KR20150002653A - 5α-안드로스탄-3β,5,6β-트리올의 결정형 및 그 제조 방법 - Google Patents
5α-안드로스탄-3β,5,6β-트리올의 결정형 및 그 제조 방법 Download PDFInfo
- Publication number
- KR20150002653A KR20150002653A KR1020147028362A KR20147028362A KR20150002653A KR 20150002653 A KR20150002653 A KR 20150002653A KR 1020147028362 A KR1020147028362 A KR 1020147028362A KR 20147028362 A KR20147028362 A KR 20147028362A KR 20150002653 A KR20150002653 A KR 20150002653A
- Authority
- KR
- South Korea
- Prior art keywords
- solvent
- dilution
- triol
- crystal
- ethanol
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000013078 crystal Substances 0.000 title claims abstract description 131
- 238000000034 method Methods 0.000 title claims description 10
- 150000001875 compounds Chemical class 0.000 title description 2
- 238000004519 manufacturing process Methods 0.000 claims abstract description 6
- IFRIPYPBJCUNAG-OTMXHXQLSA-N (3s,5r,6r,8s,9s,10r,13s,14s)-10,13-dimethyl-1,2,3,4,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthrene-3,5,6-triol Chemical compound C([C@]1(O)[C@H](O)C2)[C@@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CCC[C@@]2(C)CC1 IFRIPYPBJCUNAG-OTMXHXQLSA-N 0.000 claims description 73
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 60
- 239000002904 solvent Substances 0.000 claims description 52
- 238000010790 dilution Methods 0.000 claims description 45
- 239000012895 dilution Substances 0.000 claims description 45
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 44
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 claims description 39
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 claims description 34
- 239000002244 precipitate Substances 0.000 claims description 22
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 claims description 17
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 12
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 12
- 238000004090 dissolution Methods 0.000 claims description 12
- 230000007704 transition Effects 0.000 claims description 12
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 claims description 8
- 125000004836 hexamethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[*:1] 0.000 claims description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 8
- 239000003208 petroleum Substances 0.000 claims description 6
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 claims description 4
- 238000001816 cooling Methods 0.000 claims description 3
- 238000010438 heat treatment Methods 0.000 claims description 2
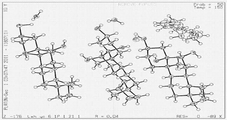
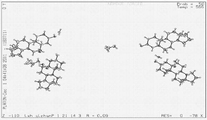
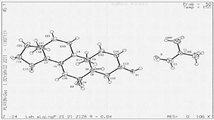
- 238000000634 powder X-ray diffraction Methods 0.000 abstract description 22
- 238000002844 melting Methods 0.000 abstract description 2
- 230000008018 melting Effects 0.000 abstract description 2
- 238000010586 diagram Methods 0.000 description 32
- 238000000113 differential scanning calorimetry Methods 0.000 description 20
- 238000002360 preparation method Methods 0.000 description 15
- 239000003814 drug Substances 0.000 description 6
- 229940079593 drug Drugs 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 238000004455 differential thermal analysis Methods 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 238000001514 detection method Methods 0.000 description 3
- 238000004467 single crystal X-ray diffraction Methods 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 2
- 239000008186 active pharmaceutical agent Substances 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- QNRATNLHPGXHMA-XZHTYLCXSA-N (r)-(6-ethoxyquinolin-4-yl)-[(2s,4s,5r)-5-ethyl-1-azabicyclo[2.2.2]octan-2-yl]methanol;hydrochloride Chemical compound Cl.C([C@H]([C@H](C1)CC)C2)CN1[C@@H]2[C@H](O)C1=CC=NC2=CC=C(OCC)C=C21 QNRATNLHPGXHMA-XZHTYLCXSA-N 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000012159 carrier gas Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 230000000324 neuroprotective effect Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07J—STEROIDS
- C07J1/00—Normal steroids containing carbon, hydrogen, halogen or oxygen, not substituted in position 17 beta by a carbon atom, e.g. estrane, androstane
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07J—STEROIDS
- C07J1/00—Normal steroids containing carbon, hydrogen, halogen or oxygen, not substituted in position 17 beta by a carbon atom, e.g. estrane, androstane
- C07J1/0003—Androstane derivatives
- C07J1/0007—Androstane derivatives not substituted in position 17
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/13—Crystalline forms, e.g. polymorphs
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Steroid Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201210060611.6 | 2012-03-08 | ||
| CN201210060611.6A CN102617685B (zh) | 2012-03-08 | 2012-03-08 | 雄甾-3β,5α,6β-三醇的晶型化合物及其制备方法 |
| PCT/CN2012/073854 WO2013131305A1 (zh) | 2012-03-08 | 2012-04-11 | 雄甾-3β,5α,6β-三醇的晶型化合物及其制备方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150002653A true KR20150002653A (ko) | 2015-01-07 |
Family
ID=46557937
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020147028362A Ceased KR20150002653A (ko) | 2012-03-08 | 2012-04-11 | 5α-안드로스탄-3β,5,6β-트리올의 결정형 및 그 제조 방법 |
Country Status (13)
| Country | Link |
|---|---|
| US (3) | US9688716B2 (enExample) |
| EP (2) | EP3112374A1 (enExample) |
| JP (1) | JP2015511587A (enExample) |
| KR (1) | KR20150002653A (enExample) |
| CN (4) | CN103626817B (enExample) |
| AU (1) | AU2012372640B2 (enExample) |
| CA (2) | CA2866398C (enExample) |
| IL (1) | IL234492A (enExample) |
| IN (1) | IN2014DN08198A (enExample) |
| RU (1) | RU2608894C2 (enExample) |
| SG (1) | SG11201405376TA (enExample) |
| TW (1) | TW201336861A (enExample) |
| WO (1) | WO2013131305A1 (enExample) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9815258B2 (en) | 2012-05-07 | 2017-11-14 | The Procter & Gamble Company | Flexible containers |
| CN109985048B (zh) * | 2017-12-29 | 2021-06-29 | 广州市赛普特医药科技股份有限公司 | 2β,3α,5α-三羟基雄甾-6-酮用于炎症反应的治疗 |
| US11532903B2 (en) * | 2021-04-01 | 2022-12-20 | Ideal Industries, Inc. | Universal electrical connector |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2191576A (en) * | 1936-11-21 | 1940-02-27 | Soc Of Chemical Ind | 3,5,6-trihydroxy androstane and pregnane compounds |
| US2191578A (en) | 1939-07-24 | 1940-02-27 | Crosley Corp | Ice-cream tray |
| ATE15206T1 (de) * | 1981-09-21 | 1985-09-15 | Schering Ag | 3-beta,7-beta,15-alpha-trihydroxy-5-androsten-1 - one, ihre 3,15-trimethylessigsaeure-ester und ihre herstellung. |
| US6046185A (en) * | 1996-07-11 | 2000-04-04 | Inflazyme Pharmaceuticals Ltd. | 6,7-oxygenated steroids and uses related thereto |
| CN101884638B (zh) * | 2010-07-09 | 2011-11-09 | 中山大学 | 5α-雄甾(烷)-3β,5,6β-三醇在制备神经元保护药物中的应用 |
| CN101961311B (zh) * | 2010-09-21 | 2012-11-21 | 中山大学 | 一种5α-雄甾(烷)-3β,5,6β-三醇注射剂及其制备方法 |
| CN102180928B (zh) * | 2011-03-15 | 2013-01-16 | 广州市赛普特医药科技有限公司 | 3β,5α,6β-三羟基甾体化合物及其合成方法和应用 |
-
2012
- 2012-03-08 CN CN201310392426.1A patent/CN103626817B/zh active Active
- 2012-03-08 CN CN201310393026.2A patent/CN103626818B/zh active Active
- 2012-03-08 CN CN201310393373.5A patent/CN103626819B/zh active Active
- 2012-03-08 CN CN201210060611.6A patent/CN102617685B/zh active Active
- 2012-04-11 JP JP2014560211A patent/JP2015511587A/ja active Pending
- 2012-04-11 KR KR1020147028362A patent/KR20150002653A/ko not_active Ceased
- 2012-04-11 IN IN8198DEN2014 patent/IN2014DN08198A/en unknown
- 2012-04-11 EP EP16184231.5A patent/EP3112374A1/en not_active Ceased
- 2012-04-11 CA CA2866398A patent/CA2866398C/en active Active
- 2012-04-11 SG SG11201405376TA patent/SG11201405376TA/en unknown
- 2012-04-11 WO PCT/CN2012/073854 patent/WO2013131305A1/zh not_active Ceased
- 2012-04-11 CA CA2909998A patent/CA2909998A1/en not_active Abandoned
- 2012-04-11 AU AU2012372640A patent/AU2012372640B2/en active Active
- 2012-04-11 EP EP12870566.2A patent/EP2889303A4/en not_active Withdrawn
- 2012-04-11 US US14/383,405 patent/US9688716B2/en active Active
- 2012-04-11 RU RU2014136891A patent/RU2608894C2/ru active
-
2013
- 2013-03-07 TW TW102108093A patent/TW201336861A/zh unknown
-
2014
- 2014-09-07 IL IL234492A patent/IL234492A/en active IP Right Grant
-
2017
- 2017-05-08 US US15/589,228 patent/US9944672B2/en active Active
- 2017-05-08 US US15/589,268 patent/US9944673B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN103626819A (zh) | 2014-03-12 |
| US20170305957A1 (en) | 2017-10-26 |
| US9688716B2 (en) | 2017-06-27 |
| IN2014DN08198A (enExample) | 2015-05-01 |
| CA2866398A1 (en) | 2013-09-12 |
| CN102617685A (zh) | 2012-08-01 |
| IL234492A (en) | 2017-02-28 |
| CN103626818A (zh) | 2014-03-12 |
| CN103626817A (zh) | 2014-03-12 |
| CN102617685B (zh) | 2014-01-22 |
| US20170305958A1 (en) | 2017-10-26 |
| CA2909998A1 (en) | 2013-09-12 |
| CN103626818B (zh) | 2016-03-30 |
| RU2014136891A (ru) | 2016-05-10 |
| WO2013131305A1 (zh) | 2013-09-12 |
| US9944673B2 (en) | 2018-04-17 |
| EP3112374A1 (en) | 2017-01-04 |
| CA2866398C (en) | 2016-06-28 |
| SG11201405376TA (en) | 2014-11-27 |
| EP2889303A1 (en) | 2015-07-01 |
| US20150045566A1 (en) | 2015-02-12 |
| US9944672B2 (en) | 2018-04-17 |
| TW201336861A (zh) | 2013-09-16 |
| JP2015511587A (ja) | 2015-04-20 |
| AU2012372640A1 (en) | 2014-09-25 |
| RU2608894C2 (ru) | 2017-01-26 |
| EP2889303A4 (en) | 2016-02-17 |
| AU2012372640B2 (en) | 2015-07-09 |
| CN103626817B (zh) | 2016-01-20 |
| CN103626819B (zh) | 2016-01-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| ES2911186T3 (es) | Formas cristalinas de aminolípidos | |
| KR102162208B1 (ko) | 7-((3s,4s)-3-[(사이클로프로필아미노)메틸]-4-플루오로피롤리딘-1-일)-6-플루오로-1-(2-플루오로에틸)-8-메톡시-4-옥소-1,4-다이하이드로퀴놀린-3-카복실산 결정 | |
| US20230295121A1 (en) | Solid forms of pralsetinib | |
| KR20150002653A (ko) | 5α-안드로스탄-3β,5,6β-트리올의 결정형 및 그 제조 방법 | |
| Du et al. | Investigation of the solid forms of deferasirox: solvate, co-crystal, and amorphous form | |
| WO2015196926A1 (en) | Process for the preparation of polymorphs of imidacloprid | |
| CN101318964B (zh) | 盐酸伊立替康新晶型及其制备方法 | |
| EP3372602B1 (en) | Pyrroloquinoline quinone b crystal form and preparation method therefor | |
| CA3087474A1 (en) | Crystalline forms of acalabrutinib, processes for preparation and use thereof | |
| AU2018355472A1 (en) | Crystalline form of alkynyl pyridine prolyl hydroxylase inhibitor and method for preparing same | |
| Harris et al. | Structure determination of organic molecular solids from powder x-ray diffraction data: current opportunities and state of the art | |
| KR20170060035A (ko) | 나트륨 글루코스 공동운반체 2 억제제의 l- 프롤린 화합물, 및 l- 프롤린 화합물의 모노하이드레이트 및 결정 | |
| RU2792620C2 (ru) | Кристаллическая форма ингибитора parp-1 и способ ее получения | |
| CN102633775B (zh) | 一种甲磺酸伊马替尼α晶型的制备方法 | |
| Suenaga et al. | Thermal transition behavior and crystal structure of octakis (phenylsulfanyl) naphthalene | |
| Wang et al. | Seven New Irbesartan Salts: Significantly Improved Dissolution, Excellent Hygrothermal Stability, and Characteristic Supramolecular Synthons. | |
| KR20250056611A (ko) | Cftr 조절제의 결정형 및 이의 제조방법 | |
| KR101724301B1 (ko) | 바레니클린 살리실산염의 i형 결정 및 그 제조방법 | |
| JP2016507569A (ja) | ラミブジン結晶塩 | |
| WO2024255833A1 (zh) | 吡啶氮氧化合物与富马酸的共晶体及其组合物、用途和制备方法 | |
| Renou et al. | Synthesis and X-ray structural studies of the dextro-rotatory enantiomer of methyl α-5 (4, 5, 6, 7-tetrahydro (3, 2-c) thieno pyridyl)(2-chlorophenyl)-acetate isopropylsulfate | |
| CN103534248A (zh) | 制备无水阿立哌唑晶型ii的方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A201 | Request for examination | ||
| PA0105 | International application |
Patent event date: 20141008 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20151120 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20160524 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20151120 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |