KR20160141727A - Cd19-항체 약물 컨쥬게이트의 최적의 주입 - Google Patents
Cd19-항체 약물 컨쥬게이트의 최적의 주입 Download PDFInfo
- Publication number
- KR20160141727A KR20160141727A KR1020167027123A KR20167027123A KR20160141727A KR 20160141727 A KR20160141727 A KR 20160141727A KR 1020167027123 A KR1020167027123 A KR 1020167027123A KR 20167027123 A KR20167027123 A KR 20167027123A KR 20160141727 A KR20160141727 A KR 20160141727A
- Authority
- KR
- South Korea
- Prior art keywords
- dose
- cd19a
- sgn
- administered
- treatment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 229940049595 antibody-drug conjugate Drugs 0.000 title claims abstract description 35
- 239000000611 antibody drug conjugate Substances 0.000 title claims abstract description 34
- 238000000034 method Methods 0.000 claims abstract description 23
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 claims description 83
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 claims description 83
- 238000011282 treatment Methods 0.000 claims description 69
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 41
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 claims description 40
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 claims description 36
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 claims description 36
- 201000010099 disease Diseases 0.000 claims description 29
- 238000012423 maintenance Methods 0.000 claims description 22
- 230000037396 body weight Effects 0.000 claims description 10
- 238000002347 injection Methods 0.000 abstract description 7
- 239000007924 injection Substances 0.000 abstract description 7
- BXTJCSYMGFJEID-XMTADJHZSA-N (2s)-2-[[(2r,3r)-3-[(2s)-1-[(3r,4s,5s)-4-[[(2s)-2-[[(2s)-2-[6-[3-[(2r)-2-amino-2-carboxyethyl]sulfanyl-2,5-dioxopyrrolidin-1-yl]hexanoyl-methylamino]-3-methylbutanoyl]amino]-3-methylbutanoyl]-methylamino]-3-methoxy-5-methylheptanoyl]pyrrolidin-2-yl]-3-met Chemical compound C([C@H](NC(=O)[C@H](C)[C@@H](OC)[C@@H]1CCCN1C(=O)C[C@H]([C@H]([C@@H](C)CC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)CCCCCN1C(C(SC[C@H](N)C(O)=O)CC1=O)=O)C(C)C)OC)C(O)=O)C1=CC=CC=C1 BXTJCSYMGFJEID-XMTADJHZSA-N 0.000 description 73
- 210000004027 cell Anatomy 0.000 description 53
- 206010028980 Neoplasm Diseases 0.000 description 29
- 238000004088 simulation Methods 0.000 description 17
- 210000001185 bone marrow Anatomy 0.000 description 14
- 201000011510 cancer Diseases 0.000 description 14
- 239000003814 drug Substances 0.000 description 14
- 210000001167 myeloblast Anatomy 0.000 description 14
- 229940079593 drug Drugs 0.000 description 13
- 208000035475 disorder Diseases 0.000 description 12
- ORFNVPGICPYLJV-YTVPMEHESA-N (2s)-2-[[(2r,3r)-3-[(2s)-1-[(3r,4s,5s)-4-[[(2s)-2-[[(2s)-2-[6-(2,5-dioxopyrrol-1-yl)hexanoyl-methylamino]-3-methylbutanoyl]amino]-3-methylbutanoyl]-methylamino]-3-methoxy-5-methylheptanoyl]pyrrolidin-2-yl]-3-methoxy-2-methylpropanoyl]amino]-3-phenylpropan Chemical compound C([C@H](NC(=O)[C@H](C)[C@@H](OC)[C@@H]1CCCN1C(=O)C[C@H]([C@H]([C@@H](C)CC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)CCCCCN1C(C=CC1=O)=O)C(C)C)OC)C(O)=O)C1=CC=CC=C1 ORFNVPGICPYLJV-YTVPMEHESA-N 0.000 description 10
- 230000027455 binding Effects 0.000 description 10
- 206010020718 hyperplasia Diseases 0.000 description 9
- 230000003285 pharmacodynamic effect Effects 0.000 description 9
- 230000014759 maintenance of location Effects 0.000 description 8
- 206010025323 Lymphomas Diseases 0.000 description 6
- 210000003719 b-lymphocyte Anatomy 0.000 description 6
- 238000012417 linear regression Methods 0.000 description 6
- 210000004881 tumor cell Anatomy 0.000 description 6
- MFRNYXJJRJQHNW-DEMKXPNLSA-N (2s)-2-[[(2r,3r)-3-methoxy-3-[(2s)-1-[(3r,4s,5s)-3-methoxy-5-methyl-4-[methyl-[(2s)-3-methyl-2-[[(2s)-3-methyl-2-(methylamino)butanoyl]amino]butanoyl]amino]heptanoyl]pyrrolidin-2-yl]-2-methylpropanoyl]amino]-3-phenylpropanoic acid Chemical compound CN[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MFRNYXJJRJQHNW-DEMKXPNLSA-N 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 239000008280 blood Substances 0.000 description 5
- 230000010354 integration Effects 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 210000001519 tissue Anatomy 0.000 description 5
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 4
- 102000004243 Tubulin Human genes 0.000 description 4
- 108090000704 Tubulin Proteins 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 230000015556 catabolic process Effects 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 108020003175 receptors Proteins 0.000 description 4
- 102000005962 receptors Human genes 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 208000004736 B-Cell Leukemia Diseases 0.000 description 3
- 241000880493 Leptailurus serval Species 0.000 description 3
- 102000029749 Microtubule Human genes 0.000 description 3
- 108091022875 Microtubule Proteins 0.000 description 3
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 3
- 150000001413 amino acids Chemical group 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 238000001574 biopsy Methods 0.000 description 3
- 208000032839 leukemia Diseases 0.000 description 3
- 230000003211 malignant effect Effects 0.000 description 3
- 210000004688 microtubule Anatomy 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 230000000306 recurrent effect Effects 0.000 description 3
- 230000035945 sensitivity Effects 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- ZDILXFDENZVOTL-BPNCWPANSA-N Ala-Val-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZDILXFDENZVOTL-BPNCWPANSA-N 0.000 description 2
- GCACQYDBDHRVGE-LKXGYXEUSA-N Asp-Thr-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@H](O)C)NC(=O)[C@@H](N)CC(O)=O GCACQYDBDHRVGE-LKXGYXEUSA-N 0.000 description 2
- 208000003950 B-cell lymphoma Diseases 0.000 description 2
- 101710112752 Cytotoxin Proteins 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 101100383038 Homo sapiens CD19 gene Proteins 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000025084 cell cycle arrest Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000021615 conjugation Effects 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 239000002619 cytotoxin Substances 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 210000000265 leukocyte Anatomy 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 210000003712 lysosome Anatomy 0.000 description 2
- 230000001868 lysosomic effect Effects 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 239000013610 patient sample Substances 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 230000036470 plasma concentration Effects 0.000 description 2
- 230000007065 protein hydrolysis Effects 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 108010072986 threonyl-seryl-lysine Proteins 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 1
- BTYTYHBSJKQBQA-GCJQMDKQSA-N Ala-Asp-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](C)N)O BTYTYHBSJKQBQA-GCJQMDKQSA-N 0.000 description 1
- MAZZQZWCCYJQGZ-GUBZILKMSA-N Ala-Pro-Arg Chemical compound [H]N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O MAZZQZWCCYJQGZ-GUBZILKMSA-N 0.000 description 1
- QLSRIZIDQXDQHK-RCWTZXSCSA-N Arg-Val-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QLSRIZIDQXDQHK-RCWTZXSCSA-N 0.000 description 1
