KR20180098000A - 피부 미용액을 이용한 화장료 조성물의 제조방법 - Google Patents
피부 미용액을 이용한 화장료 조성물의 제조방법 Download PDFInfo
- Publication number
- KR20180098000A KR20180098000A KR1020170024898A KR20170024898A KR20180098000A KR 20180098000 A KR20180098000 A KR 20180098000A KR 1020170024898 A KR1020170024898 A KR 1020170024898A KR 20170024898 A KR20170024898 A KR 20170024898A KR 20180098000 A KR20180098000 A KR 20180098000A
- Authority
- KR
- South Korea
- Prior art keywords
- extraction
- less
- extract
- glycerin
- skin
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 36
- 239000002537 cosmetic Substances 0.000 title claims abstract description 34
- 238000000034 method Methods 0.000 title claims abstract description 27
- 238000000605 extraction Methods 0.000 claims abstract description 79
- 239000002904 solvent Substances 0.000 claims abstract description 60
- 239000000419 plant extract Substances 0.000 claims abstract description 9
- 239000000284 extract Substances 0.000 claims description 54
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 26
- 241000196324 Embryophyta Species 0.000 claims description 25
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 claims description 19
- 210000002966 serum Anatomy 0.000 claims description 18
- 238000004519 manufacturing process Methods 0.000 claims description 16
- 235000011187 glycerol Nutrition 0.000 claims description 13
- WMBWREPUVVBILR-WIYYLYMNSA-N (-)-Epigallocatechin-3-o-gallate Chemical compound O([C@@H]1CC2=C(O)C=C(C=C2O[C@@H]1C=1C=C(O)C(O)=C(O)C=1)O)C(=O)C1=CC(O)=C(O)C(O)=C1 WMBWREPUVVBILR-WIYYLYMNSA-N 0.000 claims description 12
- WMBWREPUVVBILR-UHFFFAOYSA-N GCG Natural products C=1C(O)=C(O)C(O)=CC=1C1OC2=CC(O)=CC(O)=C2CC1OC(=O)C1=CC(O)=C(O)C(O)=C1 WMBWREPUVVBILR-UHFFFAOYSA-N 0.000 claims description 12
- ACCCMOQWYVYDOT-UHFFFAOYSA-N hexane-1,1-diol Chemical compound CCCCCC(O)O ACCCMOQWYVYDOT-UHFFFAOYSA-N 0.000 claims description 12
- ULWHHBHJGPPBCO-UHFFFAOYSA-N propane-1,1-diol Chemical compound CCC(O)O ULWHHBHJGPPBCO-UHFFFAOYSA-N 0.000 claims description 12
- 150000002314 glycerols Chemical class 0.000 claims description 8
- NCZPCONIKBICGS-UHFFFAOYSA-N 3-(2-ethylhexoxy)propane-1,2-diol Chemical group CCCCC(CC)COCC(O)CO NCZPCONIKBICGS-UHFFFAOYSA-N 0.000 claims description 6
- 229940100524 ethylhexylglycerin Drugs 0.000 claims description 6
- 238000001914 filtration Methods 0.000 claims description 5
- DNIAPMSPPWPWGF-VKHMYHEASA-N (+)-propylene glycol Chemical compound C[C@H](O)CO DNIAPMSPPWPWGF-VKHMYHEASA-N 0.000 claims description 4
- 229940015975 1,2-hexanediol Drugs 0.000 claims description 4
- YPFDHNVEDLHUCE-UHFFFAOYSA-N 1,3-propanediol Substances OCCCO YPFDHNVEDLHUCE-UHFFFAOYSA-N 0.000 claims description 4
- 229940030275 epigallocatechin gallate Drugs 0.000 claims description 4
- FHKSXSQHXQEMOK-UHFFFAOYSA-N hexane-1,2-diol Chemical compound CCCCC(O)CO FHKSXSQHXQEMOK-UHFFFAOYSA-N 0.000 claims description 4
- 229920000166 polytrimethylene carbonate Polymers 0.000 claims description 4
- 229940058015 1,3-butylene glycol Drugs 0.000 claims description 3
- 235000019437 butane-1,3-diol Nutrition 0.000 claims description 3
- 239000008406 cosmetic ingredient Substances 0.000 claims description 3
- 238000001816 cooling Methods 0.000 claims description 2
- 235000019225 fermented tea Nutrition 0.000 claims description 2
- 238000010992 reflux Methods 0.000 claims description 2
- 238000002137 ultrasound extraction Methods 0.000 claims description 2
- 241001122767 Theaceae Species 0.000 claims 1
- 235000013616 tea Nutrition 0.000 claims 1
- 230000002708 enhancing effect Effects 0.000 abstract 1
- 230000001737 promoting effect Effects 0.000 abstract 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 19
- 230000000052 comparative effect Effects 0.000 description 18
- 244000269722 Thea sinensis Species 0.000 description 13
- 235000009569 green tea Nutrition 0.000 description 13
- 230000000694 effects Effects 0.000 description 12
- 239000007788 liquid Substances 0.000 description 11
- 230000014509 gene expression Effects 0.000 description 10
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 9
- 230000003078 antioxidant effect Effects 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 239000000126 substance Substances 0.000 description 8
- 238000012360 testing method Methods 0.000 description 7
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 239000003963 antioxidant agent Substances 0.000 description 6
- 230000003247 decreasing effect Effects 0.000 description 6
- 238000000855 fermentation Methods 0.000 description 6
- 230000004151 fermentation Effects 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 6
- 239000002994 raw material Substances 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- 102100027456 Cytochrome c oxidase subunit 2 Human genes 0.000 description 5
- 101710091264 Cytochrome c oxidase subunit 2 Proteins 0.000 description 5
- 235000006708 antioxidants Nutrition 0.000 description 5
- 239000000470 constituent Substances 0.000 description 5
- 102000016938 Catalase Human genes 0.000 description 4
- 108010053835 Catalase Proteins 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- 101000588302 Homo sapiens Nuclear factor erythroid 2-related factor 2 Proteins 0.000 description 4
- 102000011782 Keratins Human genes 0.000 description 4
- 108010076876 Keratins Proteins 0.000 description 4
- 102100031701 Nuclear factor erythroid 2-related factor 2 Human genes 0.000 description 4
- 230000003712 anti-aging effect Effects 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 239000006210 lotion Substances 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 230000037394 skin elasticity Effects 0.000 description 4
- 238000000638 solvent extraction Methods 0.000 description 4
- PRAKJMSDJKAYCZ-UHFFFAOYSA-N squalane Chemical compound CC(C)CCCC(C)CCCC(C)CCCCC(C)CCCC(C)CCCC(C)C PRAKJMSDJKAYCZ-UHFFFAOYSA-N 0.000 description 4
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 230000003110 anti-inflammatory effect Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 230000003013 cytotoxicity Effects 0.000 description 3
- 231100000135 cytotoxicity Toxicity 0.000 description 3
- 229940093499 ethyl acetate Drugs 0.000 description 3
- 235000019439 ethyl acetate Nutrition 0.000 description 3
- 235000016709 nutrition Nutrition 0.000 description 3
- 239000003960 organic solvent Substances 0.000 description 3
- 238000000956 solid--liquid extraction Methods 0.000 description 3
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 2
- POJWUDADGALRAB-UHFFFAOYSA-N allantoin Chemical compound NC(=O)NC1NC(=O)NC1=O POJWUDADGALRAB-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 239000013522 chelant Substances 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 235000013399 edible fruits Nutrition 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- 235000019634 flavors Nutrition 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 238000011081 inoculation Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000000813 microbial effect Effects 0.000 description 2
- JXTPJDDICSTXJX-UHFFFAOYSA-N n-Triacontane Natural products CCCCCCCCCCCCCCCCCCCCCCCCCCCCCC JXTPJDDICSTXJX-UHFFFAOYSA-N 0.000 description 2
- 230000035764 nutrition Effects 0.000 description 2
- 239000013641 positive control Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 239000008213 purified water Substances 0.000 description 2
- 238000003753 real-time PCR Methods 0.000 description 2
- 229930002330 retinoic acid Natural products 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 230000036548 skin texture Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000008247 solid mixture Substances 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- 229940032094 squalane Drugs 0.000 description 2
- 229960001727 tretinoin Drugs 0.000 description 2
- 229940035437 1,3-propanediol Drugs 0.000 description 1
- 208000002874 Acne Vulgaris Diseases 0.000 description 1
- POJWUDADGALRAB-PVQJCKRUSA-N Allantoin Natural products NC(=O)N[C@@H]1NC(=O)NC1=O POJWUDADGALRAB-PVQJCKRUSA-N 0.000 description 1
- 201000004624 Dermatitis Diseases 0.000 description 1
- 206010013786 Dry skin Diseases 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 238000009017 Fluorometric Assay Kit Methods 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 102100039696 Glutamate-cysteine ligase catalytic subunit Human genes 0.000 description 1
- 108010018924 Heme Oxygenase-1 Proteins 0.000 description 1
- 102100028006 Heme oxygenase 1 Human genes 0.000 description 1
- 101001034527 Homo sapiens Glutamate-cysteine ligase catalytic subunit Proteins 0.000 description 1
- 101000973778 Homo sapiens NAD(P)H dehydrogenase [quinone] 1 Proteins 0.000 description 1
- 101000741967 Homo sapiens Presequence protease, mitochondrial Proteins 0.000 description 1
- 102000004125 Interleukin-1alpha Human genes 0.000 description 1
- 108010082786 Interleukin-1alpha Proteins 0.000 description 1
- 102000004034 Kelch-Like ECH-Associated Protein 1 Human genes 0.000 description 1
- 108090000484 Kelch-Like ECH-Associated Protein 1 Proteins 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- 102100022365 NAD(P)H dehydrogenase [quinone] 1 Human genes 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 238000010222 PCR analysis Methods 0.000 description 1
- 229920001214 Polysorbate 60 Polymers 0.000 description 1
- 102100038632 Presequence protease, mitochondrial Human genes 0.000 description 1
- 238000002123 RNA extraction Methods 0.000 description 1
- 101150032199 Rplp0 gene Proteins 0.000 description 1
- 206010040914 Skin reaction Diseases 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 206010000496 acne Diseases 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229930013930 alkaloid Natural products 0.000 description 1
- 229960000458 allantoin Drugs 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000003064 anti-oxidating effect Effects 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 125000004429 atom Chemical group 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 230000007541 cellular toxicity Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 238000007398 colorimetric assay Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 229930003935 flavonoid Natural products 0.000 description 1
- 150000002215 flavonoids Chemical class 0.000 description 1
- 235000017173 flavonoids Nutrition 0.000 description 1
- LNTHITQWFMADLM-UHFFFAOYSA-N gallic acid Chemical compound OC(=O)C1=CC(O)=C(O)C(O)=C1 LNTHITQWFMADLM-UHFFFAOYSA-N 0.000 description 1
- 229960001031 glucose Drugs 0.000 description 1
- 229960005150 glycerol Drugs 0.000 description 1
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical compound C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 1
- 229940094952 green tea extract Drugs 0.000 description 1
- 235000020688 green tea extract Nutrition 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000006651 lactation Effects 0.000 description 1
- 238000002386 leaching Methods 0.000 description 1
- 229940057995 liquid paraffin Drugs 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 238000000622 liquid--liquid extraction Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- 230000003020 moisturizing effect Effects 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- GSGDTSDELPUTKU-UHFFFAOYSA-N nonoxybenzene Chemical compound CCCCCCCCCOC1=CC=CC=C1 GSGDTSDELPUTKU-UHFFFAOYSA-N 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 1
- 230000036542 oxidative stress Effects 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 235000010989 polyoxyethylene sorbitan monostearate Nutrition 0.000 description 1
- 239000001818 polyoxyethylene sorbitan monostearate Substances 0.000 description 1
- 229940113124 polysorbate 60 Drugs 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- IZTQOLKUZKXIRV-YRVFCXMDSA-N sincalide Chemical compound C([C@@H](C(=O)N[C@@H](CCSC)C(=O)NCC(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(N)=O)NC(=O)[C@@H](N)CC(O)=O)C1=CC=C(OS(O)(=O)=O)C=C1 IZTQOLKUZKXIRV-YRVFCXMDSA-N 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 230000035483 skin reaction Effects 0.000 description 1
- 231100000430 skin reaction Toxicity 0.000 description 1
- 235000010378 sodium ascorbate Nutrition 0.000 description 1
- PPASLZSBLFJQEF-RKJRWTFHSA-M sodium ascorbate Substances [Na+].OC[C@@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RKJRWTFHSA-M 0.000 description 1
- 229960005055 sodium ascorbate Drugs 0.000 description 1
- PPASLZSBLFJQEF-RXSVEWSESA-M sodium-L-ascorbate Chemical compound [Na+].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RXSVEWSESA-M 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 230000035882 stress Effects 0.000 description 1
- 150000003505 terpenes Chemical class 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 238000002723 toxicity assay Methods 0.000 description 1
- LADGBHLMCUINGV-UHFFFAOYSA-N tricaprin Chemical compound CCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCC)COC(=O)CCCCCCCCC LADGBHLMCUINGV-UHFFFAOYSA-N 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 230000037303 wrinkles Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/34—Alcohols
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/34—Alcohols
- A61K8/345—Alcohols containing more than one hydroxy group
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/49—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds
- A61K8/4973—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with oxygen as the only hetero atom
- A61K8/498—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing heterocyclic compounds with oxygen as the only hetero atom having 6-membered rings or their condensed derivatives, e.g. coumarin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/96—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution
- A61K8/97—Cosmetics or similar toiletry preparations characterised by the composition containing materials, or derivatives thereof of undetermined constitution from algae, fungi, lichens or plants; from derivatives thereof
- A61K8/9783—Angiosperms [Magnoliophyta]
- A61K8/9789—Magnoliopsida [dicotyledons]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/08—Anti-ageing preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/52—Stabilizers
- A61K2800/522—Antioxidants; Radical scavengers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/59—Mixtures
- A61K2800/592—Mixtures of compounds complementing their respective functions
- A61K2800/5922—At least two compounds being classified in the same subclass of A61K8/18
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/80—Process related aspects concerning the preparation of the cosmetic composition or the storage or application thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/80—Process related aspects concerning the preparation of the cosmetic composition or the storage or application thereof
- A61K2800/82—Preparation or application process involves sonication or ultrasonication
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/80—Process related aspects concerning the preparation of the cosmetic composition or the storage or application thereof
- A61K2800/85—Products or compounds obtained by fermentation, e.g. yoghurt, beer, wine
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Birds (AREA)
- Epidemiology (AREA)
- Emergency Medicine (AREA)
- Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Botany (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Dermatology (AREA)
- Gerontology & Geriatric Medicine (AREA)
- Cosmetics (AREA)
Abstract
Description
도 2는 본 발명의 일 측면에 따라 추출한 추출물을 사용했을 경우 피부보습 효과가 향상됨을 나타낸 것이다.
도 3은 본 발명의 일 측면에 따라 추출한 추출물을 사용했을 경우 피부탄력이 증진됨을 나타낸 것이다.
도 4는 본 발명의 일 측면에 따라 추출한 추출물을 사용했을 경우 피부 투명도가 개선됨을 나타낸 것이다.
도 5는 본 발명의 일 측면에 따라 추출한 추출물을 사용했을 경우 피부각질에서 항산화 효소(catalase)의 활성이 증진되어 피부 항산화 효과가 있음을 나타낸 것이다.
도 6은 본 발명의 일 측면에 따른 실시예 1과 비교예 1의 미용액(용매)에 의해 추출한 추출물의 세포독성을 비교한 것이다.
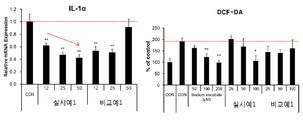
도 7은 본 발명의 일 측면에 따른 실시예 1과 비교예 1의 미용액(용매)으로 추출한 추출물을 사용한 경우 IL-1α 및 DCF-DA 발현 정도를 측정한 결과를 나타낸 것이다.
도 8은 본 발명의 일 측면에 따른 실시예의 미용액(용매)에 의해 추출한 추출물을 사용한 경우 MMP-1, COX2, Nrf2 및 Keap-1을 평가한 결과를 나타낸 것이다.
| 구분 | 성분명 | 실시예 1 | 실시예 2 | 비교예 1 | 비교예 2 |
| 1 | 정제수 | 잔량 | 잔량 | 100(열수) | - |
| 2 | 에탄올 | - | - | - | 100 |
| 3 | 글리세린 | 15 | 15 | - | - |
| 4 | 1,3-부틸렌글라이콜 | 20 | 20 | - | - |
| 5 | 1,3-프로판다이올 | 10 | - | - | - |
| 6 | 1,2-헥산다이올 | 1 | 1 | - | - |
| 7 | 에틸헥실글리세린 | 0.1 | 0.1 | - | - |
| 총중량 | 100 | 100 | 100 | 100 |
| 추출용매 | 추출효율 | 비고 |
| 열수(비교예 1) | 66% | 녹차 1g에 대해 실시예 1을 사용했을 경우(즉, 추출물 전체를 100g으로 보았을 때)의 EGCG 추출량(42mg) 기준 |
| 에탄올(비교예 2) | 82% | |
| 실시예1 | 100% | |
| 실시예2 | 94% |
| 시험 대상자 | 39~49세 여성 (평균 약 45.7세) |
| 시험 기간 | 4주 |
| 시험 부위 | 안면,전박 |
| 시험 항목 | 피부결, 수분, 탄력, 투명도, 항산화 수준 |
| 측정 시기 | 제품 사용 직후, 1주, 2주, 4주 시점에서 측정 및 평가 |
Claims (19)
- 피부 미용액을 이용하여 식물로부터 추출물을 추출하는 단계; 및
상기 단계에서 추출된 추출물에 부가 성분을 가하여 화장료 조성물을 제조하는 단계를 포함하는, 식물 추출물을 함유하는 화장료 조성물의 제조 방법. - 제1항에 있어서, 상기 식물은 차나무 잎인, 제조 방법.
- 제1항에 있어서, 상기 식물은 발효시킨 차나무 잎인, 제조 방법.
- 제1항에 있어서, 상기 추출은 초음파추출, 상온추출, 냉침 추출, 또는 환류 냉각 추출인, 제조 방법.
- 제1항에 있어서, 상기 추출은 10 내지 50℃에서 실시하는 것인, 제조 방법.
- 제1항에 있어서, 상기 추출은 3 내지 30시간 동안 실시하는 것인, 제조 방법.
- 제1항에 있어서, 상기 추출은 5 내지 100 rpm에서 실시하는 것인, 제조 방법.
- 제1항에 있어서, 상기 추출하는 단계는 필터링하는 단계를 더 포함하는 것인, 제조 방법.
- 식물 추출물을 함유하는 화장료 조성물의 제조에 있어서 식물 추출에 사용하기 위한, 피부 미용액으로 구성된 추출 용매.
- 제9항에 있어서, 상기 피부 미용액은 부틸렌글라이콜, 프로판다이올 및 헥산다이올로 이루어진 군에서 선택된 하나 이상, 및 글리세린과 글리세린 유도체 중 하나 이상을 포함하는 것인, 추출 용매.
- 제10항에 있어서 상기 부틸렌글라이콜은 1,3-부틸렌글라이콜이고, 상기 프로판다이올은 1,3-프로판다이올이며, 상기 헥산다이올은 1,2-헥산다이올인, 추출 용매.
- 제10항에 있어서, 상기 글리세린 유도체는 에틸헥실글리세린인, 추출 용매.
- 제9항에 있어서, 상기 피부 미용액은 글리세린, 부틸렌글라이콜, 프로판다이올, 헥산다이올, 및 에틸헥실글리세린을 포함하는 것인, 추출 용매.
- 제10항에 있어서, 상기 부틸렌글라이콜의 함량은 상기 추출 용매 총중량 대비 10 내지 25 중량%;
상기 프로판다이올의 함량은 상기 추출 용매 총중량 대비 1 내지 20 중량%; 또는
상기 헥산다이올의 함량은 상기 추출 용매 총중량 대비 0.1 내지 5 중량%인, 추출 용매. - 제10항에 있어서, 상기 글리세린의 함량은 상기 추출 용매 총중량 대비 5 내지 20 중량%; 또는 상기 글리세린 유도체의 함량은 상기 추출 용매 총중량 대비 0.01 내지 10 중량%인, 추출 용매.
- 제10항에 있어서, 상기 글리세린:부틸렌글라이콜의 중량 비율은 1 : 0.5 내지 5;
상기 부틸렌글라이콜:프로판다이올의 중량 비율은 1 : 0.04 내지 2;
상기 프로판다이올:헥산다이올의 중량 비율은 1 : 0.005 내지 5; 또는
상기 헥산다이올:글리세린 유도체의 중량 비율은 1 : 0.002 내지 100인, 추출 용매. - 제9항 내지 제16항에 따른 추출 용매 및 그에 의해 식물로부터 추출된 성분을 포함하는 추출물.
- 제17항에 있어서, 상기 추출물은 상기 식물 1g 대비 에피갈로카테킨 갈레이트(Epigallocatechin gallate, EGCG)의 함량이 20 내지 60 mg인, 추출물.
- 제17항에 따른 추출물을 포함하는, 화장료 조성물.
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020170024898A KR102610940B1 (ko) | 2017-02-24 | 2017-02-24 | 피부 미용액을 이용한 화장료 조성물의 제조방법 |
| US16/479,052 US11179319B2 (en) | 2017-02-24 | 2018-01-31 | Method for producing cosmetic composition using skin serum |
| AU2018226144A AU2018226144B2 (en) | 2017-02-24 | 2018-01-31 | Method for producing cosmetic composition using skin cosmetic solution |
| PCT/KR2018/001310 WO2018155829A1 (ko) | 2017-02-24 | 2018-01-31 | 피부 미용액을 이용한 화장료 조성물의 제조방법 |
| TW107105936A TWI827537B (zh) | 2017-02-24 | 2018-02-22 | 使用皮膚化妝品溶液製備化妝品組合物的方法 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020170024898A KR102610940B1 (ko) | 2017-02-24 | 2017-02-24 | 피부 미용액을 이용한 화장료 조성물의 제조방법 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20180098000A true KR20180098000A (ko) | 2018-09-03 |
| KR102610940B1 KR102610940B1 (ko) | 2023-12-08 |
Family
ID=63253298
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020170024898A Active KR102610940B1 (ko) | 2017-02-24 | 2017-02-24 | 피부 미용액을 이용한 화장료 조성물의 제조방법 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US11179319B2 (ko) |
| KR (1) | KR102610940B1 (ko) |
| AU (1) | AU2018226144B2 (ko) |
| TW (1) | TWI827537B (ko) |
| WO (1) | WO2018155829A1 (ko) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20200137432A (ko) * | 2019-05-30 | 2020-12-09 | (주)아모레퍼시픽 | 피부 미용액을 추출용매로 하여 추출한 장미꽃 추출물을 유효성분으로 포함하는 피부 보습용 조성물 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20050013352A (ko) * | 2003-07-28 | 2005-02-04 | 그린텍이십일 주식회사 | 초임계 유체를 이용한 녹차 추출물의 추출 방법 |
| KR20090114513A (ko) | 2008-04-30 | 2009-11-04 | 세현씨엠티(주) | 저저항 탄탈 고분자 캐패시터의 제조방법 |
| KR20130088912A (ko) * | 2012-01-31 | 2013-08-09 | (주)아모레퍼시픽 | 텐저레틴 및 egcg를 함유하는 피부 외용제 조성물 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001172160A (ja) * | 1999-12-20 | 2001-06-26 | Katakura Chikkarin Co Ltd | 皮膚外用剤 |
| JP2003204787A (ja) * | 2002-01-15 | 2003-07-22 | San Field:Kk | 糖分生産酵素抑制剤及びそれを含む組成物 |
| US7198807B2 (en) * | 2003-12-01 | 2007-04-03 | Triarco Industries, Inc. | Inhibition of P. acnes using botanical extracts |
| US20080103103A1 (en) * | 2006-10-30 | 2008-05-01 | Bahram Memarzadeh | Reagents and methods to treat ocular diseases and infection |
| WO2010062106A2 (ko) | 2008-11-25 | 2010-06-03 | (주)아모레퍼시픽 | 바실러스속 균주를 이용한 발효차 제조 방법 |
| KR101757255B1 (ko) | 2009-11-13 | 2017-07-13 | (주)아모레퍼시픽 | 고압 효소 분해 기법을 이용한 식물 추출물의 제조방법 및 이 추출물을 함유하는 화장료 조성물 |
| US20140004210A1 (en) * | 2012-06-28 | 2014-01-02 | Shiseido Company, Ltd. | Degradation inhibitor of hyaluronic acid, comprising rosemary extract and retinol acetate |
| KR20160015705A (ko) * | 2014-07-31 | 2016-02-15 | 주식회사 아미코스메틱 | 탈염 용암해수 및 풍난 식물세포배양추출물을 유효성분으로 포함하는 모공 축소용 화장료 조성물 |
| KR20160103756A (ko) * | 2015-02-25 | 2016-09-02 | 울산과학대학교 산학협력단 | 식물 추출물을 포함하는 화장료 조성물 |
| KR20160146136A (ko) * | 2015-06-11 | 2016-12-21 | 주식회사 아미코스메틱 | 피부 모공 축소용 화장료 조성물 |
-
2017
- 2017-02-24 KR KR1020170024898A patent/KR102610940B1/ko active Active
-
2018
- 2018-01-31 US US16/479,052 patent/US11179319B2/en active Active
- 2018-01-31 WO PCT/KR2018/001310 patent/WO2018155829A1/ko not_active Ceased
- 2018-01-31 AU AU2018226144A patent/AU2018226144B2/en active Active
- 2018-02-22 TW TW107105936A patent/TWI827537B/zh active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20050013352A (ko) * | 2003-07-28 | 2005-02-04 | 그린텍이십일 주식회사 | 초임계 유체를 이용한 녹차 추출물의 추출 방법 |
| KR20090114513A (ko) | 2008-04-30 | 2009-11-04 | 세현씨엠티(주) | 저저항 탄탈 고분자 캐패시터의 제조방법 |
| KR20130088912A (ko) * | 2012-01-31 | 2013-08-09 | (주)아모레퍼시픽 | 텐저레틴 및 egcg를 함유하는 피부 외용제 조성물 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20200137432A (ko) * | 2019-05-30 | 2020-12-09 | (주)아모레퍼시픽 | 피부 미용액을 추출용매로 하여 추출한 장미꽃 추출물을 유효성분으로 포함하는 피부 보습용 조성물 |
Also Published As
| Publication number | Publication date |
|---|---|
| US11179319B2 (en) | 2021-11-23 |
| AU2018226144B2 (en) | 2023-09-28 |
| AU2018226144A1 (en) | 2019-08-15 |
| TW201840304A (zh) | 2018-11-16 |
| KR102610940B1 (ko) | 2023-12-08 |
| WO2018155829A1 (ko) | 2018-08-30 |
| US20190365638A1 (en) | 2019-12-05 |
| TWI827537B (zh) | 2024-01-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10849840B2 (en) | Compositions and methods for stimulation MAGP-1 to improve the appearance of skin | |
| KR101387308B1 (ko) | 황칠나무 발효산물을 이용한 피부 미백 화장료 조성물 | |
| US20180110720A1 (en) | Skin external composition for skin moisturization containing red yeast rice extract | |
| JPH09328410A (ja) | 化粧料及びその製造方法 | |
| WO2018002338A1 (en) | Novel silybum marianum achene extract and uses thereof in dermatology and dermo-cosmetics | |
| JP2011088845A (ja) | インボルクリン発現抑制剤 | |
| KR101736714B1 (ko) | 천연 추출물을 포함하는 피부 개선용 화장료 조성물 | |
| KR101616284B1 (ko) | 식물 추출물을 함유하는 피부 노화 방지 또는 주름 개선용화장료 조성물 | |
| KR101805872B1 (ko) | 엽록소가 제거된 식물추출물을 유효성분으로 함유하는 주름 개선용 화장료 조성물 | |
| KR102610940B1 (ko) | 피부 미용액을 이용한 화장료 조성물의 제조방법 | |
| US11077159B2 (en) | Compositions from halophyte plants and methods of use thereof | |
| KR100712249B1 (ko) | 생약재 추출물을 함유하는 지성피부 개선용 화장료 조성물및 그 제조방법 | |
| JP2011088854A (ja) | インボルクリン発現抑制剤 | |
| CN110859788A (zh) | 一种塔纳卡组合物及其制备方法和应用 | |
| KR102504487B1 (ko) | 피부장벽 보호 기능이 있는 금전초 추출물 및 이의 제조방법 | |
| JP2015086168A (ja) | リパーゼ阻害剤および皮脂コントロール用皮膚化粧料 | |
| KR102685167B1 (ko) | 소나무, 마누카, 수국, 은행나무, 구아바 및 서양머위의 복합 천연물 추출물을 유효성분으로 함유하는 항산화, 피부 탄력 증진 및 피부 진정용 화장료 조성물 | |
| KR102849900B1 (ko) | 화장료 조성물 | |
| KR102898422B1 (ko) | 바쿠치올이 함유된 보골지 추출물 및 그 제조 방법, 이 추출물을 유효성분으로 하는 주름개선 및 염증개선용 화장료 조성물 | |
| KR101117563B1 (ko) | 여지 추출물을 함유하는 항산화용 조성물 | |
| JP6385533B2 (ja) | リパーゼ阻害剤および皮脂分解抑制用皮膚化粧料 | |
| FR3150440A1 (fr) | Composition comprenant différents extraits | |
| KR20250030108A (ko) | 블루플래그아이리스, 양까막까치밥나무열매 및 블랙커민씨 혼합 추출물을 유효성분으로 함유하는 피부 보습용 화장료 조성물 | |
| KR20160123628A (ko) | 윤판나물 추출물을 포함하는 화장료 조성물 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0109 | Patent application |
Patent event code: PA01091R01D Comment text: Patent Application Patent event date: 20170224 |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20211126 Comment text: Request for Examination of Application Patent event code: PA02011R01I Patent event date: 20170224 Comment text: Patent Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230704 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20231117 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20231204 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20231205 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration |