KR20120052939A - 화학적으로 조절된 산화환원 상태를 사용한 단백질의 재폴딩 - Google Patents
화학적으로 조절된 산화환원 상태를 사용한 단백질의 재폴딩 Download PDFInfo
- Publication number
- KR20120052939A KR20120052939A KR1020127001716A KR20127001716A KR20120052939A KR 20120052939 A KR20120052939 A KR 20120052939A KR 1020127001716 A KR1020127001716 A KR 1020127001716A KR 20127001716 A KR20127001716 A KR 20127001716A KR 20120052939 A KR20120052939 A KR 20120052939A
- Authority
- KR
- South Korea
- Prior art keywords
- protein
- thiol
- refolding
- pair
- buffer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 102000004169 proteins and genes Human genes 0.000 title claims abstract description 209
- 108090000623 proteins and genes Proteins 0.000 title claims abstract description 209
- 238000000034 method Methods 0.000 claims abstract description 80
- 239000006176 redox buffer Substances 0.000 claims abstract description 11
- 235000018102 proteins Nutrition 0.000 claims description 204
- 239000000872 buffer Substances 0.000 claims description 91
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims description 45
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 claims description 34
- 230000014509 gene expression Effects 0.000 claims description 32
- 210000003000 inclusion body Anatomy 0.000 claims description 29
- 239000000203 mixture Substances 0.000 claims description 29
- 235000018417 cysteine Nutrition 0.000 claims description 22
- 229960002433 cysteine Drugs 0.000 claims description 20
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 claims description 20
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 claims description 19
- 239000004202 carbamide Substances 0.000 claims description 17
- OOTFVKOQINZBBF-UHFFFAOYSA-N cystamine Chemical compound CCSSCCN OOTFVKOQINZBBF-UHFFFAOYSA-N 0.000 claims description 17
- 229940099500 cystamine Drugs 0.000 claims description 17
- 238000011534 incubation Methods 0.000 claims description 16
- 238000004220 aggregation Methods 0.000 claims description 14
- 230000002776 aggregation Effects 0.000 claims description 14
- 239000003398 denaturant Substances 0.000 claims description 14
- 239000003112 inhibitor Substances 0.000 claims description 14
- 239000011159 matrix material Substances 0.000 claims description 13
- 239000004475 Arginine Substances 0.000 claims description 12
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 claims description 12
- 229940124272 protein stabilizer Drugs 0.000 claims description 12
- 238000000926 separation method Methods 0.000 claims description 11
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 10
- 229930006000 Sucrose Natural products 0.000 claims description 10
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 claims description 10
- 239000005720 sucrose Substances 0.000 claims description 10
- ZRALSGWEFCBTJO-UHFFFAOYSA-N Guanidine Chemical class NC(N)=N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 claims description 9
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 claims description 9
- 239000002202 Polyethylene glycol Substances 0.000 claims description 9
- 239000007983 Tris buffer Substances 0.000 claims description 9
- 229920001223 polyethylene glycol Polymers 0.000 claims description 9
- 108010024636 Glutathione Proteins 0.000 claims description 8
- 230000001580 bacterial effect Effects 0.000 claims description 8
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 claims description 7
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 claims description 7
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 claims description 7
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 claims description 7
- 239000000600 sorbitol Substances 0.000 claims description 7
- 108010053070 Glutathione Disulfide Proteins 0.000 claims description 6
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 claims description 6
- YPZRWBKMTBYPTK-BJDJZHNGSA-N glutathione disulfide Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@H](C(=O)NCC(O)=O)CSSC[C@@H](C(=O)NCC(O)=O)NC(=O)CC[C@H](N)C(O)=O YPZRWBKMTBYPTK-BJDJZHNGSA-N 0.000 claims description 6
- YPZRWBKMTBYPTK-UHFFFAOYSA-N oxidized gamma-L-glutamyl-L-cysteinylglycine Natural products OC(=O)C(N)CCC(=O)NC(C(=O)NCC(O)=O)CSSCC(C(=O)NCC(O)=O)NC(=O)CCC(N)C(O)=O YPZRWBKMTBYPTK-UHFFFAOYSA-N 0.000 claims description 6
- OTYBMLCTZGSZBG-UHFFFAOYSA-L potassium sulfate Chemical compound [K+].[K+].[O-]S([O-])(=O)=O OTYBMLCTZGSZBG-UHFFFAOYSA-L 0.000 claims description 6
- 229910052939 potassium sulfate Inorganic materials 0.000 claims description 6
- 235000011151 potassium sulphates Nutrition 0.000 claims description 6
- 229910052938 sodium sulfate Inorganic materials 0.000 claims description 6
- 235000011152 sodium sulphate Nutrition 0.000 claims description 6
- 150000005846 sugar alcohols Polymers 0.000 claims description 6
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 5
- LEVWYRKDKASIDU-IMJSIDKUSA-N L-cystine Chemical compound [O-]C(=O)[C@@H]([NH3+])CSSC[C@H]([NH3+])C([O-])=O LEVWYRKDKASIDU-IMJSIDKUSA-N 0.000 claims description 5
- MGJKQDOBUOMPEZ-UHFFFAOYSA-N N,N'-dimethylurea Chemical compound CNC(=O)NC MGJKQDOBUOMPEZ-UHFFFAOYSA-N 0.000 claims description 5
- 240000004808 Saccharomyces cerevisiae Species 0.000 claims description 5
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 claims description 5
- UFULAYFCSOUIOV-UHFFFAOYSA-N cysteamine Chemical compound NCCS UFULAYFCSOUIOV-UHFFFAOYSA-N 0.000 claims description 5
- 229960003067 cystine Drugs 0.000 claims description 5
- 239000008103 glucose Substances 0.000 claims description 5
- 239000002563 ionic surfactant Substances 0.000 claims description 5
- 229960003151 mercaptamine Drugs 0.000 claims description 5
- 239000002736 nonionic surfactant Substances 0.000 claims description 5
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 claims description 5
- RYECOJGRJDOGPP-UHFFFAOYSA-N Ethylurea Chemical compound CCNC(N)=O RYECOJGRJDOGPP-UHFFFAOYSA-N 0.000 claims description 4
- XGEGHDBEHXKFPX-UHFFFAOYSA-N N-methylthiourea Natural products CNC(N)=O XGEGHDBEHXKFPX-UHFFFAOYSA-N 0.000 claims description 4
- XGEGHDBEHXKFPX-NJFSPNSNSA-N methylurea Chemical compound [14CH3]NC(N)=O XGEGHDBEHXKFPX-NJFSPNSNSA-N 0.000 claims description 4
- 239000011347 resin Substances 0.000 claims description 3
- 229920005989 resin Polymers 0.000 claims description 3
- 238000005342 ion exchange Methods 0.000 claims description 2
- 238000001914 filtration Methods 0.000 claims 3
- 239000012562 protein A resin Substances 0.000 claims 1
- 210000004962 mammalian cell Anatomy 0.000 abstract description 8
- 125000003396 thiol group Chemical class [H]S* 0.000 abstract 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 32
- 239000001301 oxygen Substances 0.000 description 32
- 229910052760 oxygen Inorganic materials 0.000 description 32
- 241000894007 species Species 0.000 description 28
- 238000006243 chemical reaction Methods 0.000 description 27
- 150000003573 thiols Chemical class 0.000 description 27
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 26
- 239000000243 solution Substances 0.000 description 25
- 210000004027 cell Anatomy 0.000 description 20
- 230000008569 process Effects 0.000 description 18
- 239000000047 product Substances 0.000 description 17
- 239000000126 substance Substances 0.000 description 15
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 14
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 14
- 238000009826 distribution Methods 0.000 description 14
- 230000000694 effects Effects 0.000 description 13
- 229960005150 glycerol Drugs 0.000 description 13
- 229910052757 nitrogen Inorganic materials 0.000 description 13
- 229960003121 arginine Drugs 0.000 description 10
- 239000003638 chemical reducing agent Substances 0.000 description 9
- 108090000765 processed proteins & peptides Proteins 0.000 description 9
- 230000015572 biosynthetic process Effects 0.000 description 8
- 238000005063 solubilization Methods 0.000 description 8
- 230000007928 solubilization Effects 0.000 description 8
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 7
- 125000000539 amino acid group Chemical group 0.000 description 7
- 150000001875 compounds Chemical class 0.000 description 7
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 7
- 238000005457 optimization Methods 0.000 description 7
- 230000003381 solubilizing effect Effects 0.000 description 7
- 238000001042 affinity chromatography Methods 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 239000003599 detergent Substances 0.000 description 6
- 239000006166 lysate Substances 0.000 description 6
- 238000004007 reversed phase HPLC Methods 0.000 description 6
- 241000894006 Bacteria Species 0.000 description 5
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 5
- 238000005273 aeration Methods 0.000 description 5
- 229940024606 amino acid Drugs 0.000 description 5
- 235000001014 amino acid Nutrition 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 229960003589 arginine hydrochloride Drugs 0.000 description 5
- 239000013592 cell lysate Substances 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 230000030788 protein refolding Effects 0.000 description 5
- 150000003839 salts Chemical class 0.000 description 5
- 230000035945 sensitivity Effects 0.000 description 5
- 230000014616 translation Effects 0.000 description 5
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 4
- 241000588724 Escherichia coli Species 0.000 description 4
- 238000004925 denaturation Methods 0.000 description 4
- 230000036425 denaturation Effects 0.000 description 4
- 238000010790 dilution Methods 0.000 description 4
- 239000012895 dilution Substances 0.000 description 4
- 239000011261 inert gas Substances 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 238000012546 transfer Methods 0.000 description 4
- 229940045136 urea Drugs 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- 108091006020 Fc-tagged proteins Proteins 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- CHJJGSNFBQVOTG-UHFFFAOYSA-N N-methyl-guanidine Natural products CNC(N)=N CHJJGSNFBQVOTG-UHFFFAOYSA-N 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 150000001413 amino acids Chemical class 0.000 description 3
- SWSQBOPZIKWTGO-UHFFFAOYSA-N dimethylaminoamidine Natural products CN(C)C(N)=N SWSQBOPZIKWTGO-UHFFFAOYSA-N 0.000 description 3
- 238000010828 elution Methods 0.000 description 3
- 230000007613 environmental effect Effects 0.000 description 3
- 238000000855 fermentation Methods 0.000 description 3
- 230000004151 fermentation Effects 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 230000003278 mimic effect Effects 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 239000000376 reactant Substances 0.000 description 3
- 238000003998 size exclusion chromatography high performance liquid chromatography Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 2
- 229910052786 argon Inorganic materials 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 230000003196 chaotropic effect Effects 0.000 description 2
- 150000001945 cysteines Chemical class 0.000 description 2
- 238000006471 dimerization reaction Methods 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 239000007789 gas Substances 0.000 description 2
- 238000001502 gel electrophoresis Methods 0.000 description 2
- 229960003180 glutathione Drugs 0.000 description 2
- -1 glycine; Polyols Chemical class 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 239000000543 intermediate Substances 0.000 description 2
- 150000002500 ions Chemical class 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 230000002934 lysing effect Effects 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 239000007800 oxidant agent Substances 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 210000001236 prokaryotic cell Anatomy 0.000 description 2
- 238000001742 protein purification Methods 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 239000000523 sample Substances 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 239000002002 slurry Substances 0.000 description 2
- 239000011537 solubilization buffer Substances 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- UMCMPZBLKLEWAF-BCTGSCMUSA-N 3-[(3-cholamidopropyl)dimethylammonio]propane-1-sulfonate Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCC[N+](C)(C)CCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 UMCMPZBLKLEWAF-BCTGSCMUSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- 229920001450 Alpha-Cyclodextrin Polymers 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 101001065501 Escherichia phage MS2 Lysis protein Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 230000004308 accommodation Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000003570 air Substances 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- HFHDHCJBZVLPGP-RWMJIURBSA-N alpha-cyclodextrin Chemical compound OC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](CO)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)CO)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1CO HFHDHCJBZVLPGP-RWMJIURBSA-N 0.000 description 1
- 229940043377 alpha-cyclodextrin Drugs 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 238000011021 bench scale process Methods 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 238000009295 crossflow filtration Methods 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 238000011026 diafiltration Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 125000002228 disulfide group Chemical group 0.000 description 1
- 150000002019 disulfides Chemical class 0.000 description 1
- NLEBIOOXCVAHBD-QKMCSOCLSA-N dodecyl beta-D-maltoside Chemical compound O[C@@H]1[C@@H](O)[C@H](OCCCCCCCCCCCC)O[C@H](CO)[C@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 NLEBIOOXCVAHBD-QKMCSOCLSA-N 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- ZRALSGWEFCBTJO-UHFFFAOYSA-O guanidinium Chemical compound NC(N)=[NH2+] ZRALSGWEFCBTJO-UHFFFAOYSA-O 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000005462 in vivo assay Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 238000009776 industrial production Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 230000009465 prokaryotic expression Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000012846 protein folding Effects 0.000 description 1
- 230000006920 protein precipitation Effects 0.000 description 1
- 239000012521 purified sample Substances 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000013341 scale-up Methods 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 108020005087 unfolded proteins Proteins 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/107—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides
- C07K1/113—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides without change of the primary structure
- C07K1/1136—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides without change of the primary structure by reversible modification of the secondary, tertiary or quarternary structure, e.g. using denaturating or stabilising agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/107—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides
- C07K1/113—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides without change of the primary structure
- C07K1/1133—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length by chemical modification of precursor peptides without change of the primary structure by redox-reactions involving cystein/cystin side chains
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Analytical Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Gastroenterology & Hepatology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Immunology (AREA)
- Peptides Or Proteins (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Investigating Or Analysing Biological Materials (AREA)
Abstract
Description
도 2는 고정된 티올-쌍 비율 및 티올-쌍 완충액 강도하에 종 분포에 대한 통기 정도(degree of aeration)의 효과를 도시하는 일련의 플롯이다.
도 3은 6g/ℓ에서 수행되고 1ℓ 및 2000ℓ에서 수행된 기재된 방법의 양태를 사용하여 최적화된, 화학적으로 조절된, 비-호기성 재폴딩의 분석학적 오버레이(overlay)이다.
Claims (24)
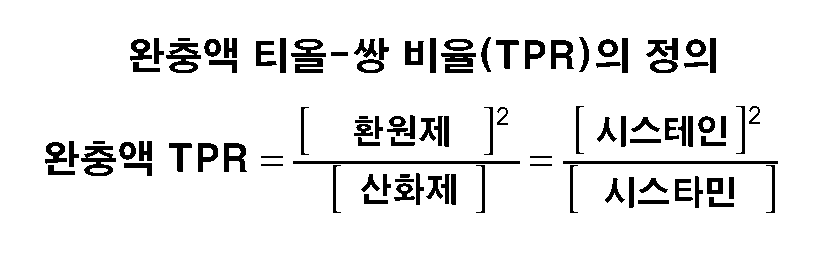
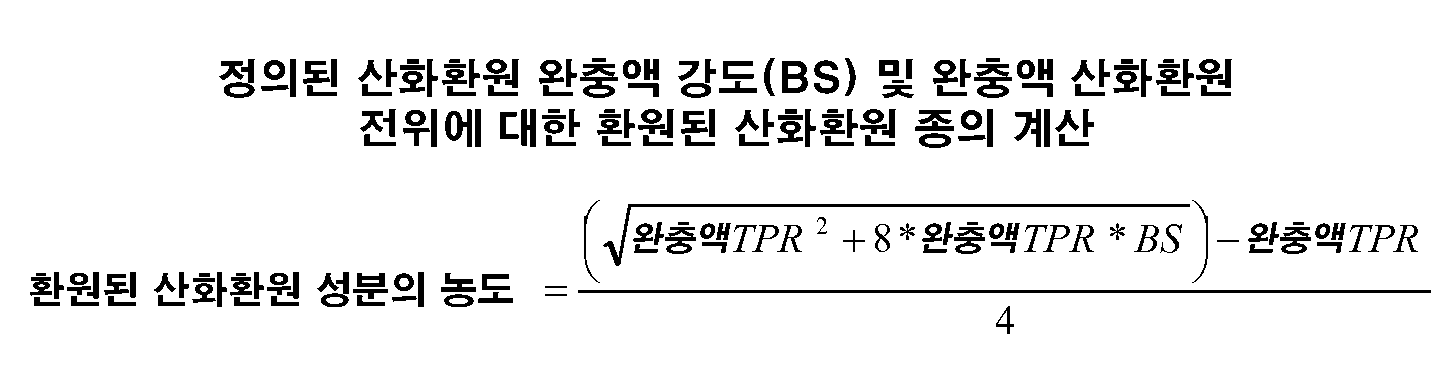
- (a) 비-포유동물 발현 시스템에서 발현되고 2.0g/ℓ 이상의 농도로 용적중에 존재하는 단백질을, 최종 티올-쌍 비율이 0.001 내지 100의 범위이고 산화환원 완충액 강도가 2mM 이상인 산화환원 성분, 및
(i) 변성제;
(ii) 응집 억제제 및
(iii) 단백질 안정화제
중에서 한 가지 이상을 포함하는 재폴딩 완충액과 접촉시켜 재폴딩 혼합물을 형성하는 단계;
(b) 재폴딩 혼합물을 항온처리하는 단계; 및
(c) 재폴딩 혼합물로부터 단백질을 분리하는 단계를 포함하는,
비-포유동물 발현 시스템에서 발현되고 2.0g/ℓ 이상의 농도로 용적중에 존재하는 단백질을 재폴딩시키는 방법. - 제1항에 있어서, 최종 티올-쌍 비율이 0.05 내지 50, 0.1 내지 50, 0.25 내지 50, 0.5 내지 50, 0.75 내지 40, 1.0 내지 50 또는 1.5 내지 50, 2 내지 50, 5 내지 50, 10 내지 50, 15 내지 50, 20 내지 50, 30 내지 50 또는 40 내지 50으로 구성된 군으로부터 선택되는 것인 방법.
- 제1항에 있어서, 티올-쌍 완충액 강도가 2.25 mM, 2.5 mM, 2.75 mM, 3 mM, 5 mM, 7.5 mM, 10 mM 또는 15 mM 이상으로 구성된 군으로부터 선택되는 것인 방법.
- 제1항에 있어서, 단백질이 비-자연 제한된 가용성 형태로 용적중에 존재하는 것인 방법.
- 제4항에 있어서, 비-자연 제한된 가용성 형태가 봉입체(inclusion body)인 방법.
- 제1항에 있어서, 단백질이 가용성 형태로 용적중에 존재하는 것인 방법.
- 제1항에 있어서, 단백질이 재조합체인 방법.
- 제1항에 있어서, 단백질이 내인성 단백질인 방법.
- 제1항에 있어서, 단백질이 항체인 방법.
- 제1항에 있어서, 단백질이 복합 단백질인 방법.
- 제1항에 있어서, 단백질이 다량체 단백질인 방법.
- 제1항에 있어서, 단백질이 Fc 단백질 접합체인 방법.
- 제1항에 있어서, 비-포유동물 발현 시스템이 세균 발현 시스템 및 효모 발현 시스템 중에서 한 가지인 방법.
- 제1항에 있어서, 변성제가 우레아, 구아니디늄 염, 디메틸 우레아, 메틸우레아 및 에틸우레아로 구성된 군으로부터 선택되는 것인 방법.
- 제1항에 있어서, 단백질 안정화제가 아르기닌, 프롤린, 폴리에틸렌 글리콜, 비이온성 계면활성제, 이온성 계면활성제, 다가 알콜, 글리세롤, 슈크로스, 소르비톨, 글루코스, 트리스, 황산나트륨, 황산칼륨 및 삼투용해물로 구성된 군으로부터 선택되는 것인 방법.
- 제1항에 있어서, 응집 억제제가 아르기닌, 프롤린, 폴리에틸렌 글리콜, 비이온성 계면활성제, 이온성 계면활성제, 다가 알콜, 글리세롤, 슈크로스, 소르비톨, 글루코스, 트리스, 황산나트륨, 황산칼륨 및 삼투용해물로 구성된 군으로부터 선택되는 것인 방법.
- 제1항에 있어서, 티올-쌍이 환원된 글루타티온, 산화된 글루타티온, 시스테인, 시스틴, 시스테아민, 시스타민 및 베타-머캅토에탄올로 구성된 군으로부터 선택되는 한 가지 이상의 성분을 포함하는 것인 방법.
- 제1항에 있어서, 항온처리가 비-호기성 조건하에서 수행되는 것인 방법.
- 제1항에 있어서, 분리가 혼합물을 친화성 분리 매트릭스와 접촉시킴을 포함하는 것인 방법.
- 제19항에 있어서, 친화성 분리 매트릭스가 단백질 A 수지인 방법.
- 제19항에 있어서, 친화성 수지가 혼합 방식 분리 매트릭스인 방법.
- 제1항에 있어서, 분리가 혼합물을 이온 교환 분리 매트릭스와 접촉시킴을 포함하는 것인 방법.
- 제1항에 있어서, 분리가 여과 단계를 추가로 포함하는 것인 방법.
- 제23항에 있어서, 여과 단계가 심층 여과를 포함하는 것인 방법.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US21925709P | 2009-06-22 | 2009-06-22 | |
| US61/219,257 | 2009-06-22 | ||
| PCT/US2010/039390 WO2011005488A1 (en) | 2009-06-22 | 2010-06-21 | Refolding proteins using a chemically controlled redox state |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20120052939A true KR20120052939A (ko) | 2012-05-24 |
| KR101741859B1 KR101741859B1 (ko) | 2017-06-15 |
Family
ID=42710673
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127001716A Active KR101741859B1 (ko) | 2009-06-22 | 2010-06-21 | 화학적으로 조절된 산화환원 상태를 사용한 단백질의 재폴딩 |
Country Status (15)
| Country | Link |
|---|---|
| US (5) | US8952138B2 (ko) |
| EP (2) | EP3366692A1 (ko) |
| JP (2) | JP5808323B2 (ko) |
| KR (1) | KR101741859B1 (ko) |
| CN (1) | CN102482321B (ko) |
| AU (1) | AU2010270986B2 (ko) |
| BR (1) | BRPI1011940B8 (ko) |
| CA (1) | CA2765881C (ko) |
| CL (1) | CL2011003278A1 (ko) |
| EA (1) | EA020621B1 (ko) |
| IL (1) | IL216954A (ko) |
| MX (1) | MX2011013898A (ko) |
| SG (1) | SG176963A1 (ko) |
| WO (1) | WO2011005488A1 (ko) |
| ZA (1) | ZA201200512B (ko) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EA020621B1 (ru) | 2009-06-22 | 2014-12-30 | Амген Инк. | Рефолдинг белков с использованием химически контролируемого окислительно-восстановительного состояния |
| JP2012531428A (ja) | 2009-06-25 | 2012-12-10 | アムジエン・インコーポレーテツド | 哺乳類以外の系で発現されるタンパク質の捕獲精製プロセス |
| EP2417982A1 (en) * | 2010-07-30 | 2012-02-15 | Ferring B.V. | Stabilization of gonadotropins |
| US20150376228A1 (en) * | 2013-02-22 | 2015-12-31 | Biogenomics Limited | Process for high efficiency refolding of recombinant proteins |
| WO2017019776A1 (en) * | 2015-07-27 | 2017-02-02 | Purdue Research Foundation | Tandem folding methods to improve protein folding yield |
| WO2017040363A1 (en) | 2015-09-02 | 2017-03-09 | Merck Sharp & Dohme Corp. | A process for obtaining insulin with correctly formed disulfide bonds |
| EP3487867A2 (en) * | 2016-07-22 | 2019-05-29 | Amgen Inc. | Methods of purifying fc-containing proteins |
| CN108276470A (zh) * | 2017-01-06 | 2018-07-13 | 深圳市新产业生物医学工程股份有限公司 | 细胞裂解液、提取胞内蛋白的方法、制备弓形虫抗原的方法和试剂盒 |
| US11406576B2 (en) | 2017-04-10 | 2022-08-09 | Kao Corporation | Method for removing keratotic plugs |
| WO2019183334A1 (en) | 2018-03-21 | 2019-09-26 | Waters Technologies Corporation | Non-antibody high-affinity-based sample preparation, sorbents, devices and methods |
| DK3650037T3 (da) * | 2018-11-07 | 2022-05-02 | Applied Molecular Transport Inc | Indgivelseskonstrukter til transcytose og tilhørende fremgangsmåder |
| HRP20251064T1 (hr) | 2020-12-18 | 2025-11-07 | Richter Gedeon Nyrt. | Metode za pročišćavanje ponovno savijenog fc-peptidnog fuzionog proteina |
| CN115894604B (zh) * | 2022-12-16 | 2024-01-23 | 康日百奥生物科技(苏州)有限公司 | 重组蛋白澄清纯化方法 |
Family Cites Families (82)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4237224A (en) | 1974-11-04 | 1980-12-02 | Board Of Trustees Of The Leland Stanford Jr. University | Process for producing biologically functional molecular chimeras |
| US4468464A (en) | 1974-11-04 | 1984-08-28 | The Board Of Trustees Of The Leland Stanford Junior University | Biologically functional molecular chimeras |
| JPH06102034B2 (ja) | 1983-03-25 | 1994-12-14 | セルテク リミテツド | タンパク質の生産方法 |
| US4468454A (en) | 1983-06-10 | 1984-08-28 | E. I. Du Pont De Nemours And Company | Antifoggant process |
| US4572798A (en) * | 1984-12-06 | 1986-02-25 | Cetus Corporation | Method for promoting disulfide bond formation in recombinant proteins |
| GB8508340D0 (en) | 1985-03-29 | 1985-05-09 | Creighton T E | Production of protein |
| US4810643A (en) | 1985-08-23 | 1989-03-07 | Kirin- Amgen Inc. | Production of pluripotent granulocyte colony-stimulating factor |
| IL86090A (en) | 1987-04-16 | 1993-03-15 | Cetus Oncology Corp | Production of purified, biologically active, bacterially produced recombinant human csf-1 |
| CA1339757C (en) | 1987-04-16 | 1998-03-17 | Robert F. Halenbeck | Production of purified biologically active, bacterially produced recombinant human csf-1 |
| CA1329119C (en) | 1988-03-29 | 1994-05-03 | Milton David Goldenberg | Cytotoxic therapy |
| GB8807673D0 (en) | 1988-03-31 | 1988-05-05 | Lingner & Fischer Gmbh | Novel article |
| EP0347041A3 (en) | 1988-05-13 | 1990-11-22 | Amgen Inc. | Compositions and method for treating or preventing infections in animals |
| GB8927546D0 (en) * | 1989-12-06 | 1990-02-07 | Ciba Geigy | Process for the production of biologically active tgf-beta |
| US5986070A (en) | 1990-04-06 | 1999-11-16 | Amgen Inc. | Production of biologically active NGF proteins |
| EP0547102B1 (en) * | 1990-09-05 | 1998-07-08 | Southern Cross Biotech Pty.Ltd. | Solubilization of proteins in active forms |
| WO1993018136A1 (en) | 1992-03-05 | 1993-09-16 | Cytomed, Inc. | Process for supporting hematopoietic progenitor cells |
| US5663304A (en) | 1993-08-20 | 1997-09-02 | Genentech, Inc. | Refolding of misfolded insulin-like growth factor-I |
| DK0657466T3 (da) | 1993-11-10 | 1997-07-14 | Pfizer | Fremgangsmåde til refoldning af (pro-)chymosin, som omfatter recirkulering af urea |
| US5466377A (en) | 1994-01-19 | 1995-11-14 | Grandics; Peter | Chromatography media and their uses |
| IL113497A (en) | 1994-04-28 | 2001-01-11 | Amgen Inc | Method for controlling metallophosphate precipitation in high cell density fermentations |
| AU2603995A (en) | 1994-05-25 | 1995-12-18 | University Of Nebraska Board Of Regents | Biologically active glycoprotein hormones produced in procaryotic cells |
| CA2223433C (en) | 1995-06-07 | 2003-11-18 | Amgen Inc. | Ob protein compositions and methods |
| WO1997027219A1 (en) | 1996-01-23 | 1997-07-31 | Ortho Pharmaceutical Corporation | Methods for purification and use of erythropoietin binding protein |
| US5935824A (en) | 1996-01-31 | 1999-08-10 | Technologene, Inc. | Protein expression system |
| US20020052026A1 (en) | 1997-10-08 | 2002-05-02 | Steven M. Vicik | Methods of refolding proteins |
| US6180391B1 (en) | 1998-01-28 | 2001-01-30 | Amgen Inc. | Highly efficient controlled expression of exogenous genes in e. coli |
| US6653098B1 (en) * | 1998-02-23 | 2003-11-25 | G. D. Searle & Co. | Method of producing mouse and human endostatin |
| WO1999060120A2 (en) | 1998-05-19 | 1999-11-25 | Avidex Limited | Soluble t cell receptor |
| US6660843B1 (en) * | 1998-10-23 | 2003-12-09 | Amgen Inc. | Modified peptides as therapeutic agents |
| JP2003505471A (ja) | 1999-07-21 | 2003-02-12 | アムジエン・インコーポレーテツド | Vgfポリペプチドおよびvgf関連障害の治療方法 |
| US6808902B1 (en) | 1999-11-12 | 2004-10-26 | Amgen Inc. | Process for correction of a disulfide misfold in IL-1Ra Fc fusion molecules |
| JP4873818B2 (ja) | 2000-05-16 | 2012-02-08 | ボルダー バイオテクノロジー, インコーポレイテッド | 遊離システイン残基を含有するタンパク質をリフォールディングする方法 |
| ATE431405T1 (de) | 2000-09-05 | 2009-05-15 | Amgen Inc | Tnf-rezeptor-ähnliche moleküle und deren anwendungen |
| ES2370235T3 (es) | 2001-02-23 | 2011-12-13 | Immunex Corporation | Recuperación eficaz de proteínas replegadas correctamente. |
| US6972327B1 (en) | 2001-05-08 | 2005-12-06 | Immunex Corporation | Regeneration of chromatography material |
| US7138370B2 (en) * | 2001-10-11 | 2006-11-21 | Amgen Inc. | Specific binding agents of human angiopoietin-2 |
| ITMI20012345A1 (it) | 2001-11-08 | 2003-05-08 | Internat Ct For Genetic En Gin | Procedimento per la produzione di interferone alfa di grado terapeutico |
| SI21102A (sl) | 2001-12-19 | 2003-06-30 | LEK, tovarna farmacevtskih in kemi�nih izdelkov, d.d. | Postopek za izolacijo biološko aktivnega granulocitne kolonije stimulirajočega dejavnika |
| WO2004001056A1 (en) | 2002-06-24 | 2003-12-31 | Dr. Reddy's Laboratories Ltd. | Process for preparing g-csf |
| US7067279B1 (en) | 2002-08-23 | 2006-06-27 | Immunex Corporation | Cell culture performance with betaine |
| EA009056B1 (ru) | 2002-12-20 | 2007-10-26 | Амген, Инк. | Связывающие агенты, ингибирующие миостатин |
| US7083948B1 (en) | 2002-12-24 | 2006-08-01 | Immunex Corporation | Polypeptide purification reagents and methods for their use |
| EP1449848A1 (en) | 2003-02-20 | 2004-08-25 | GBF German Research Centre for Biotechnology | Method for the production of cystine-knot proteins |
| GB0304068D0 (en) | 2003-02-22 | 2003-03-26 | Avidex Ltd | Substances |
| US7427659B2 (en) | 2003-10-24 | 2008-09-23 | Amgen Inc. | Process for purifying proteins in a hydrophobic interaction chromatography flow-through fraction |
| AU2004315196B2 (en) | 2004-01-30 | 2008-10-02 | Amgen Inc. | Process for purifying proteins |
| NZ548893A (en) | 2004-02-04 | 2009-09-25 | Biogernerix Ag | Methods of refolding mammalian glycosyltransferases |
| US20050209441A1 (en) | 2004-03-22 | 2005-09-22 | Lile Jackson D | Process for promoting proper folding of human serum albumin using a human serum albumin ligand |
| FR2874318B1 (fr) | 2004-08-19 | 2006-11-24 | Oreal | Utilisation en cosmetique de composes polysaccharidiques amphoteres a chaine(s) polymerique(s) cationique(s) |
| CN101123978B (zh) | 2004-08-19 | 2012-12-12 | 比奥根艾迪克Ma公司 | 神经胚素变体 |
| DE102004041639A1 (de) | 2004-08-27 | 2006-03-02 | Bioceuticals Arzneimittel Ag | Verfahren zur Gewinnung von biologisch aktivem humanen G-CSF aus Inclusion Bodies |
| AU2005289685B2 (en) | 2004-09-24 | 2009-07-16 | Amgen Inc. | Modified Fc molecules |
| US7435804B2 (en) | 2004-10-19 | 2008-10-14 | Phage Biotechnology, Inc. | Method for obtaining single chain antibodies to human interferon α2b |
| CA2584211C (en) | 2004-10-22 | 2014-07-08 | Amgen Inc. | Methods for refolding of recombinant antibodies |
| AU2005314438B2 (en) | 2004-12-10 | 2012-03-29 | Zymogenetics, Inc. | FGF18 production in prokaryotic hosts |
| JP2008528039A (ja) | 2005-01-28 | 2008-07-31 | ザイモジェネティクス,インコーポレイティド | Il−31の均質調製物 |
| WO2006097944A2 (en) | 2005-03-17 | 2006-09-21 | Zenotech Laboratories Limited | Process for the purification of recombinant granulocyte-colony stimulating factor |
| US7344962B2 (en) | 2005-06-21 | 2008-03-18 | International Business Machines Corporation | Method of manufacturing dual orientation wafers |
| DE102005033250A1 (de) | 2005-07-15 | 2007-01-18 | Bioceuticals Arzneimittel Ag | Verfahren zur Reinigung von G-CSF |
| US8008453B2 (en) | 2005-08-12 | 2011-08-30 | Amgen Inc. | Modified Fc molecules |
| WO2007059494A2 (en) | 2005-11-14 | 2007-05-24 | Amgen, Inc. | Thermally insulated apparatus for liquid chromatographic analysis |
| EP1963367A4 (en) | 2005-12-06 | 2009-05-13 | Amgen Inc | POLISHING STEPS IN MULTI-STAGE PROTEIN PURIFICATION TREATMENTS |
| EP1973942B1 (en) | 2005-12-22 | 2011-02-09 | Genentech, Inc. | Recombinant production of heparin binding proteins |
| US7612273B2 (en) * | 2006-03-20 | 2009-11-03 | Roland Corporation | Electronic percussion instrument |
| EP1845103B1 (en) * | 2006-04-10 | 2015-02-25 | Boehringer Ingelheim RCV GmbH & Co KG | Method for refolding a protein |
| AU2007260787A1 (en) | 2006-06-13 | 2007-12-21 | Zymogenetics, Inc | IL-17 and IL-23 antagonists and methods of using the same |
| CN101506222B (zh) | 2006-07-14 | 2013-10-02 | 健泰科生物技术公司 | 重组蛋白的重折叠 |
| WO2008011132A2 (en) | 2006-07-21 | 2008-01-24 | Amgen, Inc. | Rupture valve |
| US8629250B2 (en) | 2007-02-02 | 2014-01-14 | Amgen Inc. | Hepcidin, hepcidin antagonists and methods of use |
| WO2008097829A2 (en) | 2007-02-02 | 2008-08-14 | Neose Technologies, Inc. | Large scale production of eukaryotic n-acetylglucosaminyltransferase i in bacteria |
| WO2008096370A2 (en) | 2007-02-05 | 2008-08-14 | Natco Pharma Limited | An efficient and novel purification method of recombinant hg-csf |
| WO2008100578A2 (en) | 2007-02-14 | 2008-08-21 | Amgen Inc. | Method of isolating antibodies by precipitation |
| BRPI0815416A2 (pt) | 2007-08-15 | 2014-10-21 | Amunix Inc | Composições e métodos para modificar propriedades de polipeptídeos biologicamente ativos |
| JP2011514161A (ja) | 2008-02-29 | 2011-05-06 | ラモット・アット・テル・アビブ・ユニバーシテイ・リミテッド | 免疫グロブリン組成物およびその製造方法 |
| KR102095257B1 (ko) | 2008-06-25 | 2020-04-01 | 노바르티스 아게 | Vegf를 억제하는 안정하고 가용성인 항체 |
| EA020621B1 (ru) * | 2009-06-22 | 2014-12-30 | Амген Инк. | Рефолдинг белков с использованием химически контролируемого окислительно-восстановительного состояния |
| JP2012531428A (ja) * | 2009-06-25 | 2012-12-10 | アムジエン・インコーポレーテツド | 哺乳類以外の系で発現されるタンパク質の捕獲精製プロセス |
| TWI772897B (zh) | 2011-08-29 | 2022-08-01 | 美商安美基公司 | 用於非破壞性檢測-流體中未溶解粒子之方法及裝置 |
| US8945872B2 (en) | 2013-01-25 | 2015-02-03 | Warsaw Orthopedic, Inc. | Methods of purifying human recombinant growth and differentiation factor-5 (rhGDF-5) protein |
| AU2014228423A1 (en) | 2013-03-15 | 2015-11-05 | Amgen Inc. | Myostatin antagonism in human subjects |
| US9632095B2 (en) | 2014-12-01 | 2017-04-25 | University Of Delaware | Device and method for determining reaction kinetics |
| US9704239B1 (en) | 2016-09-02 | 2017-07-11 | Amgen Inc. | Video trigger synchronization for improved particle detection in a vessel |
-
2010
- 2010-06-21 EA EA201270015A patent/EA020621B1/ru not_active IP Right Cessation
- 2010-06-21 AU AU2010270986A patent/AU2010270986B2/en active Active
- 2010-06-21 EP EP18167168.6A patent/EP3366692A1/en active Pending
- 2010-06-21 EP EP10747092.4A patent/EP2445923B1/en active Active
- 2010-06-21 JP JP2012517634A patent/JP5808323B2/ja active Active
- 2010-06-21 MX MX2011013898A patent/MX2011013898A/es active IP Right Grant
- 2010-06-21 KR KR1020127001716A patent/KR101741859B1/ko active Active
- 2010-06-21 CA CA2765881A patent/CA2765881C/en active Active
- 2010-06-21 US US12/820,087 patent/US8952138B2/en active Active
- 2010-06-21 SG SG2011095411A patent/SG176963A1/en unknown
- 2010-06-21 CN CN201080028756.7A patent/CN102482321B/zh active Active
- 2010-06-21 BR BRPI1011940A patent/BRPI1011940B8/pt active IP Right Grant
- 2010-06-21 WO PCT/US2010/039390 patent/WO2011005488A1/en not_active Ceased
-
2011
- 2011-12-13 IL IL216954A patent/IL216954A/en active IP Right Grant
- 2011-12-22 CL CL2011003278A patent/CL2011003278A1/es unknown
-
2012
- 2012-01-20 ZA ZA2012/00512A patent/ZA201200512B/en unknown
-
2015
- 2015-01-30 US US14/611,037 patent/US20150315232A1/en not_active Abandoned
- 2015-07-07 US US14/793,590 patent/US20150329586A1/en not_active Abandoned
- 2015-09-07 JP JP2015175422A patent/JP6026608B2/ja active Active
-
2017
- 2017-02-01 US US15/422,327 patent/US9856287B2/en active Active
-
2018
- 2018-02-06 US US15/889,559 patent/US12269843B2/en active Active
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12269843B2 (en) | Refolding proteins using a chemically controlled redox state | |
| Qoronfleh et al. | Confronting high-throughput protein refolding using high pressure and solution screens | |
| US11345722B2 (en) | High pH protein refolding methods | |
| US11053278B2 (en) | Tangential flow filtration based protein refolding methods | |
| HK1259915A1 (en) | Refolding proteins using a chemically controlled redox state | |
| HK1170245A (en) | Refolding proteins using a chemically controlled redox state | |
| HK1170245B (en) | Refolding proteins using a chemically controlled redox state |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20120120 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20150612 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20160621 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20170224 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
Comment text: Registration of Establishment Patent event date: 20170524 Patent event code: PR07011E01D |
|
| PR1002 | Payment of registration fee |
Payment date: 20170524 End annual number: 3 Start annual number: 1 |
|
| PG1601 | Publication of registration | ||
| PR1001 | Payment of annual fee |
Payment date: 20200504 Start annual number: 4 End annual number: 4 |
|
| PR1001 | Payment of annual fee |
Payment date: 20210428 Start annual number: 5 End annual number: 5 |
|
| PR1001 | Payment of annual fee |
Payment date: 20220422 Start annual number: 6 End annual number: 6 |
|
| PR1001 | Payment of annual fee |
Payment date: 20240424 Start annual number: 8 End annual number: 8 |