- UZFHNLYQWMGUHU-DCAQKATOSA-N Asp-Lys-Arg Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O UZFHNLYQWMGUHU-DCAQKATOSA-N 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000028564 B-cell non-Hodgkin lymphoma Diseases 0.000 description 1
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- AMRLSQGGERHDHJ-FXQIFTODSA-N Cys-Ala-Arg Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O AMRLSQGGERHDHJ-FXQIFTODSA-N 0.000 description 1
- CMYVIUWVYHOLRD-ZLUOBGJFSA-N Cys-Ser-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O CMYVIUWVYHOLRD-ZLUOBGJFSA-N 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- MADFVRSKEIEZHZ-DCAQKATOSA-N Gln-Gln-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)N)N MADFVRSKEIEZHZ-DCAQKATOSA-N 0.000 description 1
- SMLDOQHTOAAFJQ-WDSKDSINSA-N Gln-Gly-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)NCC(=O)N[C@@H](CO)C(O)=O SMLDOQHTOAAFJQ-WDSKDSINSA-N 0.000 description 1
- ZFBBMCKQSNJZSN-AUTRQRHGSA-N Gln-Val-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZFBBMCKQSNJZSN-AUTRQRHGSA-N 0.000 description 1
- AVZHGSCDKIQZPQ-CIUDSAMLSA-N Glu-Arg-Ala Chemical compound C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCC(O)=O)C(O)=O AVZHGSCDKIQZPQ-CIUDSAMLSA-N 0.000 description 1
- INGJLBQKTRJLFO-UKJIMTQDSA-N Glu-Ile-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCC(O)=O INGJLBQKTRJLFO-UKJIMTQDSA-N 0.000 description 1
- YWAQATDNEKZFFK-BYPYZUCNSA-N Gly-Gly-Ser Chemical compound NCC(=O)NCC(=O)N[C@@H](CO)C(O)=O YWAQATDNEKZFFK-BYPYZUCNSA-N 0.000 description 1
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 1
- HHRODZSXDXMUHS-LURJTMIESA-N Gly-Met-Gly Chemical compound CSCC[C@H](NC(=O)C[NH3+])C(=O)NCC([O-])=O HHRODZSXDXMUHS-LURJTMIESA-N 0.000 description 1
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 1
- ZZWUYQXMIFTIIY-WEDXCCLWSA-N Gly-Thr-Leu Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O ZZWUYQXMIFTIIY-WEDXCCLWSA-N 0.000 description 1
- XHVONGZZVUUORG-WEDXCCLWSA-N Gly-Thr-Lys Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCCN XHVONGZZVUUORG-WEDXCCLWSA-N 0.000 description 1
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 1
- WZBLRQQCDYYRTD-SIXJUCDHSA-N His-Ile-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](CC3=CN=CN3)N WZBLRQQCDYYRTD-SIXJUCDHSA-N 0.000 description 1
- QCBYAHHNOHBXIH-UWVGGRQHSA-N His-Pro-Gly Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)NCC(O)=O)C1=CN=CN1 QCBYAHHNOHBXIH-UWVGGRQHSA-N 0.000 description 1
- FOCSWPCHUDVNLP-PMVMPFDFSA-N His-Trp-Tyr Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC3=CC=C(C=C3)O)C(=O)O)NC(=O)[C@H](CC4=CN=CN4)N FOCSWPCHUDVNLP-PMVMPFDFSA-N 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 108090000144 Human Proteins Proteins 0.000 description 1
- 102000003839 Human Proteins Human genes 0.000 description 1
- ASCFJMSGKUIRDU-ZPFDUUQYSA-N Ile-Arg-Gln Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O ASCFJMSGKUIRDU-ZPFDUUQYSA-N 0.000 description 1
- PXKACEXYLPBMAD-JBDRJPRFSA-N Ile-Ser-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PXKACEXYLPBMAD-JBDRJPRFSA-N 0.000 description 1
- HXIDVIFHRYRXLZ-NAKRPEOUSA-N Ile-Ser-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)O)N HXIDVIFHRYRXLZ-NAKRPEOUSA-N 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 108010065920 Insulin Lispro Proteins 0.000 description 1
- ZTLGVASZOIKNIX-DCAQKATOSA-N Leu-Gln-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N ZTLGVASZOIKNIX-DCAQKATOSA-N 0.000 description 1
- HPBCTWSUJOGJSH-MNXVOIDGSA-N Leu-Glu-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HPBCTWSUJOGJSH-MNXVOIDGSA-N 0.000 description 1
- OGUUKPXUTHOIAV-SDDRHHMPSA-N Leu-Glu-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N OGUUKPXUTHOIAV-SDDRHHMPSA-N 0.000 description 1
- FAELBUXXFQLUAX-AJNGGQMLSA-N Leu-Leu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(C)C FAELBUXXFQLUAX-AJNGGQMLSA-N 0.000 description 1
- VCHVSKNMTXWIIP-SRVKXCTJSA-N Leu-Lys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O VCHVSKNMTXWIIP-SRVKXCTJSA-N 0.000 description 1
- SBANPBVRHYIMRR-GARJFASQSA-N Leu-Ser-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N SBANPBVRHYIMRR-GARJFASQSA-N 0.000 description 1
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 1
- BRTVHXHCUSXYRI-CIUDSAMLSA-N Leu-Ser-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O BRTVHXHCUSXYRI-CIUDSAMLSA-N 0.000 description 1
- LINKCQUOMUDLKN-KATARQTJSA-N Leu-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N)O LINKCQUOMUDLKN-KATARQTJSA-N 0.000 description 1
- LCNASHSOFMRYFO-WDCWCFNPSA-N Leu-Thr-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O LCNASHSOFMRYFO-WDCWCFNPSA-N 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- YKIRNDPUWONXQN-GUBZILKMSA-N Lys-Asn-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N YKIRNDPUWONXQN-GUBZILKMSA-N 0.000 description 1
- GQFDWEDHOQRNLC-QWRGUYRKSA-N Lys-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN GQFDWEDHOQRNLC-QWRGUYRKSA-N 0.000 description 1
- MYZMQWHPDAYKIE-SRVKXCTJSA-N Lys-Leu-Ala Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O MYZMQWHPDAYKIE-SRVKXCTJSA-N 0.000 description 1
- YTJFXEDRUOQGSP-DCAQKATOSA-N Lys-Pro-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YTJFXEDRUOQGSP-DCAQKATOSA-N 0.000 description 1
- 206010064912 Malignant transformation Diseases 0.000 description 1
- SJDQOYTYNGZZJX-SRVKXCTJSA-N Met-Glu-Leu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O SJDQOYTYNGZZJX-SRVKXCTJSA-N 0.000 description 1
- WYBVBIHNJWOLCJ-UHFFFAOYSA-N N-L-arginyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCCN=C(N)N WYBVBIHNJWOLCJ-UHFFFAOYSA-N 0.000 description 1
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- YYKZDTVQHTUKDW-RYUDHWBXSA-N Phe-Gly-Gln Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)NCC(=O)N[C@@H](CCC(=O)N)C(=O)O)N YYKZDTVQHTUKDW-RYUDHWBXSA-N 0.000 description 1
- BPCLGWHVPVTTFM-QWRGUYRKSA-N Phe-Ser-Gly Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)NCC(O)=O BPCLGWHVPVTTFM-QWRGUYRKSA-N 0.000 description 1
- VXCHGLYSIOOZIS-GUBZILKMSA-N Pro-Ala-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1 VXCHGLYSIOOZIS-GUBZILKMSA-N 0.000 description 1
- JMVQDLDPDBXAAX-YUMQZZPRSA-N Pro-Gly-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1 JMVQDLDPDBXAAX-YUMQZZPRSA-N 0.000 description 1
- IOVHBRCQOGWAQH-ZKWXMUAHSA-N Ser-Gly-Ile Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O IOVHBRCQOGWAQH-ZKWXMUAHSA-N 0.000 description 1
- KDGARKCAKHBEDB-NKWVEPMBSA-N Ser-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CO)N)C(=O)O KDGARKCAKHBEDB-NKWVEPMBSA-N 0.000 description 1
- UBRMZSHOOIVJPW-SRVKXCTJSA-N Ser-Leu-Lys Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(O)=O UBRMZSHOOIVJPW-SRVKXCTJSA-N 0.000 description 1
- ADJDNJCSPNFFPI-FXQIFTODSA-N Ser-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO ADJDNJCSPNFFPI-FXQIFTODSA-N 0.000 description 1
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 1
- PYTKULIABVRXSC-BWBBJGPYSA-N Ser-Ser-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PYTKULIABVRXSC-BWBBJGPYSA-N 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- KRPKYGOFYUNIGM-XVSYOHENSA-N Thr-Asp-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O KRPKYGOFYUNIGM-XVSYOHENSA-N 0.000 description 1
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 1
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 1
- VEYXZZGMIBKXCN-UBHSHLNASA-N Trp-Asp-Asp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)O)C(=O)O)N VEYXZZGMIBKXCN-UBHSHLNASA-N 0.000 description 1
- KIMOCKLJBXHFIN-YLVFBTJISA-N Trp-Ile-Gly Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(O)=O)=CNC2=C1 KIMOCKLJBXHFIN-YLVFBTJISA-N 0.000 description 1
- ABRICLFKFRFDKS-IHPCNDPISA-N Trp-Ser-Tyr Chemical compound C([C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)N)C(O)=O)C1=CC=C(O)C=C1 ABRICLFKFRFDKS-IHPCNDPISA-N 0.000 description 1
- VTFWAGGJDRSQFG-MELADBBJSA-N Tyr-Asn-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC2=CC=C(C=C2)O)N)C(=O)O VTFWAGGJDRSQFG-MELADBBJSA-N 0.000 description 1
- BVDHHLMIZFCAAU-BZSNNMDCSA-N Tyr-Cys-Phe Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O BVDHHLMIZFCAAU-BZSNNMDCSA-N 0.000 description 1
- LMKKMCGTDANZTR-BZSNNMDCSA-N Tyr-Phe-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 LMKKMCGTDANZTR-BZSNNMDCSA-N 0.000 description 1
- NUQZCPSZHGIYTA-HKUYNNGSSA-N Tyr-Trp-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N NUQZCPSZHGIYTA-HKUYNNGSSA-N 0.000 description 1
- JVYIGCARISMLMV-HOCLYGCPSA-N Val-Gly-Trp Chemical compound CC(C)[C@@H](C(=O)NCC(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N JVYIGCARISMLMV-HOCLYGCPSA-N 0.000 description 1
- HWNYVQMOLCYHEA-IHRRRGAJSA-N Val-Ser-Tyr Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N HWNYVQMOLCYHEA-IHRRRGAJSA-N 0.000 description 1
- CEKSLIVSNNGOKH-KZVJFYERSA-N Val-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](C(C)C)N)O CEKSLIVSNNGOKH-KZVJFYERSA-N 0.000 description 1
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 1
- PMKQKNBISAOSRI-XHSDSOJGSA-N Val-Tyr-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N2CCC[C@@H]2C(=O)O)N PMKQKNBISAOSRI-XHSDSOJGSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 108010081404 acein-2 Proteins 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000002491 angiogenic effect Effects 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 108010010430 asparagine-proline-alanine Proteins 0.000 description 1
- 108010068265 aspartyltyrosine Proteins 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 238000007470 bone biopsy Methods 0.000 description 1
- 238000009583 bone marrow aspiration Methods 0.000 description 1
- 208000015322 bone marrow disease Diseases 0.000 description 1
- -1 caproyl Chemical group 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000022534 cell killing Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000015861 cell surface binding Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 229920000547 conjugated polymer Polymers 0.000 description 1
- 108010081447 cytochrophin-4 Proteins 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 230000000857 drug effect Effects 0.000 description 1
- 239000003596 drug target Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 108010089804 glycyl-threonine Proteins 0.000 description 1
- 108010020688 glycylhistidine Proteins 0.000 description 1
- 108010015792 glycyllysine Proteins 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 230000036212 malign transformation Effects 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 108010059074 monomethylauristatin F Proteins 0.000 description 1
- 210000003098 myoblast Anatomy 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000002399 phagocytotic effect Effects 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 229910000160 potassium phosphate Inorganic materials 0.000 description 1
- 235000011009 potassium phosphates Nutrition 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 208000037821 progressive disease Diseases 0.000 description 1
- 108010070643 prolylglutamic acid Proteins 0.000 description 1
- 235000018102 proteins Nutrition 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 108010045269 tryptophyltryptophan Proteins 0.000 description 1
- 108010077037 tyrosyl-tyrosyl-phenylalanine Proteins 0.000 description 1
- 230000004222 uncontrolled growth Effects 0.000 description 1
- 108010073969 valyllysine Proteins 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- A61K47/4863—
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- C07K16/3061—Blood cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
- A61K47/6817—Toxins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6851—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a determinant of a tumour cell
- A61K47/6867—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a determinant of a tumour cell the tumour determinant being from a cell of a blood cancer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Cell Biology (AREA)
- Molecular Biology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Hematology (AREA)
- Epidemiology (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Oncology (AREA)
- Toxicology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201461976790P | 2014-04-08 | 2014-04-08 | |
| US61/976,790 | 2014-04-08 | ||
| PCT/US2015/024732 WO2015157297A1 (en) | 2014-04-08 | 2015-04-07 | Optimal dosing of a cd19-antibody drug conjugate |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160141727A true KR20160141727A (ko) | 2016-12-09 |
Family
ID=54288332
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167027123A Withdrawn KR20160141727A (ko) | 2014-04-08 | 2015-04-07 | Cd19-항체 약물 컨쥬게이트의 최적의 주입 |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US20170182178A1 (enExample) |
| EP (1) | EP3129052B1 (enExample) |
| JP (1) | JP2017512801A (enExample) |
| KR (1) | KR20160141727A (enExample) |
| CN (1) | CN106170300A (enExample) |
| AU (1) | AU2015244004A1 (enExample) |
| CA (1) | CA2941154A1 (enExample) |
| EA (1) | EA201692016A1 (enExample) |
| IL (1) | IL247526A0 (enExample) |
| MX (1) | MX2016012258A (enExample) |
| SG (1) | SG11201607133QA (enExample) |
| WO (1) | WO2015157297A1 (enExample) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3481868A1 (en) * | 2016-07-08 | 2019-05-15 | Genmab A/S | New dosage regimens for antibody drug conjugates based on anti-axl antibodies |
| WO2018229222A1 (en) * | 2017-06-14 | 2018-12-20 | Adc Therapeutics Sa | Dosage regimes for the administration of an anti-cd19 adc |
| WO2019000327A1 (zh) * | 2017-06-29 | 2019-01-03 | 成都华创生物技术有限公司 | 一种trail类蛋白持续抑制肿瘤细胞生长的给药方法 |
| CN111683971A (zh) * | 2017-12-23 | 2020-09-18 | 宇越生医科技股份有限公司 | 医药重组受体组成物及方法 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103394083B (zh) * | 2003-11-06 | 2017-07-18 | 西雅图基因公司 | 能够与配体偶联的单甲基缬氨酸化合物 |
| LT2211904T (lt) * | 2007-10-19 | 2016-11-25 | Seattle Genetics, Inc. | Cd19 surišantys agentai ir jų panaudojimas |
| JP6055404B2 (ja) * | 2010-06-15 | 2016-12-27 | ゲンマブ エー/エス | 組織因子に対するヒト抗体薬物結合体 |
| EP2629801B3 (en) * | 2010-10-22 | 2019-11-27 | Seattle Genetics, Inc. | Synergistic effects between auristatin-based antibody drug conjugates and inhibitors of the pi3k-akt mtor pathway |
| DK2714738T3 (en) * | 2011-05-24 | 2019-01-28 | Zyngenia Inc | MULTIVALENT AND MONOVALENT MULTISPECIFIC COMPLEXES AND THEIR APPLICATIONS |
-
2015
- 2015-04-07 CA CA2941154A patent/CA2941154A1/en not_active Abandoned
- 2015-04-07 CN CN201580019099.2A patent/CN106170300A/zh active Pending
- 2015-04-07 EP EP15775944.0A patent/EP3129052B1/en not_active Not-in-force
- 2015-04-07 WO PCT/US2015/024732 patent/WO2015157297A1/en not_active Ceased
- 2015-04-07 MX MX2016012258A patent/MX2016012258A/es unknown
- 2015-04-07 AU AU2015244004A patent/AU2015244004A1/en not_active Abandoned
- 2015-04-07 JP JP2016559853A patent/JP2017512801A/ja active Pending
- 2015-04-07 US US15/129,727 patent/US20170182178A1/en not_active Abandoned
- 2015-04-07 EA EA201692016A patent/EA201692016A1/ru unknown
- 2015-04-07 SG SG11201607133QA patent/SG11201607133QA/en unknown
- 2015-04-07 KR KR1020167027123A patent/KR20160141727A/ko not_active Withdrawn
-
2016
- 2016-08-29 IL IL247526A patent/IL247526A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| EP3129052A1 (en) | 2017-02-15 |
| WO2015157297A1 (en) | 2015-10-15 |
| IL247526A0 (en) | 2016-11-30 |
| EA201692016A1 (ru) | 2017-01-30 |
| JP2017512801A (ja) | 2017-05-25 |
| EP3129052B1 (en) | 2020-06-03 |
| AU2015244004A1 (en) | 2016-09-15 |
| SG11201607133QA (en) | 2016-09-29 |
| EP3129052A4 (en) | 2017-11-15 |
| CN106170300A (zh) | 2016-11-30 |
| CA2941154A1 (en) | 2015-10-15 |
| MX2016012258A (es) | 2017-01-06 |
| US20170182178A1 (en) | 2017-06-29 |
Similar Documents
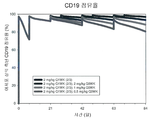
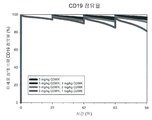
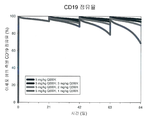
| Publication | Publication Date | Title |
|---|---|---|
| Cura et al. | Phase I and pharmacokinetics study of crotoxin (cytotoxic PLA2, NSC-624244) in patients with advanced cancer | |
| Oldroyd et al. | Interferon-γ inhibits experimental renal fibrosis | |
| US7794713B2 (en) | Compositions and methods for the treatment and prevention of hyperproliferative diseases | |
| CN112546217B (zh) | 用于治疗肿瘤的抗b7-h1抗体 | |
| JP2019142961A (ja) | B細胞悪性腫瘍の症状の治療のための抗cd19メイタンシンノイドイムノコンジュゲート抗体の使用 | |
| JP2016509582A5 (enExample) | ||
| US20210196835A1 (en) | Anti-cd37 immunoconjugate dosing regimens | |
| Aung et al. | Locally delivered GLP-1 analogues liraglutide and exenatide enhance microvascular perfusion in individuals with and without type 2 diabetes | |
| RU2454245C2 (ru) | Фармацевтические композиции, способные вызывать апоптоз опухолевых клеток, для диагностики и лечения в-клеточной хронической лимфоцитарной лейкемии | |
| KR20180121571A (ko) | Liv1-adc와 화학요법제를 사용한 병용 요법 | |
| KR20160141727A (ko) | Cd19-항체 약물 컨쥬게이트의 최적의 주입 | |
| IL178042A (en) | Pharmaceutical compositions containing anti-alpha5beta1 antibodies for inhibiting cancer cell proliferation | |
| Lu et al. | Folate receptor-targeted aminopterin therapy is highly effective and specific in experimental models of autoimmune uveitis and autoimmune encephalomyelitis | |
| CN113613678A (zh) | 使用抗cd30抗体药物缀合物疗法治疗外周t细胞淋巴瘤的方法 | |
| TR201806960T4 (tr) | Lupus eri̇tematozus tedavi̇si̇ i̇çi̇n bi̇r i̇lacin üreti̇mi̇ amaciyla atacicept gi̇bi̇ taci-ig füzyon protei̇ni̇ni̇n kullanimi | |
| Yawalkar et al. | Successful treatment of recalcitrant palmoplantar pustular psoriasis with sequential use of infliximab and adalimumab | |
| KR20070036035A (ko) | 자가면역 및 염증성 질환의 치료 방법 | |
| US20150218286A1 (en) | Methods of Treating Pediatric Acute Lymphoblastic Leukemia with an Anti-CD22 Immunotoxin | |
| AU2005325227B2 (en) | Compositions and methods for the treatment and prevention of hyperproliferative diseases | |
| Kim | Daclizumab treatment for multiple sclerosis | |
| US20200230254A1 (en) | Combination therapy using a cd19-adc and rchp | |
| Zhang et al. | A CD6-Targeted Antibody-Drug Conjugate As a Potential Therapy for T Cell-Mediated Disorders | |
| CN117015386A (zh) | 细胞周转因子用于增加组织再生的用途 | |
| CN117083073A (zh) | 用于治疗t细胞和b细胞介导的障碍以及t细胞和b细胞癌症的抗cd6抗体缀合物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20160929 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination |