids and methods of making and using thereof |
| CN101027318B (zh) | 2004-07-19 | 2016-05-25 | 比奥孔有限公司 | 胰岛素-低聚物共轭物,制剂及其用途 |
| EP1772149A1 (en) | 2004-07-27 | 2007-04-11 | Kowa Company. Ltd. | Drug for prevention or treatment of diabetes |
| JP2008520739A (ja) | 2004-11-19 | 2008-06-19 | マーテック・バイオサイエンシーズ・コーポレーション | 長鎖多価不飽和脂肪酸由来のオキシリピンならびにその作製および使用方法 |
| US20070032420A1 (en) | 2005-02-09 | 2007-02-08 | Entelos, Inc. | Treating diabetes with glucagon-like peptide-1 secretagogues |
| US9274129B2 (en) | 2006-05-31 | 2016-03-01 | Lpath, Inc. | Methods and reagents for detecting bioactive lipids |
| WO2008008767A2 (en) | 2006-07-14 | 2008-01-17 | Cedars-Sinai Medical Center | Methods of using ppar-gamma agonists and caspase-dependent chemotherapeutic agents for the treatment of cancer |
| RU2009101324A (ru) | 2006-07-19 | 2010-07-27 | Ризолвикс Фармасьютикалз, Инк. (Us) | Композиции и способы лечения воспаления слизистой оболочки |
| US20110190389A1 (en) | 2007-02-20 | 2011-08-04 | Linda Arterburn | Oxylipins from long chain polyunsaturated fatty acids and methods of making and using the same |
| US8324277B2 (en) | 2007-08-01 | 2012-12-04 | University of Pittsburgh—of the Commonwealth System of Higher Education | Nitrated-fatty acids modulation of type II diabetes |
| AU2008301895A1 (en) | 2007-09-14 | 2009-03-26 | Resolvyx Pharmaceuticals, Inc. | Oxylipin compounds for treating autoimmune diseases |
| EP2271929B1 (en) | 2008-04-18 | 2015-11-25 | The University of Utah Research Foundation | Use of nitrated lipids for treatment of lipid disorders and obesity, and lipid- and obesity-related conditions |
| ES2692291T3 (es) | 2008-05-01 | 2018-12-03 | Complexa Inc. | Ácidos grasos vinil sustituidos |
| WO2009149496A1 (en) | 2008-06-10 | 2009-12-17 | Central Northern Adelaide Health Service | Treatment of diabetes and complications thereof and related disorders |
| EP2299997A4 (en) | 2008-06-19 | 2012-01-11 | Univ Utah Res Found | USE OF NITRATED LIPIDS FOR THE TREATMENT OF SIDE EFFECTS OF TOXIC MEDICAL THERAPIES |
| US20140024713A1 (en) | 2008-06-19 | 2014-01-23 | University Of Utah Research Foundation | Use of nitrated lipids for treatment of side effects of toxic medical therapies |
| CN102105152B (zh) | 2008-07-29 | 2012-10-17 | 内尔维阿诺医学科学有限公司 | 包含cdks抑制剂和抗肿瘤剂的治疗组合 |
| EP3225621A1 (en) | 2008-10-09 | 2017-10-04 | Arbutus Biopharma Corporation | Improved amino lipids and methods for the delivery of nucleic acids |
| CN102307464A (zh) | 2008-12-31 | 2012-01-04 | 尼特罗米加公司 | 含有硝基脂肪酸的营养制品 |
| US8937194B2 (en) | 2008-12-31 | 2015-01-20 | Nitromega Corp. | Topical compositions containing nitro fatty acids |
| US20100286257A1 (en) | 2009-05-08 | 2010-11-11 | Perricone Nicholas V | Methods Of Use Of Nitroalkane Compositions In Dermatologic Applications To Prevent or Treat Skin Aging |
| US20100286272A1 (en) | 2009-05-08 | 2010-11-11 | Perricone Nicholas V | Methods Of Use Of Nitroalkene Compositions In Dermatologic Applications |
| US20100286271A1 (en) | 2009-05-08 | 2010-11-11 | Perricone Nicholas V | Nitro-alkyl Compound Compositions |
| RU2012106896A (ru) | 2009-07-29 | 2013-09-10 | Феноменом Дискавериз Инк. | Гидроксилированные жирные кислоты и их применение для лечения и диагностики заболеваний |
| JP2013500966A (ja) | 2009-07-31 | 2013-01-10 | ユニバーシティー オブ ピッツバーグ − オブ ザ コモンウェルス システム オブ ハイヤー エデュケーション | 抗炎症剤としての脂肪酸 |
| EP2483233A4 (en) | 2009-10-02 | 2013-08-14 | Complexa Inc | HETEROATOMA CONTAINING SUBSTITUTED FATTY ACIDS |
| US20130005730A1 (en) | 2009-11-09 | 2013-01-03 | Piaoyang Sun | Novel 1,3-oxazolidine compounds and their use as renin inhibitors |
| WO2011098746A1 (en) | 2010-02-09 | 2011-08-18 | Pulmagen Therapeutics (Inflammation) Limited | Crystalline acid addition salts of ( 5r) -enanti0mer of pioglitazone |
| CN102970972B (zh) * | 2010-03-15 | 2015-12-16 | 安雷戚·迪兹 | 硝基羧酸用于治疗、诊断和预防侵袭性愈合模式的用途 |
| WO2011143587A1 (en) * | 2010-05-13 | 2011-11-17 | Nitromega Corp. | Nitro fatty acids - neuroprotection and/or inhibition of cognitive decline |
| US20110319325A1 (en) | 2010-06-28 | 2011-12-29 | Complexa, Inc. | Multi-component pharmaceuticals for treating diabetes |
| EP2744491B1 (en) | 2011-08-19 | 2020-07-29 | The University of Utah Research Foundation | Combination therapy with nitrated lipids and inhibitors of the renin-angiotensin-aldosterone system |
| US9271952B2 (en) | 2011-10-11 | 2016-03-01 | Complexa, Inc. | Compositions and methods for treating nephropathy |
| DE102012008730A1 (de) | 2011-12-02 | 2013-06-06 | Dr. Budz GmbH | Orale Zusammensetzungen mit ungesättigten C18-Fettsäuren und ihre Verwendung |
| US20150018417A1 (en) | 2012-02-03 | 2015-01-15 | University Of Pittsburgh - Of The Commonwealth System Of Higher Education | Fatty acids as anti-inflammatory agents |
| US20140271844A1 (en) * | 2013-03-15 | 2014-09-18 | Nitromega Corp. | Compositions containing nitro fatty acids |
| EP2994165A4 (en) | 2013-05-10 | 2017-01-04 | Nitromega Corp. | Nutritional or dietary supplements containing fatty acids and nitrite |
| WO2014204872A2 (en) | 2013-06-14 | 2014-12-24 | Complexa, Inc. | Composition and method for inhibition of pkng from mycobacterium tuberculosis |
| WO2015073527A1 (en) | 2013-11-12 | 2015-05-21 | Complexa, Inc. | Nitroalkene tocopherols and analogs thereof for use in the treatment and prevention of inflammation related conditions |
| WO2016161285A1 (en) * | 2015-04-02 | 2016-10-06 | Aobiome Llc | Production of nitro-fatty acids and nitro-hydrocarbons by ammonia oxidizing bacteria |
| KR20180098222A (ko) * | 2015-10-02 | 2018-09-03 | 컴플렉사, 인코포레이티드 | 활성화된 지방산의 치료학적 유효량을 이용한 질환의 예방, 치료 및 역행 |
-
2016
- 2016-10-03 KR KR1020187012337A patent/KR20180098222A/ko not_active Ceased
- 2016-10-03 MX MX2018004043A patent/MX2018004043A/es unknown
- 2016-10-03 EP EP16852846.1A patent/EP3355879A4/en not_active Withdrawn
- 2016-10-03 HK HK19101519.9A patent/HK1259031A1/zh unknown
- 2016-10-03 CN CN202110744035.6A patent/CN113440506A/zh active Pending
- 2016-10-03 IL IL297844A patent/IL297844A/en unknown
- 2016-10-03 WO PCT/US2016/055206 patent/WO2017059451A1/en not_active Ceased
- 2016-10-03 CR CR20180246A patent/CR20180246A/es unknown
- 2016-10-03 CN CN201680070797.XA patent/CN108430466A/zh active Pending
- 2016-10-03 CA CA3000842A patent/CA3000842A1/en not_active Abandoned
- 2016-10-03 IL IL258476A patent/IL258476B2/en unknown
- 2016-10-03 US US15/283,887 patent/US10537541B2/en not_active Expired - Fee Related
- 2016-10-03 JP JP2018536704A patent/JP2018529779A/ja not_active Withdrawn
- 2016-10-03 EA EA201890859A patent/EA201890859A1/ru unknown
- 2016-10-03 BR BR112018006687A patent/BR112018006687A2/pt not_active Application Discontinuation
- 2016-10-03 AU AU2016331314A patent/AU2016331314A1/en not_active Abandoned
- 2016-10-03 SG SG10201913953UA patent/SG10201913953UA/en unknown
-
2018
- 2018-03-29 CL CL2018000835A patent/CL2018000835A1/es unknown
- 2018-04-27 CO CONC2018/0004576A patent/CO2018004576A2/es unknown
-
2019
- 2019-10-18 US US16/657,028 patent/US20200046669A1/en not_active Abandoned
-
2021
- 2021-03-19 US US17/207,380 patent/US20220000824A1/en not_active Abandoned
- 2021-07-14 AU AU2021205027A patent/AU2021205027A1/en not_active Abandoned
- 2021-08-27 JP JP2021139287A patent/JP2021183640A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| CN113440506A (zh) | 2021-09-28 |
| EP3355879A1 (en) | 2018-08-08 |
| EP3355879A4 (en) | 2019-05-22 |
| CA3000842A1 (en) | 2017-04-06 |
| JP2021183640A (ja) | 2021-12-02 |
| US20220000824A1 (en) | 2022-01-06 |
| CR20180246A (es) | 2018-11-22 |
| JP2018529779A (ja) | 2018-10-11 |
| HK1259031A1 (zh) | 2019-11-22 |
| EA201890859A1 (ru) | 2018-11-30 |
| SG10201913953UA (en) | 2020-03-30 |
| CN108430466A (zh) | 2018-08-21 |
| AU2021205027A1 (en) | 2021-08-12 |
| IL258476B (en) | 2022-12-01 |
| AU2016331314A1 (en) | 2018-05-17 |
| IL297844A (en) | 2023-01-01 |
| US20170095437A1 (en) | 2017-04-06 |
| CL2018000835A1 (es) | 2018-09-14 |
| WO2017059451A1 (en) | 2017-04-06 |
| CO2018004576A2 (es) | 2018-07-19 |
| IL258476B2 (en) | 2023-04-01 |
| IL258476A (en) | 2018-06-28 |
| US20200046669A1 (en) | 2020-02-13 |
| MX2018004043A (es) | 2018-11-09 |
| BR112018006687A2 (pt) | 2018-10-09 |
| US10537541B2 (en) | 2020-01-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10537541B2 (en) | Treatment of focal segmental glomerular sclerosis (FSGS) using therapeutically effective oral doses of 10-nitro-9(E)-octadec-9-enoic acid | |
| JP6986022B2 (ja) | Fxrアゴニストを使用するための方法 | |
| US10537537B2 (en) | Methods of treatment of inflammation related conditions using pluripotent anti-inflammatory and metabolic modulators | |
| US20230321022A1 (en) | Reversibly protected thiolated electrophilic fatty acids as prodrugs | |
| US20250099411A1 (en) | Methods of Treatment of Inflammation Related Conditions Using Pluripotent Anti-Inflammatory and Metabolic Modulators | |
| JP6878596B2 (ja) | Fxrアゴニストの組合せ | |
| EP3440045B1 (en) | Prevention, treatment and reversal of disease using therapeutically effective amounts of dicarboxylic acid compounds | |
| KR20210110407A (ko) | Fxr 작용제의 신규 요법 | |
| US11491128B2 (en) | Reversible nitroxide derivatives of nitroalkenes that mediate nitrosating and alkylating reactions | |
| JP2020533339A (ja) | Fxrアゴニストを含む組合せ | |
| US11400066B2 (en) | Methods of treatment of inflammation related conditions using pluripotent anti-inflammatory and metabolic modulators |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20180430 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210930 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20231201 Patent event code: PE09021S01D |
|
| PE0601 | Decision on rejection of patent |
Patent event date: 20240701 Comment text: Decision to Refuse Application Patent event code: PE06012S01D |