KR20150145225A - 변경된 글리코실화를 갖는 세툭시맙 및 이의 용도 - Google Patents
변경된 글리코실화를 갖는 세툭시맙 및 이의 용도 Download PDFInfo
- Publication number
- KR20150145225A KR20150145225A KR1020157024950A KR20157024950A KR20150145225A KR 20150145225 A KR20150145225 A KR 20150145225A KR 1020157024950 A KR1020157024950 A KR 1020157024950A KR 20157024950 A KR20157024950 A KR 20157024950A KR 20150145225 A KR20150145225 A KR 20150145225A
- Authority
- KR
- South Korea
- Prior art keywords
- antibody
- glycosylated
- seq
- galactose
- alpha
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 229960005395 cetuximab Drugs 0.000 title claims abstract description 60
- 230000013595 glycosylation Effects 0.000 title abstract description 24
- 238000006206 glycosylation reaction Methods 0.000 title abstract description 24
- 238000000034 method Methods 0.000 claims abstract description 93
- 238000004519 manufacturing process Methods 0.000 claims abstract description 25
- 241000282414 Homo sapiens Species 0.000 claims description 71
- 239000000203 mixture Substances 0.000 claims description 60
- 238000004113 cell culture Methods 0.000 claims description 55
- QIGJYVCQYDKYDW-SDOYDPJRSA-N alpha-D-galactosyl-(1->3)-D-galactose Chemical group O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@H]1[C@@H](O)[C@@H](CO)OC(O)[C@@H]1O QIGJYVCQYDKYDW-SDOYDPJRSA-N 0.000 claims description 54
- 210000002919 epithelial cell Anatomy 0.000 claims description 49
- 230000009261 transgenic effect Effects 0.000 claims description 45
- 235000013336 milk Nutrition 0.000 claims description 43
- 239000008267 milk Substances 0.000 claims description 43
- 210000004080 milk Anatomy 0.000 claims description 43
- 241000699666 Mus <mouse, genus> Species 0.000 claims description 36
- 150000007523 nucleic acids Chemical class 0.000 claims description 34
- 239000000460 chlorine Substances 0.000 claims description 31
- 229910052801 chlorine Inorganic materials 0.000 claims description 31
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 claims description 30
- 102000039446 nucleic acids Human genes 0.000 claims description 28
- 108020004707 nucleic acids Proteins 0.000 claims description 28
- 241000283707 Capra Species 0.000 claims description 27
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 22
- 210000004962 mammalian cell Anatomy 0.000 claims description 21
- 241000124008 Mammalia Species 0.000 claims description 20
- 239000003814 drug Substances 0.000 claims description 17
- 241000283690 Bos taurus Species 0.000 claims description 13
- 241001494479 Pecora Species 0.000 claims description 13
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 13
- 201000000050 myeloid neoplasm Diseases 0.000 claims description 13
- 206010028980 Neoplasm Diseases 0.000 claims description 12
- 201000011510 cancer Diseases 0.000 claims description 11
- 238000001727 in vivo Methods 0.000 claims description 11
- 210000005075 mammary gland Anatomy 0.000 claims description 10
- 241000283973 Oryctolagus cuniculus Species 0.000 claims description 9
- 241000700159 Rattus Species 0.000 claims description 9
- 241000282898 Sus scrofa Species 0.000 claims description 9
- 241000283073 Equus caballus Species 0.000 claims description 8
- 239000003937 drug carrier Substances 0.000 claims description 8
- 229940124597 therapeutic agent Drugs 0.000 claims description 8
- 235000002198 Annona diversifolia Nutrition 0.000 claims description 7
- 241000283726 Bison Species 0.000 claims description 7
- 241000282836 Camelus dromedarius Species 0.000 claims description 7
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 claims description 7
- 229960002949 fluorouracil Drugs 0.000 claims description 7
- 206010009944 Colon cancer Diseases 0.000 claims description 5
- 201000010536 head and neck cancer Diseases 0.000 claims description 5
- 208000014829 head and neck neoplasm Diseases 0.000 claims description 5
- 238000000338 in vitro Methods 0.000 claims description 5
- 230000001131 transforming effect Effects 0.000 claims description 5
- KVUAALJSMIVURS-ZEDZUCNESA-L calcium folinate Chemical compound [Ca+2].C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC([O-])=O)C([O-])=O)C=C1 KVUAALJSMIVURS-ZEDZUCNESA-L 0.000 claims description 3
- 235000008207 calcium folinate Nutrition 0.000 claims description 3
- 239000011687 calcium folinate Substances 0.000 claims description 3
- 125000001309 chloro group Chemical group Cl* 0.000 claims description 3
- 208000029742 colonic neoplasm Diseases 0.000 claims description 3
- 229960004768 irinotecan Drugs 0.000 claims description 3
- 229960002293 leucovorin calcium Drugs 0.000 claims description 3
- 241000282842 Lama glama Species 0.000 claims 3
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 claims 1
- 210000004027 cell Anatomy 0.000 description 60
- 241001465754 Metazoa Species 0.000 description 46
- 108090000623 proteins and genes Proteins 0.000 description 36
- 235000017168 chlorine Nutrition 0.000 description 28
- 239000012634 fragment Substances 0.000 description 23
- 108010076119 Caseins Proteins 0.000 description 19
- 239000008194 pharmaceutical composition Substances 0.000 description 19
- 102000004169 proteins and genes Human genes 0.000 description 19
- 102000011632 Caseins Human genes 0.000 description 18
- 235000018102 proteins Nutrition 0.000 description 18
- 239000013598 vector Substances 0.000 description 17
- 108700019146 Transgenes Proteins 0.000 description 16
- 239000000427 antigen Substances 0.000 description 15
- 108091007433 antigens Proteins 0.000 description 15
- 102000036639 antigens Human genes 0.000 description 15
- 235000021247 β-casein Nutrition 0.000 description 15
- 108020004414 DNA Proteins 0.000 description 13
- 108090000765 processed proteins & peptides Proteins 0.000 description 13
- 150000003839 salts Chemical class 0.000 description 13
- 108091028043 Nucleic acid sequence Proteins 0.000 description 11
- 150000001875 compounds Chemical class 0.000 description 11
- 210000000287 oocyte Anatomy 0.000 description 11
- 238000012546 transfer Methods 0.000 description 11
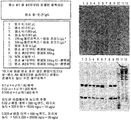
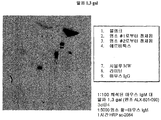
- 238000001262 western blot Methods 0.000 description 11
- 238000010367 cloning Methods 0.000 description 10
- 239000013612 plasmid Substances 0.000 description 10
- 206010020751 Hypersensitivity Diseases 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 239000000725 suspension Substances 0.000 description 9
- 230000001225 therapeutic effect Effects 0.000 description 9
- 108091026890 Coding region Proteins 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 8
- 239000002245 particle Substances 0.000 description 8
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 7
- 241000282412 Homo Species 0.000 description 7
- 241000699670 Mus sp. Species 0.000 description 7
- 125000003275 alpha amino acid group Chemical group 0.000 description 7
- 208000003455 anaphylaxis Diseases 0.000 description 7
- 239000003085 diluting agent Substances 0.000 description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- 206010002198 Anaphylactic reaction Diseases 0.000 description 6
- 108060003951 Immunoglobulin Proteins 0.000 description 6
- 230000036783 anaphylactic response Effects 0.000 description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
- 239000013604 expression vector Substances 0.000 description 6
- 102000018358 immunoglobulin Human genes 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 239000007788 liquid Substances 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 239000003826 tablet Substances 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 108060006698 EGF receptor Proteins 0.000 description 5
- 102000001301 EGF receptor Human genes 0.000 description 5
- 241000699660 Mus musculus Species 0.000 description 5
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 5
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 5
- 208000030961 allergic reaction Diseases 0.000 description 5
- 150000001720 carbohydrates Chemical class 0.000 description 5
- 229930182830 galactose Natural products 0.000 description 5
- 235000020251 goat milk Nutrition 0.000 description 5
- 210000004216 mammary stem cell Anatomy 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 108091033319 polynucleotide Proteins 0.000 description 5
- 102000040430 polynucleotide Human genes 0.000 description 5
- 239000002157 polynucleotide Substances 0.000 description 5
- 230000001105 regulatory effect Effects 0.000 description 5
- 230000028327 secretion Effects 0.000 description 5
- 238000011830 transgenic mouse model Methods 0.000 description 5
- 238000011282 treatment Methods 0.000 description 5
- 239000003981 vehicle Substances 0.000 description 5
- 244000303258 Annona diversifolia Species 0.000 description 4
- 241000880493 Leptailurus serval Species 0.000 description 4
- 241001529936 Murinae Species 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 4
- 241000288906 Primates Species 0.000 description 4
- 241000282887 Suidae Species 0.000 description 4
- 239000002246 antineoplastic agent Substances 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- 235000014633 carbohydrates Nutrition 0.000 description 4
- 229940127089 cytotoxic agent Drugs 0.000 description 4
- 239000012636 effector Substances 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 210000004602 germ cell Anatomy 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 239000003550 marker Substances 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 4
- 230000009885 systemic effect Effects 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- 229920002307 Dextran Polymers 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- 102000009109 Fc receptors Human genes 0.000 description 3
- 108010087819 Fc receptors Proteins 0.000 description 3
- 102000003886 Glycoproteins Human genes 0.000 description 3
- 108090000288 Glycoproteins Proteins 0.000 description 3
- 101000851181 Homo sapiens Epidermal growth factor receptor Proteins 0.000 description 3
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 3
- 102000014171 Milk Proteins Human genes 0.000 description 3
- 108010011756 Milk Proteins Proteins 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 239000000443 aerosol Substances 0.000 description 3
- 108010087924 alanylproline Proteins 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 3
- 210000000349 chromosome Anatomy 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- JYEFSHLLTQIXIO-SMNQTINBSA-N folfiri regimen Chemical compound FC1=CNC(=O)NC1=O.C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 JYEFSHLLTQIXIO-SMNQTINBSA-N 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 108010089804 glycyl-threonine Proteins 0.000 description 3
- 230000003053 immunization Effects 0.000 description 3
- 238000003780 insertion Methods 0.000 description 3
- 230000037431 insertion Effects 0.000 description 3
- 210000000936 intestine Anatomy 0.000 description 3
- GURKHSYORGJETM-WAQYZQTGSA-N irinotecan hydrochloride (anhydrous) Chemical compound Cl.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 GURKHSYORGJETM-WAQYZQTGSA-N 0.000 description 3
- 230000006651 lactation Effects 0.000 description 3
- 239000008101 lactose Substances 0.000 description 3
- 210000004072 lung Anatomy 0.000 description 3
- 235000021239 milk protein Nutrition 0.000 description 3
- 229920001542 oligosaccharide Polymers 0.000 description 3
- 150000002482 oligosaccharides Chemical class 0.000 description 3
- 238000007911 parenteral administration Methods 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 235000020989 red meat Nutrition 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- 238000012552 review Methods 0.000 description 3
- 238000010374 somatic cell nuclear transfer Methods 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 210000002784 stomach Anatomy 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 238000004885 tandem mass spectrometry Methods 0.000 description 3
- 230000009466 transformation Effects 0.000 description 3
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- MEFILNJXAVSUTO-JXUBOQSCSA-N Ala-Leu-Thr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MEFILNJXAVSUTO-JXUBOQSCSA-N 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 102000011022 Chorionic Gonadotropin Human genes 0.000 description 2
- 108010062540 Chorionic Gonadotropin Proteins 0.000 description 2
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 2
- OHLLDUNVMPPUMD-DCAQKATOSA-N Cys-Leu-Val Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N OHLLDUNVMPPUMD-DCAQKATOSA-N 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- 101001035782 Gallus gallus Hemoglobin subunit beta Proteins 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- SYZZMPFLOLSMHL-XHNCKOQMSA-N Gln-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N)C(=O)O SYZZMPFLOLSMHL-XHNCKOQMSA-N 0.000 description 2
- WGYHAAXZWPEBDQ-IFFSRLJSSA-N Glu-Val-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGYHAAXZWPEBDQ-IFFSRLJSSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 2
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 2
- 102000007547 Laminin Human genes 0.000 description 2
- 108010085895 Laminin Proteins 0.000 description 2
- BRTVHXHCUSXYRI-CIUDSAMLSA-N Leu-Ser-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O BRTVHXHCUSXYRI-CIUDSAMLSA-N 0.000 description 2
- NRQRKMYZONPCTM-CIUDSAMLSA-N Lys-Asp-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O NRQRKMYZONPCTM-CIUDSAMLSA-N 0.000 description 2
- 230000004988 N-glycosylation Effects 0.000 description 2
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 2
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 2
- HNDMFDBQXYZSRM-IHRRRGAJSA-N Ser-Val-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HNDMFDBQXYZSRM-IHRRRGAJSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- MNYNCKZAEIAONY-XGEHTFHBSA-N Thr-Val-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O MNYNCKZAEIAONY-XGEHTFHBSA-N 0.000 description 2
- 101710087237 Whey acidic protein Proteins 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 208000026935 allergic disease Diseases 0.000 description 2
- 230000007815 allergy Effects 0.000 description 2
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 108010052670 arginyl-glutamyl-glutamic acid Proteins 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 239000003183 carcinogenic agent Substances 0.000 description 2
- 239000001913 cellulose Substances 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 238000003795 desorption Methods 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 210000001198 duodenum Anatomy 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 230000006862 enzymatic digestion Effects 0.000 description 2
- 210000000981 epithelium Anatomy 0.000 description 2
- -1 fatty acid esters Chemical class 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- 235000019634 flavors Nutrition 0.000 description 2
- 235000003599 food sweetener Nutrition 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 210000002980 germ line cell Anatomy 0.000 description 2
- 108010049041 glutamylalanine Proteins 0.000 description 2
- 108010050848 glycylleucine Proteins 0.000 description 2
- 239000000938 histamine H1 antagonist Substances 0.000 description 2
- 229940084986 human chorionic gonadotropin Drugs 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 238000002649 immunization Methods 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 238000007913 intrathecal administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 210000004940 nucleus Anatomy 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 108010073101 phenylalanylleucine Proteins 0.000 description 2
- 108010051242 phenylalanylserine Proteins 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 2
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 238000002203 pretreatment Methods 0.000 description 2
- 108010031719 prolyl-serine Proteins 0.000 description 2
- 230000002685 pulmonary effect Effects 0.000 description 2
- 229950010131 puromycin Drugs 0.000 description 2
- 238000001959 radiotherapy Methods 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 108010026333 seryl-proline Proteins 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 239000003765 sweetening agent Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 108010073969 valyllysine Proteins 0.000 description 2
- DDMOUSALMHHKOS-UHFFFAOYSA-N 1,2-dichloro-1,1,2,2-tetrafluoroethane Chemical compound FC(F)(Cl)C(F)(F)Cl DDMOUSALMHHKOS-UHFFFAOYSA-N 0.000 description 1
- QNNUQDYYZAMNPV-UHFFFAOYSA-N 1,4-oxathiocane Chemical compound C1CCSCCOC1 QNNUQDYYZAMNPV-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- XKTYXVDYIKIYJP-UHFFFAOYSA-N 3h-dioxole Chemical compound C1OOC=C1 XKTYXVDYIKIYJP-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 1
- SUMYEVXWCAYLLJ-GUBZILKMSA-N Ala-Leu-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O SUMYEVXWCAYLLJ-GUBZILKMSA-N 0.000 description 1
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 1
- DPNZTBKGAUAZQU-DLOVCJGASA-N Ala-Leu-His Chemical compound C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N DPNZTBKGAUAZQU-DLOVCJGASA-N 0.000 description 1
- OINVDEKBKBCPLX-JXUBOQSCSA-N Ala-Lys-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OINVDEKBKBCPLX-JXUBOQSCSA-N 0.000 description 1
- MDNAVFBZPROEHO-DCAQKATOSA-N Ala-Lys-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O MDNAVFBZPROEHO-DCAQKATOSA-N 0.000 description 1
- MDNAVFBZPROEHO-UHFFFAOYSA-N Ala-Lys-Val Natural products CC(C)C(C(O)=O)NC(=O)C(NC(=O)C(C)N)CCCCN MDNAVFBZPROEHO-UHFFFAOYSA-N 0.000 description 1
- XWFWAXPOLRTDFZ-FXQIFTODSA-N Ala-Pro-Ser Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O XWFWAXPOLRTDFZ-FXQIFTODSA-N 0.000 description 1
- VJVQKGYHIZPSNS-FXQIFTODSA-N Ala-Ser-Arg Chemical compound C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCN=C(N)N VJVQKGYHIZPSNS-FXQIFTODSA-N 0.000 description 1
- YYAVDNKUWLAFCV-ACZMJKKPSA-N Ala-Ser-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O YYAVDNKUWLAFCV-ACZMJKKPSA-N 0.000 description 1
- DEAGTWNKODHUIY-MRFFXTKBSA-N Ala-Tyr-Trp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O DEAGTWNKODHUIY-MRFFXTKBSA-N 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 235000019489 Almond oil Nutrition 0.000 description 1
- 102000009366 Alpha-s1 casein Human genes 0.000 description 1
- 108050000244 Alpha-s1 casein Proteins 0.000 description 1
- 108050001786 Alpha-s2 casein Proteins 0.000 description 1
- 206010002199 Anaphylactic shock Diseases 0.000 description 1
- 208000028185 Angioedema Diseases 0.000 description 1
- AUFHLLPVPSMEOG-YUMQZZPRSA-N Arg-Gly-Glu Chemical compound NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O AUFHLLPVPSMEOG-YUMQZZPRSA-N 0.000 description 1
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 1
- ZJBUILVYSXQNSW-YTWAJWBKSA-N Arg-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)O ZJBUILVYSXQNSW-YTWAJWBKSA-N 0.000 description 1
- NVGWESORMHFISY-SRVKXCTJSA-N Asn-Asn-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O NVGWESORMHFISY-SRVKXCTJSA-N 0.000 description 1
- BVLIJXXSXBUGEC-SRVKXCTJSA-N Asn-Asn-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O BVLIJXXSXBUGEC-SRVKXCTJSA-N 0.000 description 1
- FTCGGKNCJZOPNB-WHFBIAKZSA-N Asn-Gly-Ser Chemical compound NC(=O)C[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O FTCGGKNCJZOPNB-WHFBIAKZSA-N 0.000 description 1
- WQLJRNRLHWJIRW-KKUMJFAQSA-N Asn-His-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CC(=O)N)N)O WQLJRNRLHWJIRW-KKUMJFAQSA-N 0.000 description 1
- KMCRKVOLRCOMBG-DJFWLOJKSA-N Asn-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(=O)N)N KMCRKVOLRCOMBG-DJFWLOJKSA-N 0.000 description 1
- RCFGLXMZDYNRSC-CIUDSAMLSA-N Asn-Lys-Ala Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O RCFGLXMZDYNRSC-CIUDSAMLSA-N 0.000 description 1
- HPBNLFLSSQDFQW-WHFBIAKZSA-N Asn-Ser-Gly Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O HPBNLFLSSQDFQW-WHFBIAKZSA-N 0.000 description 1
- HNXWVVHIGTZTBO-LKXGYXEUSA-N Asn-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O HNXWVVHIGTZTBO-LKXGYXEUSA-N 0.000 description 1
- ANRZCQXIXGDXLR-CWRNSKLLSA-N Asn-Trp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CNC3=CC=CC=C32)NC(=O)[C@H](CC(=O)N)N)C(=O)O ANRZCQXIXGDXLR-CWRNSKLLSA-N 0.000 description 1
- MJIJBEYEHBKTIM-BYULHYEWSA-N Asn-Val-Asn Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N MJIJBEYEHBKTIM-BYULHYEWSA-N 0.000 description 1
- KBQOUDLMWYWXNP-YDHLFZDLSA-N Asn-Val-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CC(=O)N)N KBQOUDLMWYWXNP-YDHLFZDLSA-N 0.000 description 1
- HSWYMWGDMPLTTH-FXQIFTODSA-N Asp-Glu-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HSWYMWGDMPLTTH-FXQIFTODSA-N 0.000 description 1
- WSGVTKZFVJSJOG-RCOVLWMOSA-N Asp-Gly-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O WSGVTKZFVJSJOG-RCOVLWMOSA-N 0.000 description 1
- USNJAPJZSGTTPX-XVSYOHENSA-N Asp-Phe-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O USNJAPJZSGTTPX-XVSYOHENSA-N 0.000 description 1
- CUQDCPXNZPDYFQ-ZLUOBGJFSA-N Asp-Ser-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O CUQDCPXNZPDYFQ-ZLUOBGJFSA-N 0.000 description 1
- YUELDQUPTAYEGM-XIRDDKMYSA-N Asp-Trp-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CC(=O)O)N YUELDQUPTAYEGM-XIRDDKMYSA-N 0.000 description 1
- NJLLRXWFPQQPHV-SRVKXCTJSA-N Asp-Tyr-Asn Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O NJLLRXWFPQQPHV-SRVKXCTJSA-N 0.000 description 1
- KNDCWFXCFKSEBM-AVGNSLFASA-N Asp-Tyr-Glu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O KNDCWFXCFKSEBM-AVGNSLFASA-N 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 206010052358 Colorectal cancer metastatic Diseases 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- YMBAVNPKBWHDAW-CIUDSAMLSA-N Cys-Asp-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CS)N YMBAVNPKBWHDAW-CIUDSAMLSA-N 0.000 description 1
- WVLZTXGTNGHPBO-SRVKXCTJSA-N Cys-Leu-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O WVLZTXGTNGHPBO-SRVKXCTJSA-N 0.000 description 1
- SMEYEQDCCBHTEF-FXQIFTODSA-N Cys-Pro-Ala Chemical compound [H]N[C@@H](CS)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O SMEYEQDCCBHTEF-FXQIFTODSA-N 0.000 description 1
- KSMSFCBQBQPFAD-GUBZILKMSA-N Cys-Pro-Pro Chemical compound SC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 KSMSFCBQBQPFAD-GUBZILKMSA-N 0.000 description 1
- ALTQTAKGRFLRLR-GUBZILKMSA-N Cys-Val-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N ALTQTAKGRFLRLR-GUBZILKMSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 239000004338 Dichlorodifluoromethane Substances 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 108010042407 Endonucleases Proteins 0.000 description 1
- 102000004533 Endonucleases Human genes 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 241000282324 Felis Species 0.000 description 1
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 1
- DTMLKCYOQKZXKZ-HJGDQZAQSA-N Gln-Arg-Thr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(O)=O DTMLKCYOQKZXKZ-HJGDQZAQSA-N 0.000 description 1
- WLODHVXYKYHLJD-ACZMJKKPSA-N Gln-Asp-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CO)C(=O)O)N WLODHVXYKYHLJD-ACZMJKKPSA-N 0.000 description 1
- CITDWMLWXNUQKD-FXQIFTODSA-N Gln-Gln-Asn Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N CITDWMLWXNUQKD-FXQIFTODSA-N 0.000 description 1
- NVEASDQHBRZPSU-BQBZGAKWSA-N Gln-Gln-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O NVEASDQHBRZPSU-BQBZGAKWSA-N 0.000 description 1
- ZNZPKVQURDQFFS-FXQIFTODSA-N Gln-Glu-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZNZPKVQURDQFFS-FXQIFTODSA-N 0.000 description 1
- HMIXCETWRYDVMO-GUBZILKMSA-N Gln-Pro-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O HMIXCETWRYDVMO-GUBZILKMSA-N 0.000 description 1
- BETSEXMYBWCDAE-SZMVWBNQSA-N Gln-Trp-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)N)N BETSEXMYBWCDAE-SZMVWBNQSA-N 0.000 description 1
- ZFBBMCKQSNJZSN-AUTRQRHGSA-N Gln-Val-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZFBBMCKQSNJZSN-AUTRQRHGSA-N 0.000 description 1
- HUFCEIHAFNVSNR-IHRRRGAJSA-N Glu-Gln-Tyr Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 HUFCEIHAFNVSNR-IHRRRGAJSA-N 0.000 description 1
- IVGJYOOGJLFKQE-AVGNSLFASA-N Glu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N IVGJYOOGJLFKQE-AVGNSLFASA-N 0.000 description 1
- YHOJJFFTSMWVGR-HJGDQZAQSA-N Glu-Met-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O YHOJJFFTSMWVGR-HJGDQZAQSA-N 0.000 description 1
- AAJHGGDRKHYSDH-GUBZILKMSA-N Glu-Pro-Gln Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCC(=O)O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O AAJHGGDRKHYSDH-GUBZILKMSA-N 0.000 description 1
- BFEZQZKEPRKKHV-SRVKXCTJSA-N Glu-Pro-Lys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CCC(=O)O)N)C(=O)N[C@@H](CCCCN)C(=O)O BFEZQZKEPRKKHV-SRVKXCTJSA-N 0.000 description 1
- SYAYROHMAIHWFB-KBIXCLLPSA-N Glu-Ser-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O SYAYROHMAIHWFB-KBIXCLLPSA-N 0.000 description 1
- BPCLDCNZBUYGOD-BPUTZDHNSA-N Glu-Trp-Glu Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=CNC2=C1 BPCLDCNZBUYGOD-BPUTZDHNSA-N 0.000 description 1
- HGJREIGJLUQBTJ-SZMVWBNQSA-N Glu-Trp-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(C)C)C(O)=O HGJREIGJLUQBTJ-SZMVWBNQSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- UGVQELHRNUDMAA-BYPYZUCNSA-N Gly-Ala-Gly Chemical compound [NH3+]CC(=O)N[C@@H](C)C(=O)NCC([O-])=O UGVQELHRNUDMAA-BYPYZUCNSA-N 0.000 description 1
- XCLCVBYNGXEVDU-WHFBIAKZSA-N Gly-Asn-Ser Chemical compound NCC(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O XCLCVBYNGXEVDU-WHFBIAKZSA-N 0.000 description 1
- FMNHBTKMRFVGRO-FOHZUACHSA-N Gly-Asn-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CN FMNHBTKMRFVGRO-FOHZUACHSA-N 0.000 description 1
- XBWMTPAIUQIWKA-BYULHYEWSA-N Gly-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CN XBWMTPAIUQIWKA-BYULHYEWSA-N 0.000 description 1
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 1
- DHDOADIPGZTAHT-YUMQZZPRSA-N Gly-Glu-Arg Chemical compound NCC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N DHDOADIPGZTAHT-YUMQZZPRSA-N 0.000 description 1
- BUEFQXUHTUZXHR-LURJTMIESA-N Gly-Gly-Pro zwitterion Chemical compound NCC(=O)NCC(=O)N1CCC[C@H]1C(O)=O BUEFQXUHTUZXHR-LURJTMIESA-N 0.000 description 1
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 1
- GMTXWRIDLGTVFC-IUCAKERBSA-N Gly-Lys-Glu Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O GMTXWRIDLGTVFC-IUCAKERBSA-N 0.000 description 1
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 1
- GGAPHLIUUTVYMX-QWRGUYRKSA-N Gly-Phe-Ser Chemical compound OC[C@@H](C([O-])=O)NC(=O)[C@@H](NC(=O)C[NH3+])CC1=CC=CC=C1 GGAPHLIUUTVYMX-QWRGUYRKSA-N 0.000 description 1
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 1
- JSLVAHYTAJJEQH-QWRGUYRKSA-N Gly-Ser-Phe Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 JSLVAHYTAJJEQH-QWRGUYRKSA-N 0.000 description 1
- FFJQHWKSGAWSTJ-BFHQHQDPSA-N Gly-Thr-Ala Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O FFJQHWKSGAWSTJ-BFHQHQDPSA-N 0.000 description 1
- FULZDMOZUZKGQU-ONGXEEELSA-N Gly-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)CN FULZDMOZUZKGQU-ONGXEEELSA-N 0.000 description 1
- ZVXMEWXHFBYJPI-LSJOCFKGSA-N Gly-Val-Ile Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZVXMEWXHFBYJPI-LSJOCFKGSA-N 0.000 description 1
- 239000012981 Hank's balanced salt solution Substances 0.000 description 1
- 108091005904 Hemoglobin subunit beta Proteins 0.000 description 1
- 102100021519 Hemoglobin subunit beta Human genes 0.000 description 1
- HVCRQRQPIIRNLY-IUCAKERBSA-N His-Gln-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)NCC(=O)O)N HVCRQRQPIIRNLY-IUCAKERBSA-N 0.000 description 1
- SDTPKSOWFXBACN-GUBZILKMSA-N His-Glu-Asp Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O SDTPKSOWFXBACN-GUBZILKMSA-N 0.000 description 1
- TTYKEFZRLKQTHH-MELADBBJSA-N His-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CC2=CN=CN2)N)C(=O)O TTYKEFZRLKQTHH-MELADBBJSA-N 0.000 description 1
- TVMNTHXFRSXZGR-IHRRRGAJSA-N His-Lys-Val Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O TVMNTHXFRSXZGR-IHRRRGAJSA-N 0.000 description 1
- ALPXXNRQBMRCPZ-MEYUZBJRSA-N His-Thr-Phe Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O ALPXXNRQBMRCPZ-MEYUZBJRSA-N 0.000 description 1
- 238000012450 HuMAb Mouse Methods 0.000 description 1
- VSZALHITQINTGC-GHCJXIJMSA-N Ile-Ala-Asp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CC(=O)O)C(=O)O)N VSZALHITQINTGC-GHCJXIJMSA-N 0.000 description 1
- DFJJAVZIHDFOGQ-MNXVOIDGSA-N Ile-Glu-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N DFJJAVZIHDFOGQ-MNXVOIDGSA-N 0.000 description 1
- DFFTXLCCDFYRKD-MBLNEYKQSA-N Ile-Gly-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)O)N DFFTXLCCDFYRKD-MBLNEYKQSA-N 0.000 description 1
- RKQAYOWLSFLJEE-SVSWQMSJSA-N Ile-Thr-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)O)N RKQAYOWLSFLJEE-SVSWQMSJSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- PWWVAXIEGOYWEE-UHFFFAOYSA-N Isophenergan Chemical compound C1=CC=C2N(CC(C)N(C)C)C3=CC=CC=C3SC2=C1 PWWVAXIEGOYWEE-UHFFFAOYSA-N 0.000 description 1
- 101150105104 Kras gene Proteins 0.000 description 1
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 1
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 1
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 1
- 102000004407 Lactalbumin Human genes 0.000 description 1
- 108090000942 Lactalbumin Proteins 0.000 description 1
- 108010060630 Lactoglobulins Proteins 0.000 description 1
- 102000008192 Lactoglobulins Human genes 0.000 description 1
- HYIFFZAQXPUEAU-QWRGUYRKSA-N Leu-Gly-Leu Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(C)C HYIFFZAQXPUEAU-QWRGUYRKSA-N 0.000 description 1
- BKTXKJMNTSMJDQ-AVGNSLFASA-N Leu-His-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N BKTXKJMNTSMJDQ-AVGNSLFASA-N 0.000 description 1
- HNDWYLYAYNBWMP-AJNGGQMLSA-N Leu-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(C)C)N HNDWYLYAYNBWMP-AJNGGQMLSA-N 0.000 description 1
- IEWBEPKLKUXQBU-VOAKCMCISA-N Leu-Leu-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O IEWBEPKLKUXQBU-VOAKCMCISA-N 0.000 description 1
- ZGUMORRUBUCXEH-AVGNSLFASA-N Leu-Lys-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZGUMORRUBUCXEH-AVGNSLFASA-N 0.000 description 1
- VCHVSKNMTXWIIP-SRVKXCTJSA-N Leu-Lys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O VCHVSKNMTXWIIP-SRVKXCTJSA-N 0.000 description 1
- YESNGRDJQWDYLH-KKUMJFAQSA-N Leu-Phe-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CS)C(=O)O)N YESNGRDJQWDYLH-KKUMJFAQSA-N 0.000 description 1
- UHNQRAFSEBGZFZ-YESZJQIVSA-N Leu-Phe-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N UHNQRAFSEBGZFZ-YESZJQIVSA-N 0.000 description 1
- MAXILRZVORNXBE-PMVMPFDFSA-N Leu-Phe-Trp Chemical compound C([C@H](NC(=O)[C@@H](N)CC(C)C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)C1=CC=CC=C1 MAXILRZVORNXBE-PMVMPFDFSA-N 0.000 description 1
- WMIOEVKKYIMVKI-DCAQKATOSA-N Leu-Pro-Ala Chemical compound [H]N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O WMIOEVKKYIMVKI-DCAQKATOSA-N 0.000 description 1
- DPURXCQCHSQPAN-AVGNSLFASA-N Leu-Pro-Pro Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DPURXCQCHSQPAN-AVGNSLFASA-N 0.000 description 1
- MVHXGBZUJLWZOH-BJDJZHNGSA-N Leu-Ser-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O MVHXGBZUJLWZOH-BJDJZHNGSA-N 0.000 description 1
- QWWPYKKLXWOITQ-VOAKCMCISA-N Leu-Thr-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(C)C QWWPYKKLXWOITQ-VOAKCMCISA-N 0.000 description 1
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 1
- VUBIPAHVHMZHCM-KKUMJFAQSA-N Leu-Tyr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 VUBIPAHVHMZHCM-KKUMJFAQSA-N 0.000 description 1
- XOEDPXDZJHBQIX-ULQDDVLXSA-N Leu-Val-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 XOEDPXDZJHBQIX-ULQDDVLXSA-N 0.000 description 1
- QESXLSQLQHHTIX-RHYQMDGZSA-N Leu-Val-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QESXLSQLQHHTIX-RHYQMDGZSA-N 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- WSXTWLJHTLRFLW-SRVKXCTJSA-N Lys-Ala-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O WSXTWLJHTLRFLW-SRVKXCTJSA-N 0.000 description 1
- NTSPQIONFJUMJV-AVGNSLFASA-N Lys-Arg-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O NTSPQIONFJUMJV-AVGNSLFASA-N 0.000 description 1
- YKIRNDPUWONXQN-GUBZILKMSA-N Lys-Asn-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N YKIRNDPUWONXQN-GUBZILKMSA-N 0.000 description 1
- KWUKZRFFKPLUPE-HJGDQZAQSA-N Lys-Asp-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KWUKZRFFKPLUPE-HJGDQZAQSA-N 0.000 description 1
- NTBFKPBULZGXQL-KKUMJFAQSA-N Lys-Asp-Tyr Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NTBFKPBULZGXQL-KKUMJFAQSA-N 0.000 description 1
- GOVDTWNJCBRRBJ-DCAQKATOSA-N Lys-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCCCN)N GOVDTWNJCBRRBJ-DCAQKATOSA-N 0.000 description 1
- ODTZHNZPINULEU-KKUMJFAQSA-N Lys-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCCCN)N ODTZHNZPINULEU-KKUMJFAQSA-N 0.000 description 1
- MIFFFXHMAHFACR-KATARQTJSA-N Lys-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CCCCN MIFFFXHMAHFACR-KATARQTJSA-N 0.000 description 1
- CAVRAQIDHUPECU-UVOCVTCTSA-N Lys-Thr-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAVRAQIDHUPECU-UVOCVTCTSA-N 0.000 description 1
- QLFAPXUXEBAWEK-NHCYSSNCSA-N Lys-Val-Asp Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O QLFAPXUXEBAWEK-NHCYSSNCSA-N 0.000 description 1
- GILLQRYAWOMHED-DCAQKATOSA-N Lys-Val-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCCN GILLQRYAWOMHED-DCAQKATOSA-N 0.000 description 1
- DTICLBJHRYSJLH-GUBZILKMSA-N Met-Ala-Val Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O DTICLBJHRYSJLH-GUBZILKMSA-N 0.000 description 1
- RKIIYGUHIQJCBW-SRVKXCTJSA-N Met-His-Glu Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(O)=O RKIIYGUHIQJCBW-SRVKXCTJSA-N 0.000 description 1
- FWAHLGXNBLWIKB-NAKRPEOUSA-N Met-Ile-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCSC FWAHLGXNBLWIKB-NAKRPEOUSA-N 0.000 description 1
- LBSWWNKMVPAXOI-GUBZILKMSA-N Met-Val-Ser Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(O)=O LBSWWNKMVPAXOI-GUBZILKMSA-N 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 101710160107 Outer membrane protein A Proteins 0.000 description 1
- JDMKQHSHKJHAHR-UHFFFAOYSA-N Phe-Phe-Leu-Tyr Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)CC1=CC=CC=C1 JDMKQHSHKJHAHR-UHFFFAOYSA-N 0.000 description 1
- QARPMYDMYVLFMW-KKUMJFAQSA-N Phe-Pro-Glu Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(O)=O)C1=CC=CC=C1 QARPMYDMYVLFMW-KKUMJFAQSA-N 0.000 description 1
- NJJBATPLUQHRBM-IHRRRGAJSA-N Phe-Pro-Ser Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)N)C(=O)N[C@@H](CO)C(=O)O NJJBATPLUQHRBM-IHRRRGAJSA-N 0.000 description 1
- SJRQWEDYTKYHHL-SLFFLAALSA-N Phe-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC3=CC=CC=C3)N)C(=O)O SJRQWEDYTKYHHL-SLFFLAALSA-N 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- NXEYSLRNNPWCRN-SRVKXCTJSA-N Pro-Glu-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O NXEYSLRNNPWCRN-SRVKXCTJSA-N 0.000 description 1
- VPEVBAUSTBWQHN-NHCYSSNCSA-N Pro-Glu-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O VPEVBAUSTBWQHN-NHCYSSNCSA-N 0.000 description 1
- FMLRRBDLBJLJIK-DCAQKATOSA-N Pro-Leu-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1 FMLRRBDLBJLJIK-DCAQKATOSA-N 0.000 description 1
- ULWBBFKQBDNGOY-RWMBFGLXSA-N Pro-Lys-Pro Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCCN)C(=O)N2CCC[C@@H]2C(=O)O ULWBBFKQBDNGOY-RWMBFGLXSA-N 0.000 description 1
- SPLBRAKYXGOFSO-UNQGMJICSA-N Pro-Phe-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@@H]2CCCN2)O SPLBRAKYXGOFSO-UNQGMJICSA-N 0.000 description 1
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 1
- SEZGGSHLMROBFX-CIUDSAMLSA-N Pro-Ser-Gln Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O SEZGGSHLMROBFX-CIUDSAMLSA-N 0.000 description 1
- MKGIILKDUGDRRO-FXQIFTODSA-N Pro-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 MKGIILKDUGDRRO-FXQIFTODSA-N 0.000 description 1
- KHRLUIPIMIQFGT-AVGNSLFASA-N Pro-Val-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O KHRLUIPIMIQFGT-AVGNSLFASA-N 0.000 description 1
- YDTUEBLEAVANFH-RCWTZXSCSA-N Pro-Val-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 YDTUEBLEAVANFH-RCWTZXSCSA-N 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- UEJYSALTSUZXFV-SRVKXCTJSA-N Rigin Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O UEJYSALTSUZXFV-SRVKXCTJSA-N 0.000 description 1
- YUSRGTQIPCJNHQ-CIUDSAMLSA-N Ser-Arg-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O YUSRGTQIPCJNHQ-CIUDSAMLSA-N 0.000 description 1
- WDXYVIIVDIDOSX-DCAQKATOSA-N Ser-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CO)CCCN=C(N)N WDXYVIIVDIDOSX-DCAQKATOSA-N 0.000 description 1
- HZWAHWQZPSXNCB-BPUTZDHNSA-N Ser-Arg-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O HZWAHWQZPSXNCB-BPUTZDHNSA-N 0.000 description 1
- UBRXAVQWXOWRSJ-ZLUOBGJFSA-N Ser-Asn-Asp Chemical compound C([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](CO)N)C(=O)N UBRXAVQWXOWRSJ-ZLUOBGJFSA-N 0.000 description 1
- VAUMZJHYZQXZBQ-WHFBIAKZSA-N Ser-Asn-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O VAUMZJHYZQXZBQ-WHFBIAKZSA-N 0.000 description 1
- UGJRQLURDVGULT-LKXGYXEUSA-N Ser-Asn-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O UGJRQLURDVGULT-LKXGYXEUSA-N 0.000 description 1
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 1
- BLPYXIXXCFVIIF-FXQIFTODSA-N Ser-Cys-Arg Chemical compound C(C[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)[C@H](CO)N)CN=C(N)N BLPYXIXXCFVIIF-FXQIFTODSA-N 0.000 description 1
- MOVJSUIKUNCVMG-ZLUOBGJFSA-N Ser-Cys-Ser Chemical compound C([C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)O)N)O MOVJSUIKUNCVMG-ZLUOBGJFSA-N 0.000 description 1
- HJEBZBMOTCQYDN-ACZMJKKPSA-N Ser-Glu-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O HJEBZBMOTCQYDN-ACZMJKKPSA-N 0.000 description 1
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 1
- IOVHBRCQOGWAQH-ZKWXMUAHSA-N Ser-Gly-Ile Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)CC)C(O)=O IOVHBRCQOGWAQH-ZKWXMUAHSA-N 0.000 description 1
- KDGARKCAKHBEDB-NKWVEPMBSA-N Ser-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CO)N)C(=O)O KDGARKCAKHBEDB-NKWVEPMBSA-N 0.000 description 1
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 1
- XXXAXOWMBOKTRN-XPUUQOCRSA-N Ser-Gly-Val Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O XXXAXOWMBOKTRN-XPUUQOCRSA-N 0.000 description 1
- LQESNKGTTNHZPZ-GHCJXIJMSA-N Ser-Ile-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O LQESNKGTTNHZPZ-GHCJXIJMSA-N 0.000 description 1
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 1
- XNCUYZKGQOCOQH-YUMQZZPRSA-N Ser-Leu-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O XNCUYZKGQOCOQH-YUMQZZPRSA-N 0.000 description 1
- MUJQWSAWLLRJCE-KATARQTJSA-N Ser-Leu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MUJQWSAWLLRJCE-KATARQTJSA-N 0.000 description 1
- GZSZPKSBVAOGIE-CIUDSAMLSA-N Ser-Lys-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O GZSZPKSBVAOGIE-CIUDSAMLSA-N 0.000 description 1
- XUDRHBPSPAPDJP-SRVKXCTJSA-N Ser-Lys-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CO XUDRHBPSPAPDJP-SRVKXCTJSA-N 0.000 description 1
- GDUZTEQRAOXYJS-SRVKXCTJSA-N Ser-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GDUZTEQRAOXYJS-SRVKXCTJSA-N 0.000 description 1
- RHAPJNVNWDBFQI-BQBZGAKWSA-N Ser-Pro-Gly Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O RHAPJNVNWDBFQI-BQBZGAKWSA-N 0.000 description 1
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 1
- JURQXQBJKUHGJS-UHFFFAOYSA-N Ser-Ser-Ser-Ser Chemical compound OCC(N)C(=O)NC(CO)C(=O)NC(CO)C(=O)NC(CO)C(O)=O JURQXQBJKUHGJS-UHFFFAOYSA-N 0.000 description 1
- PYTKULIABVRXSC-BWBBJGPYSA-N Ser-Ser-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PYTKULIABVRXSC-BWBBJGPYSA-N 0.000 description 1
- BEBVVQPDSHHWQL-NRPADANISA-N Ser-Val-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O BEBVVQPDSHHWQL-NRPADANISA-N 0.000 description 1
- HSWXBJCBYSWBPT-GUBZILKMSA-N Ser-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CO)C(C)C)C(O)=O HSWXBJCBYSWBPT-GUBZILKMSA-N 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- PXQUBKWZENPDGE-CIQUZCHMSA-N Thr-Ala-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](C)NC(=O)[C@H]([C@@H](C)O)N PXQUBKWZENPDGE-CIQUZCHMSA-N 0.000 description 1
- JVTHIXKSVYEWNI-JRQIVUDYSA-N Thr-Asn-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O JVTHIXKSVYEWNI-JRQIVUDYSA-N 0.000 description 1
- LAFLAXHTDVNVEL-WDCWCFNPSA-N Thr-Gln-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)O)N)O LAFLAXHTDVNVEL-WDCWCFNPSA-N 0.000 description 1
- KGKWKSSSQGGYAU-SUSMZKCASA-N Thr-Gln-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O KGKWKSSSQGGYAU-SUSMZKCASA-N 0.000 description 1
- SXAGUVRFGJSFKC-ZEILLAHLSA-N Thr-His-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SXAGUVRFGJSFKC-ZEILLAHLSA-N 0.000 description 1
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 1
- KZSYAEWQMJEGRZ-RHYQMDGZSA-N Thr-Leu-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O KZSYAEWQMJEGRZ-RHYQMDGZSA-N 0.000 description 1
- JLNMFGCJODTXDH-WEDXCCLWSA-N Thr-Lys-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O JLNMFGCJODTXDH-WEDXCCLWSA-N 0.000 description 1
- SPVHQURZJCUDQC-VOAKCMCISA-N Thr-Lys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O SPVHQURZJCUDQC-VOAKCMCISA-N 0.000 description 1
- LKJCABTUFGTPPY-HJGDQZAQSA-N Thr-Pro-Gln Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(O)=O LKJCABTUFGTPPY-HJGDQZAQSA-N 0.000 description 1
- QJIODPFLAASXJC-JHYOHUSXSA-N Thr-Thr-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O QJIODPFLAASXJC-JHYOHUSXSA-N 0.000 description 1
- OGOYMQWIWHGTGH-KZVJFYERSA-N Thr-Val-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(O)=O OGOYMQWIWHGTGH-KZVJFYERSA-N 0.000 description 1
- UJGDFQRPYGJBEH-AAEUAGOBSA-N Trp-Ser-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CO)C(=O)NCC(=O)O)N UJGDFQRPYGJBEH-AAEUAGOBSA-N 0.000 description 1
- PKZIWSHDJYIPRH-JBACZVJFSA-N Trp-Tyr-Gln Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKZIWSHDJYIPRH-JBACZVJFSA-N 0.000 description 1
- SSSDKJMQMZTMJP-BVSLBCMMSA-N Trp-Tyr-Val Chemical compound C([C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)C1=CC=C(O)C=C1 SSSDKJMQMZTMJP-BVSLBCMMSA-N 0.000 description 1
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- QJBWZNTWJSZUOY-UWJYBYFXSA-N Tyr-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N QJBWZNTWJSZUOY-UWJYBYFXSA-N 0.000 description 1
- LGEYOIQBBIPHQN-UWJYBYFXSA-N Tyr-Ala-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 LGEYOIQBBIPHQN-UWJYBYFXSA-N 0.000 description 1
- QYSBJAUCUKHSLU-JYJNAYRXSA-N Tyr-Arg-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O QYSBJAUCUKHSLU-JYJNAYRXSA-N 0.000 description 1
- SMLCYZYQFRTLCO-UWJYBYFXSA-N Tyr-Cys-Ala Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O SMLCYZYQFRTLCO-UWJYBYFXSA-N 0.000 description 1
- HDSKHCBAVVWPCQ-FHWLQOOXSA-N Tyr-Glu-Phe Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O HDSKHCBAVVWPCQ-FHWLQOOXSA-N 0.000 description 1
- JJNXZIPLIXIGBX-HJPIBITLSA-N Tyr-Ile-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N JJNXZIPLIXIGBX-HJPIBITLSA-N 0.000 description 1
- GZUIDWDVMWZSMI-KKUMJFAQSA-N Tyr-Lys-Cys Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CS)C(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 GZUIDWDVMWZSMI-KKUMJFAQSA-N 0.000 description 1
- MQGGXGKQSVEQHR-KKUMJFAQSA-N Tyr-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 MQGGXGKQSVEQHR-KKUMJFAQSA-N 0.000 description 1
- OJCISMMNNUNNJA-BZSNNMDCSA-N Tyr-Tyr-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 OJCISMMNNUNNJA-BZSNNMDCSA-N 0.000 description 1
- ANHVRCNNGJMJNG-BZSNNMDCSA-N Tyr-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CS)C(=O)O)N)O ANHVRCNNGJMJNG-BZSNNMDCSA-N 0.000 description 1
- 208000024780 Urticaria Diseases 0.000 description 1
- CGGVNFJRZJUVAE-BYULHYEWSA-N Val-Asp-Asn Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N CGGVNFJRZJUVAE-BYULHYEWSA-N 0.000 description 1
- BMGOFDMKDVVGJG-NHCYSSNCSA-N Val-Asp-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N BMGOFDMKDVVGJG-NHCYSSNCSA-N 0.000 description 1
- SCBITHMBEJNRHC-LSJOCFKGSA-N Val-Asp-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](C(C)C)C(=O)O)N SCBITHMBEJNRHC-LSJOCFKGSA-N 0.000 description 1
- OACSGBOREVRSME-NHCYSSNCSA-N Val-His-Asn Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(N)=O)C(O)=O OACSGBOREVRSME-NHCYSSNCSA-N 0.000 description 1
- FTKXYXACXYOHND-XUXIUFHCSA-N Val-Ile-Leu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O FTKXYXACXYOHND-XUXIUFHCSA-N 0.000 description 1
- HGJRMXOWUWVUOA-GVXVVHGQSA-N Val-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N HGJRMXOWUWVUOA-GVXVVHGQSA-N 0.000 description 1
- BTWMICVCQLKKNR-DCAQKATOSA-N Val-Leu-Ser Chemical compound CC(C)[C@H]([NH3+])C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C([O-])=O BTWMICVCQLKKNR-DCAQKATOSA-N 0.000 description 1
- FMQGYTMERWBMSI-HJWJTTGWSA-N Val-Phe-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](C(C)C)N FMQGYTMERWBMSI-HJWJTTGWSA-N 0.000 description 1
- BCBFMJYTNKDALA-UFYCRDLUSA-N Val-Phe-Phe Chemical compound N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O BCBFMJYTNKDALA-UFYCRDLUSA-N 0.000 description 1
- QZKVWWIUSQGWMY-IHRRRGAJSA-N Val-Ser-Phe Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 QZKVWWIUSQGWMY-IHRRRGAJSA-N 0.000 description 1
- GBIUHAYJGWVNLN-AEJSXWLSSA-N Val-Ser-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N GBIUHAYJGWVNLN-AEJSXWLSSA-N 0.000 description 1
- GBIUHAYJGWVNLN-UHFFFAOYSA-N Val-Ser-Pro Natural products CC(C)C(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O GBIUHAYJGWVNLN-UHFFFAOYSA-N 0.000 description 1
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 1
- SDHZOOIGIUEPDY-JYJNAYRXSA-N Val-Ser-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](N)C(C)C)C(O)=O)=CNC2=C1 SDHZOOIGIUEPDY-JYJNAYRXSA-N 0.000 description 1
- NZYNRRGJJVSSTJ-GUBZILKMSA-N Val-Ser-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NZYNRRGJJVSSTJ-GUBZILKMSA-N 0.000 description 1
- UVHFONIHVHLDDQ-IFFSRLJSSA-N Val-Thr-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N)O UVHFONIHVHLDDQ-IFFSRLJSSA-N 0.000 description 1
- GVNLOVJNNDZUHS-RHYQMDGZSA-N Val-Thr-Lys Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O GVNLOVJNNDZUHS-RHYQMDGZSA-N 0.000 description 1
- BGTDGENDNWGMDQ-KJEVXHAQSA-N Val-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](C(C)C)N)O BGTDGENDNWGMDQ-KJEVXHAQSA-N 0.000 description 1
- JVGDAEKKZKKZFO-RCWTZXSCSA-N Val-Val-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](C(C)C)N)O JVGDAEKKZKKZFO-RCWTZXSCSA-N 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 238000012452 Xenomouse strains Methods 0.000 description 1
- ZYHGIAPHLSTGMX-WCQYABFASA-N [(4r,6s)-2,2,6-trimethylpiperidin-4-yl] benzoate Chemical compound C1C(C)(C)N[C@@H](C)C[C@H]1OC(=O)C1=CC=CC=C1 ZYHGIAPHLSTGMX-WCQYABFASA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 108010041407 alanylaspartic acid Proteins 0.000 description 1
- 239000013566 allergen Substances 0.000 description 1
- 239000008168 almond oil Substances 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 108010008355 arginyl-glutamine Proteins 0.000 description 1
- 108010072041 arginyl-glycyl-aspartic acid Proteins 0.000 description 1
- 108010009111 arginyl-glycyl-glutamic acid Proteins 0.000 description 1
- 108010047857 aspartylglycine Proteins 0.000 description 1
- 108010092854 aspartyllysine Proteins 0.000 description 1
- 108010068265 aspartyltyrosine Proteins 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 229940088623 biologically active substance Drugs 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 201000008275 breast carcinoma Diseases 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000000337 buffer salt Substances 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 239000003560 cancer drug Substances 0.000 description 1
- 230000005907 cancer growth Effects 0.000 description 1
- 229940022399 cancer vaccine Drugs 0.000 description 1
- 238000009566 cancer vaccine Methods 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 229960004424 carbon dioxide Drugs 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 125000002843 carboxylic acid group Chemical class 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 210000003855 cell nucleus Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 239000013599 cloning vector Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 230000004154 complement system Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- PXBRQCKWGAHEHS-UHFFFAOYSA-N dichlorodifluoromethane Chemical compound FC(F)(Cl)Cl PXBRQCKWGAHEHS-UHFFFAOYSA-N 0.000 description 1
- 235000019404 dichlorodifluoromethane Nutrition 0.000 description 1
- 229940042935 dichlorodifluoromethane Drugs 0.000 description 1
- 229940087091 dichlorotetrafluoroethane Drugs 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- FSXRLASFHBWESK-UHFFFAOYSA-N dipeptide phenylalanyl-tyrosine Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FSXRLASFHBWESK-UHFFFAOYSA-N 0.000 description 1
- ZZVUWRFHKOJYTH-UHFFFAOYSA-N diphenhydramine Chemical group C=1C=CC=CC=1C(OCCN(C)C)C1=CC=CC=C1 ZZVUWRFHKOJYTH-UHFFFAOYSA-N 0.000 description 1
- 229960000520 diphenhydramine Drugs 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 230000006334 disulfide bridging Effects 0.000 description 1
- 210000001755 duct epithelial cell Anatomy 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 210000002257 embryonic structure Anatomy 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940079360 enema for constipation Drugs 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 210000004920 epithelial cell of skin Anatomy 0.000 description 1
- 229940082789 erbitux Drugs 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 230000012173 estrus Effects 0.000 description 1
- 235000019441 ethanol Nutrition 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000010685 fatty oil Substances 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 235000011389 fruit/vegetable juice Nutrition 0.000 description 1
- 230000007849 functional defect Effects 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 108010013768 glutamyl-aspartyl-proline Proteins 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 1
- 108010051307 glycyl-glycyl-proline Proteins 0.000 description 1
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 1
- 108010010147 glycylglutamine Proteins 0.000 description 1
- 108010015792 glycyllysine Proteins 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 230000009931 harmful effect Effects 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 244000038280 herbivores Species 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 238000001794 hormone therapy Methods 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 1
- 210000003405 ileum Anatomy 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 239000000367 immunologic factor Substances 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 239000012212 insulator Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 229960000779 irinotecan hydrochloride Drugs 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 1
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 1
- 210000002429 large intestine Anatomy 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 238000001294 liquid chromatography-tandem mass spectrometry Methods 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 108010064235 lysylglycine Proteins 0.000 description 1
- 108010017391 lysylvaline Proteins 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 238000001819 mass spectrum Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 238000000520 microinjection Methods 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 239000002687 nonaqueous vehicle Substances 0.000 description 1
- 230000009972 noncorrosive effect Effects 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 238000012510 peptide mapping method Methods 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 108010024607 phenylalanylalanine Proteins 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 239000013600 plasmid vector Substances 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 108010077112 prolyl-proline Proteins 0.000 description 1
- 108010070643 prolylglutamic acid Proteins 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical class CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 1
- 230000012846 protein folding Effects 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 210000005000 reproductive tract Anatomy 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 108010069117 seryl-lysyl-aspartic acid Proteins 0.000 description 1
- 108010048397 seryl-lysyl-leucine Proteins 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 206010041823 squamous cell carcinoma Diseases 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 229940032147 starch Drugs 0.000 description 1
- 239000002511 suppository base Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 108010033670 threonyl-aspartyl-tyrosine Proteins 0.000 description 1
- 108010071097 threonyl-lysyl-proline Proteins 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- CYRMSUTZVYGINF-UHFFFAOYSA-N trichlorofluoromethane Chemical compound FC(Cl)(Cl)Cl CYRMSUTZVYGINF-UHFFFAOYSA-N 0.000 description 1
- 229940029284 trichlorofluoromethane Drugs 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 108010079202 tyrosyl-alanyl-cysteine Proteins 0.000 description 1
- 108010051110 tyrosyl-lysine Proteins 0.000 description 1
- 108010052774 valyl-lysyl-glycyl-phenylalanyl-tyrosine Proteins 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 235000021249 α-casein Nutrition 0.000 description 1
- 235000021241 α-lactalbumin Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2863—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against receptors for growth factors, growth regulators
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K67/00—Rearing or breeding animals, not otherwise provided for; New or modified breeds of animals
- A01K67/027—New or modified breeds of vertebrates
- A01K67/0275—Genetically modified vertebrates, e.g. transgenic
- A01K67/0278—Knock-in vertebrates, e.g. humanised vertebrates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/513—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim having oxo groups directly attached to the heterocyclic ring, e.g. cytosine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
- A61K31/52—Purines, e.g. adenine
- A61K31/522—Purines, e.g. adenine having oxo groups directly attached to the heterocyclic ring, e.g. hypoxanthine, guanine, acyclovir
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2227/00—Animals characterised by species
- A01K2227/10—Mammal
- A01K2227/102—Caprine
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2267/00—Animals characterised by purpose
- A01K2267/01—Animal expressing industrially exogenous proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
- C07K2317/12—Immunoglobulins specific features characterized by their source of isolation or production isolated from milk
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
- C07K2317/14—Specific host cells or culture conditions, e.g. components, pH or temperature
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/40—Immunoglobulins specific features characterized by post-translational modification
- C07K2317/41—Glycosylation, sialylation, or fucosylation
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Immunology (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Engineering & Computer Science (AREA)
- Environmental Sciences (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Biodiversity & Conservation Biology (AREA)
- Animal Husbandry (AREA)
- Zoology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361764446P | 2013-02-13 | 2013-02-13 | |
| US61/764,446 | 2013-02-13 | ||
| PCT/IB2014/000867 WO2014125382A2 (en) | 2013-02-13 | 2014-02-13 | Cetuximab with modified glycosylation and uses thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150145225A true KR20150145225A (ko) | 2015-12-29 |
Family
ID=50980328
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157024950A Withdrawn KR20150145225A (ko) | 2013-02-13 | 2014-02-13 | 변경된 글리코실화를 갖는 세툭시맙 및 이의 용도 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US20160002330A1 (enExample) |
| EP (1) | EP2956484B1 (enExample) |
| JP (1) | JP2016513105A (enExample) |
| KR (1) | KR20150145225A (enExample) |
| CN (1) | CN105358577A (enExample) |
| AR (1) | AR094779A1 (enExample) |
| AU (1) | AU2014217569B2 (enExample) |
| BR (1) | BR112015019339A2 (enExample) |
| CA (1) | CA2900915A1 (enExample) |
| IL (1) | IL240442A0 (enExample) |
| MX (1) | MX2015010429A (enExample) |
| TW (1) | TW201444871A (enExample) |
| WO (1) | WO2014125382A2 (enExample) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PH12013501365A1 (en) | 2010-12-30 | 2013-09-16 | Laboratoire Francais Du Fractionnment Et Des Biotechnologies | Glycols as pathogen inactivating agents |
| BR112015019341A2 (pt) | 2013-02-13 | 2017-08-22 | Lab Francais Du Fractionnement | Anticorpo anti-tnf-alfa, composição que compreende o anticorpo, método para produzir uma população de anticorpos, células epiteliais da glândula mamária, mamífero não humano transgênico, e, composição de anticorpo anti-tnf monoclonal |
| EP2956003A2 (en) | 2013-02-13 | 2015-12-23 | Laboratoire Français du Fractionnement et des Biotechnologies | Proteins with modified glycosylation and methods of production thereof |
| JP2016532100A (ja) | 2013-07-05 | 2016-10-13 | ラボラトワール・フランセ・デュ・フラクシオンマン・エ・デ・ビョテクノロジーLaboratoire Francais Du Fractionnement Et Des Biotechnologies | アフィニティークロマトグラフィーマトリックス |
| US10927384B2 (en) | 2014-04-09 | 2021-02-23 | Dna Twopointo Inc. | DNA vectors, transposons and transposases for eukaryotic genome modification |
| FR3038517B1 (fr) | 2015-07-06 | 2020-02-28 | Laboratoire Francais Du Fractionnement Et Des Biotechnologies | Utilisation de fragments fc modifies en immunotherapie |
| EP3974524A1 (en) | 2015-10-08 | 2022-03-30 | Dna Twopointo Inc. | Dna vectors, transposons and transposases for eukaryotic genome modification |
| WO2017120613A1 (en) * | 2016-01-10 | 2017-07-13 | Sorrento Therapeutics, Inc. | Improved safety for treating cancers with a glycosylated chimeric antibody to egfr |
| US11649291B2 (en) * | 2016-05-24 | 2023-05-16 | Insmed Incorporated | Antibodies and methods of making same |
| KR102376545B1 (ko) * | 2017-08-01 | 2022-03-21 | 삼성디스플레이 주식회사 | 플렉시블 디스플레이 윈도우 |
| US10537585B2 (en) | 2017-12-18 | 2020-01-21 | Dexcel Pharma Technologies Ltd. | Compositions comprising dexamethasone |
| WO2022148549A1 (en) | 2020-01-14 | 2022-07-14 | Babylat Gmbh | Apparatus and method for obtaining protein-enriched fractions from breast milk |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8823869D0 (en) | 1988-10-12 | 1988-11-16 | Medical Res Council | Production of antibodies |
| US5175384A (en) | 1988-12-05 | 1992-12-29 | Genpharm International | Transgenic mice depleted in mature t-cells and methods for making transgenic mice |
| US6150584A (en) | 1990-01-12 | 2000-11-21 | Abgenix, Inc. | Human antibodies derived from immunized xenomice |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| EP0690452A3 (en) | 1994-06-28 | 1999-01-07 | Advanced Micro Devices, Inc. | Electrically erasable memory and method of erasure |
| US5945577A (en) | 1997-01-10 | 1999-08-31 | University Of Massachusetts As Represented By Its Amherst Campus | Cloning using donor nuclei from proliferating somatic cells |
| DE60237282D1 (de) | 2001-06-28 | 2010-09-23 | Domantis Ltd | Doppelspezifischer ligand und dessen verwendung |
| ATE464389T1 (de) * | 2002-05-17 | 2010-04-15 | Kyowa Hakko Kirin Co Ltd | Transgene huftiere, die zur produktion von menschlichen antikörpern fähig sind |
| CA2506629A1 (en) * | 2002-11-27 | 2004-06-17 | Gtc Biotherapeutics, Inc. | Modified antibodies stably produced in milk and methods of producing same |
| CA2450289A1 (en) * | 2003-03-20 | 2005-05-19 | Imclone Systems Incorporated | Method of producing an antibody to epidermal growth factor receptor |
| WO2005111627A2 (en) | 2004-04-15 | 2005-11-24 | Massachusetts Institute Of Technology | Methods and products related to the improved analysis of carbohydrates |
| US20060127950A1 (en) | 2004-04-15 | 2006-06-15 | Massachusetts Institute Of Technology | Methods and products related to the improved analysis of carbohydrates |
| BRPI0518151A2 (pt) | 2004-10-13 | 2009-06-16 | Ablynx Nv | polipetìdeos contra amiloide-beta, ácido nucléico que codifica tal polipetìdeo, composição compreendendo tal polipetìdeo, método para produzir um polipetìdeo e uso do mesmo |
| CN103254309B (zh) | 2005-05-18 | 2017-09-26 | 埃博灵克斯股份有限公司 | 针对肿瘤坏死因子α的改进的纳米体TM |
| NZ568433A (en) | 2005-10-21 | 2012-07-27 | Gtc Biotherapeutics Inc | Milk-derived antibodies with enhanced antibody-dependent cellular cytoxicity activity, methods of their production and use |
| FR2901707B1 (fr) * | 2006-05-31 | 2017-09-29 | Lab Francais Du Fractionnement | Composition de facteur vii recombinant ou transgenique, chaque molecule de facteur vii possedant deux sites de n-glycosylation a motifs glycanniques definis |
| HRP20150307T1 (hr) * | 2006-09-10 | 2015-04-24 | Glycotope Gmbh | Upotreba ljudskih stanica podrijetlom iz ljudske leukemije za eksprimiranje protutijela |
| WO2008101177A2 (en) * | 2007-02-16 | 2008-08-21 | University Of Virginia Patent Foundation | Ige antibodies to chimeric or humanized igg therapeutic monoclonal antibodies as a screening test for anaphylaxis |
| EP2389586B2 (en) * | 2009-01-22 | 2018-01-24 | Momenta Pharmaceuticals, Inc. | Galactose-alpha-1,3-galactose-containing n-glycans in glycoprotein products derived from cho cells |
| KR20120101059A (ko) * | 2009-11-11 | 2012-09-12 | 모멘타 파머슈티컬스 인코포레이티드 | 중국 햄스터 유래의 글리코실 전이효소 및 관련 방법 |
-
2014
- 2014-02-13 MX MX2015010429A patent/MX2015010429A/es unknown
- 2014-02-13 BR BR112015019339A patent/BR112015019339A2/pt not_active Application Discontinuation
- 2014-02-13 AU AU2014217569A patent/AU2014217569B2/en not_active Ceased
- 2014-02-13 US US14/767,089 patent/US20160002330A1/en not_active Abandoned
- 2014-02-13 AR ARP140100458A patent/AR094779A1/es unknown
- 2014-02-13 KR KR1020157024950A patent/KR20150145225A/ko not_active Withdrawn
- 2014-02-13 TW TW103104778A patent/TW201444871A/zh unknown
- 2014-02-13 CA CA2900915A patent/CA2900915A1/en not_active Abandoned
- 2014-02-13 CN CN201480018266.7A patent/CN105358577A/zh active Pending
- 2014-02-13 EP EP14732006.3A patent/EP2956484B1/en not_active Not-in-force
- 2014-02-13 WO PCT/IB2014/000867 patent/WO2014125382A2/en not_active Ceased
- 2014-02-13 JP JP2015557534A patent/JP2016513105A/ja active Pending
-
2015
- 2015-08-09 IL IL240442A patent/IL240442A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| JP2016513105A (ja) | 2016-05-12 |
| US20160002330A1 (en) | 2016-01-07 |
| AR094779A1 (es) | 2015-08-26 |
| WO2014125382A3 (en) | 2014-11-06 |
| WO2014125382A2 (en) | 2014-08-21 |
| MX2015010429A (es) | 2016-05-16 |
| BR112015019339A2 (pt) | 2017-08-22 |
| EP2956484B1 (en) | 2018-11-14 |
| CN105358577A (zh) | 2016-02-24 |
| AU2014217569A1 (en) | 2015-08-27 |
| CA2900915A1 (en) | 2014-08-21 |
| EP2956484A2 (en) | 2015-12-23 |
| AU2014217569B2 (en) | 2018-10-25 |
| TW201444871A (zh) | 2014-12-01 |
| IL240442A0 (en) | 2015-09-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2014217569B2 (en) | Cetuximab with modified glycosylation and uses thereof | |
| KR102698738B1 (ko) | B-세포 성숙화 항원을 표적화하는 키메라 항원 수용체 및 그의 용도 | |
| AU2019315440B2 (en) | Improving the efficacy and safety of adoptive cellular therapies | |
| AU2016229000B2 (en) | Recombinant Glut1 adeno-associated viral vector constructs and related methods for restoring Glut1 expression | |
| CN114585641B (zh) | 间皮素car及其用途 | |
| DK2831244T3 (en) | TRANSGENE MOUSE EXPRESSING HUMAN LAMBDA AND CAPTAIN IMMUNOGLOBULIN EASY VARIABLE DOMAINS | |
| AU2018221171B2 (en) | Targeted ligand-payload based drug delivery for cell therapy | |
| AU2016232146B2 (en) | Optimized liver-specific expression systems for FVIII and FIX | |
| CN108753824B (zh) | 用于治疗视网膜营养不良的病毒载体 | |
| KR102880569B1 (ko) | B형 간염 바이러스를 중화하는 항체 및 이의 용도 | |
| KR20120086297A (ko) | 골 상실-관련된 질환의 치료에서 siglec 15 항체 | |
| TW200940563A (en) | Improved mammalian expression vectors and uses thereof | |
| KR101682040B1 (ko) | Msrv 관련 질환에서 특이적 리간드의 치료적 용도 | |
| CN115768890A (zh) | 通过分子和物理启动对t细胞免疫疗法的热控制 | |
| CN116970083B (zh) | 结合Trop2的抗体或Trop2抗原结合片段以及抗Trop2CAR和应用 | |
| CN108472364B (zh) | 抗cd43抗体及其在癌症治疗中的应用 | |
| KR102070176B1 (ko) | 신경회로 연결망을 검출할 수 있는 이중표지 바이러스 벡터 및 이의 용도 | |
| CN112203697A (zh) | 编码氨基己糖苷酶alpha和beta亚基的双顺反子AAV载体及其用途 | |
| CN115335396A (zh) | 靶向cd33的嵌合抗原受体 | |
| CN112138150A (zh) | 基于黑猩猩腺病毒载体的治疗性hpv疫苗、其制备方法及应用 | |
| CN107988259B (zh) | SmartBac杆状病毒表达系统及其应用 | |
| RU2822193C2 (ru) | Мезотелиновые car-рецепторы и их применение | |
| CN114945673A (zh) | 无限增殖化成肌细胞系及其用途 | |
| HK40056051B (zh) | 中和乙型肝炎病毒的抗体和其用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20150911 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |