KR20120012823A - 신경보호 및 퇴행성 신경 질환 치료용 클라블라네이트 제제 - Google Patents
신경보호 및 퇴행성 신경 질환 치료용 클라블라네이트 제제 Download PDFInfo
- Publication number
- KR20120012823A KR20120012823A KR1020117028280A KR20117028280A KR20120012823A KR 20120012823 A KR20120012823 A KR 20120012823A KR 1020117028280 A KR1020117028280 A KR 1020117028280A KR 20117028280 A KR20117028280 A KR 20117028280A KR 20120012823 A KR20120012823 A KR 20120012823A
- Authority
- KR
- South Korea
- Prior art keywords
- clavulanate
- formulation
- tablets
- potassium
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 208000012902 Nervous system disease Diseases 0.000 title claims abstract description 28
- 208000025966 Neurological disease Diseases 0.000 title claims abstract description 28
- 230000003412 degenerative effect Effects 0.000 title claims abstract description 27
- 238000002360 preparation method Methods 0.000 title description 25
- 230000000324 neuroprotective effect Effects 0.000 title description 15
- 239000000203 mixture Substances 0.000 claims abstract description 180
- HZZVJAQRINQKSD-PBFISZAISA-N clavulanic acid Chemical compound OC(=O)[C@H]1C(=C/CO)/O[C@@H]2CC(=O)N21 HZZVJAQRINQKSD-PBFISZAISA-N 0.000 claims abstract description 145
- HZZVJAQRINQKSD-UHFFFAOYSA-N Clavulanic acid Natural products OC(=O)C1C(=CCO)OC2CC(=O)N21 HZZVJAQRINQKSD-UHFFFAOYSA-N 0.000 claims abstract description 144
- 229940090805 clavulanate Drugs 0.000 claims abstract description 144
- 238000000034 method Methods 0.000 claims abstract description 58
- 238000013265 extended release Methods 0.000 claims abstract description 45
- 239000012729 immediate-release (IR) formulation Substances 0.000 claims abstract description 32
- 210000002569 neuron Anatomy 0.000 claims abstract description 30
- 230000004112 neuroprotection Effects 0.000 claims abstract description 14
- 208000018737 Parkinson disease Diseases 0.000 claims abstract description 10
- 230000030833 cell death Effects 0.000 claims abstract description 8
- 230000006727 cell loss Effects 0.000 claims abstract description 6
- 208000024827 Alzheimer disease Diseases 0.000 claims abstract description 5
- 201000006417 multiple sclerosis Diseases 0.000 claims abstract description 5
- 239000003826 tablet Substances 0.000 claims description 92
- 238000009472 formulation Methods 0.000 claims description 64
- ABVRVIZBZKUTMK-JSYANWSFSA-M potassium clavulanate Chemical group [K+].[O-]C(=O)[C@H]1C(=C/CO)/O[C@@H]2CC(=O)N21 ABVRVIZBZKUTMK-JSYANWSFSA-M 0.000 claims description 54
- 238000011282 treatment Methods 0.000 claims description 50
- 239000002253 acid Substances 0.000 claims description 40
- -1 troches Substances 0.000 claims description 34
- 239000000945 filler Substances 0.000 claims description 32
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 claims description 30
- 239000000314 lubricant Substances 0.000 claims description 27
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 27
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 25
- 239000000843 powder Substances 0.000 claims description 21
- 150000003839 salts Chemical class 0.000 claims description 21
- 239000000243 solution Substances 0.000 claims description 21
- 229920000168 Microcrystalline cellulose Polymers 0.000 claims description 20
- 239000008108 microcrystalline cellulose Substances 0.000 claims description 20
- 229940016286 microcrystalline cellulose Drugs 0.000 claims description 20
- 235000019813 microcrystalline cellulose Nutrition 0.000 claims description 20
- 235000019359 magnesium stearate Nutrition 0.000 claims description 15
- 239000011159 matrix material Substances 0.000 claims description 15
- 239000000454 talc Substances 0.000 claims description 14
- 229910052623 talc Inorganic materials 0.000 claims description 14
- 239000002775 capsule Substances 0.000 claims description 13
- 238000004108 freeze drying Methods 0.000 claims description 13
- 229920001577 copolymer Chemical group 0.000 claims description 12
- 206010010904 Convulsion Diseases 0.000 claims description 11
- 239000000377 silicon dioxide Substances 0.000 claims description 11
- SERLAGPUMNYUCK-DCUALPFSSA-N 1-O-alpha-D-glucopyranosyl-D-mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O SERLAGPUMNYUCK-DCUALPFSSA-N 0.000 claims description 10
- 230000015556 catabolic process Effects 0.000 claims description 10
- 238000006731 degradation reaction Methods 0.000 claims description 10
- 239000000905 isomalt Substances 0.000 claims description 10
- 235000010439 isomalt Nutrition 0.000 claims description 10
- HPIGCVXMBGOWTF-UHFFFAOYSA-N isomaltol Natural products CC(=O)C=1OC=CC=1O HPIGCVXMBGOWTF-UHFFFAOYSA-N 0.000 claims description 10
- 235000012239 silicon dioxide Nutrition 0.000 claims description 10
- 229920003091 Methocel™ Polymers 0.000 claims description 9
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical group O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 claims description 7
- 229920003134 Eudragit® polymer Polymers 0.000 claims description 7
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 claims description 7
- 239000003795 chemical substances by application Substances 0.000 claims description 7
- JVTIXNMXDLQEJE-UHFFFAOYSA-N 2-decanoyloxypropyl decanoate 2-octanoyloxypropyl octanoate Chemical group C(CCCCCCC)(=O)OCC(C)OC(CCCCCCC)=O.C(=O)(CCCCCCCCC)OCC(C)OC(=O)CCCCCCCCC JVTIXNMXDLQEJE-UHFFFAOYSA-N 0.000 claims description 4
- 206010044565 Tremor Diseases 0.000 claims description 4
- 229960004977 anhydrous lactose Drugs 0.000 claims description 4
- 230000016273 neuron death Effects 0.000 claims description 4
- 239000006187 pill Substances 0.000 claims description 4
- WLAMNBDJUVNPJU-UHFFFAOYSA-N 2-methylbutyric acid Chemical group CCC(C)C(O)=O WLAMNBDJUVNPJU-UHFFFAOYSA-N 0.000 claims description 3
- 239000000725 suspension Substances 0.000 claims description 3
- 208000000044 Amnesia Diseases 0.000 claims description 2
- 208000026139 Memory disease Diseases 0.000 claims description 2
- 230000006984 memory degeneration Effects 0.000 claims description 2
- 208000023060 memory loss Diseases 0.000 claims description 2
- 230000004770 neurodegeneration Effects 0.000 claims description 2
- 239000007935 oral tablet Substances 0.000 claims description 2
- 239000006190 sub-lingual tablet Substances 0.000 claims description 2
- 239000010409 thin film Substances 0.000 claims description 2
- 239000007787 solid Substances 0.000 abstract description 28
- 239000008194 pharmaceutical composition Substances 0.000 abstract description 17
- 239000004480 active ingredient Substances 0.000 abstract description 15
- 239000007909 solid dosage form Substances 0.000 abstract description 7
- 241001465754 Metazoa Species 0.000 description 30
- 239000002552 dosage form Substances 0.000 description 19
- 239000011780 sodium chloride Substances 0.000 description 19
- 239000007884 disintegrant Substances 0.000 description 18
- 108091000117 Tyrosine 3-Monooxygenase Proteins 0.000 description 17
- 102000048218 Tyrosine 3-monooxygenases Human genes 0.000 description 17
- 238000012360 testing method Methods 0.000 description 17
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 17
- 229910052700 potassium Inorganic materials 0.000 description 16
- 239000011591 potassium Substances 0.000 description 16
- 238000004090 dissolution Methods 0.000 description 15
- 230000000694 effects Effects 0.000 description 15
- 238000003860 storage Methods 0.000 description 15
- AAWZDTNXLSGCEK-LNVDRNJUSA-N (3r,5r)-1,3,4,5-tetrahydroxycyclohexane-1-carboxylic acid Chemical compound O[C@@H]1CC(O)(C(O)=O)C[C@@H](O)C1O AAWZDTNXLSGCEK-LNVDRNJUSA-N 0.000 description 14
- 239000011230 binding agent Substances 0.000 description 13
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 13
- 239000003814 drug Substances 0.000 description 13
- 235000012222 talc Nutrition 0.000 description 13
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- ZHAFUINZIZIXFC-UHFFFAOYSA-N [9-(dimethylamino)-10-methylbenzo[a]phenoxazin-5-ylidene]azanium;chloride Chemical compound [Cl-].O1C2=CC(=[NH2+])C3=CC=CC=C3C2=NC2=C1C=C(N(C)C)C(C)=C2 ZHAFUINZIZIXFC-UHFFFAOYSA-N 0.000 description 12
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 11
- 239000003085 diluting agent Substances 0.000 description 10
- 201000010099 disease Diseases 0.000 description 10
- 239000004615 ingredient Substances 0.000 description 10
- 239000006186 oral dosage form Substances 0.000 description 10
- 229920000642 polymer Polymers 0.000 description 10
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 9
- 239000007864 aqueous solution Substances 0.000 description 9
- 239000011324 bead Substances 0.000 description 9
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 9
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 8
- 238000010171 animal model Methods 0.000 description 8
- 238000000576 coating method Methods 0.000 description 8
- 230000006870 function Effects 0.000 description 8
- 238000004128 high performance liquid chromatography Methods 0.000 description 8
- 230000008569 process Effects 0.000 description 8
- 241000699666 Mus <mouse, genus> Species 0.000 description 7
- 229930006000 Sucrose Natural products 0.000 description 7
- 239000003782 beta lactam antibiotic agent Substances 0.000 description 7
- 239000011248 coating agent Substances 0.000 description 7
- 238000000338 in vitro Methods 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 239000005720 sucrose Substances 0.000 description 7
- 229940124597 therapeutic agent Drugs 0.000 description 7
- 239000002132 β-lactam antibiotic Substances 0.000 description 7
- 229940124586 β-lactam antibiotics Drugs 0.000 description 7
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 101000945318 Homo sapiens Calponin-1 Proteins 0.000 description 6
- 101000652736 Homo sapiens Transgelin Proteins 0.000 description 6
- VLSMHEGGTFMBBZ-OOZYFLPDSA-M Kainate Chemical compound CC(=C)[C@H]1C[NH2+][C@H](C([O-])=O)[C@H]1CC([O-])=O VLSMHEGGTFMBBZ-OOZYFLPDSA-M 0.000 description 6
- 102100031013 Transgelin Human genes 0.000 description 6
- 239000008186 active pharmaceutical agent Substances 0.000 description 6
- 210000004556 brain Anatomy 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 230000000971 hippocampal effect Effects 0.000 description 6
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical group CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 6
- JJAHTWIKCUJRDK-UHFFFAOYSA-N succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate Chemical compound C1CC(CN2C(C=CC2=O)=O)CCC1C(=O)ON1C(=O)CCC1=O JJAHTWIKCUJRDK-UHFFFAOYSA-N 0.000 description 6
- 239000003981 vehicle Substances 0.000 description 6
- 235000021355 Stearic acid Nutrition 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 210000003169 central nervous system Anatomy 0.000 description 5
- 239000000975 dye Substances 0.000 description 5
- 239000003623 enhancer Substances 0.000 description 5
- 238000005469 granulation Methods 0.000 description 5
- 230000003179 granulation Effects 0.000 description 5
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 5
- 238000007912 intraperitoneal administration Methods 0.000 description 5
- 238000002156 mixing Methods 0.000 description 5
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 5
- 230000001012 protector Effects 0.000 description 5
- 239000008117 stearic acid Substances 0.000 description 5
- 241000282472 Canis lupus familiaris Species 0.000 description 4
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 4
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 4
- 206010029350 Neurotoxicity Diseases 0.000 description 4
- 241000700159 Rattus Species 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- 206010044221 Toxic encephalopathy Diseases 0.000 description 4
- 229960003022 amoxicillin Drugs 0.000 description 4
- 238000009227 behaviour therapy Methods 0.000 description 4
- 230000003542 behavioural effect Effects 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 239000002702 enteric coating Substances 0.000 description 4
- 238000009505 enteric coating Methods 0.000 description 4
- 238000003304 gavage Methods 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 238000003364 immunohistochemistry Methods 0.000 description 4
- 238000010348 incorporation Methods 0.000 description 4
- 239000011777 magnesium Substances 0.000 description 4
- 230000014759 maintenance of location Effects 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 230000037023 motor activity Effects 0.000 description 4
- 210000000653 nervous system Anatomy 0.000 description 4
- 230000007135 neurotoxicity Effects 0.000 description 4
- 231100000228 neurotoxicity Toxicity 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 210000001048 venom Anatomy 0.000 description 4
- 239000002435 venom Substances 0.000 description 4
- 231100000611 venom Toxicity 0.000 description 4
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- 108090000204 Dipeptidase 1 Proteins 0.000 description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 3
- SJRJJKPEHAURKC-UHFFFAOYSA-N N-Methylmorpholine Chemical compound CN1CCOCC1 SJRJJKPEHAURKC-UHFFFAOYSA-N 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical group C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 150000001298 alcohols Chemical class 0.000 description 3
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 3
- 239000012736 aqueous medium Substances 0.000 description 3
- 230000006399 behavior Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 102000006635 beta-lactamase Human genes 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 229920002678 cellulose Polymers 0.000 description 3
- 239000001913 cellulose Substances 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 230000003111 delayed effect Effects 0.000 description 3
- 239000002274 desiccant Substances 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 238000004043 dyeing Methods 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 239000010408 film Substances 0.000 description 3
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 3
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 3
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 3
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 3
- 238000010253 intravenous injection Methods 0.000 description 3
- 239000008101 lactose Substances 0.000 description 3
- 229960001375 lactose Drugs 0.000 description 3
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 3
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 3
- 230000000704 physical effect Effects 0.000 description 3
- 230000003389 potentiating effect Effects 0.000 description 3
- 230000004224 protection Effects 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 239000000600 sorbitol Substances 0.000 description 3
- 235000010356 sorbitol Nutrition 0.000 description 3
- 238000010186 staining Methods 0.000 description 3
- 239000008107 starch Substances 0.000 description 3
- 235000019698 starch Nutrition 0.000 description 3
- 238000007619 statistical method Methods 0.000 description 3
- 239000007916 tablet composition Substances 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- 238000005550 wet granulation Methods 0.000 description 3
- 206010003591 Ataxia Diseases 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 241000283707 Capra Species 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- 208000012661 Dyskinesia Diseases 0.000 description 2
- 208000014094 Dystonic disease Diseases 0.000 description 2
- 229920003160 Eudragit® RS PO Polymers 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 229920000544 Gore-Tex Polymers 0.000 description 2
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 2
- 208000019430 Motor disease Diseases 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 229930040373 Paraformaldehyde Natural products 0.000 description 2
- QGMRQYFBGABWDR-UHFFFAOYSA-M Pentobarbital sodium Chemical compound [Na+].CCCC(C)C1(CC)C(=O)NC(=O)[N-]C1=O QGMRQYFBGABWDR-UHFFFAOYSA-M 0.000 description 2
- SMWDFEZZVXVKRB-UHFFFAOYSA-N Quinoline Chemical compound N1=CC=CC2=CC=CC=C21 SMWDFEZZVXVKRB-UHFFFAOYSA-N 0.000 description 2
- 201000001880 Sexual dysfunction Diseases 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- 150000003973 alkyl amines Chemical class 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000000845 anti-microbial effect Effects 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 239000012267 brine Substances 0.000 description 2
- 229940084030 carboxymethylcellulose calcium Drugs 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 229920002301 cellulose acetate Polymers 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000004040 coloring Methods 0.000 description 2
- 230000002860 competitive effect Effects 0.000 description 2
- 229960000913 crospovidone Drugs 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 230000006735 deficit Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 125000004985 dialkyl amino alkyl group Chemical group 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 238000000835 electrochemical detection Methods 0.000 description 2
- 238000011067 equilibration Methods 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- 235000019634 flavors Nutrition 0.000 description 2
- 235000013350 formula milk Nutrition 0.000 description 2
- 210000001035 gastrointestinal tract Anatomy 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 210000001320 hippocampus Anatomy 0.000 description 2
- 230000036732 histological change Effects 0.000 description 2
- 238000010191 image analysis Methods 0.000 description 2
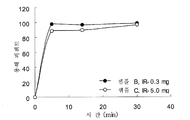
- 239000012728 immediate-release (IR) tablet Substances 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 239000007928 intraperitoneal injection Substances 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 229920003145 methacrylic acid copolymer Polymers 0.000 description 2
- 230000001537 neural effect Effects 0.000 description 2
- 230000006576 neuronal survival Effects 0.000 description 2
- 230000003287 optical effect Effects 0.000 description 2
- 229920002866 paraformaldehyde Polymers 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 229960002275 pentobarbital sodium Drugs 0.000 description 2
- 230000010412 perfusion Effects 0.000 description 2
- 239000000049 pigment Substances 0.000 description 2
- 229940068196 placebo Drugs 0.000 description 2
- 239000000902 placebo Substances 0.000 description 2
- 231100000614 poison Toxicity 0.000 description 2
- 239000002574 poison Substances 0.000 description 2
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 2
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 2
- 229920003124 powdered cellulose Polymers 0.000 description 2
- 235000019814 powdered cellulose Nutrition 0.000 description 2
- 210000002763 pyramidal cell Anatomy 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 231100000872 sexual dysfunction Toxicity 0.000 description 2
- 210000003625 skull Anatomy 0.000 description 2
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 210000003523 substantia nigra Anatomy 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 230000009747 swallowing Effects 0.000 description 2
- 208000016686 tic disease Diseases 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- GPRLSGONYQIRFK-MNYXATJNSA-N triton Chemical compound [3H+] GPRLSGONYQIRFK-MNYXATJNSA-N 0.000 description 2
- 210000002700 urine Anatomy 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 238000005303 weighing Methods 0.000 description 2
- LXJXRIRHZLFYRP-VKHMYHEASA-L (R)-2-Hydroxy-3-(phosphonooxy)-propanal Natural products O=C[C@H](O)COP([O-])([O-])=O LXJXRIRHZLFYRP-VKHMYHEASA-L 0.000 description 1
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-UHFFFAOYSA-N 2-(hydroxymethyl)-6-[4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxane-3,4,5-triol Chemical compound OCC1OC(OC2C(O)C(O)C(O)OC2CO)C(O)C(O)C1O GUBGYTABKSRVRQ-UHFFFAOYSA-N 0.000 description 1
- QZCLKYGREBVARF-UHFFFAOYSA-N Acetyl tributyl citrate Chemical compound CCCCOC(=O)CC(C(=O)OCCCC)(OC(C)=O)CC(=O)OCCCC QZCLKYGREBVARF-UHFFFAOYSA-N 0.000 description 1
- 229920002126 Acrylic acid copolymer Polymers 0.000 description 1
- 229920000178 Acrylic resin Polymers 0.000 description 1
- 239000004925 Acrylic resin Substances 0.000 description 1
- 229910002012 Aerosil® Inorganic materials 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 206010003547 Asterixis Diseases 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 206010008748 Chorea Diseases 0.000 description 1
- 241001573498 Compacta Species 0.000 description 1
- 229920002261 Corn starch Polymers 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- LXJXRIRHZLFYRP-VKHMYHEASA-N D-glyceraldehyde 3-phosphate Chemical compound O=C[C@H](O)COP(O)(O)=O LXJXRIRHZLFYRP-VKHMYHEASA-N 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- BWLUMTFWVZZZND-UHFFFAOYSA-N Dibenzylamine Chemical compound C=1C=CC=CC=1CNCC1=CC=CC=C1 BWLUMTFWVZZZND-UHFFFAOYSA-N 0.000 description 1
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical compound CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 229920003141 Eudragit® S 100 Polymers 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 229920002907 Guar gum Polymers 0.000 description 1
- 208000002972 Hepatolenticular Degeneration Diseases 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 229920002774 Maltodextrin Polymers 0.000 description 1
- 239000005913 Maltodextrin Substances 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 229920003093 Methocel™ K100 LV Polymers 0.000 description 1
- 208000002033 Myoclonus Diseases 0.000 description 1
- HTLZVHNRZJPSMI-UHFFFAOYSA-N N-ethylpiperidine Chemical compound CCN1CCCCC1 HTLZVHNRZJPSMI-UHFFFAOYSA-N 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 206010033296 Overdoses Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- 229920002845 Poly(methacrylic acid) Polymers 0.000 description 1
- 229920001030 Polyethylene Glycol 4000 Polymers 0.000 description 1
- 229920002594 Polyethylene Glycol 8000 Polymers 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 208000005793 Restless legs syndrome Diseases 0.000 description 1
- 229920001800 Shellac Polymers 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 208000027520 Somatoform disease Diseases 0.000 description 1
- 241000187747 Streptomyces Species 0.000 description 1
- 241000187433 Streptomyces clavuligerus Species 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 1
- 206010057040 Temperature intolerance Diseases 0.000 description 1
- 206010044074 Torticollis Diseases 0.000 description 1
- 208000000323 Tourette Syndrome Diseases 0.000 description 1
- 208000016620 Tourette disease Diseases 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 208000018839 Wilson disease Diseases 0.000 description 1
- 208000013142 Writer cramp Diseases 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- MZVQCMJNVPIDEA-UHFFFAOYSA-N [CH2]CN(CC)CC Chemical group [CH2]CN(CC)CC MZVQCMJNVPIDEA-UHFFFAOYSA-N 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- NWVHJWLIIAEGQF-UHFFFAOYSA-N acetic acid;cyclohex-3-ene-1,2-dicarboxylic acid Chemical compound CC(O)=O.OC(=O)C1CCC=CC1C(O)=O NWVHJWLIIAEGQF-UHFFFAOYSA-N 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 125000005041 acyloxyalkyl group Chemical group 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 1
- 125000005205 alkoxycarbonyloxyalkyl group Chemical group 0.000 description 1
- 125000000278 alkyl amino alkyl group Chemical group 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- CEGOLXSVJUTHNZ-UHFFFAOYSA-K aluminium tristearate Chemical compound [Al+3].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CEGOLXSVJUTHNZ-UHFFFAOYSA-K 0.000 description 1
- 229940063655 aluminum stearate Drugs 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- HOPRXXXSABQWAV-UHFFFAOYSA-N anhydrous collidine Natural products CC1=CC=NC(C)=C1C HOPRXXXSABQWAV-UHFFFAOYSA-N 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 230000002924 anti-infective effect Effects 0.000 description 1
- 229960005475 antiinfective agent Drugs 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 230000000949 anxiolytic effect Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 230000001174 ascending effect Effects 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 239000003781 beta lactamase inhibitor Substances 0.000 description 1
- 229940126813 beta-lactamase inhibitor Drugs 0.000 description 1
- 125000003460 beta-lactamyl group Chemical group 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 206010005159 blepharospasm Diseases 0.000 description 1
- 230000000744 blepharospasm Effects 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 125000002057 carboxymethyl group Chemical group [H]OC(=O)C([H])([H])[*] 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 229920003086 cellulose ether Polymers 0.000 description 1
- 208000012601 choreatic disease Diseases 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 229940032122 claris Drugs 0.000 description 1
- AGOYDEPGAOXOCK-KCBOHYOISA-N clarithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@](C)([C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)OC)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 AGOYDEPGAOXOCK-KCBOHYOISA-N 0.000 description 1
- 229960002626 clarithromycin Drugs 0.000 description 1
- 208000010877 cognitive disease Diseases 0.000 description 1
- 230000001149 cognitive effect Effects 0.000 description 1
- UTBIMNXEDGNJFE-UHFFFAOYSA-N collidine Natural products CC1=CC=C(C)C(C)=N1 UTBIMNXEDGNJFE-UHFFFAOYSA-N 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000008120 corn starch Substances 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 239000006059 cover glass Substances 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 125000006264 diethylaminomethyl group Chemical group [H]C([H])([H])C([H])([H])N(C([H])([H])*)C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000006222 dimethylaminomethyl group Chemical group [H]C([H])([H])N(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 210000001198 duodenum Anatomy 0.000 description 1
- 208000016570 early-onset generalized limb-onset dystonia Diseases 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 239000013020 final formulation Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 201000002865 focal hand dystonia Diseases 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 235000011389 fruit/vegetable juice Nutrition 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229960001031 glucose Drugs 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 150000002314 glycerols Chemical class 0.000 description 1
- 239000000665 guar gum Substances 0.000 description 1
- 235000010417 guar gum Nutrition 0.000 description 1
- 229960002154 guar gum Drugs 0.000 description 1
- 230000008543 heat sensitivity Effects 0.000 description 1
- 210000001661 hippocampal ca3 region Anatomy 0.000 description 1
- 239000010514 hydrogenated cottonseed oil Substances 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 229920013819 hydroxyethyl ethylcellulose Polymers 0.000 description 1
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 description 1
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 description 1
- 229920000639 hydroxypropylmethylcellulose acetate succinate Polymers 0.000 description 1
- 210000003405 ileum Anatomy 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 208000018197 inherited torticollis Diseases 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 125000000686 lactone group Chemical group 0.000 description 1
- 229910003002 lithium salt Inorganic materials 0.000 description 1
- 159000000002 lithium salts Chemical class 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000000845 maltitol Substances 0.000 description 1
- 235000010449 maltitol Nutrition 0.000 description 1
- VQHSOMBJVWLPSR-WUJBLJFYSA-N maltitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-WUJBLJFYSA-N 0.000 description 1
- 229940035436 maltitol Drugs 0.000 description 1
- 229940035034 maltodextrin Drugs 0.000 description 1
- 229960001855 mannitol Drugs 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 238000002552 multiple reaction monitoring Methods 0.000 description 1
- ACTNHJDHMQSOGL-UHFFFAOYSA-N n',n'-dibenzylethane-1,2-diamine Chemical compound C=1C=CC=CC=1CN(CCN)CC1=CC=CC=C1 ACTNHJDHMQSOGL-UHFFFAOYSA-N 0.000 description 1
- 230000032405 negative regulation of neuron apoptotic process Effects 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 230000001272 neurogenic effect Effects 0.000 description 1
- 230000000926 neurological effect Effects 0.000 description 1
- 230000004693 neuron damage Effects 0.000 description 1
- 230000007557 neuronal destruction Effects 0.000 description 1
- 230000003961 neuronal insult Effects 0.000 description 1
- 239000002858 neurotransmitter agent Substances 0.000 description 1
- 210000002589 oculomotor nerve Anatomy 0.000 description 1
- 235000019645 odor Nutrition 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 239000008203 oral pharmaceutical composition Substances 0.000 description 1
- 208000027753 pain disease Diseases 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- PNJWIWWMYCMZRO-UHFFFAOYSA-N pent‐4‐en‐2‐one Natural products CC(=O)CC=C PNJWIWWMYCMZRO-UHFFFAOYSA-N 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 210000001428 peripheral nervous system Anatomy 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 125000005498 phthalate group Chemical class 0.000 description 1
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 1
- 125000005633 phthalidyl group Chemical group 0.000 description 1
- 229920000191 poly(N-vinyl pyrrolidone) Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229940100467 polyvinyl acetate phthalate Drugs 0.000 description 1
- 229920001592 potato starch Polymers 0.000 description 1
- 238000011533 pre-incubation Methods 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 201000002212 progressive supranuclear palsy Diseases 0.000 description 1
- 210000001176 projection neuron Anatomy 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 229960004063 propylene glycol Drugs 0.000 description 1
- 235000013772 propylene glycol Nutrition 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 208000020016 psychiatric disease Diseases 0.000 description 1
- 239000008213 purified water Substances 0.000 description 1
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000011808 rodent model Methods 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 239000004208 shellac Substances 0.000 description 1
- 229940113147 shellac Drugs 0.000 description 1
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 1
- 235000013874 shellac Nutrition 0.000 description 1
- 150000003378 silver Chemical class 0.000 description 1
- 208000019116 sleep disease Diseases 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000008279 sol Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 239000001593 sorbitan monooleate Substances 0.000 description 1
- 235000011069 sorbitan monooleate Nutrition 0.000 description 1
- 229940035049 sorbitan monooleate Drugs 0.000 description 1
- 229960002920 sorbitol Drugs 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 238000013112 stability test Methods 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 229940071138 stearyl fumarate Drugs 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 150000003890 succinate salts Chemical class 0.000 description 1
- 150000005846 sugar alcohols Chemical class 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- IHCDKJZZFOUARO-UHFFFAOYSA-M sulfacetamide sodium Chemical compound O.[Na+].CC(=O)[N-]S(=O)(=O)C1=CC=C(N)C=C1 IHCDKJZZFOUARO-UHFFFAOYSA-M 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- GFYHSKONPJXCDE-UHFFFAOYSA-N sym-collidine Natural products CC1=CN=C(C)C(C)=C1 GFYHSKONPJXCDE-UHFFFAOYSA-N 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 235000019640 taste Nutrition 0.000 description 1
- 229960003865 tazobactam Drugs 0.000 description 1
- LPQZKKCYTLCDGQ-WEDXCCLWSA-N tazobactam Chemical compound C([C@]1(C)S([C@H]2N(C(C2)=O)[C@H]1C(O)=O)(=O)=O)N1C=CN=N1 LPQZKKCYTLCDGQ-WEDXCCLWSA-N 0.000 description 1
- OHKOGUYZJXTSFX-KZFFXBSXSA-N ticarcillin Chemical compound C=1([C@@H](C(O)=O)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)C=CSC=1 OHKOGUYZJXTSFX-KZFFXBSXSA-N 0.000 description 1
- 229960004659 ticarcillin Drugs 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- 229960005196 titanium dioxide Drugs 0.000 description 1
- 235000010215 titanium dioxide Nutrition 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 235000019731 tricalcium phosphate Nutrition 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229940100445 wheat starch Drugs 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1682—Processes
- A61K9/1694—Processes resulting in granules or microspheres of the matrix type containing more than 5% of excipient
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/397—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having four-membered rings, e.g. azetidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/42—Oxazoles
- A61K31/424—Oxazoles condensed with heterocyclic ring systems, e.g. clavulanic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/2027—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2054—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2095—Tabletting processes; Dosage units made by direct compression of powders or specially processed granules, by eliminating solvents, by melt-extrusion, by injection molding, by 3D printing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2806—Coating materials
- A61K9/2833—Organic macromolecular compounds
- A61K9/284—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone
- A61K9/2846—Poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Psychology (AREA)
- Hospice & Palliative Care (AREA)
- Pain & Pain Management (AREA)
- Psychiatry (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines Containing Plant Substances (AREA)
- Nitrogen And Oxygen Or Sulfur-Condensed Heterocyclic Ring Systems (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US17384109P | 2009-04-29 | 2009-04-29 | |
| US61/173,841 | 2009-04-29 | ||
| PCT/US2010/032983 WO2010127125A1 (en) | 2009-04-29 | 2010-04-29 | Clavulanate formulation for neuroprotection and treatment of neurodegenerative disorders |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20120012823A true KR20120012823A (ko) | 2012-02-10 |
Family
ID=42261971
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117028280A Withdrawn KR20120012823A (ko) | 2009-04-29 | 2010-04-29 | 신경보호 및 퇴행성 신경 질환 치료용 클라블라네이트 제제 |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US20100255099A1 (enExample) |
| EP (1) | EP2424498A1 (enExample) |
| JP (1) | JP2012525427A (enExample) |
| KR (1) | KR20120012823A (enExample) |
| CN (1) | CN102413814A (enExample) |
| AU (1) | AU2010242948A1 (enExample) |
| BR (1) | BRPI1013901A2 (enExample) |
| CA (1) | CA2758029A1 (enExample) |
| IL (1) | IL215940A0 (enExample) |
| MX (1) | MX2011011459A (enExample) |
| WO (1) | WO2010127125A1 (enExample) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BR0013366A (pt) * | 1999-08-16 | 2002-07-23 | Revaax Pharmaceuticals Llc | Métodos para tratar um distúrbio comportamental, um paciente humano, doença de próstata, distúrbios de ansiedade e cognitivos em um paciente humano afligido com uma condição ou disposto ao desenvolvimento de uma condição distinguida pelo menos em parte pela concentração de glutamato extracelular anormal no cérebro ou em outro tecido nervoso, distúrbio comportamental nas espécies humana, canina, felina e equina e um paciente afligido com ou disposto a desenvolver uma doença compreendendo concentrações de glutamato anormalmente elevadas em tecido neuronal ou nìveis de naaladase elevados em tecido prostático e com esclerose múltipla e para realçar a função cognitiva, formulação farmacêutica e usos de um inibidor da atividade de peptidase de uma dipeptidase ácida ligada em alfa n-acetilada e de um composto de beta-lactama |
| US6426342B2 (en) * | 1999-08-16 | 2002-07-30 | Revaax Pharmaceuticals, Llc | Use of β-lactamase inhibitors as neuroprotectants |
| MX2010004556A (es) * | 2007-10-26 | 2010-07-01 | Rexahn Pharmaceuticals Inc | Formulacion farmaceutica de acido clavulanico. |
| WO2013006808A2 (en) * | 2011-07-06 | 2013-01-10 | Rexahn Pharmaceuticals, Inc. | Clavulanic acid for treatment of restless legs syndrome |
| US8978166B2 (en) * | 2012-08-27 | 2015-03-17 | Well & David Corp. | Multi-function garment |
| KR102541110B1 (ko) | 2014-08-25 | 2023-06-08 | 에이뮨 테라퓨틱스, 인코퍼레이티드 | 난 단백질 제형 및 이의 제조 방법 |
| AU2016278846B2 (en) * | 2015-06-19 | 2021-08-05 | Biotie Therapies, Inc. | Controlled-release tozadenant formulations |
| WO2020212418A1 (en) * | 2019-04-15 | 2020-10-22 | Nanologica Ab | Empty porous particles for use in treatment, prevention and/or postponement of degeneration of neurodegenerative diseases, neurons and glia. |
| TWI739220B (zh) * | 2019-11-26 | 2021-09-11 | 亞瑟瑞智科技管理顧問股份有限公司 | 包含克拉維酸與丙戊酸之組合物及其用途 |
| CN112843034B (zh) * | 2019-11-26 | 2022-08-23 | 亚瑟瑞智科技管理顾问股份有限公司 | 包含克拉维酸与丙戊酸的组合物及其用途 |
Family Cites Families (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4110165A (en) * | 1974-04-20 | 1978-08-29 | Beecham Group Limited | Process for the production of clavulanic acid |
| CA1085392A (en) * | 1976-03-25 | 1980-09-09 | Masayuki Narisada | Arylmalonamido-1-oxadethiacephalosporins |
| US4127118B1 (en) * | 1977-03-16 | 1995-12-19 | Alvaro Latorre | Method of effecting and enhancing an erection |
| US4234579A (en) * | 1977-06-07 | 1980-11-18 | Pfizer Inc. | Penicillanic acid 1,1-dioxides as β-lactamase inhibitors |
| JO984B1 (en) * | 1977-10-11 | 1979-12-01 | بيتشام غروب ليمتد | A dry pharmaceutical compound with a suitable dosage unit for oral administration |
| NZ189022A (en) * | 1977-12-08 | 1981-11-19 | Beecham Group Ltd | Pharmaceutically acceptable particles of clavulanates dispersed in a polymeric binder |
| US4273763A (en) * | 1978-01-23 | 1981-06-16 | Efamol Limited | Pharmaceutical and dietary compositions |
| US4268503A (en) * | 1978-09-14 | 1981-05-19 | Fujisawa Pharmaceutical Co., Ltd. | Antibacterial composition |
| DE3001961C2 (de) * | 1980-01-21 | 1984-08-16 | Didier Engineering Gmbh, 4300 Essen | Anströmboden für einen Wirbelschichtreaktor |
| NZ198241A (en) * | 1980-09-27 | 1983-12-16 | Beecham Group Ltd | Tablet containing amoxycillin and potassium clavulanate |
| US4594247A (en) * | 1981-12-21 | 1986-06-10 | Eli Lilly And Company | Synergistic antibacterial compositions and method of treatment of infections caused by multiple antibiotic-resistant organisms |
| JPS59104389A (ja) * | 1982-12-06 | 1984-06-16 | Shionogi & Co Ltd | オキサセファム誘導体 |
| JPS62106015A (ja) * | 1985-10-31 | 1987-05-16 | Sumitomo Pharmaceut Co Ltd | 抗痴呆薬 |
| US5256652A (en) * | 1987-11-12 | 1993-10-26 | Pharmedic Co. | Topical compositions and methods for treatment of male impotence |
| GB9201639D0 (en) * | 1992-01-25 | 1992-03-11 | Smithkline Beecham Plc | Pharmaceutical formulations |
| GB9215908D0 (en) * | 1992-07-27 | 1992-09-09 | Wellcome Found | Water dispersible tablets |
| US5643909A (en) * | 1993-04-19 | 1997-07-01 | Syntex (U.S.A.) Inc. | 10,11-Methanodibenzosuberane derivatives |
| JP3233407B2 (ja) * | 1993-11-06 | 2001-11-26 | 大鵬薬品工業株式会社 | 結晶性ペニシリン誘導体並びにその製造及び使用 |
| US5827537A (en) * | 1995-05-04 | 1998-10-27 | Smithkline Beecham Corporation | Pharmaceutical thermal infusion process |
| GB9515411D0 (en) * | 1995-07-27 | 1995-09-27 | Pharmacia Spa | N-(4-substituted-benzyl)-2-aminolactam derivatives |
| GB9525697D0 (en) * | 1995-12-15 | 1996-02-14 | Pharmacia Spa | Cephem derivatives |
| ES2248840T3 (es) * | 1996-02-23 | 2006-03-16 | Eli Lilly And Company | Antagonistas de vasopresina v1a no peptidilicos. |
| US5905076A (en) * | 1996-04-10 | 1999-05-18 | National Research Council Of Canada | 6-substituted amino-4-oxa-1-azabicyclo 3,2,0! heptan-7-one derivatives as cysteine protease inhibitors |
| US5795877A (en) * | 1996-12-31 | 1998-08-18 | Guilford Pharmaceuticals Inc. | Inhibitors of NAALADase enzyme activity |
| US5824662A (en) * | 1996-09-27 | 1998-10-20 | Guilford Pharmaceuticals Inc. | Treatment of global and focal ischemia using naaladase inhibitors |
| US5977090A (en) * | 1996-09-27 | 1999-11-02 | Guilford Pharmaceuticals Inc. | Pharmaceutical compositions and methods of treating compulsive disorders using NAALADase inhibitors |
| US5863536A (en) * | 1996-12-31 | 1999-01-26 | Guilford Pharmaceuticals Inc. | Phosphoramidate derivatives |
| US6017903A (en) * | 1996-09-27 | 2000-01-25 | Guilford Pharmaceuticals Inc. | Pharmaceutical compositions and methods of treating a glutamate abnormality and effecting a neuronal activity in an animal using NAALADase inhibitors |
| US5672592A (en) * | 1996-06-17 | 1997-09-30 | Guilford Pharmaceuticals Inc. | Certain phosphonomethyl-pentanedioic acid derivatives thereof |
| JP2002504122A (ja) * | 1997-06-13 | 2002-02-05 | ノースウエスタン ユニバーシティー | ベータラクタマーゼ阻害剤及びその使用方法 |
| US6015809A (en) * | 1998-08-17 | 2000-01-18 | American Home Products Corporation | Photocyclized rapamycin |
| US6177421B1 (en) * | 1999-05-04 | 2001-01-23 | Fuisz International Ltd. | Amoxicillin and clavulanate composition |
| WO2000045793A1 (en) * | 1999-02-04 | 2000-08-10 | Abbott Laboratories | Ph independent extended release pharmaceutical formulation |
| IE990159A1 (en) * | 1999-02-26 | 2000-09-20 | Fuisz Internat Ltd | Storage Stable Amoxycillin and Clavulanate Suspension Composition. |
| US6472406B1 (en) * | 1999-07-06 | 2002-10-29 | Methylgene, Inc. | Sulfonamidomethyl phosphonate inhibitors of beta-lactamase |
| BR0013366A (pt) * | 1999-08-16 | 2002-07-23 | Revaax Pharmaceuticals Llc | Métodos para tratar um distúrbio comportamental, um paciente humano, doença de próstata, distúrbios de ansiedade e cognitivos em um paciente humano afligido com uma condição ou disposto ao desenvolvimento de uma condição distinguida pelo menos em parte pela concentração de glutamato extracelular anormal no cérebro ou em outro tecido nervoso, distúrbio comportamental nas espécies humana, canina, felina e equina e um paciente afligido com ou disposto a desenvolver uma doença compreendendo concentrações de glutamato anormalmente elevadas em tecido neuronal ou nìveis de naaladase elevados em tecido prostático e com esclerose múltipla e para realçar a função cognitiva, formulação farmacêutica e usos de um inibidor da atividade de peptidase de uma dipeptidase ácida ligada em alfa n-acetilada e de um composto de beta-lactama |
| US6489319B2 (en) * | 1999-08-16 | 2002-12-03 | Revaax Pharmaceuticals, Llc | Neurotherapeutic use of carboxypeptidase inhibitors |
| US6426342B2 (en) * | 1999-08-16 | 2002-07-30 | Revaax Pharmaceuticals, Llc | Use of β-lactamase inhibitors as neuroprotectants |
| NZ519467A (en) * | 1999-12-22 | 2004-02-27 | Pharmacia Corp | Dual-release compositions of a cyclooxygenase-2- inhibitor |
| US20020013270A1 (en) * | 2000-06-05 | 2002-01-31 | Bolte Ellen R. | Method for treating a mental disorder |
| ATE286057T1 (de) * | 2000-10-20 | 2005-01-15 | Sandoz Ag | Pharmazeutische zubereitungen enthaltend clavulansäure |
| US7166626B2 (en) * | 2001-06-18 | 2007-01-23 | Revaax Pharmaceuticals, Llc | Therapeutic treatment for sexual dysfunction |
| PL371407A1 (en) * | 2002-01-10 | 2005-06-13 | Biovail Laboratories Inc. | Sedative non-benzodiazepine formulations |
| IL154370A0 (en) * | 2003-02-10 | 2003-09-17 | Chemagis Ltd | Solid amorphous mixtures, processes for the preparation thereof and pharmaceutical compositions containing the same |
| SI21402A (sl) * | 2003-02-12 | 2004-08-31 | LEK farmacevtska dru�ba d.d. | Obloženi delci in farmacevtske oblike |
| US7833998B2 (en) * | 2003-08-25 | 2010-11-16 | Revaax Pharmaceuticals, Llc | Oral neurotherapeutic cephalosporin sulfoxide and sulfone-containing compositions |
| PE20060489A1 (es) * | 2004-08-13 | 2006-06-19 | Schering Plough Ltd | Formulacion farmaceutica que comprende un antibiotico, un triazol y un corticosteroide |
| US20060088591A1 (en) * | 2004-10-22 | 2006-04-27 | Jinghua Yuan | Tablets from a poorly compressible substance |
| SI21912A (sl) * | 2004-12-24 | 2006-06-30 | Lek Farmacevtska Druzba D.D. | Stabilne farmacevtske oblike, ki vsebujejo amoksicilin in klavulansko kislino |
| DE102006007830A1 (de) * | 2006-02-17 | 2007-08-30 | Grünenthal GmbH | Lagerstabile orale Darreichungsform von Amoxicillin und Clavulansäure |
| CA2644911A1 (en) * | 2006-03-24 | 2007-10-04 | Panacea Biotec Ltd. | Antibiotic compositions of modified release and process of production thereof |
| US20080014257A1 (en) * | 2006-07-14 | 2008-01-17 | Par Pharmaceutical, Inc. | Oral dosage forms |
| MX2010004556A (es) * | 2007-10-26 | 2010-07-01 | Rexahn Pharmaceuticals Inc | Formulacion farmaceutica de acido clavulanico. |
-
2010
- 2010-04-29 US US12/770,304 patent/US20100255099A1/en not_active Abandoned
- 2010-04-29 MX MX2011011459A patent/MX2011011459A/es unknown
- 2010-04-29 WO PCT/US2010/032983 patent/WO2010127125A1/en not_active Ceased
- 2010-04-29 EP EP10717387A patent/EP2424498A1/en not_active Withdrawn
- 2010-04-29 AU AU2010242948A patent/AU2010242948A1/en not_active Abandoned
- 2010-04-29 CA CA2758029A patent/CA2758029A1/en not_active Abandoned
- 2010-04-29 CN CN2010800184471A patent/CN102413814A/zh active Pending
- 2010-04-29 JP JP2012508734A patent/JP2012525427A/ja active Pending
- 2010-04-29 BR BRPI1013901A patent/BRPI1013901A2/pt not_active IP Right Cessation
- 2010-04-29 KR KR1020117028280A patent/KR20120012823A/ko not_active Withdrawn
-
2011
- 2011-10-26 IL IL215940A patent/IL215940A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| BRPI1013901A2 (pt) | 2019-09-24 |
| AU2010242948A1 (en) | 2011-11-24 |
| IL215940A0 (en) | 2012-01-31 |
| MX2011011459A (es) | 2011-11-18 |
| EP2424498A1 (en) | 2012-03-07 |
| CA2758029A1 (en) | 2011-11-04 |
| US20100255099A1 (en) | 2010-10-07 |
| WO2010127125A1 (en) | 2010-11-04 |
| CN102413814A (zh) | 2012-04-11 |
| JP2012525427A (ja) | 2012-10-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20120012823A (ko) | 신경보호 및 퇴행성 신경 질환 치료용 클라블라네이트 제제 | |
| US4404183A (en) | Sustained release pharmaceutical composition of solid medical material | |
| US9572781B2 (en) | Orally disintegrating tablet compositions comprising combinations of non-opioid and opioid analgesics | |
| US10441585B2 (en) | Formulations containing nalbuphine and uses thereof | |
| US20100291225A1 (en) | Stabilized Sustained Release Composition of Bupropion Hydrochloride and Process For Preparing the Same | |
| CA2742680C (en) | Pharmaceutical compositions for release control of methylphenidate | |
| SK369292A3 (en) | Orally administerable drugs for the treatment of central dopamine deficiency conditions | |
| ES2560052T3 (es) | Nueva combinación | |
| US20090270358A1 (en) | Pharmaceutical formulation of clavulanic acid | |
| MXPA06000529A (es) | Formulaciones farmaceuticas utiles para inhibir la secrecion de acido y metodos para elaborarlas y utilizarlas. | |
| US11234962B2 (en) | Pharmaceutical composition and method of manufacturing the same | |
| KR20050009983A (ko) | 트라마돌의 서방성 제제 | |
| EP4268816A1 (en) | Oral solid preparation | |
| US20050181055A1 (en) | Pharmaceutical compositions of quinapril | |
| EP4313155A1 (fr) | Formulation par voie orale d'ivermectine et utilisations | |
| JPH0236571B2 (ja) | Jizokuseiseizai | |
| HK40099642A (en) | Oral solid preparation | |
| NZ625506B2 (en) | Compositions For Treatment of Heart Failure in Dogs. | |
| KR20080071286A (ko) | 암로디핀 함유 입자 및 그것을 포함하는 구강 내 붕괴정 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20111128 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |